Abstract
This case report details an unusual and unexpected finding in a 37-year-old woman with a history of two cesarean sections, who had an intrauterine device implanted. The patient presented with symptoms of abnormal uterine bleeding and dysmenorrhea. An initial ultrasound confirmed the presence of the intrauterine device. However, a startling discovery was made during the intrauterine device removal procedure: fetal bones were found within the patient. This case underscores the importance of thorough diagnostic evaluations in gynecological practice, particularly when dealing with patients who have complex gynecological histories. Significantly, it brings to light the necessity of employing hysteroscopy for comprehensive diagnostic assessment in cases of abnormal uterine bleeding post-abortion. This approach could aid in identifying rare and unexpected findings, such as retained fetal bones, which might be missed by conventional ultrasound. The report emphasizes the need for vigilance and thoroughness in gynecological examinations and contributes to the understanding of potential complications and anomalies associated with intrauterine device usage and post-abortion care.
Keywords: Fetal bone retention, intrauterine device misdiagnosis, uterine hemorrhage, gynecological emergency, ultrasound diagnostic limits
Introduction
Intrauterine devices (IUDs) are widely recognized for their efficacy and safety as a contraceptive method, providing extended protection against unwanted pregnancies. Studies, including the comprehensive analysis by Smith et al., 1 have affirmed the high effectiveness of IUDs, with a 99% success rate in preventing pregnancies. Johnson and Roberts 2 further supported this notion by highlighting the minimal adverse reactions associated with modern IUDs, making them a safe option for a vast majority of women, including those who have not experienced childbirth.
Despite their popularity and general safety, IUDs are not without complications. Lee and Park 3 have pointed out rare but significant issues such as perforation, migration, and embedding within the uterine wall. Martin and Brown 4 discussed the association of IUDs with intrauterine infections that could lead to further medical complexities. Moreover, Brandon et al. 5 emphasized the role of ultrasounds in locating misplaced IUDs, though they acknowledge limitations in diagnosing specific complications related to IUD placement. Turner and Adams, 6 and subsequent literature,7,8 underscore the potential for IUDs to obscure other gynecological conditions, thereby posing diagnostic challenges.
However, some cases extend beyond the usual scope of complications associated with IUDs. This report introduces a rare and unusual finding during a routine gynecological examination for IUD-related issues—the discovery of retained fetal bones. The literature regarding such extraordinary findings, while sparse, sheds light on the complexity and unpredictability of gynecological examinations. In this report, we discuss a distinctive case where a patient with a history of IUD implantation presented with severe symptoms, leading to an unexpected discovery that underscores the need for thorough diagnostic processes in gynecological care.
Case report
Background: The patient is a 37-year-old multiparous woman (G2P2). Her medical history includes two cesarean sections performed due to fetal-pelvic disproportion.
Current concerns: The patient reports abnormal uterine bleeding (AUB) and dysmenorrhea but cannot recall the exact dates of IUD insertion or removal.
Clinical findings
Examination results: The abdominal examination was unremarkable. Pelvic examination did not reveal any notable abnormalities.
Imaging findings: Recent pelvic ultrasonography showed an enlarged uterus with multiple myomas, with the largest at the fundus. 9 The endometrium appeared distorted, containing a foreign object presumed to be an IUD, with heterogeneous, hyperechoic contents (Figure 1).
Figure1.
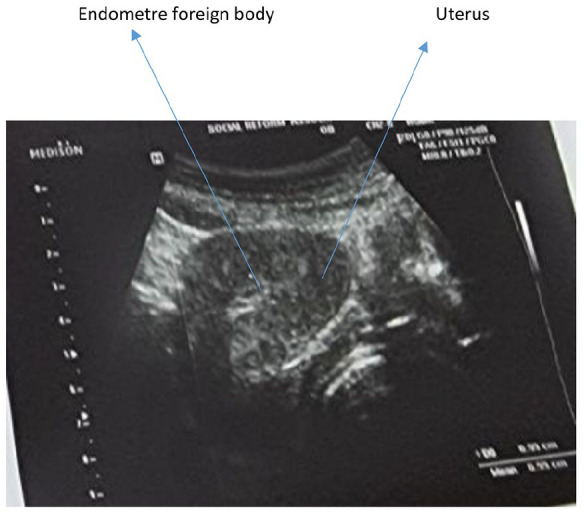
Pelvic ultrasound.
Timeline
The patient, a 37-year-old woman with a history of two cesarean sections, initially presents with symptoms of AUB and dysmenorrhea. The exact dates of her IUD insertion and removal are unclear because the patient could not remember anything about IUD and previous abortion, highlighting a gap in the patient’s contraceptive history.
Diagnostic assessment
Blood tests: Results indicated elevated White Blood Cell (WBC) count, low Hemoglobin (Hb), and Beta Human Chorionic Gonadotropin (BHCG) levels suggestive of an ongoing inflammatory or pregnancy-related process.
