Abstract
Objective
Using the Manhattan Lupus Surveillance Program (MLSP), a multi-racial/ethnic population-based registry, we compared three commonly used classification criteria for Systemic Lupus Erythematosus (SLE) to identify unique cases and determine the incidence and prevalence of SLE using the EULAR/ACR criteria.
Methods
SLE cases were defined as fulfilling 1997 ACR, SLICC, or EULAR/ACR classification criteria. We quantified the number of cases uniquely associated with each and the number fulfilling all three. Prevalence and incidence using the EULAR/ACR classification criteria and associated 95% confidence intervals (CI) were calculated.
Results
1,497 cases fulfilled at least one of the three classification criteria, with 1,008 (67.3%) meeting all three classifications, 138 (9.2%) fulfilling only SLICC criteria, 35 (2.3%) fulfilling only ACR criteria and 34 (2.3%) uniquely fulfilling EULAR/ACR criteria. Patients solely satisfying EULAR/ACR criteria had fewer than four manifestations. The majority classified only by the ACR criteria did not meet any of the defined immunologic criteria. Patients fulfilling only SLICC criteria did so based on the presence of features unique to this system. Using the EULAR/ACR classification criteria, age-adjusted overall prevalence and incidence rates of SLE in Manhattan were 59.6 (95%CI:55.9-63.4) and 4.9 (95%CI 4.3-5.5) per 100,000 population, with age-adjusted prevalence and incidence rates highest among non-Hispanic Black females.
Conclusion
Applying the three commonly used classification criteria to a population-based registry identified patients with SLE fulfilling only one validated definition. The most recently developed EULAR/ACR classification criteria revealed similar prevalence and incidence estimates to those previously established for the ACR and SLICC classification schemes.
Introduction
Systemic lupus erythematosus (SLE) is a heterogeneous disease with manifestations that range from mild to life-threatening illness (1). In an effort to standardize clinical studies of SLE, classification criteria were developed. In the early 1970s, the American Rheumatism Association (ARA) published preliminary classification criteria for SLE (2), which did not contain the serologic evaluation that would eventually be used in the diagnosis and management of SLE (3). In 1982, the ARA-revised classification criteria for SLE were published and shown to be 96% sensitive and 96% specific compared with race- and sex-matched controls with connective tissue diseases (3). In 1997, further updates were made to these SLE classification criteria by the American College of Rheumatology (1997 ACR), but these new criteria were not tested for sensitivity or specificity at the time (4).
Although several classification criteria for SLE have been developed since, two are regularly used in clinical research practice. The first was developed by the Systemic Lupus International Collaborating Clinics (SLICC), which required the presence of at least one clinical criterion and one immunologic criterion among the four required criteria, or biopsy-proven lupus nephritis with the appropriate serology (5) without requiring four criteria. The SLICC criteria expanded in particular on the neurologic and cutaneous manifestations found in the revised 1997 ACR classification criteria and, during its validation, performed with greater sensitivity and lower specificity (5). More recently, the European League Against Rheumatism (EULAR) and the ACR developed SLE classification criteria that employed weighting based on criterion performance during derivation and required a positive antinuclear antibody (ANA) as an entry criterion (6). This new definition had higher sensitivity than the ACR and higher specificity than the SLICC criteria (6). The EULAR/ACR 2019 SLE classification criteria perform well among patients with early disease, and among men, women, and all four of the key racial/ethnic demographics (7).
The Manhattan Lupus Surveillance Program (MLSP) was initiated in September 2010 as a collaboration between the New York City Department of Health and Mental Hygiene (NYC DOHMH) and New York University School of Medicine (NYUSoM), with the primary goal of determining the prevalence of SLE in 2007 and incidence of SLE during 2007–2009 in patients residing in the New York City borough of Manhattan. Manhattan was unique in being the only CDC-funded surveillance site with substantial populations of Black, Hispanic, Asian, and White patients, with > 1,000 cases of SLE fulfilling ≥4 1997 ACR criteria. We have published results for incidence and prevalence using both the 1997 ACR and SLICC classification criteria and an analysis of patients meeting both schema and those fulfilling each alone (8). In this study, we use the newly-devised EULAR/ACR classification criteria to perform an analysis of cases fulfilling all classification criteria and those meeting only one schema to analyze which components account for discordance between schema. We also determine the incidence and prevalence of SLE in Manhattan in 2007–2009 using the EULAR/ACR classification criteria.
Patients and Methods
The Manhattan Lupus Surveillance Program
We have previously described the MLSP (8–10). In brief, under the health surveillance exemption to HIPAA privacy rules as authorized by the New York City Charter, medical records were reviewed with no potential patients being contacted for this project. IRBs at the participating institutions deemed the MLSP to qualify as a surveillance study, and additional IRB applications were completed and submitted for independent case-finding sources when requested. The DOHMH IRB reviewed and approved secondary analyses on the de-identified MLSP dataset including the analyses presented here. The incidence and prevalence period for the MLSP was January 1, 2007–December 31, 2009, with Manhattan chosen for reasons previously described (8). There were 1,585,873 persons residing in Manhattan (48% non-Latino White, 25% Latino, 13% non-Latino Black, 11% non-Latino Asian) based on 2010 US Census data (11).
Case-finding sources for the MLSP included rheumatologists’ practices, hospitals, and administrative databases (8). Sources were queried retrospectively to identify patients with International Classification of Disease Ninth Revision Clinical Modification (ICD-9CM) billing codes for SLE and related connective tissue diseases living in Manhattan (8). Charts for every patient with one of the respective ICD-9CM codes and confirmed to live in Manhattan were fully abstracted for clinical manifestations found in the 1997 ACR and SLICC classification criteria, final diagnosis, date of diagnosis, and type of physician (e.g. rheumatologist, dermatologist) making the diagnosis. Abstraction was performed by individuals with medical degrees who underwent extensive training and routine quality assurance (8). Abstraction was completed in 90.5% of hospitals and 75.8% of rheumatologists’ practices (8).
