Abstract
Cancer stem cells (CSCs) are a subset of cells with self-renewal ability and tumor generating potential. Accumulated evidence has revealed that CSCs were shown to contribute to tumorigenesis, metastasis, recurrence and resistance to chemoradiotherapy. Therefore, CSCs were regarded as promising therapeutic targets in cancer. This study is the first to reveal the development process, research hotspots, and trends of entire CSCs research field through bibliometric methods. All relevant publications on CSCs with more than 100 citations (notable papers) and the 100 most cited papers (top papers) during 1997 to 2023 were extracted and analyzed. Cancer research published the largest number of papers (184 papers). The USA accounted for the most publications (1326 papers). Rich, JN was the author with the most publications (56 papers) and the highest M-index (3.111). The most contributive institution was the University of Texas System (164 papers). Before 2007, research mainly focused on the definition and recognition of CSCs. Between 2007 and 2016, with the emergence of the terms such as “sonic hedgehog,” “metabolism,” “oxidative phosphorylation,” and “epithelial mesenchymal transition,” research began to shift toward exploring the mechanisms of CSCs. In 2016, the focus transitioned to the tumor microenvironment and the ecological niches. The analysis of papers published in major journals since 2021 showed that “transcription,” “inhibition,” and “chemoresistance” emerged as new focused issues. In general, the research focus has gradually shifted from basic biology to clinical transformation. “Tumor microenvironment” and “chemo-resistance” should be given more attention in the future.
Keywords: cancer stem cell (CSC), chemoresistance, hotspots, identification, mechanisms, tumor microenvironment, visualization
1. Introduction
Stem cells (SCs) are undifferentiated cells which have the ability to self-renew and proliferate for longer periods of time compared to non-stem cells, and can produce various types of cells in the body.[1,2] Despite its potential for proliferation, SCs typically remain inactive and stationary, which protects them from cell damage and mutations.[3] According to the different levels of differentiation potential exhibited by SCs, they can be divided into totipotency (maximum differentiation potential), pluripotency, multipotency, oligopotency, and monopotency (stem cells can only produce one cell type). Similar to normal SCs, cancer stem cells (CSCs) can also self-renew and differentiate into tumor cells.[4] In 1997, Bonnet and Dick first identified the existence and isolation of CSCs in human acute myeloid leukemia.[5] The classic definition of CSCs is a rare subset of cells with self-renewal ability and tumor-generating potential. CSCs represent the most aggressive cell groups within the tumor cluster, usually defined as the tumor-initiating cells.[6] However, unlike normal SCs, which are generally in a dormant state, CSCs are able to maintain their undifferentiated state through self-renewal and have very active high differentiation potential, thereby maintaining the stem cell pool and producing heterogeneous progeny of differentiated tumor cells.[7]
Two different theories have been proposed to explain the role of CSCs, namely the hierarchical model and the stochastic model. In the hierarchical theory, tumors originate from SCs that evade normal growth control and regulation. Therefore, they can be transformed into CSCs, making them the root cause of heterogeneous growth tumors and forming the biological basis of tumors. In the stochastic model, tumors originate from random mutations that occur in normal cells, and each cell in the tumor can trigger further lesions into CSCs.[8–10] As is well known, most cancer patients often have to cope with cancer recurrence and metastasis and develop drug resistance after one or more treatment failures, so the role of CSCs may explain this phenomenon. An increasing number of studies have shown that CSCs are key participants in tumor development, and the presence of their markers is often considered a negative prognostic factor and is associated with poor overall-free survival and poor disease-free survival among various tumor types.[11–15] In this case, specific patterns have been identified for identifying CSCs markers in some solid tumors. For example, CD133 is used as a CSCs marker in a range of tumors, including hepatocellular carcinoma, glioblastoma, colon cancer, and ovarian cancer.[16–19] Specifically, the CD133+CD44+ population can recognize human colon CSCs,[20] and ovarian CSCs are further enriched in ALDH+CD133+ cells.[21] Several transcription factors, including Oct4, Sox2, Klf4, and c-Myc, have been found to convert differentiated cells back into pluripotent stem phenotypes, therefore these transcription factors with the ability to self-renew and differentiate in CSCs can be considered as CSC markers.[22] Moreover, side population (SP) cells are used to identify CSCs in a series of solid tumors,[23–25] and SP analysis is increasingly being used as an indicator of stemness and therapeutic resistance.[26–29]
Bibliometrics is a new data-driven method that applies statistical methods to scientific outputs, which has knowledge-oriented quantitative functions. It can find out the knowledge association between publications through the filtering and processing of massive information, therefore dig out the potential knowledge value.[30] Bibliometric citation analysis can map the literature, which summarizes the work done in the field and reveals research trends. The citation scale of an article illustrates its importance and reflects its direct impact on research in this area. Comments by Gates et al on Nature commemorating its 150th anniversary even highly recognize the help of bibliometrics for contemporary scientific inspiration and trend identification.[31] Obviously, it is undoubtedly scientific and effective for revealing and digging the development law of a certain research field and providing new academic views by using bibliometric methods.
CSCs are becoming a hot field for studying tumor metastasis and recurrence. New strategies for CSCs and clinical trials against CSCs are receiving increasing attention from researchers. Over the last few decades, there has been an increasing amount of literature on CSCs. Currently, several articles have conducted bibliometric analysis on SCs or CSCs. In bibliometric studies of SCs, Liu et al retrieved 552 publications related to SCs and conducted a comprehensive analysis of the existing research in SCs precision medicine from 2018 to 2022.[32] Another bibliometric analysis summarized the global trends in the field of extracellular vesicles based on SCs research from 1991 to 2021.[33] In bibliometric analysis of CSCs, Guo et al revealed the current status and hotspots of CSCs-derived exosomes and tumor microenvironments globally from 2009 to 2022.[34] Moreover, a report of Song et al identified current state and hotspots of glioma stem cells research during 2003 to 2021 through bibliometric methods.[35] However, the current bibliometric research on CSCs mainly focused on a specific type of cancer or the components within cancer cells, and there is no bibliometric analysis of the research progress and hotspots covering the entire field of CSCs. In addition, previous studies have only been updated until 2022. In our study, we filtered and evaluated the notable papers (i.e., papers cited more than 100 times) and top papers (i.e., the 100 most cited papers) in the entire field of CSCs from 1997 to 2023 (i.e., from the year CSCs first been identified to the very recent year) using bibliometric citation analysis to provide researchers with an overview of research progress in CSCs and valuable insights into future research directions.
2. Materials and methods
2.1. Data sources and search strategies
In light of the necessity for high-quality literature and adherence to proper citation formats, we selected the Science Citation Index-Expanded from the Web of Science Core Collection (WoSCC) as our data source. The WoSCC, which encompasses over 12,000 premier scientific journals, is recognized for its reliable database.[36] It has been deemed the superior choice for bibliometric studies in previous research due to its reliability and breadth of coverage.[37]
Scientific output data downloaded from SCI-EXPANDED of WoSCC was conducted on May 1, 2023. The retrieval time span was 1997 to 2023, and the publication type was limited to articles and reviews in English. The author designed a retrieval strategy that considered various writing formats and tested it several times to ensure its accuracy. The detailed retrieval strategy is presented in Data S1, Supplemental Digital Content, http://links.lww.com/MD/M540. Then sorted the above results by citations and screened the results to exclude irrelevant papers to obtain the data of papers with more than 100 citations in CSCs. We used Web of Science to extract and analyze publication dates, journals, countries/regions, institutions, total citations, and average citations per year. To evaluate the most influential in the field, we selected the 100 most cited studies and defined them as top papers for analysis. The authors then identified the journals which published top-papers and calculated the top-cited paper rates (TPR) of the journals (percentage of top-papers among all papers with more than 100 citations in CSCs in a journal). Journals with a TPR > 5% were considered as major journals on CSCs.[38] The papers published in these journals since 2021 were analyzed to evaluate recent research hotspots.
Two independent and trained researchers reviewed all the articles. They recorded the following information for each article: The CSC type, research contents, and research topic. If they disagreed on an article, the senior researcher read it again and made a final decision. This study does not contain any research involving humans or animals, therefore ethical approval is not required.
