Abstract
Polyamines are required for optimal growth in most cells; however, polyamine accumulation leads to inhibition of cellular growth. To reduce intracellular polyamine levels, spermidine is monoacetylated in both prokaryotes and eukaryotes. In Escherichia coli, the speG gene encodes the spermidine acetyltransferase, which transfers the acetyl group to either the N-1 or N-8 position. In addition to polyamine accumulation, stress conditions, such as cold shock, cause an increase in the level of spermidine acetylation, suggesting an adaptive role for reduced polyamine levels under stressful growth conditions. The effect of spermidine accumulation on the growth of E. coli at low temperature was examined using a speG mutant. At 37°C, growth of the speG mutant was normal in the presence of 0.5 or 1 mM spermidine. However, following a shift to 7°C, the addition of 0.5 or 1 mM spermidine resulted in inhibition of cellular growth or cell lysis, respectively. Furthermore, at 7°C, spermidine accumulation resulted in a decrease in total protein synthesis accompanied by an increase in the synthesis of the major cold shock proteins CspA, CspB, and CspG. However, the addition of 50 mM Mg2+ restored growth and protein synthesis in the presence of 0.5 mM spermidine. The results indicate that the level of spermidine acetylation increases at low temperature to prevent spermidine toxicity. The data suggest that the excess spermidine replaces the ribosome-bound Mg2+, resulting in ribosome inactivation at low temperatures.
Polyamines, such as spermidine and putrescine, are present in virtually all cells (25, 33). These polycations have pleiotropic properties, which can influence several cellular processes. They can bind to nucleic acids, stabilize membranes, and stimulate the activity of several enzymes, such as RNA polymerase (1, 31, 34). Found in the ribosomal fraction, polyamines enhance the synthesis of several proteins and stimulate the in vivo assembly of the Escherichia coli 30S ribosomal subunit (7, 13, 21, 35).
Although intracellular polyamine is required for optimal growth, polyamine accumulation can lead to inhibition of cellular growth (11, 25, 26, 33). The addition of spermidine to cell cultures of mouse FM3A cells results in a decrease in cell growth accompanied by inhibition of protein synthesis (11). To prevent polyamine toxicity in eukaryotes, polyamine catabolism is initiated by the monoacetylation of spermidine and spermine catalyzed by spermidine/spermine N1-acetyltransferase (SSAT) (4). The acetylpolyamines are then either further oxidized by polyamine oxidase or excreted from the cell.
In E. coli, the speG gene encodes spermidine acetyltransferase (SAT), which transfers the acetyl group to either the N-1 or N-8 position of the polyamine (2, 8, 20). SAT is required to reduce the spermidine level, since the addition of exogenous spermidine to a speG mutant results in intracellular accumulation of spermidine (9). Furthermore, the excess spermidine causes decreased protein synthesis and cell viability during the stationary phase of growth (9). Acetylation serves to convert the polyamine to a physiologically inert form; acetylpolyamines cannot substitute for polyamines in RNA binding, in the enhancement of growth of an E. coli polyamine-deficient mutant, or in the stimulation of in vitro translation (18). Neither spermidine-deacetylating activity nor polyamine oxidase activity has been detected in E. coli, suggesting that the N-acetylspermidine is either excreted or kept in the inert acetylated form (14, 24). SAT is induced under nutrient-poor conditions, and its level is higher in cells growing in minimal medium than in those growing in rich medium (8). In addition to high polyamine levels, chemical and physical stresses result in an increased level of spermidine acetylation in E. coli and mammalian cells, suggesting a role for polyamine acetylation in adaptation to stress (2, 4, 10, 27, 32).
Shifting E. coli to low temperature results in inhibition of growth and protein synthesis accompanied by the onset of the cold shock response (16). The cold shock response consists of the induction of a set of proteins that has been proposed to aid in cellular adaptation to the low temperature. E. coli also responds to a downshift in temperature by increasing the level of spermidine acetylation (2, 32). However, the role of reduced spermidine levels in growth at low temperature is not known. In this study, we examined the effect of spermidine accumulation on adaptation to low temperatures. We found that the addition of spermidine to a speG mutant resulted in inhibition of growth and protein synthesis at low temperature. However, the inhibition of growth or protein synthesis caused by the addition of 0.5 mM spermidine could be suppressed by the presence of a speG+ multicopy plasmid or the inclusion of magnesium in the growth medium. The data indicate that spermidine acetylation at low temperatures occurs to reduce the toxic effect of spermidine accumulation.
MATERIALS AND METHODS
Bacterial strains and plasmids.
E. coli strains C600 (supE44 hsdR thi thr leu lacY1 tonA21) and CAG2242 (speG supE44 hsdR thi thr leu lacY1 tonA21) were obtained from E. W. Gerner (2). The speG+ low-copy-number plasmid, pMWSAT (9), and the vector pMW119 (37) were provided by K. Igarashi. Cultures were grown in Luria-Bertani (LB) medium or in M9 medium (19) supplemented with 19 amino acids (no methionine) and 4 bases. For experiments with plasmids, ampicillin (25 μg/ml) was added.
