ABSTRACT
Tuberculous (TB) involvement of the vascular system has been reported in the preantibiotic era. We, hereby, report a case involving a teenage boy who presented to us with left upper limb pain followed by gradually progressive motor and sensory deficit over 1 month with preceding history of tuberculosis. Examination revealed a palpable, noncompressible, nonpulsatile swelling superior to the lateral third of the clavicle. Imaging through ultrasonography, computed tomography, and magnetic resonance imaging confirmed the presence of a pseudoaneurysm with compression of the underlying nerves. The child underwent surgical thrombectomy with pseudoaneurysm repair and arteriorrhaphy along with antitubercular medications with complete recovery at 6 months. The tissue staining, nucleic acid amplification tests, and histopathology confirmed TB etiology. Tuberculosis continues to remain a major health concern, especially in the developing world. High index of suspicion is necessary to diagnose such manifestations to avoid catastrophic sequelae.
Keywords: Pseudoaneurysm repair, tuberculosis complications, vascular surgery
INTRODUCTION
Vascular involvement in tuberculosis is a rare manifestation. Pattern of vascular involvement corresponds to the organ involved, possibly due to the direct extension[1] of the granulomatous process occurring in the lymph nodes draining these organs, namely, the lung, spine, and the intestines. Rarely, tuberculous (TB) pseudoaneurysms may form at a location remote from the primary organ after hematogenous dissemination of the tubercle bacilli, like that seen with mycotic aneurysms. We report a case of a large TB pseudoaneurysm of axillary artery in an immunocompetent adolescent male presenting as monoparesis. The case is being reported for its atypical presentation and successful management.
CASE REPORT
A young adolescent boy presented to us with complains of pain and weakness in the left arm for the preceding 1 month. It was a dull aching pain that started around his left shoulder after sustaining a trivial injury during hand combat training. The pain had gradually progressed over the next 2 weeks, resulting in marked restriction of the movement of left shoulder joint.
Subsequently, he began to perceive weakness of the left arm, which initially was limited to the proximal muscle group but later progressed to involve the distal muscle groups of the hands and fingers. This was also accompanied by loss of sensation over the dorsal and lateral aspect of the palm and elbow. Three months prior to the onset of these symptoms, he developed high-grade fever without any localizing signs and unresponsive to a short course of oral antibiotics. It was also associated with anorexia and weight loss. A physician had initiated the boy on anti-TB therapy based on radiological evidence to which he had responded appropriately. On evaluation, he had a palpable, noncompressible, nonpulsatile swelling superior to the lateral one-third of the clavicle. The muscle power across the joints of the digits and wrist were Grade I, while at the elbow and shoulder, it was grade II. The child had enophthalmos, ptosis, and miosis.
The deep tendon reflexes were absent in the involved limb. An ultrasound of the swelling revealed a large lobulated pseudoaneurysm measuring 5.5 cm × 6 cm originating from the proximal axillary artery with reduced velocity monophasic flow in the distal arteries. A computed tomography (CT) angiography confirmed the presence of a saccular outpouching in the initial part of the axillary artery measuring 7.6 cm × 7 cm × 5.7 cm and causing luminal compression proximal axillary artery with thrombus seen within its lumen. In addition, centrilobular nodules were noticed in the upper lobes of the bilateral lungs suggestive of tuberculosis [Figures 1 and 2]. There was no other vessel involved. A noncontrast magnetic resonance imaging was suggestive of compression of adjacent neural structures [Figure 3]. Nerve conduction studies demonstrated inexcitability of the ulnar and median nerve.
Figure 1.

(a) Coronal maximum intensity projection computed tomography (CT) image showing partially thrombosed pseudoaneurysm (patent part shown by red arrow) arising from the 2nd and 3rd part of the left subclavian artery (yellow dotted line). (b) Curved reformat CT image showing partially thrombosed pseudoaneurysm (patent part shown by red arrow) arising from the 2nd and 3rd part of the left subclavian artery
Figure 2.
