Abstract
Objective:
Colon cancer is affluent among many people, and having cancer greatly impacts the lives of many. Ginger is a common food, particularly in Asian cuisine. However, the health benefits of ginger as a whole food and 6-gingerol, its bioactive compound in prevention of colon cancer have not been fully addressed. This experiment investigated effects of ginger juice and 6-gingerol on colon cancer cell growth and death.
Methods:
Fresh ginger roots were homogenized for juice preparation. Total phenolic contents of ginger juice were measured using Folin-C assay. Colon cancer SW480 cells and normal colon epithelial cells CCD-18Co were treated with ginger juice and/or 6-gingerol. Cell metabolic activity was assessed by MTT assay. Cell apoptosis and cell cycle arrest were accessed by immunoblotting. Data were analyzed by 2-way ANOVA with a Tukey post-hoc test and statistical significance was set at P < .05.
Results:
The results showed that ginger juice selectively inhibited SW480 cell growth at 25 µL/mL for 40 hours. High doses of ginger juice (at 50 and 100 µL/mL for 40 hours) inhibited the growth of both cell types. This was independent of caspase-3 activation. Six-gingerol specifically inhibited SW480 cell growth starting at 0.5 µmoL/L (P < .01). More than 1 µmoL/L 6-gingerol did not give more power to inhibit SW480 cell growth. The results also showed that CCD-18Co cell growth rates were not changed after 6-gingerol treatments (up to 10 µmoL/L, P > .1). Immunoblotting results revealed that the elevation of Myt1 levels and decreases in CDK1, p21 Wafl/Cip1 and pSer642-Wee1 only occurred in SW480 but not CCD-18Co cells when treated with 1 and/or 2.5 µmoL/L 6-gingerol for 40 hours.
Conclusion:
6-gingerol can specifically inhibit SW480 cancer cells without killing normal CCd-18Co cells, through cell cycle arrest. Ginger juice can selectively inhibit colon cancer cell growth in a narrow window at ~25 µL/mL.
Keywords: 6-Gingerol, apoptosis, cell cycle arrest, colorectal cancer, epithelial cell, phenol
Introduction
Colon cancer is the third most common type of cancer in the US, and it affects the lives of many, particularly older people. 1 The causes of the disease can be related to genetic factors, unhealthy lifestyles and eating habits, and others.2-4 High intakes of foods high in fat, salt, sugar, but low in fruits and vegetables, are considered significant contributors to the disease etiology and progression.4,5 If the cancer is diagnosed at an early stage, the 5-year survival rate is expected to be high (~90%). 6 For cancer prevention purposes, there are several FDA-approved colon cancer treatment regimens, including changes in dietary patterns.
Ginger roots (Zingiber officinale Roscoe) are common in Asian cuisine.7,8 It is also becoming popular in Western foods, for example, gingerbread (fortification in cookie), ginger tea (fortification in beverage), and ginger powder (fortification as spice). According to the database published by USDA, 100 g fresh ginger roots contain 1.8 g proteins, 17.8 g carbohydrates, 0.7 g lipids, and various minerals and vitamins. As previously reported, ginger roots are rich in phytochemicals, such as gingerols and phenols.7,8 In vitro and in vivo studies also present that ginger roots have antioxidant, anti-inflammatory and anticancer properties,7,8 though the mechanisms of action are not well elucidated.
Gingerols, such as 6-gingerol, are a biologically active compound found rich in ginger roots. The estimated content of 6-gingerol is about 1 mg/100 g fresh ginger roots. 9 There are numbers of publications showing that 6-gingerol inhibits colon cancer cell growth, through apoptosis and cell cycle arrest, in culture at higher doses (~30-100 μmoL/L).10-13 However, there is no data to confirm whether 6-gingerol at such high doses is safe to normal colon cells.
In this study, we sought to investigate the protective roles of low doses of ginger root juice and its bioactive 6-gingerol compound in colon cancer by specifically reducing colon cancer cell growth while keeping normal colon cells safe.
