Abstract
Background.
No human rabies post-exposure prophylaxis (PEP) failure has been documented in the United States using modern cell culture–based vaccines. In January 2021, an 84-year-old male died from rabies 6 months after being bitten by a rabid bat despite receiving timely rabies PEP. We investigated the cause of breakthrough infection.
Methods.
We reviewed medical records, laboratory results, and autopsy findings and performed whole-genome sequencing (WGS) to compare patient and bat virus sequences. Storage, administration, and integrity of PEP biologics administered to the patient were assessed; samples from leftover rabies immunoglobulin were evaluated for potency. We conducted risk assessments for persons potentially exposed to the bat and for close patient contacts.
Results.
Rabies virus antibodies present in serum and cerebrospinal fluid were nonneutralizing. Antemortem blood testing revealed that the patient had unrecognized monoclonal gammopathy of unknown significance. Autopsy findings showed rabies meningoencephalitis and metastatic prostatic adenocarcinoma. Rabies virus sequences from the patient and the offending bat were identical by WGS. No deviations were identified in potency, quality control, administration, or storage of administered PEP. Of 332 persons assessed for potential rabies exposure to the case patient, 3 (0.9%) warranted PEP.
Conclusions.
This is the first reported failure of rabies PEP in the Western Hemisphere using a cell culture–based vaccine. Host-mediated primary vaccine failure attributed to previously unrecognized impaired immunity is the most likely explanation for this breakthrough infection. Clinicians should consider measuring rabies neutralizing antibody titers after completion of PEP if there is any suspicion for immunocompromise.
Keywords: rabies, post-exposure prophylaxis, vaccine failure, whole-genome sequencing, bat
Rabies is a zoonotic, vaccine-preventable viral disease that affects mammals [1]. Rabies virus is typically transmitted via saliva from an infected mammal bite. With a fatality rate >99% upon symptom onset, rabies causes an estimated 59 000 deaths worldwide annually [2]. Rabies post-exposure prophylaxis (PEP) is highly effective at preventing disease if administered before symptom onset [3]. The US Advisory Committee on Immunization Practices (ACIP) recommends that PEP include immediate wound cleaning, infiltration of human rabies immunoglobulin (HRIG) within and around the wound (in unvaccinated persons), and intramuscular administration of modern cell culture–based rabies vaccines. The regimen depends on the immunity and vaccination history of the exposed person [4].
In the United States, approximately 60 000 people receive PEP annually following a confirmed or suspected rabies exposure. During 2000–2021, an average of 2.5 persons (median, 2; range, 0–8) died from rabies each year [5-7], none of whom received pre- or post-exposure prophylaxis before symptom onset. We describe the first reported failure of rabies PEP in the Western Hemisphere using modern cell culture–based vaccine in a patient who received PEP promptly after a confirmed exposure.
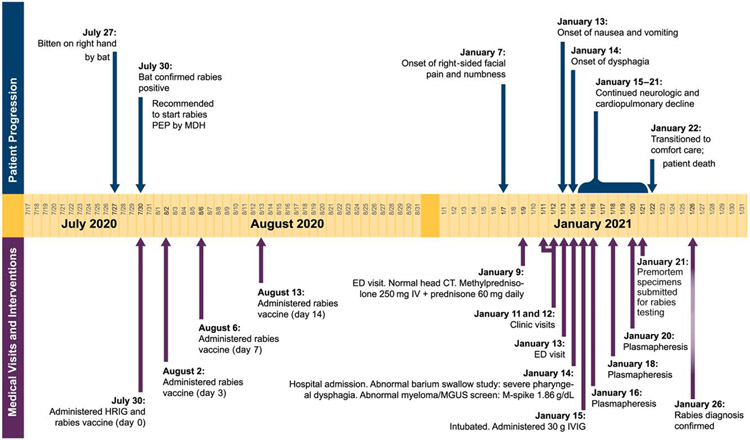
On 27 July 2020, an 84-year-old male in Minnesota was awoken by a bat biting his right hand. The bat tested positive for rabies on 30 July at the Minnesota Department of Health (MDH) (Figure 1) prompting initiation of PEP that day. The patient was previously unvaccinated against rabies. Though there was no visible wound, he washed his hands with soap and water after the exposure. The patient received HRIG (total dose of 20 IU/kg with as much as possible infiltrated at the bite site and the remaining administered into the right thigh) and rabies vaccine at an emergency department (ED). The patient’s medical history included coronary artery disease with prior coronary artery bypass and automatic defibrillator placement, controlled diabetes mellitus type II, hypertension, hyperlipidemia, chronic kidney disease (stage 2/5), and benign prostatic hyperplasia. He received 3 additional doses of rabies vaccine (days 3, 7, 14), as recommended for previously unvaccinated immunocompetent persons [4]. The patient’s wife received PEP for a possible unrecognized exposure during sleep. She received the same regimen, with the same dates and at the same healthcare facility as her husband, with both completing PEP on 13 August. The patient received 4 vaccines from 2 different lots. The patient’s wife received 4 vaccines from 4 different lots, including 2 lots in common with the patient (Supplementary Section 1). The bat was subsequently identified as a silver-haired bat (Lasionycteris noctivagans) by 12S rRNA gene sequencing (Supplementary Section 4).