2.2. Bibliometric and visualized analysis
Descriptive statistical analysis and graphical analysis were conducted using Excel software (Microsoft, Redmond, WA). Bibliometric analysis and data visualization were performed using the “bibliometrix” packages of the R software and the software VOSviewer (v1.6.14). VOSviewer is capable of generating 3 distinct types of visualization maps: network, density, and overlay, each carrying unique implication.[39] In these knowledge maps, each node represents an element such as a country, institution, or author. The links between nodes depict the relationships among these elements. The size of a node can be determined by various factors, including the number of publications and the frequency of citations or occurrences. In this paper, the size of nodes is mainly determined by the total link strength (TLS). The nodes and links are color-coded to differentiate clusters or to indicate the corresponding average appearing year.[40] In this study, we established a literature co-occurrence network based on journals, countries, authors, and keywords using VOSviewer, and conducted co-occurrence analysis and visualization.
CiteSpace, a Java-based bibliometric tool developed by Chaomei Chen of Drexel University, offers another avenue for analyzing the evolution and research clusters within a given topic.[41] In this study, we utilized CiteSpace (v6.2.R3) to conduct visual analyses such as burst words analysis and reference co-citation analysis. These analyses allow us to identify references or keywords that have garnered significant attention within a specific timeframe, a process also known as burst detection. The parameters set for CiteSpace in this study were as follows: time span (from July 1, 1997 to May 1, 2023), slice length (3 years), selection criterion (g-index with k = 25), link retention factor, lookback years, and pruning method.
In summary, we first conducted an academic contribution evaluation using the “bibliometrix” packages of the R software, then used software VOSviewer to conduct co-occurrence analysis to evaluate the research status in the field of CSCs, and finally evaluated the research hotspots and development trends in the field of CSCs by using Citespace software.
3. Results
3.1. General analysis
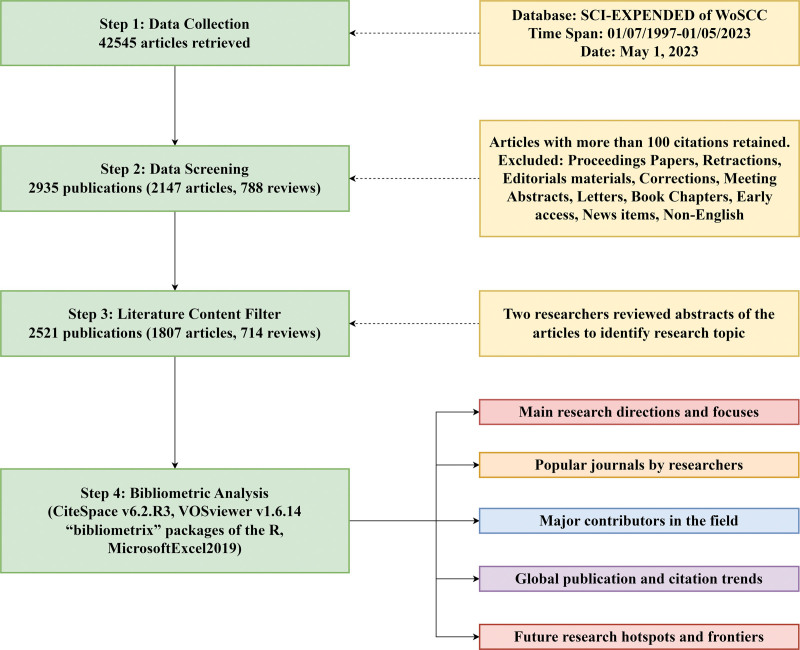
We retrieved 42,545 publications and selected papers that had been cited more than 100 times, limiting the document type to articles and reviews, with a total of 2935 records. The specific retrieval process is shown in Figure 1. After the initial search, we manually filtered the records by checking the content of the articles and excluded literature that was irrelevant to the topic. Finally, we retained 2521 papers, including 1807 articles and 714 reviews. These papers had a total of 657,611 citations and a median number of 160 citations (range, 100–7786). The paper with the highest number of total citations (7786 times) was “Prospective identification of tumorigenic breast cancer cells” by Al-Hajj et al (2003), published in Proceedings of the National Academy of Sciences of the United States of America (PNAS). The most recent paper among those with more than 100 citations in CSCs was “Cancer stem cell-immune cell crosstalk in tumor progression” by Bayik and Lathia (2021), published in Nature Reviews Cancer.
Figure 1.
Flowchart of literature screening and data analysis.
3.2. Publication time and type
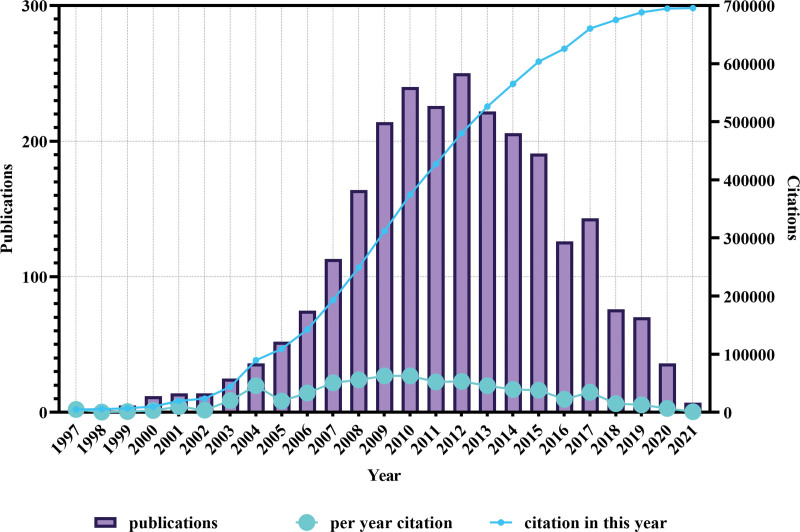
The literature search yielded 2521 papers on CSCs published since 1997 (Fig. 2), and the year with the most papers was 2012 (250 papers), followed by 2010 (240 papers). In terms of citations, papers published in 2010 (62,814/657,611) contributed the greatest citations. According to WOS categories, the majority of publications mainly focused on “oncology” (1115), followed by “cell biology” (689), “biochemistry & molecular Biology” (352), “hematology” (222) and “research & experimental medicine” (148).
Figure 2.
Time of publication and distribution of citations of the papers on CSCs. The cyan line indicates the total citations of papers published each year. The blue line indicates the total citations of all papers each year.
3.3. Top papers
The identified 100 most cited articles (top papers) were listed in Table S1, Supplemental Digital Content, http://links.lww.com/MD/M541. These 100 articles accounted for only 4% of the total number of publications but their citation number accounted for 24.9% of citations for all articles on CSCs (164,043/657,611). The median number of citations of the top papers was 1076.5 (range: 811–7786). The 10 most cited papers in CSCs were listed in Table 1. Among the 10 most cited papers, there were 3 studies on glioma stem cells, 3 studies on colon cancer, and one study on breast cancer and leukemia. The paper with the highest average citations per year (282.5) was “Prospective identification of tumorigenic breast cancer cells” by Al-Hajj et al (2003), published in PNAS. The paper titled “New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer” by Dongre and Weinberg (2019), published in Nature Reviews Molecular Cell Biology, was the most recent publication among the 100 most cited papers.
Table 1.
The 10 most cited papers in CSCs between 1997 and 2023.