Methods
Cell culture
Colon cancer SW480 cells and CCD-18Co normal colon epithelial cells were purchased from the American Type Culture Collection (CCL-228 and CRL-1459, Manassas, VA, USA). SW480 cells were grown in DMEM with 4.5 g/L glucose supplemented with 10% fetal bovine serum (FBS), penicillin, and streptomycin, and normal colon CCD-18Co cells were maintained in EMEM with 4.5 g/L glucose and glutamine with 10% FBS. Cells were seeded in either flasks or 96-well plates with initial cell density about 35%.
Ginger roots and ginger juice preparation and total phenolic content assay
Ginger roots were purchased from a local grocery store and maintained in a refrigerator till use. One hundred grams ginger roots were homogenized, and the juice was collected through 3 layers chess cloth filtering, followed by centrifugation and 0.2 μm filter sterilization. After freeze-drying, ginger root powders were completely dissolved in 750 μL DMSO. Finally, the solution was reconstituted to 75 mL with cell culture media, followed by 0.2 μm filter sterilization. The sterile ginger root juice was used for total phenol content measurement and cell culture treatments. The phenolic content in the ginger juice was measured using the Folin-Ciocalteu assay as previously reported, expressed as gallic acid equivalent. 14
6-Gingerol
6-gingerol was obtained from Sigma-Aldrich (St. Louis, MO, USA. catalog # G1046). It was dissolved in DMSO in a stock of 100 mmol/L and was diluted in cell culture media for experimental uses.
Cell treatments and metabolic activity assessment
Cell numbers were examined using Trypan blue stain. Cells were treated with ginger juice and/or 6-gingerol at various doses for 20, 40, and 80 hours. Cell metabolic activity was analyzed using a MTT cell proliferation assay kit (Cayman Chemical, Ann Arbor, MI, USA, catalog # 10009365), by following the manufacturer’s introduction. Relative metabolic activity was normalized to the SW480 control (the “0” group).
Immunoblotting and cell apoptosis and cycle arrest
After treatments, cells were harvested and whole cell lysates were collected in RIPA lysis buffer solutions (catalog # 89901, Thermo Fisher, Waltham, MA, USA), with 1% protease and phosphatase inhibitors. Protein concentration was measured using the Pierce BCA protein assay kit (catalog # 23225, Thermo Fisher, Waltham, MA, USA). Cell apoptosis and/or cycle arrest were determined by immunoblotting using antibodies against key proteins in cell cycles. 15 Immunoblotting images were quantified using ImageJ software. Antibodies used were purchased from Cell Signaling Technology (with catalog #, Danvers, MA, USA), beta-actin (4970), cleaved caspase-3 (9961), p21 Waf1/Cip1 (12D1) (2947), phospho-Histone H3 (Ser10) (3377), cyclin A2 (BF683) (4656), cyclin B1 (D5C10) (12231), phospho-Wee1 (Ser642) (4910), Cyclin Dependent Kinase 1 (CDK1) (77055), phospho-Tyr 15 CDK1 (4539), and Myt1 (4282). Specificity of antibodies were validated by the vender.
Statistical analysis
Data were analyzed by 2-way ANOVA with a Tukey post-hoc test and statistical significance was set at P < .05. Experiments were repeated 4 times. Values were expressed as means ± standard deviation.
Results
Ginger juice inhibited cell growth
In the ginger juice prepared in this study, there were 0.8 ± 0.1 mg garlic acid equivalent/mL total phenolics. Time course (20, 40, or 80 hours) and dosage curve (1, 10, 25, 50, or 100 μL/mL) experiments showed that ginger juice significantly inhibited the growth of both cell types at a higher dose (data not shown). The results in Figure 1A indicated that for the 40 hour treatment, ginger juice at 25 μL/mL specifically inhibited SW480 cancer cells but not normal colon cell CCD-18Co. Yet, at higher doses, for example, 50 and 100 μL/mL, ginger juice inhibited growth of both cell types. Immunoblotting results in Figure 1B showed that cleaved caspase-3 levels were not altered by the treatments, regardless of doses of ginger juice. It suggested no caspase-3 activation by ginger juice.