Figure 1.
Timeline of bat exposure, rabies post-exposure prophylaxis, and clinical course of patient infected with rabies virus. Abbreviations: CT, computed tomography; ED, emergency department; HRIG, human rabies immunoglobulin; IV, intravenous; IVIG, intravenous immune globulin; MDH, Minnesota Department of Health; MGUS, monoclonal gammopathy of undetermined significance; PEP, post-exposure prophylaxis.
On 7 January 2021, approximately 5 months after exposure and PEP administration, the patient developed right-sided facial paroxysms of severe pain with excessive right eye lacrimation. He presented to an ED on 9 January and, with an elevated erythrocyte sedimentation rate (110 mm/h), was discharged with oxycodone, carbamazepine, and corticosteroids for suspected trigeminal neuralgia or temporal arteritis. The patient’s symptoms persisted; he was evaluated at the clinic on 11 January for surgical clearance and during a telehealth appointment on 12 January for preoperative evaluation for a temporal artery biopsy. He returned to the ED on 13 January with facial paresthesia, dysphagia, bilateral shoulder and arm myalgias, right arm paresthesia, nausea, and vomiting. He was discharged with ondansetron for nausea attributed to oxycodone. On 14 January, he returned to the ED and was hospitalized with worsening facial pain and paresthesia, generalized weakness, and decreased oral intake secondary to dysphagia. He had dysarthria, night sweats, right eye redness and discomfort, right-sided facial paralysis, and left ear pain. Computed tomography of the head was unremarkable. Temporal artery biopsy showed no arteritis. The clinical team considered rabies due to the clinical presentation and confirmed exposure. However, as is customary prior to pursuing rabies diagnostic testing and because the patient was given timely and appropriate PEP, other infectious, autoimmune, and paraneoplastic diagnoses were explored.
On 15 January, cerebrospinal fluid (CSF) analysis revealed 10 nucleated cells with lymphocytic predominance, consistent with viral encephalitis (Supplementary Section 2). The patient was intubated due to hypoxia and inability to protect his airway. On 16 January, he developed fever that continued until his death, with a maximum recorded temperature of 103.1°F (39.5°C). Signs of autonomic dysfunction included labile blood pressures that required norepinephrine. Serum protein electrophoresis revealed immunoglobulin (Ig) M monoclonal gammopathy of undetermined significance (MGUS) with an elevated gamma monoclonal protein and elevated IgM in the presence of reduced IgA and IgG. Testing for MYD88 L265P alteration, a mutation highly associated with IgM-producing lymphoplasmacytic lymphoma, and IgM MGUS was negative. Other diagnostic tests were noncontributory, and the patient did not improve with empiric treatment. Premortem specimens were submitted for rabies testing to the Centers for Disease Control and Prevention (CDC).
Supportive care was withdrawn, and the patient died 15 days after symptom onset on 22 January. The CDC confirmed rabies virus (RABV) infection [8] on 26 January (Table 1; Supplementary Section 5). Detection of viral RNA by real-time reverse-transcription polymerase chain reaction (RT-PCR) in saliva and detection of antirabies antibodies in CSF confirmed the laboratory criteria for rabies diagnosis. Although RABV IgG was detected in the CSF and serum by indirect fluorescence antibody test, no RABV neutralizing antibodies were detected in CSF or serum by rapid fluorescent focus inhibition test (RFFIT), indicating absence of an immune response to rabies vaccine administered during PEP and suggesting immunocompromise. No RABV RNA was detected in a nuchal skin biopsy by RT-PCR.
Table 1.
