Abstract
Cytoreductive surgery is a surgical treatment approach that has been applied over the last 3 decades in patients with peritoneal metastases originating from intraabdominal organs. Goal-directed fluid therapy (GDFT) is an approach in which a patient fluid therapy during a medical procedure or surgery is carefully managed based on a specific goal. In this study, we aimed to present the results of GDFT in patients who underwent cytoreductive surgery for peritoneal carcinomatosis (PC) during the perioperative period. This retrospective study included 398 patients patient who underwent cytoreductive surgery + hyperthermic intraperitoneal chemotherapy (CRS + HIPEC) due to PC originating from intraabdominal malignancies. Of the cases, 233 (58.6%) were female, and 165 (41.4%) were male patients. The mean age was 58.9. Perioperative findings revealed an average PC score of 12 (3–24), average lactate levels of 3 (2–7) mmol/L, Pao2/fio2 of 3.3 (2.4–4.1) mm Hg, mean arterial pressure (MAP) of 60 (55–70), average surgery duration of 6.5 hours (3–14), and average blood loss of 400 (200–4000) cc. The mean intraoperative fluid rate was 6.4 mL/kg/h (IQR 5.8–7.1). Sixteen (16.3%) patients experienced Clavien-Dindo Grade 3–4 adverse events. Within 30 days, 25 patients (6.3%) died. CRS + HIPEC procedures utilizing perioperative GDFT along with advanced anesthesia monitoring devices have shown successful application, offering an alternative to traditional and restrictive fluid management approaches.
Keywords: cytoreductive surgery, goal-directed fluid therapy, hyperthermic intraperitoneal chemotherapy
1. Introduction
Cytoreductive surgery (CRS) is a surgical treatment approach that has been applied over the last 3 decades in patients with peritoneal metastases originating from intraabdominal organs (such as the colorectum, appendix, ovarian, and stomach). The aim was to reduce or completely remove tumor masses within the abdominal cavity. This procedure primarily focuses on surgical removal of most tumors. Hyperthermic Intraperitoneal Chemotherapy (HIPEC) is a treatment method administered immediately after CRS.[1–7] Chemotherapy is applied to the abdominal cavity, making cancer cells more sensitive due to hyperthermia effects (at 41–43°C). HIPEC targets small tumor cells that cannot be removed during surgery, and aims to eliminate the remaining cancer cells.[8–14] In the past, high-volume fluid therapies were administered during these complex surgeries, and later, restrictive fluid management was employed. However, both high-volume and restrictive fluid management are not ideal treatment methods for these challenging cases and are insufficient for fluid management.
Goal-directed fluid Therapy (GDFT) is a medical approach in which a patient fluid therapy during a medical procedure or surgery is carefully managed according to specific goals. The primary aim is to optimize a patient fluid balance by tailoring the administration of fluids to meet individual needs. This approach involves continuous monitoring of patient vital signs, such as blood pressure, heart rate, urine output, and other relevant parameters, during a medical procedure. By doing so, healthcare professionals can make real-time adjustments to the administration of fluids to ensure that a patient fluid levels are within the optimal range. The goals of GDFT can vary depending on the specific medical procedure or patient condition. This study aimed to prevent the complications associated with excessive (hypervolemia) and inadequate fluid administration (hypovolemia). The key benefits of GDFT include reducing the risk of complications during and after surgery, improving patient outcomes, and enhancing the overall efficiency and safety of medical procedures. This approach is especially valuable in complex surgeries where precise fluid management can significantly impact patient recovery.[15–29]
In this study, we aimed to present the results of GDFT in patients who underwent cytoreductive surgery + hyperthermic intraperitoneal chemotherapy (CRS + HIPEC) for peritoneal carcinomatosis (PC) during the perioperative period.
2. Materials and methods
The data was collected prospectively and analyzed retrospectively from 456 patients who were analyzed, 58 patients were excluded from the study. Among these 58 patients, 40 of them were considered inoperable and for the other 18 patients, operation was ended due to patient hemodynamic instability. These patients were excluded from the study. The other 398 patients underwent CRS and HIPEC for PC originating from intraabdominal malignancies at the Surgical Oncology Clinic of the University Health Sciences, Istanbul Umraniye Training and Research Hospital between June 2016 and 2023. All patients provided informed consent for the use of their data during hospitalization and ethical approval was obtained from the hospital ethics committee (approval number: 2023/114). All patients were evaluated by a multidisciplinary tumor board during the preoperative period. Demographic data of the patients (age, sex, diagnosis, body surface area [BSA], American Society of Anesthesiologists [ASA] score, Eastern Cooperative Oncology Group score) and intraoperative variables, including intravenous fluid volume, blood product transfusions, estimated blood loss, urine output, blood gas analysis, inotropic and chemotherapeutic agents, operative time, duration of intensive care unit (ICU) stay, and hospital stay, were evaluated in terms of postoperative morbidity, mortality, and median overall survival.
