Abstract
Schistosomiasis is a neglected tropical disease that is prevalent in low- and middle-income countries. There are five human pathogenic species, of which Schistosoma haematobium, Schistosoma mansoni and Schistosoma japonicum are the most prevalent worldwide and cause the greatest burden of disease in terms of mortality and morbidity. In addition, hybrid schistosomes have been identified through molecular analysis. Human infection occurs when cercariae, the larval form of the parasite, penetrate the skin of people while bathing in contaminated waters such as lakes and rivers. Schistosomiasis can cause both urogenital and intestinal symptoms. Urogenital symptoms include haematuria, bladder fibrosis, kidney damage, and an increased risk of bladder cancer. Intestinal symptoms may include abdominal pain, sometimes accompanied by diarrhoea and blood in the stool. Schistosomiasis affects more than 250 million people and causes approximately 70 million Disability-Adjusted Life Years (DALYs), mainly in Africa, South America, and Asia. To control infection, it is essential to establish sensitive and specific diagnostic tests for epidemiological surveillance and morbidity reduction. This review provides an overview of schistosomiasis, with a focus on available diagnostic tools for Schistosoma spp. Current molecular detection methods and progress in the development of new diagnostics for schistosomiasis infection are also discussed.
Introduction
Schistosomiasis, also known as bilharzia, is a neglected tropical disease caused by blood flukes (trematode worms) that affects low-income countries in tropical and subtropical regions, with approximately 250 million people infected annually [1, 2]. The disease is prevalent in tropical regions worldwide and it is the second most important human parasitic disease in terms of socio-economic impact, after malaria [3]. According to World Health Organization (WHO) data, schistosomiasis is endemic in 78 countries, 51 of which require preventive drug administration for moderate to severe transmission [4]. In 2016, the disease burden of schistosomiasis was estimated to be 2.5 million Disability-Adjusted Life Years (DALYs) with approximately 24,000 deaths globally [5]. These numbers highlight the severity of schistosomiasis as a public health issue, and its elimination is a key goal of the Neglected Tropical Diseases (NTD) roadmap 2021–2030 [6]. The infection is caused by a trematode belonging to the genus Schistosoma. People contract the larval forms of the parasite through contaminated fresh water [7]. The infection is widespread in Africa, South America and Asia, where access to safe drinking water and sanitation is often inadequate [8]. Infection rates are high among rural communities engaged in water-related agricultural activities, as well as among women and children who use contaminated water for household chores [9]. However, the rise of ecotourism and intercontinental travel has led to the spread of schistosomiasis to new areas, including industrialised countries [10]. The parasite was first identified by Theodor Bilharz in 1851. In 1915 Leiper discovered that freshwater snails serve as intermediate hosts in the parasite's life cycle [11]. In 1970, Bayer introduced praziquantel, the first and still the most important drug to treat schistosomiasis [12].
Epidemiology
Schistosomiasis in humans is caused by five main pathogenic species: Schistosoma mansoni, Schistosoma japonicum, Schistosoma intercalatum, Schistosoma mekongi, and Schistosoma haematobium. Among these, S. mansoni is one of the three most common species worldwide, along with S. japonicum and S. haematobium [13]. Molecular analysis has identified hybrid schistosomes, which are new hybrid species resulting from the hybridization of different Schistosoma species [79]. The intermediate host for S. mansoni is the freshwater snail of the genus Biomphalaria [14]. Reservoirs include primates, marsupials, and rodents. Schistosoma mansonii adult parasites establish themselves in the lower mesenteric veins, where they lay between 200 and 300 eggs daily [15]. The eggs that are not excreted with faeces cause a granulomatous inflammatory response. This condition is prevalent in Africa, the Middle East, the Caribbean, Brazil, Venezuela, and South America [16]. S. japonicum has a large number of reservoir hosts, including cattle, dogs, cats, rodents, pigs, horses, and goats [17]. To reproduce, it temporarily infects freshwater snails of the genus Oncomelania. Adult S. japonicum worms inhabit mainly the upper mesenteric veins and lay eggs that can easily reach the liver or intestinal tissues [18]. Compared with other hepatotropic species of Schistosoma spp, S. japonicum causes more rapid progression to liver fibrosis and occasionally liver failure [19]. This parasite is found in China, the Philippines and the Indonesian island of Sulawesi. Schistosoma haematobium is responsible for urogenital pathology [20]. It is a parasitic roundworm found in Africa and the Middle East. Primates serve as the primary host and act as a reservoir for the infection. The adult female of the parasite lays her eggs in the veins that supply blood to the main organs of the pelvis [21]. S. haematobium has been classified as a probable human carcinogen by the International Agency for Research on Cancer (IARC) [21]. S. intercalatum and S. mekongi are two species that are locally widespread, but to a lesser extent than the other species [22]. S. intercalatum is common in the Democratic Republic of Congo, while S. mekongi is common in certain areas of Cambodia and Laos [23]. Notably, S. intercalatum shares the same gastropod as S. haematobium, whereas S. mekongi infects snails of the genus Neotricula [17]. In recent decades, clinically significant hybrid species have been discovered with increasing frequency. Hybridisation between human and animal pathogenic species poses a potential threat due to the easier transmission and spread of new strains [24]. This could potentially render current control measures ineffective. Hybrid Schistosoma species include S. haematobium-bovis, which share an intermediate host, S. haematobium-mattheei, found in children in Malawi and infected travelling populations in South Africa, and S. haematobium-mansoni, discovered in France by a migrant from the Ivory Coast [25–26].
Life cycle
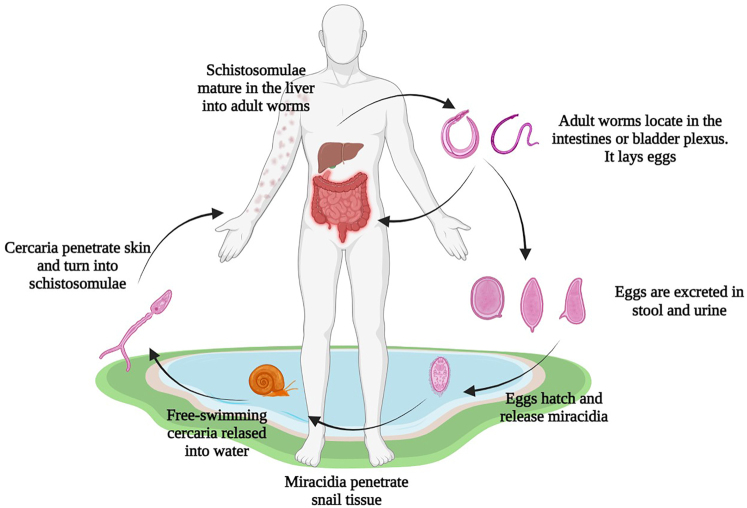
The biological cycle of Schistosoma begins with eggs shed in the faeces and urine of infected individuals (Fig. 1) [23]. These eggs hatch and release miracids that infect freshwater snails, which act as intermediate hosts. The miracids metamorphose inside the mollusc into cercariae, the form that infects humans (or other mammals) by penetrating the skin. The cercariae are attracted to hydrocarbons and fatty acids present on the epidermal surface [13, 24]. Schistosomula develop into adult worms after the parasite enters the final host through the skin and travels through the bloodstream to the liver (Fig. 1) [18]. At this stage, the trematodes absorb antigens produced by the host onto their integument using molecular mimicry, which enables them to evade the host's immune system and survive for several years [25]. Depending on the species, schistosomes colonise either the intestinal plexus or the bladder plexus [4]. Females lay eggs approximately 1–3 months after cercariae invasion [25]. While some of the eggs are excreted in faeces and urine, those that do not pass through the mucous membrane cause inflammation, resulting in the appearance of granulomas.