Case Definitions and Analyses
Our case definition for the incidence and prevalence estimates used in this analysis was the EULAR/ACR classification criteria (6). Given the requirement of a positive ANA at a minimum of 1:80 or an equivalent positive ANA test for the EULAR/ACR classification criteria, we limited the primary analyses to those cases in which an ANA was documented in the chart. In addition, given the retrospective nature of the MLSP, ANA titers were not always available. If an ANA titer was available, it was only counted as entry criteria if the ANA titer was ≥1:80. If the chart just stated ANA positive without further details on methodology, the ANA was considered as having met inclusion criteria under an equivalent positive test. Given the EULAR/ACR classification criteria were developed after the data dictionary and database for the MLSP had been finalized, the criterion of fever was not included as it had not been captured. To fulfill the EULAR/ACR classification criteria for SLE after having a positive ANA, a case needs ≥10 points from a group of additive weighted criteria in six clinical (given fever was not collected) and three immunologic domains, with at least one clinical criterion present (6). For comparison of the EULAR/ACR criteria to the 1997 ACR and SLICC classification criteria we used the definitions as previously described (8). Cases only meeting one individual set of classification criteria were further analyzed and categorized to evaluate the reasons they did not meet the other definitions. Secondary analysis was performed for the cases in which an ANA test result could not be found during chart review for MLSP data collection, given that an ANA test result is not required to fulfill either the 1997 ACR or SLICC classification criteria, to evaluate the possibility of meeting 1 of those criteria.
Statistical Analysis
Prevalent cases were new or existing cases of SLE fulfilling the EULAR/ACR criteria and residing in Manhattan January 1–December 31, 2007. Incident cases were those fulfilling the EULAR/ACR criteria, residing in Manhattan, and first diagnosed with SLE during January 1, 2007–December 31, 2009. Denominators were calculated from DOHMH intercensal population estimates for Manhattan (11). Rates overall, by sex, and by race/ethnicity were calculated per 100,000 person-years and age-adjusted to the standard 2000 projected US population (12).
Data on race and Latino ethnicity were recorded separately but used to assign cases into five mutually exclusive race/ethnicity categories: Latino, non-Latino White, non-Latino Black, non-Latino Asian, and non-Latino other (including multiple races). Differences by sex and race/ethnicity were assessed using Chi-square tests or Fisher’s exact tests. For significant differences by race/ethnicity, we further evaluated pairwise differences using z-tests assuming the Poisson distribution and statistical significance at 0.05, with Bonferroni correction to 0.008. A secondary analysis combined all cases fulfilling the 1997 ACR, SLICC and EULAR/ACR classification criteria and calculated the incidence and prevalence using the same methodology.
All analyses were completed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Role of the Funding Source
Data collection for the MLSP was supported by the CDC, which had general input into its design to ensure consistency among the CDC-funded SLE registries. Cooperative agreements between the NYC DOHMH and NYUSoM provided support for this analysis. Neither the CDC nor the NYC DOHMH had a role in the design of the study but both institutions reviewed and approved the manuscript.
Results
Overall 1,497 cases fulfilled at least one of the three classification criteria. Of those, 1,008 (67.3%) cases fulfilled all three, 138 (9.2%) only fulfilled the SLICC classification, 35 (2.3%) only fulfilled the 1997 ACR classification, and 34 (2.3%) fulfilled the EULAR/ACR classification criteria only, with the remaining 282 (18.8%) cases fulfilling a combination of two criteria, Figure 1.
Figure 1.
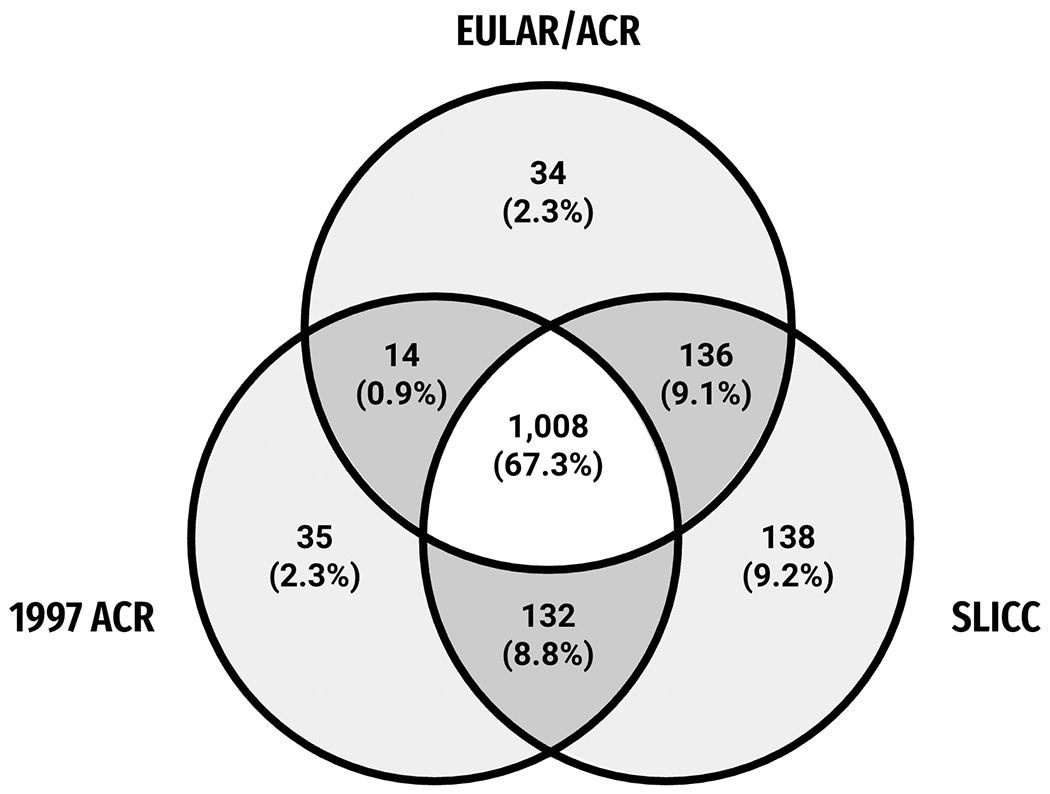
Number of 1,497 SLE cases among NYC Manhattan residents, by EULAR, ACR, or SLICC classification criteria. All cases with available antinuclear antibody result. ACR = American College of Rheumatology, EULAR = European Alliance of Associations for Rheumatology, SLICC = Systemic Lupus International Collaborating Clinics.