2.1. CRS and HIPEC procedure
Patients with intraabdominal acidity were admitted to the interventional radiology unit for controlled acid drainage by placing a peritoneal fluid catheter in the abdomen before surgery. In all the cases, an incision was made from the xiphoid to the pubis in the modified lithotomy position. First, the peritoneal cancer index (PCI) score was calculated. Organ resections were performed to leave macroscopically tumor-free areas. Anastomoses or stoma maturation were performed before the HIPEC procedure. After the placement of HIPEC and heat probes, the abdomen was closed, and intraabdominal intraperitoneal injections of cisplatin (75 mg/m² BSA) and doxorubicin (15 mg/m² BSA) in 0.9% NaCl solution were administered for 60 minutes at 42 to 30 minutes. 43°C and 1200 cc/h, targeting the stomach, ovarian, and mesothelioma. In cases of PC originating from colorectal sources, intraabdominal intraperitoneal injections of Mitomycin C (35 mg/m² BSA) in 0.9% NaCl solution were administered for 60 minutes. A Belmont Hyperthermia Pump (Belmont Instrument Corporation, Billerica, MA) was used as the HIPEC machine. Closed technique was applied in all HIPEC procedures.
2.2. Anesthesia management
During the preoperative period, a central venous catheter was opened in all the patients in the interventional radiology department. Pneumatic compression stockings were applied in the operating room and normothermia was maintained with a warming blanket. A nasogastric tube was then inserted. Prior to induction, all patients had a thoracic epidural catheter and temperature probe, and thoracic epidural analgesia was initiated before incision with 10 mL of chirocaine 0.25%, after which continuous administration was started with bupivacaine 0.125% and sufentanil 1 µg/mL (6–10 mL/h). Routine perioperative antimicrobial prophylaxis was administered to all patients 30 minutes before incision with 2 g of intravenous cefazolin, and repeated every 4 hours with 1 g of cefazolin. General anesthesia was induced with propofol (3–5 mg/kg), fentanyl (1µg/kg), and atracurium (0.5 mg/kg). Anesthesia was maintained with the continuous administration of propofol after induction. After placement of the endotracheal tube, ventilation was provided at a tidal volume of 6 to 8 mL/kg body weight. Radial artery catheterization was performed in all patients after the induction of general anesthesia. FloTrac was used to measure the cardiac index in all patients, and arterial line FloTrac sensors (FloTrac/Vigileo system (Edwards Lifesciences, Irvine, CA) or Pulse Indicating Continuous Cardiac Output (PICCO) catheter (PulsioCath PV2015 L20; Pulsion Medical Systems, Munich, Germany) were used for hemodynamic monitoring. Central venous catheter was applied to all patients and scvO2 was measured from the catheter. Intraoperative fluid management (volume status, resuscitation goals, and choice of crystalloid, colloid, vasopressors, and blood products) was determined through coordinated decision-making between experienced anesthesia specialists and the surgical team for CRS + HIPEC cases through the central venous catheter. In perioperative fluid therapy, balanced salt solutions such as lactated Ringer and acetate-based solutions were used as crystalloids, while only albumin was used as a colloid. As a standard procedure, a small fluid bolus was administered to each patient during induction, and the fluid rate was adjusted according to the hemodynamic parameters. Urine output was targeted at 0.5 to 1 mL/kg. We used intraoperative near-infrared spectroscopy to assess blood flow to the kidneys and measure the renal oxygen saturation. After the operation, all patients were sent to the ICU and were intubated. Patients aged <18 years, those with distant organ metastases, and those who did not undergo surgery due to high perioperative PCI scores were excluded from the study.
2.3. Statistical analyzes
Data were processed and analyzed using the IBM SPSS Statistics 22 software (IBM Corp., Armonk, NY). Qualitative variables are expressed as absolute and relative frequencies. Quantitative variables were expressed as mean and standard deviation and as median and interquartile range if the distribution was not normal. The chi-squared test or Fisher exact test, if necessary, was used.