| Rank | Title | Corresponding author | Journal | Year | Total citations | Average citations per year (rank) |
|---|---|---|---|---|---|---|
| 1 | Prospective identification of tumorigenic breast cancer cells | Al-Hajj, M[42] | PNAS | 2003 | 7786 | 282.5 (1) |
| 2 | Stem cells, cancer, and cancer stem cells | Reya, T[43] | Nature | 2001 | 7206 | 183.2 (2) |
| 3 | The epithelial-mesenchymal transition generates cells with properties of stem cells | Mani, SA[44] | Cell | 2008 | 6478 | 135.2 (7) |
| 4 | Identification of human brain tumor initiating cells | Singh, SK | Nature | 2004 | 5666 | 172.7 (5) |
| 5 | Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell | Bonnet, D[5] | Nature Medicine | 1997 | 5010 | 182.4 (3) |
| 6 | Glioma stem cells promote radioresistance by preferential activation of the DNA damage response | Bao, S | Nature | 2015 | 4614 | 148.7 (6) |
| 7 | Identification of a cancer stem cell in human brain tumors | Singh, SK[45] | Cancer Research | 2003 | 3866 | 102.3 (10) |
| 8 | Identification of stem cells in small intestine and colon by marker gene Lgr5 | Barker, N | Nature | 2007 | 3815 | 110.4 (9) |
| 9 | A human colon cancer cell capable of initiating tumor growth in immunodeficient mice | O’Brien, CA[46] | Nature | 2007 | 3202 | 117.2 (8) |
| 10 | ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome | Ginestier, C | Cell Stem Cell | 2007 | 2999 | 178.6 (4) |
3.4. Journals
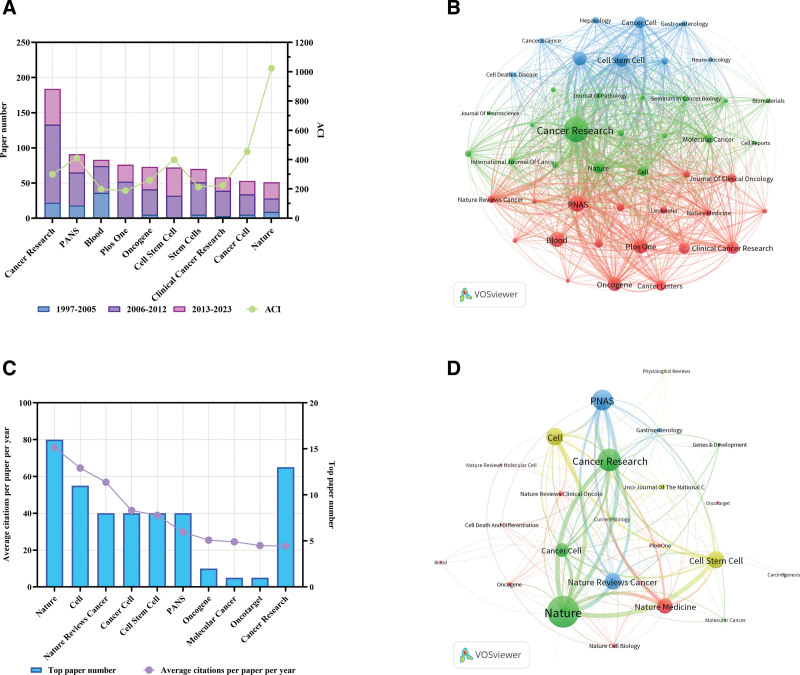
A total of 483 journals participated in 2521 articles with over 100 citations published in CSCs research. The top 10 journals with the highest publications were shown in Table 2. Cancer Research published the largest number of papers (184 papers), followed by PNAS (91 papers) (Fig. 3A), and these 2 journals were at the center of the citation network based on the co-occurrence analysis (Fig. 3B). In addition, several other journals had also published over 50 articles with over 100 citations, including Blood (83 papers), Plos One (76 papers), Oncogene (73 papers), Cell Stem Cell (72 papers), Stem Cells (70 papers), Clinical Cancer Research (58 papers), Cancer Cell (53 papers) and Nature (51 papers) (Table 2). It is worth noting that Nature had the highest average citations per item (ACI) at 1023.41, despite ranking tenth out of articles with over 100 citations published in the field of CSCs.
Table 2.
The top 10 productive journals in CSCs between 1997 and 2023.
| Journals with most papers | Paper number | Total citations | ACI* | Citations per paper per year | IF (2023) |
|---|---|---|---|---|---|
| Cancer research | 184 | 55,003 | 298.93 | 22.3 | 11.2 |
| PNAS | 91 | 37,422 | 411.23 | 29.9 | 11.1 |
| Blood | 83 | 16,450 | 198.19 | 14.1 | 20.3 |
| Plos One | 76 | 14,291 | 188.04 | 16.2 | 3.7 |
| Oncogene | 73 | 18,963 | 259.77 | 25.4 | 8.0 |
| Cell stem cell | 72 | 28,703 | 398.65 | 38.9 | 23.9 |
| Stem cells | 70 | 14,982 | 214.03 | 17.3 | 5.2 |
| Clinical cancer research | 58 | 12,974 | 223.69 | 19.2 | 11.5 |
| Cancer cell | 53 | 24,082 | 454.38 | 41.7 | 50.3 |
| Nature | 51 | 52,194 | 1023.41 | 77.5 | 64.8 |
Average citations per item.
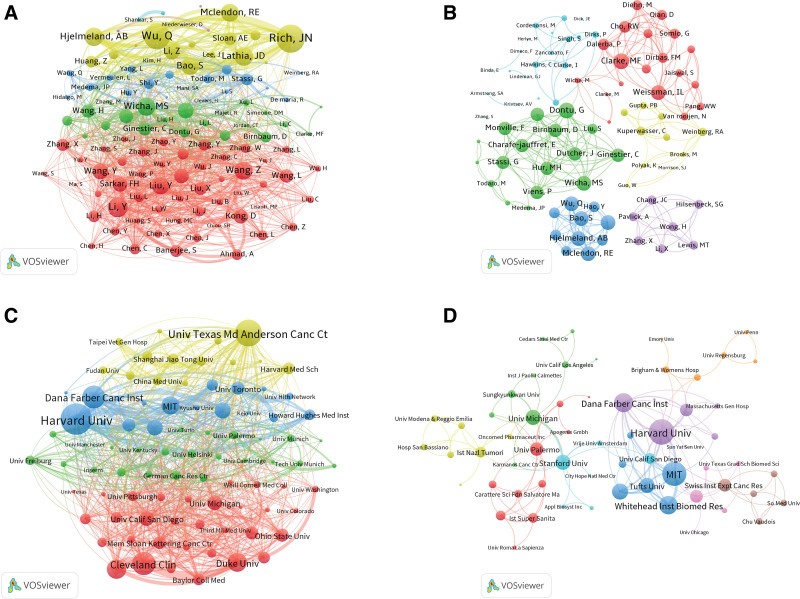
Figure 3.
Analysis of journals related to CSCs research. (A) Paper numbers and ACI of the top 10 productive journals. (B) Co-occurrence network of journals related to CSCs by using VOSviewer. The circle size represents the total link strength. The width of the curved line represents the strength of the connection. The journals in the same color are similar research areas. (C) Top 10 journals with the most citations per paper per year. (D) Co-occurrence network of journals with top-papers related to CSCs by using VOSviewer. The circle size represents the total link strength. The width of the curved line represents the strength of the connection. The journals in the same color are of similar research areas.
After ranking the journals with average citations per paper per year in CSCs, the top 10 journals were shown in Table 3 and Figure 3C. Nature had the most significant average number of citations per paper per year (75.7), followed by Cell (64.6) and Nature Reviews Cancer (54.9). The co-occurrence analysis of the journals of top papers showed that the TLS of Nature was the highest (Fig. 3D). The Nature, Cell and the other 5 journals had a TPR of >5% (Table 3). Thus, the authors considered these journals as major journals on CSCs. A total of 187 papers with more than 100 citations published in these journals since 2021 were identified (Table S2, Supplemental Digital Content, http://links.lww.com/MD/M542). Among the major journals, Cancer Research published the most papers (161 papers) since 2021.
Table 3.
The top 10 journals with most citations per paper per year in CSCs between 1997 and 2023.
| Journals | Paper number | Top-paper number | TPR* | Total citations | ACI | Citation per paper per year | IF (2023) |
|---|---|---|---|---|---|---|---|
| Nature | 51 | 16 | 31.4% | 52,194 | 1023.4 | 75.7 | 64.8 |
| Cell | 37 | 11 | 29.7% | 28,103 | 759.5 | 64.6 | 64.5 |
| Nature reviews cancer | 27 | 8 | 29.6% | 18,463 | 683.8 | 56.9 | 78.5 |
| Cancer cell | 53 | 8 | 15.1% | 24,082 | 454.4 | 41.5 | 50.3 |
| Cell stem cell | 72 | 8 | 11.1% | 28,703 | 398.7 | 38.9 | 23.9 |
| PNAS | 91 | 8 | 8.8% | 37,422 | 411.2 | 29.8 | 11.1 |
| Oncogene | 73 | 2 | 2.7% | 18,963 | 259.8 | 25.4 | 8.0 |
| Molecular cancer | 37 | 1 | 2.7% | 7267 | 196.4 | 24.5 | 37.3 |
| Oncotarget | 51 | 1 | 2% | 9211 | 180.6 | 22.5 | N |
| Cancer research | 184 | 13 | 7.1% | 55,003 | 298.9 | 22.1 | 11.2 |
Percentage of top-papers among all papers with more than 100 citations in CSCs in a journal.