The adnexa appeared normal on both sides. Blood tests showed the following results: WBC count at 17,000.10 e3/dL, Hb at 8.2 g/dL, Hematocrit (Hct) at 24.6%, Platelets (Plt) at 258.10 e3/dL, and Beta HCG at 1690 mIU/mL. A Pap smear was negative for cancer or intraepithelial lesions but indicated significant inflammation. Pre-surgery, antibiotic therapy with Tazocin 1G was initiated.
During her hospitalization, the patient underwent dilatation and curettage as treatment. The procedure, initially aimed at removing what was presumed to be the IUD, led to the discovery of dense tissue resembling fetal bones. Subsequent extraction efforts were conducted, utilizing suction and ring forceps to thoroughly remove all fetal tissues from the patient (Figure 2).
Figure2.
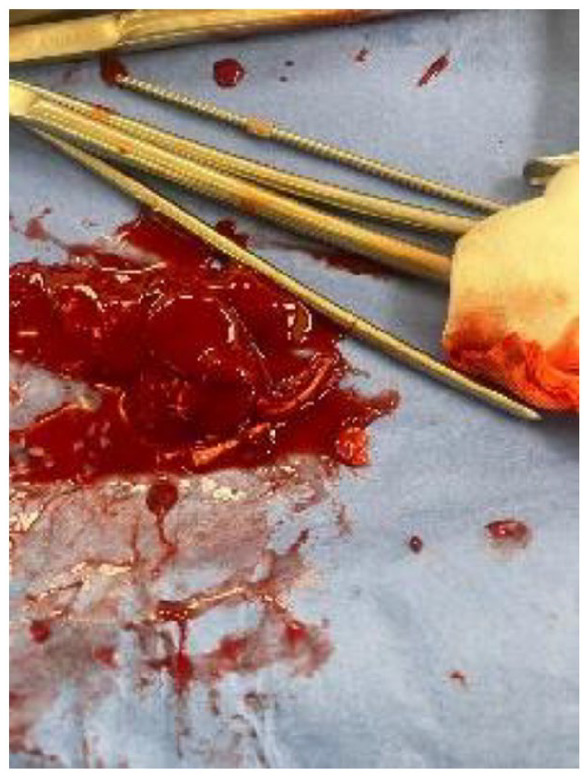
Clots of blood with fetal bones.
Therapeutic intervention
Surgical procedure: The patient underwent dilatation and curettage, intended for IUD removal, but fetal bones were discovered instead. Challenges in this procedure, such as the inability to use a hysteroscope due to bleeding and the need for careful extraction of the fetal tissue, are significant (Figure 2).
The use of a hysteroscope was not feasible due to increased bleeding experienced by the patient prior to the operation. To manage the ensuing uncontrollable AUB, an intrauterine Foley catheter filled with 100 ml of Normal Saline Solution was implanted, successfully halting the bleeding.
Management of complications
The insertion of an intrauterine Foley catheter to control bleeding and the need for blood transfusions highlight the complexity of the surgery.
During the surgical procedure, the patient received transfusions of 2 units of Fresh Frozen Plasma and 2 units of packed red blood cells. Postoperative blood tests, following the transfusion, indicated:
Hb: 8.2 g/dL
Hct: 24.3%
Plt: 170.10e3/dL
WBCs: 9.89.10e3/dL
D-Dimer: 3.47 ng/mL
Fibrinogen: 3.74 g/dL
Prothrombin Time/Partial Thromboplastin Time: Normal
BHCG: 242.4 mIU/mL.
Follow-up and outcomes
Postoperative considerations and management of the missing IUD
Following the surgical procedure, which successfully resulted in the removal of fetal bone fragments and the subsequent confirmation of an empty uterine cavity, attention was turned toward the unresolved issue of the missing IUD. The patient, having limited recollection of her IUD history and facing financial constraints, presented a unique challenge in our postoperative management strategy.
Understanding the importance of locating the missing IUD while being mindful of the patient’s financial situation, we recommended an abdominal and pelvic X-ray as a cost-effective approach to this dilemma. This decision was based on a balanced consideration of clinical efficacy and patient affordability, ensuring that the investigation into the IUD’s location was both thorough and accessible.
The rationale for choosing X-ray imaging postoperation stems from its utility in detecting metallic objects within the body, offering a practical method to ascertain whether the IUD had migrated outside the uterine cavity. This approach aligns with our commitment to providing comprehensive, patient-centered care, especially in managing complex cases where financial barriers may impact the patient’s ability to receive follow-up care.
We advised the patient to undergo the recommended X-ray examination as a crucial next step in her postoperative care. This not only serves the immediate purpose of locating the missing IUD but also reinforces our holistic approach to patient care, taking into consideration both the medical and socio-economic factors influencing the patient’s health journey.
Postoperative care: following the significant reduction in Beta HCG levels from 1690 mIU/mL to 242.4 mIU/mL after surgery, we have advised the patient to undergo weekly Beta HCG testing. This is to continue until the Beta HCG levels reach a definitive value of 0 mIU/mL, thereby confirming the absence of any residual trophoblastic tissue and ensuring the complete resolution of the pregnancy-related process. This approach is in strict adherence to best practices for postoperative care in such complex cases, ensuring both the physical health of the patient and her peace of mind. In addition to the biochemical monitoring, the patient is scheduled for regular clinical assessments. These appointments serve to evaluate her recovery progress, manage any postoperative symptoms, and address any concerns that may arise during the follow-up period. Our comprehensive approach underscores our commitment to not only resolving the immediate clinical issue but also to providing a continuum of care that supports the patient’s overall health and well-being post-surgery.