3. Results
Owing to PC, 398 patients underwent CRS and HIPEC. Of these, 233 (58.6%) were female, and 165 (41.4%) were male patients. The average age was 58.9 (interquartile range 17–77). ASA scores were calculated as follows: ASA 1, 44 (11%); ASA 2, 220 (55.2%); ASA 3, 131 (33%); and ASA 4, 3 (0.75%). The average BSA was 177 (136–202), and the Eastern Cooperative Oncology Group score was 0–3 (1.3) (Table 1).
Table 1.
Demographics and clinical characteristics of the patients.
| Variables | Total (n = 398) |
|---|---|
| Age (yr) | 58.9 (17–77) |
| Sex | |
| Male | 165 (41.4%) |
| Female | 233 (58.6%) |
| ASA score | |
| I | 44 (11%) |
| II | 220 (55.2%) |
| III | 131 (33%) |
| IV | 3 (0.75%) |
| BSA (m2) | 177 (136–202) |
| ECOG score | 1.3 (0–3) |
Data are presented as median (min-max) or n (%).
ASA = American Society of Anesthesiologists score, BSA = body surface area, ECOG = Eastern Cooperative Oncology Group performance status.
Of the patients, 164 (41.2%) had colorectal, 65 (16.3%) had ovarian, 47 (11.8%) had gastric, 35 (8.8%) had sarcomatosis, 18 (4.5%) had appendiceal, 14 (3.5%) had mesothelioma, 13 (3.2%) had cervical, 8 (2%) had pancreatic, 7 (1.75%) had uterine, and 27 (6.8%) had other (gastrointestinal stromal tumor, bladder, small intestine, peritoneum, and renal origin) origins (Table 2).
Table 2.
Tumor origin.
| Origin | n |
|---|---|
| Colorectal | 164 (41.2%) |
| Ovarian | 65 (16.3%) |
| Gastric | 47 (11.8%) |
| Sarcomatosis | 35 (8.8%) |
| Appendix | 18 (4.5%) |
| Mesothelioma | 14 (3.5%) |
| Cervical | 13 (3.2%) |
| Pancreas | 8 (2%) |
| Uterus | 7 (1.75%) |
| Other* | 27 (6.8%) |
Data are presented as n (%).
Gastrointestinal stromal tumor, bladder, small intestine, peritoneum and renal.
Perioperative findings revealed an average PCI score of 12 (3–24), intraabdominal ascites measured 150 (0–3000) mL, average body temperature was 36.1°C (35–37), average lactate levels were 3 (2–7) mmol/L, Pao2/fio2 was measured as 3.3 (2.4–4.1) mm Hg, mean arterial pressure (MAP) was 60 (55–70), average surgery duration was 6.5 hours (3–14), and average blood loss was 400 (200–4000) cc. The ICU stay duration was 1.5 days (0–20), and the average hospital stay was 8.8 days (4–46). The mean intraoperative fluid (IOF) rate was 6.4 mL/kg/h (IQR, 5.8–7.1). The cell saver autotransfusion system was used in 152 patients (38.2%). Red blood cell transfusion was required in 296 (74.4%) patients, with an average of 3 units. Fresh frozen plasma (FFP) transfusion was required in 304 (76.4%) cases, with an average of 4 units. Among other transfused blood products, platelets were administered to 5 (1.25%) cases, and no cryoprecipitate was used. 120 (30%) patients received a 4% human albumin solution, averaging 2 units during CRS. Additional inotropic support or vasopressors were required in 169 (42.5%) patients during CRS; however, no additional inotropes or vasopressors were required during HIPEC. Prolonged ventilation lasting more than 24 hours occurred in 75 (18.8%) cases. The average urine output was 600 (300–1800) mL (Table 3).
Table 3.
Perioperative findings.
