Abstract
Background:
Atherosclerosis (AS), as a complex chronic inflammatory disease, is 1 of the main causes of cardiovascular and cerebrovascular diseases. This study aimed to confirm the direct interaction between miR-146a-3p and NF-κB, and explore the role of miR-146a-3p/NF-κB in the regulation of inflammation in AS.
Methods:
Bioinformatic prediction and dual-luciferase reporter assay were used to confirm the interaction between miR-146a-3p and NF-κB. Lipopolysaccharides stimulation was performed to establish AS inflammatory cell model, and the levels of pro-inflammatory cytokines were estimated using an enzyme-linked immunosorbent assay. miR-146a-3p and NF-κB expression were evaluated using reverse transcription quantitative PCR, and their clinical value was examined using a receiver operating characteristic curve.
Results:
Inflammatory cell model showed increased IL-1β, IL-6, and TNF-α. NF-κB was a target gene of miR-146a-3p, and mediated the inhibitory effects of miR-146a-3p on inflammatory responses in the cell model. In patients with AS, miR-146a-3p/NF-κB was associated with patients’ clinical data and inflammatory cytokine levels, and aberrant miR-146a-3p and NF-κB showed diagnostic accuracy to distinguish AS patients from healthy populations.
Conclusion:
miR-146a-3p might inhibit inflammation by targeting NF-κB in AS progression, and miR-146a-3p/ NF-κB might provide novel biomarkers and therapeutic targets for the prevention of AS and related vascular events.
Keywords: atherosclerosis, diagnosis, inflammatory responses, microRNA-146a-3p, NF-κB
1. Introduction
With the aging of society, the incidence of cerebrovascular disease is increasing, and has become 1 of the main causes of death among Chinese residents.[1] According to the record data in recent years, stroke has risen to third place in the global burden of disease.[2] China is the country with the highest lifetime risk of stroke in the world, and at least 1 out of every 5 deaths is related to stroke.[3] The number of stroke deaths accounts for about 1/3 of the global total, and the number of patients with stroke in China has reached 13 million.[4] Atherosclerotic ischemic stroke accounts for first place among all stroke cases, and atherosclerotic cerebrovascular disease has the characteristics of high incidence, disability, and recurrence, which brings a heavy burden to human health and society, and is a medical and social problem to be solved urgently.[5,6] Atherosclerosis (AS) is a complex chronic inflammatory disease characterized by plaque accumulation on the arterial wall, which often occurs in carotid arteries, coronary arteries, and lower extremity arteries.[7] Notably, carotid AS and plaque formation are the main causes of cerebrovascular stenosis, occlusion and distal blood flow reduction, and are considered to be independent risk factors for ischemic cerebrovascular disease, which can significantly increase the risk of ischemic stroke.[8,9]
MicroRNA-146 (miR-146) is the first microRNA found to play a regulatory role in the immune system, which can induce the proliferation and differentiation of megakaryocytes, including miR-146a and miR-146b.[10] miR-146a is 1 of the major regulatory miRNAs of inflammatory responses, which is widely involved in the pathogenesis of inflammation, immune-related diseases, and neoplastic diseases.[11–13] Recent studies have shown that deregulated expression of miR-146a was found in AS plaques, indicating that miR-146a is closely related to AS.[14] However, the specific mechanism is still unclear, so it is of great significance to study the mechanism of miR-146a in the formation of AS. NF-activated B cell Kappa-light-chain enhancer (NF-κB) has been proven to induce the gene expression of many cytokines, adhesion factors, chemokines, acute phase response proteins, and enzymes involved in inflammatory responses, which is involved in the formation, progression, and plaque rupture of AS, and can affect the progression of AS through multiple pathways.[15–17]
In this study, we found the complementary sequences of miR-146a-3p in the 3’-untranslated region (3’-UTR) of NF-κB, which indicated that there might be an interaction between the 2 molecules. Therefore, we speculated that miR-146a-3p might be involved in the progression of AS by regulating the expression of NF-κB. To verify our hypothesis, this study intends to explore the relationship between miR-146a-3p and NF-κB through clinical data analysis and cell experiments, and prove the clinical significance and biological function of miR-146a-3p/NF-κB in the progression of AS.
2. Materials and methods
2.1. Cell culture and treatment
HEK293T cells and THP-1 monocytes were purchased from Shanghai Cell Bank (Shanghai, China). HEK293T was cultured in Dulbecco-modified Eagle medium (Gibco, Thermo Fisher Scientific, Waltham), and THP-1 cells were cultured in PRMI 1640 medium (Gibco) in a humidified chamber with 5% CO2 at 37°C. Both 2 kinds of mediums contain 10% fetal bovine serum (HyClone, Logan, UT) and 1% penicillin/streptomycin (100 U/mL penicillin and 100 µg/mL streptomycin) for cell culture.
