Abstract
BACKGROUND:
The prognostic impact of left atrial appendage (LAA) patency, including those with and without visible peri-device leak (PDL), post–LAA closure in patients with atrial fibrillation, remains elusive.
METHODS:
Patients with atrial fibrillation implanted with the WATCHMAN 2.5 device were prospectively enrolled. The device surveillance by cardiac computed tomography angiography was performed at 3 months post-procedure. Adverse events, including stroke/transient ischemic attack (TIA), major bleeding, cardiovascular death, all-cause death, and the combined major adverse events (MAEs), were compared between patients with complete closure and LAA patency.
RESULTS:
Among 519 patients with cardiac computed tomography angiography surveillance at 3 months post–LAA closure, 271 (52.2%) showed complete closure, and LAA patency was detected in 248 (47.8%) patients, including 196 (37.8%) with visible PDL and 52 (10.0%) without visible PDL. During a median of 1193 (787–1543) days follow-up, the presence of LAA patency was associated with increased risks of stroke/TIA (adjusted hazard ratio for baseline differences, 3.22 [95% CI, 1.17–8.83]; P=0.023) and MAEs (adjusted hazard ratio, 1.12 [95% CI, 1.06–1.17]; P=0.003). Specifically, LAA patency with visible PDL was associated with increased risks of stroke/TIA (hazard ratio, 3.66 [95% CI, 1.29–10.42]; P=0.015) and MAEs (hazard ratio, 3.71 [95% CI, 1.71–8.07]; P=0.001), although LAA patency without visible PDL showed higher risks of MAEs (hazard ratio, 3.59 [95% CI, 1.28–10.09]; P=0.015). Incidences of stroke/TIA (2.8% versus 3.0% versus 6.7% versus 22.2%; P=0.010), cardiovascular death (0.9% versus 0% versus 1.7% versus 11.1%; P=0.005), and MAEs (4.6% versus 9.0% versus 11.7% versus 22.2%; P=0.017) increased with larger PDL (0, >0 to ≤3, >3 to ≤5, or >5 mm). Older age and discontinuing antiplatelet therapy at 6 months were independent predictors of stroke/TIA and MAEs in patients with LAA patency.
CONCLUSIONS:
LAA patency detected by cardiac computed tomography angiography at 3 months post–LAA closure is associated with unfavorable prognosis in patients with atrial fibrillation implanted with WATCHMAN 2.5 device.
REGISTRATION:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03788941.
Keywords: atrial fibrillation, cardiac computed tomography angiography, incomplete device endothelialization, left atrial appendage closure, peri-device leak
WHAT IS KNOWN
Peri-device leak detected by transesophageal echocardiogram is correlated with thromboembolic events post–left atrial appendage (LAA) closure.
Cardiac computed tomography angiography, rather than transesophageal echocardiogram, might more accurately detect LAA patency, especially those without visible peri-device leak.
WHAT THE STUDY ADDS
LAA patency detected by cardiac computed tomography angiography at 3 months, even without visible peri-device leak, increases the risks of stroke/transient ischemic attack and major adverse events.
A dose-response relationship between peri-device leak severity and major adverse events is detected, emphasizing the importance of pursuing complete closure at the time of implantation.
Continuing antiplatelet therapy post–LAA closure reduces the risks of stroke/transient ischemic attack and major adverse events in patients with LAA patency.
Atrial fibrillation (AF) is increasingly prevalent with population aging and causes nearly 13% to 26% of ischemic stroke.1,2 Oral anticoagulation can effectively reduce the risk of AF-related stroke, while is also associated with increased bleeding risks.3 Left atrial appendage (LAA) closure (LAAC) prevents LAA-derived thromboembolism, serving as a valuable alternative therapy to long-term oral anticoagulation in patients vulnerable to both stroke and bleeding.4 However, residual risks of stroke, as well as other adverse outcomes, still exist after LAAC device implantation.5 Although with controversy, risks of adverse events post-LAAC might be associated with LAA patency, including those with and without visible peri-device leak (PDL).6–8 The latter is caused either by incomplete device endothelialization (IDE) in which residual LAA permeability could be assessed by continuous contrast enhancement from left atrium to LAA through the fabric membrane on cardiac computed tomography angiography (CCTA), or invisible microleaks in the circumference of the device.7,8
A postimplantation PDL<5 mm detected by a transesophageal echocardiogram (TEE) is empirically considered as sufficient LAA closure, which was supported by the initial results from the PROTECT-AF study (Percutaneous Closure of the Left Atrial Appendage Versus Warfarin Therapy for Prevention of Stroke in Patients With Atrial Fibrillation) suggesting minimal correlation between PDL and later thromboembolic events.9,10 However, the recently updated data combining PROTECT-AF, PREVAIL (Evaluation of the Watchman Left Atrial Appendage LAA Closure Device in Patients With Atrial Fibrillation vs Long Term Warfarin Therapy), and CAP2 (Continued Access to PREVAIL) studies demonstrated that PDL<5 mm at 1-year TEE surveillance was still associated with increased stroke risk during the 5-year follow-up, suggesting complete LAA closure, rather than sufficient closure, is wished for LAAC.10 It is thus of important to monitor device closure status post-LAAC. In real-world practice, however, the compliance of TEE follow-up was limited due to the invasive feature and patients’ acceptance.11 The noninvasive CCTA is widely accepted as an alternative modality to TEE for post-LAAC device surveillance and might even be more sensitive in detecting PDL.12 Whether PDL, assessed by CCTA is correlated with post-LAAC adverse events remains unclear. Besides, LAA patency without visible PDL might only be accurately detected by CCTA rather than TEE.12,13 There have been cases reported in which delayed endothelialization was observed in patients who had a transient ischemic attack (TIA).14,15 However, the clinical consequences of LAA patency without visible PDL (or termed as IDE in previous literature) on subsequent thromboembolism are poorly characterized to date. Herein, we sought to explore the prognostic impacts and risk factors of LAA patency detected by CCTA, including those with and without visible PDL, in a prospective cohort of patients with AF who underwent WATCHMAN LAAC device implantation.
METHODS
Study Population
Among 1055 patients with AF enrolled in the LAACablation registry (NCT03788941), 519 who underwent post-LAAC device surveillance by CCTA at 3 months were prospectively included in this study (Figure S1). The LAACablation registry, as a physician-initiated prospective observational study, is continuously recruiting patients undergoing the LAAC procedures with the WATCHMAN 2.5 device (Boston Scientific Corporation, Natick, MA) following catheter ablation in Xinhua Hospital, Shanghai, China.14 The study was approved by the Ethics Committee of Xinhua Hospital Affiliated with Shanghai Jiao Tong University School of Medicine (approval number: XH-18-015) and complies with the Declaration of Helsinki. Written informed consent was provided by all participants. The detailed inclusion and exclusion criteria of the LAACablation registry are shown in Table S1. The data that support the findings of this study are available from the corresponding author upon reasonable request.