| Variables | |
|---|---|
| PCI score | 12 (3–24) |
| İntraabdominal ascites (mL) | 150 (0–3000) |
| Body temperature (°C) | 36.1 (35–37) |
| Lactate (mmol/L) | 3 (2–7) |
| PaO2/FiO2 (mm Hg) | 3,3 (2,4–4,1) |
| MAP | 60 (55–70) |
| Operation time (h) | 6.5 (3–14) |
| Blood loss (cc) | 400 (200–4000) |
| İCU stay time (d) | 1.5 (0–20) |
| Hospitalization stay time (d) | 8.8 (4–46) |
| İntraoperative fluid rate (IOF) (mL/kg/h) | 6.4 (5.8–7.1) |
| Cell saver autotransfusion system | 152 (38.2%) |
| RBC transfusion requirement | 296 (74.4%) |
| RBC transfusion (unit) | 3 (0–8) |
| FFP transfusion requirement | 304 (76.4%) |
| FFP transfusion (unit) | 4 (0–7) |
| Platelet transfusion requirement | 5 (1.25%) |
| Use of human albumin solution | 120 (30%) |
| Use of vasopressors and inotropes during CRS | 169 (42.5%) |
| Use of vasopressors and inotropes during HIPEC | 0 (0%) |
| PMV more than 24 h | 75 (18.8%) |
| Urine output (mL) | 600 (300–1800) |
Data are presented as median (IQR), median (min-max) or n (%).
CRS = cytoreductive surgery, FFP = fresh frozen plasma, HIPEC = hyperthermic intraperitoneal chemotherapy, ICU = intensive care unit, MAP = mean arterial pressure, PCI = peritoneal cancer index, PMV = prolonged mechanical ventilation, RBC = red blood cell.
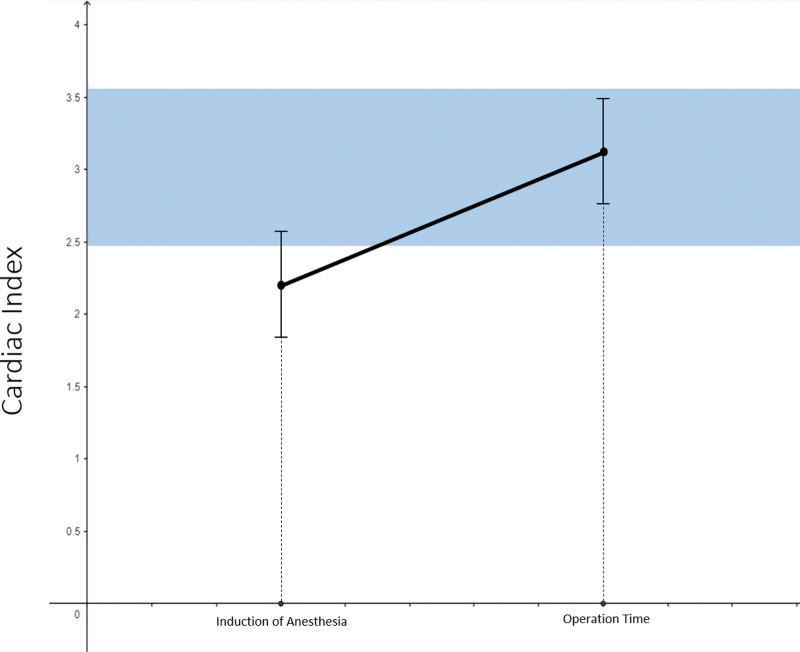
After anesthesia induction, the cardiac index was low, but returned to the normal range during surgery. During HIPEC, the cardiac index exceeded the upper normal range. The systemic vascular resistance index increased after anesthesia induction, normalized during surgery, and dropped slightly below the normal range during HIPEC (Fig. 1).
Figure 1.
The change of cardiac index over time.
Sixteen (16.3%) patients experienced Clavien-Dindo Grade 3–4 adverse events. In 22 cases (5.5%), reoperation was required, and 43 cases (10.8%) were treated by interventional radiology and gastroenterology. Within 30 days, 25 (6.3%) patients died. In 25 cases with mortality, sepsis due to anastomotic leakage occurred in 12 (48%), intraabdominal bleeding in 5 (20%), pulmonary embolism in 4 (16%), cardiac failure in 3 (12%), and COVID-19 in 1 (4%) case (Table 4).
Table 4.
Morbidity and mortality.
| Morbidity and mortality | n |
|---|---|
| Reoperation | 22 (5,5%) |
| Endoscopic or radiological intervention | 43 (10,8%) |
| Total Clavien-Dindo 3–4 morbidity | 65 (16%) |
| 30-day mortality | 25 (6,3%) |
Data are presented as n (%).