Two-dimensional electrophoresis of proteins.
Steady-state cultures of bacterial strains were grown at 37°C to an optical density at 420 nm of ca. 0.5. The cultures were shifted to 7°C, and 1 mM spermidine was added. At 68 h postshift, a 1-ml portion of the culture was labeled with [35S]methionine (1,175 Ci/mmol, 100 μCi/ml; ICN Pharmaceuticals) for 2 h. Equal portions of the extracts were processed by two-dimensional gel electrophoresis (23).
Preparation of the ribosome.
Steady-state cultures were grown at the indicated temperatures in LB medium. Ribosomal particles were isolated as described previously (6) from extracts prepared by the freeze-thaw method (29). The lysates corresponding to equal amounts of protein were layered on top of a 5 to 30% sucrose gradient in 10 mM Tris-HCl (pH 7.6)–10 mM MgCl2–60 mM NH4Cl–6 mM β-mercaptoethanol. The ribosomal particles were isolated by centrifugation at 151,000 × g.
Measurement of protein synthesis.
At various times, 0.5-ml samples were pulse-labeled with [35S]methionine (1,175 Ci/mmol, 150 μCi/ml). Following precipitation, trichloroacetic acid-insoluble radioactivity was counted using 0.1-ml aliquots.
RESULTS
Spermidine accumulation inhibits exponential growth at low temperatures.
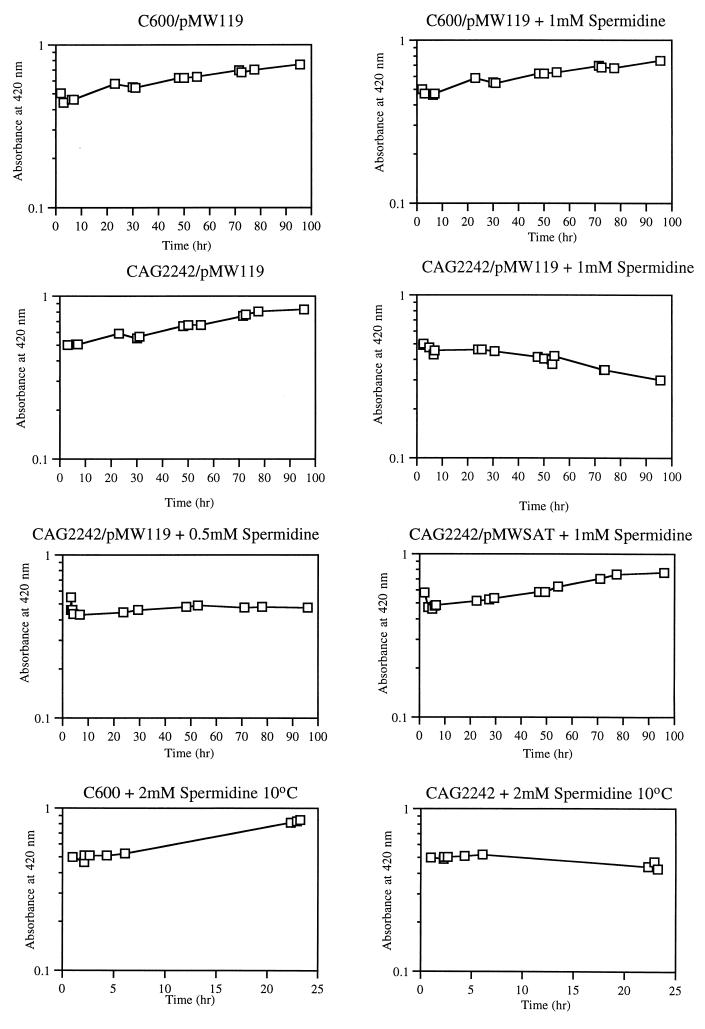
The speG gene encodes SAT, which is required to prevent the intracellular accumulation of spermidine (8, 9). The addition of 0.5 mM spermidine to the speG mutant results in a threefold-increased level of intracellular spermidine without having any appreciable affect on exponential growth at 37°C (9). Introduction of the speG+ plasmid, however, restores normal intracellular levels. Cold shock is a stress condition that increases the level of spermidine acetylation (2, 32). To determine the role of spermidine acetylation in adaptation of E. coli to low temperature, the effect of exogenous spermidine on the parental (C600) and speG mutant (CAG2242) strains was examined following a shift from 37 to 7°C. As shown in Fig. 1, the addition of 1 mM spermidine to the parental strain C600 did not have any noticeable effect on cellular growth at 7°C. In contrast, the addition of 1 mM spermidine to the speG mutant, strain CAG2242, resulted in inhibition of cellular growth accompanied by cell lysis. Furthermore, the addition of 0.5 mM spermidine to the speG mutant resulted in growth inhibition. However, as shown in Fig. 1, cellular growth in the presence of 1 mM spermidine was fully restored by the presence of the speG+ multicopy plasmid pMWSAT. We also found that the growth inhibition caused by spermidine accumulation at 7°C could be reversed by shifting the mutant back to 37°C (data not shown). This is consistent with the previous observation that exponential growth of the speG mutant is not inhibited by spermidine accumulation at 37°C (9). Therefore, the data indicate that spermidine accumulation specifically inhibits exponential growth upon a shift to low temperatures. Although the addition of 1 mM spermidine inhibited the growth of the speG mutant at 7°C, we found that the addition of 1 mM spermidine did not inhibit the growth of the mutant following the shift from 37 to 10°C (data not shown). However, the addition of 2 mM spermidine to the mutant at 10°C resulted in inhibition of growth, in contrast to the effect on the parent (Fig. 1). The addition of 2 mM spermidine did not inhibit the exponential growth of the parent and speG mutant at 37°C or of the mutant transformed with pMWSAT at 10°C (data not shown). Therefore, at the lower temperatures, the toxic effect of spermidine accumulation increases as the temperature decreases. The data further indicate that SAT is required to prevent spermidine toxicity at low temperatures.