LAAC Procedure
LAAC implantation of the WATCHMAN 2.5 device was performed under light sedation, following catheter ablation during the same procedure. The device size was decided according to the intraprocedural LAA angiography. The device was released only when the position-anchor-size-seal principle was achieved and evaluated by intraoperative TEE and angiography. The procedural details were described in our previous report.11
Device Surveillance by CCTA at 3-Month Post-LAAC
Patients underwent device surveillance by CCTA (Somatom Definition, Siemens Medical Solutions, Forchheim, Germany) at 3 months post-LAAC, with 100 mL of loversol injected with 50-mL saline flush followed via elbow vein at a rate of 5 mL/s. An end-systolic image acquisition was applied for device surveillance independently from the heart rate to acquire the image data during the maximum distension of the left atrium and LAA, similar to previously reported.15 Imaging of the left atrium, LAA, and adjacent structures were obtained (Figure S2). The temporal resolution was 330 ms, with a detector collimation of 64×0.6 mm, the tube voltage of 120 kV, and the tube current of 380 mA.
Image analyses were performed by the Extended Brilliance Workspace version 4.5 (Philips Healthcare Cleveland, OH). Images were analyzed by 2 experienced physicians independently, who were blinded to patients’ clinical characteristics. The measurement results should be confirmed by both physicians. In case of disagreement in the evaluation, a third physician was invited to review the image, and the final consensus was achieved based on the opinions of at least 2 physicians.
LAA patency, either with or without visible PDL, was evaluated. The CT workspace was applied to reconstruct the original CCTA images in 3-dimensional multiplanar, and the axial plane should be at the level of the left atrium. Analyses were performed in the following sequence: (1) observing the position of the nitinol skeleton of the device; (2) moving the coronal axis within the transverse window perpendicular to the coves of the parachute of the device; (3) aligning the axes on the 2 other viewers also perpendicular to the coves of the parachute of the device; and (4) placing the center of the axes to the center of the screw-hub.15
Quantitative contrast assessment of LAA postimplantation was carried out by measuring the average linear attenuation coefficient (Hounsfield Units [HU]) in the LAA distal to the WATCHMAN device, using a 3-mm diameter circle for the region of interest. According to the 3-month results of the contrast assessment, patients were divided into the complete closure (Figure S2B) and LAA patency groups, which were further divided into 2 forms, that is, the LAA patency with visible PDL (Figure S2C) and that without visible PDL (Figure S2D). Complete closure was defined as the average linear attenuation coefficient of LAA <100 HU, while the average linear attenuation coefficient of LAA in LAA patency was >100 HU. Continuous contrast enhancement could be observed from left atrium to LAA alongside the device (LAA patency with visible PDL) and through the fabric of the device (LAA patency without visible PDL).7 The width of the visible PDL was defined as the maximum diameter of the contrast gap adjacent to the device on a reconstructed plane parallel to the LAA orifice. Therefore, the visible PDL was further divided into 3 types according to the severity of the PDL width, that is, mild PDL (>0 to ≤3 mm), moderate PDL (>3 to ≤5 mm), and severe PDL (>5 mm).
Postprocedural Management and Events Evaluation
Patients were generally followed every 3 months. For the initial 3 months, oral anticoagulants were prescribed if there were no contraindications. Anticoagulants were switched to dual antiplatelet agents (aspirin 100 mg+clopidogrel 75 mg) until 6 months, if no PDL>5 mm or device-related thrombus, otherwise, anticoagulants were prescribed until the repeated device surveillance. After 6 months, aspirin monotherapy was recommended. In addition, ECG and Holter monitoring were advised at every follow-up visit to detect recurrence of atrial tachyarrhythmias. Clinical adverse events were evaluated during every follow-up visit.
The outcome events evaluated were as follows: (1) stroke or TIA; (2) major bleeding, which meets at least 1 of the following criteria: a drop in the hemoglobin level of at least 30 g/L; requiring transfusion of 2 or 3 units of whole blood/red blood cells; causing hospitalization or permanent injury, or requiring surgery; (3) cardiovascular death; (4) all-cause death; and (5) major adverse events (MAEs), including stroke, TIA, major bleeding, and all-cause death. The definitions of events followed the Munich consensus document for LAAC procedures.16 Events occurring before the postoperative CCTA were censored during analyses. Strokes were confirmed by neuroimaging. Stroke severity was determined by comparing the modified Rankin Scale score at 3 months after the stroke with that at baseline and was classified as nondisabling (score <2 points) and disabling or fatal strokes (score ≥2 points). Death was treated as a competing event if other adverse events occurred during the period between the operation and death.
Statistical Analyses
Data were shown as n (%) for categoric variables and as mean±SD (n) or median (25th–75th quartiles) for continuous variables. Intergroup comparisons were made by χ2 test for categorical variables, Student t test or Mann-Whitney U test for continuous variables, as appropriate. Time to adverse events were assessed by Kaplan-Meier estimates and log-rank tests. Unadjusted hazard ratios (HRs) between complete closure and LAA patency groups were calculated using a Cox proportional hazard regression model without covariate. They were further adjusted hazard ratios (aHRs) using a Cox proportional hazard regression model that included patients’ baseline characteristics. Bonferroni correction was used for multiple comparisons among groups. Univariate and multivariable analyses of predictors of adverse events were assessed using a Cox hazard regression model for the following variables: age, sex, paroxysmal AF (versus nonparoxysmal AF), hypertension, diabetes, coronary artery disease, history of stroke or TIA, congestive heart failure, NT-proBNP (N-terminal pro-B-type natriuretic peptide, pg/mL), left atrial diameter (LAD, mm), left ventricular ejection fraction (LVEF, %), size of the WATCHMAN device (mm), antiplatelet therapy or not at 6 months, recurrence of atrial tachyarrhythmia after ablation, and the width of PDL if any (millimeter). The threshold for entry of independent variables into the multivariable model was set at 0.10. Age and sex were also included in the multivariable model. To evaluate the degrees of PDL on outcomes, clinical outcomes were also assessed by severity of PDL, that is, no PDL, mild PDL, moderate PDL, and severe PDL, using a Cox hazard regression. Besides, logistic regression analyses were used to assess the predictors of device patency. Statistical analyses were performed by SPSS V22.0 (IBM Software, Armonk, NY). A 2-sided P<0.05 was considered statistically significant unless otherwise noted.