Test Results for Rabies Virus Diagnostics and Post-Exposure Prophylaxis Biologics
| Sample Type | Date Collected | Reverse-Transcriptase Polymerase Chain Reaction Rabies Virus RNA |
Direct Fluorescent Antibody Rabies Virus Antigen |
IFA RABV Specific IgM |
IFA RABV Specific IgG |
RFFIT RVNA/Titer (CVS.11a) |
RFFIT RVNA/Titer (Minnesota Patient, Silver-Haired Bat Variantb,c) |
|---|---|---|---|---|---|---|---|
| Serum | 15 Jan 2021 | Not detected | Detected | Not detected | Not detected | ||
| CSF | 15 Jan 2021 | Not detected | Detected | Not detected | |||
| CSF | 20 Jan 2021 | Not detected | Detected | Not detected | |||
| Saliva | 21 Jan 2021 | Detected | |||||
| Saliva | 21 Jan 2021 | Indeterminate | |||||
| Skin | 22 Jan 2021 | Not detected | Not detected | ||||
| Brain stem | 23 Jan 2021 | Detected | Detected | ||||
| Vermis | 23 Jan 2021 | Detected | Detected | ||||
| Right cerebellum | 23 Jan 21 | Detected | Detected | ||||
| Left cerebellum | 23 Jan 2021 | Detected | Detected | ||||
| HRIG (hospital) lot no. R2MBD00113 | Detected 1:53 887 (399 IU/mL) | Detected 1:31 950 | |||||
| HRIG (Grifols) lot no. R2MBD00113 | Detected 1:45 687 (366 IU/mL) | Detected 1:33 489 | |||||
| HRIG (Grifols) lot no. R2MFD00163 | Detected 1:67 491 (482 IU/mL) | Detected 1:40 269 | |||||
| Serum (wife) | 2 Feb 2021 | Detected 1:45 (0.33 IU/mL) |
Abbreviations: CSF, cerebrospinal fluid; HRIG, human rabies immunoglobulin; IFA, indirect fluorescent antibody; Ig, immunoglobulin; RABV, rabies virus; RFFIT, rapid fluorescent foci inhibition test; RVNA, rabies virus neutralizing antibodies.
Virus dose FFD40 52.
Virus dose FFD40 42.
Silver-haired bat virus MN Hu A21-0686 P3d4 Snate 2-13-21.
A public health investigation was initiated to understand the reason for rabies breakthrough infection and identify family members and healthcare personnel (HCP) potentially exposed to RABV from the patient.
METHODS
Patient Investigation
The patient’s medical record was reviewed, and an autopsy was performed to identify underlying conditions and obtain postmortem CNS specimens. Whole-genome sequencing (WGS) was performed on RABV isolated from the offending bat and the patient to confirm the bat was the source of infection (Supplementary Section 6). Serum obtained from the patient during hospitalization was assessed for antibodies to influenza, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and seasonal human coronavirus (HCoV) as a proxy measure to evaluate the patient’s immune response to recent influenza vaccination and for potential prior infections with influenza, SARS-CoV-2, and HCoV (Supplementary Section 7).
Evaluation of Rabies Biologics
All relevant lot numbers were shared with the manufacturers of the vaccine (Bavarian Nordic, Morrisville, NC) and HRIG (Grifols, Los Angeles, CA). Records were reviewed to evaluate for manufacturing deviations, adverse events, and product failure. MDH reviewed clinic records to ensure that PEP storage and administration practices were compliant with manufacturer and ACIP guidelines.
Available HRIG vials from the implicated lots were obtained from the hospital (R2MBD00113) and the manufacturer (R2MBD00113 and R2MFD00163) for potency testing. HRIG potency was determined by the CDC using RFFIT against the CVS-11 RABV variant and the RABV variant isolated from the patient, with slight modification as previously described [9] (Supplementary Section 8). Serum obtained from the patient’s wife was tested using RFFIT to confirm adequate antibody response to RABV because some of the lots administered to her were also given to the patient and both received PEP products that were stored in and administered at the same healthcare locations.
Epidemiologic Investigation and Exposure Assessments
MDH interviewed the patient’s family to investigate for additional animal exposures. MDH administered exposure risk assessments to family members and HCP with known contact with the patient during his infectious period, that is, 14 days before symptom onset to cremation. An online assessment algorithm facilitated rapid HCP assessments to determine who should receive rabies PEP (Supplementary Section 9). HCP whose answers indicated no risk for rabies exposures received immediate notification of no further required action, while those with possible rabies exposures received an in-person assessment.
RESULTS
Patient Investigation
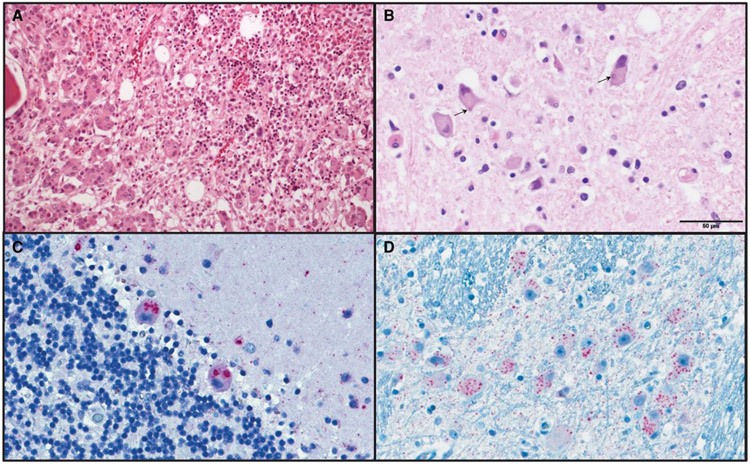
The patient’s records did not identify any immunocompromising conditions associated with lack of seroconversion after rabies vaccination. Postmortem rabies testing of brain stem and cerebellum specimens by direct fluorescent antibody and RT-PCR further established the diagnosis. At autopsy, the prostate was enlarged, and histopathology revealed a previously undiagnosed prostatic adenocarcinoma (Gleason pattern, 5 + 5 = 10; grade group 5) metastatic to bone marrow. No morphologic or immunophenotypic evidence of lymphoma or plasma cell neoplasm was visible in lymph nodes, spleen, or bone marrow. Brain tissue histopathology revealed meningoencephalitis, and immunohistochemistry for RABV showed extensive viral antigen labeling (Figure 2; Supplementary Section 5.2).