Figure 1.
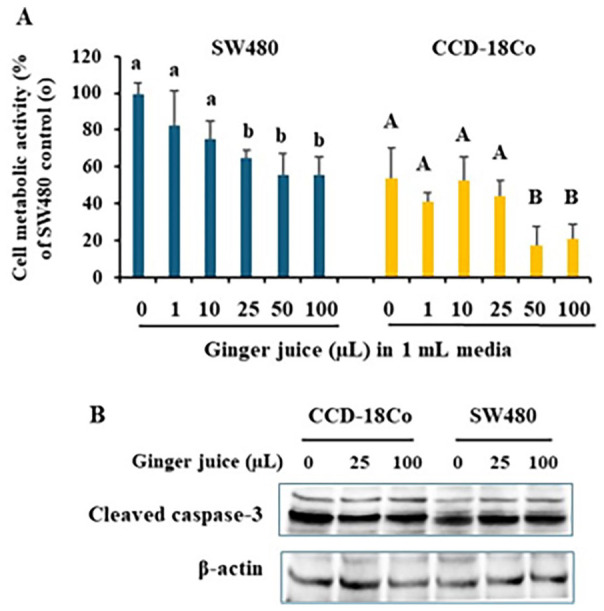
Ginger juice selectively inhibited SW480 cell growth at 25 µL/mL Colon cancer SW480 and normal colon epithelial CCD-18Co cells were treated with ginger juice for 40 hours. Cell metabolic activity was measured by MTT assay, data was normalized to the group of SW480 “0” (A). Whole cell lysates were subject to immunoblotting to detect caspase-3 activation as shown in cleaved caspase-3 (B). Representative immunoblotting images are shown. Data are from 4 independent replicates. Values with different letters are statistically different.
N = 4. P < .05.
6-Gingerol specifically inhibited colon cancer SW480 cell growth
Nest, we selected 6-gingerol, one of the major phenols in the ginger juice, to treat cells. The results in Figure 2A revealed that 40 hours 6-gingerol treatment significantly inhibited SW480 cell growth starting at 0.5 µmol/L (P < .01). More than 1 µmol/L 6-gingerol did not give more power to inhibit SW480 cell growth. The results also showed that CCD-18Co cell growth were not changed after 6-gingerol treatments (P > .1). Immunoblotting results further revealed that 6-gingerol at 1 and 2.5 µmol/L cleaved caspase-3 levels were consistent among groups, suggesting there were no caspase-3 dependent cell apoptosis (Figure 2B).
Figure 2.
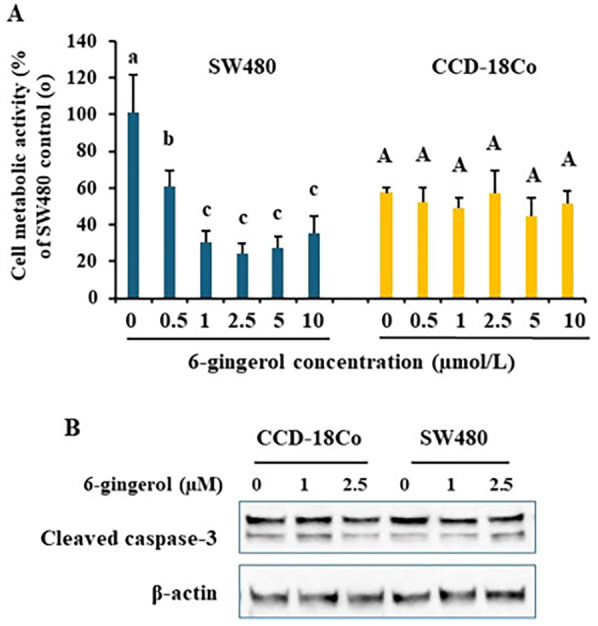
6-gingerol specifically inhibited SW480 cell growth Colon cancer SW480 and normal colon epithelial CCD-18Co cells were treated with various doses of 6-gingerol for 40 hours. Cell metabolic activity was measured by MTT assay, data was normalized to the group of SW480 “0” (A). Whole cell lysates were subject to immunoblotting to detect caspase-3 activation as shown in cleaved caspase-3 (B). Representative immunoblotting images are shown. Data are from 4 independent replicates. Values with different letters are statistically different.
