Abstract
Background and Objectives
A subgroup of patients with multiple sclerosis (MS) presents focal paramagnetic rims at the border between cortex and white matter (juxtacortical paramagnetic rims [JPRs]). We investigated the presence of this finding in our in vivo MS cohort and explored its potential clinical relevance. Moreover, we exploited postmortem MRI of fixed whole MS brains to (1) detect those rims and (2) investigate their histologic correlation.
Methods
Quantitative susceptibility mapping (QSM) and magnetization-prepared 2 rapid acquisition gradient-echo (MP2RAGE) images at 3T-MRI of 165 patients with MS from the in vivo cohort were screened for JPRs and the presence of cortical lesions. Five postmortem brains from patients with MS were imaged with 3T-MRI to obtain QSM and MP2RAGE sequences. Tissue blocks containing JPRs were excised and paraffin-embedded slices stained by immunohistochemistry for myelin basic protein (for myelin) and anti-CR3/43 (for major histocompatibility complex II–positive microglia/macrophages). DAB-Turnbull stain was performed to detect iron.
Results
JPRs are present in approximately 10% of in vivo patients and are associated with increased cortical lesion load. One of the 5 postmortem brains showed JPRs. Histologically, JPRs correspond to an accumulation of activated iron-laden phagocytes and are associated with demyelination of the whole overlying cortical ribbon.
Discussion
JPRs are a novel potential MRI biomarker of focal cortical demyelination, which seems related to global cortical pathology and might be useful for diagnostic and stratification purposes in a clinical setting.
Introduction
Multiple sclerosis (MS) is a chronic immune-mediated and degenerative disease of the CNS, which affects both white and grey matter.1 In the past few decades, histologic2 and neuroimaging studies3 showed extensive focal and diffuse cortical involvement in MS. The most frequent form of focal cortical damage is represented by subpial cortical demyelination, which is specific to MS,4 and is predominant in the progressive phase of the disease. The overall volume of cortical lesions (CLs) represents 1 contributor of clinical disability and cognitive impairment.5 As such, detecting cortical involvement more accurately might be useful for disease prognostication.6
The detection of CLs in clinical practice is challenging because of (1) the lack of contrast sensitivity and a relatively low signal-to-noise ratio for CLs in conventional MRI protocols, (2) the paucity of gadolinium enhancement in the cortex due to the relatively low frequency of blood-brain barrier disruption, and (3) the small size of CL.7
Nonconventional MRI protocols might improve the evaluation of cortical alterations. Susceptibility-based MRI has already been proven useful in detecting MS-specific tissue changes.8 In the white matter, paramagnetic rim lesions (PRLs) have been extensively investigated: PRLs are detectable in susceptibility-based imaging because they harbor an edge of iron-laden activated microglia/macrophages, they are highly specific to MS, and they represent the imaging correlate of the histologically defined chronic active lesions.9 A subgroup of patients with MS presents peculiar paramagnetic rims extending along the border between white matter and cortex on susceptibility-sensitive MRI, which we will hereafter refer to as juxtacortical paramagnetic rims (JPRs). In contrast to the classic juxtacortical PRLs, JPRs are adjacent to the cortex and do not surround white matter lesions (WMLs).
After having observed the presence of these rims in quantitative susceptibility mapping (QSM) images in 1 postmortem brain, we undertook an investigation to understand their nature and implications. The aim of this study was (1) to assess the frequency of JPRs, (2) to explore their clinical relevance in patients with MS in vivo, and (3) to characterize them histologically.
Methods
Clinical Study
To assess the prevalence and clinical significance of JPRs, we selected 165 MS patients with availability of susceptibility-based MRI from an ongoing monocentric cohort study that enrolled patients with active relapsing remitting MS (RRMS) and nonactive progressive MS. We screened QSM maps (obtained from segmented 3D-echo planar imaging [3D-EPI]), magnetization-prepared 2 rapid acquisition gradient-echo (MP2RAGE), and 3D-fluid–attenuated inversion recovery (FLAIR) to assess for the presence of JPRs (QSM), PRLs (QSM), WMLs (FLAIR), and CL count/CL volume (CLV) (MP2RAGE). All scans were performed on a 3T-MRI clinical scanner.