Fig. 1.
Schistosoma life cycle. The life cycle of Schistosoma begins with the excretion of eggs by humans in water through stools and urine. Once in the aquatic environment, the eggs hatch and release immature larvae known as miracidia. These miracidia penetrate the tissues of freshwater snails, where they transform into cercariae. The snails then release the cercariae into the water, which infest humans by penetrating their tissues. Finally, the cercaria loses its tail and becomes schistosomulae. The schistosomulae reach the liver and mature into the adult form. The male and female worms mate and migrate into either the intestine or bladder veins, depending on the species. Once in their final location, the females begin to lay eggs.
Pathogenesis and clinical disease
Schistosomiasis can manifest as either an acute or chronic condition [23]. The acute form includes cercarial dermatitis and Katayama fever or syndrome. The latter is named after the Katayama District of Hiroshima prefecture in Japan, where the first human case of S. japonicum was reported in 1904 [26]. Cercarial dermatitis, which is a maculopapular skin rash, appears at the site of cercarial entry and typically occurs within 2–7 days of infection [27]. The condition is usually caused by a hypersensitive immune response to the parasite and typically resolves within 15 days [28]. Katayama syndrome is characterised by fever, abdominal pain, diarrhoea, diffuse myalgia, haematuria (in individuals infected with S. haematobium), asthma, non-productive cough, pulmonary infiltrates, and marked eosinophilia [29]. This disease is commonly observed in individuals who have been infected while travelling to endemic areas or living in areas with high transmission rates. These symptoms are usually caused by systemic hypersensitivity to antigens released by schistosomes or eggs [29]. The acute form of the disease may develop in non-immune individuals several weeks or months after exposure [28]. Acute schistosomiasis is a condition that is not well understood and is often not diagnosed [30]. Symptoms typically improve within 2–10 weeks, but in severe cases, patients may experience persistent symptoms such as breathlessness, weight loss, and hepatomegaly [31].
Chronic infection
In endemic areas, most infections progress directly to the chronic phase of the disease due to repeated exposure, and acute schistosomiasis is rare [23, 28]. This form of schistosomiasis is characterised by the presence of adult worms producing large numbers of eggs. In contrast to adult worms, schistosome eggs lack surface antigens and are therefore susceptible to the host's immune system [32]. Symptoms arise from the inflammatory responses generated against the eggs. Soluble glycoproteins secreted by schistosome eggs react with CD4+ and T-helper-2 (Th-2) lymphocytes [33]. The eggs are shed into the bladder lumen and form granulomas at sites of maximum infestation, leading to fibrotic lesions in the host tissue [15]. Treatment with drugs that kill the adult forms can partially reverse infection in patients with active schistosomiasis [34]. As the disease progresses to a chronic state, there is a progressive decrease in the proliferative responses of the lymphocytes induced by the soluble egg antigens [33]. Due to its chronic nature, bilharzia is a significant public health problem. The severity of the disease is directly related to the number of eggs infiltrating the tissues [15]. Intestinal and urogenital schistosomiasis are distinguished by the species involved [23].
Intestinal and urogenital schistosomiasis
Intestinal infection caused by S. mansoni, S. japonicum, S. intercalatum and S. mekongi begins with bloody diarrhoea and sideropenic anaemia due to mucosal ulceration [35]. This condition can progress to a more severe state, which is characterized by portal hypertension, polyposis, microabscess formation, organomegaly, and hepatic fibrosis. In some cases, this may eventually lead to cirrhosis [36]. Urogenital schistosomiasis is characterised by the presence of numerous calcified eggs in the bladder wall and genital organs, resulting in polyposis, hyperplasia and severe ulceration that can lead to haemorrhage [21]. In advanced stages of the disease, the bladder may calcify, leading to urethral obstruction, hydronephrosis and ultimately renal failure [21]. Women with genital schistosomiasis experience pain and bleeding during sexual intercourse, while men have elevated levels of pro-inflammatory cytokines and leukocytes in the seminal fluid [28]. Possible factors contributing to the schistosomal carcinogenic process include inflammatory gene damage, β-glucuronidase and nitrosamines [37]. Although S. haematobium lesions may increase the exposure of the bladder epithelium to mutagenic substrates such as tobacco, it is important to note that this is still under investigation [33]. In addition, female genital schistosomiasis has been found to increase the risk of human immunodeficiency virus (HIV) transmission [38].
IARC has classified S. haematobium as a class 1 carcinogen due to its association with squamous cell carcinoma (SCC), a form of bladder cancer [39]. Chronic schistosomiasis causes bladder cancer by inducing inflammation of the bladder wall. Eggs that are not excreted trigger a strong inflammatory response, leading to the formation of granulomas [40]. Bladder fibrosis may result in bacterial infection, which can convert nitrite and dietary nitrates into nitrosamines. Nitrosamines are carcinogenic and affect metaplastic squamous epithelium, leading to the development of SCC [39]. Numerous epidemiological studies have demonstrated that the prevalence of cancer decreases in areas where schistosomiasis control interventions are implemented and infection prevalence decreases [41, 42]. As bladder cancer remains a common problem in regions where schistosomiasis is endemic, it is advisable to monitor individuals testing positive for schistosomiasis for prognostic indicators of bladder cancer [41]. The observed association between Schistosoma infection and infertility in both men and women may be explained by chronic inflammation, scar tissue formation, and severe granulomatous reactions [43]. In women, schistosomiasis can cause progressive fibrosis in the ovaries and fallopian tubes, leading to tubal occlusion and affecting the reproductive system [44]. In contrast, male infertility is usually caused by infections of the prostate, testes, and seminal vesicles, which are promoted by schistosomiasis [45]. S. haematobium infection may increase the risk of HIV infection, especially in women. Female genital schistosomiasis can cause significant damage to the cervix and vaginal epithelium, resulting in inflammation, ulceration, and trauma due to the calcification of uncleared eggs. The fragile epithelium, which is prone to frequent bleeding, can facilitate HIV transmission [38]. Observational studies have shown that schistosomiasis may modify the immune response and potentially facilitate HIV infection [46]. In individuals infected with schistosomes, there is a decrease in the Th-1 immune response and an increase in CD4+ receptors and chemokines, which are co-receptors used by the virus to infect cells [46]. Therefore, praziquantel therapy is considered critical in regions with a high prevalence of HIV. Studies suggest that praziquantel may be effective in reducing HIV viral load [47, 48].