Figure 2.
Metastatic prostatic adenocarcinoma and rabies viral encephalitis. A, Hematoxylin and eosin (H&E) staining of vertebral bone marrow revealed prostatic adenocarcinoma with no evidence of a hematologic malignancy. B, H&E stained medulla showed widespread neuronal viral cytopathic effect and eosinophilic cytoplasmic exclusion bodies (Negri bodies, indicated by arrows). Cerebellum (C) and brain stem (D) showed extensive labeling of rabies viral antigen by immunohistochemistry.
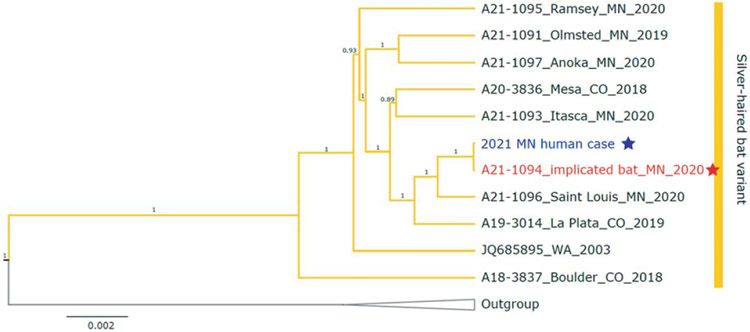
WGS of RABV obtained from the patient and the bat were identical (Figure 3; Supplementary Section 6). The patient received high-dose quadrivalent influenza vaccine on 25 August 2020. Serum collected on 15 January 2021 (143 days post-vaccination) was seropositive (≥40) to 3 of the 4 vaccine antigens by hemagglutination inhibition assay (Supplementary Section 7). There were elevated levels of Pan Ig and total IgG to multiple influenza antigens (including vaccine-like antigens) and human coronavirus OC-43 spike protein but negative to spike (s) and nucleoproteins (N) of SARS-CoV-2 viruses. The patient had no known SARS-CoV-2 infection and had not received the SARS-CoV-2 vaccine. The serum contained moderate levels of IgM to several influenza antigens but was negative for IgA to all antigens tested.
Figure 3.
Phylogenetic tree generated with rabies virus (RABV) whole-genome sequences from the patient isolate and selected bats infected with silver-haired bat (Lasionycteris noctivagans) RABV variant. Isolate from the human patient (blue star) and offending bat (red star) confirming sequences were 100% identical. Silver-haired bat variant is shown by yellow branches.
Evaluation of Rabies Biologics
No concerns were noted in PEP storage procedures and administration practices. Neither manufacturer reported deviations in product manufacturing, packaging, or distribution of implicated lots. No serious adverse events or product complaints were reported to the manufacturers or the Vaccine Adverse Event Reporting Systems as of 28 January 2022. All rabies vaccines underwent National Institute of Health (NIH) potency testing prior to release [10]. HRIG lots underwent routine RFFIT potency testing before release; implicated lots contained rabies Ig above the minimum stated concentration (>300 IU/mL) (Supplementary Section 3).
The patient received HRIG from 2 vials (Grifols lots R2MBD00113 and R2MFD00163). Samples from lot R2MBD00113 were obtained from the ED, and retention samples of both lots were obtained from the manufacturer and sent to the CDC. RFFIT using both standard challenge RABV (CVS-11) and silver-haired bat RABV isolated from the patient demonstrated that all samples contained adequate RABV neutralizing antibody (RVNA) titers, above the stated minimal concentration (Table 1).
The wife’s serum was collected on 2 February 2021, and RFFIT showed complete neutralization at 1:45 serum dilution (0.33 IU/mL).
Epidemiologic Investigation and Exposure Assessments
The patient and his wife lived in a log home. The wife reported occasional intrusions from bats and flying squirrels but did not recall if her husband had any additional exposures to wild animals.
Eight family members were assessed for rabies exposure during the patient’s infectious period, and only the patient’s wife was exposed. A total of 324 HCP had contact with the patient during his infectious period. Of these, 174 (54%) completed the online risk assessment within 72 hours of it being operational, 312 (96%) within 7 days, and all within 14 days. Two (0.6%) HCP received PEP due to lack of eye protection during aerosol-generating procedures with the patient.