PMA (100 ng/mL) was used for macrophage induction of THP-1 for 24 hours. To induce an inflammatory cell model, THP-1 cells were stimulated with 1 µg/mL lipopolysaccharides (LPS) for 24 hours. In order to regulate the expression of miR-146a-3p and NF-κB, miR-146a-3p mimic, miR-146a-3p inhibitor, mimic NC, inhibitor NC, and pcDNA3.1-NF-κB were synthesized by GenePharma (Shanghai, China). Lipofectamine 3000 reagent (Invitrogen Life Technologies Inc., Carlsbad, CA) was mixed with the synthesized vectors at room temperature for 20 minutes, then the mixture was added in the THP-1 cells, which were cultured with serum-free RPMI 1640 medium. After 6 hours of incubation, the medium was replaced using RPMI 1640 containing 10% fetal bovine serum, and the cells were continued cultured for 18 hours.
2.2. Dual-luciferase reporter assay
According to the TargetScan website (https://www.targetscan.org/vert_72/), the complementary sequence of miR-146a-3p on the 3’-UTR of NF-κB was obtained. Then, the wild-type and mutant-type 3’-UTR fragments were synthesized and cloned into a luciferase reporter vector (GenePharma). The reporter vectors were co-transfected into HEK293T cells with miR-146a-3p mimic, miR-146a-3p inhibitor, mimic NC, or inhibitor NC following the manufacturer’s protocols. After 48 hours, the relative luciferase activity was evaluated by Dual-Luciferase Reporter Assay System (Promega), and the results were normalized to the Renilla luciferase activity.
2.3. RNA extraction and reverse transcription quantitative PCR
Total RNA was extracted by RNeasy Mini Kit (Qiagen, Valencia, CA), and the RNA was reversely transcribed into cDNA using a reverse transcription kit (Takara, Tokyo, Japan) according to the manufacturer’s instructions. qPCR was conducted using SYBR Premix Ex TaqTM II Kit (Takara) and a real-time fluorescent qPCR 7500 system (ABI, Foster City, CA). The primer sequences used in the reactions were synthesized by GenePharma (Shanghai, China) and listed in Table 1. The final relative expression values were calculated using 2-ΔΔCT method and normalized to U6 or GAPHD.
Table 1.
Nucleotide fragments and primer sequences used in study.
| Gene | Sequences | |
|---|---|---|
| miR-146a-3p | Forward | 5’-GCCGAGCCTCTGAAATTCA-3’ |
| Reverse | 5’-CTCAACTGGTGTCGTGGA-3’ | |
| NF-κB | Forward | 5’-GAAGCACGAATGACAGAGGC-3’ |
| Reverse | 5’-GCTTGGCGGATTAGCTCTTTT-3’ | |
| U6 | Forward | 5’-CTCGCTTCGGCAGCACA-3’ |
| Reverse | 5’-AACGCTTCACGAATTGG-3’ | |
| GAPDH | Forward | 5’-AAGAAGGTGGTGAAGCAGGC-3’ |
| Reverse | 5’-TCCACCACCCAGTTGCTGTA-3’ | |
| miR-146a-3p mimic | 5’-CCUCUGAAAUUCAGUUCUUCAG-3’ | |
| miR-146a-3p inhibitor | 5’-CUGAAGAACUGAAUUUCAGAGG-3’ | |
miR-146 = microRNA-146, NF-κB = NF-activated B cell Kappa-light-chain enhancer.
2.4. Enzyme-linked immunosorbent assay
The inflammatory responses were evaluated by measuring the levels of pro-inflammatory cytokines, including interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α. Enzyme-linked immunosorbent assay kits (Beyotime, Shanghai, China) according to the manufacturer’s instructions.
2.5. Study population and serum samples
This study enrolled 22 cerebral AS patients and 22 healthy controls from the Taizhou Central Hospital (Taizhou University Hospital). The patients were diagnosed with AS based on the examination results from cerebrovascular transcranial Doppler sonography, magnetic resonance angiography, and arterial CT angiography. None of the controls had AS phenotypes or examination results. The cases with a history of stroke, severe heart diseases, severe infection diseases, dissection, liver and nephrosis disease, or any malignant tumors were excluded from our study. Venous blood was collected from the participants at the time of admission, and serum samples were isolated using centrifugation and stored at −80°C for subsequent application. The protocols of this study were approved by the Ethics Committee of the Taizhou Central Hospital (Taizhou University Hospital), and written informed consent was obtained from each participant.