FIG. 1.
Effect of spermidine on exponential growth following a shift from 37 to 7°C. The various cultures were grown in LB medium at 37°C to an optical density at 420 nm of ca. 0.5 followed by a shift to 7°C. Where indicated, various concentrations of spermidine were added at the time of the shift. Time zero represents the time of the shift to 7°C. Strains were transformed with pMWSAT (9), the speG+ multicopy plasmid, or the vector pMW119 (36).
Spermidine accumulation results in the preferential synthesis of cold shock proteins at low temperatures.
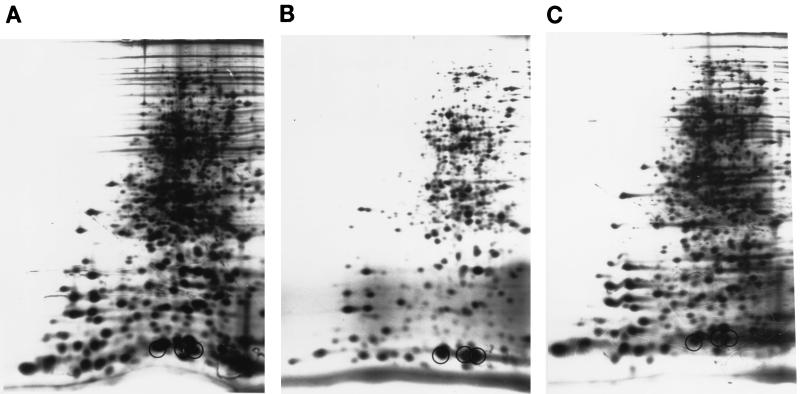
Spermidine accumulation in the speG mutant causes inhibition of protein synthesis at the stationary phase of growth (9). Furthermore, spermidine accumulation in mouse cells also results in a decrease in protein synthesis (11). Similarly, we found that the addition of 1 mM spermidine to the mutant at 7°C resulted in a dramatic decrease in the synthesis of several polypeptides, as shown in Fig. 2A and B. The presence of speG+ plasmid pMWSAT restored normal protein synthesis (Fig. 2C). However, we found that spermidine addition resulted in the preferential synthesis of certain proteins (Fig. 2, circles). These proteins are the major cold shock proteins CspA, CspB, and CspG (17, 38). The data indicate that spermidine accumulation in the speG mutant results in increased synthesis of the major cold shock proteins at low temperatures.
FIG. 2.
Effect of spermidine on the synthesis of proteins following a shift from 37 to 7°C. Strains CAG2242/pMW119 (A), CAG2242/pMW119 in the presence of 1 mM spermidine (B), and CAG2242/pMWSAT in the presence of 1 mM spermidine (C) were labeled with [35S]methionine at 68 h postshift, and extracts were processed by two-dimensional gel electrophoresis as described in Materials and Methods. Circles indicate cold shock proteins (from left to right) CspB, CspG, and CspA.
Spermidine accumulation inhibits protein synthesis at low temperatures.