SLE cases who fulfilled EULAR/ACR classification only
Among the 34 patients who fulfilled only the EULAR/ACR classification criteria, all met fewer than four individual criteria and did not meet either the SLICC or 1997 ACR classification criteria, as both require at least four clinical or immunologic features to be classified as SLE (excluding the SLICC exemption for a biopsy consistent with lupus nephritis and a positive ANA or anti-dsDNA antibodies) (Table 1). All 34 patients met only three individual SLICC criteria—a positive ANA and two additional manifestations. Similarly, 31 patients met only three individual 1997 ACR criteria, with the remaining three patients fulfilling only two of the 1997 ACR criteria. Patients were found to meet fewer 1997 ACR criteria due to the absence of certain manifestations (e.g. low complement) in that scheme and the combination of criteria into a single domain (e.g. lymphopenia and thrombocytopenia) in the 1997 ACR criteria.
Table 1.
Patients meeting EULAR/ACR classification criteria only (N=34).
| REASONS FOR NOT MEETING SLICC CLASSIFICATION CRITERIA | Number | Example |
|---|---|---|
| 3 criteria only (ANA + 2 additional criteria): | N=34 | e.g. ANA, anti-dsDNA antibody, arthritis |
| REASONS FOR NOT MEETING ACR CLASSIFICATION CRITERIA | ||
|
| ||
| 3 criteria only (ANA + 2 additional criteria): | N=31 | e.g. ANA, thrombocytopenia, arthritis |
| 2 criteria only (ANA + 1 additional criteria): | N=3 | e.g. ANA arthritis, [low complements] |
Abbreviations: ACR-American College of Rheumatology, EULAR- European Alliance of Associations for Rheumatology, SLICC- Systemic Lupus International Collaborating Clinics, ANA-antinuclear antibody
[Brackets]: criteria not part of, or does not meet, the specified classification criteria
SLE cases who fulfilled 1997 ACR classification only
Thirty-five cases in the MLSP met only the 1997 ACR classification criteria (Table 2). Of these, 19 did not have an immunologic manifestation to meet the SLICC classification criteria. The remaining 16 cases fulfilled the requirement of an immunologic criterion but did not meet SLICC definitions for lymphopenia, antiphospholipid antibodies, and renal disease, or they had malar rash and photosensitivity as two separate ACR criteria.
Table 2.
Patients meeting 1997 ACR classification criteria only (N=35).
| REASONS FOR NOT MEETING SLICC CRITERIA: | Number | Example |
|---|---|---|
| ANA negative | N=20 | |
| No Serologic Criteria: | N=19 | e.g. malar, discoid, photo, arthritis |
| Serologic criteria met but < 4 criteria overall: |
N=1 | e.g. Anti-Cardiolipin antibody, serositis [lymphopenia, non RBC cast] |
| ANA positive | N=15 | |
| Lymphopenia not met by SLICC: | N=4 | e.g. ANA, arthritis, seizure, [lymphopenia] |
| Malar/photosensitivity combined in SLICC: |
N=1 | e.g ANA, anti-dsDNA antibody, malar, photosensitivity |
| APL not met by SLICC: | N=4 | e.g. ANA, photosensitivity, arthritis [Anti-Cardiolipin antibody] |
| Renal not met by SLICC: | N=2 | e.g. ANA, arthritis, lymphopenia [non RBC cast] |
| Combinations of the above | N=4 | e.g. ANA, malar, photosensitivity, [lymphopenia] |
| REASONS FOR NOT MEETING EULAR/ACR CLASSIFICATION CRITERIA: | ||
|
| ||
| ANA negative: | N=20 | |
| Sufficient points to meet EULAR/ACR if ANA positive: |
N=18 [range 10-21, mean 14.38] | e.g. malar, oral ulcer, arthritis, pericarditis |
| Insufficient points to meet EULAR/ACR if ANA positive: |
N=2 [range 6-8, mean 7] | e.g. malar, oral ulcer, discoid, [photosensitivity] |
| ANA <1:80, not sufficient for entry: | N=6 | |
| Sufficient points to meet EULAR/ACR if ANA titer was sufficient: |
N=4 [range 10-12, mean 10.75] | e.g. ANA, malar, [photosensitivity], dsDNA |
| Insufficient points to meet EULAR/ACR if ANA titer was sufficient: |
N=2 [range 5-6, mean 5.5] | e.g. ANA, arthritis, [Anti-Cardiolipin antibody], [lymphopenia] |
| ANA positive/sufficient titer for entry, not meeting points threshold > 10: | N=9 [range 0-9, mean 6.1] | e.g. ANA, discoid, seizure, [lymphopenia] |
Abbreviations:: ACR-American College of Rheumatology, EULAR- European Alliance of Associations for Rheumatology, SLICC- Systemic Lupus International Collaborating Clinics, ANA-antinuclear antibody, aPL = antiphospholipid antibody, RBC = red blood cell
Bolded font in Examples column: overlapping criteria falling within the same domain, [Brackets]: criteria not part of, or does not meet, the specified classification criteria
Of the 35 patients meeting only the 1997 ACR classification, the same 20 patients who were ANA negative, of whom 19 did not meet the SLICC system due to absence of an immunologic criterion, were unable to fulfill EULAR/ACR classification as they never had a positive ANA. Of these 20 patients, 18 would have met EULAR/ACR classification had their ANA been positive. The remaining two patients did not achieve the points required by the EULAR/ACR system either due to the presence of a unique manifestation not accounted for by this classification schema (e.g. lymphopenia), or due to the presence of overlapping criteria within the same domain (e.g. malar rash, discoid rash and oral ulcers), thus limiting the number of achievable points. Six of the 35 patients in this cohort had a positive ANA, though below the threshold of 1:80 required for entry by the EULAR/ACR system. Two of these six would not have had sufficient points to fulfill EULAR/ACR classification had they met the entry criterion, again due to the presence of manifestations absent from the EULAR/ACR system (e.g. lymphopenia). Lastly, nine patients in this cohort had a positive ANA of sufficient titer for entry in the EULAR/ACR classification criteria, but did not achieve the points required to fulfill classification criteria. Again, this was largely due to the presence of manifestations not included within the EULAR/ACR criteria (e.g., lymphopenia and photosensitivity).