3.5. Countries
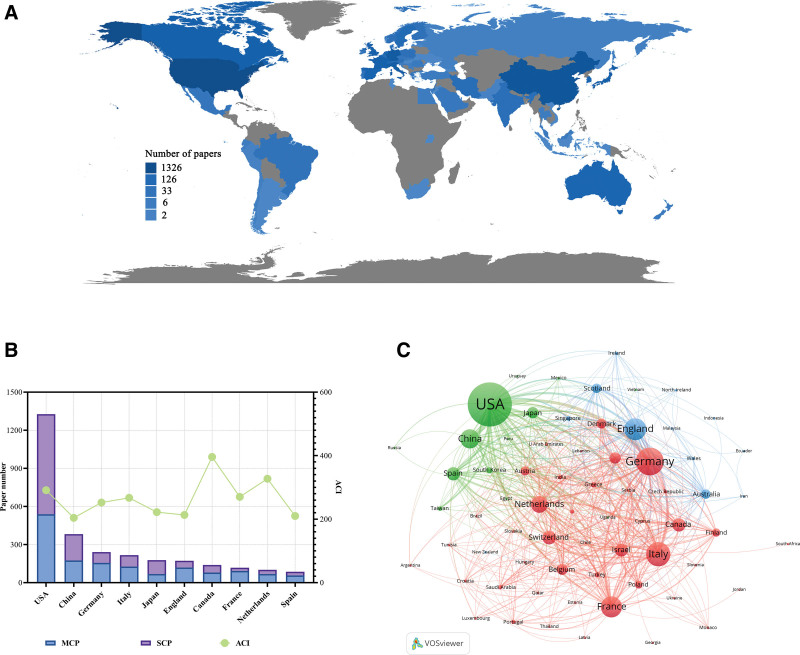
The cited more than 100 times papers in CSCs were written by authors from 67 countries or regions, and corresponding authors were from 62 countries or regions (Fig. 4A). The top 10 high-output countries or regions were listed in Figure 4B and Table 4. USA stood out as the most prolific contributor with a total of 1326 publications (52.6%), underscoring its dominant role in the field. Followed by the People’s Republic of China with 382 publications (15.2%), and Germany with 241 publications (9.6%). Other notable contributors included Italy, Japan, England, Canada, France, Netherlands, and Spain, each with publication counts ranging from 86 to 217. In terms of average citations, Canada had the highest ACI at 396.01. Netherlands (327.27) and USA (291.17) followed closely behind. Collaboration in scientific research often manifests as multi-country publications (MCP). The USA had the highest number of MCP at 538 (Table 4). Figure 4C displayed a visual map of the co-occurrence network between countries. A node of each color represents a country, and a thicker line between 2 nodes indicates closer cooperation between countries. The USA was at the center of its cooperation with other countries, most closely with China, Japan, Italy, and Germany.
Figure 4.
Analysis of countries related to CSCs research. (A) Visualization world map of paper number. (B) Total number of papers and ACI in the corresponding author’s countries. MCP, multiple-country publications; SCP, single-country publications. (C) Co-occurrence network of countries related to CSCs by using VOSviewer. The circle size represents the total link strength. The width of the curved line represents the strength of the connection.
Table 4.
The top 10 productive countries in CSCs between 1997 and 2023.
| Country | Articles | Percentage (N/2521) | Total citations | ACI | Top papers count | MCP* |
|---|---|---|---|---|---|---|
| USA | 1326 | 52.60% | 386,088 | 291.17 | 35 | 538 |
| China | 382 | 15.15% | 77,947 | 204.05 | 24 | 175 |
| Germany | 241 | 9.56% | 60,793 | 252.25 | 11 | 155 |
| Italy | 217 | 8.61% | 57,989 | 267.23 | 7 | 126 |
| Japan | 177 | 7.02% | 39,327 | 222.19 | 5 | 68 |
| England | 172 | 6.82% | 36,673 | 213.22 | 1 | 119 |
| Canada | 139 | 5.51% | 55,046 | 396.01 | 2 | 80 |
| France | 118 | 4.68% | 31,876 | 270.14 | 1 | 92 |
| Netherlands | 101 | 4.01% | 33,054 | 327.27 | 4 | 68 |
| Spain | 86 | 3.41% | 18,047 | 209.85 | 2 | 56 |
Multi-country publications.
3.6. Authors and institutions
A total of 14,387 authors participated in the research of CSCs. Table 5 listed the top 10 productive authors in the field of CSCs according to the number of papers. The most published author in this field was Rich, JN from the University of Pittsburgh School of Medicine (56 papers), followed by Wicha, MS from the University of Michigan (45 papers), and Wu, QL from the University of California (28 papers) (Table 5). M-index is often utilized to assess academic productivity. Rich, JN led with an impressive M-index of 3.111, followed by Wicha, MS and Lathia, JD with indices of 2.143 and 1.563, respectively. Of the 10 most productive authors, 7 were from the USA, and the other 3 were from Italy, China, and Australia. Meanwhile, the number of citations of an article can often evaluate the influence of a researcher in a certain discipline. The papers of Clarke, MF from the Stanford University had the highest total number of citations (28,558 citations) and the highest ACI (28,558/20 = 1427.9 citations per paper), although he had published only 20 papers. Followed by Wicha, MS from the University of Michigan (24,891 citations), Rich, JN from the University of Pittsburgh School of Medicine (20,114 citations). The author co-occurrence network in Figure 5A showed that Rich, JN had the highest TLS, and the analysis of the author co-occurrence network of authors of top papers (Fig. 5B) showed that Clarke, MF was in the most critical position.
Table 5.
The top 10 productive Authors in CSCs between 1997 and 2023.
| Name | Country | Paper number | Total citations | M-index | ACI | Articales fractionalized* | Local citation† | Top-paper number |
|---|---|---|---|---|---|---|---|---|
| Rich, JN | USA | 56 | 20,114 | 3.111 | 374.79 | 9.11 | 1315 | 4 |
| Wicha, MS | USA | 45 | 24,891 | 2.143 | 592.84 | 8.42 | 2180 | 5 |
| Wu, QL | USA | 28 | 11,221 | 1.556 | 418.86 | 2.24 | 792 | 2 |
| Lathia, JD | USA | 25 | 6660 | 1.563 | 274.96 | 2.79 | 378 | 1 |
| Stassi, G | Italy | 24 | 9464 | 1.412 | 413.96 | 2.48 | 746 | 4 |
| Liu, Y | China | 23 | 4296 | 1.438 | 205.45 | 2.4 | 112 | 0 |
| Sarkar, FH | USA | 22 | 4256 | 1.375 | 203.5 | 3.39 | 230 | 0 |
| Hjelmeland, AB | USA | 21 | 11,177 | 1.167 | 558.1 | 2.09 | 966 | 3 |
| Li, Y | Australia | 21 | 3323 | 1.313 | 220.66 | 2 | 63 | 0 |
| Weinberg, RA | USA | 21 | 18,766 | 1.313 | 934.38 | 5.48 | 958 | 5 |
Articles fractionalized = paper number/total number of authors of the papers.
Citation number in the current dataset (papers with more than 100 citations in CSCs between 1997 and 2023).
Figure 5.
Analysis of authors and institutions related to CSCs research by using VOSviewer. The circle size represents total link strength. The width of the curved line represents the strength of the connection. (A) Network visualization of authors related to CSCs. The authors in the same color have stronger collaboration with each other. (B) Network visualization of authors with top papers related to CSCs. The authors in the same color have stronger collaboration with each other. (C) Network visualization of institutions related to CSCs. The institutions in the same color have stronger collaboration with each other. (D) Network visualization of institutions with top papers related to CSCs. The institutions in the same color have stronger collaboration with each other.
Table 6 listed the top 10 institutions that published CSCs publications. Eight are located in the USA, one in Canada, and one in France. The University of Texas System stood out with the most publications (164), followed by Harvard University (145) and MD Anderson Cancer Center (131). The institutional co-occurrence analysis was depicted in Figure 5C. Harvard University, Dana-Farber Cancer Institute, and MD Anderson Cancer Center emerged as central nodes. Furthermore, among the top 100 cited articles, Harvard University, MIT and Dana-Farber Cancer Institute were central nodes (Fig. 5D).
Table 6.