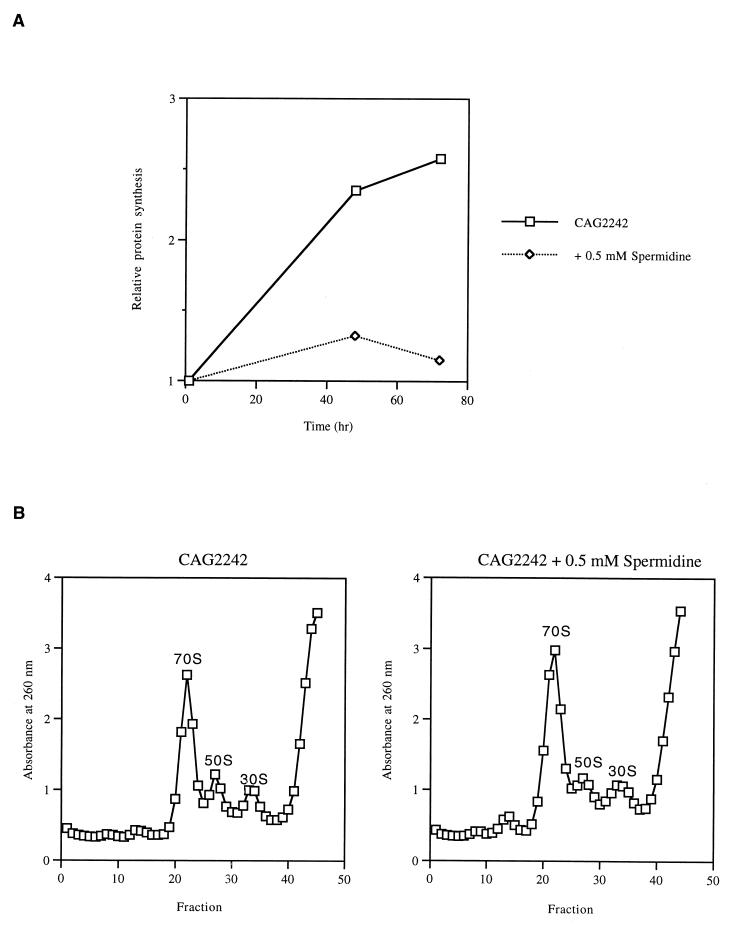
Our data suggest that spermidine accumulation inhibits protein synthesis at low temperatures. Following the shift to 7°C, total protein synthesis in the presence and absence of spermidine was monitored by the incorporation of [35S]methionine into trichloroacetic acid-insoluble material. As shown in Fig. 3A, spermidine accumulation resulted in inhibition of total protein synthesis at the low temperature.
FIG. 3.
Effect of 0.5 mM spermidine on protein synthesis (A) and on the levels of ribosomal particles (B) in the speG mutant. Strain CAG2242 was grown in LB medium to mid-log phase and shifted to 7°C. (A) Following the shift to 7°C, strain CAG2242 in the absence and presence of spermidine was pulse-labeled with [35S]methionine (1, 48, and 72 h postshift) and radioactivity in the trichloroacetic acid-insoluble material was counted as described in Materials and Methods. The counts were normalized to the amount of protein present. The values were normalized to the value obtained at 1 h. Time zero represents the time of the shift to 7°C and/or the addition of 0.5 mM spermidine. (B) Sedimentation profiles of extracts of strain CAG2242 in the absence and presence of 0.5 mM spermidine were prepared as described in Materials and Methods. Extracts were prepared from cells growing for 72 h at 7°C.
Spermidine has been demonstrated in vitro to shift the equilibrium towards the formation of the 70S ribosome (30). Ribosome profiles of the speG mutant were analyzed to determine if excess spermidine results in an increase in the 70S ribosome level in vivo. Because spermidine accumulation inhibits growth at low temperatures, lysates corresponding to equal amounts of protein were loaded on the 5 to 30% sucrose gradient to compare the level of the 70S ribosome in the presence and absence of spermidine at 72 h postshift. As shown in Fig. 3B, the addition of 0.5 mM spermidine resulted in a relatively higher 70S ribosome level, suggesting that excess spermidine stabilizes 70S ribosomes in vivo, as has been previously demonstrated in vitro (30).
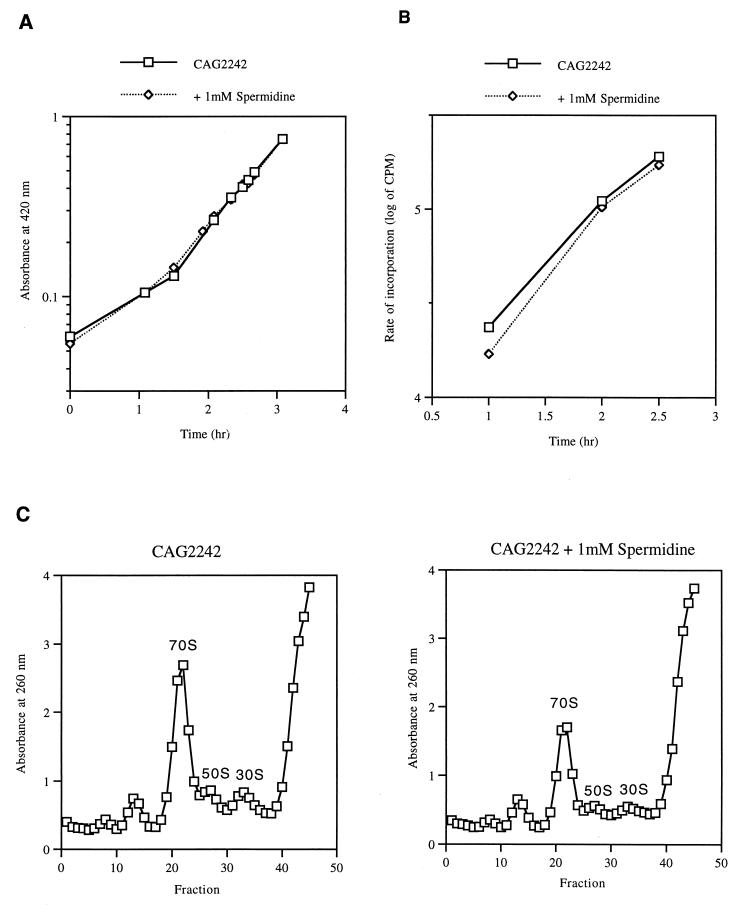
It has been previously shown that spermidine accumulation in the speG mutant does not inhibit exponential growth at 37°C (9). The effects of spermidine accumulation on protein synthesis and the 70S ribosome level were also analyzed at 37°C. As shown in Fig. 4A and B, the exponential growth and protein synthesis of the speG mutant were not negatively affected by the addition of 1 mM spermidine. The doubling time of the speG mutant, regardless of the presence of spermidine, was 45 min. The addition of 1 mM spermidine to the mutant at 37°C also resulted in a decrease in the 70S ribosome level (Fig. 4C). The data indicate that spermidine accumulation inhibits exponential growth and protein synthesis only at low temperatures.