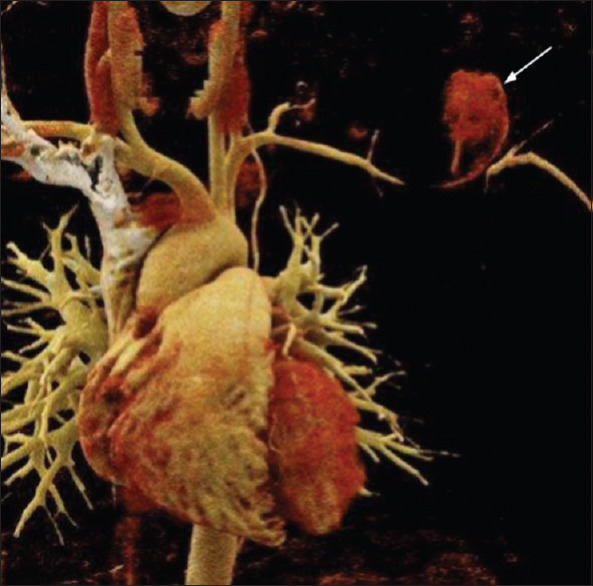
Volume rendered computed tomography image showing patent part of the pseudoaneurysm (arrow)
Figure 3.
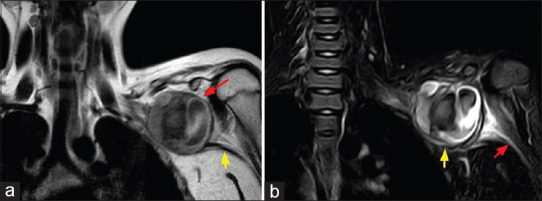
(a) Coronal T1 magnetic resonance imaging image showing round-shaped pseudoaneurysm (red arrow) arising from the left subclavian artery, continuation of left axillary artery (yellow arrow). (b) Coronal short tau inversion recovery (STIR) image showing pseudoaneurysm compressing brachial plexus (yellow arrow), continuation of brachial plexus shown (red arrow)
After obtaining proximal control of the artery, the wall and contents of the pseudoaneurysm were debrided. The neck of the pseudoaneurysm was sutured after confirming distal pulse and saturation. Acid fast bacilli were seen on Ziehl - Neelsen staining of debrided pseudoaneurysm wall. The Nucleic Acid Amplification Test of the tissue samples turned out positive for Rifampicin sensitive Mycobacteriae. The histopathology specimen revealed acid-fast bacilli in the arterial wall adjacent to the vasa vasorum. Antitubercular medications were continued. Physical therapy for the right upper limb was instituted.
The child had an uneventful postoperative recovery. The postoperative arterial Doppler revealed biphasic flow. The power of proximal and distal group of muscles normalized over the next 6 months.
DISCUSSION
TB involvement of the arterial system is usually caused either due to contiguous extension of granulomatous process from an adjoining TB lymph node[1] or hematogenous seeding of the vascular intima from a remote focus, the former mechanism being more common. In the hematogenous form of vessel involvement, the tubercle bacilli may either lodge in an atheromatous plaque or may involve the vessel wall by way of vasa vasorum.
Furthermore, other pathological mechanisms such as tubercular polyps from the vascular intima and aneurysm formation have been described by Haythorn.[2] In addition, a stenosing variety of aortoarteritis, possibly due to tuberculosis, has also been described.[3] Due to early detection and treatment, the contiguous focus may not be necessarily seen by radiologic means as in our case. Aneurysms of the arterial system may contain all the layers of vessel wall (true aneurysm) or none with surrounding structures encapsulating the contents (pseudoaneurysm). Majority of the aneurysms due to mycobacteria are pseudoaneurysms (87%).
Although true aneurysms or dissecting aneurysms are seen only in <10% and 5% of cases, respectively, rupture into the surrounding viscera is noted in two-thirds of these cases.[4] Presentation of TB pseudoaneurysm typically involves persistent pain related to the location of the aneurysm due to compression of adjoining structures by a rapidly expanding pulsatile mass [Figure 4]. The order of vessel involvement usually follows the same scheme of organ involvement in tuberculosis viz. bronchial artery in pulmonary tuberculosis, vertebral artery in spinal tuberculosis, and mesenteric artery in intestinal tuberculosis. Although the symptoms of compression were similar in our patient, the location was not typical of a TB pseudoaneurysm. Tubercular aneurysms are most often solitary, and calcification is conspicuously absent.[4]
Figure 4.