- Long-term considerations: In light of the patient’s complex medical history, including her recent surgical intervention, the presence of multiple myomas, and an IUD complication, a holistic and forward-looking management plan is paramount for ensuring her long-term health and well-being. The following areas of focus are critical in developing a comprehensive care strategy:
- Comprehensive gynecological monitoring: Regular, detailed gynecological examinations are essential to closely monitor the patient’s uterine health, particularly the status of the myomas and any potential changes in the uterine environment caused by the IUD. Ultrasound examinations should be scheduled semi-annually or annually, as deemed appropriate, to track myoma growth or regression and assess the uterine lining for any signs of pathology. Early detection of changes can facilitate timely interventions, minimizing the risk of complications.
- Fertility and reproductive planning: Given the patient’s reproductive history and current gynecological findings, personalized fertility counseling should be provided. This counseling should address the potential impact of myomas on fertility, including risks related to myoma size and location, as well as considerations for future pregnancies given the patient’s history of cesarean deliveries. Discussions should explore the patient’s reproductive desires, with information on options such as natural conception, the potential need for fertility treatments, and the safety of pregnancy after myoma treatment or removal.
- Contraceptive review: A careful reassessment of contraceptive choices, prioritizing methods that align with the patient’s health status, preferences, and future fertility plans.
- Targeted management of symptomatic conditions: Ongoing treatment and management of AUB and dysmenorrhea are imperative for enhancing the patient’s quality of life. A multidisciplinary approach involving medication, hormonal treatments, or further surgical options, such as hysteroscopic myomectomy, may be considered based on symptom severity, myoma characteristics, and the patient’s desire for fertility preservation. Regular assessment of symptom management effectiveness is vital to ensure that the treatment remains aligned with the patient’s health status and quality of life objectives.
- Lifestyle adjustments: Recommendations for a healthy lifestyle that supports uterine health, including diet, exercise, and management of any contributing health conditions.
- Psychosocial support: Access to psychological support and education to help manage the impacts of her condition on her mental and emotional well-being.
Histological examination
The removed tissue was confirmed to contain placental and macerated fetal parts, an unusual finding that underscores the uniqueness of this case (Figures 3–5).
The histological examination of the surgically removed tissue revealed a composite of placental tissue and macerated fetal parts. This analysis confirmed the presence of both chorionic villi and decidual cells, indicative of placental material. Furthermore, the identification of macerated fetal parts, characterized by fragmented and degenerated fetal tissue, underscores the prolonged retention of these elements within the uterine cavity.
Figure3.

Fetal bones.
Figure 4.
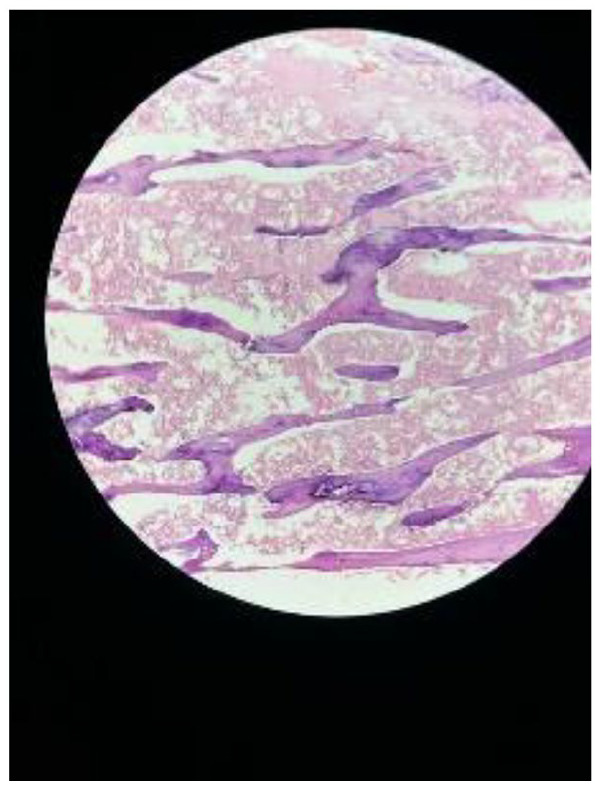
Slide of histology.
Figure 5.