2.6. Collection of clinical data
The demographic data of the participants, including age, gender, history of smoking and drinking, hypertension, and diabetes, were recorded for analysis. The laboratory parameters were also obtained and analyzed, including hypersensitivity C response protein (hs-CRP), total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol.
2.7. Statistical analysis
SPSS 26.0 software (IBM Corp., Armonk, NY) and GraphPad Prism 9.0 (GraphPad Software Inc., San Diego, CA) were adopted for statistical analyses. The data obtained were described as mean ± SD. Student t test was used for the data comparison between 2 groups, and 1-way ANOVA followed by Turkey test was used for the comparison between multiple groups. The Receiver operating characteristic (ROC) curves were plotted to evaluate the diagnostic performance of miR-146a-3p in distinguishing AS patients from healthy controls. A Pearson correlation coefficient was adopted to analyze the relationship between serum miR-146a-3p with inflammatory cytokines in AS patients. A P value of <.05 was considered as statistical significance.
3. Results
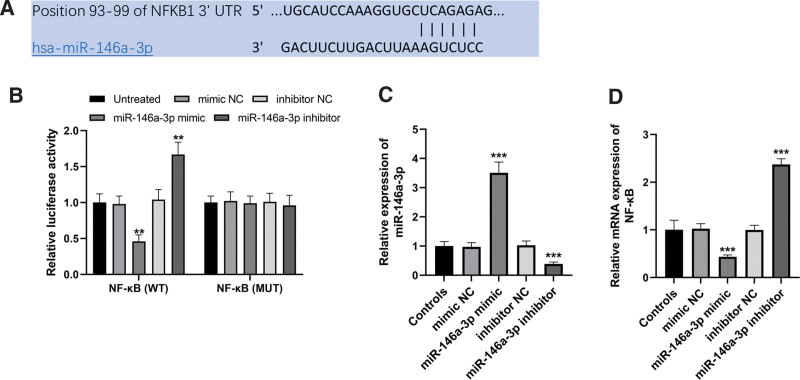
3.1. miR-146a-3p directly inhibited the expression of NF-κB
The sequences of the 3’-UTR of NF-κB, which contained the complementary sequences of miR-146a-3p, were predicted according to the TargetScan (Fig. 1A). The following dual-luciferase reporter assay showed that miR-146a-3p overexpression inhibited the relative luciferase activity, while the reduction of miR-146a-3p performed reversed function in the wild-type group in HEK293T cells (both P < .01, Fig. 1B). However, no significant changes were observed in the luciferase activities of mutant-type group (all P > .05). In THP-1 cells, miR-146a-3p was successfully increased by miR-146a-3p mimic, and was decreased by miR-146a-3p inhibitor (both P < .001, Fig. 1C). By the regulation of miR-146a-3p, the mRNA expression of NF-κB was hopefully inhibited by the overexpression of miR-146a-3p, and was promoted by the knockdown of miR-146a-3p (both P < .001, Fig. 1D).
Figure 1.
NF-κB was a direct target of miR-146a-3p. (A) Complementary sequences of miR-146a-3p in the 3’-UTR of NF-κB. (B) Results of dual-luciferase reporter assay. (C) miR-146a-3p expression was successfully regulated by cell transfection. (D) NF-κB expression was inhibited by miR-146a-3p overexpression but was promoted by miR-146a-3p inhibition. **P < .01, ***P < .001 compared to untreated or controls. 3’-UTR = 3’-untranslated region, miR-146 = microRNA-146, NF-κB = NF-activated B cell Kappa-light-chain enhancer.
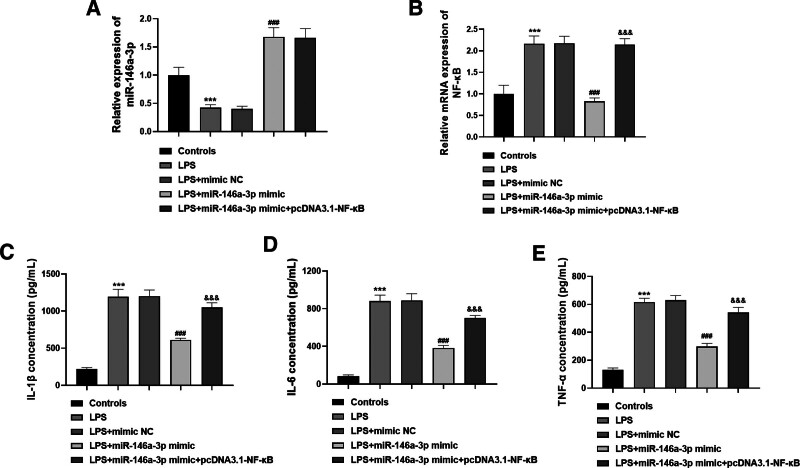
3.2. Regulatory effects of miR-146a-3p/NF-κB on the inflammatory response in THP-1 cells
By using LPS stimulation, an inflammatory cell model was established. The expression of miR-146a-3p was markedly inhibited, but NF-κB expression was significantly promoted in the cell model (both P < .001, Fig. 2A and B). Additionally, miR-146a-3p mimic upregulated the expression of miR-146a-3p, and inhibited the expression of NF-κB in the cell model, but the inhibited NF-κB was reversed with the co-transfection of pcDNA3.1-NF-κB (all P < .001). For the inflammatory responses in the cell model, LPS-induced significantly increased release of IL-1β, IL-6, and TNF-α (all P < .001, Fig. 2C–E). By upregulating the expression of miR-146a-3p, the increased inflammatory cytokine levels were all reduced (all P < .001), but this inhibition was rescued by the promotion of NF-κB expression (all P < .001).
