Abstract
Background and Objectives
It is not possible to fully establish the safety of a disease-modifying drug (DMD) for multiple sclerosis (MS) from randomized controlled trials as only very common adverse events occurring over the short-term can be captured, and the quality of reporting has been variable. We examined the relationship between the DMDs for MS and potential adverse events in a multiregion population-based study.
Methods
We identified people with MS using linked administrative health data from 4 Canadian provinces. MS cases were followed from the most recent of first MS or related demyelinating disease event on January 1, 1996, until the earliest of emigration, death, or December 31, 2017. DMD exposure primarily comprised β-interferon, glatiramer acetate, natalizumab, fingolimod, dimethyl fumarate, teriflunomide, and alemtuzumab. We examined associations between DMD exposure and infection-related hospitalizations and physician visits using recurrent events proportional means models and between DMD exposure and 15 broad categories of incident adverse events using stratified multivariate Cox proportional hazard models.
Results
We identified 35,894 people with MS. While virtually all DMDs were associated with a 42%–61% lower risk of infection-related hospitalizations, there was a modest increase in infection-related physician visits by 10%–33% for select DMDs. For incident adverse events, most elevated risks involved a second-generation DMD, with alemtuzumab's hazard of thyroid disorders being 19.42 (95% CI 9.29–36.51), hypertension 4.96 (95% CI 1.78–13.84), and cardiovascular disease 3.72 (95% CI 2.12–6.53). Natalizumab's highest risk was for cardiovascular disease (adjusted hazard ratio [aHR] 1.61; 95% CI 1.24–2.10). For the oral DMDs, fingolimod was associated with higher hazards of cerebrovascular (aHR 2.04; 95% CI 1.27–3.30) and ischemic heart diseases (aHR 1.64; 95% CI 1.10–2.44) and hypertension (aHR 1.73; 95% CI 1.30–2.31); teriflunomide with higher hazards of thyroid disorders (aHR 2.30; 95% CI 1.11–4.74), chronic liver disease (aHR 1.94; 95% CI 1.19–3.18), hypertension (aHR 1.76; 95% CI 1.32–2.37), and hyperlipidemia (aHR 1.61; 95% CI 1.07–2.44); and from complementary analyses (in 1 province), dimethyl fumarate with acute liver injury (aHR 6.55; 95% CI 1.96–21.87).
Discussion
Our study provides an extensive safety profile of several different DMDs used to treat MS in the real-world setting. Our findings not only complement those observed in short-term clinical trials but also provide new insights that help inform the risk-benefit profile of the DMDs used to treat MS in clinical practice. The results of this study highlight the continued need for long-term, independent safety studies of the DMDs used to treat MS.
Classification of Evidence
This study provides Class III evidence that for patients with MS, while DMD exposure reduces the risk of infection-related hospitalizations, there are increased risks of infection-related physician visits and incident adverse events for select DMDs.
Introduction
Disease-modifying drugs (DMDs) for multiple sclerosis (MS) are typically approved after short-term randomized controlled trials (RCTs) conducted over 2–3 years in a limited number of carefully selected individuals and are designed and powered to examine efficacy, not safety. It is not possible to fully establish DMD safety from such studies as only very common adverse events occurring over the short-term are captured, and the quality of reporting has been variable.1-4 Individuals treated in routine clinical practice, such as older persons or those with comorbidities, are often excluded from RCTs, limiting the generalizability of findings.5
Adverse drug reactions have been reported as one of the top 10 leading causes of death in the United States (pre-severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]/coronavirus disease 2019 pandemic), resulting in >100,000 deaths each year.6 While the first-generation MS DMDs (β-interferon and glatiramer acetate) have been considered relatively safe, to date, surprisingly, few population-based safety studies have been conducted for those or indeed for any of the DMDs.7-11 Although cancer risk is a concern for all DMDs,12 just a few population-based studies have examined this and for a limited number of DMDs.13-15 Very few safety-related studies are independent of the pharmaceutical companies that sell these products, a recognized conflict of interest and a problem in the MS field.4
Our primary research question was “what is the relationship between the MS DMDs and risk of adverse events?” We examined this in an MS population using linked administrative data from 4 Canadian provinces collected over 22 years within a universal health care setting.
Methods
Data Sources and Study Population
Canada has a universal, publicly funded health care system; health services are delivered to >98% of the population provincially.16 We used linked administrative data from British Columbia, Manitoba, Saskatchewan, and Nova Scotia. Combined, these regions covered >8 million residents, representing ∼25% of Canada's population (2016).17 Linked data included the Discharge Abstract Database (hospital admissions/discharges and International Classification of Diseases [ICD] codes),e1 physician claims (medical services, including laboratory-related visits, and ICD codes),e2 provincial health insurance data (demographics and residency confirmation [through enrollment in the mandatory provincial health program],e3 and for Manitoba/Saskatchewan, death dates), and Vital Statistics (capturing death dates in British Columbia/Nova Scotia).e4 Prescription data from British Columbia/Manitoba/Saskatchewane5 and the Dalhousie MS Research database, Nova Scotia, provided DMD-related information. Together, data captured all medically necessary hospitalizations, physician services, and MS DMD prescriptions filled, unaffected by ability to pay.
We identified all MS cases (described previously), as any individual with ≥3 MS diagnostic codes (ICD-9/10:340/G35) or ≥1 DMD record.17,18 The index date was the most recent of the first MS or related demyelinating disease code recorded in the hospital/physician claims data or first DMD prescription filled; a person's 18th birthday; or the first availability of the DMD prescription data in each province (January 1, 1996/1997/1998 [British Columbia/Saskatchewan/Nova Scotia] and April 1, 1996 [Manitoba]).
MS cases had ≥1 year of provincial residency to allow for baseline characterization at the index date (sex, age, calendar year, socioeconomic status [SES], and comorbidity). SES was based on neighborhood-level income, using Statistics Canada's algorithm linking census-derived family income with each individual's residential postcode.17 Comorbidity was measured by the Charlson Comorbidity Index (CCI), using diagnostic codes in hospital/physician (primary/secondary care) claims data in the year pre-index date, excluding hemiplegia/paraplegia.19 This index has been widely used in MS and other populations, including the examination of long-term mortality,16 other DMD-related outcomes,20,21 and adverse drug events.22,23 The index also captures several common comorbidities, including those most relevant to specific contraindications/cautions for DMD use, such as cardiovascular, cerebrovascular, chronic lung and liver diseases, diabetes, and malignancy. We followed MS cases from their index date until the earliest of emigration, death, or study end (December 31, 2017 [British Columbia/Manitoba/Nova Scotia], and March 31, 2018 [Saskatchewan]).
DMD Exposure
The DMDs approved for MS in Canada during the study period included the first-generation DMDs (β-interferons [all products combined] and glatiramer acetate) and second-generation DMDs (natalizumab, fingolimod, dimethyl fumarate, teriflunomide, alemtuzumab, daclizumab [withdrawn March 2018], and ocrelizumab). DMDs were assessed individually (except for daclizumab and ocrelizumab due to insufficient exposure in our cohorts).
We examined DMD exposure grouped as any DMD, then by generation, and by individual DMD. DMD exposure was defined using 2 approaches (“current” and minimum cumulative exposure) and was updated over time (treated as a time-varying variable). Exposure periods were calculated based on the numbers of days/quantity dispensed or start/stop dates for each DMD. A person could be exposed to 1 or more individual DMDs during the follow-up period, and exposures to different DMDs (e.g., due to switching) were accounted for in the analysis by separate DMD exposure indicators.
“Current” exposure was based on the duration of each DMD used during follow-up. Discontinuation occurred if no dispensations for that DMD for ≥90 consecutive days, plus a 30-day grace period.20 For alemtuzumab, exposure was “current” for 12 months from the first prescription filled. We used a similar approach for ocrelizumab, except with a 6-month period. Each of these DMDs was then considered discontinued if no further prescriptions were filled for that DMD (plus a 30-day grace period). Individuals could only be exposed to 1 DMD at a time (i.e., once a subsequent DMD prescription was filled, then the previous DMD was considered discontinued).
For the minimum cumulative approach, exposure was based on contiguous use of a DMD, defined as ≥6 months for β-interferon and glatiramer acetate; ≥3 months for natalizumab, fingolimod, dimethyl fumarate, and teriflunomide; and after 3 months following the first prescription filled for alemtuzumab or ocrelizumab.16 These definitions were based on the minimum time needed for a DMD to yield a clinical response.16 Once the definition of minimum cumulative exposure for a DMD was reached, a person was considered exposed to that DMD for the remainder of their study follow-up. If the minimum cumulative exposure was not reached for any individual DMD, then that person was considered not exposed to that DMD.
Adverse Event-Related Outcomes
All adverse safety signals (adverse event-related outcomes) were identified using diagnostic codes (ICD-9/10).
First, infection-related outcomes captured in the physician and hospital data were assessed as recurrent events (eTable 1, links.lww.com/WNL/D339). As serious infections are of particular concern and hospitalizations can serve as a proxy for severity, the hospital and physician visit data were examined separately (this was performed for infections only, not for other outcomes). To minimize double counting, any overlapping hospital stays or stays beginning ≤1 day of the previous hospitalization were considered as 1 event.20,24 Similarly, multiple infection-related physician visits falling within a 30-day period were considered as 1 event.24 The duration of an infection-related hospitalization was excluded from follow-up as someone could not be at risk of another infection-related hospitalization during the existing event, by definition. A 29-day period after an infection-related physician claim was excluded, based on the same reasoning.
Second, incident adverse events were defined as the presence of ≥1 ICD code for each event of interest captured in physician/hospital data (eTable 2, links.lww.com/WNL/D339). These were identified a priori based on the literature and relevant product monographs7,24-30 and guided by their occurrence and potential importance in MS. They included autoimmune diseases, cancer, cerebrovascular diseases, chronic liver diseases, chronic kidney diseases, diabetes, hyperlipidemia, hypertension, any mental condition (anxiety, depression, or bipolar disorders), migraine, and thyroid disorders. Cardiovascular disorders were assessed as either ischemic heart disease or “other” forms of heart diseases (e.g., pericarditis, cardiac arrhythmias). Incident was defined as not present in the year pre-index date. For each adverse event, persons with evidence of that event in the year pre-index date were not included such that the cohorts could naturally vary for each event examined.
Statistical Analyses
We examined the association between “current” DMD exposure and recurrent infection-related events using a proportional means model with robust sandwich variance estimates. This allowed each individual to have repeated events while accounting for dependence of events. The models were adjusted for sex, SES (quintiles), and age (continuous) at the index date and Charlson comorbidity score (categorized as 0, 1, 2, or ≥3 and updated over time). The index year was also included as a continuous variable to account for secular changes in health care use over time.20,24
For all other adverse events (i.e., not infection-related), the association between minimum cumulative DMD exposure and the incident event was examined using a stratified multivariate Cox proportional hazard model. The proportional hazards assumptions were examined by an interaction term between covariates and log (follow-up). Follow-up was defined as time from the index date to the adverse event date of interest or study end. The same model adjustments were applied, except for the index year where models were stratified (grouped as calendar years: 1996–1999, 2000–2005, 2006–2011, or 2012–2017/18) to account for potential differences in health care use over time, baseline risk, and unequal follow-up across each stratum.
As per data access/privacy requirements, analyses were performed within each province, and the results were combined using random-effects meta-analyses16,20 and reported as adjusted hazard ratios (aHRs) and 95% CIs.
We conducted 3 complementary analyses in the largest province, British Columbia, including (1) 2 additional incident adverse events—acute liver injury and abnormal hematologic findings (eTable 2, links.lww.com/WNL/D339),7 using the “current” exposure approach; (2) an “intention-to-treat” analysis, by defining the minimum cumulative DMD exposure as at least 1 day; and (3) an assessment of potential incident adverse events by using previously published or validated case definitions (9 were found; eTable 3). Sensitivity analyses included (1) computing E-values to assess how likely the findings were due to unobserved confounding,31 (2) computing summary measures to assess the susceptibility of findings to selection bias,32 (3) comparing the results of unadjusted and adjusted models to examine the effects of selected confounders,33 (4) using each individual comorbidity (captured in the CCI) as separate covariates, and (5) using propensity score as a covariate adjustment whereby propensity scores for DMD exposure status (never vs ever) for each outcome were generated separately using logistic regression and included sex, SES (quintiles), individual comorbidity (captured in the CCI), calendar year, and age (modeled with a cubic spline) at index date. The latter 2 sensitivity analyses were conducted in the largest province. These are recognized approaches to assess the impact of biases, including unobserved confounders, in observational studies.31-33 Statistical analyses were performed using SAS version 9.4 and R version 4.0.2.
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by Research Ethics Boards at the Universities of British Columbia/Saskatchewan/Manitoba (H18-00407/HS21764) and Nova Scotia Health (1023555) and registered with ClinicalTrials.gov (NCT04472975).
