SUMMARY:
Dysfunction in sodium channels and their ankyrin scaffolding partners have both been implicated in neurodevelopmental disorders, including autism spectrum disorder (ASD). In particular, the genes SCN2A, which encodes the sodium channel NaV1.2, and ANK2, which encodes ankyrin-B, have strong ASD association. Recent studies indicate that ASD-associated haploinsufficiency in Scn2a impairs dendritic excitability and synaptic function in neocortical pyramidal cells, but how NaV1.2 is anchored within dendritic regions is unknown. Here, we show that ankyrin-B is essential for scaffolding NaV1.2 to the dendritic membrane of mouse neocortical neurons, and that haploinsufficiency of Ank2 phenocopies intrinsic dendritic excitability and synaptic deficits observed in Scn2a+/− conditions. These results establish a direct, convergent link between two major ASD risk genes and reinforce an emerging framework suggesting that neocortical pyramidal cell dendritic dysfunction can contribute to neurodevelopmental disorder pathophysiology.
eTOC Blurb:
Autism spectrum disorder (ASD) is associated with dysfunction in hundreds of genes. How dysfunction in ASD-associated genes converge on shared biological mechanisms remains an open question. Here, Nelson et al., show that one ASD-associated gene, Ank2, scaffolds another, Scn2a, in neocortical pyramidal cell dendrites, with shared effects on dendritic integration.
INTRODUCTION:
A decade of gene discovery has identified hundreds of genes whose dysfunction is associated with autism spectrum disorder (ASD)1–6. A key challenge remains to translate these findings into an understanding of pathophysiology at the cellular and circuit level. Loss-of-function in SCN2A, which encodes the neuronal sodium channel NaV1.2, has the strongest evidence of ASD association based on exome sequencing6–9. Given this critical role in ASD etiology, alterations in cellular function due to SCN2A loss may illuminate common causes of dysfunction shared with other ASD-associated genes.
A novel role for NaV1.2 was identified recently in neocortical pyramidal cells, a cell class whose dysfunction is implicated in ASD6,10. In contrast to well-characterized roles for sodium channels (NaVs) in axonal action potential (AP) electrogenesis and propagation, NaV1.2 was found to be critical for dendritic excitability, with ASD-associated Scn2a haploinsufficiency impairing postsynaptic features of synaptic function and plasticity9. This dendritic NaV1.2 localization is presumably controlled by ankyrins, which are a family of scaffolding proteins that link ion channels to the actin cytoskeleton11–13. Ankyrin-NaV interactions have been studied extensively in the axon, where NaVs are anchored by ankyrin-G (ANK3)14–17, but how NaVs are scaffolded to dendritic domains is unknown.
Insight may come from ASD gene discovery, which implicates dysfunction in another ankyrin family member, ankyrin-B (ANK2), in ASD6,8. Immunostaining for ankyrin-G and ankyrin-B in cultured neurons indicates that they occupy largely non-overlapping domains, with ankyrin-G enriched in the axon initial segment (AIS) and nodes of Ranvier, and ankyrin-B enriched in other regions, including dendrites18. Thus, ankyrin-B is well-positioned to scaffold dendritic NaV1.2 channels. In this way, loss-of-function in either SCN2A or ANK2 could impair dendritic excitability, either directly through reduced NaV density or indirectly through reduced NaV scaffolding.
Here, we show that the protein products of these two ASD risk genes, SCN2A and ANK2, interact in neocortical pyramidal cell dendrites to mutually regulate dendritic excitability. Using epitope-tagged NaV1.2, we found that NaV1.2 co-localizes with ankyrin-B in the dendrites of neocortical neurons. Removal of ankyrin-B eliminated NaV1.2 dendritic localization. Furthermore, dendritic ankyrin-B loss was not compensated for by other ankyrin family members, indicating that ankyrin-B has a unique scaffolding role in this neuronal compartment. Ex vivo electrophysiology revealed that Ank2 haploinsufficiency results in intrinsic and synaptic dendritic deficits that closely phenocopy those observed in Scn2a heterozygous neurons. The direct convergence between SCN2A and ANK2 shown here therefore further implicate deficits in dendritic excitability in ASD.
RESULTS:
NaV1.2 and ankyrin-B colocalize in neocortical neuron dendrites after the first postnatal week
To determine whether NaVs and ankyrins physically converge in dendrites, we first aimed to visualize their subcellular distribution patterns within a single cell. While NaVs can be observed in the AIS and nodes of Ranvier using conventional immunostaining approaches, they are often too diffuse to detect reliably in other regions19. To visualize NaV1.2 across development in all neuronal compartments, we first generated a cDNA encoding full-length NaV1.2 with a 3xFLAG epitope tag on the carboxyl-terminal, in addition to IRES-mediated expression of freely diffusible GFP. Voltage-clamp recordings from HEK293 cells transfected with either wild type (WT) NaV1.2 or NaV1.2–3xFLAG indicated that the introduction of this epitope tag did not alter channel biophysics (Figure S1A) or its ability to interact with β1 subunits (Figure S1B). For all experiments in cultured neurons, transfection efficiency was purposely held to low levels, often with only a single neuron transfected within a 1×1 mm field of view, to ensure that effects observed were due to cell autonomous manipulations (Figure S2).
Immunostaining and electrophysiological measurements indicate that NaV1.2 is enriched in the AIS of neocortical pyramidal cells in early development20–23 (Figure 1A). Consistent with staining of native channels, NaV1.2–3xFLAG was similarly restricted to the AIS at day in vitro 7 (DIV7) in cultured neocortical neurons, where it colocalized with ankyrin-G (Figure 1C). Later in development, NaV1.2 is largely displaced from the AIS and instead increases in density throughout somatodendritic domains9,24–27 (Figure 1A). Consistent with this shift in NaV1.2 subcellular localization, NaV1.2–3xFLAG was visualized at high levels throughout dendrites at DIV21 (Figure 1C). Dendritic NaV1.2 was not co-localized with ankyrin-G (ANK3) (Figure 1C), suggesting that another ankyrin may be important for NaV scaffolding in dendritic regions. Based on genetic and co-expression data related to ASD6,8,10 (Figure 1B), we hypothesized that this dendritic scaffold is ankyrin-B (ANK2).
Figure 1: Ankyrin-B is colocalized with NaV1.2 in the dendrites of mature pyramidal neurons.
(A) Schematic of NaV channel distribution with ankyrins throughout neocortical pyramidal neuron development.
(B) Manhattan plot of ASD-associated genes identified by whole-exome sequencing. Red dotted line indicates false discovery rate threshold of ≤ 0.1.
(C) Left: Confocal images of WT cultured neocortical neurons transfected with NaV1.2–3xFLAG-IRES-eGFP, fixed at DIV7 or DIV21. Cells were immunostained for ankyrin-G (white), FLAG (magenta), and GFP (green). Arrowheads denote AIS of transfected cell. Top right: zoomed image of dendrite labeled with yellow box. Middle right: Plot profiles of dendritic NaV1.2–3xFLAG, ankyrin-G, and GFP fluorescence intensity (a.u.) at DIV7 and DIV21. Dendritic region used to generate plot profile indicated by white line in above figure.
Quantification of mean fluorescence intensity of NaV1.2–3xFLAG in dendrites or AIS at DIV7 versus DIV21. Circles represent individual neurons. Dendrites – (DIV7: 237.4±36.0, n=10 cells; DIV21: 1046±111.8, n=13 cells) ****p<0.0001. AIS – (DIV7: 3134±444.5, n=10 cells; DIV21: 2798±334.7, n=13 cells) p=0.83. No significant differences.
(D) Left: Confocal images of NaV1.2–3xFLAG-IRES-eGFP-transfected cells, as in C, but immunostained for ankyrin-B instead of ankyrin-G.
(E) Linear regression of mean NaV1.2–3xFLAG dendrite:AIS fluorescent signal versus GFP at DIV7 and DIV21.
To compare the subcellular localization patterns of ankyrin-B and NaV1.2 across development, we transfected cultured neocortical neurons with NaV1.2–3xFLAG-IRES-GFP and immunostained with antibodies against ankyrin-B at DIV7 and DIV21. At DIV7, ankyrin-B was localized to the distal axon (Figure 1D). At DIV21, however, ankyrin-B was enriched along dendritic shafts, colocalizing with NaV1.2 (Figure 1D). NaV1.2 expression remained in distal axons (Figure 1C-D), likely because neurons were not co-cultured with myelinating oligodendrocytes28–30. Importantly, there was no correlation between dendritic NaV1.2–3xFLAG and plasmid expression levels, inferred from GFP fluorescence intensity, suggesting that NaV1.2 expression is tightly regulated and that its dendritic localization is not an off-target effect of overexpression (Figure 1E). These data indicate ankyrin-B is well-positioned to scaffold NaV1.2 to neocortical pyramidal cell dendrites after early development.
Ankyrin-B localizes NaV1.2 to the dendritic membrane of neocortical neurons
To determine whether ankyrin-B directly scaffolds NaV1.2 to the dendritic membrane, we performed knockout-and-rescue experiments in cultured neocortical neurons generated from Ank2fl/fl mice, which contain loxP sites flanking exon 24 of the Ank2 gene31. In WT conditions, NaV1.2–3xFLAG was highly enriched in the dendritic membrane with endogenous ankyrin-B in DIV21 neurons (Figure 2A, S2A). Co-transfection of NaV1.2–3xFLAG-IRES-GFP with Cre-2A-BFP (blue fluorescent protein) in Ank2fl/fl neurons resulted in the complete loss of ankyrin-B, with a corresponding loss of dendritic NaV1.2 (Figure 2A). Simultaneous knockout of endogenous ankyrin-B via Cre-2A-BFP and rescue with the canonical wild-type ankyrin-B restored NaV1.2 to dendritic membranes (Figure 2A). Similar reductions in dendritic NaV1.2 localization were observed in Ank2 heterozygous cultured neurons as seen with full knockout, suggesting that even the loss of one functional Ank2 allele is enough to decrease NaV1.2 dendritic density to levels below detection threshold (Figure S4A-C).
Figure 2: 220 kDa ankyrin-B scaffolds NaV1.2 to the dendritic membrane.
(A) Top left: Confocal images of DIV21 Ank2flox/flox cultured neocortical neurons co-transfected with NaV1.2–3xFLAG-IRES-eGFP and TagBFP (WT) or Cre-2A-BFP (Ank2 null) or rescued with WT or FF/QQ mutant 220 kDa ankyrin-B. Cells were immunostained for ankyrin-B (white), FLAG (magenta), and GFP (green). Top right: zoomed images of dendrite labeled with yellow box. Bottom left: Quantification of mean fluorescence intensity of NaV1.2–3xFLAG in dendrites. Circles represent individual neurons. Dendrites – (WT: 933.0±218.8, n=18 cells; Ank2−/−: 246.0±51.16, n=16 cells; Ank2−/− + WT 220 kDa AnkB: 945.1±191.8, n=16 cells; Ank2−/− + FF/QQ 220 kDa AnkB: 237.3±57.9, n=15 cells). WT vs. Ank2−/− *p=0.016, Ank2−/− vs 220 AnkB *p=0.016, WT vs. FF/QQ AnkB *p=0.016, 220 AnkB vs. FF/QQ AnkB *p=0.016. Bottom right: Quantification of mean fluorescence intensity of NaV1.2–3xFLAG in the AIS. AIS – (WT: 2046±309.2, n=18 cells; Ank2−/−: 2123±282.9, n=16 cells; Ank2−/− + WT 220 kDa AnkB: 1900±241.3, n=16 cells; Ank2−/− + FF/QQ 220 kDa AnkB: 1627±981.3, n=15 cells). No significant differences. Arrowhead marks the AIS.
(B) Left: Western blot of WT mouse neocortical lysates at P0, P7, P14, P21, and P30. Blots were probed with antibodies to endogenous total ankyrin-B (labeling both 440 kDa (grey) and 220 kDa (green) ankyrin-B isoforms), NaV1.2 (cyan), and α-tubulin. Right: Western blot data, normalized to expression at P30. N=3 mice per age. Data shown as mean ± SEM.
(C) Confocal images of WT cultured neurons transfected with 220 kDa AnkB-GFP or 440 kDa AnkB-GFP, fixed at DIV21. Neurons were immunostained for endogenous ankyrin-G (magenta) and GFP (green).
(D) Mean fluorescence intensity (a.u.) ratio of dendritic and distal axon GFP from 220 kDa ankyrin-B (green) versus 440 kDa ankyrin-B (grey) in (C). (220 AnkB: 1.12±0.14, n=11 cells; 440 AnkB: 0.51±0.06, n=6 cells). ***p=0.0003.
(E) Plot profiles of mean fluorescence intensity (a.u.) of transfected 220 kDa ankyrin-B-GFP versus endogenous total ankyrin-G across different neuronal domains in (C). White dashes indicate regions measured.
Ankyrin-B is highly homologous with ankyrin-G, especially throughout the ankyrin repeat domain that contains the canonical NaV binding site32. Therefore, we examined whether ankyrin-B requires this same sequence to localize NaV1.2. We generated a double mutant (F131Q, F164Q) in the ankyrin repeats of ankyrin-B, which has been shown previously to reduce binding affinity between ankyrins and NaV1.2 by >40-fold33. Knockout-and-rescue with the FF/QQ mutant failed to scaffold NaV1.2 to the dendrites, demonstrating the importance of this site for the proper localization of NaV1.2 to adult neocortical dendrites (Figure 2A). Importantly, NaV1.2 clustered appropriately to the AIS in Ank2 null and FF/QQ mutant neurons (Figure 2A). This suggests that the presence of NaV1.2 in the dendrites is not an artifact of NaV1.2–3xFLAG overexpression and requires ankyrin-B. In addition, the localization patterns of NaV1.2–3xFLAG across these conditions are consistent with previous studies using two distinct methods to label endogenous NaV1.229,34.
Ankyrin-B is expressed as two splice variants in the brain that both contain the conserved NaV-binding site 35: a 220 kDa isoform and a giant 440 kDa isoform 36. The 440 kDa ankyrin-B has been shown to be the primary isoform in the distal axon18,37,38, but which splice variant functions in the dendrites is less clear. Western blot analysis of mouse neocortical lysates from P0 to P30 revealed parallel increases in NaV1.2 and ankyrin-B expression throughout development (Figure 2B). Expression of the 220 kDa ankyrin-B, which has been shown to be more prominent later in development18,39, correlated more closely with NaV1.2 expression than the 440 ankyrin-B, suggesting that the 220 kDa ankyrin-B may be the main isoform that functions in the dendrites (Figure 2B). To evaluate localization patterns of the 220 versus the 440 kDa ankyrin-B, we cloned full-length 220 kDa ankyrin-B-GFP and 440 kDa ankyrin-B-GFP. Constructs were expressed in cultured neocortical neurons and immunostained with antibodies against GFP and ankyrin-G. As previously shown, the 440 kDa ankyrin localized almost exclusively to the distal axon (Figure 2C-D). By contrast, the 220 kDa isoform localized throughout the entire dendritic arbor, the soma, and the axon (Figure 2C-E). Overall, these data indicate that the 220 kDa ankyrin-B is the predominant ankyrin that targets NaV1.2 channels to pyramidal cell dendrites.
