Abstract
Objective
Patients with impaired kidney function and increased albuminuria are at risk of developing cardiovascular disease (CVD). Previous research has revealed that a substantial proportion of patients with chronic kidney disease (CKD) do not get a registered diagnosis in the electronic health record of the general practitioner. The aim of this study was to investigate the association between non-registration of CKD and all-cause mortality and cardiovascular outcome.
Design and setting
A retrospective study in primary care.
Methods
The analyses were carried out in the INTEGO database, a general practice-based morbidity registration network in Flanders, Belgium. The study used INTEGO data from the year 2018 for all patients ≥18 years old, including 10 551 patients. To assess the risk of mortality and CVD, a time-to-event analysis was performed. Cox proportional hazard model was used to evaluate the association between non-registration and incidence of all-cause mortality and cardiovascular events with mortality as a competing risk. Subgroup analyses were performed for estimated glomerular filtration rate stages (3A, 3B, 4 and 5). Multiple imputation was done following the methodology of Mamouris et al.
Results
Mortality was higher in patients with non-registered CKD compared with patients with registered CKD (HR 1.29, 95% CI 1.19 to 1.41). Non-registration of CKD was not associated with an increased risk for the development of CVD (HR 0.92, 95% CI 0.77 to 1.11).
Conclusion
An association between non-registration and all-cause mortality was identified, although no such association was apparent for CVD.
Keywords: chronic renal failure, mortality, cardiology
STRENGTHS AND LIMITATIONS OF THIS STUDY.
To assess the risk of cardiovascular disease and mortality, Cox-proportional hazard models were used and a competing risk analysis was performed to account for the presence of competing event (mortality).
For the missing variables, we used multiple imputation.
The presence of proteinuria was not taken into account in our chronic kidney disease population due to the lack of data.
The study used healthcare data, which may under-represent the healthy and asymptomatic that do not seek healthcare.
The participating general practitioners are a selected group of high-quality registering practitioners that use a specific electronic health record, although the patient population is representative for the Flemish population.
Introduction
Chronic kidney disease (CKD) is a progressive condition that describes the gradual loss of kidney function over time. A reduced estimated glomerular filtration rate (eGFR) and elevated albuminuria are the two key measures in patients with CKD.1 Multiple studies have documented suboptimal albuminuria testing in CKD patients in primary care.2 3 However, both reduced eGFR and the presence of albuminuria are associated with an increased risk of cardiovascular disease (CVD), hospitalisation and premature death.4–9 The most common causes of CKD in high-income and middle-income countries are glomerulonephritis, diabetes mellitus and hypertension (the latter being also a consequence of CKD).10–12 The increased cardiovascular risk (CVR) in patients with CKD was therefore assumed to be the result of these underlying diseases. However, meta-analyses showed that impaired kidney function and increased albuminuria are CVR factors, independently of the presence of hypertension or diabetes mellitus.6 13 Kidney specific mechanisms that make significant contributions to the CVR were documented.4
Previous research revealed that a substantial proportion of patients did not have a registered CKD diagnosis in the general practitioner’s (GP) electronic health record (EHR).14 15 In addition, mainly patients with early-stage CKD (stage 3) remained without official diagnosis.15 Although we know that patients with CKD are more at risk, the impact of not registering a diagnosis has not been investigated, neither on cardiovascular outcome nor on mortality.4–6
The aim of this study was to evaluate the impact of non-registration on all-cause mortality and cardiovascular outcomes in Flanders, Belgium.
Methods
Study setting and data source
This study was conducted following on from previous work.15 In that research, the prevalence of non-registered CKD, the diagnostic delay (time between abnormal eGFR and diagnosis) and the baseline characteristics of the non-registered patient group were examined in a Belgian GP population. The same study population was used.
The analyses were carried out in the INTEGO database, a general practice-based computerised morbidity and mortality registration network in Flanders, Belgium, managed at the Department of General Practice of the University of Leuven since 1994. Data collection is regulated by an opting-out procedure. More than 100 GP centres applied for inclusion in this registry. Only the data of the 86 practices (representing 454 GPs) with optimal registration performance (80% coded diagnoses) were included in the database. Patient characteristics and diagnoses are encoded and classified using the International Classification of Primary Care (ICPC-2; WHO FIC Collaborating Centre). All laboratory tests performed by GPs are included in the database.
The methodology of data collection, study design and analyses in the INTEGO registry have been previously reported.16
Study population
Guidelines for CKD management recommend that patients should be diagnosed with CKD if the reduction in kidney function (eGFR <60 mL/min/1.73m2) is present for more than 3 months.1 17 18 All patients ≥18 years old with two consecutive eGFR laboratory measurements indicating CKD (eGFR <60 mL/min/1.73m2) recorded >90 and ≤730 days apart during the baseline period were included. The current study used INTEGO data from the year 2018. Selected patients had at least one eGFR measurement <60 mL/min/1.73m2 in 2018 and belonged to the GP’s yearly contact group. There must be at least 12 months of continuous presence in the database prior to the first qualifying eGFR. Patients were excluded if they had a solid kidney transplant (ICD-10 Z94.0) before the date of the second qualifying eGFR (index date).