The top 10 productive institutions in CSCs between 1997 and 2023.
| Affiliation | Country | Articles | Percentage (N/2521, %) | Top-paper number | Top-paper number rank |
|---|---|---|---|---|---|
| University of Texas System | USA | 164 | 6.505% | 6 | 17 |
| Harvard University | USA | 145 | 5.752% | 13 | 1 |
| MD Anderson Cancer Center | USA | 131 | 5.196% | 3 | 53 |
| University of California System | USA | 127 | 5.038% | 7 | 11 |
| University of Michigan | USA | 101 | 4.006% | 12 | 2 |
| Harvard Medical School | USA | 97 | 3.848% | 9 | 5 |
| Udice French Research Universities | Franch | 87 | 3.451% | 5 | 31 |
| National Institutes of Health | USA | 83 | 3.292% | 4 | 37 |
| University of Toronto | Canada | 69 | 2.737% | 8 | 7 |
| National Cancer Institute | USA | 67 | 2.658% | 4 | 37 |
3.7. Research heat and focus of different types of CSCs
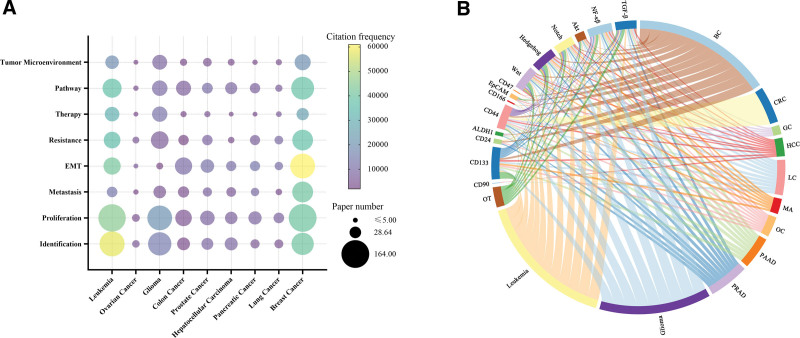
The research heat of different CSCs was shown in Figure 6A. Citation frequency (302,926 citations) and paper number (781 papers) of breast cancer were higher than those of other cancers. The research topic of tumor proliferation received the most attention from articles (663 papers), and the most cited research topic was epithelial-mesenchymal transition (EMT) (168,069 citations). The surface markers and signaling pathways of CSCs have always been the focus of research, which are related to the identification, proliferation, and therapeutic targets of CSCs. The chord diagram of the relationships between different types of CSCs, signal pathways and surface markers was shown in Figure 6B. CD133 and CD44 were the most studied, with 128 and 97 papers, respectively, playing an important role in the identification of CSCs. NF-κβ (98 times), Wnt (94 times) and Hedgehog (93 times) were the most extensively studied signaling pathways in CSCs.
Figure 6.
Research heat and focus of different types of CSCs. (A) Distribution of research topic and citations of different CSCs. The node size represents the number of publications. the color represents the citation frequency of the papers. (B) Chordal diagram of different types of CSCs, signaling pathways and surface markers of CSCs. BC = breast cancer, CRC = colorectal cancer, EC = esophageal cancer, GC = gastric cancer, HCC = hepatocellular carcinoma, LC = lung cancer, MA = melanoma, OC = ovarian cancer, OT = other tumors, including bladder cancer, cervical cancer, esophageal cancer, lymphoma, myeloma, thyroid cancer and kidney cancer, PAAD = pancreatic adenocarcinoma, PRAD = prostate adenocarcinoma, SCLC = small-cell lung cancer.
3.8. Co-cited references analysis
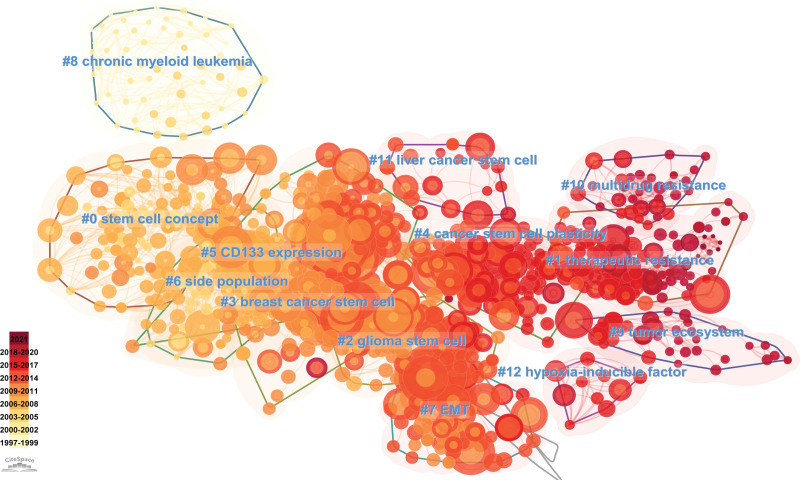
We generated a visual representation of the field of CSCs research using latent semantic indexing technology via CiteSpace and clustering techniques based on title words. Co-cited references cluster revealed that the primary research focus in the field of CSCs included cancer stem cell plasticity, EMT, and therapeutic resistance (Fig. 7). Among various tumor types, glioma, liver cancer, breast cancer, and leukemia had emerged as the most extensively studied. It is noteworthy that these research hotspots had evolved over time. The initial research mainly focused on stem cell concept, expression of CD133, and SP. While recent research had shifted to tumor ecosystem and therapeutic resistance.
Figure 7.
Citespace reference co-citation analysis network (Cluster View) in CSCs research from 1997 to 2021. The network has a modularity of 0.7239 and an average silhouette score of 0.8819. The labels of each cluster are exhibited beside the blocks. The color of the clusters shows when the co-citation links happened. The yellow color means the citing year is relatively early, and the red color indicates that the citation time is relatively recent.
3.9. Keywords analysis and research trends
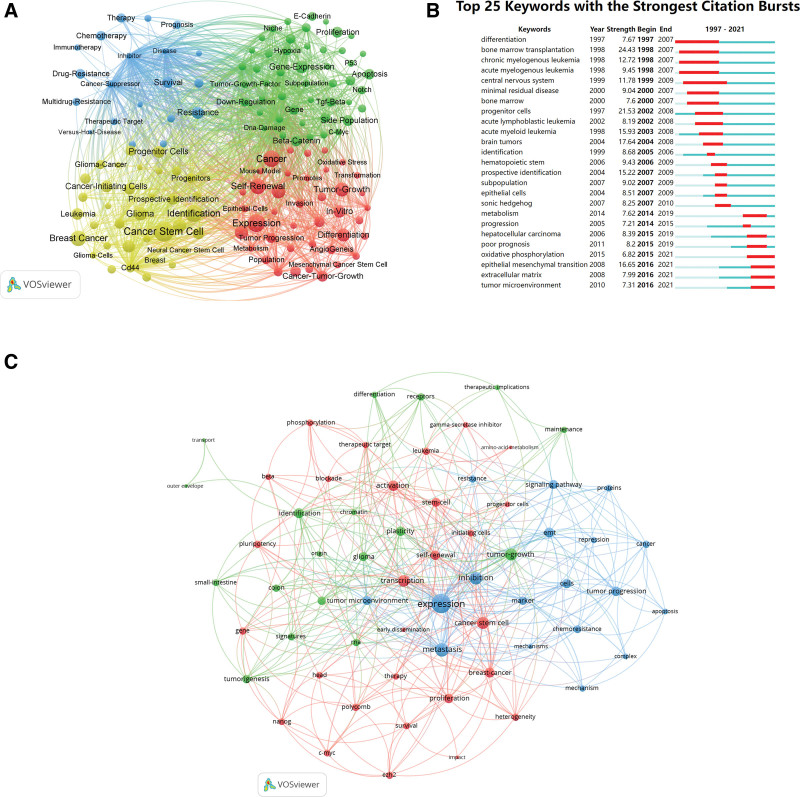
Utilizing VOSviewer software, we extracted 60 keywords with a frequency of 20 or more from a pool of 3838 keywords for analysis (Fig. 8A). These keywords were categorized into 4 main clusters: “CSCs properties” (represented by red nodes), “molecular biology properties” (green nodes), “tumor therapy” (blue nodes), and “CSCs identification (yellow nodes).” The keyword “cancer stem cell” emerged as the most TLS, appearing 4289, followed by “expression” (3089), and “identification” (2649). Within the “CSCs properties” cluster, the standout keywords were “expression” (3089), “cancer” (2441), and “self-renewal” (1935). In the “molecular biology properties” cluster, the most TLS words were “gene-expression” (1012), “proliferation” (720), “side population” (703), and “beta-catenin” (651). In the “tumor therapy” cluster, studies frequently focused on “resistance” (920), “survival” (687), “drug-resistance” (518), and “chemotherapy” (467). In the “CSCs identification” cluster, studies frequently focused on “cancer stem cell” (4289), “identification” (2649), “breast cancer” (1981), and “glioma” (1329).
Figure 8.
Analysis of keywords and research trends related to CSCs research. (A) Co-occurrence network of keywords that occurred at least 20 times in the papers by using VOSviewer. The circle size represents the total link strength. The width of the curved line represents the strength of the connection. The keywords in the same color are similar areas. (B) Top 25 keywords with the strongest citation bursts in papers of CSCs research. The green line indicates the timeline. The intervals in which bursts were found are indicated by red sections on the timeline, indicating the start year, end year, and burst duration according to the average year of publication. (C) Co-occurrence network of keywords published in major journals between 2021 and 2023 based on cluster analysis by using VOSviewer. The circle size represents the total link strength. The width of the curved line represents the link strength. The distance between 2 keywords approximates the relatedness of the nodes.