RESULTS
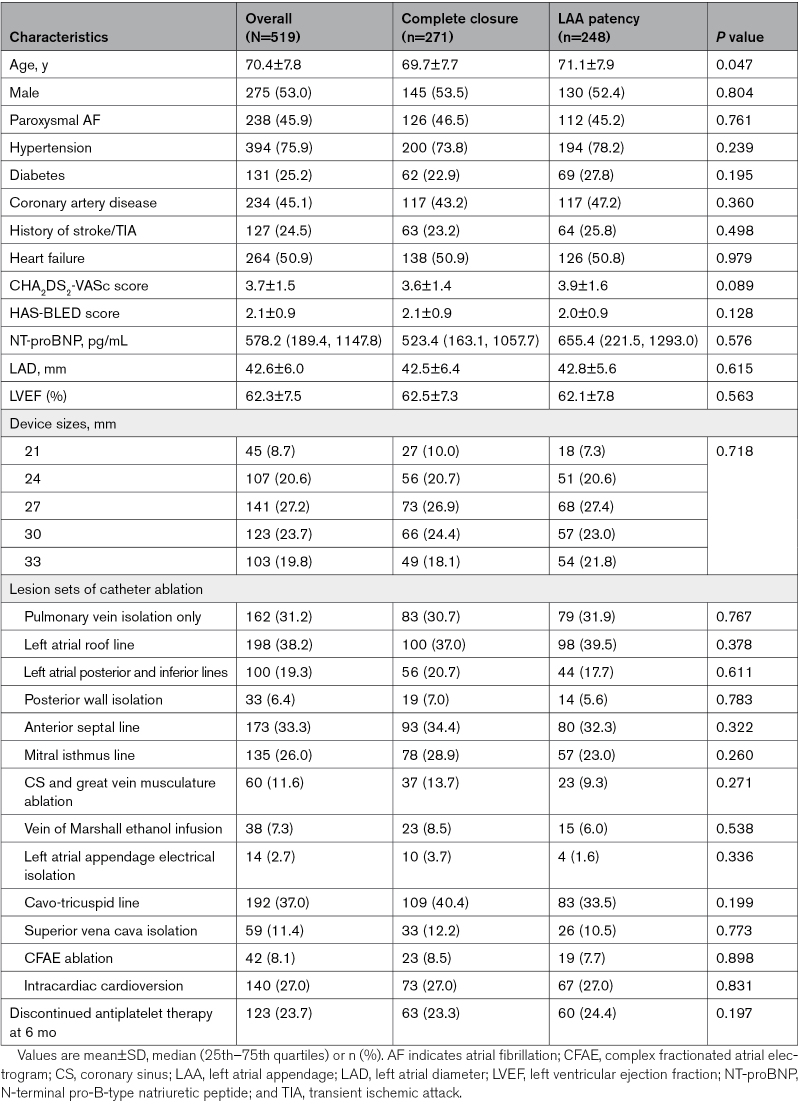
A total of 519 patients (mean age, 70.4±7.8 years, 275 males, and 238 with paroxysmal AF) from LAACablation registry with 3-month post-LAAC CCTA imaging evaluation were included. The mean CHA2DS2-VASc score was 3.7±1.5 and HAS-BLED score was 2.1±0.9. The CCTA imaging showed 271 (52.2%) with complete closure and 248 (47.8%) with LAA patency, including 196 (37.8%) with visible PDL and 52 (10.0%) without visible PDL. The median follow-up duration was 1193 days (interquartile range, 787–1543 days), which were comparable between groups (1195 days [interquartile range, 787–1596 days] in the complete closure group and 1188 days [interquartile range, 784–1527 days] in the LAA patency group). Age was the only baseline difference between the complete closure and LAA patency groups, while the other baseline features were similar. The detailed clinical characteristics are listed in Table 1.
Table 1.
Clinical Characteristics of Patients With Complete Closure and With LAA Patency
Further comparisons were made among the complete closure group and the 2 forms of LAA patency, that is, LAA patency with and without visible PDL groups, showing similar baseline characteristics (Table S2). The antithrombotic therapy at each follow-up time point was further listed in Table S3. Besides, of patients with PDL, 67 (34.2%) had mild PDL, 120 (61.2%) had moderate PDL, and 9 (4.6%) had severe PDL. Baseline characteristics were similar among patients with different PDL severity (Table S4).
Adverse Events Post-LAAC Procedures
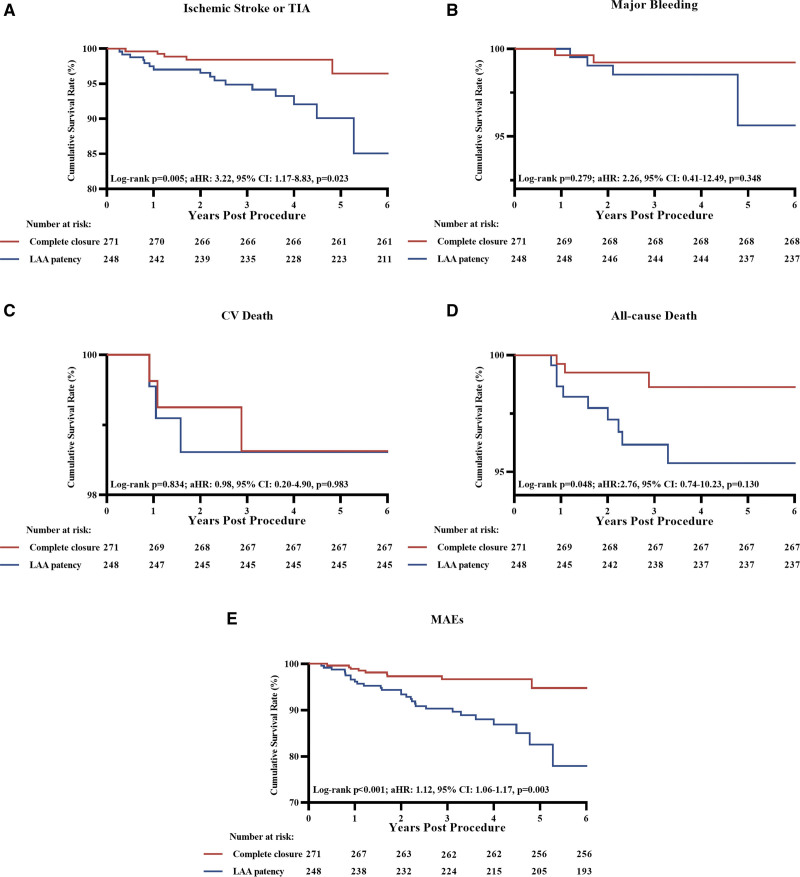
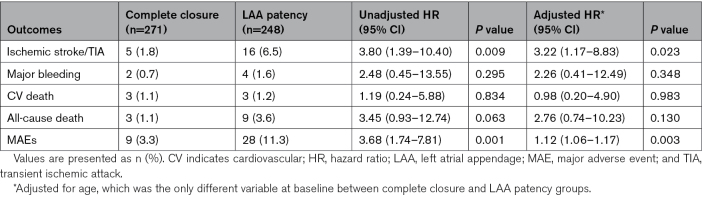
Time-to-events curves of the complete closure and the LAA patency groups are shown in Figure 1. Compared with subjects with complete closure, patients with LAA patency showed increased risks of ischemic stroke/TIA (6.5% versus 1.8%; log-rank P=0.005), all-cause death (3.6% versus 1.1%; log-rank P=0.048), and MAEs (11.3% versus 3.3%; log-rank P<0.001). The Cox hazard regression analyses showed HRs for stroke/TIA and MAEs were 3.80 ([95% CI, 1.39–10.40]; P=0.009) and 3.68 ([95% CI, 1.74–7.81]; P=0.001), but that for all-cause death was 3.45 ([95% CI, 0.93–12.74]; P=0.063). After adjustment for the baseline age differences, aHRs for stroke/TIA and MAEs were 3.22 ([95% CI, 1.17–8.83]; P=0.023) and 1.12 ([95% CI, 1.06–1.17]; P=0.003). The rates for all-cause death (aHR, 2.76 [95% CI, 0.74–10.23]; P=0.130), major bleeding (aHR, 2.26 [95% CI, 0.41–12.49]; P=0.348), and cardiovascular death (aHR, 0.98 [95% CI, 0.20–4.90]; P=0.983) did not differ between the complete closure and the LAA patency groups (Table 2).
Figure 1.