Diagnosis
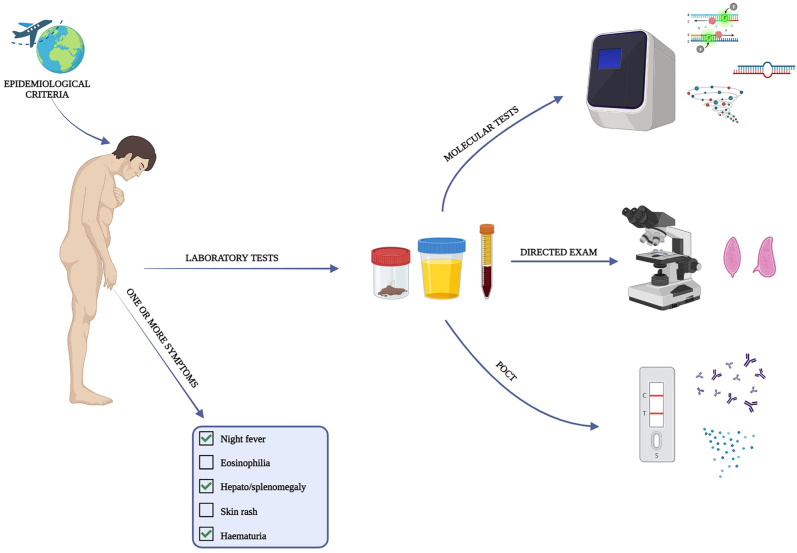
The diagnosis of schistosomiasis relies on specific epidemiological criteria and symptoms associated with the acute or chronic phase of the disease [49]. In recent years, the incidence of schistosomiasis has increased even in areas that are not considered endemic [1]. To diagnose acute schistosomiasis, healthcare professionals should consider persons who have recently visited endemic countries and present with night fever, non-productive cough, headache, asthenia, rash, gastrointestinal symptoms and hepatosplenomegaly [50]. These symptoms are similar to those of acute viral, bacterial, or malarial illnesses. However, there are distinguishing features from malaria, including generalized urticaria, a pruritic rash at the site of cercarial penetration (often on the legs) and eosinophilia [50]. Acute illness may be missed unless schistosomiasis is suspected. In cases of acute schistosomiasis, patients may present with focal neurologic deficits. Neuroschistosomiasis, neurocysticercosis, and coccidiomycosis are potential differential diagnoses for eosinophilia and neurological complications. To differentiate between these conditions, clinical history, radiography, and cerebrospinal fluid examination can be used [51]. Individuals who have lived in or come from endemic areas and present with eosinophilia, gastrointestinal or urogenital symptoms (haematuria, dysuria, hemospermia, dyspareunia) may be suspected of having chronic schistosomiasis, which can be contracted from contaminated fresh water [52]. Co-infections are not unusual in people living in endemic areas [52]. Eosinophilia and fever may also be caused by parasitic helminth infections such as clonorchiasis, fascioliasis, trichinosis, paragonimus, strongyloidiasis, hookworm or Ascaris infections [53]. Haematuria caused by urinary schistosomiasis, especially S. haematobium, must be differentiated from other potential causes such as urinary tract infection, acute nephritis, renal tuberculosis and cancer of the urogenital tract. It is also important to consider alternative causes of infertility when diagnosing genital schistosomiasis [21]. National and international guidelines recommend screening for HIV-Antibody (HIV-Ab), HCV-Ab, HBsAb, HAV, Strongyloides-Ab, and latent tuberculosis infection in individuals with bilharzia infection [54]. Laboratory tests that support the diagnosis of schistosomiasis include eosinophilia, IgE assay, calprotectin, fecal occult blood, and proteinuria [13]. The diagnosis of schistosomiasis can be made using direct or indirect laboratory methods. Direct methods include parasitological examinations, antigenic and molecular tests for specific DNA, while indirect methods involve the search for antibodies in serum [49]. Parasitological tests aim to detect Schistosoma spp. eggs in stool or urine samples using a light microscope (Fig. 2) [49]. Eggs can also be found in biopsy samples of tissues such as the intestine or bladder [35]. Schistosoma spp. can be identified by their different morphological characteristics, such as the size and location of the spur (Table 1) [55]. S. mansoni eggs are oval, and have a prominent lateral spine. S. japonicum eggs are small and round, with a barely visible spine. S. haematobium eggs are large and have a prominent terminal spine. They are found in urine samples (Fig. 2). The size of S. mekongi eggs is smaller than that of S. japonicum [17]. Although morphologically similar to S. haematobium, S. intercalatum eggs are found in faeces [23]. Copro-parasitological examination can be conducted using methods such as the Kato-Katz method, FLOTAC, and HELMINTEX, which indicate the number of eggs present in the feces (Fig. 3) [56]. Quantification can be achieved through conversion factors that multiply the number of eggs per gram of feces [57]. The parasitological examination is highly specific, making it the reference diagnostic method and a screening tool in endemic areas. However, the sensitivity of the test varies due to the discontinuous and sometimes insufficient emission of eggs (Table 2) [57]. According to guidelines, negative test results should be confirmed by analyzing three different samples collected on alternate days [28]. For urine samples, it is recommended to analyze the terminal micturition collected preferably between 10:00 a.m. and 2:00 p.m. or within the previous 24 h [58]. To increase egg shedding in urine, patients can jump shortly before urinating. This facilitates the release of eggs from the bladder mucosa. Samples may also be centrifuged to increase the likelihood of finding eggs and should be kept away from direct light [57]. Antigen tests can detect the presence of eggs, schistosomuli, or adult worms in the body by analyzing serum or urine samples for the presence of intestinal antigens [59]. The search for two primary antigens, circulating cathodic antigen (CCA) and circulating anodic antigen (CAA), takes place in urine [60]. These antigens are named based on their migratory characteristics during testing. Currently, ELISA or immunochromatographic assays are in use. The Point-of-care circulating cathodic antigen immunochromatographic assay is the most widely used point of care test in endemic areas [61]. To enhance accuracy, it is advisable to conduct multiple tests every other day to overcome variability in antigen excretion [61]. The UP-LF CAA test, developed by Leiden University Medical Center in the Netherlands, is a recent development [62]. It is an immunochromatographic assay that searches for antigens in serum and urine. The sensitivity is higher because the sample is concentrated, and the reading of a fluorescent signal is done digitally [62]. However, these features do not currently allow for its use as a point-of-care test. Molecular diagnosis of schistosomiasis is not yet widely available due to its high cost and the need for highly specialised personnel and equipment. Molecular testing involves the detection of parasitic DNA in biological samples through the amplification of specific regions (Fig. 3) [13]. The methods tested so far include real-time PCR, ELISA-PCR, loop-mediated isothermal amplification (LAMP), or recombinant polymerase amplification (RPA) [49]. Real-time PCR based on amplification of the genus-specific ITS-2 sequence of Schistosoma spp. is the molecular assay that has found greater use and standardisation in routine diagnostic practice for schistosomiasis [63]. Another molecular technique that can be used for diagnosis is the search for cell-free DNA, which can be actively released by the parasite or can be derived from dead or decaying worms [64]. Serum, plasma, and other body fluids are the most commonly used samples for this purpose. Currently, many of the molecular tests available in Europe may be subject to re-evaluation and re-approval to meet the requirements of the IDV Regulation (EU 2017/746). This process could potentially limit the availability of diagnostic solutions. Animal models have been studied to analyze the potential of microRNAs (miRNAs), cytokines, and other metabolic products as new diagnostic markers for the disease [65]. These models are important because serological diagnosis can be complicated by hybrids between human and animal schistosomes. These hybrids can result in undetectable antibody responses using available assays. In addition to studying Schistosoma-specific miRNAs, research has been conducted on the dysregulation of host miRNA profiles following Schistosoma infection [65]. It has been shown that this alteration correlates with the onset of various diseases in humans [66]. The immune response in individuals infected with Schistosoma spp. varies depending on the stage of infection [67]. Initially, a Th -1-like response is predominant. This response is subsequently reduced and gives way to a Th2-like response as the schistosome matures. Around 12–16 weeks after infection, granulomas start to form, leading to a gradual decrease in the Th2-like response [68]. IgM antibodies reach their peak levels approximately 12–16 weeks after infection, followed by IgG antibodies at around 20 weeks [69]. During the acute phase of the infection, IgG is primarily produced, while IgA is associated with the onset of chronic granuloma [70]. Indirect diagnostic methods, such as immunochromatography, ELISA, Western blot, and indirect haemagglutination, can be used to differentiate between active and past infections [71]. These are rapid diagnostic tests for Schistosoma-specific antibodies that can be performed at the point-of-care. However, confirmatory tests should be performed in patients with other helminth infections due to potential cross-reactivity [71].