Non-registered CKD case definition
Patients with non-registered CKD were identified if they had no diagnostic CKD code for any time during the ≥12-month lookback period before the first eGFR measurement and up to 6 months postindex date. ICPC-2 codes are used more frequently in general practice than ICD-10, so we chose to use the ICPC-2 code U99. Those with a documented U99 during this time period were considered as having registered CKD. Since the U99 code is a collective code for unspecified kidney disease—like CKD, renal cyst—we manually checked both the code and the written diagnosis whether the code merged with CKD. It was assumed that patients with at least one diagnostic code for CKD during the above specified time window had registered CKD.
Statistical analysis
R software (V.4.0.4) was used.19 A descriptive analysis was performed, calculating incidences of all-cause mortality, myocardial infarction, stroke, peripheral vascular disease and heart failure among those with registered versus non-registered CKD. The follow-up period for these adverse clinical outcomes started 6 months after the index date until observation end date (follow-up end date or end of data coverage up to 17 July 2023, whichever came first). The variables were summarised using patient counts with percentages. The χ2 was calculated. P values less than 0.05 were considered significant. Subgroup analyses were performed for eGFR stages (3A, 3B, 4 and 5) and visualised using Kaplan-Meier curves.
To assess the risk of CVD and mortality, Cox-proportional hazard model was used. A competing risk analysis was performed to account for the presence of competing event (mortality).20 We estimated the HRs and derived the sub-distribution HRs (sHRs) from the Fine and Gray model. Their 95% CI was calculated. P values less than 0.05 were considered significant. We adjusted for all possible confounders (age, gender, hypertension, diabetes, smoking status, hypercholesterolaemia, history of CVD). We fitted the models by including and excluding covariates one-by-one (sequential method) and we did not find significant change in the estimate and significance of covariates which were already in the model after adding new covariate. We calculated the variance inflation factor to check for multicollinearity.21
Variables were chosen based on the risk factors for CVD, defined by the Framingham Heart Study.22 Cardiovascular events were defined as myocardial infarction (ICPC-2 K75), stroke (ICPC-2 K90), peripheral vascular disease (ICPC-2 K92) and heart failure (ICPC-2 K77). Hypertension or hypercholesterolaemia included patients with a diagnosis of hypertension (ICPC-2 K86) or hypercholesterolaemia (ICPC-2 T93) in the EHR. Antihypertensive, lipid lowering and antidiabetic medication were defined by the ATC-codes (online supplemental file 1). Patients with a diagnosis of diabetes type 1 or 2 in the EHR (ICPC-2 T89 and T90) and patients taking antidiabetic drugs were merged into the diabetes group. Since there was multicollinearity between total cholesterol, high-density lipoprotein and low-density lipoprotein, we chose to include total cholesterol.
bmjopen-2023-081115supp001.pdf (58.4KB, pdf)
For the missing variables, we used the methodology developed by Mamouris et al.23 Concisely, in their work, they developed a three-stage approach to impute longitudinal covariates so as complexities such as convergence and collinearity are resolved.23 We imputed body mass index (BMI), total cholesterol, systolic blood pressure (SBP) and smoking status longitudinally for years 2017–2023, thus using the previous and earlier information of the same patient (online supplemental file 2). We then extracted the observed year 2018. The dataset was imputed 20 times and model analysis was performed for each imputation separately. We finally pooled the results together using Rubin’s rules.24
bmjopen-2023-081115supp002.pdf (37.7KB, pdf)
Patient and public involvement
None.
Results
As reported in our first research, 231 702 patients ≥18 years old were detected in the INTEGO database in 2018 (online supplemental file 3). The maximum follow-up was 3.97 years. Since the general practice didn’t meet the criteria for best quality register, 40 216 patients were excluded. Among included patients, there were 10 551 patients (5.5%) with two consecutive eGFR laboratory measurements indicating CKD (eGFR <60 mL/min/1.73m2), recorded at least 3 months apart during the baseline period. Out of them, 7176 patients (68%) had no U99 at any time. The other 3375 patients (32%) had a registered diagnosis.15
bmjopen-2023-081115supp003.pdf (219.2KB, pdf)
Descriptive analysis
Incidences
Incidences of all-cause mortality, myocardial infarction, stroke, peripheral vascular disease and heart failure associated with CKD diagnosis status as of index date, are being displayed in table 1.
Table 1.