Ankyrin-B directly interacts with NaV1.2 in mouse brain
We next evaluated the molecular basis underlying the interaction between ankyrin-B and dendritic NaV1.2. Due to their large size, it is difficult to study direct protein-protein interactions between full-length ankyrins and ion channels12,40. Therefore, we evaluated binding between ankyrin-B-GFP and an epitope-tagged fragment of NaV1.2 that contains a highly conserved core nine amino acid motif necessary for ankyrin binding (NaV1.2 II-III loop-HA)12 (Figure 3A). We co-transfected these constructs in HEK293 cells and performed a proximity ligation assay (PLA), finding that ankyrin-B and the NaV1.2 II-III loop associate within 10 nanometers of each other (Figure 3B). To assess direct binding between ankyrin-B and NaV1.2, we then transfected HEK293 cells with a mutant NaV1.2 II-III loop (termed Δ9-mutant NaV1.2) from which we excised the core 9 amino acid ankyrin-binding motif. Expression of the Δ9-mutant NaV1.2 completely abolished PLA signal, highlighting this motif’s importance for ankyrin-B—NaV1.2 binding (Figure 3B). Of note, deletion of the Δ9 sequence in the NaV1.2–3xFLAG construct did not affect channel biophysical properties (Figure S1C). We further validated their interaction by immunoprecipitating ankyrin-B-GFP and NaV1.2 II-III loop from HEK293 cells (Figure 3C and S5). Again, we failed to detect any Δ9-mutant NaV1.2 following immunoprecipitation of ankyrin-B (Figure 3C and S5).
Figure 3: Ankyrin-B directly interacts with NaV1.2 in adult mouse brain.
(A) NaV1.2 schematic highlighting the ankyrin-binding motif (purple) located within the intracellular loop between domains II and III (cyan). Core nine amino acids (Δ9 motif) within the II-III loop are essential for ankyrin binding.
(B) Left: Representative images of proximity ligation assay (PLA) signal (cyan) between anti-HA and anti-ankyrin-B antibodies from HEK293 cells transfected with 220 kDa ankyrin-B-GFP (green) and HA-tagged NaV1.2 II-III loop (left) or the HA-tagged Δ9 mutant loop (right). Right: Quantification of PLA signal (a.u.) between ankyrin-B and WT NaV1.2 II-III loop versus ankyrin-B and Δ9 NaV1.2 II-III loop. (WT: 11.6±1.5, n=26 cells; Δ9: 1.4±0.2, n=24 cells). ****p<0.0001.
(C) Co-immunoprecipitation of ankyrin-B-GFP with WT NaV1.2 II-III loop or Δ9 NaV1.2 II-III loop. Western blots were probed with antibodies against anti-GFP (to label ankyrin-B-GFP) and anti-HA (to label NaV1.2 II-III loop-HA). Non-immune IgG used as a negative control.
(D) Left: Confocal images of cultured neocortical neurons transfected with WT NaV1.2–3xFLAG-IRES-eGFP or Δ9 NaV1.2–3xFLAG-IRES-eGFP. Cells were immunostained with anti-GFP (green) and anti-FLAG (magenta) antibodies. Right: Mean fluorescence intensity (a.u.) of NaV1.2–3xFLAG in the AIS (top) and dendrites (bottom). AIS: (WT: 1445±233.5, n=5 cells; Δ9: 17.06±15.2, n=4 cells). *p=0.016. Dendrites: (WT: 620±71.0, n=5 cells; Δ9: 46.1±9.7, n=4 cells). *p=0.016.
(E) Left: IP of endogenous ankyrin-B and western blot of IP lysates probed with antibodies to ankyrin-B or endogenous NaV1.2 from P60-P75 mice. Right: IP of endogenous NaV1.2 and western blot of IP lysates probed with antibodies to NaV1.2 or ankyrin-B from P60-P75 mice. Black arrows highlight bands of ankyrin-B or NaV1.2. Non-immune IgG used as a negative control. Note: 440 kDa ankyrin-B band consistently runs anomalously high as reported previously14.
We next tested whether ankyrin-B localized full-length NaV1.2 in pyramidal cell dendrites if NaV1.2 lacked the ankyrin-binding motif. Cultured neurons were transfected with plasmids encoding either WT NaV1.2–3xFLAG-IRES-GFP or Δ9 NaV1.2–3xFLAG-IRES-GFP. At DIV21, WT NaV1.2–3xFLAG properly localized to the dendrites and the AIS; however, the Δ9-mutant NaV1.2–3xFLAG-IRES-GFP, which is unable to interact with ankyrin-B, failed to localize to the dendrites (Figure 3D). Since excising the nine amino acid motif prevents all ankyrins from binding, NaV1.2–3xFLAG clustering at the AIS was also lost (Figure 3D). These Δ9-mutant NaV1.2 data are consistent with those obtained with the FF/QQ mutant ankyrin-B, further confirming that dendritic localization of NaV1.2–3xFLAG is not an artifact of overexpression.
The above experiments demonstrate that ankyrin-B is in complex with NaV1.2 in pyramidal neuron dendrites when each protein is overexpressed in cultured neocortical neurons. To determine if this interaction occurs with endogenous ankyrin-B and NaV1.2 in native adult neocortex, we immunoprecipitated ankyrin-B using antibodies that detect both the 220 kDa and 440 kDa isoforms. Western blot and immunoblotting with antibodies against NaV1.2 revealed that ankyrin-B is in complex with NaV1.2 in the adult neocortex (Figure 3E and S7). Since ankyrin-B is expressed in almost every cell-type within the neocortex39, we wanted to confirm their interaction in pyramidal cell dendrites, where NaV1.2 predominantly resides, by immunoprecipitating NaV1.2 from adult brain. Immunoprecipitation of NaV1.2 resulted in co-IP of the dendritic 220 kDa ankyrin-B (Figure 2C), but not the 440 kDa isoform, which is predominantly axonal (Figure 3E). These data provide further evidence that the 220 kDa ankyrin-B is the primary isoform that interacts with NaV1.2 in adult mouse brain and demonstrates that endogenous NaV1.2 is associated in complex with ankyrin-B in adult neocortex.
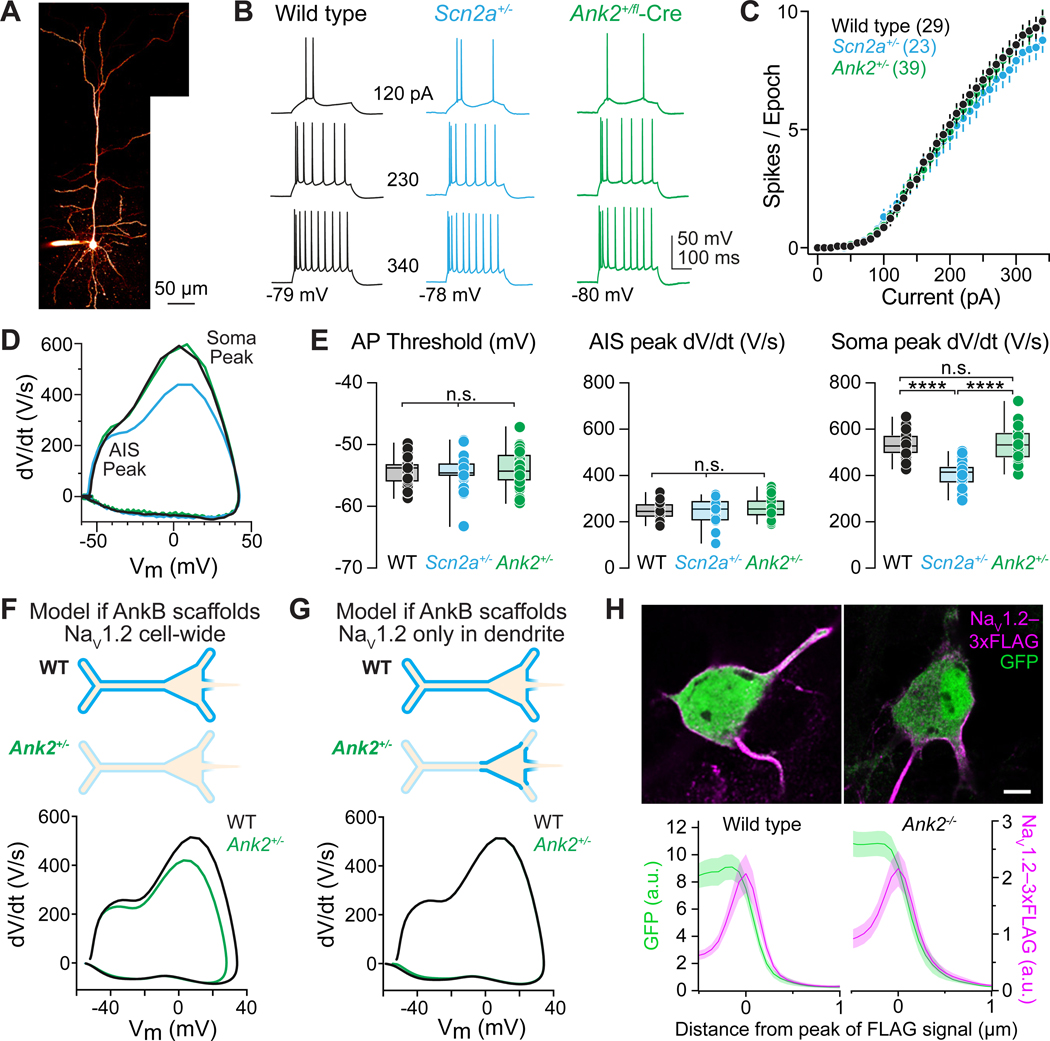
Ank2 haploinsufficiency impairs dendritic, but not somatic, excitability
NaV1.2 channels are expressed on both somatic and dendritic membranes in neocortical pyramidal neurons. Data above indicates that ankyrin-B is important for dendritic NaV scaffolding; however, previous reports have demonstrated that both ankyrin-B and ankyrin-G are present on the soma of mature pyramidal neurons14,41,42. As such, Ank2 haploinsufficiency is expected to impair measures of dendritic excitability, but whether somatic excitability is also affected may depend instead on how channels interact with ankyrin-G at the soma. Previously, we used the peak velocity of the rising phase of the AP (peak dV/dt) and AP-evoked dendritic calcium imaging as functional proxies for NaV1.2 membrane density in the somatic and dendritic compartments, respectively9,26. Thus, these approaches may help reveal functional changes in NaV1.2 density in somatic and dendritic compartments in cases of Ank2 loss.
In prior studies on Scn2a, we found that haploinsufficiency, either throughout life or induced after P16, resulted in identical deficits in dendritic excitability and excitatory synaptic function9. Furthermore, we leveraged genetic approaches in ~P30 Scn2a+/− mice to restore Scn2a expression and rescue downstream excitability deficits to WT levels43. This suggests that Scn2a has a life-long role in regulating dendritic excitability, and that our studies here should focus on this developmental period to assess potential convergent function between Scn2a and Ank2. Therefore, we examined AP waveform properties from layer 5 (L5) thick-tufted neurons in acute slices of P43–75 Ank2+/fl::CaMKIIɑ-Cre mice, which express Cre in all neocortical pyramidal neurons after ~P169,44 (Figure 4A-E, S6). Western blots of neocortical lysates generated from adult Ank2+/fl::CaMKIIɑ-Cre mice revealed a reduction in the 440 kDa and 220 kDa isoforms of ankyrin-B without any change in NaV1.2 or ankyrin-G expression(Figure S7). While interleaved experiments in Scn2a+/− cells revealed expected reductions in peak somatic dV/dt, Ank2+/fl cells were not different than WT (Figure 4D-E). In addition, we observed no difference in the F/I curves of Ank2+/fl neurons compared to WT and Scn2a+/− neurons, or in measures of AIS excitability (threshold and AIS-associated peak dV/dt) (Figure 4B-E).
Figure 4: Ank2 haploinsufficiency has no effect on axonal or somatic excitability.
(A) 2-photon maximum intensity z-stack of a layer 5 thick-tufted pyramidal neuron in mPFC, filled with Alexa-594 via whole-cell pipette.
(B) APs generated by current injection (0–340 pA, 10 pA intervals, 300 ms) in WT, Scn2a+/−, and Ank2+/fl-Cre in P43–75 L5 neurons.
(C) APs per 300 ms epoch versus amplitude of current injection as in (B). Firing-rate slope between 0–340 pA: (WT: 0.033±0.002 Hz/pA, n=29 cells, N=14 mice; Scn2a+/−: 0.03±0.002 Hz/pA, n=23 cells, N=8 mice; Ank2+/fl-Cre: 0.033±0.001, n=39 cells, N=16 mice). No significant differences.
(D) Phase-plane plots (dV/dt vs. voltage) of somatic APs from WT (black), Scn2a+/− (cyan), and Ank2+/fl-Cre (green) neurons. Different phases of the AP correspond to AP initiation in the AIS (first peak) and soma (second peak).
(E) Left: Quantification of AP threshold (mV) from the first AP evoked by near-rheobase current in P43–75 WT, Scn2a+/−, and Ank2+/fl-Cre L5 neurons. Circles represent single cells. (WT: −54.4±0.4 mV, n=27 cells; Scn2a+/: −54.5±0.4 mV, n=23 cells; Ank2+/fl-Cre: −53.9±0.5 mV, n=39 cells). No significant differences. Middle: AIS AP peak dV/dt (V/s). (WT: 248.7±7.1 V/s, n=27 cells; Scn2a+/−: 242.2±12.3 V/s, n=23 cells; Ank2+/fl-Cre: 257.6±6.8 V/s, n=39 cells). No significant difference. Right: Somatic AP peak dV/dt (V/s). (WT: 537.8±11.3 V/s, n=27 cells; Scn2a+/−: 406.4±11.3 V/s, n=27 cells, Ank2+/fl-Cre: 535.1±10.8 V/s, n=40 cells). WT vs. Scn2a+/− ****p<0.0001, Scn2a+/− vs. Ank2+/fl-Cre ****p<0.0001, WT vs. Ank2+/fl-Cre p=0.86.
(F) Compartmental modeling of the effects of NaV1.2 loss from both somatic and dendritic domains on AP waveform. Top: Schematic of NaV1.2 distribution across soma and dendrites in WT neurons compared to loss of NaV1.2 in all compartments in Ank2+/fl-Cre neurons. Bottom: Phase-plane plots from computational model of AP somatic peak dV/dt changes with WT and heterozygous NaV1.2 membrane density. No other channels or model parameters were altered except for NaV1.2.
(G) Modeling as in F, but now restricting NaV1.2 loss to dendrites alone. Note agreement of model with empirical data from Ank2+/fl-Cre neurons in E.
(H) Top: Confocal images of DIV21 WT and Ank2-null cultured neocortical neurons transfected with NaV1.2–3xFLAG-IRES-eGFP and immunostained for FLAG (magenta) and GFP (green). Images are single optical sections through the soma. Complete z-projection images are shown in Figure S3. Bottom: Somatic NaV1.2–3xFLAG and GFP mean fluorescence intensity (a.u.) in WT (left) and Ank2-null (right) neurons. Lines are means with SEM shadows of 1 μm-wide intensity profiles drawn orthogonal to the somatic membrane (2 lines per cell, 12 cells per condition).
These empirical data suggest that loss of Ank2 does not affect somatic or axonal excitability, contrasting with previous results in Scn2a+/− neurons and with compartmental models where Ank2 haploinsufficiency induces a reduction in NaV1.2 density throughout the somatodendritic domain (Figure 4F). Instead, models indicate that peak dV/dt is dependent exclusively on somatic NaV density and is insensitive to changes in dendritic channel density (Figure 4G), and therefore suggests that NaV1.2 densities are at WT levels in Ank2+/− cells in the soma. This motivated us to examine somatic NaV1.2–3xFLAG labeling in cultured Ank2−/− neurons. Consistent with measures of excitability, NaV1.2–3xFLAG was localized to the soma in these cells at WT levels (Figure 4H). Thus, ankyrin-G may either be the primary ankyrin that localizes NaV1.2 to the soma or may be able to compensate for somatic ankyrin-B loss in both heterozygous or homozygous knockout conditions.