Fig. 2.
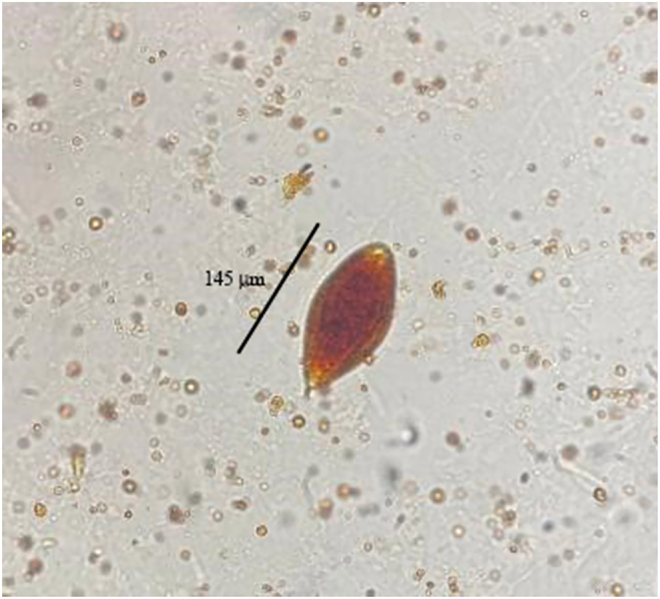
S. haematobium egg in a urine sample (40×). Image courtesy of the Laboratory of Parasitology and Mycology at the University of Messina
Table 1.
Sizes of schistosome eggs
| Species | Measures |
| S. mansoni | 114–180 µm × 45–73 µm |
| S. japonicum | 68–100 × 45–80 µm |
| S. haematobium | 112–170 µm × 40–70 µm |
| S. mekongi | 51–73 µm × 39–66 µm |
| S. intercalatum | 140–240 µm ×50–85 µm |
Fig. 3.
Differential diagnosis of schistosomiasis. The differential diagnosis of schistosomiasis relies on epidemiological criteria and/or symptoms. Laboratory tests are conducted on faeces or urine for direct diagnosis, or on serum for antibody assays. Direct diagnosis involves parasitological examinations, antigenic and molecular tests.
Table 2.
Sensitivity and specificity of diagnostic tests for schistosomiasis infection
| Test | Sensitivity | Specificity | Limitations | Ref. |
| Microscopy | ||||
| Kato-Katz Urine filtration |
<50% | 100% | Low sensitivity is often observed in cases of low-intensity infections. | [53] |
| FLOTAC | N/A 1 egg per gram of feces |
N/A | The laboratory must be equipped with the required tools to perform centrifugation using two distinct rotors. | [59] |
| HELMINTEX | 84% | 100% | More expensive than other tests. | [60] |
| Antigen detection | ||||
| POC-CCA | 63% | 93% | The diagnostic sensitivity is limited by the daily fluctuations in antigen test results. | [64] |
| UCP-LF CAA | 97% | 100% | More labour-intensive than POC-CCA, but more sensitive. | [63] |
| Antibody detection | ||||
| ELISA | 60–100% | 20–89% | Interpreting the results can be challenging because antibody titres may persist even after the infection has been resolved. | [62] |
| Indirect emoagglutination | 66–95% | 37–99% | Failure to distinguish between an active and a past infection. | [62] |
| Immunocromatography | 94–96% | 92% | High cross-reactivity results in low specificity. | [74] |
| Western blot | 94% | 97% | Cold chain logistics and other equipment required. | [62] |
| DNA detection | ||||
| real-time PCR, LAMP, RPA | >50% | 100% | Expensive methods requiring equipment. | [53] |
| qPCR | 97% | 87% | Not cost-effective for routine use in endemic countries. | [66] |
Current therapies and additional strategies to be considered
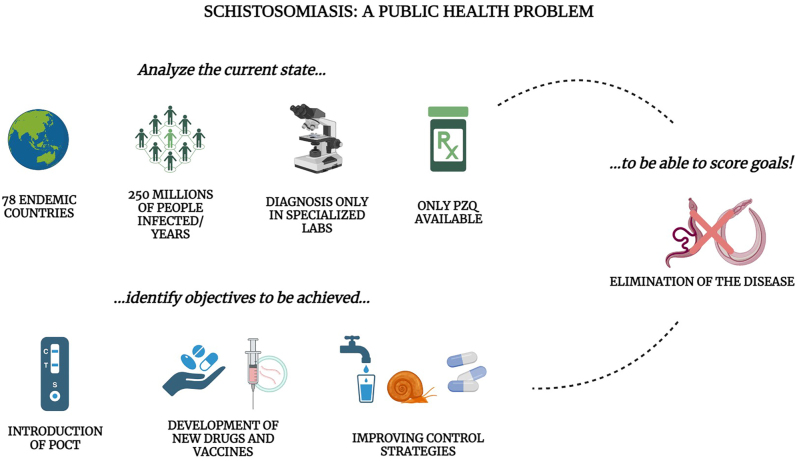
WHO recommends the use of preventive treatment as the primary approach to the control of NTDs [72]. This is achieved through the use of mass drug administration (MDA) and targeted community treatment strategies [73]. Mass drug administration involves the administration of a single dose of a drug to the entire population, while community treatment involves the administration of the drug to specific age groups, usually children between the ages of 6 and 15 years [73]. Although these strategies have reduced the impact of infections, they have limitations. They do not consider individual diagnoses and may not be effective for those recently infected. Additionally, there is a risk of reinfection once treatment is stopped, which means the overall prevalence of infection may not decrease [74]. Furthermore, the repeated use of drugs may lead to the emergence of drug-resistant forms over time [75]. Praziquantel is the drug of choice for treating schistosomiasis and was first approved in 1980 (Fig. 4). This drug targets adult parasites, causing them to become paralyzed and detach from the host's vessel wall. The host's immune response then targets the schistosoma [76]. However, it is ineffective against young schistosomulae, making early treatment less effective. The drug is administered orally in tablet form, with a recommended dosage of 40 mg kg−1 body weight. In endemic areas, treatment is administered as a single dose. However, in non-endemic countries, treatment may be extended over several consecutive days or repeated after 2–4 weeks if necessary [77]. It is believed that pregnant women are more vulnerable to the effects of the disease, which can cause inflammation of the placenta, maternal and fetal iron deficiency, and reduced intrauterine growth [78]. Although there are limited studies on this topic with conflicting results, it is important to consider the potential effects of Schistosoma infection during pregnancy [79]. The WHO recommends the use of praziquantel to treat schistosomiasis during pregnancy as the benefits outweigh the potential risks [80]. Praziquantel is also recommended for use in the paediatric population, despite limited evidence. Currently, there aren't alternative therapies to praziquantel, although some drugs and vaccines are in development (Fig. 4) [81]. Petukhova and colleagues have used cryo-electron microscopy to identify a new class of inhibitors for thioredoxin glutathione reductase, an essential schistosomal enzyme [82]. These inhibitors have the potential to be developed into new anti-schistosomal drugs, as they impair the parasite's survival in the host by inhibiting the enzyme. The compounds demonstrated schistosomicidal activity against various stages of the parasite and were effective in treating schistosome infections in mice [82]. They were found to be more effective than praziquantel in treating young worms and meet the WHO criteria for progressive parasitemia [82]. Molehin et al. (2022) reviewed the characteristics and progress of four potential vaccine candidates at various stages of clinical development [83]. The vaccines target four proteins: rSh28GST, a recombinant S. haematobium glutathione S-transferase; rSm14, a recombinant S. mansoni fatty acid binding protein (FABP); Sm-TSP-2, one of the most abundant tetraspanins of the S. mansoni tegument; and Sm-p80, a second S. mansoni surface protein expressed in all life stages of the parasite [84]. A One Health approach is also being considered, which would involve the development of a vaccine for cattle [85]. This strategy provides a stepwise approach, beginning with the use of a veterinary vaccine to block transmission from cattle, which are the main reservoirs of the infection [86]. This vaccine has the potential to decrease the transmission of infection from cattle to snails by reducing the shedding of infected eggs [33]. Developing a human vaccine to prevent schistosomiasis is a top priority [87–89]. This would limit widespread drug use and prevent the emergence of dangerous resistance. If the drugs currently used lose their effectiveness, the fight against schistosomiasis will be lost.
Fig. 4.