4. Discussion
CRS + HIPEC treatments performed for PC significantly affected the vital functions of these patients, including their previous surgical operations, history of chemoradiotherapy, sarcopenia, hypoalbuminemia, and intraabdominal acidity. This patient group required serious preoperative preparation for the surgery. During inpatient care, interventional radiology for the controlled drainage of acid by applying pleurodesis to the abdomen is essential, supported by colloids, TDP, and isotonic fluids. Studies have shown that controlled acid drainage during the preoperative period reduces perioperative hypotension and the need for vasopressor support.[13]
During surgery, an incision is made from the xiphoid to the pubis, increasing the risk of hypothermia and hypovolemia and the possibility of the patient entering metabolic acidosis, leading to a death triad (coagulopathy, acidosis, and hypovolemia) and catastrophic outcomes. At these stages, patients should be warmed with heating blankets to prevent hypothermia and supported by hemodynamic and close urine monitoring to maintain adequate fluid volume without excess or deficiency.[30,31] During the HIPEC phase, efforts should be made to avoid hyperthermia, and parameters such as end-tidal CO2, cardiac output, and intraabdominal pressure should be dynamically monitored.[7–13]
During CRS + HIPEC procedures, which typically last 6 to 12 hours and involve significant fluid and blood loss, as well as insensible losses, dynamic fluid management becomes essential in the perioperative period. Various factors, such as body temperature, heart rate, central venous pressure, and pulmonary artery pressure, make calculating the perfusion distribution challenging.[29] The non-homogeneous spread of PC, diverse surgical techniques, varying doses, and durations of HIPEC drugs coupled with perioperative fluid management contribute to complexity. Applying the same perioperative management as known surgical procedures presents challenges in these cases.
Approaches to perioperative fluid management began with traditional volume loading (high-volume perioperative fluid management, 10–12 mL/kg/h). However, due to excessive fluid distribution, fluid loading, and tissue edema, a shift toward restrictive fluid management (4–6 mL/kg/h) has been observed.[18–21] Initially, we began fluid therapy in these operations with high-volume perioperative fluid treatment. However, due to excessive fluid distribution, fluid loading, and tissue edema, we transitioned away from this method toward restrictive fluid management. This change in clinical practice is attributed to assessments indicating improvements in morbidity due to perioperative fluid restriction. One study showed that patients receiving limited fluid (5–7 mL/kg/h) during surgery exhibited improved postoperative pulmonary stress.[32] However, restrictive fluid therapy is associated with insufficient infusion volume, hemodynamic instability, tissue hypoperfusion, end-organ damage, and nephrotoxicity. Solanki et al suggested that perioperative management is a challenging process with various hemodynamic and temperature fluctuations. They proposed the need for a consensus, highlighting the insufficiency of literature in many aspects of perioperative management. They strongly recommended the use of balanced salt solutions like Ringer lactate and acetate-based solutions as crystalloids in perioperative fluid management. Additionally, they suggested using albumin as a colloid, forming a strong consensus.[33] In our own cases, we used balanced salt solutions such as Ringer lactate and acetate-based solutions as crystalloids and albumin as a colloid in perioperative fluid management, consistent with the literature.
In recent years, GDFT has become a more rational approach to fluid management in non-homogeneous cases.[19–21] While perioperative fluid volume was initially calculated using only CVP catheters and urinary output, modern technological devices such as the FloTrac/Vigileo system (Edwards Lifesciences, Irvine, CA) and pulse indicating continuous cardiac output (PICCO) PICCO catheter (PulsioCath PV2015) L20 (Pulsion Medical Systems, Munich, Germany) have enabled real-time volume optimization and successful fluid management. Studies have found that GDFT reduces major complications, length of hospital stay, and mortality rate in patients with CRS/HIPEC.[32–35] In a study involving 169 cases utilizing restrictive fluid limitation, Hendrix et al reported that restrictive fluid therapy using standard anesthesia intraoperative monitoring devices in CRS/HIPEC could be safely employed, correlating with a reduced hospital stay and complications. However, an inadequate infusion volume can lead to hemodynamic instability, tissue hypoperfusion, end-organ damage, and nephrotoxicity.[32]
In their study, Fichmann et al employed aggressive and invasive monitoring methods, such as the PICCO system, during the initial phase, which did not prevent overhydration. They suggested that monitoring and maintaining diuresis at physiological levels might be as effective as sophisticated measurement of volume status.[35]
Colantonio et al conducted a single-center prospective randomized study that compared GDFT with standard care in 80 patients who underwent CRS and HIPEC. In their study, patients in the control group received crystalloid infusions ranging from 4 mL/kg/h to 10 mL/kg/h, whereas those in the GDFT group received a baseline rate of 4 mL/kg/h with subsequent colloid boluses based on physiological variables. They found that patients in the GDFT group experienced fewer abdominal complications (10.5% vs 38.1%, P = .005), a shorter length of stay (19 days vs 29 days, P < .0001), and lower mortality rates (0% vs 9.5%, P = .12) than those in the control group. Additionally, the average total volume in the GDFT group was reported as 8.54 mL/kg/h compared to 12.30 mL/kg/h in the control group.[20] In our study, out of 398 patients receiving GDFT, 16 (16.3%) developed Clavien-Dindo Grade 3–4 adverse events, resulting in mortality in 25 cases (6.3%) within 30 days. The duration of ICU was reported as 1.5 days (range, 0–20 days), and the average hospital stay was 8.8 days (range, 4–46 days). Additionally, the mean IOF rate in the patients was determined to be 6.4 mL/kg/h (with an interquartile range [IQR] of 5.8–7.1).