While somatic recordings suggest that somatic NaV density is not affected by Ank2 haploinsufficiency, they cannot inform on changes in dendritic excitability, which modeling suggests would still be impaired in Ank2+/− conditions (Figure S6B). To evaluate the effects of ankyrin-B loss on dendritic NaV channel density and excitability, we first examined dendritic NaV function directly with AP-evoked Na+ imaging using the sodium indicator ING-245-47. In WT neurons, trains of 40 APs at 100 Hz evoked detectable sodium transients within the first 125 μm of the apical dendrite (Figure 5A). At 25 μm from the soma, Na+ transient amplitudes were comparable in WT and Ank2+/− neurons. In Scn2a+/− neurons, Na+ influx was reduced by 50%, consistent with haploinsufficiency of NaV density. Farther from the soma, however, transients in Ank2+/− neurons became significantly smaller and exhibited a 40% reduction in Na+ influx compared to WTs (Figure 5A-B). This stark change in Na+ influx between 25 and 50 microns from the soma corresponds well to the distribution of endogenous ankyrin-G, which can extend into the first tens of microns of dendrite in cultured neurons (Figure 2C)41.
Figure 5: Reduced AP-evoked Na+ apical dendrites of Ank2 haploinsufficient neurons.
(A) 2-photon Na+ imaging throughout the apical dendrite of a L5 thick-tufted neuron filled with ING-2 (500 μM) via the recording pipette. Na+ transients evoked by trains of APs (stimulus: 40× 100 Hz, 2 nA for 2 ms) every 25 microns from the soma in WT (black), Scn2a+/− (cyan) and Ank2+/fl::CaMKIIɑ-Cre (green) P46–76 mice.
Representative traces shown as mean±SEM.
(B) AP-evoked Na+ transient amplitude versus distance from soma. 25 microns – (WT: 0.013±0.001, n=11 cells, N=5 mice; Scn2a+/−: 0.008±0.0006, n=12 cells, N=3 mice; Ank2+/fl-Cre: 0.015±0.002, n=8 cells, N=3 mice) WT vs. Scn2a+/− (*) p=0.03, WT vs Ank2+/fl-Cre, p=0.49, Scn2a+/− vs. Ank2+/fl-Cre (‡) p=0.01. 50 microns – (WT: 0.014±0.001, n=11 cells; Scn2a+/−: 0.007±0.001, n=12 cells; Ank2+/fl-Cre: 0.009±0.001, n=8 cells) WT vs. Scn2a+/− (***) p=0.0004, WT vs Ank2+/fl-Cre (†) p=0.02, Scn2a+/− vs. Ank2+/fl-Cre p=0.23. 75 microns – (WT: 0.006±0.001, n=8 cells; Scn2a+/−: 0.003±0.001, n=9 cells; Ank2+/fl-Cre: 0.004±0.001, n=8 cells) WT vs. Scn2a+/− (*) p=0.04, WT vs Ank2+/fl-Cre p=0.17, Scn2a+/− vs. Ank2+/fl-Cre p=0.42. 100 microns – (WT: 0.005±0.001, n=11 cells; Scn2a+/−: 0.0018±0.0004, n=11 cells; Ank2+/fl-Cre: 0.003±0.001, n=7 cells) WT vs. Scn2a+/− (**) p=0.001, WT vs Ank2+/fl-Cre p=0.11, Scn2a+/− vs. Ank2+/fl-Cre p=0.11. 125 microns – (WT: 0.004±0.001, n=8 cells; Scn2a+/−: 0.002±0.001, n=7 cells; Ank2+/fl-Cre: 0.002±0.001, n=6 cells) WT vs.
Scn2a+/− p=0.4, WT vs Ank2+/fl-Cre p=0.4, Scn2a+/− vs. Ank2+/fl-Cre p=0.9. Data shown as mean±SEM.
(C) Na+ influx normalized to WT average at each distance.
(D) 2-photon Na+ imaging as in A-B, but in the presence of the CaV antagonists TTA-P2 (2 μM), Nifedipine (10 μM) and Conotoxin MVIIC (1 μM). Data from P63-P81 mice.
(E) AP-evoked Na+ transient amplitude in the presence of the CaV blockers. versus distance from soma. 25 microns – (WT: 0.017±0.01, n=8 cells, N=3 mice; Ank2+/fl-Cre: 0.016±0.02, n=8 cells, N=3 mice) p=0.64. 50 microns – (WT: 0.019±0.002, n=8 cells; Ank2+/fl-Cre: 0.012±0.002, n=8 cells) **p=0.007. 75 microns – (WT: 0.01±0.007, n=8 cells; Ank2+/fl-Cre: 0.006±0.002, n=8 cells) *p=0.038. 100 microns – (WT: 0.012±0.001, n=8 cells; Ank2+/fl-Cre: 0.006±0.002, n=6 cells) p=0.06. Data shown as mean±SEM.
(F) Na+ influx with CaV antagonists, normalized as in C.
Ankyrin-B has been reported to interact with CaV2 and CaV3 channels48–50. Thus, it is possible that results observed above may reflect a loss of CaV-mediated electrogenesis that, in turn, affects NaV activity. To control for this possibility, we repeated experiments in the presence of CaV2 and CaV3 antagonists conotoxin-MVIIC and TTA-P2. We further included nifedipine to antagonize CaV1 channels, as CaV1 channels interact with ankyrin-B in cardiomyocytes and are expressed in neocortical pyramidal cell dendrites51,52. Data obtained with CaV antagonists were identical to those obtained without (Figure 5D-F). Hence, these data are most consistent with a model where ankyrin-G is capable of scaffolding NaVs in the soma and proximal dendrite in Ank2 haploinsufficient conditions, but that ankyrin-B is solely responsible for NaV scaffolding in more distal dendritic domains.
Due to the low sensitivity inherent to Na+ imaging, dendritic Na+ influx could be imaged only within 150 microns of the soma. To understand how Ank2 haploinsufficiency affects excitability in more distal dendritic compartments, we took advantage of the fact that bursts of backpropagating APs (bAPs) reliably engage CaVs throughout the dendritic arbor9,53–56. Consistent with previous observations, bursts of APs (a set of 5 AP doublets at 100 Hz) evoked robust calcium transients throughout the apical dendrite of WT neurons (Figure 6B). By contrast, Ca2+ transients were reduced markedly in the Ank2+/− neurons, mirroring observations made in Scn2a+/− neurons9 (Figure 6B). Of note, dendritic arborization was unaltered in adult L5 Ank2+/− neurons, with no difference in branch number or length compared to WT neurons (Figure S8A). Taken together, these data demonstrate that Ank2 and Scn2a converge to regulate dendritic, but not somatic, intrinsic excitability.
Figure 6: Ank2 haploinsufficiency impairs dendritic backpropagation of APs and excitatory synapse function.
(A) Ank2+/fl mice were crossed with CaMKIIɑ-Cre mice or injected with AAV5-Ef1α-Cre-mCherry virus to render all mPFC pyramidal cells heterozygous for Ank2. Bottom: Max intensity z-stack of mCherry fluorescence (red) overlaid with scanning DIC image (grayscale).
(B) Left: 2-photon Ca2+ transients evoked by bursts of AP doublets in L5 thick-tufted pyramidal neuron dendrites in P52–67 WT and Ank2+/fl::CaMKIIɑ-Cre mice. Right: Ca2+ transient amplitude plotted for the first burst (top) and the area under the curve (a.u.c.) of all 5 bursts (bottom) vs. distance. (WT: n=8, N=6, Ank2+/fl-Cre: n=7, N=6). Error bars are mean ± SEM. *p<0.05.
(C) mEPSCs recorded from P54-P60 WT (black) and Ank2+/fl-Cre-mCherry-positive (green) neurons. Ticks denote detected events. Left: Cumulative probability distribution of mEPSC amplitudes. Distributions were generated per cell, then averaged. Insert: average mEPSC amplitude per cell. (WT: −9.4±0.6 pA, n=14 cells, N=3 mice; Ank2+/fl-Cre: −10.1±0.7 pA, n=15 cells, N=3 mice) p=0.5. Right: Cumulative probability distribution of mEPSC inter-event intervals (IEI). Insert: average mEPSC frequency per cell. (WT: 6.4±1.1 Hz, n=14 cells; Ank2+/fl-Cre: 3.3±0.4 Hz, n=15 cells) *p=0.01.
(D) Top: Paired-pulse ratio of evoked excitatory inputs at 25, 50, and 100 ms intervals in P50–67 WT (black) and Ank2+/fl-Cre (green) mice. Bottom: PPR grouped by inter-stimulus interval. 25 ms – (WT: 2.0±0.1, n=10 cells, N=3 mice; Ank2+/fl-Cre: 1.6±0.1, n=11 cells, N=3 mice) **p=0.0079. 50 ms – (WT: 1.9±0.1, n=13 cells; Ank2+/fl-Cre: 1.5±0.09, n=11 cells) *p=0.03. 100 ms – (WT: 1.6±0.1, n=11 cells; Ank2+/fl-Cre: 1.3±0.08, n=12 cells) *p=0.032.
(E) Top: AMPA-receptor mediated (−80 mV) and mixed AMPA-NMDA (+30) evoked EPSCs from P54–60 WT (black) and Ank2+/fl-Cre-mCherry-positive (green) neurons. Dashed line indicates when NMDA component was calculated (50 ms after stimulation onset). Bottom: Quantification of AMPA:NMDA ratio. (WT: 3.9±0.6, n=7 cells, N=3 mice; Ank2+/fl-Cre: 2.2±0.2, n=8 cells, N=3 mice) **p=0.009.
(F) Top: Voltage-clamp recordings of NMDAR-mediated EPSCs at +30 mV in P54–75 WT (black), Scn2a+/− (cyan), Ank2+/fl-Cre (green) neurons at baseline (dark shade) and after bath application of 1 μM Ro 25–6981 (light shade). Bottom: Normalized weighted decay time-constant from baseline and in Ro 25–6981 (WT: 0.898±0.04, n=11 cells, N=4 mice; Scn2a+/−: 0.696±0.02, n=9 cells, N=3 mice; Ank2+/fl-Cre: 0.717±0.03, n=9 cells, N=3 mice). WT vs. Scn2a+/− ***p=0.0007; WT vs. Ank2+/fl-Cre **p=0.0015; Scn2a+/− vs. Ank2+/fl-Cre p=0.67.
Ank2 heterozygous mice demonstrate impaired excitatory synaptic function
In Scn2a+/− cells, impaired dendritic excitability weakens postsynaptic aspects of excitatory synaptic transmission by reducing the relative number of functionally mature, AMPA receptor-containing synapses9. We hypothesized that similar impairments may result from Ank2 loss. To test this, we evaluated pre- and postsynaptic components of synaptic function in Ank2+/fl mice injected with a Cre-expression adeno-associated virus (AAV-EF1ɑ-Cre-mCherry, injections at P30, experiments at P52–60) (Figure 6A). Whole-cell voltage-clamp recordings of miniature excitatory and inhibitory postsynaptic currents (mEPSC, mIPSC) revealed a 50% reduction in mEPSC frequency with no change in mEPSC amplitude (Figure 6C). mIPSC frequency and amplitude were unaffected (Figure S9).
These data are consistent with a reduction in the number of functionally mature, AMPA receptor-containing excitatory synapses that were observed in Scn2a+/− conditions; however, reductions in release probability can also contribute to such observations. We therefore examined paired-pulse ratio (PPR) of evoked, AMPA receptor-mediated currents, as this ratio is often inversely correlated with release probability. We observed a small decrease, rather than an increase, in PPR (Figure 6D). Taken together, these data support a model where a large fraction of synapses lack AMPA receptors and are silent in mEPSC recordings, and that release probability may be higher, at least at synapses that have AMPA receptors. To further test for the presence of silent synapses, we performed three additional experiments. First, we measured AMPA:NMDA ratio, finding that it was reduced in Ank2+/−-Cre neurons (Figure 6E). This is consistent with prior observations in Scn2a+/− mice and with an increased number of silent synapses9. Second, we tested for the presence of NR2B-containing NMDA receptors more commonly localized to immature, silent synapses57. We found that evoked NMDA receptor-mediated EPSCs were more sensitive to the NR2B-selective antagonist RO 25–6981 (1 μM) in both Ank2+/− and Scn2a+/− conditions, compared to WT controls (Figure 6F). Lastly, we examined spine morphology in Ank2+/− pyramidal cells. Similar to observations in Scn2a+/− cells9, spines along apical dendritic shafts had smaller heads relative to their total volume, an anatomical feature typically observed in immature synapses (Figure S8B).
These experiments indicate that ankyrin-B heterozygosity has differential roles in dendritic and axonal compartments, regulating release probability presynaptically and dendritic excitability postsynaptically. But since transmitter release can only be detected at synapses with postsynaptic receptors, the net effect may be a reduction in excitatory transmission. To test this, we first isolated the postsynaptic contributions of ankyrin-B to and compared them to loss in both pre- and postsynaptic compartments. A dilute AAV-EF1ɑ-Cre-mCherry virus was injected into mPFC of Ank2+/fl mice at P30 to render only a few cells heterozygous for Ank2. (Figure 7A). In these conditions, cell-autonomous effects of Ank2 haploinsufficiency in dendrites could be assessed by recording from one of the few mCherry-positive neurons that receive input from largely mCherry-negative (e.g., WT) inputs (Figure 7A). Consistent with a lack of presynaptic effect in this experimental design, PPR was not different between mCherry-positive and negative cells (Figure 7C), and matched PPR values obtained from uninfected WT littermates (Figure 6E). By contrast, postsynaptic components of transmission were affected to the same degree as observed in Ank2+/fl::CaMKII-Cre conditions. The sparsely labeled Cre-positive Ank2 heterozygous neurons showed a reduction in mEPSC frequency and in AMPA:NMDA ratio compared to interleaved Cre-negative neurons recorded in the same slice (Figure 7B, D). The relative reduction in mEPSC frequency and AMPA:NMDA ratio, normalized to each condition’s WT comparison, was no different between full conditional Ank2 heterozygosity, cell-autonomous postsynaptic Ank2 heterozygosity, or prior experiments focused on Scn2a heterozygosity [AMPA:NMDA ratio (norm. to WT avg.): Ank2+/fl-Cre high-titer: 0.57±0.06 n=8 cells; Ank2+/fl-Cre low-titer: 0.74±0.09, n=9 cells; Scn2a+/−: 0.6±0.09, n=11 cells. No significant differences. mEPSC frequency (norm. to WT avg.): Ank2+/fl-Cre high-titer: 0.52±0.07 n=15 cells; Ank2+/fl-Cre low-titer: 0.35±0.07, n=5 cells; Scn2a+/−: 0.5±0.09, n=19 cells. No significant differences].
Figure 7: Cell-autonomous Ank2 haploinsufficiency results in postsynaptic excitatory synaptic dysfunction.
(A) Top: Neurons were sparsely transduced by injecting a diluted AAV5-Ef1α-Cre-mCherry virus in mPFC of P30 Ank2+/fl mice. Bottom: Max intensity z-stack of mCherry fluorescence (red) overlaid with scanning DIC image (grayscale) showing Ef1α-Cre-mCherry expression in a subset of Ank2+/fl L5 pyramidal neurons.
(B) mEPSCs from P55-P65 interleaved mCherry-negative (black) or Ank2+/fl-Cre-mCherry-positive (purple) neurons. Data quantified as in Fig. 6C. mEPSC amplitude: mCherry-: −9.5±1.1 pA, n=7 cells, N=3 mice; mCherry+: −7.7±1.4 pA, n=5 cells, N=3 mice; p=0.43. mEPSC frequency: mCherry-: 4.9 ±0.8 Hz, n=7 cells; Ank2+/fl-Cre: 1.7±0.3 Hz, n=5 cells; **p=0.0025.