Schistosomiasis: A public health issue requiring a one health approach. The elimination of schistosomiasis can be achieved by controlling the intermediate snail hosts, improving sanitation, managing livestock and developing new drugs and diagnostics for schistosomiasis infection.
Conclusions and future perspectives
Despite the implementation of mass anti-parasitic drug treatment programmes and integrated control approaches, the transmission rate of schistosomiasis has not decreased, and the infection continues to spread, particularly in the world's poorest countries. Treating schistosomiasis requires integrating various interventions with existing drug therapies. It is important to recognize schistosomiasis as a public health issue and address its environmental and social causes. The WHO roadmap outlines the goals of improving sanitation, expanding access to clean water, and controlling vectors through molluscicide, physical removal, and environmental modification to achieve the goal of eliminating schistosomiasis as a public health problem by 2030. Adequate sanitation is harmful to miracidia and cercariae, thus preventing miracidia infection of intermediate host snails. Eliminating this ancient multi-host, multi-species infectious disease will require a change of course from the traditional approach based on large-scale human-only control measures to a One Health approach that recognises the inextricable link between human health, animal health and the environment in which they all live.
Funding
This research received no external funding.
Author contributions
Conceptualization, C.B., E.P and G.M.; figures and preparation of the original draft, M.P., A.Mi. and A.M.; proofreading and editing, C.B. and G.M. All authors have read and agreed to the published version of the manuscript.
Conflict of interest
The authors declare no conflict of interest.
Abbreviations
- CAA
Circulating anodic antigen
- CCA
Circulating cathodic antigen
- CSF
Cerebrospinal fluid
- DALYs
Disability-adjusted life year
- DNA
DeoxyriboNucleic Acid
- ELISA
Enzyme Linked ImmunoSorbent Assay
- HAV
Hepatitis A virus
- HBsAb
Anti-Hepatitis B surface
- HCV-Ab
Hepatitis C Virus - Antibody
- HIV
Human Immunodeficiency Virus
- HIV-Ab
Human Immunodeficiency Virus - Antibody
- IARC
International Agency for Research on Cancer
- LAMP
Loop-mediated isothermal amplification
- miRNAs
microRNAs
- NDTs
Neglected tropical diseases
- PCR
Polymerase chain reaction
- POC-CCA
Point of care - circulating cathodic antigen
- SCC
Squamous cell carcinoma
- Th
T-helper
- UCP-LF CAA
Up-converting phosphor lateral flow circulating anodic antigen
- WHO
World Health Organization
References
- 1.Klohe K, Koudou BG, Fenwick A, Fleming F, Garba A, Gouvras A, et al. . A systematic literature review of schistosomiasis in urban and peri-urban settings. PLoS Negl Trop Dis. 2021;15:e0008995. 10.1371/journal.pntd.0008995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verjee MA. Schistosomiasis: still a cause of significant morbidity and mortality. Res Rep Trop Med. 2019;10:153–163. 10.2147/RRTM.S204345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogongo P, Nyakundi RK, Chege GK, Ochola L. The road to elimination: current state of schistosomiasis research and progress towards the end game. Front Immunol. 2022;13:846108. 10.3389/fimmu.2022.846108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kura K, Ayabina D, Hollingsworth TD, Anderson RM. Determining the optimal strategies to achieve elimination of transmission for Schistosoma mansoni. Parasites Vectors. 2022;15:55. 10.1186/s13071-022-05178-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King CH, Galvani AP. Underestimation of the global burden of schistosomiasis. Lancet. 2018;391:307–308. 10.1016/S0140-6736(18)30098-9. [DOI] [PubMed] [Google Scholar]
- 6.Wiegand RE, Fleming FM, de Vlas SJ, Odiere MR, Kinung'hi S, King CH, et al. . Defining elimination as a public health problem for schistosomiasis control programmes: beyond prevalence of heavy-intensity infections. The Lancet. Glob Health. 2022;10:e1355–e1359. 10.1016/S2214-109X(22)00287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evan Secor W. Water-based interventions for schistosomiasis control. Pathog Glob Health. 2014;108:246–254. 10.1179/2047773214Y.0000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hajissa K, Muhajir A, Eshag HA, Alfadel A, Nahied E, Dahab R, et al. . Prevalence of schistosomiasis and associated risk factors among school children in Um-Asher Area, Khartoum, Sudan. BMC Res Notes. 2018;11:779. 10.1186/s13104-018-3871-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazigo HD, Samson A, Lambert VJ, Kosia AL, Ngoma DD, Murphy R, et al. . “We know about schistosomiasis but we know nothing about FGS”: a qualitative assessment of knowledge gaps about female genital schistosomiasis among communities living in Schistosoma haematobium endemic districts of Zanzibar and Northwestern Tanzania. PLoS Negl Trop Dis. 2021;15:e0009789. 10.1371/journal.pntd.0009789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hatz CF. Schistosomiasis: an underestimated problem in industrialized countries? J Trav Med. 2005;12:1–2. 10.2310/7060.2005.00001. [DOI] [PubMed] [Google Scholar]
- 11.Di Bella S, Riccardi N, Giacobbe DR, Luzzati R. History of schistosomiasis (bilharziasis) in humans: from Egyptian medical papyri to molecular biology on mummies. Pathog Glob Health. 2018;112:268–273. 10.1080/20477724.2018.1495357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vale N, Gouveia MJ, Rinaldi G, Brindley PJ, Gartner F, Correia da Costa JM. Praziquantel for schistosomiasis: single-drug metabolism revisited, mode of action, and resistance. Antimicrob Agents Chemother. 2017;61. 10.1128/AAC.02582-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelwan ML. Schistosomiasis: life cycle, diagnosis, and control. Curr Ther Res Clin Exp. 2019;91:5–9. 10.1016/j.curtheres.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Au MFF, Williams GA, Hui JHL. Status quo and future perspectives of molecular and genomic studies on the genus biomphalaria-the intermediate snail host of schistosoma mansoni. Int J Mol Sci. 2023:24. 10.3390/ijms24054895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costain AH, MacDonald AS, Smits HH. Schistosome egg migration: mechanisms, pathogenesis and host immune responses. Front Immunol. 2018;9:3042. 10.3389/fimmu.2018.03042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang GJ, Bergquist R. Potential impact of climate change on schistosomiasis: a global assessment attempt. Trop Med Infect Dis. 2018;3. 10.3390/tropicalmed3040117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon CA, Kurscheid J, Williams GM, Clements ACA, Li Y, Zhou XN, et al. . Asian schistosomiasis: current status and prospects for control leading to elimination. Trop Med Infect Dis. 2019;4. 10.3390/tropicalmed4010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nation CS, Da'dara AA, Marchant JK, Skelly PJ. Schistosome migration in the definitive host. PLoS Negl Trop Dis. 2020;14:e0007951. 10.1371/journal.pntd.0007951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vellere I, Mangano VD, Cagno MC, Gobbi F, Ragusa A, Bartoloni A, et al. . Imported human Schistosoma japonicum: a report on two cases in Filipino migrants present in Italy and a systematic review of literature. Trav Med Infect Dis. 2020;36:101496. 10.1016/j.tmaid.2019.101496. [DOI] [PubMed] [Google Scholar]