Burst words were used to track the evolution of research topics over time. Figure 8B showed that before 2007, the primary terms were related to the definition and identification of CSCs. The research on CSCs has undergone a transformation from leukemia to solid tumors. Between 2007 and 2016, the emergence of the terms “sonic hedgehog,” “metabolism,” “oxidative phosphorylation” and “epithelial mesenchymal transition” indicated a shift in research topics, emphasizing the mechanisms of CSCs. By 2016, the term “tumor microenvironment” became prominent, reflecting a focus on the tumor microenvironment and the ecological niches of tumor cells.
An analysis was conducted on 187 CSC papers published in major journals since 2021 by constructing a keyword co-occurrence network (Fig. 8C). The keywords which received wider attention than the previous analysis included “transcription,” “inhibition,” and “chemoresistance.”
4. Discussion
This study is the first to use bibliometric analysis to visualize the research hotspots of articles cited more than 100 times (notable papers) and the 100 most cited papers (top papers) in the entire field of CSCs and provided a comprehensive and professional analysis, including academic contributions and the evolution of subject research hotspots. We selected the WOS database as the data source and included 2521 articles into the analysis, and then used VOSviewer software and Citespace software to display the intricate bibliometric network of CSCs research. The results of bibliometric analysis showed that the research on CSCs maintained a high level of enthusiasm. Based on research evidence, researchers’ interest has been increasing over the past decade. As a result, both the number of annual publications in the field and the total citations of research articles have significantly increased.
Since Bonnet and Dick discovered CSCs in leukemia,[5] CSCs research has attracted attention. The number of articles published each year gradually increased. In 2006, the American Association for Cancer Research defined CSCs as “a cell within a tumor that possesses the capacity to self-renew and to cause the heterogeneous lineages of cancer cells that comprise the tumor.” Then, papers on CSCs began to surge. Based on the average number of citations per year for an article, most articles with higher average citations per year were published before 2010. The reason might be that with the development of the CSCs theory, the research topic became more diverse.
Among the 100 most frequently cited articles, 30 articles were about the identification of CSCs, 48 were about the mechanism exploration of CSCs, and 14 were about the significance of CSCs for tumor therapy. Tumors that received the most attention were leukemia (29 times), followed by breast cancer (19 times), glioma (9 times), colorectal cancer (7 times), and pancreatic cancer (5 times). The highest citation paper was “Prospective identification of tumorigenic breast cancer cells” published in PNAS in 2003.[42] As the first paper to identify CSCs in solid tumors, it suggested that the tumor stem cell model could be applied to various tumors. It provided solid evidence for the existence of CSCs. The second highest citation article was a review of CSCs that was published in Nature in 2001.[43] It proposed that the characteristics of CSCs were self-renewal and proposed different signaling pathways to regulate CSCs. These ideas have been widely accepted by other researchers. The article with the third highest citation was about the relationship between EMT and CSCs, which was published by Cell in 2008.[44] It confirmed that EMT could induce the conversion of differentiated tumor cells into CSCs.
In evaluating the landscape of publications on CSCs research, the journal Cancer Research emerged as a significant contributor, publishing a total of 184 articles. In contrast, Nature published only 51 articles, yet these garnered a staggering 52,194 citations, with 16 articles classified as top papers, underscoring the high quality of its contributions. Notably, while Plos One and Stem Cells might not have high impact factors, they ranked fourth and seventh in terms of publication volume, respectively, and occupied pivotal positions in the co-occurrence analysis. This underscored that a journal’s impact factor shouldn’t be the only indicator for evaluating it. To further assess the journal’s value, we calculated the TPR of journals with CSCs publication volume >20. Nature, Cell, and Nature Reviews Cancer all had TPR rates exceeding 20%, distinguishing them as leading journals in the field of CSCs. Their prominence was also reflected in the co-occurrence analysis of journals with top papers sources. Interestingly, while Cancer Research ranked second in TLS, it placed 10th in average citations per paper per year. Given its substantial publication volume, this suggested that Cancer Research not only published influential research but was also open to innovative ideas that might not always garner widespread attention.
The USA had made outstanding research contributions in this field, with more publications than the total number of countries ranked 3rd to 10th. Notably, Canada and Netherlands, early contributors in CSCs research, had demonstrated impressive ACIs of 396.01 and 327.27, respectively, despite their modest publication counts of 139 and 101 papers. Canada’s prominence in the field could be traced back to the pioneering work by Bonnet and Dick, published in Cancer Research in 1997.[5] The University of Toronto, as the first institution to identify CSCs, boasted an impressive ACI of 639.37, much higher than other institutions. This institution continued its influential contributions and groundbreaking work, such as “Identification of a cancer stem cell in human brain tumors” in 2003 and “A human colon cancer cell capable of initiating tumor growth in immunodeficient mice” in 2007,[45,46] making it the first institution to identify glioma stem cells and colon cancer stem cells. Remarkably, the publications from the University of Toronto received 34,526 citations, accounting for 62.7% of the total citations in the field of CSCs in Canada. Undoubtedly, the University of Toronto is a key factor in Canada’s high ACI in this research field. Unlike Canada, the Netherlands had multiple institutions with high ACI. The University of Amsterdam, with an ACI of 320.98 in the field of CSCs, published a famous paper in 2010 titled “Wnt activity defines colon cancer stem cells and is regulated by the microenvironment,”[47] which had been cited 1340 times. This paper was pivotal in elucidating the role of the Wnt pathway in CSCs. Additionally, the University Medical Center Utrecht and Vrije University Amsterdam had ACIs of 434 and 280, respectively, indicating the Netherlands’ substantial contributions to the field of CSCs.
Moreover, our analysis of MCP for countries with over 50 papers (15 countries/regions) revealed that 10 countries had more than 50% of their publications resulting from international collaborations. This highlights the significant role of multinational cooperative research in the field of CSCs. Among them, Switzerland accounted for the highest proportion, reaching 85.29%. In contrast, despite the significant contributions made by the USA, only 40.57% of its publications came from MCP, ranking 14th. This could be attributed to the presence of numerous influential research institutions within the country. It is also worth noting that although the number of publications in China and Japan was high, <50% of their papers came from international cooperation. It would be beneficial for these nations to enhance their international research collaborations and exchanges.
Rich, JN from the University of Pittsburgh School of Medicine and Clarke, MF from the Stanford University made the greatest contributions to the field of CSCs. Rich, JN was the most prolific author. Clarke, MF had the highest total number of citations and the highest ACI. The majority of the most productive authors and institutions were from developed nations. However, over the past decade, developing countries in Asia and South America, including China, Brazil, India, and Iran, had increased their publication output, playing an increasingly important role in this field. In addition, the top 6 productive institutions in this field were the USA, with the University of Texas System leading in publications (164). Harvard University had published the most articles among the top 100 most-cited articles, with a total of 13 papers. This dominance underscored the USA’s pivotal role in CSCs research.
In assessing the evolutionary trajectory of CSCs research, we examined 3 dimensions: co-cited clustering of title keywords, burst words, and co-occurrence analysis of subject terms. Citations are foundational in academic work, weaving a complex web of interconnected scientific literature. Co-citation arises when 2 prior studies are concurrently referenced in subsequent literature. Literature co-citation analysis, which gauges the relationship between documents based on their mutual citation frequency, is pivotal in delineating scientific frontiers and foundational research. Burst words denote the rapid emergence of articles on a specific topic within a brief period, signifying swift recognition and dissemination in the research community. Keywords in an article encapsulate its research essence, and analyzing these keywords and their interconnections can illuminate academic focal points and latent themes in a domain. Through these 3 dimensions, the research on CSCs could be divided into 3 stages. (1) 1997 to 2007: the primary focus was on the identification and definition of CSCs. (2) Since 2007: the article titled “The epithelial-mesenchymal transition generates cells with properties of stem cells” published by Mani et al (2008) in Cell marked a shift in focus towards exploring the potential mechanisms of CSCs. (3) In 2016: The article “Cancer stem cells revisited” published by Batlle and Clevers (2017) in Nature Medicine elucidated the intricate relationship between CSCs and the tumor microenvironment. After that, the spotlight turned to the tumor microenvironment.
