Abstract
Chronic graft-versus-host disease (GVHD) is an immune-mediated disorder that causes significant late morbidity and mortality following allogeneic hematopoietic cell transplantation. The “Close Assessment and Testing for Chronic GVHD (CATCH)” study is a multi-center Chronic GVHD Consortium prospective, longitudinal cohort study designed to enroll patients before hematopoietic cell transplantation and follow them closely to capture the development of chronic GVHD and to identify clinical and biologic biomarkers of chronic GVHD onset. Data are collected pre-transplant and every two months through one-year post-transplant with chart review thereafter. Evaluations include clinician assessment of chronic GVHD and its manifestations, patient-reported outcomes, multiple biospecimens (blood, saliva, tears, buccal mucosa and fecal samples, biopsies of skin and mouth), laboratory testing, and medical record abstraction. This report describes the rationale, design, and methods of the CATCH study, and invites collaboration with other investigators to leverage this resource.
trial registration: This study is registered at www.clinicaltrials.gov as NCT04188912.
Introduction
Chronic graft-versus-host disease (GVHD) is a common immune-mediated disorder following allogeneic hematopoietic cell transplantation (HCT). This complication develops in 10–30% of those who survive at least 100 days and has a median time to onset of 4–6 months after HCT [1–3]. Chronic GVHD is associated with worse quality of life [4–6], prolonged duration of immunosuppressive therapy (IST) [7, 8], and higher non-relapse mortality, but also a lower malignancy relapse rate [9]. Treatment is largely empiric although there are currently three FDA-approved treatment options: ibrutinib, belumosudil and ruxolitinib [10].
The close monitoring of patients post-HCT provides a unique opportunity to observe and characterize the development of the distinctive auto/alloimmune syndrome of chronic GVHD.
Animal models have identified inflammatory, immunologic and tissue response pathways operative in chronic GVHD, but direct applicability to humans remains unclear, especially the basis for its heterogeneous phenotypes. Previous work has been done to advance biologic understanding through infrequent and late assessment of large numbers of patients to gain sufficient power for analyses. A major limitation of previous studies was not having baseline samples before onset of systemic IST. The CATCH Study (Close Assessment and Testing for Chronic GVHD) differs because it assesses a smaller number of patients more frequently to “catch” the onset of human chronic GVHD clinical manifestations to better understand its early biology with the help of parallel collection of tears, saliva, oral and fecal microbiome, blood samples and skin and oral biopsies. Study participants are assessed before HCT to establish a baseline and provide “control” samples, and then every two months through the first year after HCT. This study design will allow participants to be their own controls as well as allowing comparison of those who do and do not develop chronic GVHD at similar timepoints after HCT. The study objective of CATCH is to test whether profile changes in blood proteins and cell subsets or bodily fluids and tissues predict chronic GVHD onset, severity and organ involvement.
This paper outlines the design and methods of the CATCH study and provides information about accessing materials and data from the study.
Methods
Population
Patients are eligible for this study if they are age ≥18 years, scheduled for allogeneic HCT from any donor for any indication with a risk of chronic GVHD of ≥25%, have ability and willingness to comply with the study assessment schedule, and ability to communicate in English or Spanish, to allow completion of patient surveys and study procedures. Patients with a chronic GVHD risk of ≤25% (umbilical cord blood, bone marrow graft with post-transplant cyclophosphamide, or use of anti-thymocyte globulin, alemtuzumab, or ex-vivo T-cell depletion) are excluded as they are unlikely to be informative to justify the intensive follow up assessments. Other exclusion criteria include hematologic malignancy with active disease at time of HCT (measurable residual disease is allowed), HCT-comorbidity index >4 [11], prior allogeneic HCT, prior autoimmune disease with ongoing symptoms or need for treatment, history of noncompliance, or inability to comply with study requirements (due to any geographic, logistic, social, or other factors). The protocol is approved by a central IRB, the Fred Hutchinson Cancer Center Institutional Review Board (initial approval date April 10, 2019, protocol #10134). All participants provide written informed consent. The study opened to accrual on September 13, 2019, and enrollment is ongoing. The consent form allows genetic studies and sharing of specimens and clinical data. Participating transplant centers and investigators are listed in the S1 Appendix.