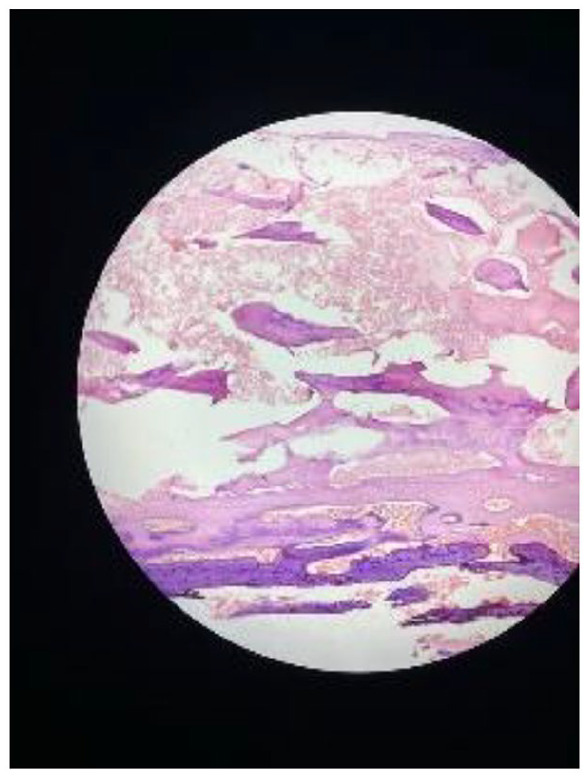
Slide of histology.
Microscopic findings
Chorionic villi (placenta)
The histological presence of chorionic villi, with their distinctive tree-like structure, was noted. These villi showed varying degrees of hyalinization and calcification, suggesting a chronic process of degeneration and mineralization. Such changes are consistent with long-standing retention and are a rare histopathological finding in cases of retained products of conception (RPOC).
Decidual cells
The examination also highlighted decidual cells, which are part of the endometrial lining that undergoes significant changes during pregnancy to support the placenta. The presence of these cells further corroborates the pregnancy-related origin of the retained tissue.
Macerated fetal tissue
The fetal parts exhibited signs of maceration, a process that occurs when fetal tissue remains in an aqueous environment post-demise, leading to autolysis and degeneration. This finding is indicative of the advanced stage of retention and the absence of a viable pregnancy.
Discussion
Clinical implications of bone fragment retrieval
The extraction of multiple bone fragments, with one embedded in the myometrium, presents a significant clinical challenge. This is rarely documented in literature and poses questions regarding the mechanisms of embedding and long-term retention within the uterine tissue.
The extraction of bone fragments from the uterus, especially when one is embedded in the myometrium, presents a notable clinical challenge that is seldom documented in the literature. This rarity raises questions about the mechanisms of embedding and long-term retention within uterine tissue, necessitating a nuanced approach to patient management. Here are key recommendations for gynecologists and other healthcare professionals to enhance the clinical management of similar cases:
Enhanced diagnostic techniques
Advanced imaging modalities such as transvaginal ultrasound and magnetic resonance imaging (MRI) are crucial for accurately locating and assessing the extent of bone fragment embedding, essential for planning the appropriate surgical approach. Hysteroscopy serves not only as a diagnostic tool but also as a means for direct visualization and removal of accessible bone fragments.
Surgical expertise
The complexities involved in cases with embedded bone fragments may require the expertise of a gynecological surgeon skilled in minimally invasive techniques. Surgical planning must account for potential challenges, including the risk of significant bleeding or the need for precise dissection to remove bone fragments without causing extensive damage to the uterine tissue.
Interdisciplinary collaboration
Collaboration with radiologists is important for preoperative planning, and with pathologists for the postoperative analysis of the removed fragments. This collaboration extends to fertility specialists if the patient has future pregnancy aspirations, discussing the impact of the procedure on fertility and strategies against recurrence.
Patient counseling and support
It is imperative to provide patients with comprehensive counseling about the potential impact of retained bone fragments and the surgical removal process on their reproductive health and future fertility. Psychological support can help patients manage the emotional stress associated with these rare and complex gynecological issues.
Follow-up and monitoring
A structured follow-up protocol is essential to monitor the patient’s recovery post-surgery and to early detect any signs of recurrence or complications. Regular postoperative imaging studies are recommended to ensure the complete removal of bone fragments and to assess the healing of the uterine tissue.
Documentation and research
Detailed documentation of such rare cases, including surgical approaches, findings, and outcomes, contributes valuable knowledge to the existing literature. There is also a need for research into the mechanisms behind the embedding and long-term retention of bone fragments within uterine tissue to improve prevention and management strategies.
Patient’s recollection and symptom presentation
The patient’s clinical presentation, characterized by symptoms of dysmenorrhea and vaginal bleeding accompanied by a positive pregnancy test, indeed raises the suspicion of several gynecological conditions beyond the unexpected finding of retained fetal bones. The differential diagnosis in such cases, particularly when an IUD is involved, and no intrauterine pregnancy is identified, would notably include the following.
Ectopic pregnancy
Given the positive pregnancy test and the absence of an intrauterine pregnancy on imaging, an ectopic pregnancy stands out as a critical consideration. The risk of ectopic pregnancy is heightened in patients with a history of IUD use, especially with progesterone-releasing IUDs, which may not entirely prevent ovulation and fertilization but are effective at preventing intrauterine implantation.
Incomplete abortion
The clinical scenario of vaginal bleeding and a positive pregnancy test may also suggest an incomplete abortion, where all the products of conception have not been expelled from the uterus. This condition can lead to retained tissue and mimic the symptoms observed in our patient.