SLE cases who fulfilled SLICC classification criteria only
There were 138 patients in the MLSP that met SLICC classification only (Table 3). All satisfied fewer than four of the 1997 ACR classification criteria. This was due to the presence of manifestations unique to the SLICC system, such as specific neuropsychiatric involvement (e.g. peripheral neuropathy), cutaneous features (e.g. bullous lesions), separating hematologic criteria, or discrepancies in other definitions between the SLICC and ACR classification, such as with lymphopenia.
Table 3.
Patients meeting SLICC classification criteria only (N=138).
| REASONS FOR NOT MEETING ACR CRITERIA: | Number | Example |
|---|---|---|
| 3 criteria only | N= 85 | |
| Low complement - not part of ACR: | N= 17 | e.g. ANA, [low complement], arthritis, oral ulcers |
| Alopecia - not part of ACR: | N= 21 | e.g. ANA, [alopecia], arthritis, lymphopenia |
| Unique NP criteria not part of ACR: | N= 13 | e.g. ANA, anti-dsDNA antibody, [transverse myelitis], lymphopenia |
| Unique cutaneous criteria not part of ACR: |
N= 2 | e.g. ANA, anti-dsDNA antibody, photosensitive rash, [Chillblains lupus] |
| Overlapping hematologic criteria: | N=6 | e.g. ANA, seizures, [lymphopenia, thrombocytopenia] |
| Lymphopenia not met by ACR: | N= 2 | e.g. ANA, APL, arthritis, [lymphopenia – NON-ACR] |
| Direct Coombs’ test (DAT) in the absence of hemolytic anemia: |
N=3 | e.g. ANA, anti-dsDNA antibody, leukopenia, +DAT |
| Combinations of above: | N=21 | e.g. ANA, anti-Smith antibody, oral ulcers, [alopecia], [peripheral neuropathy] |
| ≤ 2 criteria only: | N= 53 | |
| ANA, Renal: | N=1 | e.g. ANA, Class V |
| 2 or more described above for ≤ 3 criteria: |
N= 52 | e.g. ANA, arthritis, [alopecia, peripheral neuropathy] |
| REASONS FOR NOT MEETING EULAR/ACR CRITERIA: | ||
|
| ||
| ANA negative: | N=25 | |
| Sufficient points to meet EULAR/ACR if ANA positive: | N=21 [range 10-21, mean 13.95] | e.g. anti-dsDNA antibody, alopecia, leukopenia, thrombocytopenia |
| Insufficient points to meet EULAR/ACR if ANA positive: | N=4 [range 7-9, mean 8] | e.g. low complement, oral ulcer, [neuropathy], leukopenia, thrombocytopenia |
| ANA <1:80, not sufficient for entry: | N=17 | |
| Sufficient points to meet EULAR/ACR
if ANA titer met entry criteria : |
N=4 [range 10–12, mean 10.75] | e.g. ANA (1:40), anti-Smith antibody, alopecia, leukopenia, [lymphopenia], thrombocytopenia |
| Insufficient points to meet
EULAR/ACR if ANA titer was sufficient: |
N=13 [range 0–9, mean 6.7] | |
| Overlapping criteria within same domain: |
N=5 | e.g. ANA (1:40), malar, discoid, alopecia |
| At least one unique SLICC criterion: | N=6 | e.g. ANA (1:40), [photosensitivity], [neuropathy], [lymphopenia] |
| Overlapping criteria/Unique SLICC criteria: |
N=1 | e.g. ANA (1:40), [neuropathy], leukopenia, thrombocytopenia |
| < 10 points: | N=1 | e.g. ANA (1:40), low C3, leukopenia, alopecia |
| ANA positive/sufficient titer for entry, not meeting points threshold > 10: | N=95 [range 2–9, mean 6.9] | |
| Overlapping criteria: | N=18 | e.g. ANA, discoid, leukopenia, thrombocytopenia |
| At least one unique SLICC criteria: | N=54 | e.g. ANA, low C3, [panniculitis], alopecia, leukopenia, [lymphopenia] |
| Overlapping criteria/Unique criteria: | N=12 | e.g. ANA, anti-Smith antibody, oral ulcers, alopecia, [peripheral neuropathy] |
| < 10 points: | N=11 | e.g. ANA, Renal Class V |
| ANA positive/sufficient points, but immunologic criteria only: | N=1 | e.g. ANA, anti-dsDNA antibody, low complements, [RBC casts] |
Abbreviations: ACR-American College of Rheumatology, EULAR- European Alliance of Associations for Rheumatology, SLICC- Systemic Lupus International Collaborating Clinics, ANA-antinuclear antibody, NP-Neuropsychiatric, aPL = antiphospholipid antibody, RBC = red blood cell
Bolded font in Examples column: overlapping criteria falling within the same domain, [Brackets]: criteria not part of, or does not meet, the specified classification criteria
Among the 138 patients who met SLICC classification criteria only, 25 had a negative ANA. Of these 25 patients, four would still not have achieved sufficient points required by the EULAR/ACR system even with a positive ANA due to the presence of overlapping criteria within the same domain (e.g. leukopenia and thrombocytopenia), or due to the existence of manifestations absent from the EULAR/ACR system (e.g. photosensitivity which can only be scored in the context of a specific rash). There were an additional 17 patients with a documented positive ANA, though with a titer less than required for entry, 13 of whom would not have met the 10 point threshold by the EULAR/ACR system, again either due to the presence of overlapping criteria within the same domain (e.g. malar rash, discoid rash and alopecia), or due to the presence of manifestations unique to the SLICC criteria (e.g. peripheral neuropathy). One patient had a positive ANA sufficient for entry, and would have achieved the points required by the EULAR/ACR system; however they fulfilled no clinical criteria thus precluding them from meeting classification. Finally, the remaining 95 patients had positive ANA titers satisfying entry into the EULAR/ACR classification, but they did not meet the threshold of 10 points due to the presence of overlapping criteria within the same domain (e.g. leukopenia and thrombocytopenia) thus limiting the number of achievable points, or due to the presence of features unique to the SLICC system (e.g. panniculitis).