Data Availability
With the appropriate approvals, data may be accessed through Population Data British Columbia, Saskatchewan Health Quality Council, Manitoba Centre for Health Policy, and Health Data Nova Scotia of Dalhousie University.
Results
Cohort Characteristics
We identified 35,894 people with MS (Table 1, described previously17). These individuals formed the total source population for analyses. In brief, the mean (SD) age at the index date was 44.6 (13.6) years, and the mean (SD) follow-up from index to study end was 12.0 (7.2) years. Everyone from the source population was included in the infection-related analyses. For the incident adverse events, the total number of persons ranged from 26,513 for any mental condition to 35,709 for chronic kidney diseases, with the mean follow-up ranging from 6.3 (SD 6.2) years to 11.7 (SD 7.1) years.
Table 1.
Characteristics of Persons With Multiple Sclerosis Included in the Analysis for Each Potential Adverse Event of Interest From a Total Source Cohort of 35,894 Personsa
| Adverse event of interest | Excluded (event present in the year pre-index date) | Characteristics of persons with MS included in the analysis | ||
| Total no. of persons | Total no. of persons | Age at index date, y, mean (SD) | Follow-up from index to study end, y, mean (SD) | |
| Infection-related hospitalizations | NA | 35,894 | 44.6 (13.6) | 12.0 (7.2) |
| Infection-related physician visits | NA | 35,894 | 44.6 (13.6) | 12.0 (7.2) |
| Autoimmune diseases | 3,388 | 32,506 | 44.4 (13.5) | 8.2 (6.6) |
| Cancer | 994 | 34,900 | 44.4 (13.5) | 10.8 (7.0) |
| Cardiovascular disorders (ischemic heart diseases) | 1,074 | 34,820 | 44.2 (13.4) | 10.7 (7.1) |
| Cardiovascular disorders (other forms of heart diseases) | 1,257 | 34,637 | 44.2 (13.3) | 10.4 (7.0) |
| Cerebrovascular diseases | 1,508 | 34,386 | 44.3 (13.4) | 11.5 (7.2) |
| Chronic liver diseases | 312 | 35,582 | 44.5 (13.6) | 11.5 (7.1) |
| Chronic kidney diseases | 185 | 35,709 | 44.5 (13.5) | 11.7 (7.1) |
| Diabetes | 1,411 | 34,483 | 44.2 (13.4) | 11.0 (7.1) |
| Hyperlipidemia | 1,116 | 34,778 | 44.3 (13.6) | 10.1 (6.9) |
| Hypertension | 3,495 | 32,399 | 43.5 (13.2) | 9.2 (6.8) |
| Mental conditions (anxiety, depression, bipolar disorder) | 9,381 | 26,513 | 44.9 (14.0) | 6.3 (6.2) |
| Migraine | 1,867 | 34,027 | 44.8 (13.7) | 10.7 (7.3) |
| Thyroid disorders (autoimmune hypothyroidism and hyperthyroidism) | 211 | 35,683 | 44.5 (13.6) | 11.7 (7.2) |
| Acute liver injurya | 8 | 19,352 | 45.0 (13.5) | 11.7 (7.3) |
| Abnormal hematologic findingsa | 765 | 18,595 | 44.8 (13.4) | 11.8 (7.3) |
Abbreviation: NA = not applicable (as per the methods, a person was permitted to have an infection in the prior year; thus, no person was excluded).
Except for infections, all potential adverse events were identified as present in either the in-patient and out-patient information (hospitalization and/or physician visit data).
Total source cohort size was 35,894 from all 4 provinces, except for 2 adverse event of interest, acute liver injury, and abnormal hematologic findings (shown in italics), which was performed in British Columbia only where the total source cohort was 19,360.
Infection-Related Outcomes
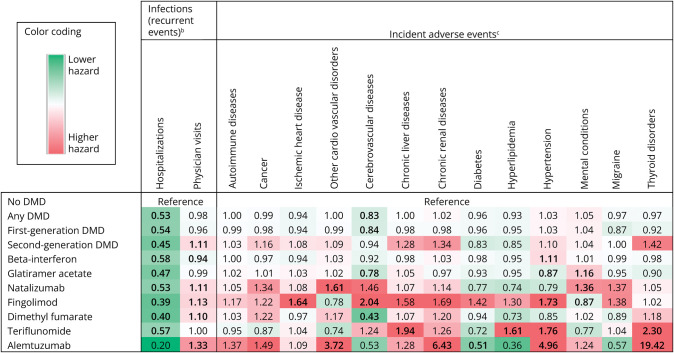
Current exposure to any DMD (vs no current exposure) was associated with a 47% lower hazard of infection-related hospitalizations (95% CI 37%–56%) (Figure, Table 2). This lower hazard was observed for the first-generation and second-generation DMDs (examined as 2 separate groups) and for each individual DMD but was not significant for alemtuzumab. For the individual DMDs, this lower hazard ranged from 42% (aHR 0.58; 95% CI 0.50–0.67) to 61% (aHR 0.39; 95% CI 0.16–0.94). By contrast, there was a modest increase in infection-related physician visits for any second-generation DMD (by 11%; 95% CI 4%–17%), and for some individual DMDs, ranging from a 33% (95% CI 13%–58%) higher hazard for alemtuzumab to 10%–13% for dimethyl fumarate (95% CI 4%–18%) and fingolimod (95% CI 3%–25%). Only β-interferon was associated with a lower hazard in both health care settings, albeit only modestly for infection-related physician visits (aHR 0.94; 95% CI 0.90–0.99). No other infection-related findings were significant in either health care setting. Crude rates of infection-related events for DMDs were highest for alemtuzumab in both settings and lowest for fingolimod (for hospitalizations) and teriflunomide (for physician visits) (Table 2).
Figure. Risk Hazarda of Potential Adverse Events Associated With Disease-Modifying Drugs Used to Treat Multiple Sclerosis in Canada (1996–2017/18).
DMD = disease-modifying drug. Bold indicates p < 0.05. Except for infections, all potential adverse events were identified as presence in either in-patient or out-patient information (hospitalizations or physician visits). aFindings from 4 Canadian provinces combined (British Columbia, Saskatchewan, Manitoba, Nova Scotia). The results were adjusted for sex, socioeconomic status (quintiles), and age (continuous) at the index date and Charlson comorbidity score (categorized as 0, 1, 2, or ≥3 and updated overtime). bRecurrent events using the “current” DMD exposure approach. cIncident adverse events using the “minimum” cumulative DMD approach. See Table 2 for full results, including the hazard ratios with 95% CI and the crude rate for each event.
Table 2.
Risk of Potential Adverse Events Associated With Disease-Modifying Drugs Used to Treat Multiple Sclerosis in Canada (1996–2017/18)
| Potential adverse event | No. of cases | Py of follow-up | Crude rate per 1,000 py | Crude hazard ratio (95% CI) | Adjusted hazard ratioa (95% CI) |
| Recurrent events using the “current” DMD exposure approachb | |||||
| Infection-related hospitalizations | |||||
| No DMD | 21,346 | 366,061 | 58.31 | Reference | Reference |
| Any DMD | 1,362 | 63,194 | 21.55 | 0.36 (0.32–0.41) | 0.53 (0.44–0.63) |
| First-generation DMD | 1,208 | 54,514 | 22.16 | 0.37 (0.33–0.43) | 0.54 (0.45–0.64) |
| Second-generation DMD | 154 | 8,680 | 17.74 | 0.27 (0.20–0.36) | 0.45 (0.33–0.62) |
| β-Interferon | 861 | 36,444 | 23.63 | 0.41 (0.36–0.46) | 0.58 (0.50–0.67) |
| Glatiramer acetate | 347 | 18,071 | 19.20 | 0.32 (0.27–0.38) | 0.47 (0.37–0.59) |
| Natalizumab | 29 | 1,747 | 16.60 | 0.30 (0.21–0.45) | 0.53 (0.36–0.78) |
| Fingolimod | 22 | 1,726 | 12.75 | 0.20 (0.07–0.54) | 0.39 (0.16–0.94) |
| Dimethyl fumarate | 48 | 3,347 | 14.34 | 0.24 (0.17–0.34) | 0.40 (0.29–0.56) |
| Teriflunomide | 36 | 1,536 | 23.44 | 0.38 (0.26–0.56) | 0.57 (0.39–0.84) |
| Alemtuzumab | 9 | 317 | 28.38 | 0.10 (0.01–1.44) | 0.20 (0.01–2.84) |
| Infection-related physician visits | |||||
| No DMD | 281,468 | 338,404 | 831.75 | Reference | Reference |
| Any DMD | 45,214 | 58,390 | 774.34 | 0.94 (0.92–0.96) | 0.98 (0.92–1.03) |
| First-generation DMD | 38,618 | 50,276 | 768.12 | 0.93 (0.90–0.95) | 0.96 (0.91–1.01) |
| Second-generation DMD | 6,596 | 8,115 | 812.85 | 1.02 (0.98–1.07) | 1.11 (1.04–1.17) |
| β-Interferon | 25,265 | 33,569 | 752.63 | 0.91 (0.88–0.94) | 0.94 (0.90–0.99) |
| Glatiramer acetate | 13,353 | 16,707 | 799.26 | 0.96 (0.91–1.01) | 0.99 (0.90–1.08) |
| Natalizumab | 1,377 | 1,630 | 845.03 | 1.05 (0.91–1.20) | 1.11 (0.95–1.31) |
| Fingolimod | 1,309 | 1,614 | 810.94 | 1.03 (0.92–1.16) | 1.13 (1.03–1.25) |
| Dimethyl fumarate | 2,561 | 3,123 | 819.98 | 1.03 (0.96–1.09) | 1.10 (1.04–1.18) |
| Teriflunomide | 1,069 | 1,446 | 739.51 | 0.94 (0.84–1.06) | 1.00 (0.91–1.10) |
| Alemtuzumab | 274 | 296 | 927.06 | 1.16 (0.97–1.38) | 1.33 (1.13–1.58) |
| Incident adverse event using the “minimum” cumulative DMD approachb | |||||
| Autoimmune diseases | |||||
| No DMD | 11,557 | 214,409 | 53.90 | Reference | Reference |
| Any DMD | 2,493 | 52,554 | 47.44 | 0.95 (0.91–0.99) | 1.00 (0.95–1.04) |
| First-generation DMD | 2,374 | 50,412 | 47.09 | 0.95 (0.91–1.00) | 0.99 (0.95–1.04) |
| Second-generation DMD | 272 | 5,472 | 49.71 | 0.99 (0.87–1.12) | 1.03 (0.91–1.17) |
| β-Interferon | 1,812 | 38,355 | 47.24 | 0.96 (0.91–1.01) | 1.00 (0.95–1.06) |
| Glatiramer acetate | 800 | 16,353 | 48.92 | 1.00 (0.93–1.08) | 1.02 (0.95–1.10) |
| Natalizumab | 71 | 1,479 | 48.00 | 1.00 (0.78–1.26) | 1.05 (0.83–1.33) |
| Fingolimod | 60 | 1,118 | 53.65 | 1.11 (0.86–1.44) | 1.17 (0.90–1.51) |
| Dimethyl fumarate | 118 | 2,328 | 50.70 | 1.01 (0.78–1.30) | 1.03 (0.81–1.31) |
| Teriflunomide | 43 | 945 | 45.51 | 0.94 (0.63–1.41) | 0.95 (0.64–1.43) |
| Alemtuzumab | 11 | 158 | 69.43 | 1.33 (0.73–2.42) | 1.37 (0.76–2.50) |
| Cancer | |||||
| No DMD | 5,801 | 297,917 | 19.47 | Reference | Reference |
| Any DMD | 1,183 | 78,421 | 15.09 | 0.77 (0.70–0.84) | 0.99 (0.92–1.06) |
| First-generation DMD | 1,132 | 75,493 | 14.99 | 0.77 (0.70–0.84) | 0.98 (0.91–1.05) |
| Second-generation DMD | 146 | 8,277 | 17.64 | 0.97 (0.81–1.15) | 1.16 (0.97–1.38) |