Kaplan-Meier curves of adverse events between patients with complete closure and left atrial appendage (LAA) patency. Kaplan-Meier curves of ischemic stroke or transient ischemic attack (TIA; A), major bleeding (B), cardiovascular (CV) death (C), all-cause death (D), and major adverse events (MAEs; E). aHR indicates adjusted hazard ratio.
Table 2.
Relative Risks of Clinical End Points for Patients With LAA Patency
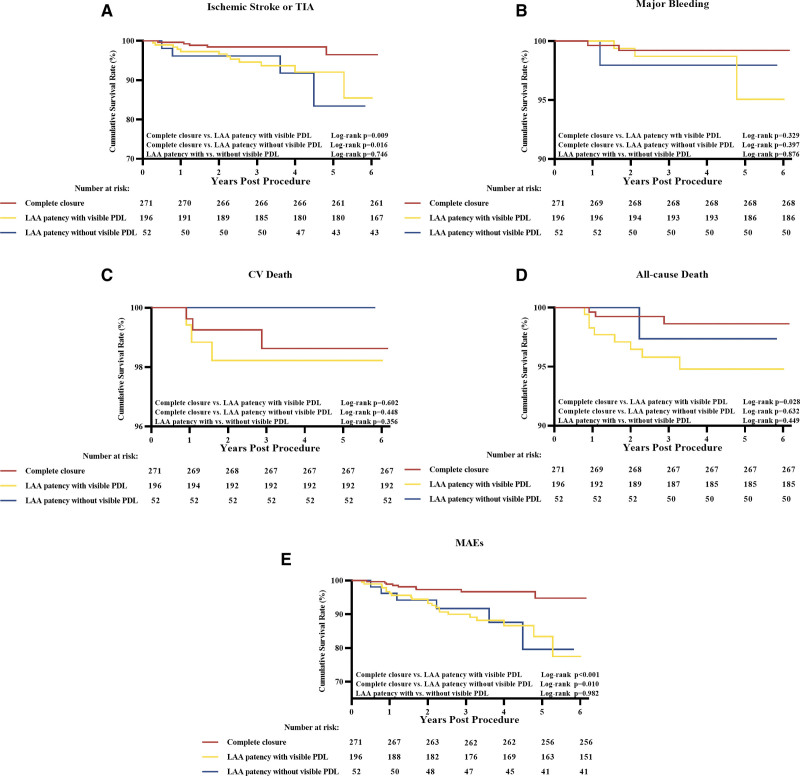
Further comparisons of adverse events among the complete closure and the LAA patency with and without visible PDL groups were shown in Figure 2. Compared with the complete closure group, patients in the LAA patency without visible PDL group were associated with increased risks of ischemic stroke/TIA (5.8% versus 1.8%; log-rank P=0.016) and MAEs (9.6% versus 3.3%; log-rank P=0.010). The Cox hazard regression analyses showed HRs for stroke/TIA and MAEs were 4.38 ([95% CI, 1.17–16.34]; P=0.028) and 3.59 ([95% CI, 1.28–10.09]; P=0.015). The rates of major bleeding (1.9% versus 0.7%; P=0.417), cardiovascular death (0.0% versus 1.1%), and all-cause death (1.9% versus 1.1%; P=0.637) did not differ between the complete closure and the LAA patency without visible PDL groups (Table S5). Compared with the complete closure group, patients in the LAA patency with visible PDL group were associated with increased risk of subsequent stroke/TIA (6.1% versus 1.8%; log-rank P=0.009) and MAEs (11.2% versus 3.3%; log-rank P<0.001), respectively with HRs of 3.66 ([95% CI, 1.29–10.42]; P=0.015) and 3.71 ([95% CI, 1.71–8.07]; P=0.001). The rates of major bleeding (1.5% versus 0.7%; P=0.329), cardiovascular death (1.5% versus 1.1%; P=0.602), and all-cause death (4.1% versus 1.1%, log-rank P=0.028) were similar between the complete closure and the LAA patency with visible PDL groups, respectively with HRs of 2.38 ([95% CI, 0.40–14.26]; P=0.344) and 1.53 ([95% CI, 0.31–7.57]; P=0.604), and 3.96 ([95% CI, 1.05–14.94]; P=0.042; Table S6). It appeared to be no prognostic difference between the 2 forms of LAA patency, including stroke/TIA (6.1% versus 5.8%; hazard ratio [HR], 1.21 [95% CI, 0.39–3.74]; P=0.747), major bleeding (1.5% versus 1.9%; HR, 1.20 [95% CI, 0.12–11.51]; P=0.876), all-cause death (4.1% versus 1.9%; HR, 0.46 [95% CI, 0.06–3.66]; P=0.461), and MAEs (11.2% versus 9.6%; HR, 0.99 [95% CI, 0.40–2.44]; P=0.982; Table S7).
Figure 2.
Kaplan-Meier curves of adverse events among the groups of complete closure and left atrial appendage (LAA) patency with and without visible peri-device leak (PDL). Kaplan-Meier curves of ischemic stroke or transient ischemic attack (TIA; A), major bleeding (B), cardiovascular (CV) death (C), all-cause death (D), and major adverse events (MAEs; E). For multiple comparisons, the Bonferroni corrected critical P value was set as 0.05/3 to 0.017.
The influence of PDL, compared with that of no PDL (combining complete closure and LAA patency without visible PDL), on stroke severity was determined (Figure S3). Compared with no PDL, mild or moderate PDL (≤5 mm) was associated with a higher incidence of disabling/fatal stroke (0.6% versus 3.2%; P=0.036), but there was no difference in the rates of nondisabling stroke (2.2% versus 2.1%; P=0.913) between patients with no PDL and those with mild or moderate PDL. Again, compared with no PDL, severe PDL (>5 mm) markedly increased the rate of disabling/fatal stroke (0.6% versus 22.2%; P<0.001).