JPRs were identified independently by 2 experienced neurologists (R.G. and A.C.), followed by a consensus review. Serum samples for measurement of serum neurofilament light chain (sNfL) were collected at the time of the MRI (±3 months) in 123 of 165 patients. sNfL Z scores were calculated as previously described by other authors.10 Since body mass index (BMI) values were missing in our data, a BMI of 25 was used. One hundred eight of the patients had a longitudinal standardized Expanded Disability Status Scale (EDSS) assessment in the context of the Swiss Multiple Sclerosis Cohort study (SMSC) (98 without JPRs and 10 with JPRs). These patients with MS were comparable with the whole original cohort (the data are listed in eTable 1, links.lww.com/WNL/D308).
We tested the following H0-hypothesis: MS patients with JPRs do not differ from patients without JPRs in demographics (age and sex); disease duration; clinical phenotype; EDSS at time of the MRI and EDSS evolution over time; sNfL Z scores; number of PRLs, WMLs, and CLs; and total CLV.
To test this hypothesis, we used a χ2 test for categorical data and a t test for normally distributed continuous data. For nonnormal data, a Wilcoxon rank-sum test was used (Table 1). To evaluate the disease progression rate in the 2 groups, we performed a Kaplan-Meier estimator for time to first worsening (defined by the EDSS score) by using the above-mentioned data from the SMSC. We also studied the association between the presence of JPRs and total CLV (independent variable) in a logistic regression model, which was adjusted for age, disease duration, and EDSS.
Table 1.
Univariate Analyses Investigating Differences Between the 2 Groups of Patients With MS In Vivo (Without and With JPRs)
| Without JPR (n = 149) | With JPR (n = 16) | p Value | |
| Age, y, median (IQR) | 47.0 (35.0–58.0) | 45.0 (32.0–53.2) | 0.251 |
| Sex, male, n (%) | 60.0 (40.3) | 5.0 (31.2) | 0.665 |
| Diagnosis, PMS, n (%) | 61.0 (40.9) | 3.0 (18.8) | 0.144 |
| EDSS score, median (IQR) | 3.0 (1.5–4.5) | 2.3 (1.5–4.5) | 0.670 |
| MSSS, median, (IQR) | 4.3 (2.6–5.9) | 4.8 (3.0–5.7) | 0.992 |
| Disease duration, median (IQR) | 5.6 (0.8–16.7) | 4.5 (0.7–8.7) | 0.553 |
| Medication, n (%) | 0.568 | ||
| Interferon | 5.0 (3.7) | 0.0 (0.0) | |
| Copaxone | 2.0 (1.5) | 0.0 (0.0) | |
| Dimethyl fumarate | 14.0 (10.4) | 2.0 (15.4) | |
| Siponimod | 2.0 (1.5) | 0.0 (0.0) | |
| Teriflunomide | 5.0 (3.7) | 0.0 (0.0) | |
| Fingolimod | 13.0 (9.6) | 4.0 (30.8) | |
| Ocrelizumab | 62.0 (45.9) | 6.0 (46.2) | |
| Rituximab | 15.0 (11.1) | 1.0 (7.7) | |
| Natalizumab | 4.0 (3.0) | 0.0 (0.0) | |
| No therapy at the time of imaging | 13.0 (9.6) | 0.0 (0.0) | |
| sNfL, pg/mL, median (IQR) | 8.6 (6.2–12.6) | 9.4 (6.7–16.3) | 0.647 |
| sNfL Z score BMI, median (IQR) | 0.4 (−0.4, 1.1), 66th percentile | 1.0 (0.2–1.5), 83rd percentile | 0.108 |
| No. of WML, median (IQR) | 40.0 (21.0–71.0) | 36.5 (10.7–63.0) | 0.509 |
| Total WML volume, mm3, median (IQR) | 6,211.0 (2,158.0–15,101.0) | 5,611.0 (1,974.0–11,341.7) | 0.498 |
| No. of periventricular lesions, median (IQR) | 8.0 (4.7–13.0) | 9.0 (2.0–10.2) | 0.484 |
| Periventricular lesion volume, mm3, median (IQR) | 575.0 (186.7–1,467.0) | 355.0 (66.7–1,301.7) | 0.300 |
| No. of juxtacortical lesions, median (IQR) | 31.0 (14.7,56.0) | 26.0 (8.7–49.5) | 0.485 |