Study design
This is a prospective, longitudinal observational study. No therapeutic interventions are mandated by the protocol. Target enrollment is approximately 200 patients at 7 institutions prior to HCT to obtain at least 180 evaluable patients, however the final enrollment numbers will be determined by the number of participants who develop chronic GVHD requiring systemic immunosuppression.
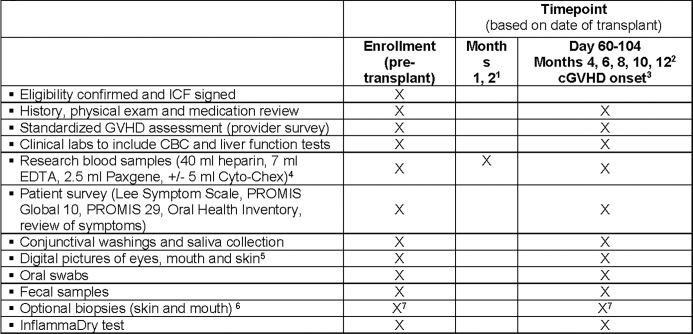
Patients are enrolled prior to HCT. Formal study assessments occur at enrollment, day 60–104 post-HCT, and then every other month from month 4 through 12 post-HCT, as well as at time of chronic GVHD diagnosis (if this is more than 14 days from other scheduled visits). Longer-term follow up beyond these assessments is done by chart review. At each time point, patients and clinicians report information on chronic GVHD, and data and samples are collected (Fig 1). Additional research blood samples are collected at months 1 and 2 post-HCT. Acceptable windows for sample collection are +/- 7 days for months 1 and 2, and then +/- 30 days for subsequent time points. This study is registered at www.clinicaltrials.gov as NCT04188912.
Fig 1. Schedule of enrollments and assessments on study.
1 Do not collect month 2 if a full study visit is conducted between days 60–74; acceptable window for these visits is +1–7 days. 2 Acceptable window for these visits is +1–30 days. To calculate target date for these visits, 1month = 30 days. 3 Onset visit only required if diagnosis date is more than 14 days before or after another study visit. 4 Cyto-Chex collected only at onset of cGVHD (per NIH consensus criteria). 5 Pretransplant for all subjects, and after transplant only if any abnormalities are detected. 6 Skin biopsy at enrollment, day 60–104, month 12, and initiation of systemic IST for chronic GVHD. Oral biopsy at day 60–104 and initiation of systemic IST for chronic GVHD.
Data and sample collection
Fig 1 shows the data and sample collection schedule. Clinical data pertaining to chronic GVHD onset, severity, treatment and organ involvement, relapse and death are collected via clinician-completed forms and chart review. In the setting of relapsed disease or participant withdrawal from study procedures, patients are given the option of ongoing data abstraction by medical record review only (no further study procedures). Patients who consent but do not proceed to HCT or who miss the day 60–104 visit are taken off study and replaced (no further follow up). Data are cleaned every 3 months using customized programs for range and logic checking. Queries to sites are made as necessary to reconcile discrepancies. Provider training occurred via written materials, in person meetings, web presentations, and ongoing discussions.
Provider assessment form
The provider reports data as recommended by the NIH Consensus Conference for skin, eye, mouth, gastrointestinal, joint, genital and pulmonary chronic GVHD involvement, as well as the presence of specific clinical manifestations such as bronchiolitis obliterans syndrome. Liver and pulmonary function test results are abstracted from the chart [12, 13].
Medical records abstraction
Information on patient, donor, and transplant characteristics, acute GVHD diagnosis, chronic GVHD presentation, and current status is collected from institutional databases or chart review. Immunosuppressive medications at the time of study visits and any given between visits are captured. Endpoint assessments (relapse, death, resolution of chronic GVHD, or discontinuation of immunosuppression) are recorded. Clinical lab values, biopsy results, and pulmonary function test results are captured.