FIG. 4.
Effect of spermidine on the growth (A), protein synthesis (B), and ribosomal levels (C) of strain CAG2242 at 37°C. (A) Strain CAG2242 was grown in LB medium in the absence and presence of 1 mM spermidine, which was added at time zero. (B) Samples were pulse-labeled to measure the rate of incorporation of [35S]methionine into protein as described in Materials and Methods. (C) Sedimentation profiles of extracts of strain CAG2242 in the absence and presence of spermidine were prepared as described in Materials and Methods.
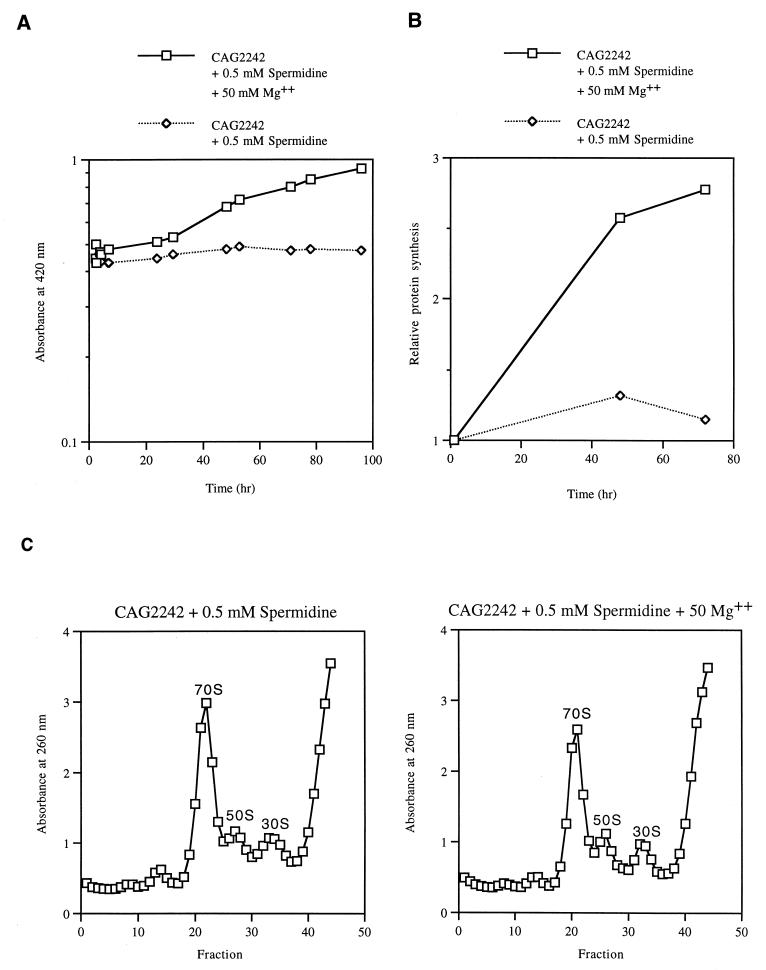
Magnesium restores cellular growth in the presence of 0.5 mM spermidine.
An effect of polyamines in in vitro translation is the lowering of the Mg2+ requirement (18). Since spermidine binds to sites on the ribosome similar to those bound by Mg2+, it can replace ribosome-bound Mg2+ (5, 28). However, the requirement for Mg2+ binding to maintain ribosome function and stability cannot be abolished by spermidine binding (12, 36). To further determine if spermidine toxicity at low temperatures could be due to the replacement of Mg2+ on the ribosome, experiments were done to ascertain if the inhibition of growth and protein synthesis could be suppressed by the inclusion of Mg2+ in the growth medium. As shown in Fig. 5A and B, the addition of 50 mM Mg2+ to the growth medium restored exponential growth and protein synthesis in the presence of 0.5 mM spermidine. As shown in Fig. 5C, the addition of 50 mM Mg2+ also resulted in a relative decrease in the 70S ribosome level in the presence of 0.5 mM spermidine. However, in the presence of 1 mM spermidine, the addition of 50 mM Mg2+ neither rescued the growth of the mutant nor restored protein synthesis (data not shown). The data suggest that spermidine accumulation at low temperatures replaces ribosome-bound Mg2+, resulting in inhibition of growth and protein synthesis.