(C) Paired-pulse ratio from in P52–73 WT (black) and Ank2+/fl-Cre-mCherry (purple) neurons, shown as in Figure 6D. PPR at 25 ms – (WT: 1.8±0.1, n=10 cells, N=5 mice; Ank2+/fl-Cre: 1.9±0.2, n=10 cells, N=3 mice) p > 0.99. At 50 ms – (WT: 1.9±0.1, n=11 cells; Ank2+/fl-Cre: 1.9±0.1, n=11 cells) p=0.7. At 100 ms – (WT: 1.6±0.9, n=10 cells; Ank2+/fl-Cre: 1.6±0.07, n=10 cells) p=0.6.
(D) AMPA:NMDA ratio from P52–73 WT (black) and Ank2+/fl-Cre-mCherry (purple) neurons, as in Figure 6E (WT: 3.3±0.2, n=10 cells, N=5 mice; Ank2+/fl-Cre: 2.5±0.3, n=9 cells, N=3 mice) *p=0.02.
(E) Left: Evoked EPSPs in response to incremental increases in stimulus intensity in P85–93 WT (black) and Ank2+/fl::CaMKIIɑ-Cre mice (green). Right: Input-output curves of EPSP amplitude versus stimulus intensity as a factor of threshold intensity. Circles are means ± SEM. Line is exponential fit. n=9 WT, 8 Ank2+/fl.
Taken together, these experiments suggest that postsynaptic effects of Ank2 heterozygosity impair excitatory transmission, despite an apparent increase in presynaptic release probability. To test this directly, we returned to Ank2+/fl::CaMKII-Cre mice and assessed excitatory transmission across a range of stimulus intensities, from threshold for EPSP generation to 5x that threshold. In WT neurons, increased stimulation intensity increased EPSP amplitude to levels where many cells fired APs. By contrast, a higher stimulus intensity was required to evoke EPSPs in Ank2+/−-Cre neurons (threshold voltage – WT: 4.9±0.3 V, Ank2+/−: 7.8±0.4 V; n=8 cells each), and resultant EPSPs were far smaller (Figure 7E).
What might be a mechanism for these changes in synaptic efficacy? Backpropagating action potentials provide instructive signals to the dendrites that are necessary to maintain synaptic strength, integration, and plasticity. We hypothesized that reductions in bAP efficacy in Ank2 haploinsufficient neurons, due to disruptions in NaV dendritic localization, could impair activity-dependent synaptic plasticity. We tested this by pairing bursts of APs, which backpropagate poorly in Ank2+/− neurons (Figure 5) with synaptic inputs impinging upon the apical tuft of layer 5 pyramidal neurons (Figure 8A, EPSP preceding APs by ~10 ms). In WT conditions, this pairing evoked long-term potentiation (LTP) reliably in mice aged <P25. Therefore, to test this in Ank2+/− neurons, we crossed Ank2+/fl mice to Rbp4-Cre-Ai14 driver lines, as this allows for expression of Cre in layer 5 pyramidal cells during early embryonic development (E13.5)58,59. As in prior studies in Scn2a+/− neurons, LTP could not be induced in Ank2+/− cells (Figure 8B-D). Altogether, these results indicate that ankyrin-B has a critical role in scaffolding of dendritic NaVs and that Ank2 haploinsufficiency phenocopies postsynaptic dendritic deficits observed in Scn2a+/− neurons.
Figure 8: Ank2 haploinsufficiency impairs synaptic plasticity.
(A) Single 2-photon optical section of Ai14-tdTomato fluorescence (red) overlaid with scanning DIC image (grayscale) showing expression of Rbp4-Cre in L5 pyramidal neurons in the mPFC of a P26 Ank2+/fl::Rbp4-Cre-Ai14 mouse. Right: Recording configuration to induce burst-dependent long-term potentiation (LTP) in L5 pyramidal neurons. A stimulating electrode was placed in L1 and synaptic stimulation was paired with bursts of APs evoked by depolarization from the somatic pipette.
(B) EPSPs before (dark) and after (light) the LTP pairing protocol from P17–23 WT (black) and Ank2+/fl::Rbp4-Cre (purple) mice. Lighter shades represent data 20–25 mins after pairing.
(C) EPSP slope (first 2 ms) versus time before and after LTP induction. Circle and bars are mean ± SEM.
(D) Average EPSP slope per cell 20–25 mins post-induction from WT and Ank2+/fl::Rbp4-Cre neurons. (WT: 1.75±0.2, n=6 cells, N=2 mice; Ank2+/fl-Cre: 1.05±0.06, n=8 cells, N=2 mice) **p=0.001.
DISCUSSION:
Ankyrin-B variants have long been known to contribute to cardiac dysfunction through their function in scaffolding a range of membrane pumps, ion exchangers, and receptors60. Here, we provide evidence that ankyrin-B (ANK2) functions as the primary scaffold for NaV1.2 (SCN2A) in the dendrites of neocortical pyramidal neurons. Haploinsufficiency of Ank2 in prefrontal neocortical neurons caused dendritic excitability and synaptic deficits, due to reduced NaV channel density within the dendritic membrane, which phenocopies Scn2a haploinsufficient conditions. These findings establish a direct, convergent mechanism between two major ASD-associated genes and add to a growing body of literature demonstrating dysfunction in dendritic excitability in ASD61–64.
Subcellular patterning of ankyrins
While ion channels and ankyrin scaffold interactions have been studied extensively in axonal domains13,65–68, the mechanisms governing dendritic localization of NaVs have received far less attention. Data here suggest that ankyrin-G and ankyrin-B have largely distinct roles in NaV scaffolding, with ankyrin-G localized to excitable parts of the axon (e.g., AIS and nodes of Ranvier), and ankyrin-B localized to other regions, including dendrites. We show that ankyrin-B is critical for scaffolding NaV1.2 to the dendritic shaft. Consistent with this, knockout of ankyrin-B in cultured neurons eliminates dendritic NaV1.2 immunostaining (Figure 1, S4), and Ank2+/− conditions decrease AP-evoked dendritic Na+ influx by ~50% (Figure 5). Interestingly, ankyrin-G is known to localize within spines, but does so in support of synapse function rather than NaV scaffolding39. Together, this suggests that dendritic sodium channels are scaffolded exclusively by ankyrin-B, and that loss of ankyrin-B cannot be compensated for by other ankyrins.
Intriguingly, one place where ankyrin-B and ankyrin-G overlap, and appear to serve compensatory NaV scaffolding roles, is at the somatic membrane. Here, we observed a disconnect between Ank2+/− and Scn2a+/− conditions, as Ank2+/− neurons did not show a decrease in peak AP velocity common to Scn2a+/− neurons9,26 (Figure 4). This indicates that somatic NaVs can be scaffolded at the soma at WT levels in Ank2+/− conditions, either by ankyrin-G, or by preferential recruitment of extant ankyrin-B to the soma over other compartments. In support of the former, we found that NaV1.2–3xFLAG constructs were still present at the soma in Ank2−/− cultured neurons, despite being absent from dendrites (Figure 4). Methods to label each ankyrin, their splice variants, and NaV subtypes scaffolded to each of these compartments in neurons will be useful to unravel these complexities.
While it appears that both ankyrins can scaffold somatic NaVs, it remains unclear why they fail to compensate for one another outside the soma. One explanation may lie in how ankyrin-G and ankyrin-B are themselves localized to different neuronal compartments. Previous studies have shown that ankyrin-G requires the post-translational modification S-palmitoylation for its membrane association at the AIS69. S-palmitoylation is mediated by a family of 23 palmitoyl acyl transferases (zDHHC PATs) that covalently adds a 16-carbon fatty acid chain to a conserved cysteine 70 (C70) that resides within the ankyrin repeats70. Two of these PATs, zDHHC5 and zDHHC8, are known to palmitoylate ankyrin-G at the AIS69. By contrast, ankyrin-B is palmitoylated at multiple cysteine residues by a distinct PAT, zDHHC17, and this S-palmitoylation is necessary for dendritic scaffolding of NaV1.271.
In addition to differential localization of ankyrin family members, individual splice variants of ankyrin-G and ankyrin-B have unique localization patterns and functions. Alternative splicing of ANK2 gives rise to two main isoforms of ankyrin-B in the brain: a canonical 220 kDa isoform and a larger 440 kDa splice variant, which contains a single 6.4-kb neuron-specific exon within the middle of the gene72–74. Here, we found that the 220 kDa ankyrin-B is expressed throughout dendrites, whereas 440 kDa isoform was dominant in distal axons (Figure 2). Using conditional Ank2 alleles, we removed Ank2 from prefrontal pyramidal cells to isolate cell-autonomous, postsynaptic roles of the 220 kDa isoform, and found that it is capable of scaffolding NaV1.2 through direct interaction. This impaired dendritic excitability and excitatory synaptic function in ways that converged with those observed in Scn2a+/− neurons9. While these effects likely contribute to ASD etiology, ASD-associated variants in ANK2 cannot only affect the 220 kDa ankyrin-B, as the 440 kDa isoform will also be impacted. Most variants in ANK2 fall in this category, resulting in heterozygous expression and haploinsufficiency of both the 220 and 440 kDa isoforms in humans6,8. Nevertheless, three ASD-associated variants have been identified in the neuronal-specific exon in ANK2 that is only found within the 440 kDa isoform38. Here, we found that a modest decrease in paired-pulse ratio was likely attributed to heterozygous loss of axonal Ank2, similar to reports examining a frameshift variant at R2608 that specifically affects the 440 kDa isoform38. How axonal ankyrin-B heterozygosity alters short-term plasticity is unclear, but in the heart, Ank2+/− enhances calcium release from intracellular stores75. Such mechanisms, if engaged at axonal boutons, could affect neurotransmitter release76. Therefore, changes in the 440 kDa ankyrin-B, which is predominantly found within the distal axon, could lead to altered presynaptic neurotransmitter release.
Convergent dendritic dysfunction in ASD
Dysfunction in layer 5 neocortical pyramidal cells has long been implicated in ASD10. These neurons have a unique structure, with a set of basal and apical dendrites that arborize to receive distinct inputs from local and long-range sources, respectively77. Dendritic integration, including supralinear dendritic spikes in the apical tuft are thought to support several computations, including sensory integration, binding of local and long-range input streams, and top-down modulation of cortical processing78–84. Indeed, these aspects of dendritic excitability are critical for detection of sensory stimuli at behavioral threshold85–88 and are some of the first neuronal signals affected with anesthesia-induced loss of consciousness89. Given these major roles in higher-order cortical processing, it is likely that dysfunction in dendritic integration is an important contributor to neurodevelopmental disorders like ASD and intellectual disability (ID). Consistent with this, SCN2A haploinsufficiency, which principally interferes with dendritic excitability, confers substantial risk for ASD4,6–8. We find that Ank2 converges with Scn2a to affect the same dendritic excitability mechanisms. This convergence is direct, as ankyrin-B is the obligate scaffold for dendritic NaV1.2. Emerging evidence indicates that other ASD-associated genes may similarly affect neocortical pyramidal cell dendritic integration, either via regulation of NaVs62, other channels that influence dendritic excitability90–95, or changes in excitation that promote dendritic non-linear events or inhibition that limits such activity96–100. These effects can be overt, with ASD-associated variants directly affecting genes encoding dendritic or synaptic proteins in question, or covert, with ASD-associated variants instead affecting gene regulatory elements that in turn alter protein expression (reviewed in 64).
NaV1.2 is critical for dendritic excitability throughout life. Conditionally induced heterozygosity of Scn2a late in development results in identical impairments in dendritic and synaptic function as observed in constitutive Scn2a heterozygotes9. Excitatory synapses appear similar to those found in immature neurons, with relatively small spine heads and low AMPA:NMDA receptor ratios, suggesting that synapses may be maintained in an immature, pre-critical-period state9. Indeed, restoration of near WT levels of Scn2a, either via Cre-induced genetic rescue or CRISPR activator-based upregulation of the residual, functional allele in Scn2a heterozygotes, restores dendritic excitability, synapse morphology, and synapse function to WT levels43. This suggests that restoration of Ank2 function would have similar benefits, at least in dendritic regions where it actively scaffolds NaV1.2. ANK genes are too large for traditional gene therapy approaches; however, several other approaches are maturing for gene regulation in neurodevelopmental disorders, with marked progress for a number of genetic conditions43,101–106. In addition, a better understanding of the unique roles of different ankyrin proteoforms in scaffolding and function of various binding partners96,98–100 may provide insight into methods that allow ankyrins to better compensate for one another in dendritic compartments. Overall, these data establish a framework that both Scn2a+/− and Ank2+/− models are forms of channelopathies contributing to ASD107, motivating future research on potential convergent impairments in channel and dendritic function associated with other ASD/ID risk genes.
Complex physiology of ankyrin-B dysfunction
Outside the brain, ankyrin-B is most highly expressed in the heart. Here, ANK2 variants are associated with ankyrin-B syndrome, a complex cardiovascular phenotype that can lead to sudden cardiac death52. Currently, it is unclear whether patients with ANK2 missense or protein truncating variants will develop either ASD/ID, cardiovascular disorders, or both, perhaps at different points in development. One of the major challenges to linking ANK2 variants to specific disease phenotypes is that, even within cohorts that share the same variant, cardiac phenotypes can range from asymptomatic to long QT syndrome to sudden cardiac arrest108,109. In addition, in our prior work on genotype-phenotype relationships in SCN2A7,110, we encountered challenges in obtaining full neurological and physiological reports, including seizure status, from clinicians. In ANK2, similar challenges are apparent between brain and cardiac specialists, compounded by the observation that most neuropsychiatric phenotypes have been reported in children whereas most cardiac phenotypes have been reported in adults. Finally, although ankyrin-B clearly plays an important role in both heart and brain, the resultant clinical phenotype of disease-associated variation is likely to depend on which isoforms of ankyrin-B are affected, the nature and severity of the variant (i.e. missense versus protein-truncating), and the specific effects of missense variants on binding to tissue-specific partners. Future clinical and basic science studies exploring the myriad roles of ANK2 will therefore need to consider its complex actions in diverse systems.
STAR METHODS:
Resource availability:
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Paul Jenkins (pjenkins@umich.edu).
Materials availability
Plasmids generated in this study are available from the lead contact upon request.
Data and code availability
Data reported in this paper are available from the lead contact upon reasonable request. This paper does not report original code. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental model details
Mouse strains
All experimental procedures were performed in accordance with UCSF and UM IACUC guidelines. All experiments were performed on mice housed under standard conditions with ad libitum access to food and water, with colonies maintained in-house. C57BL/6J mice were obtained from Jackson Laboratories (stock #000664). The Ank2 exon 24flox/flox mouse line was a gift from Dr. Peter Mohler (The Ohio State University)31. Scn2a+/− mice were provided by Drs. E. Glasscock and M. Montal111,112. Rbp4-Cre-Ai14 (Gensat K100) mice were kindly provided by Dr. John Rubenstein (University of California, San Francisco)58.
Method details
CONSTRUCTS, ANTIBODIES, AND EXPERIMENTAL MODELS:
Human NaV1.2–3xFLAG IRES eGFP was generated by Genscript (Piscataway, NJ) by the addition of a short linker (AAARG) and a triple-FLAG epitope (DYKDHDGDYKDHDIDYKDDDDK) to the carboxyl terminus of codon-optimized human NaV1.2 IRES eGFP7. Δ9 NaV1.2–3xFLAG IRES eGFP was created by Genscript by deletion of the necessary nine amino acid core ankyrin-binding motif, described previously12. The HA-tagged NaV1.2 II-III loops (wild-type and Δ9) were created by cloning the coding sequence corresponding to amino acids 991–1211 of human NaV1.2 into pENTR D-TOPO by polymerase chain reaction. The loops were shuttled into pCSF107mT-GATEWAY-3’−3HA (gift from Todd Stukenberg, Addgene plasmid # 67616) using Gateway LR clonase, according to manufacturer’s directions (Thermo Fisher). NaV β1-V5–2A-DsRed was a generous gift from Dr. Lori Isom (University of Michigan)113. 220 kDa ankyrin-B-GFP was previously described114. 440 kDa ankyrin-B-GFP was created by subcloning the additional giant exon from 440 kDa ankyrin-B-Halo38 into 220 kDa ankyrin-B-GFP using BstZ17I and SacII sites. 220 kDa ankyrin-B F131Q/F164Q33 was created by Genscript by site-directed mutagenesis. TagBFP and Cre-2A-TagBFP were previously described41. All plasmids were sequenced across the entire coding sequence by Sanger sequencing prior to use in experiments.