Cardiovascular outcome associated with registration status
| Variable | Registered CKD, n (%) | Non-registered CKD, n (%) | Total CKD, n (%) | P value |
| Total patients | 3375 | 7176 | 10 551 | |
| All-cause mortality, n (%) | 460 (13.6) | 820 (11.4) | 1280 (12.1) | 0.033 |
| Myocardial infarction, n (%) | 28 (0.8) | 35 (0.5) | 63 (0.6) | 0.067 |
| Stroke, n (%) | 70 (2.1) | 113 (1.6) | 183 (1.7) | 0.089 |
| Peripheral vascular disease, n (%) | 52 (1.5) | 82 (1.1) | 134 (1.3) | 0.004 |
| Heart failure, n (%) | 188 (5.6) | 308 (4.3) | 496 (4.7) | 0.001 |
CKD, chronic kidney disease.
Strata analyses
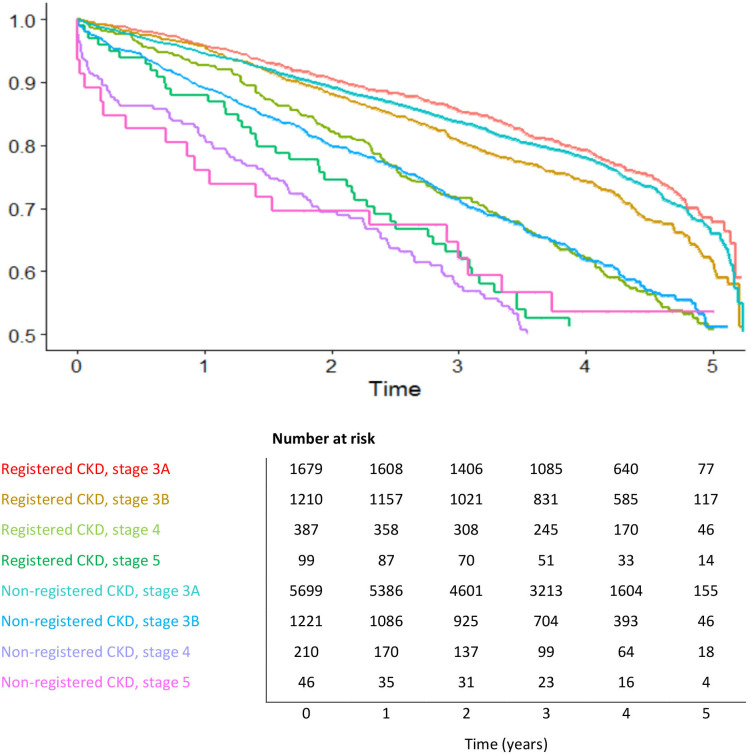
Figures 1 and 2, respectively, display the differences in survival time and time to development of CVD in patients with CKD, according to the CKD stage and presence of diagnostic code in the EHR. An informative risk set table shows the number of patients who were under observation and at risk in the specific period. It appeared that registered patients in stage 3B and 4 had a much better survival rate than non-registered patients after 3 years of follow-up, namely 82.23% (registered group, stage 3B) and 72.87% (registered group, stage 4) towards 73.05% (non-registered group, stage 3B) and 59.52% (non-registered group, stage 4) (figure 1). The same difference was documented for CKD stage 5 after 1 year of follow-up. In the registered group, 87.88% survived at that time, towards 76.09% of the non-registered patients. Only a small number of stage 5 patients were still under observation after 3 years of follow-up, making it difficult to interpret the results at that time (figure 1). Similar survival curves were reported in both registered and non-registered in stage 3A.
Figure 1.
Strata analysis for mortality. Survival probability in different years grouped by chronic kidney disease (CKD) stage and presence of diagnostic code. Risk set table with number of patients at risk per year.
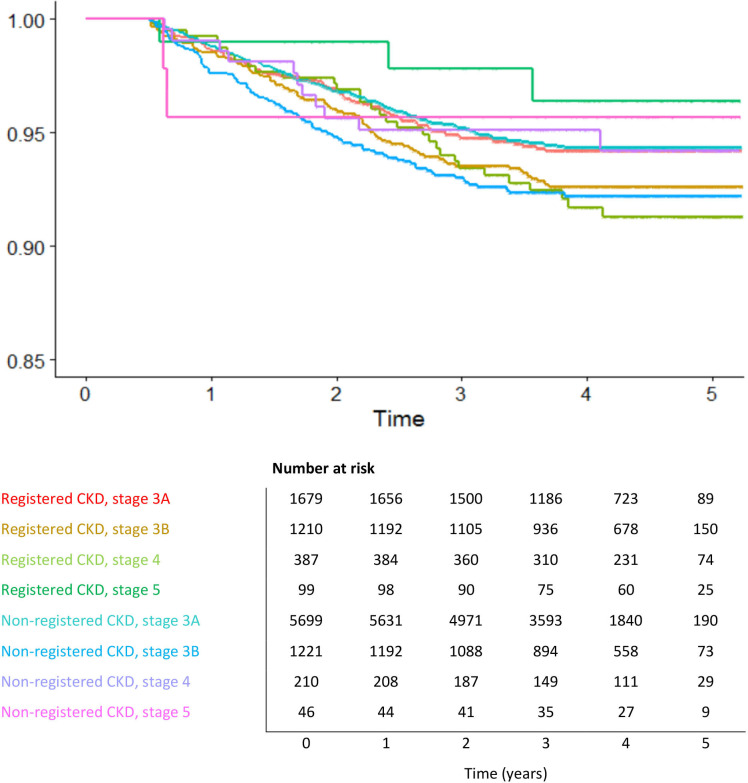
Figure 2.