| β-Interferon | 878 | 57,652 | 15.23 | 0.78 (0.71–0.87) | 0.97 (0.89–1.05) |
| Glatiramer acetate | 370 | 25,362 | 14.59 | 0.84 (0.75–0.93) | 1.01 (0.90–1.12) |
| Natalizumab | 50 | 2,355 | 21.23 | 1.21 (0.91–1.62) | 1.34 (1.00–1.78) |
| Fingolimod | 35 | 1,717 | 20.38 | 1.03 (0.73–1.45) | 1.22 (0.87–1.72) |
| Dimethyl fumarate | 59 | 3,473 | 16.99 | 1.04 (0.73–1.47) | 1.22 (0.85–1.75) |
| Teriflunomide | 20 | 1,382 | 14.48 | 0.79 (0.50–1.22) | 0.87 (0.56–1.35) |
| Alemtuzumab | 6 | 259 | 23.21 | 1.18 (0.53–2.66) | 1.49 (0.66–3.35) |
| Cardiovascular disorders (ischemic heart disease) | |||||
| No DMD | 5,329 | 291,350 | 18.29 | Reference | Reference |
| Any DMD | 875 | 80,290 | 10.90 | 0.60 (0.48–0.74) | 0.94 (0.83–1.07) |
| First-generation DMD | 839 | 77,369 | 10.84 | 0.61 (0.50–0.75) | 0.94 (0.84–1.05) |
| Second-generation DMD | 100 | 8,574 | 11.66 | 0.84 (0.57–1.24) | 1.08 (0.71–1.65) |
| β-Interferon | 655 | 59,116 | 11.08 | 0.64 (0.52–0.80) | 0.94 (0.82–1.07) |
| Glatiramer acetate | 276 | 26,046 | 10.60 | 0.74 (0.63–0.86) | 1.03 (0.91–1.17) |
| Natalizumab | 29 | 2,519 | 11.51 | 0.94 (0.65–1.36) | 1.08 (0.75–1.58) |
| Fingolimod | 27 | 1,731 | 15.60 | 1.24 (0.84–1.84) | 1.64 (1.10–2.44) |
| Dimethyl fumarate | 33 | 3,564 | 9.26 | 0.77 (0.54–1.09) | 0.97 (0.68–1.38) |
| Teriflunomide | 16 | 1,425 | 11.23 | 0.88 (0.54–1.44) | 1.04 (0.64–1.71) |
| Alemtuzumab | <6 | 252 | NR | 0.83 (0.27–2.59) | 1.09 (0.35–3.41) |
| Cardiovascular disorders (other forms of heart disease) | |||||
| No DMD | 7,267 | 284,470 | 25.55 | Reference | Reference |
| Any DMD | 1,342 | 76,835 | 17.47 | 0.65 (0.54–0.80) | 1.00 (0.89–1.12) |
| First-generation DMD | 1,278 | 74,045 | 17.26 | 0.66 (0.56–0.79) | 0.99 (0.91–1.09) |
| Second-generation DMD | 167 | 7,955 | 20.99 | 0.82 (0.54–1.24) | 1.09 (0.73–1.61) |
| β-Interferon | 1,004 | 56,682 | 17.71 | 0.72 (0.60–0.88) | 1.03 (0.90–1.18) |
| Glatiramer acetate | 431 | 24,600 | 17.52 | 0.76 (0.66–0.87) | 1.02 (0.90–1.16) |
| Natalizumab | 59 | 2,233 | 26.42 | 1.38 (1.06–1.80) | 1.61 (1.24–2.10) |
| Fingolimod | 21 | 1,681 | 12.49 | 0.64 (0.41–0.99) | 0.78 (0.50–1.21) |
| Dimethyl fumarate | 64 | 3,321 | 19.27 | 0.93 (0.72–1.19) | 1.17 (0.91–1.51) |
| Teriflunomide | 27 | 1,358 | 19.88 | 0.65 (0.22–1.89) | 0.74 (0.24–2.28) |
| Alemtuzumab | 13 | 221 | 58.92 | 2.77 (1.59–4.83) | 3.72 (2.12–6.53) |
| Cerebrovascular diseases | |||||
| No DMD | 3,398 | 311,339 | 10.91 | Reference | Reference |
| Any DMD | 448 | 82,897 | 5.40 | 0.53 (0.46–0.61) | 0.83 (0.75–0.92) |
| First-generation DMD | 433 | 80,008 | 5.41 | 0.55 (0.47–0.64) | 0.84 (0.76–0.94) |
| Second-generation DMD | 48 | 8,674 | 5.53 | 0.70 (0.52–0.94) | 0.94 (0.68–1.30) |
| β-Interferon | 357 | 61,576 | 5.80 | 0.60 (0.44–0.80) | 0.92 (0.73–1.16) |
| Glatiramer acetate | 123 | 26,665 | 4.61 | 0.55 (0.46–0.66) | 0.78 (0.65–0.94) |
| Natalizumab | 17 | 2,543 | 6.68 | 1.18 (0.38–3.68) | 1.46 (0.45–4.71) |
| Fingolimod | 18 | 1,799 | 10.01 | 1.45 (0.90–2.33) | 2.04 (1.27–3.30) |
| Dimethyl fumarate | 9 | 3,592 | 2.51 | 0.34 (0.17–0.65) | 0.43 (0.22–0.84) |
| Teriflunomide | 11 | 1,431 | 7.69 | 0.98 (0.54–1.79) | 1.24 (0.68–2.27) |
| Alemtuzumab | <6 | 264 | NR | 0.38 (0.05–2.71) | 0.53 (0.07–3.82) |
| Chronic liver diseases | |||||
| No DMD | 2,015 | 325,606 | 6.19 | Reference | Reference |
| Any DMD | 475 | 84,455 | 5.62 | 0.93 (0.84–1.04) | 1.00 (0.90–1.11) |
| First-generation DMD | 452 | 81,406 | 5.55 | 0.92 (0.83–1.02) | 0.98 (0.88–1.10) |
| Second-generation DMD | 64 | 8,988 | 7.12 | 1.26 (0.84–1.89) | 1.28 (0.87–1.90) |
| β-Interferon | 350 | 62,674 | 5.58 | 0.93 (0.83–1.04) | 0.98 (0.87–1.11) |
| Glatiramer acetate | 159 | 26,874 | 5.92 | 1.01 (0.85–1.19) | 1.05 (0.89–1.25) |
| Natalizumab | 15 | 2,639 | 5.68 | 1.09 (0.53–2.21) | 1.07 (0.52–2.17) |
| Fingolimod | 19 | 1,838 | 10.34 | 1.47 (0.73–2.97) | 1.58 (0.80–3.12) |
| Dimethyl fumarate | 25 | 3,748 | 6.67 | 1.05 (0.63–1.72) | 1.07 (0.65–1.75) |
| Teriflunomide | 17 | 1,477 | 11.51 | 1.88 (1.15–3.07) | 1.94 (1.19–3.18) |
| Alemtuzumab | <6 | 272 | NR | 1.22 (0.30–4.93) | 1.28 (0.32–5.19) |
| Chronic kidney diseases | |||||
| No DMD | 2,073 | 330,693 | 6.27 | Reference | Reference |
| Any DMD | 393 | 86,849 | 4.53 | 0.64 (0.57–0.71) | 1.02 (0.88–1.19) |
| First-generation DMD | 374 | 83,762 | 4.47 | 0.64 (0.57–0.71) | 0.98 (0.97–1.10) |
| Second-generation DMD | 54 | 9,188 | 5.88 | 0.99 (0.52–1.87) | 1.34 (0.71–2.54) |
| β-Interferon | 300 | 64,406 | 4.66 | 0.70 (0.62–0.79) | 1.03 (0.90–1.17) |
| Glatiramer acetate | 123 | 27,949 | 4.40 | 0.70 (0.58–0.84) | 0.97 (0.80–1.17) |
| Natalizumab | 12 | 2,652 | 4.52 | 0.94 (0.37–2.37) | 1.14 (0.42–3.07) |
| Fingolimod | 9 | 1,919 | 4.69 | 1.15 (0.38–3.55) | 1.69 (0.49–5.87) |
| Dimethyl fumarate | 25 | 3,818 | 6.55 | 0.94 (0.35–2.54) | 1.20 (0.44–3.26) |
| Teriflunomide | 10 | 1,537 | 6.51 | 1.02 (0.54–1.91) | 1.26 (0.67–2.37) |
| Alemtuzumab | <6 | 270 | NR | 3.87 (1.58–9.46) | 6.43 (2.60–15.95) |
| Diabetes | |||||
| No DMD | 4,260 | 300,513 | 14.18 | Reference | Reference |
| Any DMD | 827 | 79,823 | 10.36 | 0.72 (0.66–0.79) | 0.96 (0.88–1.04) |
| First-generation DMD | 796 | 76,922 | 10.35 | 0.73 (0.67–0.80) | 0.96 (0.88–1.04) |
| Second-generation DMD | 68 | 8,549 | 7.95 | 0.72 (0.55–0.93) | 0.83 (0.62–1.12) |
| β-Interferon | 632 | 58,980 | 10.72 | 0.78 (0.72–0.85) | 0.98 (0.90–1.07) |
| Glatiramer acetate | 249 | 25,709 | 9.69 | 0.76 (0.64–0.90) | 0.93 (0.80–1.09) |
| Natalizumab | 16 | 2,473 | 6.47 | 0.69 (0.42–1.13) | 0.77 (0.47–1.27) |
| Fingolimod | 19 | 1,797 | 10.57 | 1.14 (0.72–1.81) | 1.42 (0.89–2.25) |
| Dimethyl fumarate | 29 | 3,534 | 8.21 | 0.81 (0.55–1.17) | 0.94 (0.65–1.37) |
| Teriflunomide | 9 | 1,419 | 6.34 | 0.65 (0.34–1.26) | 0.72 (0.37–1.40) |
| Alemtuzumab | <6 | 275 | NR | 0.42 (0.06–3.00) | 0.51 (0.07–3.62) |
| Hyperlipidemia | |||||
| No DMD | 6,353 | 276,919 | 22.94 | Reference | Reference |
| Any DMD | 1,329 | 73,420 | 18.10 | 0.77 (0.72–0.81) | 0.93 (0.88–1.00) |
| First-generation DMD | 1,295 | 70,625 | 18.34 | 0.79 (0.74–0.84) | 0.95 (0.89–1.02) |
| Second-generation DMD | 109 | 8,031 | 13.57 | 0.75 (0.61–0.91) | 0.85 (0.70–1.03) |
| β-Interferon | 987 | 54,195 | 18.21 | 0.80 (0.75–0.86) | 0.95 (0.89–1.02) |
| Glatiramer acetate | 419 | 23,386 | 17.92 | 0.82 (0.74–0.90) | 0.95 (0.86–1.06) |
| Natalizumab | 25 | 2,385 | 10.48 | 0.68 (0.45–1.01) | 0.74 (0.49–1.10) |
| Fingolimod | 26 | 1,705 | 15.25 | 1.11 (0.75–1.65) | 1.30 (0.88–1.93) |
| Dimethyl fumarate | 38 | 3,303 | 11.50 | 0.66 (0.47–0.92) | 0.73 (0.53–1.01) |
| Teriflunomide | 30 | 1,254 | 23.92 | 1.55 (0.98–2.43) | 1.61 (1.07–2.44) |
| Alemtuzumab | <6 | 269 | NR | 0.29 (0.04–2.06) | 0.36 (0.05–2.53) |
| Hypertension | |||||
| No DMD | 8,972 | 233,816 | 38.37 | Reference | Reference |
| Any DMD | 1,872 | 65,518 | 28.57 | 0.76 (0.70–0.84) | 1.03 (0.98–1.09) |
| First-generation DMD | 1,814 | 62,876 | 28.85 | 0.77 (0.70–0.85) | 1.03 (0.98–1.09) |
| Second-generation DMD | 183 | 7,317 | 25.01 | 0.90 (0.77–1.05) | 1.10 (0.94–1.28) |
| β-Interferon | 1,451 | 47,748 | 30.39 | 0.85 (0.79–0.92) | 1.11 (1.04–1.17) |
| Glatiramer acetate | 538 | 21,212 | 25.36 | 0.71 (0.65–0.77) | 0.87 (0.80–0.95) |
| Natalizumab | 44 | 2,245 | 19.60 | 0.70 (0.52–0.95) | 0.79 (0.59–1.07) |
| Fingolimod | 50 | 1,566 | 31.92 | 1.40 (1.05–1.86) | 1.73 (1.30–2.31) |
| Dimethyl fumarate | 64 | 2,983 | 21.46 | 0.72 (0.56–0.93) | 0.85 (0.66–1.11) |
| Teriflunomide | 47 | 1,106 | 42.50 | 1.61 (1.20–2.16) | 1.76 (1.32–2.37) |
| Alemtuzumab | <6 | 225 | NR | 3.92 (1.41–10.92) | 4.96 (1.78–13.84) |
| Mental conditions (anxiety, depression, bipolar disorders) | |||||
| No DMD | 13,268 | 139,192 | 95.32 | Reference | Reference |
| Any DMD | 2,132 | 26,972 | 79.05 | 1.10 (1.05–1.16) | 1.05 (0.99–1.10) |
| First-generation DMD | 1,989 | 25,580 | 77.76 | 1.10 (1.04–1.16) | 1.04 (0.99–1.10) |
| Second-generation DMD | 237 | 2,884 | 82.18 | 1.08 (0.94–1.23) | 1.04 (0.91–1.19) |
| β-Interferon | 1,446 | 19,258 | 75.09 | 1.07 (1.00–1.14) | 1.01 (0.96–1.08) |
| Glatiramer acetate | 680 | 7,925 | 85.81 | 1.22 (1.13–1.33) | 1.16 (1.07–1.26) |
| Natalizumab | 63 | 626 | 100.59 | 1.41 (1.09–1.82) | 1.36 (1.06–1.76) |
| Fingolimod | 38 | 576 | 66.00 | 0.90 (0.65–1.24) | 0.87 (0.63–1.20) |
| Dimethyl fumarate | 112 | 1,328 | 84.36 | 1.04 (0.85–1.26) | 1.02 (0.84–1.24) |
| Teriflunomide | 28 | 490 | 57.12 | 0.79 (0.54–1.15) | 0.77 (0.51–1.15) |
| Alemtuzumab | 11 | 96 | 114.44 | 1.29 (0.71–2.34) | 1.24 (0.68–2.26) |
| Migraine | |||||
| No DMD | 3,879 | 291,676 | 13.30 | Reference | Reference |
| Any DMD | 872 | 72,969 | 11.95 | 1.14 (1.02–1.28) | 0.97 (0.90–1.05) |
| First-generation DMD | 825 | 70,326 | 11.73 | 1.13 (1.02–1.24) | 0.87 (0.89–1.06) |
| Second-generation DMD | 103 | 7,482 | 13.77 | 1.10 (0.85–1.42) | 1.00 (0.78–1.28) |