DISCUSSION
Host-mediated primary vaccine failure (ie, inability of a host to mount a protective antibody response after PEP) that results from an undiagnosed immunosuppressing comorbidity is the most parsimonious explanation for the patient’s fatal outcome. Generally, immune response in vaccinated individuals is determined by detection of neutralizing antibodies in serum, which was absent in this patient. In addition, MGUS has been associated with increased infection risk and mortality and decreased titer levels after vaccination [11-13]. In this patient, MGUS in the absence of plasma cell dyscrasias might have occurred secondary to prostate adenocarcinoma [14, 15]. The absence of lymphoma or plasma cell neoplasm at autopsy could be due to recent administration of corticosteroids (Figure 1). The patient’s advanced age and comorbidities could have affected seroconversion, although neither immunosenescence nor his comorbidities are known to be immunosuppressive with regard to rabies vaccines, which are highly immunogenic [16-18].
Other potential causes of PEP failures were ruled out. First, hyperimmune globulins such as HRIG are manufactured to slightly exceed the minimum specified potency. Potency excess beyond the minimum standard ensures that expected, relatively minor levels of IgG degradation caused by prolonged storage do not impact effectiveness (US Food and Drug Administration, written communication 29 January 2022). HRIG obtained from the same lots as administered to the patient surpassed the minimum potency standard in both manufacturer reports and independent testing at the CDC, indicating they were properly produced and potent. The patient received a dose of 30.9 IU/kg (based on RFFIT testing), yet doses up to 40 IU/kg have shown no clinically relevant impact on the immune response to rabies vaccination [19-22]. Conversely, HRIG administration without vaccination is not expected to provide complete protection against RABV infection. In one study, only 25% of unvaccinated animals survived infection when challenged with RABV after treatment with HRIG only [23]. In addition, HRIG potency testing showed complete neutralization against the silver-haired bat virus isolated from the patient, excluding the hypothesis that this specific virus would escape neutralization by HRIG [24-26]. Second, the positive RVNA titer from the patient’s wife confirmed that at least 1 of the vaccines administered was immunogenic. At the time of this investigation, the World Health Organization (WHO) and ACIP used different criteria for the minimum acceptable rabies antibody level at which serum collected 1–2 weeks after pre- or post-exposure prophylaxis completion is expected to completely neutralize challenge virus: 1:5 serum dilution per ACIP [4] and ≥ 0.5 IU/mL per WHO [27]. Although titers from the patient’s wife were lower than the WHO standard, her serum showed complete neutralization at dilution levels above the ACIP standard. Given that her serum was collected almost 6 months after PEP completion, these results suggest adequate vaccine response, as declines in antibody titers can be expected within 2 to 6 months of PEP completion [16-18]. Third, no deviations were noted in PEP manufacturing, storage, or administration. Repeat NIH potency testing and antigenic testing of vaccine product from implicated lots were not conducted because standard NIH potency testing and manufacturing records did not reveal any abnormalities. Finally, the possibility that the patient had a second cryptic exposure [25] after completing PEP could be excluded based on the indistinguishable sequences from the patient and bat isolates.
More than 29 million people worldwide and 60 000 people in the United States receive PEP each year [1, 7], yet infection with RABV after timely and appropriate administration of PEP is exceedingly rare. A systematic literature review of PEP failures worldwide identified 124 cases during 1980–2022, none of which were caused by a bat RABV variant [28]. Of these, 54 had no known deviations in PEP core practices; RIG potency tests were only conducted in 3 cases and vaccine potency tests in 2, all of which excluded a failure of PEP biologics [29-31]. The remaining 70 cases had known deviations in PEP core practices. Although reports were insufficiently detailed to conclude if potency testing was conducted, they include at most 3 RIG potency tests and 5 vaccine potency tests, none of which were found to be at fault [32-34]. Immunocompromising conditions have rarely been identified as a reason for PEP failures, possibly because this information is not routinely collected. Of the 54 PEP failures with no known deviation in core practices, 3 persons were reported to have chronic comorbidities and none were immuno-suppressed. When the definition is broadened to include 70 PEP failures with known deviations in core practices, only 2 persons were diagnosed with immunosuppression [28, 35, 36].
This investigation is notable for the few people who required PEP. The proportion of exposed persons recommended to receive PEP (0.9%) was substantially lower despite a higher number of HCP who were close contacts compared with previous US investigations (Supplementary Section 10). This could be attributed to the SARS-CoV-2 pandemic, as personal protective equipment (PPE) recommendations for SARS-CoV-2 exceeded the standard precautions recommended for treating patients with rabies [37, 38]. Considering that PEP and PEP-related fees range between $3764 and $21 754 per person [7, 39], increased use of PPE [37] likely led to substantial savings in PEP-related health expenditures.