- 20.Onyekwere AM, Rey O, Nwanchor MC, Alo M, Angora EK, Allienne JF, et al. . Prevalence and risk factors associated with urogenital schistosomiasis among primary school pupils in Nigeria. Parasite Epidemiol Control. 2022;18:e00255. 10.1016/j.parepi.2022.e00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santos LL, Santos J, Gouveia MJ, Bernardo C, Lopes C, Rinaldi G, et al. . Urogenital schistosomiasis-history, pathogenesis, and bladder cancer. J Clin Med. 2021:10. 10.3390/jcm10020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uthailak N, Adisakwattana P, Thiangtrongjit T, Limpanont Y, Chusongsang P, Chusongsang Y, et al. . Discovery of Schistosoma mekongi circulating proteins and antigens in infected mouse sera. PloS one. 2022;17:e0275992. 10.1371/journal.pone.0275992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet. 2014;383:2253–2264. 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marascio N, Loria MT, Lamberti AG, Pavia G, Adams NJ, Quirino A, et al. . Molecular characterization of Schistosoma infections in African migrants: identification of a Schistosoma haematobium-bovis hybrid in bladder biopsies. J Trav Med. 2022. taab194 10.1093/jtm/taab194. [DOI] [PubMed] [Google Scholar]
- 25.Cnops L, Huyse T, Maniewski U, Soentjens P, Bottieau E, Van Esbroeck M, et al. . Acute schistosomiasis with a schistosoma mattheei × schistosoma haematobium hybrid species in a cluster of 34 travelers infected in South Africa. Clin Infect Dis. 2021:693–1698. 10.1093/cid/ciaa312. [DOI] [PubMed] [Google Scholar]
- 26.Le Govic Y, Kincaid-Smith J, Allienne J, Rey O, de Gentile L, Boissier J. Schistosoma haematobium–schistosoma mansoni hybrid parasite in migrant boy, France, 2017. Emerg Infect Dis. 2019:365–367. 10.3201/eid2502.172028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hambrook JR, Hanington PC. Immune evasion strategies of schistosomes. Front Immunol. 2020;11:624178. 10.3389/fimmu.2020.624178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis F. Schistosomiasis. Current protocols in immunology; 2001. Chapter 19, Unit 19 11. 10.1002/0471142735.im1901s28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishii A, Tsuji M, Tada I. History of Katayama disease: schistosomiasis japonica in Katayama district, Hiroshima, Japan. Parasitol Int. 2003;52:313–319. 10.1016/s1383-5769(03)00046-1. [DOI] [PubMed] [Google Scholar]
- 30.Kolarova L, Horak P, Skirnisson K, Mareckova H, Doenhoff M. Cercarial dermatitis, a neglected allergic disease. Clin Rev Allergy Immunol. 2013;45:63–74. 10.1007/s12016-012-8334-y. [DOI] [PubMed] [Google Scholar]
- 31.McManus DP, Dunne DW, Sacko M, Utzinger J, Vennervald BJ, Zhou XN. Schistosomiasis. Nature reviews. Disease primers. 2018;4:13. 10.1038/s41572-018-0013-8. [DOI] [PubMed] [Google Scholar]
- 32.Ross AG, Vickers D, Olds GR, Shah SM, McManus DP. Katayama syndrome. The Lancet. Infect Dis. 2007;7:218–224. 10.1016/S1473-3099(07)70053-1. [DOI] [PubMed] [Google Scholar]
- 33.Giboda M, Bergquist R, Utzinger J. Schistosomiasis at the crossroad to elimination: review of eclipsed research with emphasis on the post-transmission agenda. Trop Med Infect Dis. 2022;7. 10.3390/tropicalmed7040055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carbonell C, Rodriguez-Alonso B, Lopez-Bernus A, Almeida H, Galindo-Perez I, Velasco-Tirado V, et al. . Clinical spectrum of schistosomiasis: an update. J Clin Med. 2021:10. 10.3390/jcm10235521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carson JP, Gobert GN. Modulation of the host immune response by schistosome egg-secreted proteins is a critical avenue of host-parasite communication. Pathogens. 2021;10. 10.3390/pathogens10070863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McManus DP, Bergquist R, Cai P, Ranasinghe S, Tebeje BM, You H. Schistosomiasis-from immunopathology to vaccines. Semin immunopathology. 2020;42:355–371. 10.1007/s00281-020-00789-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gouveia MJ, Brindley PJ, Gartner F, Costa J, Vale N. Drug repurposing for schistosomiasis: combinations of drugs or biomolecules. Pharmaceuticals. 2018;11. 10.3390/ph11010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elbaz T, Esmat G. Hepatic and intestinal schistosomiasis: review. J Adv Res. 2013;4:445–452. 10.1016/j.jare.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu GY, Halim MH. Schistosomiasis: progress and problems. World J Gastroenterol. 2000;6:12–19. 10.3748/wjg.v6.i1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barsoum RS, Esmat G, El-Baz T. Human schistosomiasis: clinical perspective: review. J Adv Res. 2013;4:433–444. 10.1016/j.jare.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sturt AS, Webb EL, Francis SC, Hayes RJ, Bustinduy AL. Beyond the barrier: female Genital Schistosomiasis as a potential risk factor for HIV-1 acquisition. Acta tropica. 2020;209:105524. 10.1016/j.actatropica.2020.105524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Efared B, Bako ABA, Idrissa B, Alhousseini D, Boureima HS, Sode HC, et al. . Urinary bladder Schistosoma haematobium-related squamous cell carcinoma: a report of two fatal cases and literature review. Trop Dis Trav Med Vaccin. 2022;8:3. 10.1186/s40794-022-00161-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhong J, Zhang J, Zhou Z, Pan D, Zhao D, Dong H, et al. . Novel insights into the effect of deer IGF-1 on chondrocyte viability and IL-1beta-induced inflammation response. J Biochem Mol Toxicol. 2023;37:e23227. 10.1002/jbt.23227. [DOI] [PubMed] [Google Scholar]