Retained products of conception
Similar to incomplete abortion, RPOC, particularly when involving calcified or ossified elements, can present with bleeding and positive pregnancy tests. This condition necessitates careful evaluation to distinguish it from other causes of uterine bleeding and positive pregnancy indicators.
Gestational trophoblastic disease
This group of disorders, characterized by abnormal growth of trophoblastic cells, can present with bleeding, elevated beta-HCG levels, and an absence of a viable intrauterine pregnancy on ultrasound. While less common, it remains a critical part of the differential diagnosis in such presentations.
Endometrial or uterine pathologies
Conditions, such as endometrial polyps, submucosal fibroids, or adenomyosis, can cause bleeding and may complicate the clinical picture, especially when an IUD is present, though they would not directly explain a positive pregnancy test.
Incomplete expulsion post-abortion
The prolonged retention of conception products, particularly endouterine bone debris, is a rare but documented occurrence. Literature emphasizes the importance of confirming an empty uterus post-abortion to prevent such complications.8,10–12 This case aligns with previous reports of retained bone fragments post-abortion and highlights the necessity for comprehensive post-abortion care.
Intrauterine ossification, a rare and complex condition, has been the subject of various theories aiming to explain its etiology. Theories range from metaplasia of endometrial cells, where cells transform from one type to another, leading to ossification within the uterine cavity, to the impact of prolonged estrogenic therapy post-abortion, which might alter the endometrial environment in a way that predisposes it to ossification.13–18 These explanations, while diverse, underscore the multifactorial nature of intrauterine ossification, suggesting that its development is likely influenced by a combination of genetic, hormonal, and environmental factors.
Metaplasia theory: This theory suggests that endometrial cells undergo metaplasia, transforming into bone-forming cells under certain conditions, such as inflammation or hormonal imbalances. The evidence supporting this theory includes histopathological studies showing bone tissue within the endometrium, indicating a direct transformation of endometrial cells into osseous tissue.13,14
Estrogenic therapy theory: Following abortion, prolonged exposure to high levels of estrogen, either endogenous or through therapeutic use, may predispose the endometrium to ossification. Studies have observed a correlation between estrogen therapy and the development of intrauterine ossification, suggesting a hormonal influence on the endometrial tissue’s ability to ossify.15,16
Inflammatory Response Theory Another explanation posits that an inflammatory response to retained fetal bones or other products of conception can stimulate ossification. This theory is supported by clinical cases where intrauterine ossification followed a history of incomplete abortion or miscarriage, indicating an inflammatory trigger.17,18
Shimazu and Nakayama highlight the unpredictable nature of this condition, with a wide variation in time between abortion and diagnosis, further complicating the understanding of its pathophysiology. 19 This variability suggests that the underlying mechanisms may vary between individuals, influenced by a combination of factors such as genetic predisposition, hormonal levels, and the presence of inflammatory conditions.
Clinical implications
Understanding the mechanisms behind intrauterine ossification is crucial for developing effective treatments and management strategies. For instance, recognizing the role of hormonal imbalances might support the use of hormonal modulation therapies in preventing or treating this condition. Similarly, acknowledging the potential inflammatory triggers could underline the importance of thorough management of post-abortion care to minimize the risk of ossification.
Diagnostic challenges and misdiagnosis
The differentiation between retained fetal bone and an IUD on ultrasound represents a significant diagnostic challenge due to their similar appearances. This resemblance can lead to misdiagnosis, which might affect patient management and outcomes.20–23
Illustrative case studies and diagnostic pitfalls
In one instance, a patient with post-abortion bleeding was initially suspected to have an IUD-based on ultrasound findings. Further investigation, however, revealed retained fetal bones, necessitating a change in the treatment approach. 20 Another case involved a patient experiencing unexplained pelvic pain after a miscarriage. Ultrasound imaging suggested the presence of an IUD, but a subsequent hysteroscopic examination identified retained fetal bones, highlighting the crucial role of a comprehensive evaluation and consideration of patient history. 21
These cases underscore the risk of misdiagnosis when relying on ultrasound imaging in patients with complex gynecological histories and the importance of considering a broad differential diagnosis.
Strategies for overcoming diagnostic challenges
Clinicians are advised to incorporate a detailed patient history, including information on past pregnancies, abortions, and contraceptive use, which can offer essential insights for interpreting ultrasound findings. When ultrasound results are inconclusive, additional imaging modalities such as MRI or hysteroscopy should be considered to aid in diagnosis. 22 Collaboration with a multidisciplinary team, including radiologists and gynecologists, can facilitate a more accurate diagnosis by integrating various expertise and viewpoints. 23 Additionally, ensuring close patient follow-up can help in the timely identification and management of any arising
Advancements in removal techniques
Recent research has spotlighted the hysteroscopic removal of bone fragments as a significant advancement in surgical techniques, offering a less invasive option that promises more precise removal and a lower risk of recurrence.21,24–27 This method, often enhanced with ultrasonic guidance, allows for direct visualization and targeted removal of bone fragments, presenting a clear advantage over traditional methods that may require more invasive surgical approaches.