Pearse et al conducted the OPTIMISE study, a multicenter, randomized, observer-blinded study involving 734 high-risk patients aged ≥ 50 years who underwent major gastrointestinal surgery. This study aimed to evaluate the clinical effectiveness of a hemodynamic treatment algorithm guided by cardiac output compared to standard care. The findings indicated that using a hemodynamic treatment algorithm guided by cardiac output did not reduce the composite outcomes of complications and 30-day mortality compared with standard care. However, when these data were included in the updated meta-analysis, the intervention resulted in lower complication rates.[17] Solanki et al recommended the consensus decision to implement the use of noninvasive cardiac output monitoring, such as arterial-pressure-based cardiac output monitoring, along with invasive blood pressure monitoring in their studies related to perioperative management of CRS + HIPEC.[33] Witte et al investigated whether FloTrac/Vigileo monitoring contributed to restrictive fluid management during HIPEC surgery in an observational randomized pilot study. They found that these monitoring systems did not induce fluid restriction.[36] In our study, we found that compared to the OPTIMISE and Witte studies, the FloTrac/Vigileo monitoring system led to a lower and more controlled volume rate. We believe that this difference may be attributed to the fact that the OPTIMISE study was conducted on patients undergoing gastrointestinal surgery without CRS HIPEC, and in Witte study, the results from a very low number of only 24 cases may be insufficient for a robust evaluation. In a study conducted in high-volume HIPEC-specific centers, Raspe et al reported that the boundary between hypervolemia and hypovolemia remained unclear and variable. Ultimately, they mentioned that fluid therapy has progressed toward targeted approaches in recent years.[37] Similarly, Gupta et al in their study investigating perioperative fluid management in CRS HIPEC, suggested that maintaining euvolemia (optimal fluid status) using targeted fluid therapy should be considered the standard of care.[38] Solanki et al reported in their meta-analysis study investigating fluid and hemodynamic management in CRS + HIPEC that the expert committee of the Society of Onco-Anesthesia and Perioperative Care (SOAPC) and the ERAS Society independently reached a consensus for the use of individualized GDFT during the CRS HIPEC procedure.[39]
Regarding the limitations of our study, we identified several aspects: the retrospective nature of the study, lack of homogeneity in diseases, PCI scores, CC scores, HIPEC doses, duration, and the absence of a control group. These limitations can impact the comprehensive evaluation and interpretation of the study results.
5. Conclusion
CRS/HIPEC procedures, the successful implementation of perioperative GDFT alongside advanced anesthesia monitoring devices, and offer an alternative to traditional and restrictive fluid management solutions. There is a need for multicenter, prospective, randomized controlled trials in this regard.
Acknowledgments
Thank you Hanife Şeyda Ülgür and Pirilti Özcan for statistical support and table assistance.
Author contributions
Conceptualization: Zeliha Tuncel, Özgül Düzgün.
Data curation: Zeliha Tuncel.
Formal analysis: Zeliha Tuncel.
Writing – original draft: Özgül Düzgün.
Writing – review & editing: Zeliha Tuncel.
Abbreviations:
- ASA
- American Society of Anesthesiologists score
- BSA
- body surface area
- CRS + HIPEC
- cytoreductive surgery + hyperthermic intraperitoneal chemotherapy
- CRS
- cytoreductive surgery
- GDFT
- goal-directed fluid therapy
- HIPEC
- hyperthermic intraperitoneal chemotherapy
- ICU
- intensive care unit
- PC
- peritoneal carcinomatosis
- PCI
- peritoneal cancer index
- PICCO
- pulse-modulating continuous cardiac output catheter
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Tuncel Z, Düzgün Ö. The management of goal-directed fluid therapy during cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Medicine 2024;103:20(e38187).