Lab-generated antibodies to ankyrin-B and ankyrin-G were described previously, including rabbit anti-ankyrin-G C-terminus115, goat anti-ankyrin-G C-terminus116, rabbit anti-270/480 kDa ankyrin-G14, rabbit anti-480kDa ankyrin-G14, rabbit anti-ankyrin-B C-terminus117, sheep anti-ankyrin-B C-terminus. Specificity of all lab-generated antibodies are confirmed using respective null mouse tissue. In addition, antibodies are tested for ankyrin cross-reactivity by both immunocytochemistry and western blotting in HEK293 cells expressing 220 kDa ankyrin-B-GFP or 190 kDa ankyrin-G-GFP. Commercial antibodies used in these studies include rabbit anti-Nav1.2 (Abcam, ab65163), mouse anti-FLAG-M2 (Sigma, F3165), chicken anti-GFP (Abcam, ab13970), mouse anti-HA epitope (clone 6E2, Cell Signaling Technologies), rabbit anti-HA epitope (clone C29F4, Cell Signaling Technologies), and guinea pig anti-MAP2 (Synaptic Systems, 188–004).
CO-IMMUNOPRECIPITATION:
Whole brain was dissected from C57Bl/6J adult mice (P60–75). Each brain was homogenized in 2 ml of reaction buffer (0.3 M sucrose, 10 mM Phosphate, 2 mM EDTA; pH 7.4), mixed with phosphatase inhibitor and protease inhibitor. 500 μL of 20 mM DSP (Lomant’s Reagent) was added to the sample and incubated for 2 hours on ice, after which the crosslinking reaction was quenched by adding 1x Tris to a final concentration of 50 mM and incubated on ice for 15 minutes. Samples were lysed by mixing with lysis buffer (30 mM Tris, 150 mM NaCl, 2 mM EDTA, 1% IGEPAL, 0.5% Sodium Deoxycholate; pH 6.8) and sonicating 20 times at 1-second-long pulses, followed by ultracentrifugation at 100k x g for 30 minutes. Solubilized proteins were then subjected to immunoprecipitation using magnetic beads bound to antibodies (Bio-Rad SureBeads Protein A; rabbit ankyrin-B 1:250, rabbit Nav1.2 1:100, rabbit IgG 1:250). Lysate samples were rotated with the bead mixture overnight at 4°C. Beads were collected the next day, washed 3 times with lysis buffer, and mixed 1:1 with 5x PAGE buffer (5% SDS, 25% sucrose, 50 mM Tris; pH 9, 0,5 mM EDTA) and heated to 68°C for 10 minutes.
WESTERN BLOT:
Samples were separated on a 3.5–17% gradient gel in 1x Tris buffer, pH 7.4 (40 mM Tris, 20 mM NaOAc, and 2mM NaEDTA) with 0.2% SDS. Transfer to nitrocellulose membrane was performed overnight at 300 mA at 4°C in 0.5x Tris buffer with 0.01% SDS. Membranes were blocked with 5% Bovine Serum Albumin (BSA) in TBS at room temperature for 1 hour and incubated in primary antibodies (rabbit ankyrin-B 1:1000; rabbit ankyrin-G 1:1000; rabbit Nav1.2 1:500; mouse ɑ-tubulin 1:1000) diluted in 5% BSA in TBS-T overnight at 4°C. Membranes were washed 3x for 10 minutes with TBS-T and incubated for 1 hr at room temperature with LiCor fluorescent secondaries (1:15,000) in 5% BSA in TBS-T. Membranes were then washed 3x for 10 minutes in TBS-T, 3x for 5 minutes in ddH2O) before being imaged on LiCor Odyssey Clx imager.
PROXIMITY LIGATION ASSAY:
HEK293 cells were obtained from the American Type Culture Collection and maintained in a humidified environment at 37 °C with 5% CO2. Cells were cultured in DMEM (Invitrogen #11995) with 10% fetal bovine serum, 100 units/ml penicillin, and 100 units/ml streptomycin. 100,000 cells were plated onto glass-bottomed dishes (Cellvis) and were allowed to attach for four hours. Cells were transfected with 100 ng of each plasmid (NaV1.2 II-III loop-3xHA and 220 kDa ankyrin-B-GFP) with Lipofectamine 2000, according to the manufacturer’s protocol. After 16 hours, cells were fixed with 4% paraformaldehyde for 15 minutes, permeabilized with 0.1% Triton X-100 for 10 minutes and blocked with 5% bovine serum albumin and 0.2% Tween-20 in PBS for 30 minutes. Cells were incubated with primary antibodies (mouse anti-HA and rabbit anti-ankyrin-B C terminus) in blocking buffer overnight in a humidified chamber. The next day, cells were washed with PBS containing 0.2% Tween-20 (PBS-T) three times for 10 minutes and incubated with anti-mouse minus and anti-rabbit plus PLA probes. Samples were processed for ligation amplification using red fluorescent nucleotides, and mounting, according to the manufacturer’s protocol (Duolink, Sigma-Aldrich).
NEOCORTICAL CULTURES, TRANSFECTIONS, AND IMMUNOFLUORESCENCE:
Primary neocortex was dissected from postnatal day 0 (P0) mice and treated with 0.25% trypsin and 100 μg/ml DNase I in 2 mL HBSS with 10mM HEPES, then triturated gently through a glass pipette with a fire-polished tip. The dissociated neurons were then plated on 35mm MatTek dishes, precoated with poly-D-lysine and laminin, in 0.5mL of Neurobasal-A medium containing 10% FBS, B27 supplement, 2 mM glutamine, and penicillin/streptomycin. On day in vitro 1 (DIV1), the neurons were washed with Neurobasal-A medium and fed with growth media (2.5mL of fresh Neurobasal-A medium containing 1% FBS, B27, glutamine, penicillin/streptomycin, and 2.5 μg/ml AraC. On DIV3, plasmids were introduced into neocortical neurons through lipofectamine 2000-mediated transfection. In one tube, 500 ng of each plasmid was added to 200 μL of Neurobasal-A, and in a second tube, lipofectamine 2000 (3 μL/ 1 μg plasmid) was added to 200 μL of Neurobasal-A. The two tubes were then mixed and incubated at room temperature for 15 min. The neuronal growth media was then removed from the dishes and saved, and transfection media was added to the neurons. Cells were incubated in transfection media for 1 hr at 37°C. The transfection media was aspirated, cells were washed once with warm Neurobasal-A, and growth media was added back to plates. The cells were maintained in culture until 7 DIV or 21 DIV and fixed for immunofluorescence as described below.
Dissociated neocortical neurons were fixed for 15 minutes at room temperature with 4% paraformaldehyde, followed by permeabilization with 0.2% Triton in 1X PBS pH7.4 for 10 minutes at room temperature. They were then blocked with blocking buffer (5% BSA, 0.2% Tween 20 in 1X PBS pH7.4) at room temperature for 30 minutes. Primary antibodies were diluted in blocking buffer and incubated overnight at 4 °C. The next day, cells were washed at room temperature three times for 15 minutes with PBS containing 0.2% Tween 20. Then the cells were incubated with secondary antibodies diluted in blocking buffer for one hour at room temperature. The cells were washed at room temperature three times for 15 minutes with PBS containing 0.2% Tween 20 and then mounted with ProLong Gold antifade reagent before imaging with confocal microscopy as described below.
CONFOCAL MICROSCOPY:
Samples were imaged on a Zeiss LSM 880 with Airyscan using a 63× 1.4 Plan-Apochromat objective and excitation was accomplished using 405-, 488-, 561-, and 633-nm lasers. Each experiment was repeated at least three independent times. Measurements were taken using Fiji software118. Laser power and imaging parameters were kept constant for each immunocytochemistry condition. Line profiles (Figure 4H) were calculated as described previously119.
IN VITRO CELL ELECTROPHYSIOLOGY:
Voltage-clamp recordings were performed at room temperature in standard whole-cell configuration, using Axopatch 700B amplifier and pClamp (version 10, Axon Instruments, FosterCity, CA) and a Digidata 1440A digitizer (Molecular Devices). Sodium current was recorded in the presence of external recording solution containing in mM: 120 NaCl, 4 KCl, 1 MgCl2, 1.5 CaCl2, 10 HEPES, 45 Glucose and 30 Sucrose (pH 7.35 with CsOH; osmolality was 300–305 mOsm). For the β1 subunit co-transfection experiments, the external sodium concentration was reduced to 60mM. Fire-polished patch pipettes obtained from borosilicate glass capillary (WPI) which resistance was between 1.5–3.5 MΩ, were filled with an internal solution containing in mM: 10 NaCl, 105 Cs-Aspartate, 10 CsCl, 10 EGTA, 10 HEPES, (pH 7.2 with H2SO4). To determine sodium current amplitude and voltage dependence of activation, currents were evoked by depolarization for 250 ms to different potentials (from −120 to 30 mV on 5 or 10 mV steps) from holding potential of −80 mV and a hyperpolarizing −120mV, 250 ms pre-pulse. Voltage-dependence of inactivation was determined by applying a 50 ms test pulse of 0 mV after the 250 ms pulses used for voltage dependence of activation. Series resistance was compensated no more than 40%–65% when needed, and leak subtraction was performed by application of a standard P/4 protocol. Signals were low-pass filtered at 10 kHz, and data were sampled at 40 kHz online. Current densities were determined by dividing current amplitude by the cell capacitance (Cm) measured by pClamp software. Normalized conductance and inactivation curves were generated as previously described120.
COMPARTMENTAL MODELING:
A pyramidal cell compartmental model was implemented in the NEURON environment (v7.7) based on the Blue Brain Project thick-tufted layer 5b pyramidal cell (TTPC1) model used in our previous study7,26,77,83. The TTPC1 model was adjusted to include an AIS, and the original Na channels in the TTPC1 model were replaced with NaV1.2 and NaV1.6 channels in compartments with densities as previously shown26. For phase plane comparisons, the first AP was evoked with a stimulus of 500 pA intensity (25 ms duration) in each model configuration. Threshold was defined as the membrane potential when dV/dt exceeds 15 V/s. For AP backpropagation, a single AP was evoked with a 1.2 nA, 8 ms step current applied to the somatic membrane. In model conditions with only NaV1.2 contributing Na conductance in the distal apical dendrite, NaV1.6 was replaced with NaV1.2 after ~30 microns from soma and total NaV1.2 conductance was increased by a factor of 1.9 to match total conductance levels in the mixed NaV1.2/NaV1.6 model (since NaV1.6 voltage dependence is more hyperpolarized). Voltage was recorded from the soma, shaft of the apical dendrite (460 μm from soma), and branch of the apical tuft (975 μm from soma). Conductance densities for sodium channels in different compartments across models were as in Supplemental Table 1.
EX VIVO ELECTROPHYSIOLOGY:
Mice aged P55–75 were anesthetized under isoflurane. Brains were dissected and placed in 4 °C cutting solution consisting of (in mM) 87 NaCl, 25 NaHCO3, 25 glucose, 75 sucrose, 2.5 KCl, 1.25 NaH2PO4, 0.5 CaCl2, and 7 MgCl2 and bubbled with 5% CO2/95% O2. Coronal slices 250 μm-thick were obtained that included the medial prefrontal cortex. Slices were then incubated in a holding chamber with sucrose solution for 30 mins at 33 °C, then placed at room temperature until recording. Recording solution consisted of (in mM): 125 NaCl, 2.5 KCl, 2 CaCl2, 1 MgCl2, 25 NaHCO3, 1.25 NaH2PO4, 25 glucose, bubbled with 5% CO2/95% O2. Osmolarity of the recording solution was adjusted to approximately 310 mOsm. All recordings were performed at 32–34 °C.
Neurons were identified using differential interference contrast (DIC) optics for conventional visually-guided whole-cell recording, or with two-photon-guided imaging of AAV-EF1ɑ-Cre-mCherry fluorescence overlaid on a scanning DIC image of the slice. Patch electrodes were pulled from Schott 8250 glass (3–4 MΩ tip resistance). For current-clamp recordings, patch electrodes were filled with a K-gluconate-based internal solution that contained (in mM): 113 K-Gluconate, 9 HEPES, 4.5 MgCl2, 0.1 EGTA, 14 Tris2-phosphocreatine, 4 Na2-ATP, 0.3 Tris-GTP; 290 mOSM, pH: 7.2–7.25. For Ca2+ imaging, EGTA was replaced with 250 μM Fluo-5F and 20 μM Alexa 594. For voltage-clamp recordings, a CsCl-based internal solution was used that contained (in mM): 110 CsMeSO3, 40 HEPES, 1 KCl, 4 NaCl, 4 Mg-ATP, 10 Na-phosphocreatine, 0.4 Na2-GTP, 5 QX-314, and 0.1 EGTA; ~290 mOsm, pH 7.22.
Electrophysiological recordings were collected with a Multiclamp 700B amplifier (Molecular Devices) and a custom data acquisition program in Igor Pro software (Wavemetrics). Current-clamp recordings of action potential waveform were acquired at 50 kHz and filtered at 20 kHz. Pipette capacitance was compensated by 50% of the fast capacitance measure under gigaohm seal conditions in voltage-clamp prior to establishing a whole-cell configuration, and the bridge was balanced. Voltage-clamp experiments were acquired at 10–20 kHz and filtered at 3–10 kHz. Pipette capacitance was completely compensated, and series resistance was compensated 50%. All data were corrected for measured junction potentials of 12 and 11 mV in K-gluconate and Cs-based internals, respectively. Data inclusion was based on previously established metrics9,121,122, and includes measures for recording stability and cell health [e.g., stable series resistance of <18 MΩ, stable membrane potential (Vm), and input resistance (Rin), with less than 15% change over data collection epochs]. All recordings were made using a quartz electrode holder (Sutter Instrument) to minimize electrode drift within the slice.
All acute slice recordings were made from layer 5b thick-tufted pyramidal tract (PT) neurons in the medial prefrontal cortex. In current-clamp, layer 5b neurons were characterized as those that exhibited a voltage rebound more depolarizing that Vrest in response to a strong hyperpolarizing current (−400 pA, 120 ms) that peaked within 90 ms of current offset and depolarizing (300 ms, 20–300 pA) square current pulses from a holding potential of −80 mV123. AP threshold, AIS dV/dt and peak dV/dt measurements were determined from the first AP evoked by a step current (300 ms duration; 200–300 pA) delivered to the somatic pipette within the first 2 minutes of establishing the whole-cell recording configuration. AP threshold was defined as the Vm when dV/dt first exceeded 15 V/s. AIS peak dV/dt was defined at the saddle point between two positive inflection points in the second voltage derivative that occur during the depolarizing phase of the AP.