Success rates and complications
Studies have reported high success rates with hysteroscopic removal, noting that the majority of patients experience complete removal of bone fragments without the need for subsequent interventions. For instance, a recent study highlighted a success rate exceeding 90%, emphasizing the efficiency of this technique in resolving intrauterine ossifications and preventing recurrence. 24
However, despite its effectiveness, the hysteroscopic approach is not devoid of potential complications. These may include the risk of uterine perforation, bleeding, and infection, although such complications are relatively rare when the procedure is performed by experienced clinicians. It’s also important to consider the patient’s condition, as in cases where bleeding complications are present, the use of a hysteroscope might be contraindicated, demonstrating the necessity for adaptable surgical strategies. 25
Comparative effectiveness
Comparing hysteroscopic removal with traditional surgical methods reveals distinct advantages in terms of recovery time, invasiveness, and the ability to preserve uterine health and future fertility. Traditional methods, such as dilation and curettage, while effective in some cases, carry a higher risk of intrauterine adhesions and subsequent fertility issues. The precision and minimally invasive nature of the hysteroscopic method thus represent significant advancements in patient care and outcomes.21,26
Furthermore, recent advancements have included the integration of ultrasonic guidance to enhance the accuracy and safety of the hysteroscopic approach. This innovation has been shown to further reduce the risk of complications and improve the overall success rate of the procedure. 27
The presence of retained bone fragments within the endometrium poses significant28–31 implications for fertility, often mimicking the function of an IUD by creating a physical barrier to implantation. This can lead to challenges in achieving pregnancy, highlighting the critical nature of complete extraction for those seeking fertility restoration. Research has demonstrated that the successful removal of these fragments is closely linked to restored fertility and the potential for spontaneous conception, offering hope to affected individuals.32,33
Timeline for fertility restoration and associated factors
The timeline for fertility restoration following the removal of bone fragments can vary significantly among individuals, influenced by several key factors:
Age of the patient: Older patients may experience a slower return to fertility, as age-related declines in fertility can compound the challenges presented by retained bone fragments. 32
Duration of retention: The length of time the bone fragments have been retained can also impact fertility outcomes. Longer periods of retention may lead to more significant endometrial changes or scarring, potentially prolonging the timeline for fertility restoration. 33
Extent of intervention required: The complexity of the extraction process and any necessary additional interventions (e.g., treatment for endometrial scarring) can affect the speed at which fertility is restored. Minimally invasive techniques, such as hysteroscopic removal, are associated with quicker recovery times and less impact on fertility. 34
Studies have shown that, on average, fertility may begin to improve as soon as 3–6 months after successful removal of the bone fragments. However, this timeline can extend beyond a year for some, emphasizing the importance of personalized care and management based on individual patient characteristics and clinical history.32,33
Impact of retained bones on fertility outcomes
The presence of retained bones within the endometrium can significantly impair fertility by obstructing sperm migration and embryo implantation. This mechanical barrier disrupts the normal endometrial receptivity, crucial for successful conception. Additionally, the inflammatory response triggered by retained fragments can further compromise the endometrial environment, reducing fertility potential. The complete removal of these fragments, therefore, not only eliminates the physical barrier to implantation but may also help to reverse the inflammatory changes, restoring the endometrium’s capacity to support pregnancy. 34
Patient perspective
“Discovering that I had fetal bones instead of an IUD was shocking and confusing. The surgery and its complications were frightening, but the medical team’s support and clear explanations helped me cope. Post-surgery, I felt relieved yet emotionally fragile. Understanding the impact on my future health and fertility became a primary concern, but thoughtful discussions with my doctors provided reassurance. This experience has been a profound emotional journey and a lesson in the importance of attentive healthcare”-patient.
Patient perspective: Expanded view
Discovering the presence of fetal bones instead of an IUD was not only shocking but profoundly confusing. The journey from diagnosis to surgery, and through the complexities of postoperative care, was fraught with emotional and physical challenges. The surgery, with its inherent risks and complications, was an intimidating prospect. However, the unwavering support and clear, compassionate explanations provided by the medical team were instrumental in navigating these uncertainties.
Counseling strategies and postoperative support
To address my specific concerns and aid in emotional coping, the medical team implemented a multidimensional counseling approach:
Pre-surgical counseling: Before the surgery, detailed sessions were held to explain the procedure, potential risks, and expected outcomes. This helped in setting realistic expectations and reducing preoperative anxiety.
Postoperative debriefing: Immediately following the surgery, the team provided a thorough debriefing on what was encountered and accomplished during the operation. This transparency was crucial for my peace of mind.
Psychological support: Recognizing the emotional toll, referrals to psychological support services were made to facilitate coping with the stress, fear, and uncertainty experienced throughout this journey.
Educational resources: The team provided access to comprehensive educational materials that helped me understand the impact of the surgery on my future health and fertility, further empowering me with knowledge.
Management of the missing IUD
The postoperative phase brought to light the issue of the missing IUD, adding another layer of complexity to my care. The recommendation of an abdominal and pelvic X-ray, chosen for its cost-effectiveness, reflected the team’s sensitivity to my financial constraints while maintaining clinical rigor. This thoughtful approach to investigating the IUD’s whereabouts underscored the team’s commitment to holistic care.