Strata analysis for cardiovascular disease. Morbidity probability in different years grouped by chronic kidney disease (CKD) stage and presence of diagnostic code. Risk set table with number of patients at risk per year.
Similar to the findings for mortality, less registered patients in stage 5 developed CVD compared with non-registered stage 5 patients after 1 year of follow-up (morbidity rate 98.99% in the registered group, towards 95.65% in the non-registered group) (figure 2). In stages 3B and 4 were the differences in morbidity rate between registered and non-registered smaller after 3 years of follow-up compared with what we documented for mortality, respectively 93.80% (registered, stage 3B and 4) compared with 93.28% (non-registered, stage 3B) and 95.24% (non-registered, stage 4). As for mortality, stage 3A showed similar results curves for non-registered and registered.
Time-to-event analysis
Figures 3 and 4, respectively, show the time to the occurrence of death or CVD with mortality as a competing risk. Results for analyses with and without mortality as a competing risk were similar.
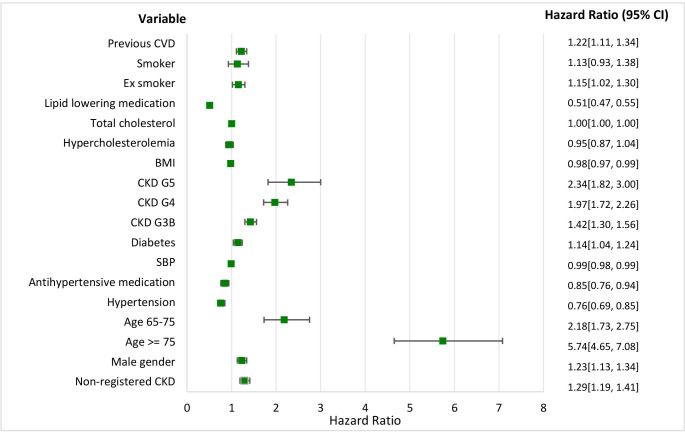
Figure 3.
Mortality and time to event. HRs and the 95% CI for different variables. BMI, body mass index; CKD, chronic kidney disease; CVD, cardiovascular disease; SBP, systolic blood pressure.
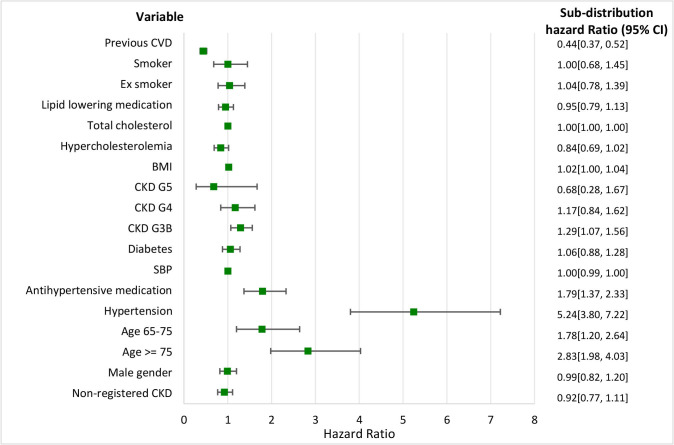
Figure 4.
Cardiovascular disease with mortality as a competing risk. Sub-distribution HRs and the 95% CI for different variables. BMI, body mass index; CKD, chronic kidney disease; CVD, cardiovascular disease; SBP, systolic blood pressure.
All-cause mortality analysis showed that patients with non-registered CKD, male gender, age ≥65, diabetes, CKD stage 3B-5, history of CVD and (ex-)smokers had a higher chance of dying. Hypertension and hypercholesterolaemia were protective factors, as was the use of antihypertensive and lipid lowering medication and BMI. The values for smoking and hypercholesterolaemia were not statistically significant. The HR for total cholesterol was equal to 1.