Research blood samples
49.5 mL peripheral blood is collected and transported directly to the processing laboratory [14]. 47 mL is collected in anticoagulant tubes (40 ml in heparin and 7 ml in EDTA) and separated as soon as possible into plasma and peripheral blood mononuclear cells (PBMC) using Ficoll. 2.5 mL of whole blood is placed in a PAXgene tube for later RNA isolation. If a patient develops chronic GVHD, an additional one-time 5 mL of blood is also collected in a Cyto-Chex tube for flow cytometry.
Patient survey
Participants complete the following patient-reported outcome surveys: Lee Symptom Scale (30 items, 2 minutes) [15–17], PROMIS (Patient-Reported Outcomes Measurement Information System [18])-Global 10 (10 items, 1 minute), PROMIS 29 (30 items, 3–5 minutes) and the Oral Health Inventory for oral health-related QOL (14 items, 1 minute) [19]. They are also asked a standardized review of systems at each study visit using eight core items (1 minute) of the Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) that are not covered in the other instruments [20, 21]. Total time to complete the battery is approximately 10 minutes.
Conjunctival washings, MMP-9 testing, and saliva collection
Tear fluid is collected by instilling approximately 50 μL preservative-free Refresh Optive artificial tears to each eye, having the subject abduct, adduct, elevate and depress the eye to rinse the ocular survace, and collecting samples 15 seconds later via microcapillary tubes, anticipating 30–35 μL recovery. Matrix metalloproteinase 9 (MMP-9) testing (InflammaDry) is performed on both eyes. Validity of the MMP-9 testing was assured with built in positive controls. Unstimulated saliva is collected into a tube over 5 minutes and immediately placed on ice for transport directly to the processing laboratory. The amount of saliva collected is recorded.
Digital pictures of eyes, mouth and skin
Digital pictures of the eyes, mouth and skin are performed pre-transplant and if any abnormalities are detected after transplant (instructions provided in manual of operations).
Oral swabs
Swabs of the oral mucosa for microbiome analysis are taken at 2 locations: dorsal tongue and buccal mucosa.
Fecal samples
Fecal samples are collected at home and transported at room temperature, then frozen at -80 degrees on day of receipt. Samples are stored for batch microbiome analysis.
Biopsies (skin and mouth)
Punch biopsies of the skin and oral mucosa are performed before HCT (skin only), at day 100 (both), one year (skin only) and if chronic GVHD develops (both), according to safety parameters at each site. Oral biopsies were performed on an affected area for those with chronic GVHD. Skin and oral biopsies are optional for the participant. Biopsies are stored in two ways: frozen in optimal cutting temperature (OCT) media (until December 2022) or Cryostor (after December 2022), and formalin-fixed, paraffin-embedded.
Biostatistical considerations
This is a longitudinal observational study with one baseline assessment and serial follow-up assessments for each participant. The target enrollment is 180 evaluable patients. Enrollment will be extended if fewer than 55 evaluable patients develop chronic GVHD. Analyses are primarily descriptive. The levels, proportions, and trajectories of cytokines, chemokines, proteins and cellular populations will be analyzed and compared between patients who develop chronic GVHD and those who do not, with attention to chronic GVHD organ manifestations and symptoms. The clinical utility of these chronic GVHD prognostic biomarkers will be evaluated. Analysis of study data will describe missing data, examine mechanisms of missingness, and attempt to account for them. These methods include a comparison of those with and without missing data, documentation of sources of missing data, and evaluations of the pattern of missingness.
Collaboration with other investigators
The Chronic GVHD Consortium invites collaboration with other investigators who wish to access the data and research samples derived from this study. Before data or samples can be provided, a concept sheet must be discussed by Consortium members and approved by the Consortium Principal Investigator. All ancillary studies require IRB approval or waiver at both the center(s) providing data/samples and the receiving institution(s), as well as a materials transfer agreement or data use agreement if a subcontract is not in place. Approval of the collaboration by the National Cancer Institute may also be required. Data and samples are de-identified, with clinical information and samples labeled with a study ID only.