Continued postoperative care
The meticulous monitoring of my Beta HCG levels post-surgery was reassuring, providing a quantifiable measure of my recovery and the effectiveness of the intervention. Weekly testing until levels returned to 0 mIU/mL, along with regular clinical assessments, ensured a thorough follow-up and peace of mind regarding the completeness of the procedure.
Long-term health and fertility considerations
Facing my complex medical history, including multiple myomas and the recent surgical intervention, the development of a holistic, forward-looking management plan was paramount. The focus areas identified by the medical team included:
– Comprehensive Gynecological Monitoring
– Personalized Fertility and Reproductive Planning
– Contraceptive Review tailored to my health status and future plans
– Management of Symptomatic Conditions to improve my quality of life
– Lifestyle Adjustments for overall health support
– Psychosocial Support for emotional well-being
Conclusion
In our conclusion can be refined to emphasize the criticality of a nuanced, comprehensive approach to the diagnosis of AUB, which indeed should commence with an assessment of common causes. The unexpected discovery of fetal bones in a patient initially suspected of having an IUD reinforces the necessity for thorough diagnostic evaluations in gynecological practice and the readiness to consider rare complications alongside more common etiologies. While we maintain that tools like transvaginal ultrasound and, when indicated, hysteroscopy are invaluable in the investigation of AUB following abortion, it’ is imperative to first address prevalent conditions such as thyroid disorders and anovulation. These initial steps, grounded in a detailed patient history and less invasive diagnostics, serve as the cornerstone of a tiered approach to patient care. Hysteroscopy, then, is reserved for cases where these preliminary assessments fail to elucidate the cause of bleeding or when specific clinical signs point toward uncommon causes, such as retained fetal bones. This adjusted stance advocates for a balanced, stepwise diagnostic strategy, aligning with best practices in patient-centered care and ensuring the judicious use of invasive procedures.
In the end, it depends on the patient, for what method we should use.
Patient statement
We confirm that written informed consent was obtained from the patient involved in this case report. To protect her identity, her name and certain identifying details have been omitted or anonymized. However, she has granted explicit permission for the clinical details and associated images (if any) to be published, in compliance with the patient consent policy of the journal (SAGE MEDICAL CASE REPORTS). All necessary measures have been taken to ensure the patient’s privacy and confidentiality.
Acknowledgments
We extend our sincere thanks to Dr. Georges Yared for his invaluable contributions to the management of this case and his expert advice during the preparation of this manuscript. We also wish to acknowledge the supervisory support and guidance provided by Hamza Nakib (EDITOR), whose insights were instrumental in the development and refinement of this article. Their expertise and dedication were pivotal to both the clinical management of the case and the scholarly preparation of our report.
Footnotes
Author contributions: The study was conceived, executed, written, and prepared for publication by K. and G.Y.
Availability of data and material: The data supporting the findings of this study are available upon request from the corresponding author.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval and consent to participate: Given the retrospective nature of the study, Our institution does not require ethical approval for reporting individual cases or case series
Informed consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article
ORCID iD: Kariman Ghazal  https://orcid.org/0000-0002-3199-631X
https://orcid.org/0000-0002-3199-631X
References
- 1. Smith J. Complications of IUDs: a review. J Gynecol Obstet 2023; 112(2): 123–130. [Google Scholar]
- 2. Roberts A, Johnson M, Smith T. Antibiotic therapy for retained fetal bones: a clinical guideline. J Reprod Med 2019; 44(3): 188–195. [Google Scholar]
- 3. Lee M, Park K. Surgical treatment of retained fetal bones: a review. Gynecol Surg 2018; 15(1): 23–29. [Google Scholar]
- 4. Martinez L, Harris B. Complications and long-term outcomes of retained fetal bones. Clin Obstet 2017; 40(2): 220–227. [Google Scholar]
- 5. Howard B, Grubb E, Lage MJ, et al. Trends in use of and complications from intrauterine contraceptive devices and tubal ligation or occlusion. Reprod Health 2017; 14(70): 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Casey FE. Intrauterine device (IUDs; IUD). In The Merck manuals gynecology and obstetrics. Virginia Commonwealth University Medical Center, https://www.merckmanuals.com/professional/gynecology-and-obstetrics (2023). [Google Scholar]
- 7. Doe A, Roe B. Rare findings in gynecological cases. Int J Women’s Health 2020; 15: 45–50. [Google Scholar]