| Juxtacortical lesion volume, mm3, median (IQR) | 2,340.0 (744.2–5,560.7) | 2,336.0 (820.7–4,241.2) | 0.672 |
| No. of cortical lesions, median (IQR) | 2.0 (0.0–8.2) | 4.0 (1.5–12.0) | 0.158 |
| Cortical lesion volume, mm3, median (IQR) | 28.5 (0.0–146.0) | 128.0 (15.0–352.0) | 0.040 |
| No. of leukocortical lesions, median (IQR) | 2.0 (0.0–7.0) | 3.0 (1.0–10.5) | 0.219 |
| Leukocortical lesion volume, mm3, median (IQR) | 25.0 (0.0–124.0) | 72.0 (13.0–196.0) | 0.150 |
| No. of rim-positive WMLs, median (IQR) | 2.0 (0.0–6.0) | 1.5 (1.0–7.5) | 0.086 |
Abbreviations: EDSS = Expanded Disability Status Scale; IQR = interquartile range; JPR = juxtacortical paramagnetic rim; MSSS = Multiple Sclerosis Severity Score; NfL = neurofilament light chain; PMS = progressive multiple sclerosis; WML = white matter lesion.
Postmortem Imaging and Histology
Five whole postmortem brains were obtained from the German MS Brain Bank and imaged on a clinical 3T-MRI (patients' characteristics are listed in eTable 2, links.lww.com/WNL/D308). The following sequences were acquired, adapted to ex vivo conditions: (1) segmented 3D-EPI to enable QSM11 and (2) MP2RAGE.12
After imaging, brains were cut by using the state-of-the-art approach described in detail elsewhere.13 Under MRI guidance, we selected and excised tissue blocks containing JPRs from the brain slabs. Slices of 4 µm thickness were stained immunohistochemically for myelin and activated microglia/macrophages; moreover, we performed iron staining (Turnbull).
Standard Protocol Approvals, Registrations, and Patient Consents
The postmortem study was approved by the ethical review committee of the University Medical Center Göttingen. The in vivo study was approved by the local ethics committee (Institutional Review Board of Northwest Switzerland), and all participants gave written consent before enrollment.
Data Availability
The data sets generated and analyzed in this study are available from the corresponding author on a reasonable request.
More details regarding methods are available in the eMethods (links.lww.com/WNL/D308).
Results
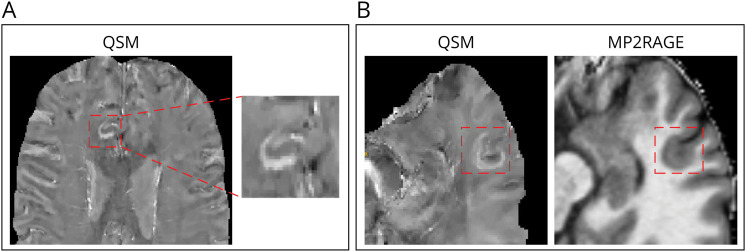
In the in vivo cohort, 16 of 165 (10%) patients with MS presented at least 1 JPR (range 1–4) (total = 26 JPRs); 15 JPRs showed an underlying focal cortical/juxtacortical hypointensity on MP2RAGE (Figure 1). The group of patients with JPRs did not differ significantly regarding demographics, disease duration, clinical phenotype, EDSS, and sNfL Z scores from those without JPRs. In total, 80% of patients with JPRs and 60% of patients without JPRs had RRMS. In the univariate analysis, total CLV was higher in the patients with JPRs (p = 0.040). In a logistic regression model, JPRs and CLV were associated, after adjusting for age (p = 0.029). Surprisingly, we did not find any association between the presence of JPRs and the number of PRLs (Table 1). Moreover, patients with JPRs did not show a different grade or evolution of clinical disability level (Figure 2). We also did not find any significant difference in cortical thickness change over a follow-up period of 2 years (±3 months) between the 2 groups (the data are given in eTable 3, links.lww.com/WNL/D308); however, we only had data available for 97 patients (89 without JPRs and 8 with JPRs).