| β-Interferon | 632 | 54,004 | 11.70 | 1.14 (1.04–1.24) | 0.99 (0.90–1.10) |
| Glatiramer acetate | 271 | 23,081 | 11.74 | 1.09 (0.96–1.24) | 0.95 (0.83–1.08) |
| Natalizumab | 30 | 2,147 | 13.97 | 1.36 (0.82–2.26) | 1.37 (0.76–2.49) |
| Fingolimod | 27 | 1,572 | 17.18 | 1.54 (0.89–2.65) | 1.38 (0.76–2.51) |
| Dimethyl fumarate | 39 | 3,089 | 12.63 | 0.96 (0.69–1.33) | 0.89 (0.64–1.23) |
| Teriflunomide | 16 | 1,245 | 12.85 | 1.05 (0.64–1.73) | 1.04 (0.63–1.72) |
| Alemtuzumab | <6 | 228 | NR | 0.66 (0.16–2.66) | 0.57 (0.14–2.28) |
| Thyroid disorders (autoimmune hypothyroidism and hyperthyroidism) | |||||
| No DMD | 1,003 | 331,520 | 3.03 | Reference | Reference |
| Any DMD | 234 | 86,309 | 2.71 | 0.94 (0.81–1.09) | 0.97 (0.83–1.13) |
| First-generation DMD | 221 | 83,208 | 2.66 | 0.90 (0.77–1.05) | 0.92 (0.79–1.08) |
| Second-generation DMD | 31 | 9,252 | 3.35 | 1.38 (0.94–2.02) | 1.42 (0.96–2.08) |
| β-Interferon | 172 | 63,912 | 2.69 | 0.95 (0.80–1.12) | 0.98 (0.83–1.16) |
| Glatiramer acetate | 73 | 27,800 | 2.63 | 0.90 (0.71–1.16) | 0.90 (0.70–1.16) |
| Natalizumab | 7 | 2,708 | 2.58 | 1.05 (0.47–2.31) | 1.05 (0.48–2.33) |
| Fingolimod | <6 | 1,933 | NR | 0.96 (0.19–4.92) | 1.02 (0.19–5.47) |
| Dimethyl fumarate | 12 | 3,823 | 3.14 | 1.14 (0.62–2.09) | 1.18 (0.64–2.16) |
| Teriflunomide | 8 | 1,537 | 5.21 | 2.29 (1.11–4.72) | 2.30 (1.11–4.74) |
| Alemtuzumab | 10 | 272 | 36.72 | 18.77 (9.51–37.05) | 19.42 (9.29–36.51) |
| Incident adverse event using the “current” DMD exposure approachc | |||||
| Acute liver injury | |||||
| No DMD | 137 | 200,004 | 0.68 | Reference | Reference |
| Any DMD | 10 | 24,925 | 0.40 | 0.65 (0.34–1.23) | 0.81 (0.42–1.56) |
| First-generation DMD | <6 | 20,988 | NR | 0.37 (0.15–0.92) | 0.47 (0.19–1.15) |
| Second-generation DMD | <6 | 3,936 | NR | 2.48 (0.96–6.37) | 3.32 (1.28–8.62) |
| β-Interferon | <6 | 15,294 | NR | 0.20 (0.05–0.80) | 0.25 (0.06–1.00) |
| Glatiramer acetate | <6 | 5,694 | NR | 0.92 (0.29–2.90) | 1.14 (0.36–3.63) |
| Natalizumab | <6 | 827 | NR | NR | NR |
| Fingolimod | <6 | 983 | NR | 1.99 (0.27–14.63) | 2.81 (0.38–20.69) |
| Dimethyl fumarate | <6 | 1,245 | NR | 4.90 (1.47–16.29) | 6.55 (1.96–21.87) |
| Teriflunomide | <6 | 657 | NR | NR | NR |
| Alemtuzumab | <6 | 222 | NR | 10.83 (1.43–81.85) | 16.18 (2.11–123.89) |
| Abnormal hematologic findings | |||||
| No DMD | 4,377 | 163,122 | 26.87 | Reference | Reference |
| Any DMD | 539 | 20,745 | 26.03 | 0.96 (0.88–1.05) | 1.15 (1.05–1.26) |
| First-generation DMD | 429 | 17,649 | 24.36 | 0.92 (0.83–1.01) | 1.09 (0.98–1.20) |
| Second-generation DMD | 110 | 3,096 | 35.52 | 1.18 (0.97–1.43) | 1.50 (1.23–1.83) |
| β-Interferon | 323 | 12,951 | 24.94 | 0.96 (0.86–1.08) | 1.13 (1.01–1.27) |
| Glatiramer acetate | 106 | 4,698 | 22.78 | 0.81 (0.67–0.98) | 0.96 (0.79–1.17) |
| Natalizumab | 25 | 634 | 39.45 | 1.37 (0.92–2.03) | 1.72 (1.16–2.56) |
| Fingolimod | 23 | 783 | 29.38 | 1.01 (0.67–1.52) | 1.33 (0.88–2.01) |
| Dimethyl fumarate | 35 | 999 | 35.03 | 1.11 (0.79–1.55) | 1.40 (1.00–1.97) |
| Teriflunomide | 17 | 509 | 33.42 | 1.08 (0.67–1.75) | 1.35 (0.84–2.18) |
| Alemtuzumab | 10 | 169 | 59.01 | 1.83 (0.98–3.41) | 2.38 (1.28–4.46) |
Abbreviations: DMD = disease-modifying drug; NR = results are not reported when a small number of events (<6) occurred in a subgroup; py = person-years
Bold indicates p < 0.05.
Except for infections, all potential adverse events were identified at present in either the in-patient and out-patient information (hospitalizations and physician visits), as presented in eTable 2 (links.lww.com/WNL/D339). E-values were calculated for statistically significant events according to recommendations,31 with relatively common events being infection-related physician visits, cardiovascular disorders, hyperlipidemia, hypertension, mental conditions, and abnormal hematologic findings (all others were considered rare). E-value point estimates and lower/upper limits of the CI were >2 for: all DMD groupings and infection-related hospitalizations (specifically: any DMD, any first-generation DMD, any second-generation DMD) and also for individual DMDs as follows: β-interferon, glatiramer acetate, and dimethyl fumarate. Estimates were also >2 for alemtuzumab and other forms of heart disease, chronic kidney disease, hypertension, thyroid disorder, acute liver injury, dimethyl fumarate, and acute liver injury. For all others, estimates were <2. Summary measures for selection bias were calculated for events reaching significance according to recommendations.32 All the summary measures for selection bias on the hazard ratios and their lower bounds exceeded 1, inferring that selection bias could not explain away our findings.
Results were adjusted for sex, socioeconomic status (quintiles), and age (continuous) at the index date and Charlson comorbidity score (categorized as 0, 1, 2, or ≥3 and updated overtime).
Findings from 4 Canadian provinces combined (British Columbia, Saskatchewan, Manitoba, Nova Scotia).
Complementary analysis was conducted in British Columbia only.
Incident Adverse Events
For other incident adverse events, when all DMDs were grouped together, “any DMD” exposure (vs none or less than the defined minimum) was not associated with an increased hazard of adverse event (Figure, Table 2). Similarly, when the first-generation or second-generation DMDs were grouped together, there were no significant increases in the hazard ratios. However, significantly elevated hazards were observed for individual DMDs. Fingolimod was the only DMD associated with an increased hazard of ischemic heart disease (aHR 1.64; 95% CI 1.10–2.44; crude rate per 1,000 person-years [CR] 15.60) and cerebrovascular disease (aHR 2.04; 95% CI 1.27–3.30; CR 10.01). Only alemtuzumab was associated with a significantly higher hazard of chronic kidney disease (aHR 6.43; 95% CI 2.60–15.95; <6 cases), while teriflunomide was associated with a significantly higher hazard of chronic liver disease (aHR 1.94; 95% CI 1.19–3.18; CR 11.51) and hyperlipidemia (aHR 1.61; 95% CI 1.07–2.44; CR 23.92). Two DMDs were associated with significantly higher hazards of thyroid disorders, reaching an aHR of 19.42 (95% CI 9.29–36.51; CR 36.72) for alemtuzumab, followed by 2.30 (95% CI 1.11–4.74; CR 5.21) for teriflunomide. Both these DMDs were also associated with a higher hazard of hypertension, being 4.96 (95% CI 1.78–13.84; <6 cases) for alemtuzumab and 1.76 (95% CI 1.32–2.37; CR 42.50) for teriflunomide, followed by 1.73 (95% CI 1.30–2.31; CR 31.92) for fingolimod and 1.11 (95% CI 1.04–1.17; CR 30.39) for β-interferon. For other forms of heart disease, alemtuzumab (aHR 3.72; 95% CI 2.12–6.53; CR 58.92) and natalizumab (aHR 1.61; 95% CI 1.24–2.10; CR 26.42) were each associated with a significantly higher hazard. Both glatiramer acetate and natalizumab increased the hazard of a mental health condition by 1.16 (95% CI 1.07–1.26; CR 85.81) and 1.36 (95% CI 1.06–1.76; CR 100.59), respectively.
A few DMDs were associated with a lower hazard of an incident adverse event: Glatiramer acetate alone was associated with a lower hazard of hypertension (aHR 0.87; 95% CI 0.80–0.95; CR 25.36), while both glatiramer acetate (aHR 0.78; 95% CI 0.65–0.94; CR 4.61) and dimethyl fumarate (aHR 0.43; 95% CI 0.22–0.84; CR 2.51) were associated with lower hazard of cerebrovascular diseases. No significant differences were observed between individual DMDs (vs no DMD) and the hazard of autoimmune diseases, cancer, diabetes, or migraine (Table 2).
Complementary/Sensitivity Analyses
Current exposure to a DMD (vs no current exposure) was associated with a higher hazard of acute liver injury and abnormal hematologic findings, with most of the significant findings being for the second-generation DMDs (Table 2). For acute liver injury, although both alemtuzumab (aHR 16.18; 95% CI 2.11–123.89) and dimethyl fumarate (aHR 6.55; 95% CI 1.96–21.87) were each associated with a significantly higher hazard, only a few such cases occurred (<6 for either DMD) resulting in wide CIs. Abnormal hematologic findings were significant for alemtuzumab (aHR 2.38; 95% CI 1.28–4.46; CR 59.01), natalizumab (aHR 1.72; 95% CI 1.16–2.56; CR 39.45), followed by β-interferon (aHR 1.13; 95% CI 1.01–1.27; CR 24.94). Both the “intention-to-treat” and incident adverse events (assessed by using available case definitions) yielded results that were generally in the same direction as those from the main analyses (Table 3). For the sensitivity analyses, the E-values and summary measures for selection bias were calculated for events reaching significance (summarized in Table 2). The direction and magnitude of findings were similar between the unadjusted and adjusted models (Table 2) and the individual comorbidities as separate covariates and propensity score adjustment (Table 3).
Table 3.