SLE cases with ANA unknown or undocumented
A total of 43 MLSP patients meeting only a single classification criteria had no ANA testing available. Fifteen of these patients did not meet a single 1997 ACR immunologic criterion but satisfied the overall 1997 ACR criteria based on the presence of 4 clinical manifestations. 28 of these patients fulfilled SLICC criteria alone by meeting at least one other immunologic criterion (18 had positive double-stranded DNA antibodies, three had positive anti-Smith antibodies, seven had positive antiphospholipid antibodies, one had a positive direct antiglobulin test and 11 patients had low complement levels).
EULAR/ACR Classification Criteria Prevalence and Incidence Estimates
Using the EULAR/ACR classification criteria, total crude prevalence was 65.1 (95%CI:61.1-69.0) and incidence was 5.2 (95%CI:4.5-5.8) per 100,000 population. The total age-adjusted prevalence and annual incidence rates of SLE in Manhattan were 59.6 (95%CI:55.9-63.4) and 4.9 (95%CI:4.3-5.5) per 100,000 population, Table 4. The age-adjusted prevalence among females was 9.0 times and incidence 6.9 times higher compared with males. The age-adjusted prevalence per 100,000 by race/ethnicity was highest among non-Latina Black females (197.1), followed by Latina females (132.5), non-Latina Asian/Pacific Islander females (97.7), and non-Latina White females (59.8). Age-adjusted incidence rates per 100,000 by race/ethnicity were highest in non-Latina Black females (15.8), followed by Latina females (7.5), non-Latina Asian/Pacific Islander females (7.3) and non-Latina White females (6.3). Prevalence and incidence rates for males followed a similar pattern.
Table 4:
Crude and age-adjusted prevalence and incidence rates of SLE among Manhattan residents, 2007, meeting case definitions for EULAR/ACR overall and by race/ethnicity and sex.
| Race/ethnicity, sex | Crude rate (95% CI) | Age-adjusted rate (95% CI) | χ2 p-value | ||
|---|---|---|---|---|---|
| Total - Prevalence | 65.1 | (61.1-69.0) | 59.6 | (55.9-63.4) | |
|
| |||||
| Male | 12.5 | (10.1-15.3) | 11.5 | (9.2-14.2) | <0.0001 |
| Female | 111.8 | (104.6-118.9) | 103.7 | (96.8-110.5) | |
| Race/ethnicity | <0.0001† | ||||
| Non-Latino White | 37.8 | (33.5-42.2) | 32.5 | (28.6-36.5) | |
| Male | 5.0 | (2.9-7.8) | 4.0 | (2.4-6.4) | |
| Female | 68.1 | (59.9-76.2) | 59.8 | (52.1-67.4) | |
| Non-Latino Black | 122.1 | (107.3-136.9) | 116.5 | (102.3-130.7) | |
| Male | 26.5 | (17.3-38.8) | 24.9 | (16.3-36.6) | |
| Female | 202.9 | (177.0-228.8) | 197.1 | (171.8-222.5) | |
| Latino | 79.3 | (70.7-88.0) | 78.4 | (69.8-87.0) | |
| Male | 16.6 | (11.3-23.4) | 16.4 | (11.1-23.3) | |
| Female | 135.6 | (120.0-151.1) | 132.5 | (117.2-147.9) | |
| Non-Latino Asian | 67.4 | (55.2-79.6) | 59.8 | (47.9-71.7) | |
| Male | 19.3 | (10.8-31.9) | 14.4 | (7.7-24.6) | |
| Female | 106.3 | (85.7-126.9) | 97.7 | (76.7-118.8) | |
| Total - Incidence | 5.2 | (4.5-5.8) | 4.9 | (4.3-5.5) | |
|
| |||||
| Male | 1.2 | (0.8-1.8) | 1.2 | (0.8-1.7) | <0.0001 |
| Female | 8.7 | (7.5-9.8) | 8.3 | (7.2-9.5) | |
| Race/ethnicity | <0.0001‡ | ||||
| Non-Latino White | 3.9 | (3.2-4.8) | 3.5 | (2.8-4.5) | |
| Male | 0.8 | (0.38-1.6) | 0.6 | (0.3-1.2) | |
| Female | 6.8 | (5.4-8.5) | 6.3 | (4.8-8.0) | |
| Non-Latino Black | 9.9 | (7.6-12.7) | 9.5 | (7.3-12.2) | |
| Male | 2.8 | (1.2-5.4) | 2.8 | (1.2-5.5) | |
| Female | 16.0 | (12.1-20.9) | 15.8 | (11.8-20.6) | |
| Latino | 4.5 | (3.4-5.9) | 4.5 | (3.4-5.8) | |
| Male | 1.0 | (0.4-2.3) | 0.5 | (0.0-3.0) | |
| Female | 7.6 | (5.6-10.1) | 7.5 | (5.5-9.9) | |
| Non-Latino Asian | 4.7 | (3.1-7.0) | 4.2 | (2.6-6.5) | |
| Male | 0.4 | (0.0-2.4) | 0.5 | (0.0-3.0) | |
| Female | 8.2 | (5.2-12.2) | 7.3 | (4.4-11.4) | |
Rates are per 100,000 Manhattan residents. Denominator data are based on 2007 intercensal population estimates from the NYC DOHMH Bureau of Epi Services (2000-2014 files). Data are age adjusted to the US 2000 Standard Population.