Figure 1. Examples of JPRs in Patients With MS In Vivo.
In vivo QSM of a patient with multiple sclerosis included in our cohort showing a JPR (A) and in vivo QSM of another patient showing a JPR and the corresponding MP2RAGE revealing an underlying focal cortical/juxtacortical hypointensity (B). JPR = juxtacortical paramagnetic rim; MP2RAGE = magnetization-prepared 2 rapid acquisition gradient-echo; MS = multiple sclerosis; QSM = quantitative susceptibility mapping.
Figure 2. Estimation of the Disease Progression Rate of Patients With and Without JPRs.
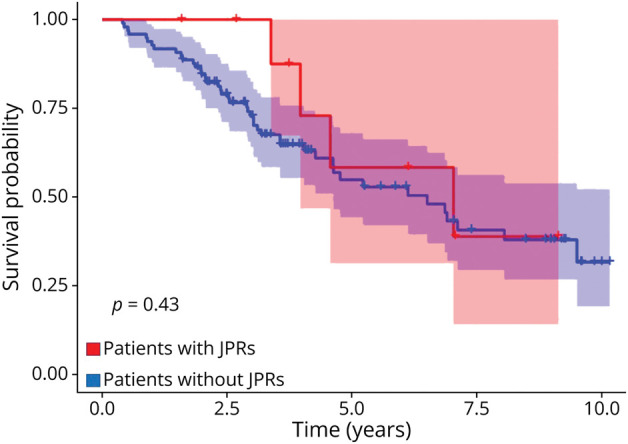
Kaplan-Meier estimate for time to first clinical worsening (EDSS progression). Number of patients with JPRs = 10, number of patients without JPRs = 98. This is a retrospective analysis, and the group (JPR) was not defined at the beginning of the observation time. EDSS = Expanded Disability Status Scale; JPR = juxtacortical paramagnetic rim; MS = multiple sclerosis.
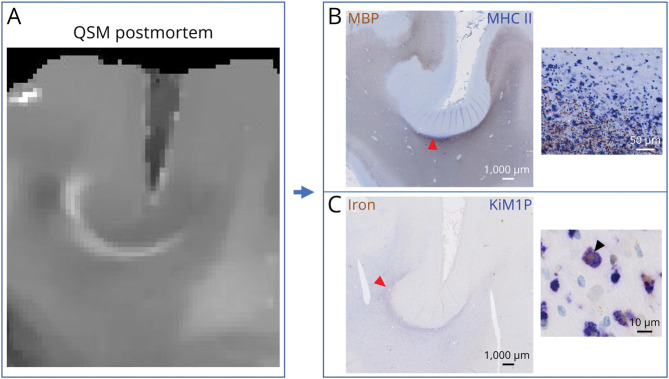
Only 1 of the 5 postmortem brains showed JPRs (patient 3, see eTable 2, links.lww.com/WNL/D308). In this brain, we observed 20 JPRs (Figure 3A) and we obtained the histologic profile of 6 of them. JPRs were associated with an infiltrate of major histocompatibility complex II–expressing amoeboid microglia, many of which were iron-laden (Figure 3, B and C). The edges of iron-laden microglia were positioned at the immediate subcortical white matter, only rarely in the cortex itself. Remarkably, those microglia rims (JPRs) were always underlying areas of demyelination extending through all cortical layers and were often located in the sulci (Figure 3B). In our postmortem patient cohort, no CLs with intracortical microglia rim were observed. The brain with JPRs was characterized by extended and confluent regions of cortical demyelination frequently involving the entire width of the cortical ribbon. In the other brains, the regions of cortical demyelination were fewer and less extensive. 3D-EPI magnetic resonance images showed 15 ± 8 whole cortical band lesions in the brains without JPR (range 6–25) and 126 whole cortical band lesions in the brain with JPRs. In contrast to the classical PRL, JPRs do not surround white matter lesions (Figure 4). By immunohistochemistry, the demyelinated cortex adjacent to JPRs did not contain relevant inflammatory infiltrates; also, we did not observe an increased presence of lymphoid aggregates in the meninges nearby. Classical PRLs were found in 3 of the 5 postmortem brains.