- 44.Yohana C, Bakuza JS, Kinung'hi SM, Nyundo BA, Rambau PF. The trend of schistosomiasis related bladder cancer in the lake zone, Tanzania: a retrospective review over 10 years period. Infect Agents Cancer. 2023;18:10. 10.1186/s13027-023-00491-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li EY, Gurarie D, Lo NC, Zhu X, King CH. Improving public health control of schistosomiasis with a modified WHO strategy: a model-based comparison study. The Lancet Glob Health. 2019;7:e1414–e1422. 10.1016/S2214-109X(19)30346-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ribeiro AR, Luis C, Fernandes R, Botelho MC. Schistosomiasis and infertility: what do we know? Trends Parasitology. 2019;35:964–971. 10.1016/j.pt.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Elias E, Silvestri V, Mushi V, Mandarano M. Ovarian schistosomiasis: challenges of a neglected ectopic involvement of blood flukes. Case report and review of literature. Pathologica. 2023;115:237–245. 10.32074/1591-951X-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abdel-Naser MB, Altenburg A, Zouboulis CC, Wollina U. Schistosomiasis (bilharziasis) and male infertility. Andrologia. 2019;51:e13165. 10.1111/and.13165. [DOI] [PubMed] [Google Scholar]
- 49.Patel P, Rose CE, Kjetland EF, Downs JA, Mbabazi PS, Sabin K, et al. . Association of schistosomiasis and HIV infections: a systematic review and meta-analysis. Int J Infect Dis: IJID: Off Publ Int Soc Infect Dis. 2021;102:544–553. 10.1016/j.ijid.2020.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ndeffo Mbah ML, Gilbert JA, Galvani AP. Evaluating the potential impact of mass praziquantel administration for HIV prevention in Schistosoma haematobium high-risk communities. Epidemics. 2014;7:22–27. 10.1016/j.epidem.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Midzi N, Mduluza T, Mudenge B, Foldager L, Leutscher PDC. Decrease in seminal HIV-1 RNA load after praziquantel treatment of urogenital schistosomiasis coinfection in HIV-positive men-an observational study. Open Forum Infect Dis. 2017;4:ofx199. 10.1093/ofid/ofx199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chala B. Advances in diagnosis of schistosomiasis: focus on challenges and future approaches. Int J Gen Med. 2023;16:983–995. 10.2147/IJGM.S391017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Comelli A, Genovese C, Gobbi F, Brindicci G, Capone S, Corpolongo A, et al. . Schistosomiasis in non-endemic areas: Italian consensus recommendations for screening, diagnosis and management by the Italian Society of Tropical Medicine and Global Health (SIMET), endorsed by the Committee for the Study of Parasitology of the Italian Association of Clinical Microbiologists (CoSP-AMCLI), the Italian Society of Parasitology (SoIPa), the Italian Society of Gastroenterology and Digestive Endoscopy (SIGE), the Italian Society of Gynaecology and Obstetrics (SIGO), the Italian Society of Colposcopy and Cervico-Vaginal Pathology (SICPCV), the Italian Society of General Medicine and Primary Care (SIMG), the Italian Society of Infectious and Tropical Diseases (SIMIT), the Italian Society of Pediatrics (SIP), the Italian Society of Paediatric Infectious Diseases (SITIP), the Italian Society of Urology (SIU). Infection. 2023;51:1249–1271. 10.1007/s15010-023-02050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Graeff-Teixeira C, da Silva AC, Yoshimura K. Update on eosinophilic meningoencephalitis and its clinical relevance. Clin Microbiol Rev. 2009;22:322–348. Table of Contents. 10.1128/CMR.00044-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roure S, Valerio L, Perez-Quilez O, Fernandez-Rivas G, Martinez-Cuevas O, Alcantara-Roman A, et al. . Epidemiological, clinical, diagnostic and economic features of an immigrant population of chronic schistosomiasis sufferers with long-term residence in a non-endemic country (North Metropolitan area of Barcelona, 2002–2016). PloS one. 2017;12:e0185245. 10.1371/journal.pone.0185245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O'Connell EM, Nutman TB. Eosinophilia in infectious diseases. Immunol Allergy Clin North America. 2015;35:493–522. 10.1016/j.iac.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marrone R, Mazzi C, Ouattara H, Cammilli M, Pontillo D, Perandin F, et al. . Screening for Neglected Tropical Diseases and other infections in African refugees and asylum seekers in Rome and Lazio region. Italy Trav Med Infect Dis. 2023;56:102649. 10.1016/j.tmaid.2023.102649. [DOI] [PubMed] [Google Scholar]
- 58.Reguera-Gomez M, Valero MA, Artigas P, De Elias-Escribano A, Fantozzi MC, Luzon-Garcia MP, et al. . Geographical influence on morphometric variability of genetically “pure” schistosoma haematobium eggs from sub-saharan migrants in Spain. Trop Med Infect Dis. 2023;8. 10.3390/tropicalmed8030144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cringoli G, Maurelli MP, Levecke B, Bosco A, Vercruysse J, Utzinger J, et al. . The Mini-FLOTAC technique for the diagnosis of helminth and protozoan infections in humans and animals. Nat Protoc. 2017;12:1723–1732. 10.1038/nprot.2017.067. [DOI] [PubMed] [Google Scholar]
- 60.Oliveira WJ, Magalhaes FDC, Elias AMS, de Castro VN, Favero V, Lindholz CG, et al. . Evaluation of diagnostic methods for the detection of intestinal schistosomiasis in endemic areas with low parasite loads: saline gradient, Helmintex, Kato-Katz and rapid urine test. PLoS Negl Trop Dis. 2018;12:e0006232. 10.1371/journal.pntd.0006232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Neal PM. Schistosomiasis--an unusual cause of ureteral obstruction: a case history and perspective. Clin Med Res. 2004;2:216–227. 10.3121/cmr.2.4.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hinz R, Schwarz NG, Hahn A, Frickmann H. Serological approaches for the diagnosis of schistosomiasis – a review. Mol Cell probes. 2017;31:2–21. 10.1016/j.mcp.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 63.Hoekstra PT, Madinga J, Lutumba P, van Grootveld R, Brienen EAT, Corstjens P, et al. . Diagnosis of schistosomiasis without a microscope: evaluating circulating antigen (CCA, CAA) and DNA detection methods on banked samples of a community-based survey from DR Congo. Trop Med Infect Dis. 2022;7. 10.3390/tropicalmed7100315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cai P, Mu Y, Weerakoon KG, Olveda RM, Ross AG, McManus DP. Performance of the point-of-care circulating cathodic antigen test in the diagnosis of schistosomiasis japonica in a human cohort from Northern Samar, the Philippines. Infect Dis poverty. 2021;10:121. 10.1186/s40249-021-00905-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Corstjens P, Hoekstra PT, de Dood CJ, van Dam GJ. Utilizing the ultrasensitive Schistosoma up-converting phosphor lateral flow circulating anodic antigen (UCP-LF CAA) assay for sample pooling-strategies. Infect Dis poverty. 2017;6:155. 