N = 4. P < .05.
6-gingerol selectively lowered p21 waf1/cip1 protein levels in colon cancer SW480 cells
Further, we examined p21 and histone H3 phosphorylation. Figure 3A and 3B (quantified data of immunoblotting) apparently indicated that 6-gingerol treatment selectively and significantly decreased p21 protein levels in SW480 cells only. Histone H3 phosphorylation on Ser10 was not altered by 6-gingerol in either cell type.
Figure 3.
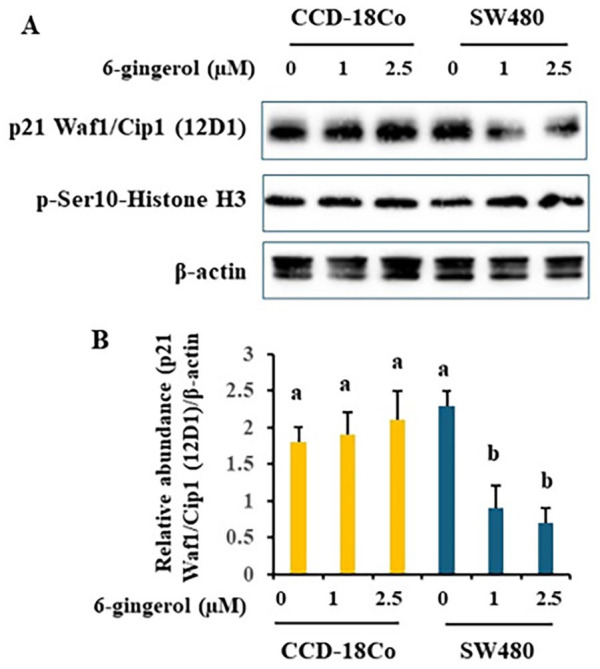
6-gingerol regulates protein levels in cell cycle arrest and cell death Colon cancer SW480 and normal colon epithelial CCD-18Co cells were treated with various doses of 6-gingerol for 40 hours. Whole cell lysates were subject to immunoblotting. Representative immunoblotting images are shown (A). ImageJ was used to quantify immunoblotting images (B). Data are from 4 independent replicates. Values with different letters are statistically different.
N = 4. P < .05.
6-gingerol altered levels of key proteins in the G2/M cell cycle checkpoint in colon cancer SW480 cells
Finally, we determined some key proteins in cell cycle checkpoints (Figure 4A representative images and B quantitative results). As expected, levels of key proteins in cell cycle checkpoints were extensively lower in normal colon epithelial cell CCD-18Co, compared to SW480, which were not much altered by 6-gingerol (Figure 4A left panels). And in SW40 cells, cyclins A2 and B1 protein levels were not changed by 6-gingerol. However, Wee1 phosphorylation on Ser642 was inhibited, but Myt1 protein levels were elevated by 6-gingerol. Further, overall CDK1 protein levels were also significantly decreased in 6-gingerol-treated SW480 cells, though the ratio of phospho-Tyr15-CDK1/CDK1 was consistent.
Figure 4.