Miniature excitatory and inhibitory postsynaptic currents (mEPSCs, mIPSCs) were acquired in voltage-clamp configuration at −80 mV and 0 mV, respectively, in the presence of 10 μM R-CPP and 400 nM TTX. Events were analyzed using a deconvolution-based event detection algorithm within IgorPro124. Detectable events were identified using a noise threshold of 3.5x with a minimum amplitude of 2 pA and a 2 ms inter-event interval. Events were subsequently manually screened to confirm appropriate event detection. Event detection code is available at benderlab.ucsf.edu/resources. Cumulative probability distribution of mEPSCs and mIPSCs event intervals were generated per cell and then averaged. Distributions were compared using the Kolmogorov-Smirnov test. A confidence interval of 95% (P < 0.05) was required for values to be considered statistically significant. In experiments measuring AMPA:NMDA ratio and paired-pulse ratio (PPR), EPSCs were evoked using a bipolar glass theta electrode placed in layer 5b ~200 μm lateral from the recording neuron. AMPA: NMDA ratio was initially measured at −80 mV to assess the AMPA contribution and then at +30 mV to evaluate the NMDA-mediated component in the presence of 25 μM picrotoxin. AMPA was defined as the peak inward current at −80 mV and NMDA as the outward current 50 ms after stimulus onset at +30 mV. PPR was acquired at −80 mV in the presence of 10 μM R-CPP and 25 μM picrotoxin. For Ro 25–6981 (Tocris cat. # 1594) experiments, external aCSF also included 10 μM NBQX and 25 μM picrotoxin to block AMPA-receptors and GABAA-receptors, respectively. Evoked NMDAR-mediated EPSCs were recorded at +30 mV before and 40–45 min after Ro 26–6981 (1 μM) application. EPSC decay was fitted with a double exponential and the normalized weighted time constant was calculated on a cell-by-cell basis.
mEPSCs, mIPSCs, and AMPA:NMDA ratio were collected from Ank2+/fl mice injected with AAV-EF1ɑ-Cre-mCherry and WT littermates. Mice were anesthetized with isofluorane and positioned in a stereotaxic apparatus. 500 nL volumes of AAV-EF1ɑ-Cre-mCherry (UNC vector core) were injected into the mPFC of Ank2+/fl mice (stereotaxic coordinates [mm]: anterior-posterior [AP] +1.7; mediolateral [ML] −0.35; dorsoventral [DV]: −2.6). Experiments were conducted four-week post-injection. Paired-pulse ratio (PPR) was recorded from Ank2+/fl::CaMKIIɑ-Cre mice and WT littermates. For AMPA:NMDA and PPR experiments in spare Cre-expressing animals, AAV-EF1ɑ-Cre-mCherry was diluted 1:3 in saline, then injected into the mPFC of Ank2+/fl mice. To generate synaptic input-output (I/O) curves, EPSPs were isolated with 25 μM picrotoxin and evoked via a glass bipolar electrode placed ~50 μm lateral from somatic recording pipette in layer 5. Threshold was defined as the voltage (200 μs duration) required to evoke an EPSP on at least 8/10 trials (10 sec inter-trial interval) and less than 3/10 trials at 0.9x threshold intensity. Stimulus intensity was then increased in intervals of 1.5x, 2x, 3x, 4x and 5x.
Spike timing-dependent plasticity experiments (STDP) were conducted in Ank2+/fl::Rbp4-Cre-Ai14 mice to induce Ank2 haploinsufficiency specifically in layer 5 cortical projection neurons during early embryonic development (E13.5)58,59. STDP was induced using an established pairing protocol, as in9,125 with EPSPs generated via a stimulating electrode placed 25–50 μm from the layer 1- layer 2/3 border, 300 μm dorsal to a line running perpendicular to the midline pia from the whole-cell pipette. Pairing was induced within 5.5 min of establishing whole-cell recordings.
TWO-PHOTON IMAGING:
Two-photon laser scanning microscopy (2PLSM) was performed as previously described122. A Coherent Ultra II was tuned to 810 nm for calcium imaging, ING-2 sodium imaging, and morphology experiments. Epi- and transfluorescence signals were captured either through a 40x, 0.8 NA objective for calcium imaging or a 60X, 1.0 NA objective for ING-2 imaging paired with a 1.4 NA oil immersion condenser (Olympus). For calcium imaging, fluorescence was split into red and green channels using dichroic mirrors and band-pass filters (575 DCXR, ET525/70 m-2p, ET620/60 m-2p, Chroma). Green fluorescence (Fluo-5F) was captured with 10770–40 photomultiplier tubes selected for high quantum efficiency and low dark counts (PMTs, Hamamatsu). Red fluorescence (Alexa 594) was captured with R9110 PMTs. Data were collected in linescan mode (2–2.4 Δ(G/R)/(G/R)max*100, where (G/R)max was the maximal fluorescence in saturating Ca2+ (2 mM)126. AP backpropagation experiments were performed in 25 μM picrotoxin, 10 μM NBQX and 10μM R-CPP. For ING-2 sodium imaging, the epifluorescence filters were removed and the transfluorescence filters were replaced with a single 535/150 bandpass filter (Semrock) and all fluorescence was collected on HA10770–40 PMTs. For ING2 imaging in the presence of CaV blockers, TTA-P2 (2 μM; Alomone Labs cat. #T-155), Nifedipine (10 μM; Tocris cat. # 1075), and ω-conotoxin-MVIIC (1 μM; Vivitide cat. # PCN-4283-s) were applied to 10 mLs aCSF along with 0.001% bovine serum albumin to minimize peptide pre-absorption. All calcium channel antagonists stock solutions were prepared in glass vials, and glass tubing and a glass syringe were used for recirculation. Dendritic spine morphology and density images were obtained using 2PLSM at 2x the Nyquist resolution limit at 810 nm excitation, with z-stacks through distal apical dendritic tuft branches (0.2 μm steps in the z-axis) through a 60X, 1.0 NA objective. Stacks were processed post-hoc with the ImageJ CANDLE denoising protocol127, then reconstructed using IMARIS v9.9 (Bitplane). Maximum intensity image projections are displayed using the ‘‘Red Hot” lookup table in FIJI. Full neuronal and dendritic reconstructions were stitched together using pairwise stitching in FIJI before generation of maximum intensity projections.
QUANTIFICATION AND STATISTICAL ANALYSIS:
Statistical analysis was performed and presented using Graphpad Prism 9 software. Data were acquired from both sexes and no sex-dependent differences were found. All data were analyzed blind to genotype. Unless otherwise noted, data are presented with box plots (medians and quartiles, with min/max tails) and individual data points overlaid. Data were quantified as mean ± standard error in figure legends and statistical tests were noted. Each data point (n) is indicative of individual neurons. Mean values per cell were compared using the Mann-Whitney test for two groups or Holm-Šídák multiple comparisons test was used to compare three or more groups unless otherwise noted. Results were considered significant at alpha value of p < 0.05. “n.s.” indicates not significant. Group sample sizes were chosen based on standards in the field and prior, similar experiments conducted by our groups.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit anti-ankyrin-G C-terminus | Lab-generated | PMID: 17620337 |
| Goat anti-ankyrin-G C-terminus | Lab-generated | PMID: 25049274 |
| Rabbit anti-270/480 kDa ankyrin-G | Lab-generated | PMID: 25552556 |
| Rabbit anti-480 kDa ankyrin-G | Lab-generated | PMID: 25552556 |
| Rabbit anti-ankyrin-B C-terminus | Lab-generated | PMID: 19109891 |
| Sheep anti-ankyrin-B C-terminus | Lab-generated | N/A |
| Rabbit anti-NaV1.2 | Abcam | ab65163 |
| Mouse anti-FLAG M2 | Sigma | F3165 |
| Chicken anti-GFP | Abcam | ab13970 |
| mouse anti-HA 6E2 | Cell Signaling Technologies | mAb #2367 |
| Rabbit anti-HA | Cell Signaling Technologies | mAb #3724 |
| Guinea pig anti-MAP2 | Synaptic Systems | 188-004 |
| Bacterial and Virus Strains | ||
| One Shot™ Top10 chemically competent E. Coli | Thermo Fisher Scientific | C404010 |
| One Shot™ BL21(DE3)pLysS Chemically Competent E. coli | Thermo Fisher Scientific | C606010 |
| AAV-EF1ɑ-Cre-mCherry | UNC Viral Core | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| NBQX disodium salt | Tocris | 0373 |
| Tetrodotoxin-citrate | Alomone | T-550 |
| (R)-CPP | Tocris | 0247 |
| Picrotoxin | Tocris | 1128 |
| Fluo-5F, Pentapotassium Salt, cell impermeant | Invitrogen | F14221 |
| Alexa Fluor 594 hydrazide | Invitrogen | A10438 |
| Prolong Gold Antifade Reagent | Invitrogen | P36930 |
| ING-2 TMA⁺ Salt | ION Biosciences | 2013C |
| TTA-P2 | Alomone | T155 |
| Nifedipine | Tocris | 1075 |
| ω-conotoxin-MVIIC | Vivitide | PCN-4283-s |
| Ro 25-6981 | Tocris | 1594 |
| Critical Commercial Assays | ||
| Duolink proximity ligation assay | Sigma-Aldrich | DUO92101 |
| Experimental Models: Cell Lines | ||
| HEK293 cells | American Type Culture Collection | CRL-1573 |
| Experimental Models: Organisms/Strains | ||
| Mouse: C57BL/6J | The Jackson Laboratory | JAX Strain #:000664 RRID:IMSR_JAX:000664 |
| Mouse: Ank2 exon 24 flox: B6.129(Cg)-Ank2tm1.1Pmoh/MnrJ | P. Mohler | JAX Strain #031428 |
| Mouse: Scn2a+/− | M. Montal/E. Glasscock | PMID: 10827969, 28334922 |
| Mouse: Rbp4-Cre-Ai14 | J. Rubenstein | PMID: 32294447 |
| Recombinant DNA | ||
| NaV1.2 IRES GFP | Genscript | PMID: 31230762 |
| NaV1.2-3xFLAG IRES GFP | This study | N/A |
| Δ9 NaV1.2-3xFLAG IRES GFP | This study | N/A |
| 220 kDa ankyrin-B-GFP | V. Bennett | PMID: 11781319 |
| 220 kDa ankyrin-B F131Q/F164Q | This study | N/A |
| 440 kDa ankyrin-B-Halo tag | V. Bennett | PMID: 31285321 |
| 440 kDa ankyrin-B-GFP | This study | N/A |
| pENTR-D-TOPO | Invitrogen | K240020 |
| NaV β1-V5-2A-DsRed | L. Isom | PMID: 33411695 |
| Δ9 NaV1.2 II-III loop-3xHA | This study | N/A |
| pCSF107mT-GATEWAY-3’−3HA | Addgene | Addgene plasmid # 67616 |
| TagBFP-N | Evrogen | #FP172 |
| Cre-2A-TagBFP | V. Bennett | PMID: 25552561 |
| Software and Algorithms | ||
| Fiji | http://fiji.sc | RRID:SCR_002285 |
| pClamp | Molecular Devices | RRID:SCR_011323; v10 |
| IGOR Pro | Wavemetrics | RRID:SCR_000325; v6.3 |
| Prism | Graphpad | RRID:SCR_002798; v9 |
| IMARIS | Bitplane | RRID:SCR_007370; v9.9 |
| Pyramidal cell compartment model | NEURON environment (v7.7) based on the Blue Brain Project thick-tufted layer 5b pyramidal cell (TTPC1) model | PMID: 28256214 |
| Postsynaptic current event detection code | benderlab.ucsf.edu/resources | PMID: 23062335 |
Highlights:
Ankyrin-B (Ank2) is expressed throughout neocortical pyramidal cell dendrites
Ankyrin-B scaffolds the sodium channel NaV1.2 (Scn2a) to dendritic membranes
Haploinsufficiency in either Ank2 or Scn2a impairs dendritic excitability
Shared function suggests that dendritic dysfunction contributes to ASD etiology
ACKNOWLEDGEMENTS:
We are grateful to members of the Jenkins and Bender labs and Drs. L. Isom, M. Roberts, and L Ptáček for comments and feedback. We thank Dr. M. Roberts for help with analysis code related to Fig. 4H. This work was supported by the Rackham Predoctoral Fellowship (JMP), Simons Foundation SFARI 675594 (PMJ) and NIH grants T32GM007315 (KJP, CCE), F32MH125536 and K99MH135209 (ADN), R01MH126960 (PMJ, KJB), and R01MH125978 (KJB).