Figure 3. Histologic Characterization of JPRs.
Postmortem QSM showing a juxtacortical rim (A) and its histologic correlate showing accumulation of activated phagocytes (B) and iron-laden macrophages (C). Cortical demyelination is reaching the cortical layer VI. JPR = juxtacortical paramagnetic rim; QSM = quantitative susceptibility mapping.
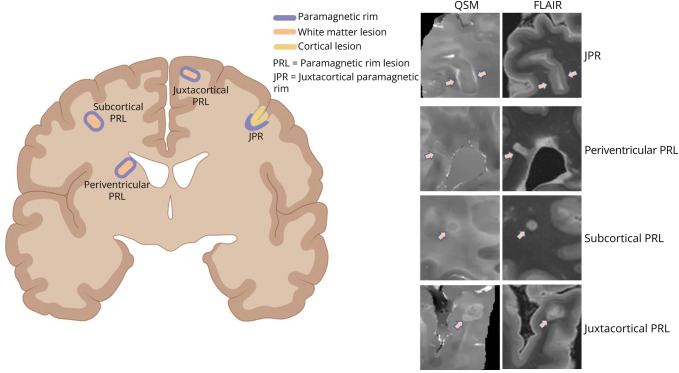
Figure 4. Different Types and Locations of Paramagnetic Rims in MS.
PRLs are lesions located in the periventricular, subcortical, and juxtacortical white matter, which are surrounded by a rim of increased susceptibility. They represent the imaging correlation of chronic active white matter lesions. JPRs are areas of local increase in susceptibility that are located in the juxtacortical white matter and that surround a cortical lesion involving the whole cortical ribbon (not a white matter lesion). FLAIR = fluid-attenuated inversion recovery; JPR = juxtacortical paramagnetic rim; MS = multiple sclerosis; PRL = paramagnetic rim lesion; QSM = quantitative susceptibility mapping.
Discussion
A subgroup of patients with MS shows rims of increased susceptibility right underneath the cortex (JPRs). This imaging feature was found in approximately 10% in our in vivo cohort of patients with MS. Histopathologically, those areas corresponded to a dense infiltrate of activated, in part, iron-laden phagocytes and were associated with demyelination involving the whole thickness of the cortical ribbon. Accordingly, the role of these rims in MS differential diagnosis should be explored because such areas of extended cortical demyelination are specific to MS4 and are difficult to detect in conventional MR images.
Since in all of our 6 specimens, the cortical demyelination adjacent to the rims also involved cortical layer VI (Figure 3B), we hypothesize that this rim of activated phagocytes represents a reaction of the immediate subcortical white matter to a pathologic process extending from the cortex.14 As such, JPRs might indicate a temporal stage of cortical demyelination. Histopathologically, no CLs with an intracortical microglia rim were observed in our postmortem patient cohort. In QSM images, we could not detect (at least with the resolution and sensitivity of our susceptibility MRIs) intracortical rims comparable with JPRs. This supports the hypothesis that JPRs originate in the subcortical white matter and that expansion of demyelination within the cortex occurs in the absence of relevant microglia/macrophage activation. Future work in samples that are acquired at higher spatial resolution should corroborate or refute this hypothesis. Furthermore, longitudinal MRI studies should aim at demonstrating whether the pathologic changes leading to JPR formation are originating from the subpial surface and hence proceed with a “surface-in” mechanism.