Considering the results for CVD, only age ≥65, hypertension, antihypertensive medication, CKD stage 3B, BMI, total cholesterol and history of CVD were statistically significant. The sHR for non-registered CKD was <1. A history of CVD and hypercholesterolaemia seemed to be protective factors for CVD, while patients with hypertension had an increased risk.
Discussion
Principal findings
This study showed that patients with a properly registered diagnosis die less quickly than non-registered ones. However, according to our results, these patients did not appear to have a lower risk of developing CVD. Besides, patients in stages 3B and 4 with a registered diagnosis had much better mortality survival rates compared with the non-registered ones. The association of non-registration and CVD was less clear in the different CKD stages.
Patients with CKD and hypercholesterolaemia were shown to be less associated with CVD and mortality, although the result was not statistically significant. In contrast, hypertensive CKD patients appeared to have a higher risk of CVD, but a lower risk of mortality. Patients with a history of CVD seemed to have a lower risk of new events, but a higher risk of dying.
Context of the results
Non-registration appears to be associated with all-cause mortality. However, the CaReMe CKD study recently showed that the rates of cardiovascular and all-cause death were 31%–49% higher in registered CKD patients than in measured CKD patients, which could not be confirmed in our study.25 Few researches have been conducted in this regard, making it difficult to compare. We must note that non-registration may be a risk factor for mortality comparable to diabetes, but outweighed by age and stage of CKD by far. An association was found, but causality was not investigated. It is unclear whether better registration will lead to a better outcome, so this should be a topic for further research.
The key research question of our results is what caused patients to die. Previous research showed that a reduced kidney function predicts both cardiovascular and non-cardiovascular mortality due to pulmonary disease, infection, cancer and other causes.26–28 The association between a reduced eGFR and the increased risk of cardiovascular events and hospitalisation was also found.9 12 29 Surprisingly, the non-registered group in our study did not have a higher risk of CVD than the registered. It is unclear why no association was found. Possibly, this group died more frequently as a result of non-CVD. On the other hand, non-registration probably extends beyond renal insufficiency and also occurs with other pathologies. Mata-Cases et al reported non-registration of diabetes mellitus in Spanish primary healthcare.30 Cardiovascular diagnosis may also be non-registered, so that no association with non-registration could be found.31
A second important question remains why the difference in mortality outcome was found between registered and non-registered patients. Is the root of the problem with the GP or the patient? Our previous research showed that there were small differences between registered and non-registered patients at baseline.15 Hypertension was more frequently present in the registered (64.4% of the registered population) compared with the non-registered (51.7% of the non-registered population). Similar results were found for type 2 diabetes (33.1%) of the registered compared with 28.2% of the non-registered). Small differences were also noticeable in the use of ACE-inhibitor (ACE-I) or angiotensin receptor blocker (ARB) (52.6% among the registered compared with 46.3% of the non-registered).15 However, these small differences do not seem to provide an adequate explanation for the difference in mortality, partly in view of the result that the non-registered group had no higher risk of CVD.
Subsequently, the follow-up of these patients should be assessed. A possible explanation for the difference in mortality between registered and non-registered groups could be that less attention was paid while prescribing and dispensing nephrotoxic (over-the-counter) medication by the GP and pharmacist, resulting in further deterioration of kidney function. There may have been less attention to the CVR factors associated with impaired renal function. In that case, we also would have expected an increase in CVD, unless, as previously described, it concerns a problem of global non-registration. On the other hand, the responsibility of the patient in the follow-up of the disease must be brought to attention. Possibly, the non-registered group contained a large proportion of patients who were not adherent to follow-up and therapy, as a result of which some did not or belatedly encountered problems. So, it is becoming increasingly important to examine these hypotheses and to involve the patient in his care and to find out what view he has in this regard.32 33 Moreover, it seems useful to investigate why the diagnosis was not registered in the EHR. Based on these results, the problem of non-registration could be addressed.
According to our research results, hypertension in CKD patients would be a risk factor in the development of CVD, although a protective factor in the development of all-cause mortality. Though, we know from previous research that hypertension is a risk factor for the development of CVD and premature death.34–36 The reasons for this difference are unclear. We need to consider the effect of antihypertensive medication on this outcome, since 48% of the patients took an ACE-I or an ARB.15 The beneficial effect of these drugs on cardiovascular events and all-cause mortality has been confirmed in the past.37 Ettehad et al described that in patients with CKD, smaller risk reductions in cardiovascular events were seen as a result of antihypertensive medication than in patients without CKD.38 However, we should also keep in mind that there may be non-registration of hypertension and SBP.