Figure 2.
miR-146a-3p inhibited inflammation by targeting NF-κB in the cell model. (A) LPS inhibited miR-146a-3p expression, but miR-146a-3p mimic promoted miR-146a-3p expression. (B) LPS promoted NF-κB expression, but miR-146a-3p overexpression inhibited NF-κB expression, while this inhibition was rescued by pcDNA3.1- NF-κB. (C–E) LPS led to increased IL-1β, IL-6, and TNF-α, but this effect was reduced by miR-146a-3p overexpression and enhanced by NF-κB overexpression. ***P < .001 compared to controls; ###P < .001 compared to LPS; &&&P < .001 compared to LPS + miR-146a-3p mimic. miR-146 = microRNA-146, IL = interleukin, LPS = lipopolysaccharides, NF-κB = NF-activated B cell Kappa-light-chain enhancer, TNF = tumor necrosis factor.
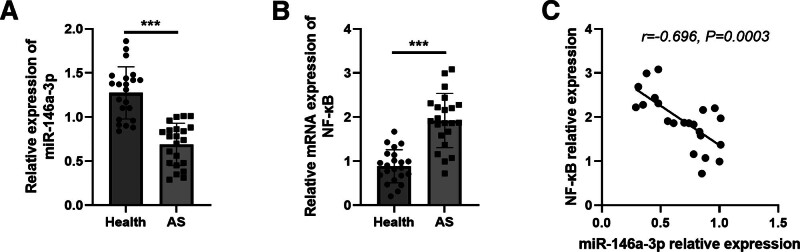
3.3. Expression of miR-146a-3p/NF-κB in the serum samples of AS patients
A total of 22 patients with AS were recruited to provide clinical data for this study, and their clinical data were listed in Table 2. As expected, the relative expression of miR-146a-3p was significantly decreased in the serum of AS patients, while NF-κB mRNA expression was increased in AS patients compared with healthy controls (both P < .001, Fig. 3A and B). According to the correlation assay, miR-146a-3p expression was negatively correlated with NF-κB expression (r = −0.696, P = .0003, Fig. 3C).
Table 2.
Clinical data of the study population.
| Variables | Health (n = 22) | AS (n = 22) | P value |
|---|---|---|---|
| Age (yr) | 61.18 ± 10.68 | 61.54 ± 7.87 | .898 |
| Males | 13 | 13 | 1.000 |
| Smoking | 10 | 11 | .763 |
| Drinking | 11 | 13 | .545 |
| Hypertension | 8 | 12 | .226 |
| Diabetes | 7 | 10 | .353 |
| hs-CRP (mg/L) | 10.89 ± 6.54 | 16.59 ± 8.50 | .017 |
| TC (mM) | 4.31 ± 0.87 | 5.14 ± 1.18 | .012 |
| TG (mM) | 1.32 ± 0.53 | 1.74 ± 0.83 | .050 |
| LDL-C (mM) | 2.386 ± 0.75 | 3.97 ± 0.77 | <.001 |
| HDL-C (mM) | 1.171 ± 0.246 | 1.13 ± 0.133 | .045 |
AS = atherosclerosis, HDL-C = high-density lipoprotein cholesterol, hs-CRP = hypersensitivity C response protein, LDL-C = low-density lipoprotein cholesterol, TC = total cholesterol, TG = triglyceride.
Figure 3.
Expression of miR-146a-3p and NF-κB in the serum of AS patients. (A) Serum miR-146a-3p was decreased in AS patients. (B) Serum NF-κB was increased in AS patients. (C) Serum miR-146a-3p levels were negatively correlated with NF-κB in AS patients. ***P < .001. AS = atherosclerosis, miR-146 = microRNA-146, NF-κB = NF-activated B cell Kappa-light-chain enhancer.