It is of interest that innate immunity activation in JPRs colocalized with WM demyelination but was not associated with WML. Therefore, JPRs represent a phenomenon distinct from that described in a previous study, where rims of increased susceptibility were reported close to the WM interface in leukocortical type I lesions.15
In our work, JPRs were associated with an increase in CL load in univariate analysis and in a logistic regression model. Our data suggest therefore that—when JPRs are present—demyelination of the whole overlying cortical ribbon is also present. Since this type of demyelination is specific to MS,4 JPRs might thus be useful for MS diagnosis; in addition, they may also be considered as an indirect sign of cortical pathology, which has been shown to be prognostic for increased disability and development of cognitive deficits.3 Consequently, the role of JPRs in MS differential diagnosis should be further explored because they underlie areas of extended cortical demyelination that are specific to MS4 and we normally cannot easily identify in conventional MRI. In addition, since CLs are a prognostic factor of conversion to progressive MS,6 JPRs might help us to identify patients deserving more aggressive treatment regimens in a first place. The identification of JPRs in clinical practice is feasible because it may be achieved using high-resolution susceptibility-based sequences such as the ones that are currently used to detect the central vein sign and PRLs.16
Remarkably, the number of JPRs was not associated with the number of PRLs in our MS cohort. This aspect might indicate that these pathologic processes are independent from each other and deserve ad hoc investigations in future studies.
A limitation of our work is that we could detect JPRs in only 1 postmortem brain. On the other hand, this reflects the frequency of this finding observed in vivo in a large cohort of patients with MS (10%). Another limitation was that we could not assess the association between the presence of JPRs and long-term disability, which is intrinsically due to the timing of the introduction of a susceptibility-based sequence in our MS protocol. New studies should aim at establishing this relationship for a better understanding of the prognostic value of this new imaging biomarker. Furthermore, since we did not administer gadolinium-based contrast agents within this study, we cannot determine with certitude whether an acute lesion was present in the JPR area at the time of the study scan. However, this scenario seems rather improbable to us. In fact, early rims have so far only been described in white matter, but not CLs by Absinta et al.,17 and they were reported after gadolinium administration. Thus, gadolinium deposits in microglia/macrophages might have contributed to it, although the nature of this early rim remains unclear to date. Moreover, in our study, JPRs were also found in patients with nonactive progressive MS, and our patients with active RRMS were all on disease-modifying treatment for at least 3 months, rendering the formation of new lesions unlikely. In addition, no patient enrolled in our study had active lesions in the location of JPR on the last conventional MRI before study inclusion.
In summary, we describe here a novel MRI feature in a subgroup of patients with MS, which has the potential to be used as a diagnostic and prognostic biomarker. Future longitudinal studies are warranted to corroborate the diagnostic and prognostic value of JPRs in patients with MS.
Acknowledgment
The authors thank Ms. Marguerite Limberg and Dr. Bettina Fischer-Barnicol for helping in the recruitment process. The authors also thank Ms. Heidelinde Brodmerkel, Mr. René Müller, and Ms. Olga Kowatsch for expert technical assistance. Figure 4 was adapted from “Brain, coronal cut, simplified,” by BioRender.com (2023). Retrieved from app.biorender.com/biorender-templates.
Glossary
- BMI
body mass index
- CL
cortical lesion
- CLV
CL volume
- EDSS
Expanded Disability Status Scale
- EPI
echo planar imaging
- FLAIR
fluid-attenuated inversion recovery
- JPR
juxtacortical paramagnetic rim
- MP2RAGE
magnetization-prepared 2 rapid acquisition gradient-echo
- MS
multiple sclerosis
- PRL
paramagnetic rim lesion
- QSM
quantitative susceptibility mapping
- RRMS
relapsing remitting MS
- SMSC
Swiss Multiple Sclerosis Cohort study
- sNfL
serum neurofilament light chain
- WML
white matter lesion
Appendix. Authors
| Name | Location | Contribution |