10.1186/s40249-017-0368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Siqueira LMV, Senra C, de Oliveira AA, Carneiro NFF, Gomes LI, Rabello A, et al. . A real-time PCR assay for the diagnosis of intestinal schistosomiasis and cure assessment after the treatment of individuals with low parasite burden. Front Immunol. 2020;11:620417. 10.3389/fimmu.2020.620417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weerakoon KG, McManus DP. Cell-free DNA as a diagnostic tool for human parasitic infections. Trends Parasitology. 2016;32:378–391. 10.1016/j.pt.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 68.Weerakoon KG, Gobert GN, Cai P, McManus DP. Advances in the diagnosis of human schistosomiasis. Clin Microbiol Rev. 2015;28:939–967. 10.1128/CMR.00137-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Condrat CE, Thompson DC, Barbu MG, Bugnar OL, Boboc A, Cretoiu D, et al. . miRNAs as biomarkers in disease: latest findings regarding their role in diagnosis and prognosis. Cells. 2020;9. 10.3390/cells9020276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Molehin AJ. Current understanding of immunity against schistosomiasis: impact on vaccine and drug development. Res Rep Trop Med. 2020;11:119–128. 10.2147/RRTM.S274518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Todt JC, Whitfield JR, Ivard SR, Boros DL. Down-regulation of interleukin-12, interleukin-12R expression/activity mediates the switch from Th1 to Th2 granuloma response during murine Schistosomiasis mansoni. Scand J Immunol. 2000;52:385–392. 10.1046/j.1365-3083.2000.00785.x. [DOI] [PubMed] [Google Scholar]
- 72.Lundy SK, Lukacs NW. Chronic schistosome infection leads to modulation of granuloma formation and systemic immune suppression. Front Immunol. 2013;4:39. 10.3389/fimmu.2013.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lica ICL, Frazao G, Nogueira RA, Lira MGS, Dos Santos VAF, Rodrigues JGM, et al. . Immunological mechanisms involved in macrophage activation and polarization in schistosomiasis. Parasitology. 2023;150:401–415. 10.1017/S0031182023000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hoermann J, Kuenzli E, Schaefer C, Paris DH, Buhler S, Odermatt P, et al. . Performance of a rapid immuno-chromatographic test (Schistosoma ICT IgG-IgM) for detecting Schistosoma-specific antibodies in sera of endemic and non-endemic populations. PLoS Negl Trop Dis. 2022;16:e0010463. 10.1371/journal.pntd.0010463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Montresor A, Gabrielli AF, Chitsulo L, Ichimori K, Mariotti S, Engels D, et al. . Preventive chemotherapy and the fight against neglected tropical diseases. Expert Rev anti-infective Ther. 2012;10:237–242. 10.1586/eri.11.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Deardorff KV, Rubin Means A, Asbjornsdottir KH, Walson J. Strategies to improve treatment coverage in community-based public health programs: a systematic review of the literature. PLoS Negl Trop Dis. 2018;12:e0006211. 10.1371/journal.pntd.0006211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aula OP, McManus DP, Jones MK, Gordon CA. Schistosomiasis with a focus on Africa. Trop Med Infect Dis. 2021:6. 10.3390/tropicalmed6030109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Greenberg RM. New approaches for understanding mechanisms of drug resistance in schistosomes. Parasitology. 2013;140:1534–1546. 10.1017/S0031182013000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bergquist R, Utzinger J, Keiser J. Controlling schistosomiasis with praziquantel: how much longer without a viable alternative? Infect Dis poverty. 2017;6:74. 10.1186/s40249-017-0286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kabuyaya M, Chimbari MJ, Mukaratirwa S. Correction: efficacy of praziquantel treatment regimens in pre-school and school aged children infected with schistosomes in sub-Saharan Africa: a systematic review. Infect Dis Poverty. 2023;12:13. 10.1186/s40249-023-01064-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Friedman JF, Olveda RM, Mirochnick MH, Bustinduy AL, Elliott AM. Praziquantel for the treatment of schistosomiasis during human pregnancy. Bull World Health Organ. 2018;96:59–65. 10.2471/BLT.17.198879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cando LFT, Perias GAS, Tantengco OAG, Dispo MD, Ceriales JA, Girasol MJG, et al. . The global prevalence of schistosoma mansoni, S. Japonicum, and S. Haematobium in pregnant women: a systematic review and meta-analysis. Trop Med Infect Dis. 2022;7. 10.3390/tropicalmed7110354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Olveda RM, Acosta LP, Tallo V, Baltazar PI, Lesiguez JL, Estanislao GG, et al. . Efficacy and safety of praziquantel for the treatment of human schistosomiasis during pregnancy: a phase 2, randomised, double-blind, placebo-controlled trial. The Lancet. Infect Dis. 2016;16:199–208. 10.1016/S1473-3099(15)00345-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hassan AS, Zelt NH, Perera DJ, Xing L, Ndao M, Ward BJ. Correction: vaccination against the digestive enzyme Cathepsin B using a YS1646 Salmonella enterica Typhimurium vector provides almost complete protection against Schistosoma mansoni challenge in a mouse model. PLoS Negl Trop Dis. 2021;15:e0009936. 10.1371/journal.pntd.0009936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Petukhova VZ, Aboagye SY, Ardini M, Lullo RP, Fata F, Byrne ME, et al. . Non-covalent inhibitors of thioredoxin glutathione reductase with schistosomicidal activity in vivo. Nat Commun. 2023;14:3737. 10.1038/s41467-023-39444-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Molehin AJ, McManus DP, You H. Vaccines for human schistosomiasis: recent progress, new developments and future prospects. Int J Mol Sci. 2022;23. 10.3390/ijms23042255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Al-Naseri A, Al-Absi S, El Ridi R, Mahana N. A comprehensive and critical overview of schistosomiasis vaccine candidates. J parasitic Dis: Off Organ Indian Soc Parasitol. 2021;45:557–580. 10.1007/s12639-021-01387-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.You H, Cai P, Tebeje BM, Li Y, McManus DP. Schistosome vaccines for domestic animals. Trop Med Infect Dis. 2018;3. 10.3390/tropicalmed3020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sander VA, Sanchez Lopez EF, Mendoza Morales L, Ramos Duarte VA, Corigliano MG, Clemente M. Use of veterinary vaccines for livestock as a strategy to control foodborne parasitic diseases. Front Cell Infect Microbiol. 2020;10:288. 10.3389/fcimb.2020.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]