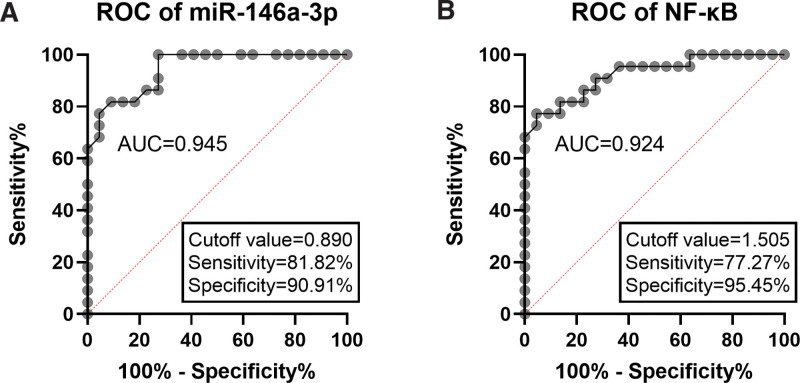
3.4. Diagnostic performance of serum miR-146a-3p and NF-κB in patients with AS
ROC curves based on serum miR-146a-3p and NF-κB levels were plotted. As presented in Figure 4A, the area under the curve (AUC) was 0.945, which indicated the diagnostic accuracy of serum miR-146a-3p in distinguishing AS patients from healthy controls. The cutoff value was 0.890 with the corresponding sensitivity and specificity of 81.82% and 90.91%. For serum NF-κB, the AUC was 0.924, and the cutoff value was 1.505 (sensitivity: 77.27%, specificity: 95.45%; Fig. 4B).
Figure 4.
ROC curves based on serum miR-146a-3p (A) and NF-κB (B). miR-146 = microRNA-146, NF-κB = NF-activated B cell Kappa-light-chain enhancer, ROC = receiver operating characteristic curve.
3.5. Association of miR-146a-3p/NF-κB with clinical data and inflammatory cytokines in AS patients
Table 3 presented the association analysis results between the miR-146a-3p/NF-κB with the clinical data and inflammatory cytokines (IL-1β, IL-6, and TNF-α) in AS patients. Serum miR-146a-3p was associated with patients’ hypertension history, hs-CRP, TC, LDL-C, and all of the inflammatory cytokine levels (all P < .05). NF-κB expression was found to be related to drinking history, hs-CRP, TC, LDL-C, and all of the inflammatory cytokine levels (all P < .05).
Table 3.
Association of miR-146a-3p/NF-κB with clinical data and inflammatory cytokines.
| Variables | miR-146a-3p expression | P | NF-κB expression | P | ||
|---|---|---|---|---|---|---|
| Low (n = 12) | High (n = 10) | Low (n = 11) | High (n = 11) | |||
| Age (yr) | 59.83 ± 6.85 | 63.60 ± 8.87 | .274 | 62.82 ± 7.40 | 60.27 ± 8.47 | .462 |
| Males | 8 | 5 | .429 | 6 | 7 | .665 |
| Smoking | 7 | 4 | .392 | 5 | 6 | .670 |
| Drinking | 9 | 4 | .096 | 4 | 9 | .030 |
| Hypertension | 9 | 3 | .035 | 4 | 8 | .087 |
| Diabetes | 6 | 4 | .639 | 4 | 6 | .392 |
| hs-CRP (mg/L) | 22.71 ± 4.90 | 9.24 ± 5.43 | .001 | 11.72 ± 6.97 | 21.46 ± 7.15 | .004 |
| TC (mM) | 5.89 ± 0.99 | 4.23 ± 0.64 | <.001 | 4.43 ± 0.72 | 5.84 ± 1.16 | .003 |
| TG (mM) | 1.89 ± 0.89 | 1.56 ± 0.74 | .343 | 1.62 ± 0.73 | 1.86 ± 0.93 | .506 |
| LDL-C (mM) | 4.44 ± 0.55 | 3.40 ± 0.60 | .001 | 3.55 ± 0.64 | 4.38 ± 0.68 | .008 |
| HDL-C (mM) | 1.10 ± 0.15 | 1.16 ± 0.11 | .277 | 1.15 ± 0.085 | 1.10 ± 0.17 | .367 |
| IL-1β (ng/mL) | 237.58 ± 27.37 | 160.21 ± 23.58 | <.001 | 175.60 ± 35.36 | 229.23 ± 41.93 | .004 |
| IL-6 (ng/mL) | 111.68 ± 17.67 | 51.93 ± 27.30 | <.001 | 65.48 ± 32.51 | 103.56 ± 33.27 | .013 |
| TNF-α (ng/mL) | 1.16 ± 0.12 | 0.84 ± 0.15 | <.001 | 0.90 ± 0.18 | 1.13 ± 0.18 | .007 |
HDL-C = high-density lipoprotein cholesterol, hs-CRP = hypersensitivity C response protein, IL = interleukin, LDL-C = low-density lipoprotein cholesterol, miR-146 = microRNA-146, NF-κB = NF-activated B cell Kappa-light-chain enhancer, TC = total cholesterol, TG = triglyceride, TNF = tumor necrosis factor.