References
- [1].Sugarbaker PH, Jablonski KA. Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy. Ann Surg. 1995;221:124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chua TC, Moran BJ, Sugarbaker PH, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012;30:2449–56. [DOI] [PubMed] [Google Scholar]
- [3].Bevan KE, Mohamed F, Moran BJ. Pseudomyxoma peritonei. World J Gastrointest Oncol. 2010;2:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Glehen O, Mohamed F, Gilly FN. Peritoneal carcinomatosis from digestive tract cancer: new management by cytoreductive surgery and intraperitoneal chemohyperthermia. Lancet Oncol. 2004;5:219–28. [DOI] [PubMed] [Google Scholar]
- [5].Koppe MJ, Boerman OC, Oyen WJ, et al. Peritoneal carcinomatosis of colorectal origin: incidence and current treatment strategies. Ann Surg. 2006;243:212–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Trimble EL, Christian MC. Intraperitoneal chemotherapy for women with advanced epithelial ovarian carcinoma. Gynecol Oncol. 2006;100:3–4. [DOI] [PubMed] [Google Scholar]
- [7].Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995;221:29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2013;21:3737–43. [DOI] [PubMed] [Google Scholar]
- [9].Schmidt U, Dahlke MH, Klempnauer J, et al. Perioperative morbidity and quality of life in long-term survivors following cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur J Surg Oncol. 2015;31:53–8. [DOI] [PubMed] [Google Scholar]
- [10].Moran B, Baratti D, Yan TD, et al. Consensus statement on the loco-regional treatment of appendiceal mucinous neoplasms with peritoneal dissemination (pseudomyxoma peritonei). J Surg Oncol. 2008;98:277–82. [DOI] [PubMed] [Google Scholar]
- [11].Konigsrainer I, Beckert S, Lehmann T, et al. Peritoneal carcinomatosis. Chirurg. 2011;82:375–80; quiz 381. [DOI] [PubMed] [Google Scholar]
- [12].Yan TD, Deraco M, Baratti D, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol. 2009;27:6237–42. [DOI] [PubMed] [Google Scholar]
- [13].Schmidt C, Creutzenberg M, Piso P, et al. Peri-operative anaesthetic management of cytoreductive surgery. Indian J Surg Oncol. 2016;7:236–43.27065715 [Google Scholar]
- [14].Chua TC, Yan TD, Deraco M, et al. Multi institutional experience of diffuse intra-abdominal multicystic peritoneal mesothelioma. Br J Surg. 2011;98:60–4. [DOI] [PubMed] [Google Scholar]
- [15].Brandstrup B, Svendsen PE, Rasmussen M, et al. Which goal for fluid therapy during colorectal surgery is followed by the best outcome: near-maximal stroke volume or zero fluid balance? Br J Anaesth. 2012;109:191–9. [DOI] [PubMed] [Google Scholar]
- [16].Mayer J, Boldt J, Mengistu AM, et al. Goal-directed intraoperative therapy based on autocalibrated arterial pressure waveform analysis reduces hospital stay in high-risk surgical patients: a randomized, controlled trial. Crit Care. 2010;14:R18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pearse RM, Harrison D, MacDonald N, et al. Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA. 2014;311:2181–90. [DOI] [PubMed] [Google Scholar]
- [18].Wrzosek A, Jakowicka-Wordliczek J, Zajaczkowska R, et al. Perioperative restrictive versus goal-directed fluid therapy for adults undergoing major non-cardiac surgery. Cochrane Database Syst Rev. 2019;12:CD012767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Esteve-Pérez N, Ferrer-Robles A, Gómez-Romero G, et al. Goal-directed therapy in cytoreductive surgery with hyperthermic intraperitoneal chemotherapy: a prospective observational study. Clin Transl Oncol. 2019;21:451–8. [DOI] [PubMed] [Google Scholar]
- [20].Colantonio L, Claroni C, Fabrizi L, et al. A randomized trial of goal directed vs. standard fluid therapy in cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. J Gastrointest Surg. 2015;19:722–9. [DOI] [PubMed] [Google Scholar]
- [21].Piccioni F, Shigeki K, Langer MJ. Goal-directed therapy for cytoreductive surgery with hyperthermic intraperitoneal chemotherapy: the right approach in the right place. J Gastrointest Surg. 2015;19:1196–7. [DOI] [PubMed] [Google Scholar]
- [22].Bezu L, Raineau M, Deloménie M, et al. Haemodynamic management during hyperthermic intraperitoneal chemotherapy: a systematic review. Anaesth Crit Care Pain Med. 2020;39:531–42. [DOI] [PubMed] [Google Scholar]