| Riccardo Galbusera, MD | Neurology Clinic and Policlinic, Departments of Medicine, Clinical Research and Biomedical Engineering, Translational Imaging in Neurology (ThINk) Basel, Department of Biomedical Engineering, and Research Center for Clinical Neuroimmunology and Neuroscience (RC2NB) Basel, University Hospital Basel and University of Basel, Switzerland | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Erik Bahn, MD | Institute of Neuropathology, University Medical Center Göttingen, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Matthias Weigel, PhD | Neurology Clinic and Policlinic, Departments of Medicine, Clinical Research and Biomedical Engineering, Translational Imaging in Neurology (ThINk) Basel, Department of Biomedical Engineering, and Research Center for Clinical Neuroimmunology and Neuroscience (RC2NB) Basel, University Hospital Basel and University of Basel; Radiological Physics, Department of Radiology, University Hospital Basel, Switzerland | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Alessandro Cagol, MD | Neurology Clinic and Policlinic, Departments of Medicine, Clinical Research and Biomedical Engineering, Translational Imaging in Neurology (ThINk) Basel, Department of Biomedical Engineering, and Research Center for Clinical Neuroimmunology and Neuroscience (RC2NB) Basel, University Hospital Basel and University of Basel, Switzerland | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Po-Jui Lu, PhD | Neurology Clinic and Policlinic, Departments of Medicine, Clinical Research and Biomedical Engineering, Translational Imaging in Neurology (ThINk) Basel, Department of Biomedical Engineering, and Research Center for Clinical Neuroimmunology and Neuroscience (RC2NB) Basel, University Hospital Basel and University of Basel, Switzerland | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Sabine A. Schaedelin, MSc | Department of Clinical Research, University Hospital Basel, Switzerland | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Jonas Franz, MD | Institute of Neuropathology | Major role in the acquisition of data; drafting/revision of themanuscript for content, including medical writing for content |
| Muhamed Barakovic, PhD | Neurology Clinic and Policlinic, Departments of Medicine, Clinical Research and Biomedical Engineering, Translational Imaging in Neurology (ThINk) Basel, Department of Biomedical Engineering, and Research Center for Clinical Neuroimmunology and Neuroscience (RC2NB) Basel, University Hospital Basel and University of Basel, Switzerland | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Reza Rahmanzadeh, MD, PhD | Institute of Diagnostic and Interventional Neuroradiology, Bern University Hospital, University of Bern, Switzerland | Drafting/revision of the manuscript for content, including medical writing for content |
| Peter Dechent, PhD | Department of Cognitive Neurology, MR-Research in Neurosciences, University Medical Center Göttingen, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Govind Nair, PhD | National Institute of Neurological Disorders and Stroke, Bethesda, MD | Drafting/revision of the manuscript for content, including medical writing for content |
| Wolfgang Brück, MD | Institute of Neuropathology, University Medical Center Göttingen, Germany | Drafting/revision of the manuscript for content, including medical writing for content |
| Jens Kuhle, MD, PhD | Neurology Clinic and Policlinic, Departments of Medicine, Clinical Research and Biomedical Engineering, and Research Center for Clinical Neuroimmunology and Neuroscience (RC2NB) Basel, University Hospital Basel and University of Basel, Switzerland | Drafting/revision of the manuscript for content, including medical writing for content |
| Ludwig Kappos, MD | Neurology Clinic and Policlinic, Departments of Medicine, Clinical Research and Biomedical Engineering, Translational Imaging in Neurology (ThINk) Basel, Department of Biomedical Engineering, and Research Center for Clinical Neuroimmunology and Neuroscience (RC2NB) Basel, University Hospital Basel and University of Basel, Switzerland | Drafting/revision of the manuscript for content, including medical writing for content |
| Christine Stadelmann, MD | Institute of Neuropathology, University Medical Center Göttingen, Germany | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Cristina Granziera, MD, PhD | Neurology Clinic and Policlinic, Departments of Medicine, Clinical Research and Biomedical Engineering, Translational Imaging in Neurology (ThINk) Basel, Department of Biomedical Engineering, and Research Center for Clinical Neuroimmunology and Neuroscience (RC2NB) Basel, University Hospital Basel and University of Basel, Switzerland | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
Footnotes
Editorial, page e208085
Study Funding