FIG. 5.
Effect of Mg2+ on the growth (A), protein synthesis (B), and ribosomal levels (C) of strain CAG2242. (A) Strain CAG2242 was grown in LB medium in the presence or absence of 50 mM Mg2+ at 37°C to mid-log phase and then shifted to 7°C. Time zero represents the time of the shift to 7°C and the addition of 0.5 mM spermidine. (B) Following the shift to 7°C, strain CAG2242 was pulse-labeled with [35S]methionine (1, 48, and 72 h postshift) and radioactivity in trichloroacetic acid-insoluble material was counted as described in Materials and Methods. The counts were normalized to the amount of protein present. The values were normalized to the value obtained at 1 h. (C) Sedimentation profiles of extracts of strain CAG2242 were prepared as described in Materials and Methods. Extracts were prepared from cells growing for 72 h at 7°C.
DISCUSSION
Spermidine accumulation in the speG mutant does not inhibit exponential growth at 37°C (9). In this study, we have found that spermidine accumulation does inhibit exponential growth following a shift to a low temperature. However, growth can be restored by the presence of a plasmid carrying speG+. Spermidine acetylation increases under cold shock conditions (2, 32). Therefore, our data indicate that an increase in the level of spermidine acetylation occurs to prevent polyamine toxicity at low temperatures.
Accompanying the inhibition of growth, spermidine accumulation in the mutant resulted in inhibition of protein synthesis at low temperatures. Spermidine accumulation has previously been shown to inhibit protein synthesis in mouse cells and during the stationary phase in E. coli (9, 11). Spermidine has also been demonstrated to cause an increase in the 70S ribosome level in vitro (30). We have found that spermidine accumulation at low temperatures results in a relatively higher 70S ribosome level, suggesting that excess spermidine stabilizes 70S ribosomes in vivo. Spermidine accumulation also resulted in the preferential synthesis of the major cold shock proteins CspA, CspB, and CspG. In addition to shifts to low temperatures, the synthesis of these cold shock proteins increases in response to other conditions where protein synthesis is inhibited (17). CspA, CspB, and CspG belong to a family of homologous proteins, which also include members that are not cold shock inducible (38). It has been proposed that these proteins, which contain RNA binding domains, function as RNA chaperones to increase the translational efficiency of the mRNA (15, 38). The data suggest that the spermidine-induced synthesis of the major cold shock proteins occurs to increase the level of protein synthesis.
A critical level of ribosome-bound Mg2+ is required for ribosomal function and stability (12, 36). We found that the addition of Mg2+ to the growth medium suppressed growth inhibition and restored protein synthesis in the presence of 0.5 mM spermidine. The data suggest that spermidine accumulation decreased the ribosomal binding of Mg2+, resulting in a decrease in protein synthesis. This is consistent with previous reports indicating that one inhibitory effect of spermidine is the replacement of ribosome-bound Mg2+ (11, 12, 22, 36). The replacement of more than 40 and 70% of the bound Mg2+ by polyamines results in a decrease in protein-synthesizing activity in vitro using polysomes from rat liver and E. coli, respectively (12, 36). In addition, spermidine or spermine accumulation in the FM3A mouse cell line results in inhibition of growth and protein synthesis, which correlates with a reduction in the Mg2+ content (11). However, cellular growth recovers on addition of Mg2+ to the growth medium, suggesting that ribosome inactivation is due to the replacement of ribosome-bound Mg2+ by the polyamines (11).
Our evidence suggests that acetylation occurs at low temperature to prevent excess spermidine from inhibiting the binding of Mg2+ to the ribosome. Consistent with this is the finding that acetylpolyamines cannot substitute for polyamines in binding to RNA (18). Although the acetylpolyamine is still cationic, it has been suggested that the acetyl group may present a steric hindrance to the binding of nucleic acids. In E. coli, the level of spermidine acetylation increases in response to other stressful conditions, such as heat shock, high pH, and ethanol treatment (2). Furthermore, spermidine and spermine acetylation also increases in response to various chemical and physical stresses in mammalian cells (4, 10). However, the physiological role of polyamine acetylation in response to these stressful conditions has not yet been clearly defined. Because the induction of human SSAT by polyamine analogs is accompanied by growth inhibition and decreased cell viability of tumor cells, it has been suggested that stress-induced acetylation may occur to specifically inhibit cellular growth (3, 27). Furthermore, expression of human SSAT in E. coli results in the conversion of the spermidine to N1-acetylspermidine, accompanied by inhibition of exponential growth at 37°C (14, 24). However, we have found that in response to spermidine accumulation at low temperature, acetylation occurs to promote exponential growth. Therefore, polyamine acetylation may be playing a similar physiological role in response to other stressful conditions.
ACKNOWLEDGMENTS
We thank E. W. Gerner and K. Igarashi for the gifts of strains and plasmids. We thank Catherine Squires, Robert Maier, and Timothy Hoover for critically reading the manuscript.