The Risk (Hazard) of Potential Adverse Events Associated With Disease-Modifying Drugs Used to Treat Multiple Sclerosis, British Columbia, Canada: Intention-to-Treat Analysis, Assessment of Incident Adverse Events Identified Using Available Case Definitions,a Sensitivity Analysis Using Each Individual Comorbidity (Captured in CCI) as Separate Covariates, and Using a Propensity Score Adjustment
| Potential adverse event by DMD exposure | Adjusted hazard ratios (95% CI) | |||
| Intention-to-treat analysis (DMD exposure defined as ≥1 d)b | Assessment of incident adverse events by using available case definitionsb | Using each individual comorbidity (captured in CCI) as separate covariatesc | Using propensity score adjustmentd (for the any DMD exposure analysis) | |
| Recurrent events using the “current” DMD exposure approach | ||||
| Infection-related hospitalizations | ||||
| No DMD | N/A | N/A | Reference | Reference |
| Any DMD | 0.64 (0.56–0.73) | 0.60 (0.53–0.69) | ||
| First-generation DMD | 0.65 (0.56–0.75) | |||
| Second-generation DMD | 0.61 (0.46–0.80) | |||
| β-Interferon | 0.66 (0.56–0.77) | |||
| Glatiramer acetate | 0.61 (0.47–0.80) | |||
| Natalizumab | 0.64 (0.40–1.02) | |||
| Fingolimod | 0.59 (0.32–1.07) | |||
| Dimethyl fumarate | 0.46 (0.28–0.78) | |||
| Teriflunomide | 0.69 (0.35–1.33) | |||
| Alemtuzumab | 1.28 (0.62–2.64) | |||
| Infection-related physician visits | ||||
| No DMD | N/A | N/A | Reference | Reference |
| Any DMD | 1.03 (0.99–1.07) | 1.01 (0.97–1.05) | ||
| First-generation DMD | 1.00 (0.97–1.04) | |||
| Second-generation DMD | 1.16 (1.09–1.24) | |||
| β-Interferon | 0.97 (0.93–1.01) | |||
| Glatiramer acetate | 1.10 (1.03–1.18) | |||
| Natalizumab | 1.34 (1.14–1.56) | |||
| Fingolimod | 1.11 (0.98–1.26) | |||
| Dimethyl fumarate | 1.14 (1.03–1.26) | |||
| Teriflunomide | 0.98 (0.86–1.12) | |||
| Alemtuzumab | 1.40 (1.15–1.70) | |||
| Incident adverse event using the “minimum” cumulative DMD approach | ||||
| Autoimmune diseases | ||||
| No DMD | Reference | N/A | Reference | Reference |
| Any DMD | 0.98 (0.92–1.04) | 0.99 (0.93–1.06) | 0.97 (0.91–1.04) | |
| First-generation DMD | 0.97 (0.91–1.04) | 1.00 (0.93–1.07) | ||
| Second-generation DMD | 1.03 (0.88–1.22) | 1.03 (0.86–1.24) | ||
| β-Interferon | 0.98 (0.92–1.05) | 0.99 (0.92–1.07) | ||
| Glatiramer acetate | 1.06 (0.95–1.17) | 1.08 (0.96–1.22) | ||
| Natalizumab | 1.15 (0.86–1.53) | 1.15 (0.84–1.59) | ||
| Fingolimod | 1.28 (0.95–1.73) | 1.26 (0.92–1.73) | ||
| Dimethyl fumarate | 0.90 (0.69–1.16) | 0.77 (0.56–1.07) | ||
| Teriflunomide | 0.89 (0.61–1.30) | 1.04 (0.69–1.57) | ||
| Alemtuzumab | 1.12 (0.60–2.09) | 1.36 (0.73–2.56) | ||
| Cancer | ||||
| No DMD | Reference | N/A | Reference | Reference |
| Any DMD | 0.94 (0.87–1.03) | 0.95 (0.87–1.04) | 0.94 (0.86–1.03) | |
| First-generation DMD | 0.92 (0.84–1.01) | 0.93 (0.85–1.03) | ||
| Second-generation DMD | 1.22 (1.00–1.49) | 1.15 (0.92–1.44) | ||
| β-Interferon | 0.91 (0.83–1.00) | 0.93 (0.84–1.03) | ||
| Glatiramer acetate | 1.13 (0.98–1.30) | 1.02 (0.87–1.21) | ||
| Natalizumab | 1.06 (0.73–1.53) | 1.17 (0.79–1.72) | ||
| Fingolimod | 1.28 (0.88–1.87) | 1.30 (0.87–1.95) | ||
| Dimethyl fumarate | 1.17 (0.85–1.59) | 1.08 (0.75–1.56) | ||
| Teriflunomide | 1.16 (0.76–1.78) | 1.00 (0.59–1.69) | ||
| Alemtuzumab | 1.41 (0.66–2.99) | 1.46 (0.65–3.29) | ||
| Cardiovascular disorders (ischemic heart disease) | ||||
| No DMD | Reference | Reference | Reference | Reference |
| Any DMD | 1.03 (0.94–1.14) | 1.06 (0.92–1.22) | 1.05 (0.95–1.16) | 1.03 (0.93–1.14) |
| First-generation DMD | 1.00 (0.90–1.10) | 1.04 (0.90–1.21) | 1.02 (0.92–1.13) | |
| Second-generation DMD | 1.47 (1.18–1.83) | 1.32 (0.89–1.95) | 1.33 (1.03–1.70) | |
| β-Interferon | 0.97 (0.88–1.08) | 1.04 (0.89–1.22) | 1.02 (0.91–1.14) | |
| Glatiramer acetate | 1.17 (1.01–1.37) | 1.18 (0.90–1.54) | 1.10 (0.92–1.32) | |
| Natalizumab | 1.28 (0.87–1.88) | 0.73 (0.32–1.66) | 1.01 (0.63–1.61) | |
| Fingolimod | 1.56 (1.02–2.38) | 1.59 (0.78–3.24) | 1.76 (1.13–2.74) | |
| Dimethyl fumarate | 1.24 (0.87–1.77) | 1.48 (0.81–2.72) | 1.03 (0.66–1.61) | |
| Teriflunomide | 1.01 (0.58–1.75) | 0.81 (0.26–2.53) | 1.19 (0.66–2.17) | |
| Alemtuzumab | 1.13 (0.42–3.04) | 1.12 (0.16–8.10) | 1.15 (0.37–3.59) | |
| Cardiovascular disorders (other forms of heart disease) | ||||
| No DMD | Reference | N/A | Reference | Reference |
| Any DMD | 1.07 (0.99–1.16) | 1.07 (0.99–1.16) | 1.02 (0.94–1.11) | |
| First-generation DMD | 1.01 (0.93–1.10) | 1.01 (0.92–1.10) | ||
| Second-generation DMD | 1.59 (1.33–1.90) | 1.60 (1.32–1.95) | ||
| β-Interferon | 0.98 (0.90–1.07) | 1.01 (0.92–1.11) | ||
| Glatiramer acetate | 1.18 (1.04–1.34) | 1.16 (1.00–1.35) | ||
| Natalizumab | 1.55 (1.14–2.10) | 1.43 (1.02–2.02) | ||
| Fingolimod | 1.02 (0.68–1.53) | 0.84 (0.52–1.36) | ||
| Dimethyl fumarate | 1.09 (0.80–1.47) | 1.28 (0.92–1.78) | ||
| Teriflunomide | 1.54 (1.06–2.24) | 1.69 (1.12–2.57) | ||
| Alemtuzumab | 3.20 (1.88–5.43) | 3.88 (2.21–6.82) | ||
| Cerebrovascular diseases | ||||
| No DMD | Reference | Reference | Reference | Reference |
| Any DMD | 0.82 (0.73–0.93) | 0.62 (0.43–0.89) | 0.83 (0.73–0.95) | 0.80 (0.70–0.91) |
| First-generation DMD | 0.80 (0.71–0.91) | 0.61 (0.42–0.88) | 0.84 (0.73–0.95) | |
| Second-generation DMD | 1.19 (0.88–1.60) | 1.34 (0.54–3.35) | 1.08 (0.76–1.51) | |
| β-Interferon | 0.89 (0.78–1.02) | 0.72 (0.49–1.04) | 0.90 (0.79–1.04) | |
| Glatiramer acetate | 0.75 (0.60–0.94) | 0.52 (0.23–1.18) | 0.75 (0.58–0.97) | |
| Natalizumab | 1.04 (0.59–1.82) | 2.30 (0.70–7.56) | 1.13 (0.63–2.01) | |
| Fingolimod | 1.94 (1.16–3.22) | 0.86 (0.11–6.60) | 2.22 (1.34–3.68) | |
| Dimethyl fumarate | 0.63 (0.34–1.15) | 1.30 (0.31–5.40) | 0.47 (0.21–1.05) | |
| Teriflunomide | 1.62 (0.91–2.89) | 1.30 (0.18–9.42) | 1.19 (0.56–2.51) | |
| Alemtuzumab | 0.93 (0.23–3.79) | 5.45 (0.70–42.41) | 0.55 (0.08–3.92) | |
| Chronic liver diseases | ||||
| No DMD | Reference | N/A | Reference | Reference |
| Any DMD | 1.05 (0.91–1.20) | 1.00 (0.86–1.16) | 0.96 (0.83–1.11) | |
| First-generation DMD | 1.07 (0.93–1.22) | 1.00 (0.86–1.17) | ||
| Second-generation DMD | 0.91 (0.64–1.30) | 1.01 (0.69–1.47) | ||
| β-Interferon | 1.11 (0.95–1.28) | 1.05 (0.89–1.23) | ||
| Glatiramer acetate | 1.00 (0.80–1.26) | 1.02 (0.78–1.33) | ||
| Natalizumab | 0.56 (0.26–1.19) | 0.61 (0.27–1.38) | ||
| Fingolimod | 1.98 (1.19–3.31) | 2.17 (1.28–3.68) | ||
| Dimethyl fumarate | 0.86 (0.49–1.50) | 0.82 (0.42–1.60) | ||
| Teriflunomide | 1.12 (0.53–2.38) | 1.37 (0.61–3.07) | ||
| Alemtuzumab | 1.06 (0.26–4.30) | 1.31 (0.32–5.30) | ||
| Chronic kidney diseases | ||||
| No DMD | Reference | Reference | Reference | Reference |
| Any DMD | 0.93 (0.80–1.08) | 0.86 (0.68–1.08) | 0.96 (0.82–1.12) | 0.93 (0.80–1.09) |
| First-generation DMD | 0.96 (0.82–1.12) | 0.89 (0.70–1.13) | 0.98 (0.83–1.16) | |
| Second-generation DMD | 0.88 (0.58–1.35) | 0.83 (0.41–1.71) | 1.00 (0.65–1.55) | |
| β-Interferon | 1.01 (0.85–1.19) | 0.91 (0.71–1.17) | 1.03 (0.87–1.23) | |
| Glatiramer acetate | 1.07 (0.83–1.38) | 1.09 (0.71–1.67) | 1.06 (0.80–1.42) | |
| Natalizumab | 0.91 (0.44–1.85) | 0.56 (0.14–2.31) | 0.82 (0.36–1.87) | |
| Fingolimod | 0.78 (0.32–1.91) | 0.38 (0.05–2.79) | 0.86 (0.35–2.11) | |
| Dimethyl fumarate | 0.64 (0.30–1.36) | 0.28 (0.04–2.00) | 0.47 (0.18–1.28) | |
| Teriflunomide | 0.92 (0.38–2.25) | 1.85 (0.59–5.84) | 1.19 (0.49–2.88) | |
| Alemtuzumab | 5.54 (2.22–13.81) | 10.04 (3.10–32.50) | 6.65 (2.69–16.44) | |
| Diabetes | ||||
| No DMD | Reference | Reference | Reference | Reference |
| Any DMD | 1.01 (0.90–1.12) | 1.04 (0.89–1.20) | 1.01 (0.90–1.12) | 0.99 (0.88–1.11) |
| First-generation DMD | 1.01 (0.91–1.13) | 1.03 (0.89–1.20) | 0.99 (0.88–1.11) | |
| Second-generation DMD | 0.90 (0.66–1.23) | 1.15 (0.76–1.75) | 1.03 (0.74–1.42) | |
| β-Interferon | 0.96 (0.86–1.08) | 0.98 (0.83–1.16) | 0.98 (0.87–1.11) | |
| Glatiramer acetate | 1.11 (0.93–1.32) | 1.22 (0.94–1.59) | 1.05 (0.86–1.29) | |
| Natalizumab | 0.98 (0.58–1.64) | 0.89 (0.42–1.90) | 0.83 (0.46–1.52) | |
| Fingolimod | 1.47 (0.87–2.48) | 2.10 (1.11–3.98) | 1.59 (0.93–2.72) | |
| Dimethyl fumarate | 0.83 (0.49–1.39) | 1.12 (0.55–2.28) | 0.97 (0.56–1.70) | |
| Teriflunomide | 0.61 (0.25–1.49) | 0.58 (0.15–2.36) | 0.81 (0.34–1.97) | |
| Alemtuzumab | 0.43 (0.06–3.05) | N/A | 0.51 (0.07–3.67) | |
| Hyperlipidemia | ||||
| No DMD | Reference | Reference | Reference | Reference |
| Any DMD | 0.89 (0.81–0.98) | 0.93 (0.80–1.07) | 0.92 (0.84–1.02) | 0.96 (0.87–1.05) |
| First-generation DMD | 0.91 (0.83–1.00) | 0.93 (0.80–1.09) | 0.94 (0.85–1.04) | |
| Second-generation DMD | 0.89 (0.68–1.16) | 0.95 (0.59–1.53) | 0.87 (0.65–1.16) | |
| β-Interferon | 0.88 (0.79–0.97) | 0.92 (0.78–1.08) | 0.92 (0.83–1.03) | |
| Glatiramer acetate | 1.04 (0.89–1.21) | 0.93 (0.70–1.25) | 1.01 (0.84–1.20) | |
| Natalizumab | 0.92 (0.58–1.46) | 0.70 (0.29–1.71) | 0.86 (0.52–1.45) | |
| Fingolimod | 1.11 (0.67–1.83) | 1.36 (0.60–3.09) | 1.18 (0.70–1.98) | |
| Dimethyl fumarate | 0.80 (0.51–1.24) | 1.03 (0.45–2.33) | 0.65 (0.38–1.14) | |