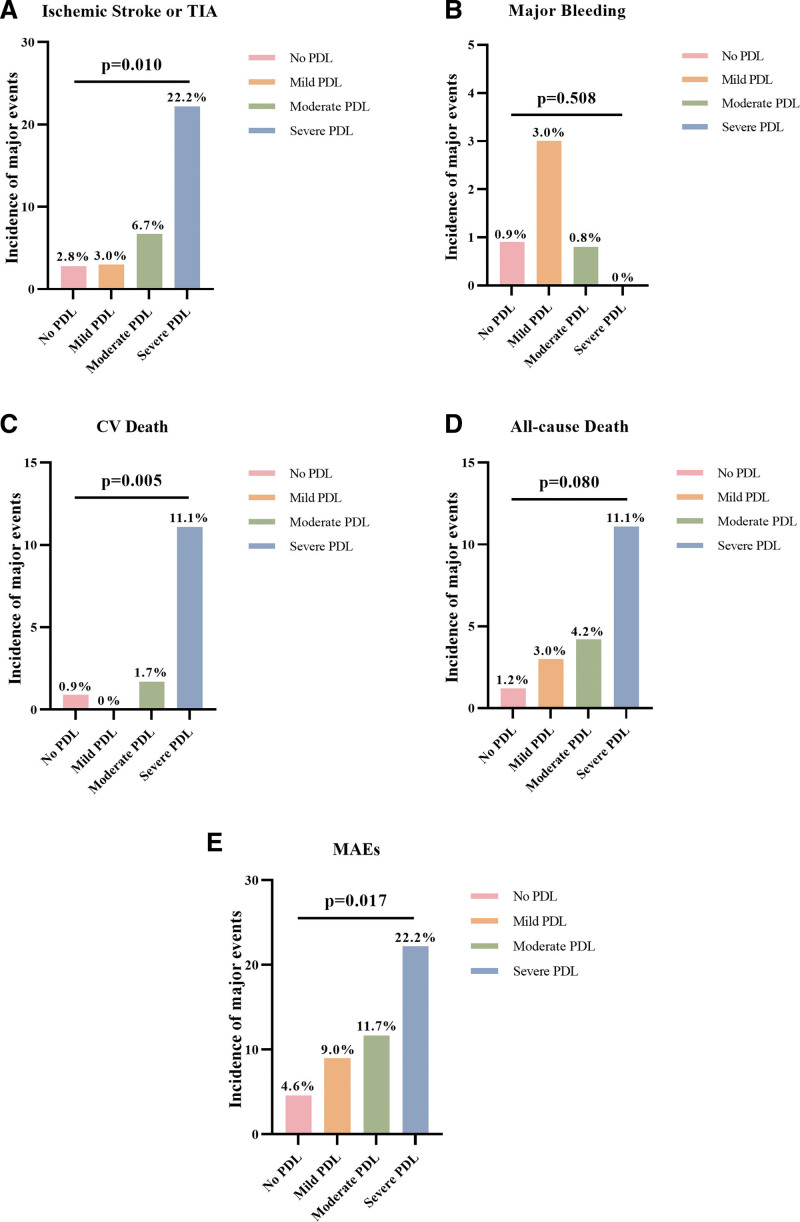
MAEs were further assessed by PDL severity to determine whether larger PDL was associated with poorer prognosis. When pooled, the complete closure and LAA patency without visible PDL as no PDL, larger PDL (no versus mild versus moderate versus severe PDL) was associated with a higher incidence of stroke/TIA (2.8% versus 3.0% versus 6.7% versus 22.2%; P=0.010), cardiovascular death (0.9% versus 0% versus 1.7% versus 11.1%; P=0.005), and MAEs (4.6% versus 9.0% versus 11.7% versus 22.2%; P=0.017, Figure 3), showing a dose-response relationship. Major bleeding and all-cause death were comparable among groups (0.9% versus 3.0% versus 0.8% versus 0%; P=0.508; 1.2% versus 3.0% versus 4.2% versus 11.1%; P=0.080; respectively). Specifically, the comparisons between no PDL and each PDL severity group were further shown in Table S8. The incidences of adverse events were similar between the no PDL versus mild PDL groups. MAEs were higher in the moderate PDL group than in the no PDL group (11.7% versus 4.6%; HR, 2.53 [95% CI, 1.22–5.25]; P=0.013). Patients with severe PDL, compared with no PDL, had markedly heightened risks of ischemic stroke/TIA (22.2% versus 2.8%; HR, 10.97 [95% CI, 2.32–51.94]; P=0.003), cardiovascular death (11.1% versus 0.9%; HR, 14.78 [95% CI, 1.53–142.49]; P=0.020), all-cause death (11.1% versus 1.2%; HR, 10.69 [95% CI, 1.19–95.88]; P=0.034), and MAEs (22.2% versus 4.6%; HR, 6.23 [95% CI, 1.41–27.48]; P=0.016). When compared with the combined no-to-mild-PDL (no PDL or PDL≤3 mm) groups, the moderate PDL group showed increased MAE risks (5.4% versus 11.7%; P=0.031) while the severe PDL group showed significantly higher risks of stroke/TIA (2.8% versus 22.2%; P=0.003), cardiovascular death (0.8% versus 11.1%; P=0.014), all-cause death (1.5% versus 11.1%; P=0.049), and MAEs (5.4% versus 22.2%; P=0.026). Similar results were noticed when separating the complete closure and LAA patency without visible PDL and comparing them with different levels of visible PDL (Figure S4).
Figure 3.
Peri-device leak (PDL) severity and incidences of adverse events. PDL severity (no PDL, mild PDL [>0 to ≤3 mm], moderate PDL [>3 to ≤5 mm], or severe PDL [>5 mm]) and incidence of adverse events post-left atrial appendage closure, including ischemic stroke or transient ischemic attack (TIA; A), major bleeding (B), cardiovascular (CV) death (C), all-cause death (D), and major adverse events (MAEs; E). Of note, no PDL includes both complete closure and left atrial appendage patency without visible PDL.
Recurrent atrial tachyarrhythmias after the procedure were observed in 124 patients during the follow-up, the rates of which were similar among groups (Figure S5; Table S9).
Predictors of Adverse Events
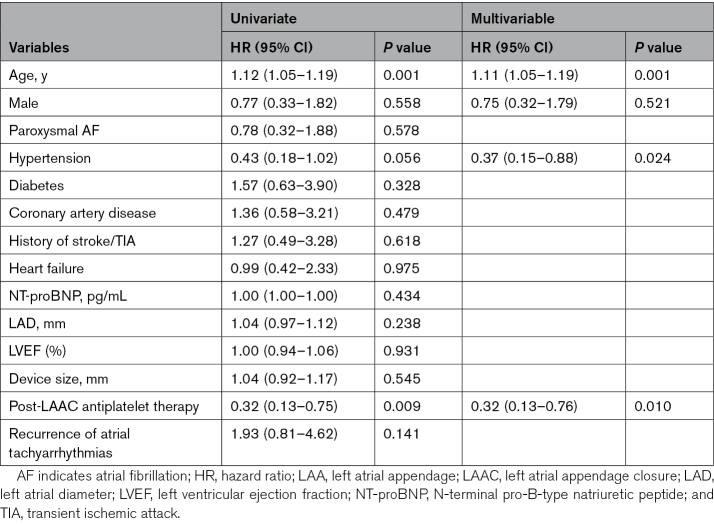
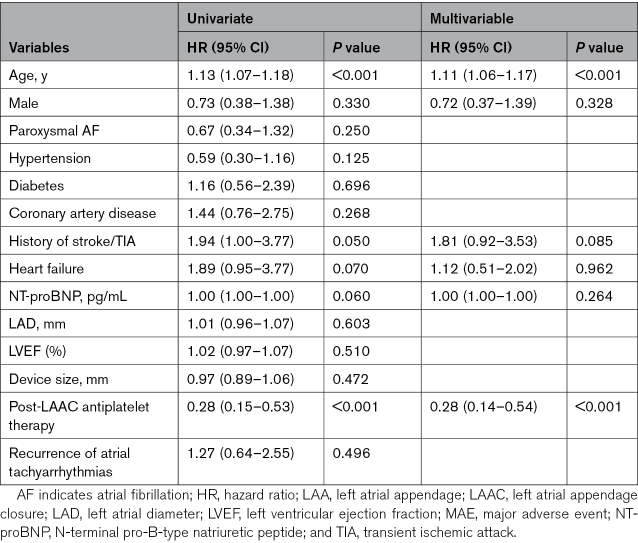
The risk factors of post-LAAC adverse events were explored. No predictor was found related to adverse events in the complete closure group. In the LAA patency group (combining those with and without visible PDL), multivariable regression analyses showed that older age (HR, 1.11 [95% CI, 1.05–1.18]; P=0.001), hypertension (HR, 0.37 [95% CI, 0.15–0.88]; P=0.024), and antiplatelet therapy relative to no therapy at 6 months (HR, 0.32 [95% CI, 0.13–0.76]; P=0.010) were independent predictors of ischemic stroke/TIA (Table 3); older age (HR, 1.11 [95% CI, 1.06–1.17]; P<0.001) and antiplatelet therapy relative to no therapy at 6 months (HR, 0.28 [95% CI, 0.14–0.54]; P<0.001) were independent predictors of MAEs (Table 4).