4. Discussion
AS is a common and frequently-occurring disease that seriously endangers human health. It is the common pathological basis and the main cause of vascular events, such as cerebral infarction, myocardial infarction, and lower extremity arterial occlusion.[18] So far, the exact pathogenesis of AS is still not completely clear. Therefore, it is of great sociological significance to further explore the etiology and pathogenesis of AS and the new mechanism of anti-AS drugs for the prevention and treatment of AS. In this study, we demonstrated the regulatory effects of miR-146a-3p and NF-κB on the inflammatory responses in an AS cell model, which was established using THP-1 cells by LPS stimulation. In addition, the direct interaction between miR-146a-3p and NF-κB was proved in both the cell model and AS patients. The clinical value of the miR-146a-3p/NF-κB in distinguishing AS patients from healthy populations was also obtained from our study.
miRNAs are a class of endogenous single-stranded mature noncoding RNAs of about 18 to 22 nucleotides in length, most of which are located in the intergenic region, and a small number of which are located in the intron or exon region of protein-coding genes.[19] The internal 2 to 8 nucleotides of miRNAs are highly conserved “seed sequences,” which play key roles in the precise matching, identification, and biological evolution of target sequences.[20] It has been found that microRNAs interact with the 3’-UTR of target mRNAs by forming RNA-induced silencing complexes, leading to mRNA degradation or translational repression, negatively regulating gene expression at the posttranscriptional level.[21] Bioinformatics target prediction is usually the first step to determine the function of microRNA and find its target genes,[22] which provides a convenient way to identify miRNA target genes. Luciferase reporter gene assay has been widely used in the detection of target molecules binding to transcription factors and miRNA by analyzing promoter DNA fragments, verifying the transactivation ability of promoter binding elements, and exploring the molecular mechanism of transcription factors in signal transduction.[23] In this study, through bioinformatics prediction by the TargetScan and a dual-luciferase reporter gene technology, it was confirmed that miR-146a-3p could target and bind to the upstream promoter region of NF-κB. Furthermore, in THP-1 cells, the overexpression of miR-146a-3p led to inhibited NF-κB, and the inhibition of miR-146a-3p led to promoted NF-κB. These results indicated that NF-κB was a direct target gene of miR-146a-3p, and might mediate the function of miR-146a-3p.
miR-146a-3p has been demonstrated to be related to immune system and innate immunity.[24] It is a typical multifunctional gene, which participates in the occurrence and development of many physiological and pathological processes such as immunity, inflammation, hematopoiesis, and cancer.[25–27] In AS mice, miR-146a-3p was found to be involved in the development of AS by regulating plaque formation.[28] Considering the crucial role of inflammation in the progression of AS and the development of vascular adverse events, this study constructed an AS-related inflammatory cell model by LPS stimulation in TPH-1 cells. According to upregulating the expression of miR-146a-3p, the concentration of IL-1β, IL-6, and TNF-α were significantly inhibited. Reversely, the levels of inflammatory cytokines were promoted by the overexpression of NF-κB. The rescued inflammatory responses by NF-κB overexpression in the cells with miR-146a-3p overexpression indicated that NF-κB might mediate the regulatory effects of miR-146a-3p on inflammation in AS model cells.
To further confirm the role of miR-146a-3p/NF-κB in AS, 20 patients with AS were enrolled to provide clinical data. In the serum samples of patients, the expression of miR-146a-3p was downregulated, but NF-κB expression was upregulated compared to that in healthy controls, and miR-146a-3p was negatively correlated with NF-κB levels. In addition, both miR-146a-3p and NF-κB were found to be associated with hs-CRP, TC, LDL-C, and inflammatory cytokines (IL-1β, IL-6, and TNF-α). These findings further demonstrated that miR-146a-3p might relate inflammation by targeting NF-κB in AS progression. Various deregulated molecules have been found to be biomarkers for AS diagnosis, especially aberrantly expressed miRNAs.[29] This study also evaluated the clinical value of serum differential miR-146a-3p and NF-κB in patients with AS. The AUC, sensitivity and specificity results of ROC revealed that miR-146a-p and NF-κB had diagnostic accuracy in distinguishing AS patients from healthy controls, indicating the biomarker roles of miR-146a-3p/NF-κB in AS. Although this study provides data for the biological function and clinical role of miR-146a-3p/NF-κB, there are still some limitations. For example, this study established cell models used only 1 cell line. In vitro analysis may be further confirmed using more cell types. Additionally, only 22 AS patients were included in the analysis, the small sample size might limit the accuracy of the clinical value of miR-146a-3p/NF-κB. Thus, the function and clinical significance of miR-146a-3p/NF-κB needs to be verified in future studies according to performing in-deep cellular experiments and clinical data analysis.