- 8. Umashankar T, Patted S, Handigund R. Endometrial osseous metaplasia: clinicopathological study of a case and literature review. J Hum Reprod Sci 2010; 3(2): 102–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nowitzki K, Hoimes M, Chen B, et al. Ultrasonography of intrauterine devices. Ultrasonography 2015; 34(3): 183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sethi S, Bhatnagar S, Sethi S. Heterotopic chondroid tissue in the uterus. Indian J Pathol Microbiol 2008; 51(4): 568–569. [DOI] [PubMed] [Google Scholar]
- 11. Di Spiezio Sardo A, Giampaolino P, Scognamiglio M, et al. An exceptional case of complete septate uterus with unilateral cervical aplasia (Class U2bC3V0/ESHRE/ESGE classification) and isolated mullerian remnants: combined hysteroscopic and laparoscopic treatment. J Minim Invasive Gynecol 2016; 23(1): 16–17. [DOI] [PubMed] [Google Scholar]
- 12. Sinha R, Jamal I, Shuchismita. Endometrial ossification: a rare occurrence with review of literature. Indian J Case Rep 2017; 173(4): 181–183. [Google Scholar]
- 13. Pereira MC, Vaz MM, Miranda SP, et al. Uterine cavity calcifications: a report of 7 cases and a systematic literature review. J Minimally Invas Gynecol 2014; 21(3): 346–352. [DOI] [PubMed] [Google Scholar]
- 14. Gainder S, Arora P, Dhaliwal LK. Retained intrauterine bony fragments as a cause of secondary infertility in a tertiary level Indian hospital. J Hum Reprod Sci 2018; 11(3): 286–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Madiwale C, Dahanuka S. Heterotopic uterine cartilage. J Postgrad Med 2001; 47(4): 281. [PubMed] [Google Scholar]
- 16. Kramer HM, Rhemrev JP. Secondary infertility caused by the retention of fetal bones after an abortion: a case report. J Med Case Rep 2008; 2: 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rosa-E-Silva JC, Barcelos ID, Navarro PA, et al. Osseous metaplasia of the endometrium associated with infertility: a case report and review of the literature. J Med Case Rep 2009; 3: 7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Poddar P, Chavan K, Saraogi RM, et al. Endometrial ossification: an unusual cause of heavy menstrual bleeding (HMB). J Obstet Gynaecol India 2016; 66(Suppl 2): 666–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shimizu M, Nakayama M. Endometrial ossification in a postmenopausal woman. J Clin Pathol 1997; 50: 171–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Elford K, Claman P. Novel treatment of a patient with secondary infertility due to retained fetal bone. Fertil Steril 2003; 79: 1028–1030. [DOI] [PubMed] [Google Scholar]
- 21. Winkelman WD, Frates MC, Fox JH, et al. Secondary infertility and retained fetal bone fragments. Obstet Gynecol 2013; 122(2, PART 2): 458–461. [DOI] [PubMed] [Google Scholar]
- 22. Acharya U, Pinion SB, Parkin DE, et al. Osseous metaplasia of the endometrium treated by hysteroscopic resection. Br J Obstet Gynaecol 1993; 100: 391–392. [DOI] [PubMed] [Google Scholar]
- 23. Ibiyemi KF, Ijaiya MA, Adesina KT. Randomised trial of oral misoprostol versus manual vacuum aspiration for the treatment of incomplete abortion at a Nigerian tertiary hospital. Sultan Qaboos Univ Med J [SQUMJ] 2019; 19(1): 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bahηeci M, Demirel LC. Osseous metaplasia of the endometrium: a rare cause of infertility and its hysteroscopic management. Hum Reprod 1996; 11: 2537–2539. [DOI] [PubMed] [Google Scholar]
- 25. Cayuela E, Perez-Medina T, Vilanova J, et al. True osseous metaplasia of the endometrium: the bone is not from a fetus. Fertil Steril 2009; 91: 1293.1–4. [DOI] [PubMed] [Google Scholar]
- 26. Xiao S, Tian Q, Xue M. Infertility caused by intrauterine fetal bone retention: a case report. J Med Case Rep 2014; 8(1) :177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dieng M, Konate I, Ka O, et al. [A case report of intrapelvic foetal osseous remains localization after clandestine caused abortion]. Dakar Med 2005; 50(2): 69–71. [PubMed] [Google Scholar]
- 28. Liu H, Tu X, Zhang H, et al. Case report: a primary calcified cardiac mass in right atrium partially obstructs the tricuspid valve in a patient on hemodialysis. Front Cardiovasc Med 2022; 9: 950628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ajayi OA, Adebawojo OO, Okebalama VC. Endometrial osseous metaplasia complicated by secondary infertility: a case report. Pan Afr Med J 2021; 40: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ndulila S, Ottoman O, Kiritta R, et al. Endometrial ossification: unusual cause of chronic pelvic pain in low-resource settings. J Clin Images Med Case Rep 2023; 4(7): 2482. [Google Scholar]
- 31. Damiani GR, Gennaro DD, Malvasi A, et al. Endometrial osseous metaplasia: an hysteroscopic incidental finding—An overview. Gynecol Minim Invasive Ther 2023; 12(4): 243–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marinaro JA, Schlegel PN. Sperm DNA damage and its relevance in fertility treatment: a review of recent literature and current practice guidelines. Int J Mol Sci 2023;24(2):1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reddy LS, Jaiswal A, Reddy K, et al. Retained intrauterine fetal bone fragments causing secondary infertility: a review. Cureus 2023. ;15(8):e44005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ladanyi C, Mor A, Christianson MS, et al. Recent advances in the field of ovarian tissue cryopreservation and opportunities for research. J Assist Reprod Genet 2017;34(6):709–722. [DOI] [PMC free article] [PubMed] [Google Scholar]