Cases were assigned to one of five mutually exclusive race/ethnicity categories: non-Latino White, non-Latino Black, non-Latino Asian, Latino, and non-Latino other. Non-Latino cases identified with more than one race were categorized as non-Latino other.
Non-Latino Whites differed from non-Latino Blacks, Latinos, and non-Latino Asians. Non-Latino Blacks also differed from Latinos and non-Latino Asians. Latinos did not differ from non-Latino Asians.
Non-Latino Blacks differed from non-Latino Whites, Latinos, and non-Latino Asians. Non-Latino Whites did not differ from Latinos or non-Latino Asians. Latinos did not differ from non-Latino Asians.
Meeting any of the three classification criteria in the MLSP yielded total age-adjusted prevalence and incidence rates of 78.0 (95%CI:73.7-82.3) and 6.7 (95%CI:5.9–7.4) per 100,000 population, respectively, Table 5. Again, age-adjusted prevalence and incidence rates followed similar patterns by sex and race/ethnicity compared with the EULAR/ACR rates.
Table 5:
Crude and age-adjusted prevalence and incidence rates of SLE among Manhattan residents, 2007, meeting case definitions for 1997 ACR, SLICC or EULAR/ACR overall and by race/ethnicity and sex
| Race/ethnicity, sex | Crude rate (95% CI) | Age-adjusted rate (95% CI) | χ2 p-value | ||
|---|---|---|---|---|---|
| Total - Prevalence | 84.86 | (80.3-89.4) | 78.01 | (73.7-82.3) | |
|
| |||||
| Male | 15.59 | (12.7-18.4) | 14.46 | (11.8-17.2) | <0.0001 |
| Female | 146.39 | (138.2-154.6) | 135.61 | (127.8-143.4) | |
| Race/ethnicity | <0.0001† | ||||
| Non-Latino White | 51.96 | (46.8-57.1) | 44.97 | (40.3-49.7) | |
| Male | 6.89 | (4.5-10.2) | 5.69 | (3.7-8.4) | |
| Female | 93.36 | (83.8-102.9) | 81.95 | (73.0-90.0) | |
| Non-Latino Black | 159.87 | (143.0-176.8) | 151.88 | (135.7-168.0) | |
| Male | 32.58 | (22.3-46.0) | 30.63 | (20.9-43.3) | |
| Female | 267.35 | (237.6-297.1) | 257.23 | (228.4-286.1) | |
| Latino | 97.94 | (88.3-107.5) | 96.93 | (87.4-106.5) | |
| Male | 21.24 | (15.2-28.8) | 21.08 | (15.0-28.8) | |
| Female | 166.69 | (149.4-183.9) | 162.65 | (145.7-179.6) | |
| Non-Latino Asian | 85.28 | (71.5-99.0) | 77.18 | (63.6-90.8) | |
| Male | 20.63 | (11.8-33.5) | 15.83 | (8.7-26.5) | |
| Female | 137.54 | (114.1-161.0) | 128.41 | (104.2-152.6) | |
| Total - Incidence | 7.05 | (6.3-7.8) | 6.68 | (5.9-7.4) | |
|
| |||||
| Male | 1.84 | (1.3-2.5) | 1.8 | (1.3-2.5) | <0.0001 |
| Female | 11.68 | (10.3-13.0) | 11.18 | (9.9-12.5) | |
| Race/ethnicity | <0.0001‡ | ||||
| Non-Latino White | 5.78 | (4.8-6.8) | 5.18 | (4.2-6.2) | |
| Male | 11.37 | (10.0-12.7) | 48.22 | (41.5-55.0) | |
| Female | 9.91 | (8.1-11.7) | 9.11 | (7.3-11.0) | |
| Non-Latino Black | 12.78 | (10.2-15.9) | 12.23 | (9.7-15.2) | |
| Male | 3.44 | (1.7-6.3) | 3.46 | (1.7-6.4) | |
| Female | 20.69 | (16.2-26.1) | 20.08 | (15.6-25.4) | |
| Latino | 5.57 | (4.3-7.1) | 5.58 | (4.3-7.1) | |
| Male | 1.39 | (0.6-2.7) | 1.57 | (0.7-3.2) | |
| Female | 9.31 | (7.1-12.0) | 9.23 | (7.0-11.9) | |
| Non-Latino Asian | 6.6 | (4.6-9.2) | 6.04 | (4.1-8.6) | |
| Male | 1.27 | (0.3-3.7) | 1.51 | (0.3-4.4) | |
| Female | 10.89 | (7.5-15.4) | 9.8 | (6.4-14.3) | |
Rates are per 100,000 Manhattan residents. Denominator data is based on 2007 intercensal population estimates from the NYC DOHMH Bureau of Epi Services (2000-2014 files). Data are age adjusted to the US 2000 Standard Population.
Cases were assigned to one of five mutually exclusive race/ethnicity categories: non-Latino White, non-Latino Black, non-Latino Asian, Latino, and non-Latino other. Non-Latino cases identified with more than one race were categorized as non-Latino other.
Non-Latino Whites differed from non-Latino Blacks, Latinos, and non-Latino Asians. Non-Latino Blacks also differed from Latinos and non-Latino Asians. Latinos did not differ from non-Latino Asians.
Non-Latino Blacks differed from non-Latino Whites, Latinos, and non-Latino Asians. Non-Latino Whites did not differ from Latinos or non-Latino Asians. Latinos did not differ from non-Latino Asians.