In conclusion, miR-146a-3p inhibited inflammatory responses by targeting NF-κB in LPS-induced AS inflammatory cell model. The decreased miR-146a-3p and increased NF-κB might provide novel biomarkers in AS patients, and the regulation of miR-146a-3p/NF-κB might be promising therapeutic methods for the prevention of AS and related vascular events.
Author contributions
Conceptualization: Taotao Tao.
Formal analysis: Chengfei Zhu.
Methodology: Taotao Tao, Linkao Chen, Xia Lin, Lingqun Mao.
Resources: Zijian Fan.
Software: Lingqun Mao, Chengfei Zhu.
Supervision: Xia Lin.
Validation: Taotao Tao.
Visualization: Zijian Fan.
Writing – original draft: Taotao Tao, Lingqun Mao.
Writing – review & editing: Lingqun Mao.
Abbreviation:
- 3’-UTR
- 3’-untranslated region
- AS
- atherosclerosis
- AUC
- area under the curve
- hs-CRP
- hypersensitivity C response protein
- IL
- interleukin
- LDL-C
- low-density lipoprotein cholesterol
- LPS
- lipopolysaccharides
- miR-146
- microRNA-146
- NF-κB
- NF-activated B cell Kappa-light-chain enhancer
- ROC
- receiver operating characteristic curve
- TC
- total cholesterol
- TG
- triglyceride
- TNF
- tumor necrosis factor
Taizhou Social Development Science and Technology Plan Project: 21ywa32.
A signed written informed consent was obtained from each patient.
The experimental procedures were all in accordance with the guidelines of the Ethics Committee of Taizhou Central Hospital (Taizhou University Hospital) and have been approved by the Ethics Committee of Taizhou Central Hospital (Taizhou University Hospital). This study complies with the Declaration of Helsinki.
The authors have no conflicts of interest to disclose.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
How to cite this article: Tao T, Chen L, Lin X, Fan Z, Zhu C, Mao L. Deregulated miR-146a-3p alleviates disease progression in atherosclerosis through inactivating NF-κB: An experimental study. Medicine 2024;103:20(e38061).
Contributor Information
Taotao Tao, Email: tuiwoyansi70@163.com.
Linkao Chen, Email: chenlk_9408@163.com.
Xia Lin, Email: linx7293@yeah.net.
Zijian Fan, Email: chaozhuo62665@126.com.
Chengfei Zhu, Email: minghuanquan6368@126.com.
References
- [1].Zhang Z, Hu Y, Liu S, et al. Trend analysis of the mortality rates of the top three causes of death among Chinese residents from 2003 to 2019. Int J Public Health. 2022;67:1604988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Owolabi MO, Thrift AG, Mahal A, et al. Primary stroke prevention worldwide: translating evidence into action. Lancet Public Health. 2022;7:e74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wu S, Wu B, Liu M, et al. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019;18:394–405. [DOI] [PubMed] [Google Scholar]
- [4].Saini V, Guada L, Yavagal DR. Global epidemiology of stroke and access to acute ischemic stroke interventions. Neurology. 2021;97(20 Suppl 2):S6–S16. [DOI] [PubMed] [Google Scholar]
- [5].Heck D, Jost A. Carotid stenosis, stroke, and carotid artery revascularization. Prog Cardiovasc Dis. 2021;65:49–54. [DOI] [PubMed] [Google Scholar]
- [6].Nguyen L, Maingard J, Jhamb A, et al. Intracranial atherosclerotic disease and acute ischaemic stroke: a review of diagnosis and management. J Med Imaging Radiat Oncol. 2022;66:391–403. [DOI] [PubMed] [Google Scholar]
- [7].Frostegard J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013;11:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Han H, Zhang R, Liu G, et al. Reduction of cerebral blood flow in community-based adults with subclinical cerebrovascular atherosclerosis: a 3.0T magnetic resonance imaging study. Neuroimage. 2019;188:302–8. [DOI] [PubMed] [Google Scholar]
- [9].Goertler M, Blaser T, Guhr S, et al. Reduced frequency of embolic signals in severe carotid stenosis with poststenotic flow velocity reduction. Cerebrovasc Dis. 2005;19:229–33. [DOI] [PubMed] [Google Scholar]