Additionally, it is surprising that hypercholesterolaemia and total cholesterol do not show a higher risk on CVD and mortality, since this is a proven risk factor for CVD.22 39 De Nicola et al showed that the CVR increases linearly with higher LDL in non-dialysis CKD patients.39 However, this result was not significant and may be explained by the use of lipid lowering medication, as 45% of patients were on this medication at baseline.15 After all, Fabbian et al determined that statins are an effective treatment in CKD patients, especially in the early stages of the disease.40 A history of CVD appears to be a protective factor in the development of new CVD, of which a properly adjusted therapy can be the reason (secondary prevention).40 41 In addition, we know that CKD is associated with adverse outcomes in those with existing CVD, which includes increased mortality after an acute coronary syndrome.42–44
In our previous work, we found that the majority of patients with renal insufficiency were in stage 3, with a higher proportion registered in stage 5 (75.7% registered) compared with stage 3A (22.9% registered).15 However, this study showed major differences in survival rates between registered and non-registered patients in both the earlier (3B) and further stages (4 and 5) of renal failure. The importance of early detection has been described many times in the past.17 45 This research must therefore be a plea for early detection of CKD and registration of the diagnostic code in the EHR. Good mutual communication between GP and nephrologist through referral letters and clear consultation reports can contribute to this. A solution to detect non-registered patients can be found in an Audit-& Feedback system, since this has proven to be effective and to have added value in primary care.46 47
Limitations
There were some limitations to note. First, we did not take the presence of proteinuria into account in our CKD population. Mainly due to the lack of data on proteinuria, which brings us straight to the problem of non-detection of proteinuria in the Flemish general practice.
Subsequently, the study used healthcare data which may under-represent the healthy and asymptomatic that do not seek healthcare. The data of care refusers were included in the research results.
Although the patient population is representative for the Flemish population, registering GPs are not representative for the GP population. It is a selected group of high-quality registering practitioners that use a specific EHR. This selection bias of GPs could eventually have an influence on some process parameters in the follow-up of patients.16 In addition, data collected in a real-world setting may lack information on specific covariates and laboratory investigations. Lab results from the hospital and specialists are automatically entered into the EHR, but their diagnoses are not. The large proportion of missingness is a limitation as well. We used multiple imputations to fill in this missingness (see the Method section).
Conclusion
An association between non-registration and all-cause mortality was identified, although no such association was apparent for CVD. Patients in stage 3B and 4 CKD with a registered diagnosis had much better survival rates compared with non-registered patients. It is unclear whether better registration will lead to a better outcome; the differences between these patient groups must be further mapped out.
Supplementary Material
Footnotes
Contributors: IVdW, PM, BV and GVP designed and conceptualised the study. EAA and PM performed the data analysis. IVDW drafted the manuscript. IVdW, EAA, PM, BV and GVP revised the manuscript. IVDW and GVP are the guarantors of this work. All authors read and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Data may be obtained from a third party and are not publicly available. All data relevant to the study are included in the article or uploaded as supplementary information. All relevant data are included in this published article (and its supplemental information files), except for the data underlying figures 1–4. As this data contains individual patient records, it can only be accessed inside a monitored analysis environment. Access to the data environment will be given to individual researchers on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
INTEGO procedures were approved by the ethical review board of the Medical School of KU Leuven (N° ML 1723) and by the Belgian Privacy Commission (no SCSZG/13/079).
References
- 1. Levin A, et al. Kidney disease: improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013;3:1–150. [Google Scholar]
- 2. Stevens PE, O’Donoghue DJ, de Lusignan S, et al. Chronic kidney disease management in the United kingdom: NEOERICA project results. Kidney Int 2007;72:92–9. 10.1038/sj.ki.5002273 [DOI] [PubMed] [Google Scholar]