Our investigation is subject to several limitations. First, we did not assess serum titers from individuals who received rabies vaccines from all the implicated lots. Second, no archived serum samples from the patient were available to ascertain the MGUS timeline. Further, although elevated antibodies to recent influenza vaccines were detected in the single serum sample collected post-vaccination, the absence of a paired serum pre-vaccination prevented differentiation of recent influenza vaccination from the cross-reactive responses of past influenza virus exposures. Despite these limitations, our investigation is, to our knowledge, the only one to investigate PEP biologics, potential for cryptic exposures, and underlying comorbidities.
This report highlights several considerations for the interpretation of rabies ACIP guidelines, especially in immunocompromised persons [4]. These guidelines acknowledge that rabies vaccines, like other vaccines, can be less effective in immunocompromised persons [40-42]. Since 2008, ACIP has recommended that immunocompromised individuals receive an additional vaccine dose, with additional doses if rabies serology demonstrates inadequate titers [4]. In this case, the challenge was that the patient was not diagnosed with nor showed overt clinical signs of an immunocompromising condition; therefore, additional doses and titer were not considered. Clinicians may consider performing a review of systems for patients where rabies PEP is administered. If an immunocompromising condition is suspected, err on the side of caution by obtaining an antibody titer. In immunocompromised patients, efforts to optimize the patient’s immune system may be needed and serial HRIG or vaccine doses may need to be administered. Patients with who are severely immunocompromised and need rabies PEP have been successfully managed through consultation with health departments and the CDC. Further research is needed to determine if additional HRIG should be administered to persons with inadequate response to PEP and if high-risk rabies exposures should systematically prompt immediate initiation of PEP prior to test results being available for the offending animal. While earlier vaccine administration would not have changed the patient’s immune dysfunction, it remains unknown if quicker administration of HRIG might have changed the outcome.
Ensuring adequate immune response to rabies vaccines is increasingly important given the rising prevalence of immunocompromised adults in the United States [43]. In this investigation, host-mediated primary vaccine failure due to immune dysfunction is the most likely explanation for the fatal outcome for this patient, and our findings do not challenge the high efficacy or safety profile of rabies PEP biologics.
Supplementary Material
Acknowledgments.
The authors thank the staff at Bavarian Nordic (Morrisville, North Carolina) and Grifols (Los Angeles, California) for contributing to the product manufacturing investigation; John R. Su, MD, PhD, MPH, Elaine R. Miller, BSN, MPH (Centers for Disease Control and Prevention [CDC], Atlanta, Georgia), and Ana E. Dayton, MD, MSHS (General Dynamics Information Technology, Falls Church, Virginia) for reviewing Vaccine Adverse Event Reporting System records; Zhunan Li, MMed, and Stacie N. Jefferson, MS (Influenza Division, CDC, Atlanta, Georgia) for assistance with the high-throughput multiplex influenza/severe acute respiratory syndrome coronavirus 2/seasonal human coronavirus antibody detection and hemagglutination inhibition assays; Ravi Goud, MD, MPH, and Laura Montague, BS (US Food and Drug Administration [FDA], Silver Spring, Maryland) for assistance with product investigation; Karen G. Esse, MD (Allina Health, Minneapolis, Minnesota) for contributing to patient evaluation and diagnostics; Lacey Towe (MidWest Medical Examiner’s Office, Ramsey, Minnesota) for assistance with pathology specimen collection and Brooke Leitgeb, MPH, Brigid Bollweg, MPM, and Pamela Fair, BS (CDC, Atlanta, Georgia) for assistance with diagnostic evaluation; and Leslie Kollmann, CVT, Anna Strain, PhD, and Amanda Beaudoin, DVM, PhD (Minnesota Department of Health, Saint Paul, Minnesota) for assistance with the public health investigation, sample collection, and diagnostic evaluation.
Financial support.
This investigation was supported in part by a cooperative agreement between the CDC and the Minnesota Department of Health as part of the Epidemiology and Laboratory Capacity for Infectious Diseases Program (U50/CDK000371).
Footnotes
Potential conflicts of interest. R. M. W. reports a role as a board member for the International Rabies Taskforce. R. L. reports roles on the ID Week Program Committee, the Council of State and Territorial Epidemiologists Executive Board, and the National Foundation for Infectious Diseases Executive Board and serving as an associate editor for AAP Red Book (Report of the Committee on Infectious Diseases) and declares support for attending meetings and/or travel from each; payment or honoraria received for their associate editor role was donated to the Minnesota Department of Health. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Availability of data. Laboratory results, biologics evaluation, and exposure interviews can be shared for analysis and review after deidentification. Requests should be directed to the corresponding author (S. M. H.).
Disclaimer. The findings and conclusions presented here are those of the authors and do not necessarily represent the official position of the CDC. FDA author comments and contributions are an informal communication and represent their own best judgment. These comments do not bind or obligate FDA. None of the individuals listed in the Acknowledgments section received compensation other than their salaries. This work was written by US government employee(s) and is in the public domain in the United States.