Table 3.
Predictors of Stroke/TIA in Patients With LAA Patency
Table 4.
Predictors of MAEs in Patients With LAA Patency
Further multivariable regression analyses were performed in the LAA patency with and without visible PDL groups. In the LAA patency with visible PDL group, older age (HR, 1.10 [95% CI, 1.01–1.21]; P=0.032) and the width of PDL (HR, 1.94 [95% CI, 1.30–2.90]; P=0.001) were independent risk factors of ischemic stroke/TIA, while antiplatelet therapy (relative to no therapy) at 6 months was the protective factor (HR, 0.27 [95% CI, 0.08–0.91]; P=0.034; Table S10). Age (HR, 1.12 [95% CI, 1.05–1.20]; P=0.001), antiplatelet therapy (HR, 0.22 [95% CI, 0.08–0.55]; P=0.001), and the width of PDL (HR, 1.80 [95% CI, 1.34–2.41]; P<0.001) were that of MAEs (Table S11). In the LAA patency without visible PDL group, no independent predictor of MAEs was found (Table S12).
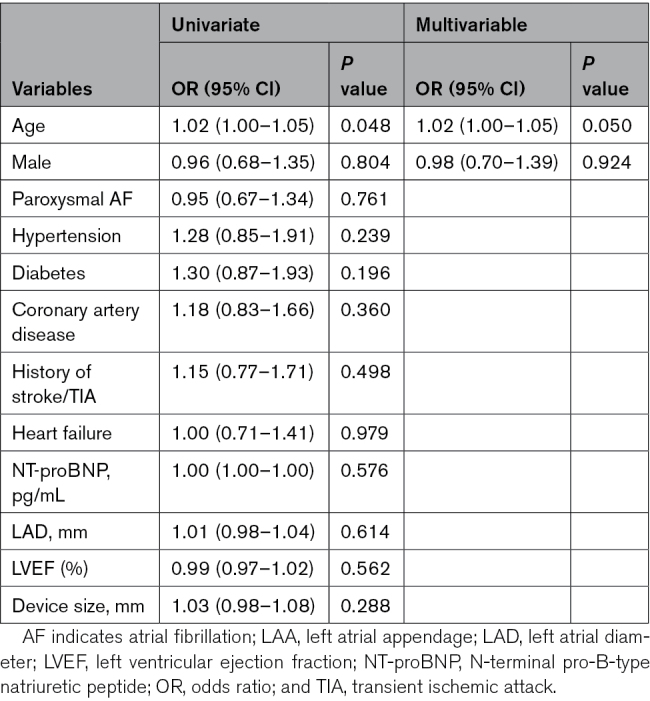
We further evaluated potential clinical variables to predict the occurrence of LAA patency and its 2 forms. Of the presence of LAA patency, the only predictor found was older age (OR, 1.02 [95% CI, 1.00–1.05]; P=0.050; Table 5). No associated factor could be defined for the presence of LAA patency with or without visible PDL (Tables S13 and S14).
Table 5.
Predictors of the Presence of LAA Patency
DISCUSSION
Results from this large prospective registry showed that the incidence of LAA patency, detected by CCTA at 3 months after WATCHMAN device implantation, was 47.8%, including 79.0% LAA patency with visible PDL and 21.0% LAA patency without visible PDL, respectively. With a median 39.8 months follow-up, LAA patency presented at 3 months was associated with ischemic stroke/TIA and MAEs. Specifically, any visible PDL was associated with increased risks of ischemic stroke/TIA and MAEs. Both mild-to-moderate PDL (≤5 mm) and severe PDL (>5 mm) markedly increased the rate of disabling or fatal stroke. In addition, the incidence of ischemic stroke/TIA, cardiovascular death, and MAEs increased in proportion to the increasing width of the PDL, and the width of PDL independently predicted ischemic stroke/TIA and MAEs. Similar to PDL, LAA patency without visible PDL (or termed as IDE) at 3 months was also associated with a heightened risk of stroke/TIA and MAEs. Our results thus highlighted the clinical importance of both forms of LAA patency surveilled by CCTA, either with or without visible PDL. Patients with LAA patency should be intensively monitored and managed, such as detachable embolization coils, vascular plugs, radiofrequency ablation, and prolonged antithrombotic therapy, to improve the outcomes.17
Both forms of LAA patency, that is, LAA patency with or without visible PDL, were not rare following LAAC device implantation.12,18,19 The association between PDL and clinical outcomes, especially ischemic stroke, has been observed, but with controversies. The initial results from the PROTECT-AF trial reported the correlations between PDL by TEE and ischemic stroke and cardiovascular death were minimal,9 which have recently been unveiled by the pooled analyses of PROTECT AF, PREVEIL, and CAP2 studies with extended follow-up periods.10 Other studies also favored that PDL was associated with worse prognoses.20,21 TEE served as the evaluation tool for PDL in all the abovementioned studies, the compliance of which, however, was low in the Asian population due to patients’ intolerance.11 CCTA thus served as an alternative imaging modality for post-LAAC device surveillance. A previous study compared the diagnostic performance for PDL between TEE and CCTA at 3 months and demonstrated that CCTA was a more sensitive way to assess PDL than TEE and what’s more, LAA patency without PDL (referred to IDE to some extent) could be detected by CCTA, but not by TEE per definition.12 This was supported by the recently published meta-analysis showing more frequent LAA patency detected by CCTA, however, failed to show prognostic significance probably due to the limited sample sizes.22 Whether both forms of LAA patency evaluated by CCTA were correlated with adverse events after the implantation of the WATCHMAN device has not been investigated by previous studies yet.
In the present study, we found the presence of LAA patency and its both forms at 3 months by CCTA negatively affected the prognosis by increasing the risks of stroke/TIA and MAEs, thus heightening the clinical importance of post-LAAC CCTA device surveillance. The timing of the post-LAAC device surveillance also matters. The previous study demonstrated that PDL detected by TEE at 1 year, but not 45 days, was associated with increased thromboembolism.10 Therefore, the results of the present study potentially suggest device surveillance by CCTA, compared with TEE, might favor early recognition of patients with high residual risks of stroke/TIA and other adverse events (3 months versus 1 year). Nevertheless, determining the optimal timing for CCTA device surveillance still warrants further investigations.