DECLARATION OF INTERESTS:
KJB and SJS receive research funding from BioMarin Pharmaceutical Inc. KJB is on the SAB for Regel Tx and receives research support from Regel Tx.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- 1.Iossifov I, O’Roak BJ, Sanders SJ, Ronemus M, Krumm N, Levy D, Stessman HA, Witherspoon KT, Vives L, Patterson KE, et al. (2014). The contribution of de novo coding mutations to autism spectrum disorder. Nature 515, 216–221. 10.1038/nature13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neale BM, Kou Y, Liu L, Ma’ayan A, Samocha KE, Sabo A, Lin CF, Stevens C, Wang LS, Makarov V, et al. (2012). Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature 485, 242–245. 10.1038/nature11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Roak BJ, Vives L, Fu W, Egertson JD, Stanaway IB, Phelps IG, Carvill G, Kumar A, Lee C, Ankenman K, et al. (2012). Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science 338, 1619–1622. 10.1126/science.1227764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanders SJ, Murtha MT, Gupta AR, Murdoch JD, Raubeson MJ, Willsey AJ, Ercan-Sencicek AG, DiLullo NM, Parikshak NN, Stein JL, et al. (2012). De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature 485, 237–241. 10.1038/nature10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanders SJ, He X, Willsey AJ, Ercan-Sencicek AG, Samocha KE, Cicek AE, Murtha MT, Bal VH, Bishop SL, Dong S, et al. (2015). Insights into Autism Spectrum Disorder Genomic Architecture and Biology from 71 Risk Loci. Neuron 87, 1215–1233. 10.1016/j.neuron.2015.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Satterstrom FK, Kosmicki JA, Wang J, Breen MS, De Rubeis S, An JY, Peng M, Collins R, Grove J, Klei L, et al. (2020). Large-Scale Exome Sequencing Study Implicates Both Developmental and Functional Changes in the Neurobiology of Autism. Cell 180, 568–584.e523. 10.1016/j.cell.2019.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben-Shalom R, Keeshen CM, Berrios KN, An JY, Sanders SJ, and Bender KJ (2017). Opposing Effects on Na(V)1.2 Function Underlie Differences Between SCN2A Variants Observed in Individuals With Autism Spectrum Disorder or Infantile Seizures. Biol Psychiatry 82, 224–232. 10.1016/j.biopsych.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu JM, Satterstrom FK, Peng M, Brand H, Collins RL, Dong S, Wamsley B, Klei L, Wang L, Hao SP, et al. (2022). Rare coding variation provides insight into the genetic architecture and phenotypic context of autism. Nat Genet 54, 1320–1331. 10.1038/s41588-022-01104-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spratt PWE, Ben-Shalom R, Keeshen CM, Burke KJ Jr., Clarkson RL, Sanders SJ, and Bender KJ (2019). The Autism-Associated Gene Scn2a Contributes to Dendritic Excitability and Synaptic Function in the Prefrontal Cortex. Neuron 103, 673–685.e675. 10.1016/j.neuron.2019.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willsey AJ, Sanders SJ, Li M, Dong S, Tebbenkamp AT, Muhle RA, Reilly SK, Lin L, Fertuzinhos S, Miller JA, et al. (2013). Coexpression networks implicate human midfetal deep cortical projection neurons in the pathogenesis of autism. Cell 155, 997–1007. 10.1016/j.cell.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett V, and Lorenzo DN (2016). An Adaptable Spectrin/Ankyrin-Based Mechanism for Long-Range Organization of Plasma Membranes in Vertebrate Tissues. Curr Top Membr 77, 143–184. 10.1016/bs.ctm.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Lemaillet G, Walker B, and Lambert S. (2003). Identification of a conserved ankyrin-binding motif in the family of sodium channel alpha subunits. J Biol Chem 278, 27333–27339. 10.1074/jbc.M303327200. [DOI] [PubMed] [Google Scholar]
- 13.Nelson AD, and Jenkins PM (2017). Axonal Membranes and Their Domains: Assembly and Function of the Axon Initial Segment and Node of Ranvier. Front Cell Neurosci 11, 136. 10.3389/fncel.2017.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jenkins PM, Kim N, Jones SL, Tseng WC, Svitkina TM, Yin HH, and Bennett V. (2015). Giant ankyrin-G: a critical innovation in vertebrate evolution of fast and integrated neuronal signaling. Proc Natl Acad Sci U S A 112, 957–964. 10.1073/pnas.1416544112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jenkins SM, and Bennett V. (2001). Ankyrin-G coordinates assembly of the spectrin-based membrane skeleton, voltage-gated sodium channels, and L1 CAMs at Purkinje neuron initial segments. J Cell Biol 155, 739–746. 10.1083/jcb.200109026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan Z, Kao T, Horvath Z, Lemos J, Sul JY, Cranstoun SD, Bennett V, Scherer SS, and Cooper EC (2006). A common ankyrin-G-based mechanism retains KCNQ and NaV channels at electrically active domains of the axon. J Neurosci 26, 2599–2613. 10.1523/jneurosci.4314-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou D, Lambert S, Malen PL, Carpenter S, Boland LM, and Bennett V. (1998). AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J Cell Biol 143, 1295–1304. 10.1083/jcb.143.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorenzo DN, Badea A, Davis J, Hostettler J, He J, Zhong G, Zhuang X, and Bennett V. (2014). A PIK3C3-ankyrin-B-dynactin pathway promotes axonal growth and multiorganelle transport. The Journal of cell biology 207, 735–752. 10.1083/jcb.201407063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inda MC, DeFelipe J, and Muñoz A. (2006). Voltage-gated ion channels in the axon initial segment of human cortical pyramidal cells and their relationship with chandelier cells. Proc Natl Acad Sci U S A 103, 2920–2925. 10.1073/pnas.0511197103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boiko T, Van Wart A, Caldwell JH, Levinson SR, Trimmer JS, and Matthews G. (2003). Functional specialization of the axon initial segment by isoform-specific sodium channel targeting. J Neurosci 23, 2306–2313. 10.1523/jneurosci.23-06-02306.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gazina EV, Leaw BT, Richards KL, Wimmer VC, Kim TH, Aumann TD, Featherby TJ, Churilov L, Hammond VE, Reid CA, and Petrou S. (2015). ‘Neonatal’ Nav1.2 reduces neuronal excitability and affects seizure susceptibility and behaviour. Hum Mol Genet 24, 1457–1468. 10.1093/hmg/ddu562. [DOI] [PubMed] [Google Scholar]
- 22.O’Brien JE, and Meisler MH (2013). Sodium channel SCN8A (Nav1.6): properties and de novo mutations in epileptic encephalopathy and intellectual disability. Front Genet 4, 213. 10.3389/fgene.2013.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Wart A, Trimmer JS, and Matthews G. (2007). Polarized distribution of ion channels within microdomains of the axon initial segment. J Comp Neurol 500, 339–352. 10.1002/cne.21173. [DOI] [PubMed] [Google Scholar]
- 24.Hu W, Tian C, Li T, Yang M, Hou H, and Shu Y. (2009). Distinct contributions of Na(v)1.6 and Na(v)1.2 in action potential initiation and backpropagation. Nat Neurosci 12, 996–1002. 10.1038/nn.2359. [DOI] [PubMed] [Google Scholar]
- 25.Lorincz A, and Nusser Z. (2010). Molecular identity of dendritic voltage-gated sodium channels. Science 328, 906–909. 10.1126/science.1187958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spratt PWE, Alexander RPD, Ben-Shalom R, Sahagun A, Kyoung H, Keeshen CM, Sanders SJ, and Bender KJ (2021). Paradoxical hyperexcitability from Na(V)1.2 sodium channel loss in neocortical pyramidal cells. Cell Rep 36, 109483. 10.1016/j.celrep.2021.109483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Chen X, Eaton M, Wu J, Ma Z, Lai S, Park A, Ahmad TS, Que Z, Lee JH, et al. (2021). Severe deficiency of the voltage-gated sodium channel Na(V)1.2 elevates neuronal excitability in adult mice. Cell Rep 36, 109495. 10.1016/j.celrep.2021.109495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boiko T, Rasband MN, Levinson SR, Caldwell JH, Mandel G, Trimmer JS, and Matthews G. (2001). Compact myelin dictates the differential targeting of two sodium channel isoforms in the same axon. Neuron 30, 91–104. 10.1016/s0896-6273(01)00265-3. [DOI] [PubMed] [Google Scholar]
- 29.Liu H, Wang HG, Pitt GS, and Liu ZJ (2022). Direct Observation of Compartment-Specific Localization and Dynamics of Voltage-Gated Sodium Channels. J Neurosci 42, 5482–5498. 10.1523/jneurosci.0086-22.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamano R, Miyazaki H, and Nukina N. (2022). The diffuse distribution of Nav1.2 on mid-axonal regions is a marker for unmyelinated fibers in the central nervous system. Neurosci Res 177, 145–150. 10.1016/j.neures.2021.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Roberts JD, Murphy NP, Hamilton RM, Lubbers ER, James CA, Kline CF, Gollob MH, Krahn AD, Sturm AC, Musa H, et al. (2019). Ankyrin-B dysfunction predisposes to arrhythmogenic cardiomyopathy and is amenable to therapy. J Clin Invest 129, 3171–3184. 10.1172/jci125538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cai X, and Zhang Y. (2006). Molecular evolution of the ankyrin gene family. Mol Biol Evol 23, 550–558. 10.1093/molbev/msj056. [DOI] [PubMed] [Google Scholar]
- 33.Wang C, Wei Z, Chen K, Ye F, Yu C, Bennett V, and Zhang M. (2014). Structural basis of diverse membrane target recognitions by ankyrins. Elife 3. 10.7554/eLife.04353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao Y, Hisey E, Bradshaw TWA, Erata E, Brown WE, Courtland JL, Uezu A, Xiang Y, Diao Y, and Soderling SH (2019). Plug-and-Play Protein Modification Using Homology-Independent Universal Genome Engineering. Neuron 103, 583–597.e588. 10.1016/j.neuron.2019.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu HC, Yamankurt G, Luo J, Subramaniam J, Hashmi SS, Hu H, and Cunha SR (2015). Identification and characterization of two ankyrin-B isoforms in mammalian heart. Cardiovasc Res 107, 466–477. 10.1093/cvr/cvv184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cunha SR, Le Scouarnec S, Schott JJ, and Mohler PJ (2008). Exon organization and novel alternative splicing of the human ANK2 gene: implications for cardiac function and human cardiac disease. J Mol Cell Cardiol 45, 724–734. 10.1016/j.yjmcc.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Creighton BA, Afriyie S, Ajit D, Casingal CR, Voos KM, Reger J, Burch AM, Dyne E, Bay J, Huang JK, et al. (2021). Giant ankyrin-B mediates transduction of axon guidance and collateral branch pruning factor sema 3A. Elife 10.10.7554/eLife.69815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang R, Walder-Christensen KK, Kim N, Wu D, Lorenzo DN, Badea A, Jiang YH, Yin HH, Wetsel WC, and Bennett V. (2019). ANK2 autism mutation targeting giant ankyrin-B promotes axon branching and ectopic connectivity. Proc Natl Acad Sci U S A 116, 15262–15271. 10.1073/pnas.1904348116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith KR, Kopeikina KJ, Fawcett-Patel JM, Leaderbrand K, Gao R, Schürmann B, Myczek K, Radulovic J, Swanson GT, and Penzes P. (2014). Psychiatric risk factor ANK3/ankyrin-G nanodomains regulate the structure and function of glutamatergic synapses. Neuron 84, 399–415. 10.1016/j.neuron.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garrido JJ, Giraud P, Carlier E, Fernandes F, Moussif A, Fache MP, Debanne D, and Dargent B. (2003). A targeting motif involved in sodium channel clustering at the axonal initial segment. Science 300, 2091–2094. 10.1126/science.1085167. [DOI] [PubMed] [Google Scholar]
- 41.Tseng WC, Jenkins PM, Tanaka M, Mooney R, and Bennett V. (2015). Giant ankyrin-G stabilizes somatodendritic GABAergic synapses through opposing endocytosis of GABAA receptors. Proc Natl Acad Sci U S A 112, 1214–1219. 10.1073/pnas.1417989112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nelson AD, Caballero-Florán RN, Rodríguez Díaz JC, Hull JM, Yuan Y, Li J, Chen K, Walder KK, Lopez-Santiago LF, Bennett V, et al. (2020). Ankyrin-G regulates forebrain connectivity and network synchronization via interaction with GABARAP. Mol Psychiatry 25, 2800–2817. 10.1038/s41380-018-0308-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tamura S, Nelson AD, Spratt PWE, Kyoung H, Zhou X, Li Z, Zhao J, Holden SS, Sahagun A, Keeshen CM, et al. (2022). CRISPR activation rescues abnormalities in SCN2A haploinsufficiency-associated autism spectrum disorder. bioRxiv, 2022.2003.2030.486483. 10.1101/2022.03.30.486483. [DOI] [Google Scholar]
- 44.Xu B, Zang K, Ruff NL, Zhang YA, McConnell SK, Stryker MP, and Reichardt LF (2000). Cortical degeneration in the absence of neurotrophin signaling: dendritic retraction and neuronal loss after removal of the receptor TrkB. Neuron 26, 233–245. 10.1016/s0896-6273(00)81153-8. [DOI] [PubMed] [Google Scholar]
- 45.Blömer LA, Canepari M, and Filipis L. (2021). Ultrafast Sodium Imaging of the Axon Initial Segment of Neurons in Mouse Brain Slices. Curr Protoc 1, e64. 10.1002/cpz1.64. [DOI] [PubMed] [Google Scholar]
- 46.Filipis L, and Canepari M. (2021). Optical measurement of physiological sodium currents in the axon initial segment. J Physiol 599, 49–66. 10.1113/jp280554. [DOI] [PubMed] [Google Scholar]
- 47.Lipkin AM, Cunniff MM, Spratt PWE, Lemke SM, and Bender KJ (2021). Functional Microstructure of Ca(V)-Mediated Calcium Signaling in the Axon Initial Segment. J Neurosci 41, 3764–3776. 10.1523/jneurosci.2843-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi CSW, Souza IA, Sanchez-Arias JC, Zamponi GW, Arbour LT, and Swayne LA (2019). Ankyrin B and Ankyrin B variants differentially modulate intracellular and surface Cav2.1 levels. Mol Brain 12, 75. 10.1186/s13041-019-0494-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garcia-Caballero A, Zhang FX, Hodgkinson V, Huang J, Chen L, Souza IA, Cain S, Kass J, Alles S, Snutch TP, and Zamponi GW (2018). T-type calcium channels functionally interact with spectrin (α/β) and ankyrin B. Mol Brain 11, 24. 10.1186/s13041-018-0368-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kline CF, Scott J, Curran J, Hund TJ, and Mohler PJ (2014). Ankyrin-B regulates Cav2.1 and Cav2.2 channel expression and targeting. J Biol Chem 289, 5285–5295. 10.1074/jbc.M113.523639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chalifoux JR, and Carter AG (2011). GABAB receptor modulation of voltage-sensitive calcium channels in spines and dendrites. J Neurosci 31, 4221–4232. 10.1523/jneurosci.4561-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koenig SN, and Mohler PJ (2017). The evolving role of ankyrin-B in cardiovascular disease. Heart Rhythm 14, 1884–1889. 10.1016/j.hrthm.2017.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gulledge AT, and Stuart GJ (2003). Action potential initiation and propagation in layer 5 pyramidal neurons of the rat prefrontal cortex: absence of dopamine modulation. J Neurosci 23, 11363–11372. 10.1523/jneurosci.23-36-11363.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Short SM, Oikonomou KD, Zhou WL, Acker CD, Popovic MA, Zecevic D, and Antic SD (2017). The stochastic nature of action potential backpropagation in apical tuft dendrites. J Neurophysiol 118, 1394–1414. 10.1152/jn.00800.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stuart GJ, and Häusser M. (2001). Dendritic coincidence detection of EPSPs and action potentials. Nat Neurosci 4, 63–71. 10.1038/82910. [DOI] [PubMed] [Google Scholar]
- 56.Larkum ME, Kaiser KM, and Sakmann B. (1999). Calcium electrogenesis in distal apical dendrites of layer 5 pyramidal cells at a critical frequency of back-propagating action potentials. Proc Natl Acad Sci U S A 96, 14600–14604. 10.1073/pnas.96.25.14600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.France G, Fernández-Fernández D, Burnell ES, Irvine MW, Monaghan DT, Jane DE, Bortolotto ZA, Collingridge GL, and Volianskis A. (2017). Multiple roles of GluN2B-containing NMDA receptors in synaptic plasticity in juvenile hippocampus. Neuropharmacology 112, 76–83. 10.1016/j.neuropharm.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fazel Darbandi S, Nelson AD, Pai EL, Bender KJ, and Rubenstein JLR (2022). LiCl treatment leads to long-term restoration of spine maturation and synaptogenesis in adult Tbr1 mutants. J Neurodev Disord 14, 11. 10.1186/s11689-022-09421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Munz M, Bharioke A, Kosche G, Moreno-Juan V, Brignall A, Rodrigues TM, Graff-Meyer A, Ulmer T, Haeuselmann S, Pavlinic D, et al. (2023). Pyramidal neurons form active, transient, multilayered circuits perturbed by autism-associated mutations at the inception of neocortex. Cell 186, 1930–1949.e1931. 10.1016/j.cell.2023.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mohler PJ, Schott JJ, Gramolini AO, Dilly KW, Guatimosim S, duBell WH, Song LS, Haurogné K, Kyndt F, Ali ME, et al. (2003). Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature 421, 634–639. 10.1038/nature01335. [DOI] [PubMed] [Google Scholar]