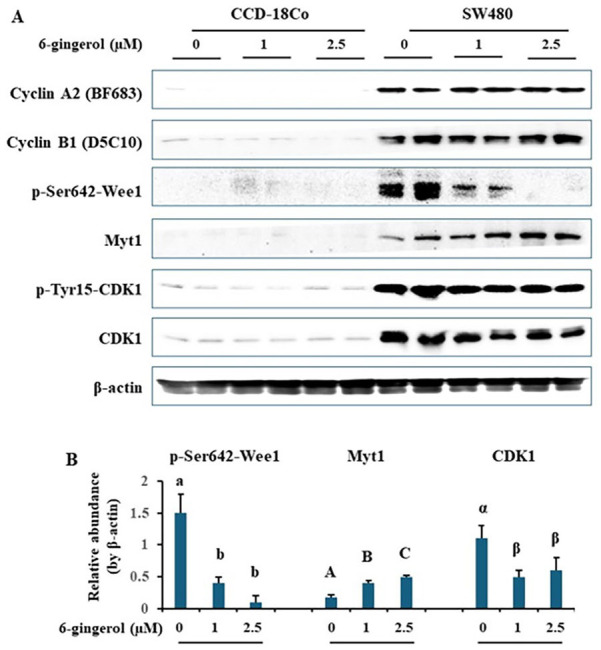
6-gingerol regulates levels of proteins involved in G2/M cell cycle arrest Colon cancer SW480 and normal colon epithelial CCD-18Co cells were treated with various doses of 6-gingerol for 40 hours. Whole cell lysates were subject to immunoblotting. Representative immunoblotting images are shown (A). ImageJ was used to quantify immunoblotting images (B). Data are from 4 independent replicates. Values with different letters are statistically different.
Abbreviations: CDK1, cyclin dependent kinase 1.
N = 4, P < .05.
Discussion
In this study, the protective roles of ginger juice and its bioactive compound 6-gingerol in cancer were investigated in cell cultures of colon cancer cells and normal colon epithelial cells. The study revealed that both ginger juice and 6-gingerol were beneficial to specifically inhibit colon cancer cell growth. As low as 1 µmoL/L 6-gingerol could effectively inhibit SW480 cancer cell growth by 64%. However, ginger juice could selectively inhibit colon cancer cell growth in a narrow window at ~25 µL/mL media. Ginger juice at larger doses was harmful because it inhibited both normal colon epithelial cells and cancer cells.
Ginger root as a whole food, is rich in nutrients, phytochemicals, and other bioactive compounds. Published work claims that health benefits of ginger are largely attributed to phenols, such as gingerols and shogaols.16,17 Among them, 6-gingerol was the most well studied. The dose of ginger has not been optimized in most human studies.7,8 Here, we prepared a ginger juice with total phenolics content at 0.8 ± 0.1 mg garlic acid equivalent/mL. The ginger juice selectively inhibited cancer cell growth at a very narrowed dose window. That suggested that special caution has been taken when using ginger juice as a whole food. It may suggest there were other compounds can possibly exert a role in cell growth and death.
6-gingerols at low doses were able to achieve the important protective effects. In the literature, high doses (at ~30 µmoL/L or higher) were used in treating cancer cells,7,8 but there were no normal colon epithelial cells as a negative control. Special caution must be taken when we implicate those data because such high doses of phenolics might cause damage to normal colon cells. Pro-caspase 3 cleavage is an essential step for caspase 3 activation shown in a form of cleaved caspase-3 by immunoblotting. 18 The immunoblotting results in this study indicated that there were no increases in activation of caspase-3, suggesting that ginger juice and 6-gingerol did not induce caspase-3-dependent apoptosis in both colon cell types.
Cell cycle arrest occurs in multiple checkpoints, under complex mechanisms.19-21 p21 Wafl/Cip1 is one of key regulators that plays a dual role in promotion of cancer cell proliferation and apoptosis. 22 In the current study, p21 protein levels were specifically lowered in cancer cells after 6-gingerol treatment, suggesting its possible role in slowing down the growth of cancer cells.
Cyclin B and CDK1 complex is another key player in transition of G2 phase to M phase in the cell cycle. 23 Phosphorylation on Tyr 14 and Tyr 15 of CDK1 by Wee1 and/or Myt1 causes CDK1 inactivation, and thereby leading to G2/M arrest.24,25 6-gingerol at lower doses also specifically decreased CDK1 protein levels, with changes in Wee1 and Myt1, though phosphorylation status on Tyr15 of CDK1 was not significantly altered. Taken together, protein levels changes suggested 6-gingerol-linked colon cancer cell cycle arrest.