Opinions also diverge when it comes to how clinical outcomes are affected by PDL severity. In a retrospective case-control study, PDL<5 mm by TEE was associated with a higher incidence of stroke/TIA compared with no PDL, but the impact of PDL≥5 mm was not discussed.21 Results from both the NCDR LAAO registry and the pooled analyses of PROTECT AF, PREVEIL, and CAP2 studies showed PDL≤5 mm was associated with a higher incidence of stroke compared with no PDL, while PDL>5 mm was not.10,20 Our research agreed to the point that mild-to-moderate PDL (≤5 mm) was associated with a higher incidence of stroke compared with no PDL, and severe PDL (>5 mm) was associated with markedly higher incidence of stroke/TIA, cardiovascular death, all-cause death and MAEs. In addition, the dose-response relationship was revealed between leak severity and clinical events including stroke/TIA, cardiovascular death, and MAEs. Even moderate PDL could increase the MAEs compared with no-to-mild PDL, which was not observed by the previous study using TEE evaluation.10 Considering the PDL at 3 months might be correlated with initial intraprocedural leaks, it suggests the necessity of striving for perfection to minimize PDL during the LAAC device implantation procedure. When further reviewing the data of patients who suffered a stroke, we found that among patients with adverse events, the 2 with severe PDL both discontinued antithrombotic (anticoagulation or antiplatelet) therapy without doctors’ permission, and one of the 2 died of stroke. No death was found due to bleeding in patients with residual leaks>5 mm. In contrast, patients with PDL>5 mm in the NCDR LAAO registry tended to keep anticoagulation therapy.20 Therefore, these results confirmed the importance of continuing antithrombotic therapy postprocedure in patients with larger PDL. Furthermore, antiplatelet was found to be protective of ischemic stroke/TIA and MAEs in patients with LAA patency, including both with and without visible PDL. Taken together, these findings hinted that antiplatelet therapy should be continued in patients with LAA patency at 6 months, especially in those with larger PDL.
The exact mechanism for LAA patency without visible PDL was not entirely clear. IDE (as known as intradevice or transfabric leak), which refers to the continuous contrast enhancement through the incomplete endothelialized fabric membrane, might be the most common mechanism.23 However, microgaps or microleaks in the circumference of the device (due to the device frame architecture), which could not be seen on CCTA, might be another potential explanation. Therefore, we used the term LAA patency without visible PDL instead of IDE under such consideration. As TEE has limited value in detecting IDE, little was known about the relationship between LAA patency without visible PDL (IDE in most cases) and long-term clinical outcomes by previous studies. There was only one short-term (6 months) study showing that delayed endothelialization conferred an increased risk for device-related thrombus, but not for stroke and other clinical adverse events.23 In this study with a larger sample size and long-term follow-up, LAA patency without visible PDL detected by 3-month CCTA increased the risks of stroke and MAEs, therefore, for the first time suggesting IDE also resulted in unfavorable long-term prognosis. Ways, both clinical- and materials-wise, should be explored to accelerate the process of device endothelialization.
Limitations
First, this prospective observational study only included patients with the WATCHMAN device. Whether LAA patency of other types of LAAC device is correlated with post-LAAC adverse events remained outside the scope of the current study. Second, dynamic CCTA observations are needed to see whether CCTA at the 3 months post-procedure is the optimal time point to evaluate LAA patency. Third, the sample size of this study, especially that of LAA patency without visible PDL group, which was only 52, might lead to unavoidable bias. Last, it remains elusive what strategies could be helpful to reduce the rates of LAA patency post the procedure, and future studies are needed to address this issue.
Conclusions
LAA patency evaluated by 3-month CCTA, whether with or without visible PDL, resulted in an unfavorable long-term prognosis. PDL was associated with increased risks of stroke/TIA and MAEs, and there was a dose-response relationship between leak severity and MAEs. Similarly, the occurrence of LAA patency without visible PDL (or termed as IDE) heightened the risks of stroke/TIA and MAEs. These findings therefore emphasize the importance of aiming for complete closure at the time of implantation. In addition, patients should undergo CCTA surveillance at 3 months post-LAAC and those with both forms of LAA patency need to be carefully monitored and managed. The continuous use of antiplatelet therapy beyond 6 months post-LAAC should be considered for patients with LAA patency to improve long-term outcomes.
ARTICLE INFORMATION
Sources of Funding
This work was supported by Clinical Research Plan of Shanghai Municipal Health Commission (No. 20224Y0078), National Science Foundation of China (No. 82130009 and No. 82070515), Shanghai City Committee of Science and Technology Research Projects (No. 201409005600), and Shanghai Leading Talent Plan 2020.
Disclosures
None.
Supplemental Material
Figures S1–S5
Tables S1–S14
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AF
- atrial fibrillation
- aHR
- adjusted hazard ratio
- CCTA
- cardiac computed tomography angiography
- HR
- hazard ratio
- IDE
- incomplete device endothelialization
- LAA
- left atrial appendage
- LAAC
- left atrial appendage closure
- MAE
- major adverse event
- PDL
- peri-device leak
- TEE
- transesophageal echocardiogram
- TIA
- transient ischemic attack
M. Chen and P.-C. Yao contributed equally.
For Sources of Funding and Disclosures, see page 355.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCINTERVENTIONS.123.013579.
Contributor Information
Mu Chen, Email: muchen56@outlook.com.
Peng-Cheng Yao, Email: yaopengcheng@sjtu.edu.cn.
Zhen-Tao Fei, Email: wmu_feizhentao@163.com.
Qun-Shan Wang, Email: wangqunshan@xinhuamed.com.cn.
Peng-Pai Zhang, Email: zhangrui@xinhuamed.com.cn.
Wei Li, Email: liyigang@xinhuamed.com.cn.
Rui Zhang, Email: zhangrui@xinhuamed.com.cn.
Bin-Feng Mo, Email: mobinfeng@xinhuamed.com.cn.
Ming-Zhe Zhao, Email: 570775403@qq.com.
Yi Yu, Email: yuyi01@xinhuamed.com.cn.
Mei Yang, Email: melva_young@126.com.
Yan Zhao, Email: 570775403@qq.com.
Chang-Qi Gong, Email: treanavor@163.com.