- 61.Brager DH, and Johnston D. (2014). Channelopathies and dendritic dysfunction in fragile X syndrome. Brain Res Bull 103, 11–17. 10.1016/j.brainresbull.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brandalise F, Kalmbach BE, Cook EP, and Brager DH (2023). Impaired dendritic spike generation in the Fragile X prefrontal cortex is due to loss of dendritic sodium channels. J Physiol 601, 831–845. 10.1113/jp283311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johnston D, Frick A, and Poolos NP (2016). Dendrites and disease. In Dendrites G. Stuart, Spruston N, and Häusser M, eds. (Oxford University Press; ), pp. 0. 10.1093/acprof:oso/9780198745273.003.0024. [DOI] [Google Scholar]
- 64.Nelson AD, and Bender KJ (2021). Dendritic Integration Dysfunction in Neurodevelopmental Disorders. Dev Neurosci 43, 201–221. 10.1159/000516657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bender KJ, and Trussell LO (2012). The physiology of the axon initial segment. Annu Rev Neurosci 35, 249–265. 10.1146/annurev-neuro-062111-150339. [DOI] [PubMed] [Google Scholar]
- 66.Huang CY, and Rasband MN (2018). Axon initial segments: structure, function, and disease. Ann N Y Acad Sci 1420, 46–61. 10.1111/nyas.13718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kole MH, and Stuart GJ (2012). Signal processing in the axon initial segment. Neuron 73, 235–247. 10.1016/j.neuron.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 68.Leterrier C. (2018). The Axon Initial Segment: An Updated Viewpoint. J Neurosci 38, 2135–2145. 10.1523/jneurosci.1922-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.He M, Jenkins P, and Bennett V. (2012). Cysteine 70 of ankyrin-G is S-palmitoylated and is required for function of ankyrin-G in membrane domain assembly. J Biol Chem 287, 43995–44005. 10.1074/jbc.M112.417501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Globa AK, and Bamji SX (2017). Protein palmitoylation in the development and plasticity of neuronal connections. Curr Opin Neurobiol 45, 210–220. 10.1016/j.conb.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 71.Gupta JP, and Jenkins PM (2023). Ankyrin-B is lipid-modified by S-palmitoylation to promote dendritic membrane scaffolding of voltage-gated sodium channel Na(V)1.2 in neurons. Front Physiol 14, 959660. 10.3389/fphys.2023.959660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chan W, Kordeli E, and Bennett V. (1993). 440-kD ankyrinB: structure of the major developmentally regulated domain and selective localization in unmyelinated axons. J Cell Biol 123, 1463–1473. 10.1083/jcb.123.6.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kunimoto M. (1995). A neuron-specific isoform of brain ankyrin, 440-kD ankyrinB, is targeted to the axons of rat cerebellar neurons. J Cell Biol 131, 1821–1829. 10.1083/jcb.131.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kunimoto M, Otto E, and Bennett V. (1991). A new 440-kD isoform is the major ankyrin in neonatal rat brain. J Cell Biol 115, 1319–1331. 10.1083/jcb.115.5.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Popescu I, Galice S, Mohler PJ, and Despa S. (2016). Elevated local [Ca2+] and CaMKII promote spontaneous Ca2+ release in ankyrin-B-deficient hearts. Cardiovasc Res 111, 287–294. 10.1093/cvr/cvw093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Galante M, and Marty A. (2003). Presynaptic ryanodine-sensitive calcium stores contribute to evoked neurotransmitter release at the basket cell-Purkinje cell synapse. J Neurosci 23, 11229–11234. 10.1523/jneurosci.23-35-11229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ramaswamy S, and Markram H. (2015). Anatomy and physiology of the thick-tufted layer 5 pyramidal neuron. Front Cell Neurosci 9, 233. 10.3389/fncel.2015.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aru J, Suzuki M, and Larkum ME (2020). Cellular Mechanisms of Conscious Processing. Trends Cogn Sci 24, 814–825. 10.1016/j.tics.2020.07.006. [DOI] [PubMed] [Google Scholar]
- 79.Branco T, Clark BA, and Häusser M. (2010). Dendritic discrimination of temporal input sequences in cortical neurons. Science 329, 1671–1675. 10.1126/science.1189664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Branco T, and Häusser M. (2010). The single dendritic branch as a fundamental functional unit in the nervous system. Curr Opin Neurobiol 20, 494–502. 10.1016/j.conb.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 81.Gidon A, Zolnik TA, Fidzinski P, Bolduan F, Papoutsi A, Poirazi P, Holtkamp M, Vida I, and Larkum ME (2020). Dendritic action potentials and computation in human layer 2/3 cortical neurons. Science 367, 83–87. 10.1126/science.aax6239. [DOI] [PubMed] [Google Scholar]
- 82.Major G, Larkum ME, and Schiller J. (2013). Active properties of neocortical pyramidal neuron dendrites. Annu Rev Neurosci 36, 1–24. 10.1146/annurev-neuro-062111-150343. [DOI] [PubMed] [Google Scholar]
- 83.Markram H, Muller E, Ramaswamy S, Reimann MW, Abdellah M, Sanchez CA, Ailamaki A, Alonso-Nanclares L, Antille N, Arsever S, et al. (2015). Reconstruction and Simulation of Neocortical Microcircuitry. Cell 163, 456–492. 10.1016/j.cell.2015.09.029. [DOI] [PubMed] [Google Scholar]
- 84.Smith SL, Smith IT, Branco T, and Häusser M. (2013). Dendritic spikes enhance stimulus selectivity in cortical neurons in vivo. Nature 503, 115–120. 10.1038/nature12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Murayama M, Pérez-Garci E, Nevian T, Bock T, Senn W, and Larkum ME (2009). Dendritic encoding of sensory stimuli controlled by deep cortical interneurons. Nature 457, 1137–1141. 10.1038/nature07663. [DOI] [PubMed] [Google Scholar]
- 86.Takahashi N, Ebner C, Sigl-Glöckner J, Moberg S, Nierwetberg S, and Larkum ME (2020). Active dendritic currents gate descending cortical outputs in perception. Nat Neurosci 23, 1277–1285. 10.1038/s41593-020-0677-8. [DOI] [PubMed] [Google Scholar]
- 87.Takahashi N, Oertner TG, Hegemann P, and Larkum ME (2016). Active cortical dendrites modulate perception. Science 354, 1587–1590. 10.1126/science.aah6066. [DOI] [PubMed] [Google Scholar]
- 88.Xu NL, Harnett MT, Williams SR, Huber D, O’Connor DH, Svoboda K, and Magee JC (2012). Nonlinear dendritic integration of sensory and motor input during an active sensing task. Nature 492, 247–251. 10.1038/nature11601. [DOI] [PubMed] [Google Scholar]
- 89.Suzuki M, and Larkum ME (2020). General Anesthesia Decouples Cortical Pyramidal Neurons. Cell 180, 666–676.e613. 10.1016/j.cell.2020.01.024. [DOI] [PubMed] [Google Scholar]
- 90.Bock T, and Stuart GJ (2016). The Impact of BK Channels on Cellular Excitability Depends on their Subcellular Location. Front Cell Neurosci 10, 206. 10.3389/fncel.2016.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gonzalez KC, Losonczy A, and Negrean A. (2022). Dendritic Excitability and Synaptic Plasticity In Vitro and In Vivo. Neuroscience 489, 165–175. 10.1016/j.neuroscience.2021.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Harnett MT, Magee JC, and Williams SR (2015). Distribution and function of HCN channels in the apical dendritic tuft of neocortical pyramidal neurons. J Neurosci 35, 1024–1037. 10.1523/jneurosci.2813-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Harnett MT, Xu NL, Magee JC, and Williams SR (2013). Potassium channels control the interaction between active dendritic integration compartments in layer 5 cortical pyramidal neurons. Neuron 79, 516–529. 10.1016/j.neuron.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Magee JC, and Johnston D. (1995). Characterization of single voltage-gated Na+ and Ca2+ channels in apical dendrites of rat CA1 pyramidal neurons. J Physiol 487, 67–90. 10.1113/jphysiol.1995.sp020862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shah MM (2014). Cortical HCN channels: function, trafficking and plasticity. J Physiol 592, 2711–2719. 10.1113/jphysiol.2013.270058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gidon A, and Segev I. (2012). Principles governing the operation of synaptic inhibition in dendrites. Neuron 75, 330–341. 10.1016/j.neuron.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 97.Megías M, Emri Z, Freund TF, and Gulyás AI (2001). Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience 102, 527–540. 10.1016/s0306-4522(00)00496-6. [DOI] [PubMed] [Google Scholar]
- 98.Ujfalussy BB, Makara JK, Branco T, and Lengyel M. (2015). Dendritic nonlinearities are tuned for efficient spike-based computations in cortical circuits. Elife 4. 10.7554/eLife.10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wilson NR, Runyan CA, Wang FL, and Sur M. (2012). Division and subtraction by distinct cortical inhibitory networks in vivo. Nature 488, 343–348. 10.1038/nature11347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang D, Li Y, Rasch MJ, and Wu S. (2013). Nonlinear multiplicative dendritic integration in neuron and network models. Front Comput Neurosci 7, 56. 10.3389/fncom.2013.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Colasante G, Lignani G, Brusco S, Di Berardino C, Carpenter J, Giannelli S, Valassina N, Bido S, Ricci R, Castoldi V, et al. (2020). dCas9-Based Scn1a Gene Activation Restores Inhibitory Interneuron Excitability and Attenuates Seizures in Dravet Syndrome Mice. Mol Ther 28, 235–253. 10.1016/j.ymthe.2019.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Derbis M, Kul E, Niewiadomska D, Sekrecki M, Piasecka A, Taylor K, Hukema RK, Stork O, and Sobczak K. (2021). Short antisense oligonucleotides alleviate the pleiotropic toxicity of RNA harboring expanded CGG repeats. Nat Commun 12, 1265. 10.1038/s41467-021-21021-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Han Z, Chen C, Christiansen A, Ji S, Lin Q, Anumonwo C, Liu C, Leiser SC, Meena Aznarez, I., et al. (2020). Antisense oligonucleotides increase Scn1a expression and reduce seizures and SUDEP incidence in a mouse model of Dravet syndrome. Sci Transl Med 12. 10.1126/scitranslmed.aaz6100. [DOI] [PubMed] [Google Scholar]
- 104.Ure K, Lu H, Wang W, Ito-Ishida A, Wu Z, He LJ, Sztainberg Y, Chen W, Tang J, and Zoghbi HY (2016). Restoration of Mecp2 expression in GABAergic neurons is sufficient to rescue multiple disease features in a mouse model of Rett syndrome. Elife 5. 10.7554/eLife.14198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Weuring W, Geerligs J, and Koeleman BPC (2021). Gene Therapies for Monogenic Autism Spectrum Disorders. Genes (Basel) 12. 10.3390/genes12111667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wolter JM, Mao H, Fragola G, Simon JM, Krantz JL, Bazick HO, Oztemiz B, Stein JL, and Zylka MJ (2020). Cas9 gene therapy for Angelman syndrome traps Ube3a-ATS long non-coding RNA. Nature 587, 281–284. 10.1038/s41586-020-2835-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ptáček LJ (2015). Episodic disorders: channelopathies and beyond. Annu Rev Physiol 77, 475–479. 10.1146/annurev-physiol-021014-071922. [DOI] [PubMed] [Google Scholar]
- 108.Mohler PJ, Splawski I, Napolitano C, Bottelli G, Sharpe L, Timothy K, Priori SG, Keating MT, and Bennett V. (2004). A cardiac arrhythmia syndrome caused by loss of ankyrin-B function. Proc Natl Acad Sci U S A 101, 9137–9142. 10.1073/pnas.0402546101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Swayne LA, Murphy NP, Asuri S, Chen L, Xu X, McIntosh S, Wang C, Lancione PJ, Roberts JD, Kerr C, et al. (2017). Novel Variant in the ANK2 Membrane-Binding Domain Is Associated With Ankyrin-B Syndrome and Structural Heart Disease in a First Nations Population With a High Rate of Long QT Syndrome. Circ Cardiovasc Genet 10. 10.1161/circgenetics.116.001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sanders SJ, Campbell AJ, Cottrell JR, Moller RS, Wagner FF, Auldridge AL, Bernier RA, Catterall WA, Chung WK, Empfield JR, et al. (2018). Progress in Understanding and Treating SCN2A-Mediated Disorders. Trends Neurosci 41, 442–456. 10.1016/j.tins.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mishra V, Karumuri BK, Gautier NM, Liu R, Hutson TN, Vanhoof-Villalba SL, Vlachos I, Iasemidis L, and Glasscock E. (2017). Scn2a deletion improves survival and brain-heart dynamics in the Kcna1-null mouse model of sudden unexpected death in epilepsy (SUDEP). Hum Mol Genet 26, 2091–2103. 10.1093/hmg/ddx104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Planells-Cases R, Caprini M, Zhang J, Rockenstein EM, Rivera RR, Murre C, Masliah E, and Montal M. (2000). Neuronal death and perinatal lethality in voltage-gated sodium channel alpha-(II)deficient mice. Biophys J 78, 2878–2891. 10.1016/s0006-3495(00)76829-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bouza AA, Edokobi N, Hodges SL, Pinsky AM, Offord J, Piao L, Zhao YT, Lopatin AN, Lopez-Santiago LF, and Isom LL (2021). Sodium channel β1 subunits participate in regulated intramembrane proteolysis-excitation coupling. JCI Insight 6. 10.1172/jci.insight.141776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mohler PJ, Gramolini AO, and Bennett V. (2002). The ankyrin-B C-terminal domain determines activity of ankyrin-B/G chimeras in rescue of abnormal inositol 1,4,5-trisphosphate and ryanodine receptor distribution in ankyrin-B (−/−) neonatal cardiomyocytes. J Biol Chem 277, 10599–10607. 10.1074/jbc.M110958200. [DOI] [PubMed] [Google Scholar]
- 115.Kizhatil K, Davis JQ, Davis L, Hoffman J, Hogan BL, and Bennett V. (2007). Ankyrin-G is a molecular partner of E-cadherin in epithelial cells and early embryos. J Biol Chem 282, 26552–26561. 10.1074/jbc.M703158200. [DOI] [PubMed] [Google Scholar]
- 116.He M, Abdi KM, and Bennett V. (2014). Ankyrin-G palmitoylation and βII-spectrin binding to phosphoinositide lipids drive lateral membrane assembly. J Cell Biol 206, 273–288. 10.1083/jcb.201401016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ayalon G, Davis JQ, Scotland PB, and Bennett V. (2008). An ankyrin-based mechanism for functional organization of dystrophin and dystroglycan. Cell 135, 1189–1200. 10.1016/j.cell.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 118.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat Methods 9, 676–682. 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schamiloglu S, Lewis E, Keeshen CM, Hergarden AC, Bender KJ, and Whistler JL (2023). Arrestin-3 Agonism at Dopamine D(3) Receptors Defines a Subclass of Second-Generation Antipsychotics That Promotes Drug Tolerance. Biol Psychiatry 94, 531–542. 10.1016/j.biopsych.2023.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Patino GA, Claes LR, Lopez-Santiago LF, Slat EA, Dondeti RS, Chen C, O’Malley HA, Gray CB, Miyazaki H, Nukina N, et al. (2009). A functional null mutation of SCN1B in a patient with Dravet syndrome. J Neurosci 29, 10764–10778. 10.1523/jneurosci.2475-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bender KJ, Ford CP, and Trussell LO (2010). Dopaminergic modulation of axon initial segment calcium channels regulates action potential initiation. Neuron 68, 500–511. 10.1016/j.neuron.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bender KJ, and Trussell LO (2009). Axon initial segment Ca2+ channels influence action potential generation and timing. Neuron 61, 259–271. 10.1016/j.neuron.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Clarkson RL, Liptak AT, Gee SM, Sohal VS, and Bender KJ (2017). D3 Receptors Regulate Excitability in a Unique Class of Prefrontal Pyramidal Cells. J Neurosci 37, 5846–5860. 10.1523/jneurosci.0310-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pernía-Andrade AJ, Goswami SP, Stickler Y, Fröbe U, Schlögl A, and Jonas P. (2012). A deconvolution-based method with high sensitivity and temporal resolution for detection of spontaneous synaptic currents in vitro and in vivo. Biophys J 103, 1429–1439. 10.1016/j.bpj.2012.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tzounopoulos T, Kim Y, Oertel D, and Trussell LO (2004). Cell-specific, spike timing-dependent plasticities in the dorsal cochlear nucleus. Nat Neurosci 7, 719–725. 10.1038/nn1272. [DOI] [PubMed] [Google Scholar]
- 126.Yasuda R, Nimchinsky EA, Scheuss V, Pologruto TA, Oertner TG, Sabatini BL, and Svoboda K. (2004). Imaging calcium concentration dynamics in small neuronal compartments. Sci STKE 2004, pl5. 10.1126/stke.2192004pl5. [DOI] [PubMed] [Google Scholar]
- 127.Coupé P, Munz M, Manjón JV, Ruthazer ES, and Collins DL (2012). A CANDLE for a deeper in vivo insight. Med Image Anal 16, 849–864. 10.1016/j.media.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data reported in this paper are available from the lead contact upon reasonable request. This paper does not report original code. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.