Results
Enrollment started in 2019 and continues. Table 1 shows characteristics of the cohort so far. As of 10/1/2023, 186 evaluable participants have been enrolled among whom 56.2% (95% CI: 43.2%, 67.2%) had developed any chronic GVHD by 26 months and 29.2% (95% CI: 21.2%, 37.7%) had developed chronic GVHD requiring IST. Table 2.
Table 1. Patient characteristics.
| Variable | Category | N | Count (%) |
|---|---|---|---|
| Study site | Fred Hutchinson Cancer Center | 186 | 101 (54.3%) |
| H. Lee Moffitt Cancer Center and Research Institute | 39 (21.0%) | ||
| Cleveland Clinic | 22 (11.8%) | ||
| Roswell Park Cancer Institute | 16 (8.6%) | ||
| University of Florida | 4 (2.2%) | ||
| National Cancer Institute | 3 (1.6%) | ||
| Vanderbilt | 1 (0.6%) | ||
| Recipient age at HCT in years | Median: IQR: |
182 | 61.5 (54, 67) |
| 18–30 | 182 | 7 (3.8%) | |
| 31–40 | 14 (7.7%) | ||
| 41–50 | 15 (8.2%) | ||
| 51–60 | 46 (25.3%) | ||
| 61–70 | 83 (45.6%) | ||
| > = 70 | 17 (9.3%) | ||
| Patient gender | Male | 185 | 111 (60.0%) |
| Female | 74 (40.0%) | ||
| Diagnosis | Acute myeloid leukemia | 170 | 74 (43.5%) |
| Myelodysplastic syndrome | 37 (21.8%) | ||
| Myeloproliferative neoplasm | 21 (12.4%) | ||
| Acute lymphocytic leukemia | 15 (8.8%) | ||
| Chronic myeloid leukemia | 6 (3.5%) | ||
| Non Hodgkins Lymphoma | 4 (2.4%) | ||
| Multiple myeloma | 3 (1.8%) | ||
| Chronic lymphocytic leukemia | 2 (1.2%) | ||
| Other | 8 (4.7%) | ||
| Disease stage at transplant | Early | 159 | 83 (52.2%) |
| Intermediate | 61 (38.4%) | ||
| Advanced | 15 (9.4%) | ||
| Graft Type | Peripheral Blood | 170 | 162 (95.3%) |
| Bone Marrow | 8 (4.7%) | ||
| Donor type | HLA-matched unrelated donor | 170 | 103 (60.6%) |
| HLA-mismatched unrelated donor | 25 (14.7%) | ||
| HLA identical sibling | 20 (11.8%) | ||
| HLA-matched other relative | 12 (7.1%) | ||
| Haploidentical related donor | 8 (4.7%) | ||
| HLA-mismatched relative | 2 (1.2%) | ||
| Conditioning Intensity | Myeloablative | 168 | 76 (45.2%) |
| Reduced intensity | 59 (35.1%) | ||
| Non-myeloablative | 33 (19.6%) | ||
| GVHD prophylaxis | CNI+MTX-based | 161 | 149 (92.6%) |
| Post-HCT Cy | 3 (1.9%) | ||
| Other | 9 (5.6%) | ||
| Donor-recipient sex match | Female into male | 173 | 44 (25.4%) |
| Other | 129 (74.6%) | ||
| Donor-recipient CMV match | Negative/negative | 167 | 61 (36.5%) |
| Negative / Positive | 36 (21.6%) | ||
| Positive/Negative | 21 (12.6%) | ||
| Positive/ Positive | 49 (29.3%) | ||
| Grade II-IV acute GVHD | Yes | 175 | 53 (30.3%) |
| No | 122 (69.7%) | ||
| Follow-up of survivors (months) | Median: IQR: |
144 | 10.5 (5, 12) |
Table 2. Study endpoints.