REFERENCES
- 1.Chen M, Li C, Liao P, Cui X, Tian W, Wang Q, Sun J, Yang M, Luo L, Wu H, et al. Epidemiology, management, and outcomes of atrial fibrillation among 30 million citizens in Shanghai, China from 2015 to 2020: a medical insurance database study. Lancet Reg Health West Pac. 2022;23:100470. doi: 10.1016/j.lanwpc.2022.100470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, Baker-Smith CM, Beaton AZ, Boehme AK, Buxton AE, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2023 update: a report from the American heart association. Circulation. 2023;147:e93–e621. doi: 10.1161/CIR.0000000000001123 [DOI] [PubMed] [Google Scholar]
- 3.Chang SH, Chou IJ, Yeh YH, Chiou MJ, Wen MS, Kuo CT, See LC, Kuo CF. Association between use of non-vitamin K oral anticoagulants with and without concurrent medications and risk of major bleeding in nonvalvular atrial fibrillation. JAMA. 2017;318:1250–1259. doi: 10.1001/jama.2017.13883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turagam MK, Osmancik P, Neuzil P, Dukkipati SR, Reddy VY. Left atrial appendage closure versus oral anticoagulants in atrial fibrillation: a meta-analysis of randomized trials. J Am Coll Cardiol. 2020;76:2795–2797. doi: 10.1016/j.jacc.2020.08.089 [DOI] [PubMed] [Google Scholar]
- 5.Fauchier L, Cinaud A, Brigadeau F, Lepillier A, Pierre B, Gras D, Mansourati J, Deharo JC, Montalescot G, Defaye P. Major adverse events with percutaneous left atrial appendage closure in patients with atrial fibrillation. J Am Coll Cardiol. 2019;73:2638–2640. doi: 10.1016/j.jacc.2019.03.468 [DOI] [PubMed] [Google Scholar]
- 6.Tholakanahalli VN. The dilemma of peri-device leaks after left atrial appendage closure. JACC Clin Electrophysiol. 2021;7:1585–1587. doi: 10.1016/j.jacep.2021.08.015 [DOI] [PubMed] [Google Scholar]
- 7.Zhao MZ, Chi RM, Yu Y, Wang QS, Sun J, Li W, Zhang PP, Liu B, Feng XF, Zhao Y, et al. Value of detecting peri-device leak and incomplete endothelialization by cardiac CT angiography in atrial fibrillation patients post WATCHMAN LAAC combined with radiofrequency ablation. J Cardiovasc Electrophysiol. 2021;32:2655–2664. doi: 10.1111/jce.15222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Granier M, Laugaudin G, Massin F, Cade S, Winum PF, Freitag C, Pasquie JL. Occurrence of incomplete endothelialization causing residual permeability after left atrial appendage closure. J Invasive Cardiol. 2018;30:245–250 [PubMed] [Google Scholar]
- 9.Viles-Gonzalez JF, Kar S, Douglas P, Dukkipati S, Feldman T, Horton R, Holmes D, Reddy VY. The clinical impact of incomplete left atrial appendage closure with the WATCHMAN device in patients with atrial fibrillation: a PROTECT AF (Percutaneous Closure of the Left Atrial Appendage Versus Warfarin Therapy for Prevention of Stroke in Patients With Atrial Fibrillation) substudy. J Am Coll Cardiol. 2012;59:923–929. doi: 10.1016/j.jacc.2011.11.028 [DOI] [PubMed] [Google Scholar]
- 10.Dukkipati SR, Holmes DR, Jr, Doshi SK, Kar S, Singh SM, Gibson D, Price MJ, Natale A, Mansour M, Sievert H, et al. Impact of peridevice leak on 5-year outcomes after left atrial appendage closure. J Am Coll Cardiol. 2022;80:469–483. doi: 10.1016/j.jacc.2022.04.062 [DOI] [PubMed] [Google Scholar]
- 11.Chen M, Sun J, Wang QS, Zhang PP, Li W, Zhang R, Mo BF, Yu YC, Cai X, Yang M, et al. Long-term outcome of combined catheter ablation and left atrial appendage closure in atrial fibrillation patients. Int J Cardiol. 2022;368:41–48. doi: 10.1016/j.ijcard.2022.08.007 [DOI] [PubMed] [Google Scholar]
- 12.Qamar SR, Jalal S, Nicolaou S, Tsang M, Gilhofer T, Saw J. Comparison of cardiac computed tomography angiography and transoesophageal echocardiography for device surveillance after left atrial appendage closure. EuroIntervention. 2019;15:663–670. doi: 10.4244/EIJ-D-18-01107 [DOI] [PubMed] [Google Scholar]
- 13.Saw J, Fahmy P, DeJong P, Lempereur M, Spencer R, Tsang M, Gin K, Jue J, Mayo J, McLaughlin P, et al. Cardiac CT angiography for device surveillance after endovascular left atrial appendage closure. Eur Heart J Cardiovasc Imaging. 2015;16:1198–1206. doi: 10.1093/ehjci/jev067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen M, Sun J, Li W, Zhang PP, Zhang R, Mo BF, Yang M, Wang QS, Li YG. Sex differences in the combined ablation and left atrial appendage closure: results from LAACablation registry. JACC Asia. 2023;3:138–149. doi: 10.1016/j.jacasi.2022.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Behnes M, Akin I, Sartorius B, Fastner C, El-Battrawy I, Borggrefe M, Haubenreisser H, Meyer M, Schoenberg SO, Henzler T. --LAA occluder view for post-implantation Evaluation (LOVE)--standardized imaging proposal evaluating implanted left atrial appendage occlusion devices by cardiac computed tomography. BMC Med Imaging. 2016;16:25. doi: 10.1186/s12880-016-0127-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tzikas A, Holmes DR, Jr, Gafoor S, Ruiz CE, Blomstrom-Lundqvist C, Diener HC, Cappato R, Kar S, Lee RJ, Byrne RA, et al. Percutaneous left atrial appendage occlusion: the Munich consensus document on definitions, endpoints, and data collection requirements for clinical studies. Europace. 2017;19:4–15. doi: 10.1093/europace/euw141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charate R, Ahmed A, Della Rocca DG, Bloom S, Garg J, Pothineni NVK, DiBiase L, Turagam M, Gopinathannair R, Horton R, et al. Evaluation of multimodality LAA leak closure methods following incomplete occlusion: the LAA leak study. JACC Cardiovasc Interv. 2022;15:2158–2170. doi: 10.1016/j.jcin.2022.08.034 [DOI] [PubMed] [Google Scholar]
- 18.Sivasambu B, Arbab-Zadeh A, Hays A, Calkins H, Berger RD. Delayed endothelialization of WATCHMAN device identified with cardiac CT. J Cardiovasc Electrophysiol. 2019;30:1319–1324. doi: 10.1111/jce.14053 [DOI] [PubMed] [Google Scholar]
- 19.Lindner S, Behnes M, Wenke A, Sartorius B, Akin M, Mashayekhi K, Gawlitza J, Weidner KJ, Ansari U, Haubenreisser H, et al. Incomplete neo-endothelialization of left atrial appendage closure devices is frequent after 6 months: a pilot imaging study. Int J Cardiovasc Imaging. 2021;37:2291–2298. doi: 10.1007/s10554-021-02192-5 [DOI] [PubMed] [Google Scholar]
- 20.Alkhouli M, Du C, Killu A, Simard T, Noseworthy PA, Friedman PA, Curtis JP, Freeman JV, Holmes DR. Clinical impact of residual leaks following left atrial appendage occlusion: insights from the NCDR LAAO registry. JACC Clin Electrophysiol. 2022;8:766–778. doi: 10.1016/j.jacep.2022.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Afzal MR, Gabriels JK, Jackson GG, Chen L, Buck B, Campbell S, Sabin DF, Goldner B, Ismail H, Liu CF, et al. Temporal changes and clinical implications of delayed peridevice leak following left atrial appendage closure. JACC Clin Electrophysiol. 2022;8:15–25. doi: 10.1016/j.jacep.2021.06.018 [DOI] [PubMed] [Google Scholar]
- 22.Samaras A, Papazoglou AS, Balomenakis C, Bekiaridou A, Moysidis DV, Patsiou V, Orfanidis A, Giannakoulas G, Kassimis G, Fragakis N, et al. Residual leaks following percutaneous left atrial appendage occlusion and outcomes: a meta-analysis. Eur Heart J. 2024;45:214–229. doi: 10.1093/eurheartj/ehad828 [DOI] [PubMed] [Google Scholar]
- 23.Xu J, Chen CZ, Xing J, Wang L, Tao YR, Yang B, Zhang Q, Shen YL, Hu JQ. Clinical relevance of incomplete device endothelialization after left atrial appendage closure. Int J Cardiovasc Imaging. 2023;39:451–459. doi: 10.1007/s10554-022-02721-w [DOI] [PubMed] [Google Scholar]