- [10].Lee HM, Kim TS, Jo EK. MiR-146 and miR-125 in the regulation of innate immunity and inflammation. BMB Rep. 2016;49:311–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cheng HS, Sivachandran N, Lau A, et al. MicroRNA-146 represses endothelial activation by inhibiting pro-inflammatory pathways. EMBO Mol Med. 2013;5:1017–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tavasolian F, Hosseini AZ, Soudi S, et al. miRNA-146a improves immunomodulatory effects of MSC-derived exosomes in rheumatoid arthritis. Curr Gene Ther. 2020;20:297–312. [DOI] [PubMed] [Google Scholar]
- [13].Hurst DR, Edmonds MD, Scott GK, et al. Breast cancer metastasis suppressor 1 up-regulates miR-146, which suppresses breast cancer metastasis. Cancer Res. 2009;69:1279–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pereira-da-Silva T, Napoleao P, Costa MC, et al. Circulating miRNAs are associated with the systemic extent of atherosclerosis: novel observations for miR-27b and miR-146. Diagnostics. 2021;11:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Karunakaran D, Nguyen MA, Geoffrion M, et al. RIPK1 expression associates with inflammation in early atherosclerosis in humans and can be therapeutically silenced to reduce NF-kappaB activation and atherogenesis in mice. Circulation. 2021;143:163–77. [DOI] [PubMed] [Google Scholar]
- [16].Altmann C, Schmidt MHH. The role of microglia in diabetic retinopathy: inflammation, microvasculature defects and neurodegeneration. Int J Mol Sci. 2018;19:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Park JH, Seo YH, Jang JH, et al. Asiatic acid attenuates methamphetamine-induced neuroinflammation and neurotoxicity through blocking of NF-kB/STAT3/ERK and mitochondria-mediated apoptosis pathway. J Neuroinflammation. 2017;14:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Taleb S. Inflammation in atherosclerosis. Arch Cardiovasc Dis. 2016;109:708–15. [DOI] [PubMed] [Google Scholar]
- [19].Lu TX, Rothenberg ME. MicroRNA. J Allergy Clin Immunol. 2018;141:1202–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mellis D, Caporali A. MicroRNA-based therapeutics in cardiovascular disease: screening and delivery to the target. Biochem Soc Trans. 2018;46:11–21. [DOI] [PubMed] [Google Scholar]
- [21].Barwari T, Joshi A, Mayr M. MicroRNAs in cardiovascular disease. J Am Coll Cardiol. 2016;68:2577–84. [DOI] [PubMed] [Google Scholar]
- [22].Chen L, Heikkinen L, Wang C, et al. Trends in the development of miRNA bioinformatics tools. Brief Bioinform. 2019;20:1836–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Campos-Melo D, Droppelmann CA, Volkening K, et al. Comprehensive luciferase-based reporter gene assay reveals previously masked up-regulatory effects of miRNAs. Int J Mol Sci. 2014;15:15592–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bogunia-Kubik K, Wysoczanska B, Piatek D, et al. Significance of polymorphism and expression of miR-146a and NFkB1 genetic variants in patients with rheumatoid arthritis. Arch Immunol Ther Exp (Warsz). 2016;64(Suppl 1):131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Testa U, Pelosi E, Castelli G, et al. miR-146 and miR-155: two key modulators of immune response and tumor development. ncRNA. 2017;3:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhai PF, Wang F, Su R, et al. The regulatory roles of microRNA-146b-5p and its target platelet-derived growth factor receptor alpha (PDGFRA) in erythropoiesis and megakaryocytopoiesis. J Biol Chem. 2014;289:22600–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tordonato C, Marzi MJ, Giangreco G, et al. miR-146 connects stem cell identity with metabolism and pharmacological resistance in breast cancer. J Cell Biol. 2021;220:e202009053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Liu H, Wang H, Ma J, et al. MicroRNA-146a-3p/HDAC1/KLF5/IKBalpha signal axis modulates plaque formation of atherosclerosis mice. Life Sci. 2021;284:119615. [DOI] [PubMed] [Google Scholar]
- [29].He L, Wang Z, Zhou R, et al. Dexmedetomidine exerts cardioprotective effect through miR-146a-3p targeting IRAK1 and TRAF6 via inhibition of the NF-kappaB pathway. Biomed Pharmacother. 2021;133:110993. [DOI] [PubMed] [Google Scholar]