Diagram depicting the relation of the pseudoaneurysm and adjoining structures
At present, combined surgery and anti-TB drug treatment should be used for the disease,[5,6] considering either of them alone may lead to very high mortality.[4,7] Occurrence of various complications in the event of delayed antitubercular medications despite surgical repair highlights the possibility of the organism meddling with the healing and the inability of surgical debridement in achieving complete eradication of infected milieu.[8] The majority of TB pseudoaneurysms are caused by inflammatory reactions surrounding the arteries. The resulting revascularization and fibrosis lead to adhesion between blood vessels and their surrounding tissues, making extensive surgical debulking difficult due to the difficulty of separating blood vessels and surrounding tissues, increasing the risk of aneurysmal rupture.[9] Institution of anti-TB medications is recommended for the improvement of the arterial wall and the surrounding soft tissues, thereby reducing the incidence of postoperative complications and mortality rate.[10] Aneurysms often spontaneously ruptured 1.5–3 months after the onset of TB symptoms without any treatment. The total rupture rate of TB aneurysms was as high as 54.6%.[8] However, surgical treatment should not be delayed once the diagnosis is confirmed, regardless of size considering the possibility of rupture with small aneurysms as well.[11] Access of anti-TB drugs in the laminated thrombus that forms the wall of the aneurysm is limited to the luminal blood flow due to thrombosis of the small vessels and risk of sudden enlargement and rupture, leading to fatal hemorrhage warranting emergent surgical intervention[12] The mortality in patients with associated miliary tuberculosis is four times higher than in those without.[13,14,15] Although endovascular stenting is a less invasive procedure for the treatment of pseudoaneurysms, it is not a preferred in cases with infective etiology since it does not involve removal of the surrounding necrotic tissue.
However, there are few reports of improved outcomes with endovascular techniques, especially after the stent or grafts are pretreated with rifampicin.[13,16,17] There is no consensus regarding the duration of the antimicrobial therapy in vascular involvement by tuberculosis. The current practice recommended is using a combination of clinical, biological, and radiographic information.[13,17] CT imaging does not help predict the recurrence. Alternative techniques such as in-labeled biotin scan are considered highly sensitive in detecting graft infection.[18]
CONCLUSIONS
With the resurgence of tuberculosis due to drug-resistance and its association with acquired immunodeficiency syndrome, the incidence of tubercular aneurysm is bound to increase. A high index of suspicion in patients with active tuberculosis if they deteriorate suddenly or report appearance of mass lesions warrants work up to identify this condition. Histologic and microbial assessment especially targeting mycobacteria should be sought in all cases of pseudoaneurysm. Surgical intervention, along with antitubercular medications, is indicated in each case independent of the size.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hara M, Bransford RM. Aneurysm of the subclavian artery associated with contiguous pulmonary tuberculosis. J Thorac Cardiovasc Surg. 1963;46:256–64. [PubMed] [Google Scholar]
- 2.Haythorn SR. Tuberculosis of the large arteries. J Am Med Assoc. 1913;60:1413–6. [Google Scholar]
- 3.Sen PK, Kinare SG, Kulkarni TP, Parulkar GB. Stenosing aortitis of unknown etiology. Surgery. 1962;51:317–25. [PubMed] [Google Scholar]