Given the heterogeneity of cancer cells in nature, 26 there is much inconsistent information available in the literature about the exact roles of ginger and/or 6-gingerol in cell cycle arrest. This study can provide some new data to support the statement that there were multiple potential mechanisms of action in terms of 6-gingerol inhibition of colon cancer cell growth. Newly developed single cell RNA sequencing can be a powerful tool to tease out mechanistically at single cell levels, on how ginger juice and the bioactive 6-gingerol can kill the cancer cells, while leaving normal colon cells unharmed.
Conclusion
Through this experiment, it can be concluded that 6-gingerol can specifically inhibit SW480 colon cancer cells without killing normal colon epithelial CCD-18Co cells, through cell cycle arrest. Ginger juice can selectively inhibit colon cancer cell growth in a relatively narrow dose window. Single cell transcriptomics can be used for future mechanistic assessment.
Acknowledgments
The authors are grateful for the support provided by the Department of Nutritional Sciences at Oklahoma State University, Stillwater, OK, including access to the internal funds and research facilities.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by internal funds from Oklahoma State University
Author Contributions: Shelley Lin, as a mentee, designed the study, conducted experiments, data analyses, and manuscript preparation. Peiran Lu was a mentor and oversee the research project, trained the mentee as needed, and revised the manuscript.
Data Availability Statement: The authors will make data available upon request after publication.
Ethical Statement: Not applicable because this is a cell culture study at BSL-1.
Grant Number: Not applicable.
Informed Consent/Patient Consent: Not applicable.
Other Journal Specific Statements Applicable: None.
Trial Registration Number/Date: Not applicable.
References
- 1. American Cancer Society, Key Statistics for Colorectal Cancer. 2024. https://www.cancer.org/cancer/types/colon-rectal-cancer/about/key-statistics.html
- 2. Willett W. The search for the causes of breast and colon cancer. Nature. 1989;338:389-394. [DOI] [PubMed] [Google Scholar]
- 3. Solomon E, Borrow J, Goddard AD. Chromosome aberrations and cancer. Science. 1991;254:1153-1160. [DOI] [PubMed] [Google Scholar]
- 4. Medema JP, Vermeulen L. Microenvironmental regulation of stem cells in intestinal homeostasis and cancer. Nature. 2011;474:318-326. [DOI] [PubMed] [Google Scholar]
- 5. Aune D, Lau R, Chan DS, et al. Nonlinear reduction in risk for colorectal cancer by fruit and vegetable intake based on meta-analysis of prospective studies. Gastroenterology. 2011;141:106-118. [DOI] [PubMed] [Google Scholar]
- 6. Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490-1502. [DOI] [PubMed] [Google Scholar]
- 7. Mao QQ, Xu XY, Cao SY, et al. Bioactive compounds and bioactivities of ginger (Zingiber officinale Roscoe). Foods. 2019;8:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nachvak SM, Soleimani D, Rahimi M, et al. Ginger as an anticolorectal cancer spice: a systematic review of in vitro to clinical evidence. Food Sci Nutr. 2022;11:651-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu M, Xia X, Chou G, et al. Variations in the contents of gingerols and chromatographic fingerprints of ginger root extracts prepared by different preparation methods. J AOAC Int. 2014;97:50-57. [DOI] [PubMed] [Google Scholar]
- 10. Luo YT, Lin LF, Liu YL, et al. [Potential molecular mechanism of banxia xiexin decoction in treatment of colon cancer based on network pharmacology and molecular docking technology]. Zhongguo Zhong Yao Za Zhi. 2020;45:5753-5761. [DOI] [PubMed] [Google Scholar]