References
- 1.World Health Organization. Rabies. Available at: https://www.who.int/news-room/fact-sheets/detail/rabies. Accessed 29 June 2022.
- 2.Wallace RM, Blanton J. Chapter 4, Epidemiology. In: Fooks AR, Jackson AC, eds. Rabies, 4th ed. Boston, MA: Academic Press, 2020:103–42. [Google Scholar]
- 3.Quiambao BP, Dimaano EM, Ambas C, Davis R, Banzhoff A, Malerczyk C. Reducing the cost of post-exposure rabies prophylaxis: efficacy of 0.1 ml PCEC rabies vaccine administered intradermally using the Thai Red Cross post-exposure regimen in patients severely exposed to laboratory-confirmed rabid animals. Vaccine 2005; 23:1709–14. [DOI] [PubMed] [Google Scholar]
- 4.Rupprecht CE, Briggs D, Brown CM, et al. Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2010; 59:1–9. [PubMed] [Google Scholar]
- 5.Kunkel A, Minhaj FS, Whitehill F, et al. Notes from the field: three human rabies deaths attributed to bat exposures—United States, August 2021. MMWR Morb Mortal Wkly Rep 2022; 71:31–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma X, Monroe BP, Wallace RM, et al. Rabies surveillance in the United States during 2019. J Am Vet Med Assoc 2021; 258:1205–20. [DOI] [PubMed] [Google Scholar]
- 7.Pieracci EG, Pearson CM, Wallace RM, et al. Vital signs: trends in human rabies deaths and exposures—United States, 1938–2018. MMWR Morb Mortal Wkly Rep 2019; 68:524–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rabies, Human 2011 Case Definition. Available at: https://ndc.services.cdc.gov/case-definitions/rabies-human-2011/. Accessed 28 June 2022.
- 9.Burgado J, Greenberg L, Niezgoda M, et al. A high throughput neutralization test based on GFP expression by recombinant rabies virus. PLoS Negl Trop Dis 2018; 12:e0007011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Recommendations for inactivated rabies vaccine for human use produced in cell substrates and embryonated eggs; 2007. Available at: https://www.who.int/publications/m/item/inactivated-rabies-vaccine-for-human-use-annex-2-trs-no-941. Accessed 28 June 2022.
- 11.Karlsson J, Andréasson B, Kondori N, et al. Comparative study of immune status to infectious agents in elderly patients with multiple myeloma, Waldenstrom’s macroglobulinemia, and monoclonal gammopathy of undetermined significance. Clin Vaccine Immunol 2011; 18:969–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kristinsson SY, Tang M, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance and risk of infections: a population-based study. Haematologica 2012; 97:854–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tete SM, Bijl M, Sahota SS, Bos NA. Immune defects in the risk of infection and response to vaccination in monoclonal gammopathy of undetermined significance and multiple myeloma. Front Immunol 2014; 5:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jehangir W, Tulpule S, Sanabria F, et al. Prostate cancer leading to monoclonal gammopathy of undetermined significance: a case report. Mol Clin Oncol 2018; 9:339–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pramanik S, Gazi MJ, Das AK, Debnath NB, Pal SK. Monoclonal gammopathy in prostate carcinoma: a case report and review of literature. J Med Case Rep 2018; 12:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goronzy JJ, Weyand CM. Understanding immunosenescence to improve responses to vaccines. Nat Immunol 2013; 14:428–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu C, Lau CL, Clark J, et al. Immunogenicity after pre- and post-exposure rabies vaccination: a systematic review and dose-response meta-analysis. Vaccine 2021; 39:1044–50. [DOI] [PubMed] [Google Scholar]
- 18.Furuya-Kanamori L, Ramsey L, Manson M, Gilbert B, Lau CL. Intradermal rabies pre-exposure vaccination schedules in older travellers: comparison of immunogenicity post-primary course and post-booster. J Travel Med 2020; 27:taaa006. [DOI] [PubMed] [Google Scholar]
- 19.Hafkin B, Alls ME, Baer GM. Human rabies globulin and human diploid vaccine dose determinations. Dev Biol Stand 1978; 40:121–7. [PubMed] [Google Scholar]
- 20.Haviv J, Rishpon S, Gdalevich M, Mimouni D, Gross E, Shpilberg O. Successful post-exposure rabies prophylaxis after erroneous starting treatment. Prev Med 1999; 29:28–31. [DOI] [PubMed] [Google Scholar]
- 21.Helmick CG, Johnstone C, Sumner J, Winkler WG, Fager S. A clinical study of Merieux human rabies immune globulin. J Biol Stand 1982; 10:357–67. [DOI] [PubMed] [Google Scholar]
- 22.Mertz GJ, Nelson KE, Vithayasai V, et al. Antibody responses to human diploid cell vaccine for rabies with and without human rabies immune globulin. J Infect Dis 1982; 145:720–7. [DOI] [PubMed] [Google Scholar]