REFERENCES
- 1.Abraham K A. Studies on DNA-dependent RNA polymerase from Escherichia coli. 1. The mechanism of polyamine induced stimulation of enzyme activity. Eur J Biochem. 1968;5:143–146. doi: 10.1111/j.1432-1033.1968.tb00348.x. [DOI] [PubMed] [Google Scholar]
- 2.Carper S W, Willis D G, Manning K A, Gerner E W. Spermidine acetylation in response to variety of stresses in Escherichia coli. J Biol Chem. 1991;266:12439–12441. [PubMed] [Google Scholar]
- 3.Casero R A, Mank A R, Xiao L, Smith J, Bergeron R J, Celano P. Steady-state messenger RNA and activity correlates with sensitivity to N1,N12-bis(ethyl)spermine in human cell lines representing the major forms of lung cancer. Cancer Res. 1992;52:5359–5363. [PubMed] [Google Scholar]
- 4.Casero R A, Pegg A E. Spermidine/spermine N1-acetyltransferase—the turning point in polyamine metabolism. FASEB J. 1993;7:653–661. [PubMed] [Google Scholar]
- 5.Cho Y S, Carr C W. Ion-binding studies of ribonucleic acid and Escherichia coli ribosomes. J Mol Biol. 1967;25:331–345. doi: 10.1016/0022-2836(67)90145-3. [DOI] [PubMed] [Google Scholar]
- 6.Dammel C S, Noller H F. Suppression of a cold-sensitive mutation in 16S rRNA by overexpression of a novel ribosome-binding factor, RbfA. Genes Dev. 1995;7:660–670. doi: 10.1101/gad.9.5.626. [DOI] [PubMed] [Google Scholar]
- 7.Echandi G, Algranati I D. Defective 30S ribosomal particles in a polyamine auxotroph of Escherichia coli. Biochem Biophys Res Commun. 1975;67:1185–1191. doi: 10.1016/0006-291x(75)90798-6. [DOI] [PubMed] [Google Scholar]
- 8.Fukuchi J, Kashiwagi K, Takio K, Igarashi K. Properties and structure of spermidine acetyltransferase in Escherichia coli. J Biol Chem. 1994;269:22581–22585. [PubMed] [Google Scholar]
- 9.Fukuchi J, Kashiwagi K, Yamagishi M, Ishihama A, Igarashi K. Decrease in cell viability due to the accumulation of spermidine in spermidine acetyltransferase-deficient mutant of Escherichia coli. J Biol Chem. 1995;270:18831–18835. doi: 10.1074/jbc.270.32.18831. [DOI] [PubMed] [Google Scholar]
- 10.Harari P M, Fuller D J, Gerner E W. Heat shock stimulates polyamine oxidation by two distinct mechanisms in mammalian cell cultures. Int J Radiat Oncol Biol Phys. 1989;16:451–457. doi: 10.1016/0360-3016(89)90341-6. [DOI] [PubMed] [Google Scholar]
- 11.He Y, Kashiwagi K, Fukuchi J, Terao K, Shirahata A, Igarashi K. Correlation between the inhibition of cell growth by accumulated polyamines and the decrease of magnesium and ATP. Eur J Biochem. 1993;217:89–96. doi: 10.1111/j.1432-1033.1993.tb18222.x. [DOI] [PubMed] [Google Scholar]
- 12.Igarashi K, Sugawara K, Hirose S. Effects of ribosomes on the substitution of spermidine or divalent cations for magnesium ions. J Biochem (Tokyo) 1975;77:753–759. doi: 10.1093/oxfordjournals.jbchem.a130779. [DOI] [PubMed] [Google Scholar]
- 13.Igarashi K, Kashiwagi K, Kishida K, Kakegawa T, Hirose S. Decrease in the S1 protein of 30-S ribosomal subunits in polyamine-requiring mutants of Escherichia coli grown in the absence of polyamines. Eur J Biochem. 1981;114:127–131. doi: 10.1111/j.1432-1033.1981.tb06182.x. [DOI] [PubMed] [Google Scholar]
- 14.Ignatenko N A, Fish J L, Shassetz L R, Woolridge D P, Gerner E W. Expression of the human spermidine/spermine N1-acetyltransferase in spermidine acetylation-deficient Escherichia coli. Biochem J. 1996;319:435–440. doi: 10.1042/bj3190435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang W, Hou Y, Inouye M. CspA, the major cold shock protein of Escherichia coli, is an RNA chaperone. J Biol Chem. 1997;272:196–202. doi: 10.1074/jbc.272.1.196. [DOI] [PubMed] [Google Scholar]
- 16.Jones P G, VanBogelen R A, Neidhardt F C. Induction of proteins in response to low temperature in Escherichia coli. J Bacteriol. 1987;169:2092–2095. doi: 10.1128/jb.169.5.2092-2095.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones P G, Inouye M. RbfA, a 30S ribosomal binding factor, is a cold shock protein whose absence triggers the cold shock response. Mol Microbiol. 1996;21:1207–1218. doi: 10.1111/j.1365-2958.1996.tb02582.x. [DOI] [PubMed] [Google Scholar]