| Endpoint | N evaluable | CI (95% CI) |
|---|---|---|
| Any chronic GVHD by 14 months | 157 | 44.9% (35.2%, 54.1%) |
| Any chronic GVHD by 26 months | 157 | 56.2% (43.2%, 67.2%) |
| Chronic GVHD requiring systemic IST by 14 months | 157 | 29.2% (21.2%, 37.7%) |
| Chronic GVHD requiring systemic IST by 26 months | 157 | 29.2% (21.2%, 37.7%) |
Summary and conclusions
The Chronic GVHD Consortium “CATCH” Study aims to improve biologic understanding of chronic GVHD development after HCT. We hope to detect the earliest clinical and biologic changes that presage chronic GVHD by collecting clinically annotated pre-transplant baseline and early post-transplant biological samples during the high-risk period for developing chronic GVHD. By prospectively collecting comprehensive data in a large population treated at many centers, with heterogeneous chronic GVHD manifestations and severity, we have a rich longitudinal database to inform these analyses. This information may be used to design pre-emptive and early intervention studies.
This study has some limitations. Enrollment continued during the COVID-19 pandemic but the speed of enrollment and completeness of data were affected. Some procedures could not be performed due to infectious disease control measures, and patients were reluctant to spend any extra time in the clinic. Although every two months is relatively frequent, we may still have missed relevant samples and symptoms that would have been informative for impending chronic GVHD. The sample size is relatively small because of the intensive follow-up procedures required.
Investigators interested in the clinical data, research forms, database structure or research samples available from this cohort study should contact the corresponding author for procedures on how to apply for access. We welcome collaboration with other investigators involved in chronic GVHD research.
Supporting information
(DOC)
(DOCX)
(DOCX)
Acknowledgments
The investigators wish to thank the patients who are participating in this study.
Data Availability
Enrollment is ongoing. Data will be available after the primary analysis is complete but requires a data use agreement.
Funding Statement
National Institutes of Health CA236229 (SJL, SZP)
References
- 1.Lee SJ. Classification systems for chronic graft-versus-host disease. Blood. 2017;129(1):30–7. doi: 10.1182/blood-2016-07-686642 ; PubMed Central PMCID: PMC5216262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saliba RM, Alousi AM, Pidala J, Arora M, Spellman SR, Hemmer MT, et al. Characteristics of Graft-Versus-Host Disease (GvHD) After Post-Transplantation Cyclophosphamide Versus Conventional GvHD Prophylaxis. Transplant Cell Ther. 2022;28(10):681–93. Epub 2022/07/20. doi: 10.1016/j.jtct.2022.07.013 ; PubMed Central PMCID: PMC10141544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolanos-Meade J, Hamadani M, Wu J, Al Malki MM, Martens MJ, Runaas L, et al. Post-Transplantation Cyclophosphamide-Based Graft-versus-Host Disease Prophylaxis. N Engl J Med. 2023;388(25):2338–48. Epub 2023/06/21. doi: 10.1056/NEJMoa2215943 ; PubMed Central PMCID: PMC10575613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pidala J, Kurland B, Chai X, Majhail N, Weisdorf DJ, Pavletic S, et al. Patient-reported quality of life is associated with severity of chronic graft-versus-host disease as measured by NIH criteria: report on baseline data from the Chronic GVHD Consortium. Blood. 2011;117(17):4651–7. Epub 2011/03/01. doi: 10.1182/blood-2010-11-319509 ; PubMed Central PMCID: PMC3099579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee SJ, Logan B, Westervelt P, Cutler C, Woolfrey A, Khan SP, et al. Comparison of Patient-Reported Outcomes in 5-Year Survivors Who Received Bone Marrow vs Peripheral Blood Unrelated Donor Transplantation: Long-term Follow-up of a Randomized Clinical Trial. JAMA Oncol. 2016;2(12):1583–9. doi: 10.1001/jamaoncol.2016.2520 ; PubMed Central PMCID: PMC5145732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee SJ, Onstad L, Chow EJ, Shaw BE, Jim HSL, Syrjala KL, et al. Patient-reported outcomes and health status associated with chronic graft-versus-host disease. Haematologica. 