- 3. Allen AS, Forman JP, Orav EJ, et al. Primary care management of chronic kidney disease. J Gen Intern Med 2011;26:386–92. 10.1007/s11606-010-1523-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 2013;382:339–52. 10.1016/S0140-6736(13)60595-4 [DOI] [PubMed] [Google Scholar]
- 5. Chronic Kidney Disease Prognosis Consortium, Matsushita K, van der Velde M, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010;375:2073–81. 10.1016/S0140-6736(10)60674-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mahmoodi BK, Matsushita K, Woodward M, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without hypertension: a meta-analysis. Lancet 2012;380:1649–61. 10.1016/S0140-6736(12)61272-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thompson S, James M, Wiebe N, et al. Cause of death in patients with reduced kidney function. J Am Soc Nephrol 2015;26:2504–11. 10.1681/ASN.2014070714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Muntner P, Coresh J, Klag MJ, et al. History of myocardial infarction and stroke among incident end-stage renal disease cases and population-based controls: an analysis of shared risk factors. Am J Kidney Dis 2002;40:323–30. 10.1053/ajkd.2002.34515 [DOI] [PubMed] [Google Scholar]
- 9. Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296–305. 10.1056/NEJMoa041031 [DOI] [PubMed] [Google Scholar]
- 10. De Bhailis ÁM, Kalra PA. Hypertension and the kidneys. Br J Hosp Med (Lond) 2022;83:1–11. 10.12968/hmed.2021.0440 [DOI] [PubMed] [Google Scholar]
- 11. Xie Y, Bowe B, Mokdad AH, et al. Analysis of the global burden of disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int 2018;94:567–81. 10.1016/j.kint.2018.04.011 [DOI] [PubMed] [Google Scholar]
- 12. Webster AC, Nagler EV, Morton RL, et al. Chronic kidney disease. Lancet 2017;389:1238–52. 10.1016/S0140-6736(16)32064-5 [DOI] [PubMed] [Google Scholar]
- 13. Fox CS, Matsushita K, Woodward M, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet 2012;380:1662–73. 10.1016/S0140-6736(12)61350-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ryan TP, Sloand JA, Winters PC, et al. Chronic kidney disease prevalence and rate of diagnosis. Am J Med 2007;120:981–6. 10.1016/j.amjmed.2007.05.012 [DOI] [PubMed] [Google Scholar]
- 15. Van den Wyngaert I, Mamouris P, Vaes B, et al. An exploration of under-registration of chronic kidney disease in Belgian general practices using logistic regression. PLoS One 2022;17:e0279291. 10.1371/journal.pone.0279291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Truyers C, Goderis G, Dewitte H, et al. The Intego database: background, methods and basic results of a Flemish general practice-based continuous morbidity registration project. BMC Med Inform Decis Mak 2014;14:48. 10.1186/1472-6947-14-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shlipak MG, Tummalapalli SL, Boulware LE, et al. The case for early identification and intervention of chronic kidney disease: conclusions from a kidney disease improving global outcomes (KDIGO) controversies conference. Kidney Int 2021;99:34–47. 10.1016/j.kint.2020.10.012 [DOI] [PubMed] [Google Scholar]
- 18. Van G, et al. Richtlijn Voor Goede Medische Praktijkvoering: Chronische Nierinsufficiëntie. Domus Medica 2012. [Google Scholar]
- 19. Team RC . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2021. [Google Scholar]
- 20. Scrucca L, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for Clinicians. Bone Marrow Transplant 2007;40:381–7. 10.1038/sj.bmt.1705727 [DOI] [PubMed] [Google Scholar]
- 21. Tsagris M, Pandis N. Multicollinearity. Am J Orthod Dentofacial Orthop 2021;159:695–6. 10.1016/j.ajodo.2021.02.005 [DOI] [PubMed] [Google Scholar]
- 22. D’Agostino RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham heart study. Circulation 2008;117:743–53. 10.1161/CIRCULATIONAHA.107.699579 [DOI] [PubMed] [Google Scholar]
- 23. Mamouris P, Nassiri V, Verbeke G, et al. A longitudinal transition imputation model for categorical data applied to a large Registry Dataset. Stat Med 2023;42:5405–18. 10.1002/sim.9919 [DOI] [PubMed] [Google Scholar]
- 24. Campion WM, Rubin DB. Multiple imputation for Nonresponse in surveys. J Market Res 1989;26:485. 10.2307/3172772 [DOI] [Google Scholar]
- 25. Sundström J, Bodegard J, Bollmann A, et al. Prevalence, outcomes, and cost of chronic kidney disease in a contemporary population of 2,4 million patients from 11 countries: the Careme CKD study. Lancet Reg Health Eur 2022;20:100438. 10.1016/j.lanepe.2022.100438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marks A, Macleod C, McAteer A, et al. Chronic kidney disease, a useful trigger for Proactive primary care? mortality results from a large UK cohort. Fam Pract 2013;30:282–9. 10.1093/fampra/cms079 [DOI] [PubMed] [Google Scholar]
- 27. James MT, Quan H, Tonelli M, et al. CKD and risk of hospitalization and death with pneumonia. Am J Kidney Dis 2009;54:24–32. 10.1053/j.ajkd.2009.04.005 [DOI] [PubMed] [Google Scholar]
- 28. Fried LF, Katz R, Sarnak MJ, et al. Kidney function as a Predictor of Noncardiovascular mortality. J Am Soc Nephrol 2005;16:3728–35. 10.1681/ASN.2005040384 [DOI] [PubMed] [Google Scholar]