| Teriflunomide | 0.99 (0.54–1.81) | 0.38 (0.05–2.69) | 1.20 (0.64–2.24) | |
| Alemtuzumab | 0.61 (0.15–2.46) | 1.35 (0.19–9.76) | 0.36 (0.05–2.57) | |
| Hypertension | ||||
| No DMD | Reference | Reference | Reference | Reference |
| Any DMD | 1.01 (0.94–1.09) | 1.06 (0.97–1.17) | 1.02 (0.94–1.11) | 1.04 (0.96–1.13) |
| First-generation DMD | 1.01 (0.93–1.09) | 1.06 (0.96–1.18) | 1.01 (0.93–1.10) | |
| Second-generation DMD | 1.10 (0.88–1.37) | 1.13 (0.84–1.51) | 1.20 (0.95–1.52) | |
| β-Interferon | 1.04 (0.96–1.14) | 1.15 (1.04–1.28) | 1.07 (0.98–1.17) | |
| Glatiramer acetate | 0.87 (0.75–1.00) | 0.76 (0.61–0.94) | 0.88 (0.75–1.04) | |
| Natalizumab | 1.00 (0.69–1.47) | 0.72 (0.41–1.29) | 0.78 (0.49–1.23) | |
| Fingolimod | 1.95 (1.35–2.81) | 2.11 (1.33–3.35) | 2.01 (1.37–2.94) | |
| Dimethyl fumarate | 0.63 (0.41–0.99) | 0.73 (0.40–1.32) | 0.74 (0.46–1.21) | |
| Teriflunomide | 1.78 (1.18–2.68) | 2.32 (1.38–3.89) | 2.03 (1.31–3.14) | |
| Alemtuzumab | N/A | N/A | N/A | |
| Mental conditions (anxiety, depression, bipolar disorders) | ||||
| No DMD | Reference | Reference | Reference | Reference |
| Any DMD | 1.11 (1.04–1.18) | 1.16 (1.06–1.26) | 1.04 (0.96–1.12) | 1.01 (0.93–1.09) |
| First-generation DMD | 1.10 (1.03–1.18) | 1.15 (1.05–1.26) | 1.03 (0.95–1.11) | |
| Second-generation DMD | 1.05 (0.88–1.26) | 1.03 (0.81–1.30) | 1.06 (0.87–1.30) | |
| β-Interferon | 1.09 (1.02–1.18) | 1.14 (1.04–1.25) | 1.02 (0.93–1.11) | |
| Glatiramer acetate | 1.16 (1.03–1.30) | 1.12 (0.95–1.33) | 1.14 (0.99–1.32) | |
| Natalizumab | 1.42 (1.02–1.99) | 1.52 (1.03–2.24) | 1.39 (0.95–2.03) | |
| Fingolimod | 0.75 (0.50–1.13) | 0.60 (0.35–1.04) | 0.88 (0.58–1.33) | |
| Dimethyl fumarate | 1.02 (0.78–1.33) | 1.05 (0.72–1.54) | 0.97 (0.70–1.33) | |
| Teriflunomide | 0.97 (0.63–1.48) | 1.17 (0.66–2.08) | 1.00 (0.60–1.66) | |
| Alemtuzumab | 1.14 (0.61–2.13) | 0.65 (0.21–2.04) | 1.04 (0.49–2.20) | |
| Migraine | ||||
| No DMD | Reference | Reference | Reference | Reference |
| Any DMD | 0.98 (0.89–1.08) | 1.10 (0.94–1.28) | 0.99 (0.89–1.11) | 0.96 (0.86–1.07) |
| First-generation DMD | 1.02 (0.92–1.12) | 1.10 (0.94–1.29) | 1.01 (0.90–1.13) | |
| Second-generation DMD | 0.85 (0.65–1.12) | 1.19 (0.82–1.71) | 0.93 (0.70–1.25) | |
| β-Interferon | 1.07 (0.96–1.19) | 1.20 (1.02–1.42) | 1.07 (0.95–1.21) | |
| Glatiramer acetate | 1.02 (0.87–1.20) | 0.94 (0.71–1.25) | 0.93 (0.76–1.14) | |
| Natalizumab | 0.73 (0.43–1.25) | 1.17 (0.62–2.22) | 0.87 (0.50–1.51) | |
| Fingolimod | 1.14 (0.71–1.83) | 1.83 (1.06–3.17) | 1.12 (0.67–1.88) | |
| Dimethyl fumarate | 0.92 (0.61–1.38) | 0.87 (0.44–1.70) | 0.89 (0.55–1.45) | |
| Teriflunomide | 0.89 (0.48–1.67) | 1.25 (0.51–3.03) | 1.04 (0.51–2.09) | |
| Alemtuzumab | 0.95 (0.35–2.56) | 0.92 (0.23–3.74) | 0.56 (0.14–2.27) | |
| Thyroid disorders (autoimmune hypothyroidism and hyperthyroidism) | ||||
| No DMD | Reference | Reference | Reference | Reference |
| Any DMD | 0.95 (0.77–1.18) | 1.11 (0.82–1.49) | 1.00 (0.80–1.26) | 0.97 (0.77–1.22) |
| First-generation DMD | 0.93 (0.74–1.16) | 0.94 (0.68–1.30) | 0.92 (0.73–1.17) | |
| Second-generation DMD | 1.13 (0.66–1.96) | 1.91 (1.02–3.57) | 1.39 (0.81–2.40) | |
| β-Interferon | 1.01 (0.80–1.28) | 1.02 (0.72–1.43) | 0.95 (0.73–1.22) | |
| Glatiramer acetate | 0.82 (0.56–1.20) | 0.93 (0.52–1.64) | 1.04 (0.69–1.57) | |
| Natalizumab | 0.56 (0.17–1.83) | 0.70 (0.16–2.93) | 0.67 (0.21–2.17) | |
| Fingolimod | 0.47 (0.11–1.94) | 0.73 (0.17–3.09) | 0.50 (0.12–2.05) | |
| Dimethyl fumarate | 0.67 (0.24–1.84) | 0.71 (0.17–2.95) | 0.68 (0.21–2.17) | |
| Teriflunomide | 0.60 (0.14–2.50) | 1.57 (0.37–6.57) | 0.97 (0.24–4.00) | |
| Alemtuzumab | 18.47 (8.86–38.51) | 23.62 (10.10–55.22) | 19.31 (9.36–39.83) | |
| Incident adverse event using the “current” DMD exposure approach | ||||
| Acute liver injury | ||||
| No DMD | N/A | N/A | Reference | Reference |
| Any DMD | 0.75 (0.39–1.44) | 0.80 (0.41–1.54) | ||
| First-generation DMD | 0.43 (0.17–1.06) | |||
| Second-generation DMD | 3.01 (1.16–7.83) | |||
| β-Interferon | 0.24 (0.06–0.99) | |||
| Glatiramer acetate | 0.89 (0.28–2.84) | |||
| Natalizumab | NR | |||
| Fingolimod | 2.53 (0.34–18.72) | |||
| Dimethyl fumarate | 6.26 (1.86–21.03) | |||
| Teriflunomide | NR | |||
| Alemtuzumab | 16.59 (2.14–128.48) | |||
| Abnormal hematologic findings | ||||
| No DMD | N/A | N/A | Reference | Reference |
| Any DMD | 1.14 (1.04–1.25) | 1.11 (1.01–1.22) | ||
| First-generation DMD | 1.08 (0.97–1.19) | |||
| Second-generation DMD | 1.49 (1.22–1.81) | |||
| β-Interferon | 1.13 (1.01–1.27) | |||
| Glatiramer acetate | 0.95 (0.78–1.15) | |||
| Natalizumab | 1.69 (1.14–2.52) | |||
| Fingolimod | 1.32 (0.87–2.00) | |||
| Dimethyl fumarate | 1.40 (1.00–1.97) | |||
| Teriflunomide | 1.33 (0.82–2.14) | |||
| Alemtuzumab | 2.41 (1.29–4.50) | |||
Abbreviations: CCI = Charlson Comorbidity Index; DMD = disease-modifying drug; N/A = not applicable.
Bold indicates p < 0.05.
Case definitions used are presented in eTable 3 (links.lww.com/WNL/D339).
Results were adjusted for sex, socioeconomic status (quintiles), and age (continuous) at the index date and Charlson comorbidity score (categorized as 0, 1, 2, or ≥3 and updated overtime).
Results were adjusted for sex, socioeconomic status (quintiles), age (continuous) at the index date, and individual comorbidity (captured in CCI) as separate covariates and updated over time.
Propensity scores for DMD exposure status (never vs ever) for each outcome were generated separately using logistic regression and included sex, socioeconomic status (quintiles), individual comorbidities (captured in CCI), calendar year, and age (modeled with a cubic spline) at the index date. The generated propensity scores were adjusted in the model as a covariate.
This study provides Class III evidence that for patients with MS, while DMD exposure reduces the risk of infection-related hospitalizations, there are increased risks of infection-related physician visits and incident adverse events for select DMDs.
Discussion
In our population-based study comprising up to 86,894 person-years of DMD exposed follow-up, we examined the association between 7 different DMDs and a range of potential adverse events. Most of the significantly elevated safety signals involved a second-generation DMDs, with some of the largest estimated risks involving alemtuzumab, where the risk (aHR) of thyroid disorder was 19.42, followed by hypertension (4.96) and other forms of cardiovascular diseases (3.72), all relative to no or less than the defined minimum DMD exposure. Natalizumab was associated with an increased risk of cardiovascular diseases (1.61) and mental conditions (1.36). For the oral DMDs, most of the elevated risks were for fingolimod and teriflunomide; fingolimod was associated with a higher hazard of cerebrovascular disease, hypertension, and ischemic heart disease ranging from 1.64 to 2.04, while teriflunomide was associated with a higher hazard of thyroid disorders, chronic liver disease, hypertension, and hyperlipidemia ranging from 1.61 to 2.30. Reassuringly (and unaffected by any SARS-CoV-2 outbreaks), there were no increases in the risk of being hospitalized for an infection for any of the DMDs studied, and instead, a 42%–61% lower risk was observed across virtually all DMDs. By including a range of safety-related events that are not always feasible to examine in modestly sized, short-term clinical trials, our study provides an important comprehensive assessment of the safety profiles of the DMDs in the real-world setting.
Although the risk of an infection-related hospitalization was lower in those exposed to DMDs, findings were also suggestive of a possible general shift in the health care setting where some DMD-related infections were being managed. This was evidenced by a higher hazard of infection-related physician visits for 3 of the DMDs—dimethyl fumarate, fingolimod, and alemtuzumab, ranging from 10% to 33%. It is also possible that infections may be less severe in nature due to prophylactic use of anti-infective agents, screening and vaccination before the initiation of some DMDs,34 with each health care setting acting as a proxy of severity (hospitalizations representing the most severe). These findings were generally consistent with our prior, smaller single province study which had been limited by the lack of widespread use of the second-generation DMDs in the timeframe examined.24 By including a much larger multiregional cohort and by accessing more contemporaneous data, we have substantially advanced those findings, including individual assessments of more second-generation DMDs. A nationwide Swedish study of 6,421 patients with MS took a different approach, comparing the risk of first infection-related hospitalization vs the general population. They observed that the risk was highest for rituximab (where unlike Canada, its off-label use for MS was common) and lowest for β-interferon and glatiramer acetate.35 Of interest, no other comparisons were made and only the first infection was assessed, whereas we were able to examine all infection-related hospitalizations and physician visits.