- 18.Kakegawa T, Guo Y, Chiba Y, Miyazaki T, Nakamura M, Hirose S, Canellakis N, Igarashi K. Effect of acetylpolyamines on in vitro protein synthesis and on the growth of polyamine-requiring mutant of Escherichia coli. J Biochem. 1991;109:627–631. doi: 10.1093/oxfordjournals.jbchem.a123431. [DOI] [PubMed] [Google Scholar]
- 19.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1982. pp. 440–442. [Google Scholar]
- 20.Matsui I, Kamei M, Otani S, Morisawa S, Pegg A E. Occurrence and induction of spermidine-N1-acetyltransferase in Escherichia coli. Biochem Biophys Res Commun. 1982;106:1155–1160. doi: 10.1016/0006-291x(82)91233-5. [DOI] [PubMed] [Google Scholar]
- 21.Mitsui K, Ohnishi R, Hirose S, Igarashi K. Necessity of polyamines for maximum in vivo synthesis of ββ' subunits of RNA polymerase. Biochem Biophys Res Commun. 1984;123:528–534. doi: 10.1016/0006-291x(84)90261-4. [DOI] [PubMed] [Google Scholar]
- 22.Morris D R. A new perspective on ornithine decarboxylase regulation: prevention of polyamine toxicity is the overriding theme. J Cell Biochem. 1991;46:102–105. doi: 10.1002/jcb.240460203. [DOI] [PubMed] [Google Scholar]
- 23.O'Farrell P H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- 24.Parry L, Lopaz-Ballester J, Wiest L, Pegg A E. Effect of expression of human spermidine/spermine N1-acetyltransferase in Escherichia coli. Biochemistry. 1995;34:2701–2709. doi: 10.1021/bi00008a038. [DOI] [PubMed] [Google Scholar]
- 25.Pegg A E. Recent advances in the biochemistry of polyamines in eukaryotes. Biochem J. 1986;234:249–262. doi: 10.1042/bj2340249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pegg A E. Polyamine metabolism and its importance in neoplastic growth and a target for chemotherapy. Cancer Res. 1988;48:759–774. [PubMed] [Google Scholar]
- 27.Porter C W, Ganis B, Libby P R, Bergeron R J. Correlations between polyamine analogue-induced increases in spermidine/spermine N1-acetyltransferase activity, polyamine pool depletion, and growth inhibition in human melanoma cell lines. Cancer Res. 1991;51:3715–3720. [PubMed] [Google Scholar]
- 28.Rodgers A. The exchange properties of magnesium in Escherichia coli ribosomes. Biochem J. 1964;90:548–555. doi: 10.1042/bj0900548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ron E Z, Kohler R E, Davis B D. Polysomes extracted from Escherichia coli by freeze-thaw-lysozyme lysis. Science. 1966;153:1119–1120. doi: 10.1126/science.153.3740.1119. [DOI] [PubMed] [Google Scholar]
- 30.Rosano C L, Hurwitz C. Antagonistic action between spermidine and putrescine on association and dissociation of purified, run-off ribosomes from Escherichia coli. J Biol Chem. 1977;252:652–654. [PubMed] [Google Scholar]
- 31.Schuber F. Influence of polyamines on membrane functions. Biochem J. 1989;260:1–10. doi: 10.1042/bj2600001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tabor C W. The effect of temperature on the acetylation of spermidine. Biochem Biophys Res Commun. 1968;30:339–342. doi: 10.1016/0006-291x(68)90747-x. [DOI] [PubMed] [Google Scholar]
- 33.Tabor C W, Tabor H. Polyamines in microorganisms. Microbiol Rev. 1985;49:81–99. doi: 10.1128/mr.49.1.81-99.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tabor H, Tabor C W. Spermidine, spermine, and related amines. Pharmacol Rev. 1964;16:245–300. [PubMed] [Google Scholar]
- 35.Watanabe Y, Igarashi K, Hirose S. Differential stimulation by polyamines of phage RNA-directed synthesis of proteins. Biochim Biophys Acta. 1981;656:134–139. doi: 10.1016/0005-2787(81)90078-2. [DOI] [PubMed] [Google Scholar]
- 36.Weiss R L, Morris D R. The inability of polyamines to maintain ribosome structure and function. Biochim Biophys Acta. 1970;204:502–511. doi: 10.1016/0005-2787(70)90170-x. [DOI] [PubMed] [Google Scholar]
- 37.Yamaguchi K, Masamune Y. Autogenous regulation of synthesis of the replication protein in plasmid pSC101. Mol Gen Genet. 1985;200:362–367. doi: 10.1007/BF00425718. [DOI] [PubMed] [Google Scholar]
- 38.Yamanaka K, Fang L, Inouye M. The CspA family in Escherichia coli: multiplication of gene duplication for stress adaptation. Mol Microbiol. 1998;27:247–255. doi: 10.1046/j.1365-2958.1998.00683.x. [DOI] [PubMed] [Google Scholar]