- 29. Foley RN, Wang CC, Collins AJ. Cardiovascular risk factor profiles and kidney function stage in the US general population: the NHANES III study. Mayo Clin Proc 2005;80:1270–7. 10.4065/80.10.1270 [DOI] [PubMed] [Google Scholar]
- 30. Mata-Cases M, Mauricio D, Real J, et al. Is diabetes mellitus correctly registered and classified in primary care? A population-based study in Catalonia, Spain. Endocrinol Nutr 2016;63:440–8. 10.1016/j.endonu.2016.07.004 [DOI] [PubMed] [Google Scholar]
- 31. Smeets M, Vaes B, Aertgeerts B, et al. Impact of an extended audit on identifying heart failure patients in general practice: baseline results of the OSCAR-HF pilot study. ESC Heart Fail 2020;7:3950–61. 10.1002/ehf2.12990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Havas K, Douglas C, Bonner A. Meeting patients where they are: improving outcomes in early chronic kidney disease with tailored self-management support (the CKD-SMS study). BMC Nephrol 2018;19:279. 10.1186/s12882-018-1075-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen S-H, Tsai Y-F, Sun C-Y, et al. The impact of self-management support on the progression of chronic kidney disease-a prospective randomized controlled trial. Nephrol Dial Transplant 2011;26:3560–6. 10.1093/ndt/gfr047 [DOI] [PubMed] [Google Scholar]
- 34. Locatelli F, Pozzoni P, Tentori F, et al. Epidemiology of cardiovascular risk in patients with chronic kidney disease. Nephrol Dial Transplant 2003;18:vii2–9. 10.1093/ndt/gfg1072 [DOI] [PubMed] [Google Scholar]
- 35. Hamrahian SM, Falkner B. Hypertension in chronic kidney disease. Adv Exp Med Biol 2017;956:307–25. 10.1007/5584_2016_84 [DOI] [PubMed] [Google Scholar]
- 36. World Health Organization . A Global Brief on Hypertension: Silent Killer, Global Public Health Crisis: World Health Day 2013. Geneva, 2013:1–39. [Google Scholar]
- 37. Xie X, Liu Y, Perkovic V, et al. Renin-angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: A Bayesian network meta-analysis of randomized clinical trials. Am J Kidney Dis 2016;67:728–41. 10.1053/j.ajkd.2015.10.011 [DOI] [PubMed] [Google Scholar]
- 38. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016;387:957–67. 10.1016/S0140-6736(15)01225-8 [DOI] [PubMed] [Google Scholar]
- 39. De Nicola L, Provenzano M, Chiodini P, et al. Prognostic role of LDL cholesterol in non-dialysis chronic kidney disease: multicenter prospective study in Italy. Nutr Metab Cardiovasc Dis 2015;25:756–62. 10.1016/j.numecd.2015.04.001 [DOI] [PubMed] [Google Scholar]
- 40. Fabbian F, De Giorgi A, Pala M, et al. Evidence-based Statin prescription for cardiovascular protection in renal impairment. Clin Exp Nephrol 2011;15:456–63. 10.1007/s10157-011-0454-9 [DOI] [PubMed] [Google Scholar]
- 41. Grundy SM. Management of blood cholesterol. J Am Col Cardiol 2019;73:E285–U87. [DOI] [PubMed] [Google Scholar]
- 42. Wright RS, Reeder GS, Herzog CA, et al. Acute myocardial infarction and renal dysfunction: A high-risk combination. Ann Intern Med 2002;137:563. 10.7326/0003-4819-137-7-200210010-00007 [DOI] [PubMed] [Google Scholar]
- 43. Shlipak MG, Heidenreich PA, Noguchi H, et al. Association of renal insufficiency with treatment and outcomes after myocardial infarction in elderly patients. Ann Intern Med 2002;137:555–62. 10.7326/0003-4819-137-7-200210010-00006 [DOI] [PubMed] [Google Scholar]
- 44. Muntner P, He J, Hamm L, et al. Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J Am Soc Nephrol 2002;13:745–53. 10.1681/ASN.V133745 [DOI] [PubMed] [Google Scholar]
- 45. Sultan AA. Inside CKD: Modelling the economic burden of chronic kidney disease in Europe using patient-level Microsimulation. Nephrol Dial Transplant 2021;36:306. [Google Scholar]
- 46. Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and Healthcare outcomes. Cochrane Database Syst Rev 2012;2012:CD000259. 10.1002/14651858.CD000259.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Van Den Bulck S, Spitaels D, Vaes B, et al. The effect of electronic audits and feedback in primary care and factors that contribute to their effectiveness: a systematic review. Int J Qual Health Care 2020;32:708–20. 10.1093/intqhc/mzaa128 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-081115supp001.pdf (58.4KB, pdf)
bmjopen-2023-081115supp002.pdf (37.7KB, pdf)
bmjopen-2023-081115supp003.pdf (219.2KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Data may be obtained from a third party and are not publicly available. All data relevant to the study are included in the article or uploaded as supplementary information. All relevant data are included in this published article (and its supplemental information files), except for the data underlying figures 1–4. As this data contains individual patient records, it can only be accessed inside a monitored analysis environment. Access to the data environment will be given to individual researchers on reasonable request.