- [23].Dranichnikov P, Semenas E, Graf W, et al. The impact on postoperative outcomes of intraoperative fluid management strategies during cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur J Surg Oncol. 2023;49:1474–80. [DOI] [PubMed] [Google Scholar]
- [24].Claroni C, Torregiani G, Covotta M, et al. Reply to authors’ letter for the manuscript entitled: “goal-directed therapy for cytoreductive surgery with hyperthermic intraperitoneal chemotherapy: the right approach in the right place”. J Gastrointest Surg. 2015;19:1198–9. [DOI] [PubMed] [Google Scholar]
- [25].White B, Dahdaleh F, Naffouje SA, et al. Impact of enhanced recovery after surgery on postoperative outcomes for patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2021;28:5265–72. [DOI] [PubMed] [Google Scholar]
- [26].Futier E, Constantin JM, Petit A, et al. Conservative vs restrictive individualized goal-directed fluid replacement strategy in major abdominal surgery: a prospective randomized trial. Arch Surg. 2010;145:1193–200. [DOI] [PubMed] [Google Scholar]
- [27].Giglio MT, Marucci M, Testini M, et al. Goal-directed haemodynamic therapy and gastrointestinal complications in major surgery: a meta-analysis of randomized controlled trials. Br J Anaesth. 2009;103:637–46. [DOI] [PubMed] [Google Scholar]
- [28].Salzwedel C, Puig J, Carstens A, et al. Perioperative goal-directed hemodynamic therapy based on radial arterial pulse pressure variation and continuous cardiac index trending reduces postoperative complications after major abdominal surgery: a multi-center, prospective, randomized study. Crit Care. 2013;17:R191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Eng OS, Dumitra S, O’Leary M, et al. Association of fluid administration with morbidity in cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. JAMA Surg. 2017;152:1156–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Al-Dardery NM, Abdelwahab OA, El-Samahy M, et al. Self-warming blankets versus active warming by forced-air devices for preventing hypothermia: a systematic review and meta-analysis. Medicine (Baltimore). 2023;102:e33579–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Melendez Rivera JG, Anjum F. Hypovolemia. In: StatPearls. 2023. [PubMed] [Google Scholar]
- [32].Hendrix RJ, Damle A, Williams C, et al. Restrictive intraoperative fluid therapy is associated with decreased morbidity and length of stay following hyperthermic intraperitoneal chemoperfusion. Ann Surg Oncol. 2019;26:490–6. [DOI] [PubMed] [Google Scholar]
- [33].Solanki SL, Mukherjee S, Agarwal V, et al. Society of Onco-Anaesthesia and Perioperative Care consensus guidelines for perioperative management of patients for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (CRS-HIPEC). Indian J Anaesth. 2019;63:972–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jiang Z, Chen J, Gao C, et al. Effects of PICCO in the guidance of goal-directed fluid therapy for gastrointestinal function after cytoreductive surgery for ovarian cancer. Am J Transl Res. 2021;13:4852–9. [PMC free article] [PubMed] [Google Scholar]
- [35].Fichmann D, Roth L, Dimitri A, et al. Standard operating procedures for anesthesia management in cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improve patient outcomes: a patient cohort analysis. Ann Surg Oncol. 2019;26:3652–62. [DOI] [PubMed] [Google Scholar]
- [36].de Witte P, de Witt CA, van de Minkelis JL, et al. Inflammatory response and optimalisation of perioperative fluid administration during hyperthermic intraoperative intraperitoneal chemotherapy surgery. J Gastrointest Oncol. 2019;10:244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Raspé C, Flöther L, Schneider R, et al. Best practice for perioperative management of patients with cytoreductive surgery and HIPEC. Eur J Surg Oncol. 2017;43:1013–27. [DOI] [PubMed] [Google Scholar]
- [38].Gupta N, Kumar V, Garg R, et al. Anesthetic implications in hyperthermic intraperitoneal chemotherapy. J Anaesthesiol Clin Pharmacol. 2019;35:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Solanki SL, Maurya I, Sharma J. Impact of fluid and haemodynamic management in cytoreductive surgery with hyperthermic intraperitoneal chemotherapy on postoperative outcomes - a systematic review. Indian J Anaesth. 2023;67:866–79. [DOI] [PMC free article] [PubMed] [Google Scholar]