Discussion
The MLSP is a diverse, multi-racial/ethnic registry initiated to obtain epidemiologic data on SLE. Leveraging this large, carefully-documented population based registry allowed a comparison of the three most commonly-used classification criteria to identify unique cases fulfilling only one set of classification criteria. In this analysis, 2.3% satisfied only the EULAR/ACR classification criteria, 2.3% fulfilled only the 1997 ACR classification and 9.2% of patients met SLICC classification only. The patients fulfilling only the EULAR/ACR criteria did so by having < 4 ACR or SLICC criteria that scored ≥10 points. The majority of patients meeting only 1997 ACR classification did not fulfill any immunologic criteria but satisfied primarily clinical criteria with definitions unique within that system, such as lymphopenia. The largest number of cases met SLICC classification criteria alone, either because the ANA was negative or below the threshold for entry into the EULAR/ACR system despite having other immunologic criteria recorded, or as a result of unique clinical components, such as those for neuropsychiatric and cutaneous disease, within the SLICC classification criteria.
The age-adjusted overall prevalence and annualized incidence rates of SLE in Manhattan using the EULAR/ACR classification criteria were 59.6 (95%CI:55.9- 63.4) and 4.9 (95%CI: 4.3–5.5) per 100,000 population. These were similar to the age-adjusted prevalence and annualized incidence rates for the 1997 ACR classification criteria (62.2 and 4.6 per 100,000 person-years) and SLICC classification criteria (73.8 and 6.2 per 100,000 person-years) (8). Additionally, the sex and racial/ethnic disparities observed using the EULAR/ACR criteria were similar to those previously published using the 1997 ACR and SLICC criteria, being highest among non-Latina Black females, followed by Latina females (8). Expanding prevalence and incidence estimates to cases fulfilling at least one set of classification criteria naturally yielded the highest rates of SLE.
Prior studies have attempted to compare the performance of these three SLE classification criteria in various special populations. The Aberle et al. study identified 3,575 subjects from the Lupus Family Registry and Repository satisfying either the ACR or SLICC classification criteria. In their analysis, 178 (5.0%) fulfilled only the SLICC system, while 85 (2.4%) met ACR criteria alone (13), similar to the present study in which a greater proportion of patients within the MLSP were found to satisfy only the SLICC criteria. Moreover, they identified certain manifestations, such as low complement levels, maculopapular rash and sensory neuropathy, as the explanation for patients meeting SLICC but not ACR criteria (13), comparable to what we observed in our population.
A smaller study by Magallares et al. compared all three classification criteria in SLE patients with longstanding disease. Among their cohort of 79 patients, only seven were found to meet just one of the classification criteria, with four patients (5.0%) meeting SLICC alone, three patients (4.0%) meeting EULAR/ACR alone, and none fulfilling only the ACR definition. Though the sample was small, all three patients satisfying only the EULAR/ACR criteria were found to have a positive ANA with just two additional manifestations (14). This is similar to what was seen in the current analysis, where all patients fulfilling only the EULAR/ACR system met three clinical or immunologic features only.
Adamichou et al. applied these definitions to 690 SLE patients and found that both the SLICC and EULAR/ACR classification criteria offer a higher sensitivity and enable earlier classification of SLE than the ACR criteria. In that study, 76.7% of SLE patients satisfied all three classification criteria, a slightly higher proportion than observed in the present analysis with predominantly established disease patients. They found that patients not meeting ACR criteria had a higher prevalence of hematologic and immunologic features, while those not fulfilling the EULAR/ACR system had more mucocutaneous disease and leukopenia, and patients not meeting SLICC criteria had skin- and joint-predominant disease (15). A recent paper by Petri et al. comparing the three criteria and a new weighting applied to the existing SLICC criteria found all the criteria had similar overall agreement with the physician diagnosis (16).
Classification criteria serve an essential role in identifying patients for inclusion in clinical trials and studies (17). The three commonly-accepted classification criteria for SLE share various clinical and immunologic features, though each system offers unique combinations allowing for the identification of patient subsets fulfilling only one of the classification criteria. We were able to identify shared features among patients satisfying only one classification criteria, which may offer insight into certain SLE phenotypes that might be relevant for specific studies such as ANA negative SLE. While including ANA as an entry criterion—as in the EULAR/ACR classification criteria—may offer improvements in specificity, it also has the potential to exclude patients with SLE who would have otherwise satisfied the classification criteria. There are technical and substrate problems, particularly with some ANA assays which could lead to a false negative ANA, thus delaying patients from being classified as SLE by this system (18). Given the many variations in the clinical and serologic presentations of SLE, some of these patients could conceivably fulfill either the SLICC and/or the 1997 classification criteria as our data have shown.
This study has several limitations, some of which have been outlined elsewhere (8). First, a significant number of patients had unknown or undocumented ANA, some of whom were noted to have other positive extractable nuclear antigens such as anti-double-stranded DNA antibodies. These patients were considered as not having an ANA in this analysis and were excluded from the EULAR/ACR criteria. Moreover, a proportion of patients had a documented ANA, though not assessed by immunofluorescence and thus with no available titer. These patients were considered as meeting the entry criterion of a positive ANA by the EULAR/ACR system, despite the absence of a titer, which may have incorrectly included patients who would not have met the ANA titer threshold for entry. Second, given the retrospective nature of the MLSP, not all information required by the three classification criteria was available, which may have contributed to an underestimation of patients satisfying each of the classification criteria. Third, the designation of SLE for this study was based on fulfilling one of the three SLE classification criteria and not validated by a panel review. Finally, fever was not captured at all as part of the MLSP data dictionary and may have resulted in an underestimation of cases that would have fulfilled EULAR criteria had fever been captured as a manifestation.
This study has several strengths as well, which have largely been described elsewhere (8). First, the MLSP is population-based, thus including the full spectrum of SLE and not just severe cases that come to attention in tertiary care centers. Second, to our knowledge, this is the first comparison of the three commonly-used classification criteria in a multi-racial/ethnic registry demonstrating unique cases which fulfilled individual classification criteria. Third, we were able to use the EULAR/ACR classification criteria to generate prevalence and incidence estimates.
In conclusion, applying the three commonly-accepted classification criteria to a multi-racial/ethnic population-based registry allowed for the identification of unique cases of SLE who only fulfilled one classification criteria. Each classification criteria was found to have certain manifestations which allowed for the inclusion of a unique subset of patients. The EULAR/ACR classification criteria revealed similar prevalence and incidence estimates and sex and racial/ethnic disparities to previously published results from the MLSP using the 1997 revised ACR and SLICC classification criteria.