2018;103(9):1535–41. doi: 10.3324/haematol.2018.192930 ; PubMed Central PMCID: PMC6119141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SJ, Nguyen TD, Onstad L, Bar M, Krakow EF, Salit RB, et al. Success of Immunosuppressive Treatments in Patients with Chronic Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2018;24(3):555–62. doi: 10.1016/j.bbmt.2017.10.042 ; PubMed Central PMCID: PMC5826880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen GL, Onstad L, Martin PJ, Carpenter P, Pidala J, Arai S, et al. Durable discontinuation of systemic therapy in patients affected by chronic graft versus host disease. Haematologica. 2022. Epub 2022/05/27. doi: 10.3324/haematol.2021.279814 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inamoto Y, Flowers ME, Lee SJ, Carpenter PA, Warren EH, Deeg HJ, et al. Influence of immunosuppressive treatment on risk of recurrent malignancy after allogeneic hematopoietic cell transplantation. Blood. 2011;118(2):456–63. Epub 2011/06/03. doi: 10.1182/blood-2011-01-330217 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeiser R, Lee SJ. Three US Food and Drug Administration-approved therapies for chronic GVHD. Blood. 2022;139(11):1642–5. Epub 2022/01/27. doi: 10.1182/blood.2021014448 ; PubMed Central PMCID: PMC8931512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sorror ML. How I assess comorbidities before hematopoietic cell transplantation. Blood. 2013;121(15):2854–63. doi: 10.1182/blood-2012-09-455063 ; PubMed Central PMCID: PMC3624933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21(3):389–401 e1. doi: 10.1016/j.bbmt.2014.12.001 ; PubMed Central PMCID: PMC4329079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SJ, Wolff D, Kitko C, Koreth J, Inamoto Y, Jagasia M, et al. Measuring therapeutic response in chronic graft-versus-host disease. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. The 2014 Response Criteria Working Group report. Biol Blood Marrow Transplant. 2015;21(6):984–99. doi: 10.1016/j.bbmt.2015.02.025 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paczesny S, Hakim FT, Pidala J, Cooke KR, Lathrop J, Griffith LM, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: III. The 2014 Biomarker Working Group Report. Biol Blood Marrow Transplant. 2015;21(5):780–92. doi: 10.1016/j.bbmt.2015.01.003 ; PubMed Central PMCID: PMC4408233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee S, Cook EF, Soiffer R, Antin JH. Development and validation of a scale to measure symptoms of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2002;8(8):444–52. doi: 10.1053/bbmt.2002.v8.pm12234170 . [DOI] [PubMed] [Google Scholar]
- 16.Merkel EC, Mitchell SA, Lee SJ. Content Validity of the Lee Chronic Graft-versus-Host Disease Symptom Scale as Assessed by Cognitive Interviews. Biol Blood Marrow Transplant. 2016;22(4):752–8. doi: 10.1016/j.bbmt.2015.12.026 ; PubMed Central PMCID: PMC4850024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teh C, Onstad L, Lee SJ. Reliability and Validity of the Modified 7-Day Lee Chronic Graft-versus-Host Disease Symptom Scale. Biol Blood Marrow Transplant. 2020;26(3):562–7. Epub 2019/11/24. doi: 10.1016/j.bbmt.2019.11.020 ; PubMed Central PMCID: PMC7031026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.PROMIS. Dynamic Tools to Measure Health Outcomes from the Patient Perspective. www.nihpromis.org Accessed November 6, 2015. [Google Scholar]
- 19.Slade GD. Assessing change in quality of life using the Oral Health Impact Profile. Community dentistry and oral epidemiology. 1998;26(1):52–61. Epub 1998/03/25. doi: 10.1111/j.1600-0528.1998.tb02084.x . [DOI] [PubMed] [Google Scholar]
- 20.Dueck AC, Mendoza TR, Mitchell SA, Reeve BB, Castro KM, Rogak LJ, et al. Validity and Reliability of the US National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). JAMA Oncol. 2015;1(8):1051–9. doi: 10.1001/jamaoncol.2015.2639 ; PubMed Central PMCID: PMC4857599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basch E, Reeve BB, Mitchell SA, Clauser SB, Minasian LM, Dueck AC, et al. Development of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst. 2014;106(9). doi: 10.1093/jnci/dju244 ; PubMed Central PMCID: PMC4200059. [DOI] [PMC free article] [PubMed] [Google Scholar]