- 11. Yusof KM, Makpol S, Fen LS, Jamal R, Wan Ngah WZ. Suppression of colorectal cancer cell growth by combined treatment of 6-gingerol and γ-tocotrienol via alteration of multiple signalling pathways. J Nat Med. 2019;73:745-760. [DOI] [PubMed] [Google Scholar]
- 12. Yim NH, Kim A, Jung YP, et al. Fermented So-Cheong-Ryong-Tang (FCY) induces apoptosis via the activation of caspases and the regulation of MAPK signaling pathways in cancer cells. BMC Complement Altern Med. 2015;15:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ryu MJ, Chung HS. [10]-gingerol induces mitochondrial apoptosis through activation of MAPK pathway in HCT116 human colon cancer cells. In Vitro Cell Dev Biol Anim. 2015;51:92-101. [DOI] [PubMed] [Google Scholar]
- 14. Kupina S, Fields C, Roman MC, Brunelle SL. Determination of total phenolic content using the folin-C Assay: single-laboratory validation, first action 2017.13. J AOAC Int. 2018;101:1466-1472. [DOI] [PubMed] [Google Scholar]
- 15. Habibi M, Goodarzi P, Shili CN, et al. A mixture of valine and isoleucine restores the growth of protein-restricted pigs likely through improved gut development, hepatic IGF-1 pathway, and plasma metabolomic profile. Int J Mol Sci. 2022;23:3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zick SM, Djuric Z, Ruffin MT, et al. Pharmacokinetics of 6-gingerol, 8-gingerol, 10-gingerol, and 6-shogaol and conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers Prev. 2008;17:1930-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jeong CH, Bode AM, Pugliese A, et al. [6]-gingerol suppresses colon cancer growth by targeting leukotriene A4 hydrolase. Cancer Res. 2009;69:5584-5591. [DOI] [PubMed] [Google Scholar]
- 18. Ribeil JA, Zermati Y, Vandekerckhove J, et al. Hsp70 regulates erythropoiesis by preventing caspase-3-mediated cleavage of GATA-1. Nature. 2007;445:102-105. [DOI] [PubMed] [Google Scholar]
- 19. Kalsbeek D, Golsteyn RM. G2/M-phase checkpoint adaptation and Micronuclei Formation as mechanisms that contribute to genomic instability in human cells. Int J Mol Sci. 2017;18:2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Senderowicz AM. Novel direct and indirect cyclin-dependent kinase modulators for the prevention and treatment of human neoplasms. Cancer Chemother Pharmacol. 2003;52(Suppl 1):S61-S73. [DOI] [PubMed] [Google Scholar]
- 21. Qi LW, Zhang Z, Zhang CF, et al. Anti-colon cancer effects of 6-shogaol through G2/M cell cycle arrest by p53/p21-cdc2/cdc25A crosstalk. Am J Chin Med. 2015;43:743-756. [DOI] [PubMed] [Google Scholar]
- 22. Moser J, Miller I, Carter D, Spencer SL. Control of the restriction point by Rb and p21. Proc Natl Acad Sci USA. 2018;115:E8219-E8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Terzoudi G, Jung T, Hain J, et al. Increased G2 chromosomal radiosensitivity in cancer patients: the role of cdk1/cyclin-B activity level in the mechanisms involved. Int J Radiat Biol. 2000;76:607-615. [DOI] [PubMed] [Google Scholar]
- 24. McGowan CH, Russell P. Human Wee1 kinase inhibits cell division by phosphorylating p34cdc2 exclusively on Tyr15. EMBO J. 1993;12:75-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wells NJ, Watanabe N, Tokusumi T, et al. The C-terminal domain of the CDC2 inhibitory kinase Myt1 interacts with CDC2 complexes and is required for inhibition of G(2)/M progression. J Cell Sci. 1999;112:3361-3371. [DOI] [PubMed] [Google Scholar]
- 26. DeBerardinis RJ, Chandel NS. Fundamentals of cancer metabolism. Sci Adv. 2016;2:e1600200. [DOI] [PMC free article] [PubMed] [Google Scholar]