This work was funded by the Swiss National Science Fund PP00P3_176984 and PP00P3_206151 and supported by the German Ministry of Education (BMBF; KKNMS German competence network for multiple sclerosis). This project was also supported by the Deutsche Forschungsgemeinschaft (DFG) transregional collaborative research center (CRC) TRR 274/1 “Checkpoints of CNS recovery,” Project ID 408885537 B01, STA 1389/5-1, the DFG under Germany's Excellence Strategy (EXC 2067/1-390729940), the Gemeinnützige Hertie Foundation, the Deutsche Multiple Sklerose Gesellschaft (DMSG), and the National MS Society (USA) to C. Stadelmann. J. Franz was supported by the clinician scientist program of the TRR 274/1.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med. 2018;378(2):169-180. doi: 10.1056/NEJMra1401483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lucchinetti CF, Popescu BFG, Bunyan RF, et al. Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med. 2011;365(23):2188-2197. doi: 10.1056/NEJMoa1100648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calabrese M, Rocca MA, Atzori M, et al. A 3-year magnetic resonance imaging study of cortical lesions in relapse-onset multiple sclerosis. Ann Neurol. 2010;67(3):376-383. doi: 10.1002/ana.21906 [DOI] [PubMed] [Google Scholar]
- 4.Junker A, Wozniak J, Voigt D, et al. Extensive subpial cortical demyelination is specific to multiple sclerosis. Brain Pathol. 2020;30(3):641-652. doi: 10.1111/bpa.12813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrison DM, Roy S, Oh J, et al. Association of cortical lesion burden on 7-T magnetic resonance imaging with cognition and disability in multiple sclerosis. JAMA Neurol. 2015;72(9):1004-1012. doi: 10.1001/jamaneurol.2015.1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beck ES, Maranzano J, Luciano NJ, et al. Cortical lesion hotspots and association of subpial lesions with disability in multiple sclerosis. Mult Scler. 2022;28(9):1351-1363. doi: 10.1177/13524585211069167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daams M, Geurts JJG, Barkhof F. Cortical imaging in multiple sclerosis: recent findings and “grand challenges.” Curr Opin Neurol. 2013;26(4):345-352. doi: 10.1097/WCO.0b013e328362a864 [DOI] [PubMed] [Google Scholar]
- 8.Sati P, Thomasson DM, Li N, et al. Rapid, high-resolution, whole-brain, susceptibility-based MRI of multiple sclerosis. Mult Scler. 2014;20(11):1464-1470. doi: 10.1177/1352458514525868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maggi P, Sati P, Nair G, et al. Paramagnetic rim lesions are specific to multiple sclerosis: an international multicenter 3T MRI study. Ann Neurol. 2020;88(5):1034-1042. doi: 10.1002/ana.25877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benkert P, Meier S, Schaedelin S, et al. Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: a retrospective modelling and validation study. Lancet Neurol. 2022;21(3):246-257. doi: 10.1016/S1474-4422(22)00009-6 [DOI] [PubMed] [Google Scholar]
- 11.Liu C, Li W, Tong KA, Yeom KW, Kuzminski S. Susceptibility-weighted imaging and quantitative susceptibility mapping in the brain. J Magn Reson Imaging. 2015;42(1):23-41. doi: 10.1002/jmri.24768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marques JP, Kober T, Krueger G, van der Zwaag W, Van de Moortele PF, Gruetter R. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage. 2010;49(2):1271-1281. doi: 10.1016/j.neuroimage.2009.10.002 [DOI] [PubMed] [Google Scholar]
- 13.Luciano NJ, Sati P, Nair G, et al. Utilizing 3D printing technology to merge MRI with histology: a protocol for brain sectioning. J Vis Exp. 2016;(118):54780. doi: 10.3791/54780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pardini M, Brown JWL, Magliozzi R, Reynolds R, Chard DT. Surface-in pathology in multiple sclerosis: a new view on pathogenesis? Brain. 2021;144(6):1646-1654. doi: 10.1093/brain/awab025 [DOI] [PubMed] [Google Scholar]
- 15.Castellaro M, Magliozzi R, Palombit A, et al. Heterogeneity of cortical lesion susceptibility mapping in multiple sclerosis. AJNR Am J Neuroradiol. 2017;38(6):1087-1095. doi: 10.3174/ajnr.A5150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sati P, Oh J, Constable RT, et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol. 2016;12(12):714-722. doi: 10.1038/nrneurol.2016.166 [DOI] [PubMed] [Google Scholar]
- 17.Absinta M, Sati P, Schindler M, et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Invest. 2016;126(7):2597-2609. doi: 10.1172/JCI86198 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated and analyzed in this study are available from the corresponding author on a reasonable request.
More details regarding methods are available in the eMethods (links.lww.com/WNL/D308).