We found a limited number of studies with which to compare our other findings. One Italian study analyzed 13,880 physician-reported MS DMD-related adverse reactions to the National Pharmacovigilance Database (2002–2020).36 Although these voluntarily reporting systems can provide important safety signals, a systematic review found that most serious or severe adverse events (95%) are never reported.37 Nonetheless, although most of the Italian findings were consistent with the literature, we were able to confirm some of the unexpected signals identified, including, for example, the risk of thyroid disorders for teriflunomide which was significantly elevated in our population-based study.36 Reassuringly, the higher potential risk of dyslipidemia for fingolimod, natalizumab, and β-interferon reported in the Italian study was not observed in our study.36
Other adverse events associated with teriflunomide in our study were chronic liver disease, hyperlipidemia, and hypertension. While the former 2 were not reported in the pivotal RCTs, drug-induced liver injury was identified in the US Food and Drug Administration (FDA)'s Adverse Event Reporting System database (2004–2016; adjusted reporting odds ratios >2).38 Cases were also found in a prospective observational study of 1,128 MS persons treated with teriflunomide in Germany (7 cases, including 4 discontinuing drug due to elevated transaminases).11 Hypertension was previously identified, with 4% of persons randomized to teriflunomide and 2% to placebo reported with this adverse event in the product monograph.29 While our observed increased risk of hyperlipidemia (primarily associated with teriflunomide) may seem unexpected, the crude event rate was similar to that occurring with no DMD use (23.92 vs 22.94/1,000 person-years). Thus, while clinicians should remain aware, there is currently insufficient information to recommend routine screening of lipid profiles in people receiving teriflunomide.39
For dimethyl fumarate, our complementary analyses found a higher hazard of acute liver injury (by 6.55), although there were few (<6) cases contributing to this observation resulting in wide CIs. Nonetheless, our findings are consistent with an FDA report aimed at alerting health care professionals of clinically significant drug-induced liver injury (14 cases were identified postmarketing in their Adverse Event Reporting System database [March 2013–February 2016])40 and the recommendation for regular biochemical test monitoring.28 Thus our study is among the first to quantify many of these risks in a real-world setting.
For fingolimod, we observed a higher hazard of 3 circulatory system disorders (hypertension, ischemic heart disease, and cerebrovascular disease). These observations are consistent with fingolimod's effect as a sphingosine-1-phosphate receptor modulator; downregulation of this receptor on endothelial cells likely leads to the increase in blood pressure, necessitating first dose-related monitoring.41 Hypertension is also a risk factor for ischemic heart and cerebrovascular diseases. Although a small number of cerebrovascular events (stroke) were reported in clinical trials and in the postmarketing setting,27 we were unable to find any population-based studies examining and quantifying these broader or longer-term effects of fingolimod. Our findings, along with others,42 underscore the ongoing need for regular cardiovascular-related assessments before and after fingolimod initiation.
For the second-generation DMDs administered by intravenous infusion, alemtuzumab was associated with some of the highest hazards. Among those identified (thyroid disorder, hypertension, and other cardiovascular disorders), thyroid disorders are the most widely established as a known risk.43 Others have reported significant increases in blood pressure during an alemtuzumab infusion in a small group of 31 people with MS,44 and cases of cardiovascular issues have been reported in the postmarketing setting.45 Although routine thyroid function testing (before initiation of alemtuzumab and every 3 months thereafter) is recommended,30 clinicians may wish to consider assessing risk factors for heart diseases, particularly given our observed high crude rate of cardiovascular disorders associated with alemtuzumab (58.92/1,000 person-years) relative to no DMD (25.55). However, the burden of additional testing should also be considered. For natalizumab, although we observed a higher risk (hazard) of mental conditions, a 2018 systematic review of clinical trials and observational studies did not.46 However, a small percentage (1.1%) of psychiatric disorders were identified as serious adverse events in the manufacturers' postmarketing study of natalizumab comprising 6,148 patients with MS.8 Finally, in the complementary analysis, both alemtuzumab and natalizumab were each associated with a higher hazard of abnormal hematologic findings which is consistent with their therapeutic effects; alemtuzumab causes rapid depletion of circulating lymphocytes and can induce autoantibodies (increasing the risk of hematologic conditions, including the rare but serious, immune thrombocytopenic purpura),30,47 and natalizumab can elevate white blood cell counts through inhibition of leukocytes adhesion to endothelial cells.26 The high alemtuzumab-associated crude rate of abnormal hematologic findings (59.0/1,000 persons-years) vs no DMD (26.87) may also reflect, in part, the intensive (monthly) safety-related laboratory testing required.30 Although a higher hazard of hematologic findings was also found for the 3 oral DMDs, the results did not reach statistical significance for these smaller subgroups. It is possible that hematologic findings associated with fingolimod, for example, might not be recorded or coded as abnormal as lymphopenia is thought to contribute to its therapeutic effects.27
For the first-generation DMDs, a previous smaller study from our group also found a higher risk of abnormal hematologic findings, although that study only evaluated β-interferon and in 1 region (British Columbia).7 Nonetheless, this elevated risk was also shown in a meta-analysis of 9 RCTs of β-interferon (vs placebo), with leukopenia and lymphopenia explicitly identified.48 Our prior smaller study found a higher risk of stroke,7 which we did not observe in the current larger study with longer follow-up, at least when evaluating the more comprehensive, broader group of cerebrovascular diseases. However, in our larger current study, we did find an increased risk of hypertension, a risk factor for stroke. We were also able to examine glatiramer acetate, the other first-generation DMD, in our current study. As with β-interferon, most of the safety signals associated with glatiramer acetate were relatively modest in magnitude, and some events (cerebrovascular disease and hypertension) were actually lower as compared with no or minimal DMD exposure. A modestly higher hazard of mental conditions was found which concurs with the modestly higher prevalence of psychiatric disorders reported across 4 of the pivotal RCTs for glatiramer acetate.25
Finally, no significant differences were observed between individual DMDs (vs no DMD) and the hazard of several other events, including cancer. Nonetheless, there remains a need for further study, especially for cancer risk, particularly as newer DMDs are used more frequently, and over extended periods in clinical practice.
The use of ICD codes to identify a range of conditions as a proxy for safety-related events has limitations, including possible underestimation or overestimation. However, the direction of findings from the complementary analyses (using case definitions) was generally consistent with the main findings. It is also possible that persons taking DMDs may visit clinicians more frequently, increasing the detection of adverse events or comorbidities (surveillance bias). Nonetheless, our previous study showed that treatment with DMD (vs no DMD) was not associated with substantial differences in physician visit rates.20 While a person stopping a DMD due to an adverse event or lack of response before they reached the definition of “exposed” would have been assigned to the “unexposed” group, our intention-to-treat analyses yielded results generally in the same direction as those from the main analyses. Furthermore, the sample size for the newer MS DMDs approved more recently may be insufficient to detect some rare adverse events. Although findings reached significance for some individual DMDs for specific adverse events, only a few such cases occurred (<6) resulting in wide CIs. The direction and magnitude of findings were largely similar between the unadjusted and adjusted models that accounted for several important characteristics including sex, age, SES, and comorbidity using a validated index, by including the individual CCI comorbidities as separate covariates, and by using propensity score adjustment. However, other relevant factors, including MS disease course and duration, are not captured in administrative data. Although no gold standard comorbidity indices exist, others have been developed using primary care, hospital, or prescription data.49,50 Each may capture a different dimension of comorbidity worthy of future consideration.49,50 Residual indication bias may remain, for example, if the presence of specific comorbidities precludes the use of some DMDs. The summary measures showed that the magnitude of selection bias was unlikely to explain away findings. Our estimated E-values indicated that the associations between most DMDs and infection-related hospitalizations and alemtuzumab (for most events) and dimethyl fumarate (liver injury) were unlikely to be explained by unobserved confounders (E-values and CI limits >2). However, other results might be less robust. Although the universal health care setting allowed us to capture data on all MS DMD prescriptions filled and medically necessary hospital and physician services, the generalizability of our results to other health care system with different insurance coverage should be explored. Finally, generalizability of our findings would not be affected by any Canada-specific initiatives related to screening for comorbidities or drug-related complications in MS, although naturally, and similar to other regions worldwide, physicians were free to develop local protocols or practices.
Our study provides an extensive safety profile of 7 different MS DMDs in the real-world setting. Adverse events can have major impacts on both patients and the health care system. Our findings not only complement those observed in short-term clinical trials but also provide new insights that help inform the risk-benefit profile of the DMDs used for treating MS in clinical practice. Our study highlights the continued need for long-term, independent safety studies of the MS DMDs.
Acknowledgment
We are grateful to the Data Services Platform of the Saskatchewan Centre for Patient-Oriented Research (SCPOR). We are also grateful to Yan Wang (Dalhousie University) for her support in performing data analyses in Nova Scotia and to Dr. Lawrence W. Svenson for supporting the team's original funding application. Access to, and use of BC data was facilitated by Population Data BC, and approved by the BC Ministry of Health, BC PharmaNet, and the BC Vital Statistics Agency. The authors acknowledge the Manitoba Centre for Health Policy for use of the Population Research Data Repository under project #2018-023 (HIPC #2018/19-13). De-identified data were provided by the Saskatchewan Ministry of Health and eHealth Saskatchewan. Data used in this report were also made available by Health Data Nova Scotia of Dalhousie University. All inferences, opinions, and conclusions drawn in this manuscript are those of the authors and do not reflect the opinions or policies of the British Columbia Data Steward(s), the Manitoba Centre for Health Policy or Manitoba Health, the Government of Saskatchewan, Saskatchewan Ministry of Health or eHealth Saskatchewan, Health Data Nova Scotia, or the Nova Scotia Department of Health and Wellness.
Glossary
- aHR
adjusted hazard ratio
- CCI
Charlson Comorbidity Index
- CR
crude rate per 1,000 person-years
- DMD
disease-modifying drug
- FDA
US Food and Drug Administration
- ICD-9/10
International Classification of Diseases, Ninth/Tenth Revision
- MS
multiple sclerosis
- RCT
randomized controlled trial
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SES
socioeconomic status
Appendix. Authors
| Name | Location | Contribution |
| Huah Shin Ng, PhD | University of British Columbia, Vancouver, Canada, Flinders University, Australia, and SA Pharmacy, Northern and Southern Adelaide Local Health Networks, Australia | Conceptualized and designed the study, performed data analysis, interpreted the results, and drafted and revised the manuscript for intellectual content |
| Feng Zhu, MSc | University of British Columbia, Vancouver, Canada | Obtained funding, conceptualized and designed the study, performed data analysis, interpreted the results, and revised the manuscript for intellectual content |
| Yinshan Zhao, PhD | University of British Columbia, Vancouver, Canada | Obtained funding, conceptualized and designed the study, interpreted the results, and revised the manuscript for intellectual content |
| Shenzhen Yao, PhD | University of Saskatchewan, and Saskatchewan Health Quality Council, Saskatoon, Canada | Performed data analysis and revised the manuscript for intellectual content |
| Xinya Lu, PhD | Saskatchewan Health Quality Council, Saskatoon, Canada | Performed data analysis and revised the manuscript for intellectual content |
| Okechukwu Ekuma, MSc | University of Manitoba, Winnipeg, Canada | Performed data analysis and revised the manuscript for intellectual content |
| Charity Evans, PhD | University of Saskatchewan, Saskatoon, Canada | Obtained funding and data, conceptualized and designed the study, interpreted the results, and revised the manuscript for intellectual content |
| John D. Fisk, PhD | Nova Scotia Health Authority, and Dalhousie University, Halifax, Canada | Obtained funding and data, conceptualized and designed the study, interpreted the results, and revised the manuscript for intellectual content |
| Ruth Ann Marrie, MD, PhD | University of Manitoba, Winnipeg, Canada | Obtained funding and data, conceptualized and designed the study, interpreted the results, and revised the manuscript for intellectual content |
| Helen Tremlett, PhD | University of British Columbia, Vancouver, Canada | Obtained funding and data, conceptualized and designed the study, interpreted the results, and drafted and revised the manuscript for intellectual content |
Study Funding
This study was supported by the Canadian Institutes of Health Research (CIHR) Project and Foundation grant (PJT-156363 and FDN-159934, PI: H. Tremlett).
Disclosure
The authors report no disclosures relevant to the manuscript. R.A. Marrie is a coinvestigator on the CanProCo study funded by Biogen & Roche Canada. Go to Neurology.org/N for full disclosures.
References
- 1.Lucchetta RC, Leonart LP, Becker J, Pontarolo R, Fernandez-Llimós F, Wiens A. Safety outcomes of disease-modifying therapies for relapsing-remitting multiple sclerosis: a network meta-analysis. Mult Scler Relat Disord. 2019;35:7-15. doi: 10.1016/j.msard.2019.06.036 [DOI] [PubMed] [Google Scholar]
- 2.Ng HS, Rosenbult CL, Tremlett H. Safety profile of ocrelizumab for the treatment of multiple sclerosis: a systematic review. Expert Opin Drug Saf. 2020;19(9):1069-1094. doi: 10.1080/14740338.2020.1807002 [DOI] [PubMed] [Google Scholar]
- 3.Liang G, Chai J, Ng HS, Tremlett H. Safety of dimethyl fumarate for multiple sclerosis: a systematic review and meta-analysis. Mult Scler Relat Disord. 2020;46:102566. doi: 10.1016/j.msard.2020.102566 [DOI] [PubMed] [Google Scholar]
- 4.Tramacere I, Del Giovane C, Salanti G, D'Amico R, Filippini G. Immunomodulators and immunosuppressants for relapsing-remitting multiple sclerosis: a network meta-analysis. Cochrane Database Syst Rev. 2015;2015(9):CD011381. doi: 10.1002/14651858.CD011381.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marrie RA, Miller A, Sormani MP, et al. The challenge of comorbidity in clinical trials for multiple sclerosis. Neurology. 2016;86(15):1437-1445. doi: 10.1212/WNL.0000000000002471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.U.S. Food and Drug Administration. Preventable adverse drug reactions: a focus on drug interactions. 2018. Accessed November 14, 2022. fda.gov/drugs/drug-interactions-labeling/preventable-adverse-drug-reactions-focus-drug-interactions.