Significance and Innovations.
There are very few studies that compared the various classification criteria for systemic lupus erythematosus (SLE) and none that were population based.
To our knowledge, this is the first comparison of the three classification criteria in a multi-racial/ethnic population-based registry demonstrating unique cases that fulfilled individual classification criteria.
We used the EULAR/ACR classification criteria to generate prevalence and incidence estimates for SLE. Expanding prevalence and incidence estimates to cases fulfilling at least one set of classification criteria yielded the highest rates of SLE.
Acknowledgements
The authors would like to acknowledge Benjamin Wainwright for his assistance in preparing the manuscript.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Grants and Other Financial Support for this Study
This study was funded by the Centers for Disease Control and Prevention (grant no. U58/DP002827) and a co-operative agreement between The New York City Department of Health and Mental Hygiene and New York University School of Medicine.
Footnotes
Disclosure of Commercial Interests
Guttmann: None, Denvir: None, Aringer: None, Buyon: None, Belmont: None, Sahl: None, Salmon: None, Askanase: None, Bathon: None, Geraldino-Pardilla: None, Ali: None, Ginzler: None, Putterman: None, Gordon: Centers for Disease Control and Prevention, AbbVie, AstraZeneca, MGP, Sanofi, UCB, grants from UCB and Sandwell and West Birmingham Hospitals NHS Trust, Helmick: None, Parton: None, Izmirly: GlaxoSmithKline, Momenta/Janssen
References
- 1.Lockshin MD, Barbhaiya M, Izmirly P, Buyon JP, Crow MK. SLE: reconciling heterogeneity. Lupus Sci Med. 2019;6(1):e000280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen AS RW, Franklin EC, Kulka JP, Ropes MW, Shlllman LE, Wallace SL. Preliminary criteria for the classification of systemic lupus erythematosus. Bull Rheum Dis. 1971;21:643–8. [Google Scholar]
- 3.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25(11):1271–7. [DOI] [PubMed] [Google Scholar]
- 4.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. [DOI] [PubMed] [Google Scholar]
- 5.Petri M, Orbai AM, Alarcon GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64(8):2677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey-Goldman R, et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheumatol. 2019;71(9):1400–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson SR, Brinks R, Costenbader KH, Daikh D, Mosca M, Ramsey-Goldman R, et al. Performance of the 2019 EULAR/ACR classification criteria for systemic lupus erythematosus in early disease, across sexes and ethnicities. Ann Rheum Dis. 2020;79(10):1333–9. [DOI] [PubMed] [Google Scholar]
- 8.Izmirly PM, Wan I, Sahl S, Buyon JP, Belmont HM, Salmon JE, et al. The Incidence and Prevalence of Systemic Lupus Erythematosus in New York County (Manhattan), New York: The Manhattan Lupus Surveillance Program. Arthritis Rheumatol. 2017;69(10):2006–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Izmirly PM, Buyon JP, Wan I, Belmont HM, Sahl S, Salmon JE, et al. The Incidence and Prevalence of Adult Primary Sjogren’s Syndrome in New York County. Arthritis Care Res (Hoboken). 2019;71(7):949–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Izmirly P, Buyon J, Belmont HM, Sahl S, Wan I, Salmon J, et al. Population-based prevalence and incidence estimates of primary discoid lupus erythematosus from the Manhattan Lupus Surveillance Program. Lupus Sci Med. 2019;6(1):e000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.New York City Department of Health and Mental Hygiene. Epiquery: NYC Interactive Health Data System: NYCDOHMH neighborhood population estimates, modified from the US Census Bureau vintage population estimates, 2007, 2008, 2009. 2016. URL: https://nyc.gov/health/epiquery. [Google Scholar]
- 12.Klein RJSC. Age adjustment using the 2000 projected U.S. population. HealthyPeople Statistical Notes Centers for Disease Control and Prevention/national Center for Health Statistics 2001;No. 20. [PubMed] [Google Scholar]
- 13.Aberle T, Bourn RL, Chen H, Roberts VC, Guthridge JM, Bean K, et al. Use of SLICC criteria in a large, diverse lupus registry enables SLE classification of a subset of ACR-designated subjects with incomplete lupus. Lupus Sci Med. 2017;4(1):e000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magallares B, Lobo-Prat D, Castellvi I, Moya P, Gich I, Martinez-Martinez L, et al. Assessment of EULAR/ACR-2019, SLICC-2012 and ACR-1997 Classification Criteria in SLE with Longstanding Disease. J Clin Med. 2021;10(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adamichou C, Nikolopoulos D, Genitsaridi I, Bortoluzzi A, Fanouriakis A, Papastefanakis E, et al. In an early SLE cohort the ACR-1997, SLICC-2012 and EULAR/ACR-2019 criteria classify non-overlapping groups of patients: use of all three criteria ensures optimal capture for clinical studies while their modification earlier classification and treatment. Ann Rheum Dis. 2020;79(2):232–41. [DOI] [PubMed] [Google Scholar]
- 16.Petri M, Goldman DW, Alarcon GS, Gordon C, Merrill JT, Fortin PR, et al. Comparison of the 2019 European Alliance of Associations for Rheumatology/American College of Rheumatology Systemic Lupus Erythematosus Classification Criteria With Two Sets of Earlier Systemic Lupus Erythematosus Classification Criteria. Arthritis Care Res (Hoboken). 2021;73(9):1231–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aggarwal R, Ringold S, Khanna D, Neogi T, Johnson SR, Miller A, et al. Distinctions between diagnostic and classification criteria? Arthritis Care Res (Hoboken). 2015;67(7):891–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pisetsky DS, Lipsky PE. New insights into the role of antinuclear antibodies in systemic lupus erythematosus. Nat Rev Rheumatol. 2020;16(10):565–79. [DOI] [PMC free article] [PubMed] [Google Scholar]