As our study revealed that “tumor microenvironment” emerged as current research hotspots of research field of CSCs, which was confirmed in another bibliometric analysis result on glioma stem cells.[35] The microenvironment of CSCs is critical to their function and is also known as the CSC niche due to its complex nature and interactions with other components and factors.[48] The CSC niche typically includes cancer cells, stromal and endothelial cells, extracellular matrix, signaling molecules, intrinsic factors, blood vessels, and other cellular and noncellular components such as exosomes.[6] Interestingly, another bibliometric study on the hotspots of cancer stem cell-derived exosomes and the tumor microenvironment suggested that cancer stem cell-derived exosomes will play an important regulatory role in the tumor microenvironment.[34] Regulatory mechanisms in the niche include intrinsic mechanisms (associated with cell-expressed transcription factors) and extrinsic mechanisms (signaling based on the microenvironment). In terms of intrinsic mechanisms, hypoxia-inducible transcription factor (HIF) is an important transcription factor involved in the niche mechanism. HIF belongs to the bHLH-PAS transcription factor family and can regulate many genes and play a role in oxygen homeostasis, glucose and iron metabolism, and erythropoiesis.[49] The HIF signaling pathway can activate the angiogenesis switch during tumor progression and maintain tumor oxygen homeostasis.[50] In CSCs, HIF has been confirmed to be related to CSC proliferation, self-renewal and induction of drug resistance.[51] Other transcription factors such as Snail and Twist affect cell connections by activating EMT, enhancing the metastasis ability of tumor cells, and inducing non-CSCs to transform into CSCs.[52] As for extrinsic mechanisms, previous studies have confirmed that multiple signaling pathways such as Wnt, TGFβ, Notch, JAK-STAT and PI3K that can regulate the niche of CSCs.[53] The Wnt signaling pathway is involved in the development and functional regulation of normal stem cells. In Eyre et al study, Wnt pathway activation increases CSC activity and promotes CSCs’ symmetrical division.[54] In addition, the Notch pathway is involved in the adhesion of CSCs in the niche and activates tumor initiation and progression.[55] The research on the tumor microenvironment in the basic research of CSCs is highly popular, and our bibliometric research has also analyzed this trend.
Although “clinical transformation” did not list among the 25 most significant burst words, it is still a research focus that needs attention. In recent years, there has also been some progress in the clinical transformation of CSCs. At present, various treatment methods for inhibiting CSCs are being tested in preclinical and clinical trials, including targeted signaling pathways, miRNA therapy, and immunotherapy. The targeted signaling pathways aim to disrupt the signal transduction pathways related to self-renewal of CSCs. Blocking these molecular pathways can inhibit proliferation and cancer progression, target TME to disrupt the interconnection between CSCs and cytokines, target CSCs surface markers to identify and severely affect CSCs, and target the metabolism of CSCs.[56,57] These signaling pathways include NF-κβ, Wnt, Notch, Hedgehog, TGFβ, PI3K/AKT and STAT3.[58] In miRNA therapy, specific miRNAs regulate different aspects of CSCs behavior and have shown potential as targets for eliminating CSCs and ultimately preventing cancer recurrence and metastasis.[58] In addition to the antibodies targeting CSCs-related signaling pathways mentioned above, new anti-CSCs immunotherapy methods such as cancer vaccines, checkpoint inhibitors, and chimeric antigen receptor T cell therapies have been developed.[59,60] However, There are several drawbacks that limit the clinic applications in currently available anti-CSCs drugs and therapies, including limited solubility, low stability, high toxicity, and lack of tissue selectivity.[61] Hence, even the CSCs transformation research is not shown in our bibliometric analysis results, it is also need more attention and development.
5. Advantages and limitations
Our study is the first to harness bibliometric analysis in exploring the intricate research landscape of CSCs, distinguishing itself from traditional literature reviews. There are several strengths to our approach. Firstly, our systematic search strategy and rigorous quantitative statistical methodologies offer a more comprehensive and insightful perspective. Secondly, the use of tools such as CiteSpace, VOSviewer, and the R package bibliometrix ensures holistic data extraction, meticulous bibliometric evaluation, and enhanced visualization. Lastly, our multifaceted approach provides a panoramic view of the CSCs research landscape, emphasizing the need for more in-depth qualitative analysis to provide a rounded understanding of the field.
However, there are limitations to our study. The exclusive reliance on the WoSCC database might have omitted certain pertinent literature, especially those with fewer citations. Additionally, the bibliometric methodologies we employed, while robust, might have overlooked nuanced insights, specific authorial perspectives, and forward-looking opinions embedded within the full texts. There are also potential inconsistencies between the results of our bibliometric analysis and the status of actual studies, given the dynamic nature of the database.
6. Conclusion
This study is the first to provide a comprehensive bibliometric analysis covering the entire CSCs research landscape. Our findings highlight a growing trend in publications, with the USA leading in this field. The University of Texas System and Harvard University have emerged as pivotal contributors, with numerous publications and citations. Key authors in the field include Rich, JN and Clarke, MF. Through keywords co-occurrence analysis, we identified 4 clusters: “CSCs properties,” “molecular biology properties,” “tumor therapy” and “CSCs identification.” Notably, there is a discernible shift from identification and definition towards the tumor microenvironment and ecological niche. Based on the latest most cited articles, future research hotspots are likely to center around chemoresistance and clinical transformation, offering potential avenues for innovative therapeutic strategies in cancer.
Author contributions
Conceptualization: Yuxian Chen, Baozhen Qu.
Methodology: Keke Zheng.
Visualization: Xiaotao Zhang.
Writing – original draft: Yuxian Chen, Baozhen Qu.
Writing – review & editing: Yanhao Liu, Linlin Lu.
Supplementary Material
Abbreviations:
- ACI
- average citations per item
- CSCs
- cancer stem cells
- EMT
- epithelial-mesenchymal transition
- HIF
- hypoxia-inducible transcription factor
- PNAS
- Proceedings of the National Academy of Sciences of the United States of America
- SCs
- stem cells
- SP
- side population
- TLS
- total link strength
- TPR
- top-cited paper rates
- WoSCC
- Web of Science Core Collection
This work was supported by Shandong Province Medical and Health Technology Development Plan Project (202203100062) and Qingdao Medical and Health Research Guidance Project (2022-WJZD100).
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental Digital Content is available for this article.
How to cite this article: Chen Y, Qu B, Zheng K, Liu Y, Lu L, Zhang X. Global research landscape and trends of cancer stem cells from 1997 to 2023: A bibliometric analysis. Medicine 2024;103:20(e38125).
YC and BQ contributed equally to this work.
Contributor Information
Baozhen Qu, Email: qubaozhen1992@163.com.
Keke Zheng, Email: 350611688@qq.com.
Yanhao Liu, Email: 1310305202@pku.edu.cn.
Linlin Lu, Email: lulinlin2007@hotmail.com.