- 23.Hanlon CA, DeMattos CA, DeMattos CC, et al. Experimental utility of rabies virus-neutralizing human monoclonal antibodies in postexposure prophylaxis. Vaccine 2001; 19:3834–42. [DOI] [PubMed] [Google Scholar]
- 24.Dietzschold B, Morimoto K, Hooper DC, Smith JS, Rupprecht CE, Koprowski H. Genotypic and phenotypic diversity of rabies virus variants involved in human rabies: implications for postexposure prophylaxis. J Hum Virol 2000; 3:50–7. [PubMed] [Google Scholar]
- 25.Messenger SL, Smith JS, Rupprecht CE. Emerging epidemiology of bat-associated cryptic cases of rabies in humans in the United States. Clin Infect Dis 2002; 35:738–47. [DOI] [PubMed] [Google Scholar]
- 26.Morimoto K, Patel M, Corisdeo S, et al. Characterization of a unique variant of bat rabies virus responsible for newly emerging human cases in North America. Proc Natl Acad Sci U S A 1996; 93:5653–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO Expert Committee on Rabies, Eighth Report. Geneva, Switzerland: World Health Organization, 1992. [Google Scholar]
- 28.Whitehouse ER, Mandra A, Bonwitt J, Beasley E, Taliano J, Rao AK. Human rabies despite post-exposure prophylaxis: a systematic review (1980–2022). Lancet Infect Dis 2022:S1473–3099(22)00641-7. 10.1016/S1473-3099(22)00641-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shantavasinkul P, Tantawichien T, Wacharapluesadee S, et al. Failure of rabies postexposure prophylaxis in patients presenting with unusual manifestations. Clin Infect Dis 2010; 50:77–9. [DOI] [PubMed] [Google Scholar]
- 30.Wilde H, Glueck R, Khawplod P, et al. Efficacy study of a new albumin-free human diploid cell rabies vaccine (Lyssavac-HDC, Berna) in 100 severely rabies-exposed Thai patients. Vaccine 1995; 13:593–6. [DOI] [PubMed] [Google Scholar]
- 31.Wilde H. Failures of post-exposure rabies prophylaxis. Vaccine 2007; 25:7605–9. [DOI] [PubMed] [Google Scholar]
- 32.Tarantola A, Blanchi S, Cappelle J, et al. Rabies postexposure prophylaxis non-completion after dog bites: estimating the unseen to meet the needs of the underserved. Am J Epidemiol 2018; 187:306–15. [DOI] [PubMed] [Google Scholar]
- 33.Devriendt J, Staroukine M, Costy F, Vanderhaeghen JJ. Fatal encephalitis apparently due to rabies. Occurrence after treatment with human diploid cell vaccine but not rabies immune globulin. JAMA 1982; 248:2304–6. [DOI] [PubMed] [Google Scholar]
- 34.Shill M, Baynes RD, Miller SD. Fatal rabies encephalitis despite appropriate post-exposure prophylaxis. A case report. N Engl J Med 1987; 316:1257–8. [DOI] [PubMed] [Google Scholar]
- 35.Mohindra R, Suri V, Chatterjee D, Rana K. Measuring antibody titres following rabies postexposure prophylaxis in immunosuppressed patients: a norm rather than the exception. BMJ Case Rep 2021; 14:e245171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rasooli A, Pourhossein B, Bashar R, et al. Investigating possible etiologies of post-exposure prophylaxis failure and deaths from rabies infection: case reports. Int J Med Toxicol Forensic Med 2020; 10:27378. [Google Scholar]
- 37.Interim infection prevention and control recommendations for healthcare personnel during the coronavirus disease 2019 (COVID-19) pandemic. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html. Accessed 1 January 2022.
- 38.Rabies exposure in healthcare settings. Available at: https://www.cdc.gov/rabies/specific_groups/hcp/exposure.html. Accessed 21 January 2022.
- 39.Johnson S, Klumb C, Holzbauer S, Scheftel J. The cost of rabies post-exposure prophylaxis in Minnesota, 2017–2018. International Conference on Emerging Infectious Diseases. Atlanta, GA: Centers for Disease Control and Prevention:190. August 2018. [Google Scholar]
- 40.Altered immunocompetence. Available at: https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/immunocompetence.html. Accessed 26 January 2022.
- 41.Kopel E, Oren G, Sidi Y, David D. Inadequate antibody response to rabies vaccine in immunocompromised patient. Emerg Infect Dis 2012; 18:1493–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodríguez-Romo R, Morales-Buenrostro LE, Lecuona L, et al. Immune response after rabies vaccine in a kidney transplant recipient. Transpl Infect Dis 2011; 13:492–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harpaz R, Dahl R, Dooling K. The prevalence of immunocompromised adults: United States, 2013. Open Forum Infect Dis 2016; 3(Suppl 1):1439. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.