- 7.de Jong HJI, Kingwell E, Shirani A, et al. Evaluating the safety of β-interferons in MS: a series of nested case-control studies. Neurology. 2017;88(24):2310-2320. doi: 10.1212/WNL.0000000000004037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butzkueven H, Kappos L, Wiendl H, et al. Long-term safety and effectiveness of natalizumab treatment in clinical practice: 10 years of real-world data from the Tysabri Observational Program (TOP). J Neurol Neurosurg Psychiatry. 2020;91(6):660-668. doi: 10.1136/jnnp-2019-322326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lebrun-Frenay C, Moulignier A, Pierrot-Deseilligny C, et al. Five-year outcome in the copaxone observatory: a nationwide cohort of patients with multiple sclerosis starting treatment with glatiramer acetate in France. J Neurol. 2019;266(4):888-901. doi: 10.1007/s00415-019-09211-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biernacki T, Sandi D, Füvesi J, et al. The safety and efficacy of fingolimod: real-world data from a long-term, non-interventional study on the treatment of RRMS patients spanning up to 5 years from Hungary. PLoS One. 2022;17(4):e0267346. doi: 10.1371/journal.pone.0267346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kallmann BA, Tiel-Wilck K, Kullmann JS, Engelmann U, Chan A. Real-life outcomes of teriflunomide treatment in patients with relapsing multiple sclerosis: TAURUS-MS observational study. Ther Adv Neurol Disord. 2019;12:1756286419835077. doi: 10.1177/1756286419835077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lebrun C, Rocher F. Cancer risk in patients with multiple sclerosis: potential impact of disease-modifying drugs. CNS Drugs. 2018;32(10):939-949. doi: 10.1007/s40263-018-0564-y [DOI] [PubMed] [Google Scholar]
- 13.Kingwell E, Evans C, Zhu F, Oger J, Hashimoto S, Tremlett H. Assessment of cancer risk with beta-interferon treatment for multiple sclerosis. J Neurol Neurosurg Psychiatry. 2014;85(10):1096-1102. doi: 10.1136/jnnp-2013-307238 [DOI] [PubMed] [Google Scholar]
- 14.Lebrun C, Vermersch P, Brassat D, et al. Cancer and multiple sclerosis in the era of disease-modifying treatments. J Neurol. 2011;258(7):1304-1311. doi: 10.1007/s00415-011-5929-9 [DOI] [PubMed] [Google Scholar]
- 15.Alping P, Askling J, Burman J, et al. Cancer risk for fingolimod, natalizumab, and rituximab in multiple sclerosis patients. Ann Neurol. 2020;87(5):688-699. doi: 10.1002/ana.25701 [DOI] [PubMed] [Google Scholar]
- 16.Ng HS, Zhu F, Kingwell E, et al. Disease-modifying drugs for multiple sclerosis and association with survival. Neurol Neuroimmunol Neuroinflamm. 2022;9(5):e200005. doi: 10.1212/NXI.0000000000200005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng HS, Zhu F, Kingwell E, et al. Characteristics of a population-based multiple sclerosis cohort treated with disease-modifying drugs in a universal healthcare setting. Expert Rev Neurother. 2021;21(1):131-140. doi: 10.1080/14737175.2021.1847085 [DOI] [PubMed] [Google Scholar]
- 18.Marrie RA, Yu N, Blanchard J, Leung S, Elliott L. The rising prevalence and changing age distribution of multiple sclerosis in Manitoba. Neurology. 2010;74(6):465-471. doi: 10.1212/WNL.0b013e3181cf6ec0 [DOI] [PubMed] [Google Scholar]
- 19.Marrie RA, Bernstein CN, Peschken CA, et al. Intensive care unit admission in multiple sclerosis: increased incidence and increased mortality. Neurology. 2014;82(23):2112-2119. doi: 10.1212/WNL.0000000000000495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng HS, Zhu F, Kingwell E, et al. Disease-modifying drugs for multiple sclerosis and subsequent health service use. Mult Scler. 2022;28(4):583-596. doi: 10.1177/13524585211063403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ng HS, Kingwell E, Zhu F, et al. Adherence to laboratory monitoring among people taking oral drugs for multiple sclerosis: a Canadian population-based study. Mult Scler. 2021;27(2):239-249. doi: 10.1177/1352458520910500 [DOI] [PubMed] [Google Scholar]
- 22.Choi E, Kim S, Suh HS. Evaluation of factors associated with adverse drug events in South Korea using a population-based database. J Clin Med. 2022;11(21):6248. doi: 10.3390/jcm11216248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parameswaran Nair N, Chalmers L, Peterson GM, Bereznicki BJ, Castelino RL, Bereznicki LR. Hospitalization in older patients due to adverse drug reactions: the need for a prediction tool. Clin Interv Aging. 2016;11:497-505. doi: 10.2147/CIA.S99097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wijnands JMA, Zhu F, Kingwell E, et al. Disease-modifying drugs for multiple sclerosis and infection risk: a cohort study. J Neurol Neurosurg Psychiatry. 2018;89(10):1050-1056. doi: 10.1136/jnnp-2017-317493 [DOI] [PubMed] [Google Scholar]
- 25.Teva Canada Limited. COPAXONE (glatiramer acetate injection 20 mg/1 mL and 40 mg/1 mL) product monograph [online]. 2021. Accessed January 20, 2023. canada.ca/en/health-canada/services/drugs-health-products/drug-products/drug-product-database.html.
- 26.Biogen Canada Inc. TYSABRI (Natalizumab intravenous infusion 300 mg/15 mL) product monograph [online]. 2017. Accessed January 20, 2023. canada.ca/en/health-canada/services/drugs-health-products/drug-products/drug-product-database.html.
- 27.Novartis Pharmaceuticals Canada Inc. GILENYA (Fingolimod capsules 0.25 mg and 0.5 mg) product monograph [online]. 2021. Accessed January 20, 2023. canada.ca/en/health-canada/services/drugs-health-products/drug-products/drug-product-database.html.
- 28.Biogen Canada Inc. TECFIDERA (Dimethyl fumarate delayed-release capsules 120 mg and 240 mg) product monograph [online]. 2021. Accessed January 20, 2023. canada.ca/en/health-canada/services/drugs-health-products/drug-products/drug-product-database.html.
- 29.Sanofi Genzyme A Division of Sanofi-Aventis Canada Inc. AUBAGIO (Teriflunomide tablets 14 mg) product monograph [online]. 2022. Accessed January 20, 2023. canada.ca/en/health-canada/services/drugs-health-products/drug-products/drug-product-database.html.
- 30.Sanofi Genzyme A Division of Sanofi-Aventis Canada Inc. LEMTRADA (Alemtuzumab 12 mg/1.2 mL) product monograph [online]. 2022. Accessed January 20, 2023. canada.ca/en/health-canada/services/drugs-health-products/drug-products/drug-product-database.html.
- 31.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268-274. doi: 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 32.Smith LH, VanderWeele TJ. Bounding bias due to selection. Epidemiology. 2019;30(4):509-516. doi: 10.1097/EDE.0000000000001032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Institute for Health and Care Excellence (NICE). NICE real-world evidence framework: methods for real-world studies of comparative studies. 2022. Accessed July 6, 2023. nice.org.uk/corporate/ecd9/chapter/methods-for-real-world-studies-of-comparative-effects.
- 34.Epstein DJ, Dunn J, Deresinski S. Infectious complications of multiple sclerosis therapies: implications for screening, prophylaxis, and management. Open Forum Infect Dis. 2018;5(8):ofy174. doi: 10.1093/ofid/ofy174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luna G, Alping P, Burman J, et al. Infection risks among patients with multiple sclerosis treated with fingolimod, natalizumab, rituximab, and injectable therapies. JAMA Neurol. 2020;77(2):184-191. doi: 10.1001/jamaneurol.2019.3365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barbieri MA, Sorbara EE, Battaglia A, et al. Adverse drug reactions with drugs used in multiple sclerosis: an analysis from the Italian Pharmacovigilance Database. Front Pharmacol. 2022;13:808370. doi: 10.3389/fphar.2022.808370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hazell L, Shakir SA. Under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2006;29(5):385-396. doi: 10.2165/00002018-200629050-00003 [DOI] [PubMed] [Google Scholar]
- 38.Antonazzo IC, Poluzzi E, Forcesi E, et al. Liver injury with drugs used for multiple sclerosis: a contemporary analysis of the FDA Adverse Event Reporting System. Mult Scler. 2019;25(12):1633-1640. doi: 10.1177/1352458518799598 [DOI] [PubMed] [Google Scholar]
- 39.Camara-Lemarroy CR, Castilló J, Sastre-Garriga J, Tintore M, Montalban X. Severe hypertriglyceridemia associated with teriflunomide in a patient with multiple sclerosis: a case report. Mult Scler. 2018;24(10):1383-1385. doi: 10.1177/1352458518761185 [DOI] [PubMed] [Google Scholar]
- 40.Muñoz MA, Kulick CG, Kortepeter CM, Levin RL, Avigan MI. Liver injury associated with dimethyl fumarate in multiple sclerosis patients. Mult Scler. 2017;23(14):1947-1949. doi: 10.1177/1352458516688351 [DOI] [PubMed] [Google Scholar]
- 41.Camm J, Hla T, Bakshi R, Brinkmann V. Cardiac and vascular effects of fingolimod: mechanistic basis and clinical implications. Am Heart J. 2014;168(5):632-644. doi: 10.1016/j.ahj.2014.06.028 [DOI] [PubMed] [Google Scholar]
- 42.Zhao Z, Lv Y, Gu ZC, Ma CL, Zhong MK. Risk for cardiovascular adverse events associated with sphingosine-1-phosphate receptor modulators in patients with multiple sclerosis: insights from a pooled analysis of 15 randomised controlled trials. Front Immunol. 2021;12:795574. doi: 10.3389/fimmu.2021.795574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coles AJ, Arnold DL, Bass AD, et al. Efficacy and safety of alemtuzumab over 6 years: final results of the 4-year CARE-MS extension trial. Ther Adv Neurol Disord. 2021;14:1756286420982134. doi: 10.1177/1756286420982134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shosha E, Casserly C, Tomkinson C, Morrow SA. Blood pressure changes during alemtuzumab infusion for multiple sclerosis patients. Eur J Neurol. 2021;28(4):1396-1400. doi: 10.1111/ene.14633 [DOI] [PubMed] [Google Scholar]
- 45.Killestein J, van Oosten B. Emerging safety issues in alemtuzumab-treated MS patients. Mult Scler. 2019;25(9):1206-1208. doi: 10.1177/1352458519851219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gasim M, Bernstein CN, Graff LA, et al. Adverse psychiatric effects of disease-modifying therapies in multiple sclerosis: a systematic review. Mult Scler Relat Disord. 2018;26:124-156. doi: 10.1016/j.msard.2018.09.008 [DOI] [PubMed] [Google Scholar]
- 47.Havrdova E, Horakova D, Kovarova I. Alemtuzumab in the treatment of multiple sclerosis: key clinical trial results and considerations for use. Ther Adv Neurol Disord. 2015;8(1):31-45. doi: 10.1177/1756285614563522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nikfar S, Rahimi R, Abdollahi M. A meta-analysis of the efficacy and tolerability of interferon-β in multiple sclerosis, overall and by drug and disease type. Clin Ther. 2010;32(11):1871-1888. doi: 10.1016/j.clinthera.2010.10.006 [DOI] [PubMed] [Google Scholar]
- 49.Stirland LE, González-Saavedra L, Mullin DS, Ritchie CW, Muniz-Terrera G, Russ TC. Measuring multimorbidity beyond counting diseases: systematic review of community and population studies and guide to index choice. BMJ. 2020;368:m160. doi: 10.1136/bmj.m160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.MacRae C, McMinn M, Mercer SW, et al. The impact of varying the number and selection of conditions on estimated multimorbidity prevalence: a cross-sectional study using a large, primary care population dataset. PLoS Med. 2023;20(4):e1004208. doi: 10.1371/journal.pmed.1004208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Access eReferences at ; links.lww.com/WNL/D338.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
With the appropriate approvals, data may be accessed through Population Data British Columbia, Saskatchewan Health Quality Council, Manitoba Centre for Health Policy, and Health Data Nova Scotia of Dalhousie University.