References
- [1].Cabarcas SM, Mathews LA, Farrar WL. The cancer stem cell niche--there goes the neighborhood? Int J Cancer. 2011;129:2315–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kordes C, Haussinger D. Hepatic stem cell niches. J Clin Invest. 2013;123:1874–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lacina L, Plzak J, Kodet O, et al. Cancer microenvironment: what can we learn from the stem cell niche. Int J Mol Sci. 2015;16:24094–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Guo W. Concise review: breast cancer stem cells: regulatory networks, stem cell niches, and disease relevance. Stem Cells Transl Med. 2014;3:942–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–7. [DOI] [PubMed] [Google Scholar]
- [6].Batlle E, Clevers H. Cancer stem cells revisited. Nat Med. 2017;23:1124–34. [DOI] [PubMed] [Google Scholar]
- [7].Marzagalli M, Fontana F, Raimondi M, Limonta P. Cancer stem cells-key players in tumor relapse. Cancers (Basel). 2021;13:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Li Y, Laterra J. Cancer stem cells: distinct entities or dynamically regulated phenotypes? Cancer Res. 2012;72:576–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cruz MH, Sidén A, Calaf GM, Delwar ZM, Yakisich JS. The stemness phenotype model. ISRN Oncol. 2012;2012:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Vermeulen L, de Sousa e Melo F, Richel DJ, Medema JP. The developing cancer stem-cell model: clinical challenges and opportunities. Lancet Oncol. 2012;13:e83–9. [DOI] [PubMed] [Google Scholar]
- [11].Fan Z, Li M, Chen X, et al. Prognostic value of cancer stem cell markers in head and neck squamous cell carcinoma: a meta-analysis. Sci Rep. 2017;7:43008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cheng B, Yang G, Jiang R, et al. Cancer stem cell markers predict a poor prognosis in renal cell carcinoma: a meta-analysis. Oncotarget. 2016;7:65862–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Suzuki H, Chen J, Xia Q, et al. Prognostic value of cancer stem cell marker ALDH1 expression in colorectal cancer: a systematic review and meta-analysis. PLoS One. 2015;10:e0145164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wei D, Peng JJ, Gao H, Zhang T, Tan Y, Hu YH. ALDH1 expression and the prognosis of lung cancer: a systematic review and meta-analysis. Heart Lung Circ. 2015;24:780–8. [DOI] [PubMed] [Google Scholar]
- [15].Ma YC, Yang JY, Yan LN. Relevant markers of cancer stem cells indicate a poor prognosis in hepatocellular carcinoma patients: a meta-analysis. Eur J Gastroenterol Hepatol. 2013;25:1007–16. [DOI] [PubMed] [Google Scholar]
- [16].Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–5. [DOI] [PubMed] [Google Scholar]
- [17].Ma S, Chan KW, Hu L, et al. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132:2542–56. [DOI] [PubMed] [Google Scholar]
- [18].Beier D, Hau P, Proescholdt M, et al. CD133+ and CD133- glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007;67:4010–5. [DOI] [PubMed] [Google Scholar]
- [19].Baba T, Convery PA, Matsumura N, et al. Epigenetic regulation of CD133 and tumorigenicity of CD133+ ovarian cancer cells. Oncogene. 2009;28:209–18. [DOI] [PubMed] [Google Scholar]
- [20].Haraguchi N, Ohkuma M, Sakashita H, et al. CD133+CD44+ population efficiently enriches colon cancer initiating cells. Ann Surg Oncol. 2008;15:2927–33. [DOI] [PubMed] [Google Scholar]
- [21].Silva IA, Bai S, McLean K, et al. Aldehyde dehydrogenase in combination with CD133 defines angiogenic ovarian cancer stem cells that portend poor patient survival. Cancer Res. 2011;71:3991–4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhang H, Wang ZZ. Mechanisms that mediate stem cell self-renewal and differentiation. J Cell Biochem. 2008;103:709–18. [DOI] [PubMed] [Google Scholar]
- [23].Wang J, Guo LP, Chen LZ, Zeng YX, Lu SH. Identification of cancer stem cell–like side population cells in human nasopharyngeal carcinoma cell line. Cancer Res. 2007;67:3716–24. [DOI] [PubMed] [Google Scholar]
- [24].Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, et al. Ovarian cancer side population defines cells with stem cell-like characteristics and mullerian inhibiting substance responsiveness. Proc Natl Acad Sci U S A. 2006;103:11154–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Fan X, Matsui W, Khaki L, et al. Notch pathway inhibition depletes stem-like cells and blocks engraftment in embryonal brain tumors. Cancer Res. 2006;66:7445–52. [DOI] [PubMed] [Google Scholar]
- [26].Li H, Zhang W, Yan M, et al. Nucleolar and spindle associated protein 1 promotes metastasis of cervical carcinoma cells by activating Wnt/beta-catenin signaling. J Exp Clin Cancer Res. 2019;38:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cho Y, Kang HG, Kim SJ, et al. Post-translational modification of OCT4 in breast cancer tumorigenesis. Cell Death Differ. 2018;25:1781–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gao M, Bai H, Jethava Y, et al. Identification and characterization of tumor-initiating cells in multiple myeloma. J Natl Cancer Inst. 2020;112:507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Islam SS, Aboussekhra A. Sequential combination of cisplatin with eugenol targets ovarian cancer stem cells through the Notch-Hes1 signalling pathway. J Exp Clin Cancer Res. 2019;38:382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ninkov A, Frank JR, Maggio LA. Bibliometrics: methods for studying academic publishing. Perspect Med Educ. 2022;11:173–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gates AJ, Ke Q, Varol O, Barabási AL. Nature’s reach: narrow work has broad impact. Nature. 2019;575:32–4. [DOI] [PubMed] [Google Scholar]
- [32].Liu M, Yang F, Xu Y. Global trends of stem cell precision medicine research (2018-2022): a bibliometric analysis. Front Surg. 2022;9:888956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lin J, Yang Z, Wang L, Xing D, Lin J. Global research trends in extracellular vesicles based on stem cells from 1991 to 2021: a bibliometric and visualized study. Front Bioeng Biotechnol. 2022;10:956058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Guo Z, Wang G, Yun Z, et al. Global research trends in tumor stem cell-derived exosomes and tumor microenvironment: visualization biology analysis. J Cancer Res Clin Oncol. 2023;149:17581–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Song S, Wu H, Wang F, et al. Global research trends and hotspots on glioma stem cells. Front Oncol. 2022;12:926025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kulkarni AV, Aziz B, Shams I, Busse JW. Comparisons of citations in Web of Science, Scopus, and Google Scholar for articles published in general medical journals. JAMA. 2009;302:1092–6. [DOI] [PubMed] [Google Scholar]
- [37].Martin-Martin A, Thelwall M, Orduna-Malea E, Delgado Lopez-Cozar E. Google Scholar, Microsoft Academic, Scopus, Dimensions, Web of Science, and OpenCitations’ COCI: a multidisciplinary comparison of coverage via citations. Scientometrics. 2021;126:871–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Liu Y, Yu L, Liang Y, et al. Research landscape and trends of melanoma immunotherapy: a bibliometric analysis. Front Oncol. 2022;12:1024179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84:523–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].van Eck NJ, Waltman L. Citation-based clustering of publications using CitNetExplorer and VOSviewer. Scientometrics. 2017;111:1053–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci U S A. 2004;101(Suppl 1):5303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. [DOI] [PubMed] [Google Scholar]
- [44].Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–8. [PubMed] [Google Scholar]
- [46].O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–10. [DOI] [PubMed] [Google Scholar]
- [47].Vermeulen L, De Sousa EMF, van der Heijden M, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468–76. [DOI] [PubMed] [Google Scholar]
- [48].Melzer C, von der Ohe J, Lehnert H, Ungefroren H, Hass R. Cancer stem cell niche models and contribution by mesenchymal stroma/stem cells. Mol Cancer. 2017;16:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Choudhry H, Harris AL. Advances in hypoxia-inducible factor biology. Cell Metab. 2018;27:281–98. [DOI] [PubMed] [Google Scholar]
- [50].Schito L, Semenza GL. Hypoxia-inducible factors: master regulators of cancer progression. Trends Cancer. 2016;2:758–70. [DOI] [PubMed] [Google Scholar]
- [51].Semenza GL. Mechanisms of breast cancer stem cell specification and self-renewal mediated by hypoxia-inducible factor 1. Stem Cells Transl. Med.. 2023;12:783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Shigdar S, Li Y, Bhattacharya S, et al. Inflammation and cancer stem cells. Cancer Lett. 2014;345:271–8. [DOI] [PubMed] [Google Scholar]
- [53].Clara JA, Monge C, Yang Y, Takebe N. Targeting signalling pathways and the immune microenvironment of cancer stem cells-a clinical update. Nat Rev Clin Oncol. 2020;17:204–32. [DOI] [PubMed] [Google Scholar]
- [54].Eyre R, Alférez DG, Santiago-Gómez A, et al. Microenvironmental IL1β promotes breast cancer metastatic colonisation in the bone via activation of Wnt signalling. Nat Commun. 2019;10:5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Yi SY, Hao YB, Nan KJ, Fan TL. Cancer stem cells niche: a target for novel cancer therapeutics. Cancer Treat Rev. 2013;39:290–6. [DOI] [PubMed] [Google Scholar]
- [56].Woosley AN, Dalton AC, Hussey GS, et al. TGFβ promotes breast cancer stem cell self-renewal through an ILEI/LIFR signaling axis. Oncogene. 2019;38:3794–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Elia I, Rossi M, Stegen S, et al. Breast cancer cells rely on environmental pyruvate to shape the metastatic niche. Nature. 2019;568:117–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Agliano A, Calvo A, Box C. The challenge of targeting cancer stem cells to halt metastasis. Semin Cancer Biol. 2017;44:25–42. [DOI] [PubMed] [Google Scholar]
- [59].Dianat-Moghadam H, Mahari A, Salahlou R, Khalili M, Azizi M, Sadeghzadeh H. Immune evader cancer stem cells direct the perspective approaches to cancer immunotherapy. Stem Cell Res Ther. 2022;13:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Walcher L, Kistenmacher AK, Suo H, et al. Cancer stem cells-origins and biomarkers: perspectives for targeted personalized therapies. Front Immunol. 2020;11:1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Eid RA, Alaa Edeen M, Shedid EM, et al. Targeting cancer stem cells as the key driver of carcinogenesis and therapeutic resistance. Int J Mol Sci. 2023;24:1786. [DOI] [PMC free article] [PubMed] [Google Scholar]