- 4.Choudhary SK, Bhan A, Talwar S, Goyal M, Sharma S, Venugopal P. Tubercular pseudoaneurysms of aorta. Ann Thorac Surg. 2001;72:1239–44. doi: 10.1016/s0003-4975(01)03002-8. [DOI] [PubMed] [Google Scholar]
- 5.Gajraj A, Victor S. Tuberculous aorta arteritis. Clin Radiol. 1981;32:461–6. doi: 10.1016/s0009-9260(81)80307-8. [DOI] [PubMed] [Google Scholar]
- 6.Zhang C, Chen B, Gu Y, Luo T, Yang S, Liang W, et al. Tuberculous abdominal aortic pseudoaneurysm with renal and vertebral tuberculosis: A case and literature review. J Infect Dev Ctries. 2014;8:1216–21. doi: 10.3855/jidc.4954. [DOI] [PubMed] [Google Scholar]
- 7.Forbes TL, Harris JR, Nie RG, Lawlor DK. Tuberculous aneurysm of the supraceliac aorta – A case report. Vasc Endovascular Surg. 2004;38:93–7. doi: 10.1177/153857440403800113. [DOI] [PubMed] [Google Scholar]
- 8.Long R, Guzman R, Greenberg H, Safneck J, Hershfield E. Tuberculous mycotic aneurysm of the aorta: Review of published medical and surgical experience. Chest. 1999;115:522–31. doi: 10.1378/chest.115.2.522. [DOI] [PubMed] [Google Scholar]
- 9.Yi S, Wang L. Clinical features of tuberculous pseudoaneurysm and risk factors for mortality. J Vasc Surg. 2022;75:1729–38.e2. doi: 10.1016/j.jvs.2021.10.048. [DOI] [PubMed] [Google Scholar]
- 10.Villegas MO, Mereles AP, Tamashiro GA, Dini AE, Mollón AP, De Cándido LV, et al. Endovascular treatment of an aortoiliac tuberculous pseudoaneurysm. Cardiovasc Intervent Radiol. 2013;36:540–4. doi: 10.1007/s00270-012-0398-9. [DOI] [PubMed] [Google Scholar]
- 11.Pimple MK, Narlawar RS, Bapat MR. Mycotic aneurysm of the descending thoracic aorta with intraspinal extension – A case report. Acta Orthop Scand. 2002;73:597–600. doi: 10.1080/000164702321022929. [DOI] [PubMed] [Google Scholar]
- 12.De Prophetis N, Armitage HV, Triboletti ED. Rupture of tuberculous aortic aneurysm into lung. Ann Surg. 1959;150:1046–51. doi: 10.1097/00000658-195912000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Labrousse L, Montaudon M, Le Guyader A, Choukroun E, Laurent F, Deville C. Endovascular treatment of a tuberculous infected aneurysm of the descending thoracic aorta: A word of caution. J Vasc Surg. 2007;46:786–8. doi: 10.1016/j.jvs.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 14.Elzein F, Qatan N, Alghamdi A, Albarrak A, Kalam K. Miliary tuberculosis presenting as bilateral superficial femoral artery mycotic aneurysm in an immunocompetent patient. Respir Med Case Rep. 2019;26:236–9. doi: 10.1016/j.rmcr.2019.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar S, Babu NS, Jaret P, Sharma A. Tubercular mycotic aortic aneurysm: A case report. Lung India. 2016;33:192–5. doi: 10.4103/0970-2113.177437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Escobar GA, Eliason JL, Hurie J, Arya S, Rectenwald JE, Coleman DM. Rifampin soaking dacron-based endografts for implantation in infected aortic aneurysms – New application of a time-tested principle. Ann Vasc Surg. 2014;28:744–8. doi: 10.1016/j.avsg.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Dogan S, Memis A, Kale A, Buket S. Endovascular stent graft placement in the treatment of ruptured tuberculous pseudoaneurysm of the descending thoracic aorta: Case report and review of the literature. Cardiovasc Intervent Radiol. 2009;32:572–6. doi: 10.1007/s00270-008-9456-8. [DOI] [PubMed] [Google Scholar]
- 18.Chiesa R, Melissano G, Castellano R, Fernandez Zamora C, Astore D, Samuel A, et al. Avidin and 111In-labelled biotin scan: A new radioisotopic method for localising vascular graft infection. Eur J Vasc Endovasc Surg. 1995;10:405–14. doi: 10.1016/s1078-5884(05)80162-5. [DOI] [PubMed] [Google Scholar]


