Abstract
INTRODUCTION:
Immune checkpoint inhibitor–mediated colitis (IMC) is commonly managed with steroids and biologics. We evaluated the efficacy of ustekinumab (UST) in treating IMC refractory to steroids plus infliximab and/or vedolizumab.
RESULTS:
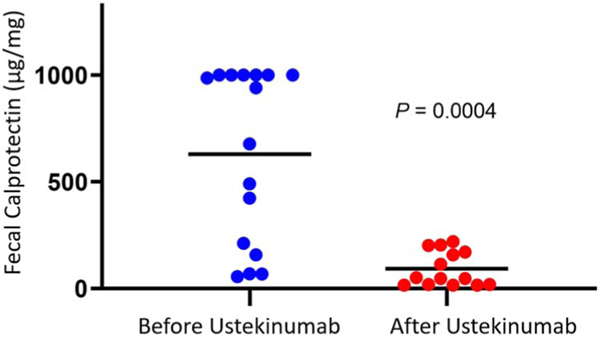
Nineteen patients were treated with UST for IMC refractory to steroids plus infliximab (57.9%) and/or vedolizumab (94.7%). Most of them had grade ≥3 diarrhea (84.2%), and colitis with ulceration was present in 42.1%. Thirteen patients (68.4%) attained clinical remission with UST, and mean fecal calprotectin levels dropped significantly after treatment (629 ± 101.5 mcg/mg to 92.0 ± 21.7 mcg/mg, P = 0.0004).
DISCUSSION:
UST is a promising therapy for the treatment of refractory IMC.
Keywords: immune checkpoint inhibitor, cancer, toxicity, immune-mediated colitis, refractory, ustekinumab
BACKGROUND
Immune checkpoint inhibitors (ICI) target regulators of the immune system and promote a highly efficacious antitumor response against several advanced cancers (1). Immune-mediated colitis (IMC) is an ICI-related toxicity that is highly reminiscent of IBD in its clinical and endoscopic presentation. Management of moderate-to-severe IMC (grade 2 or higher according to the Common Terminology Criteria for Adverse Events version 5 (CTCAE v5) typically includes weight-based systemic corticosteroids with the addition of biologics such as infliximab (IFX) or vedolizumab (VDZ) in severe or refractory cases (2,3). Approximately 12%–15% of patients have refractory disease despite the aforementioned treatments (4). Fecal microbiota transplantation (FMT), tofacitinib, and ustekinumab (UST) have been used to treat refractory IMC in select cases with encouraging preliminary efficacy in small case series (5–9). UST is a human monoclonal antibody to the interleukin (IL) 12/23 p40 subunit that has proven efficacious in the management of severe inflammatory bowel disease (IBD) (10), but data on its utility in IMC are limited to 2 case reports (11,12). Therefore, we present the largest experience to date from 2 referral centers supporting the efficacy of UST for the management of refractory IMC.
METHODS
Study design and methods
This retrospective, 2-center study was conductedwith approval from the Institutional Review Boards at The University of Texas MD Anderson Cancer Center and Memorial Sloan Kettering Cancer Center. Inclusion criteria accounted for patients who (i) developed IMC refractory to steroids and IFX and/or VDZ(ii) received UST for IMC, and (iii) had clinical or endoscopic follow-up. Demographic, oncologic, laboratory, and endoscopic data were extracted from electronic medical records and endoscopy databases.
Diarrhea was graded using the CTCAE version 5. IMC was considered refractory when (i) symptoms incompletely improved after immunosuppression and (ii) symptoms relapsed on tapering or discontinuing immunosuppression. Endoscopic findings were classified as (i) ulcerative inflammation, (ii) nonulcerative inflammation, and (iii) normal appearance. Clinical remission of symptoms was defined as sustained resolution of diarrhea to grade 1 or lower after UST. Endoscopic remission was defined as Mayo endoscopic subscore of 0 or 1 after UST (13).
Statistical analysis
Categorical variables were summarized using frequencies and percentages. Continuous variables were summarized using mean values and SDs or medians and interquartile ranges except for values of fecal calprotectin, which were presented as mean and SE of the mean. Independent and paired-sample t tests were used to compare the mean calprotectin levels between different groups after testing for normality. Logistic regression was used to test the association between different factors and response to ustekinumab. All tests were 2-sided, and P values <0.05 were considered significant.
RESULTS AND DISCUSSION
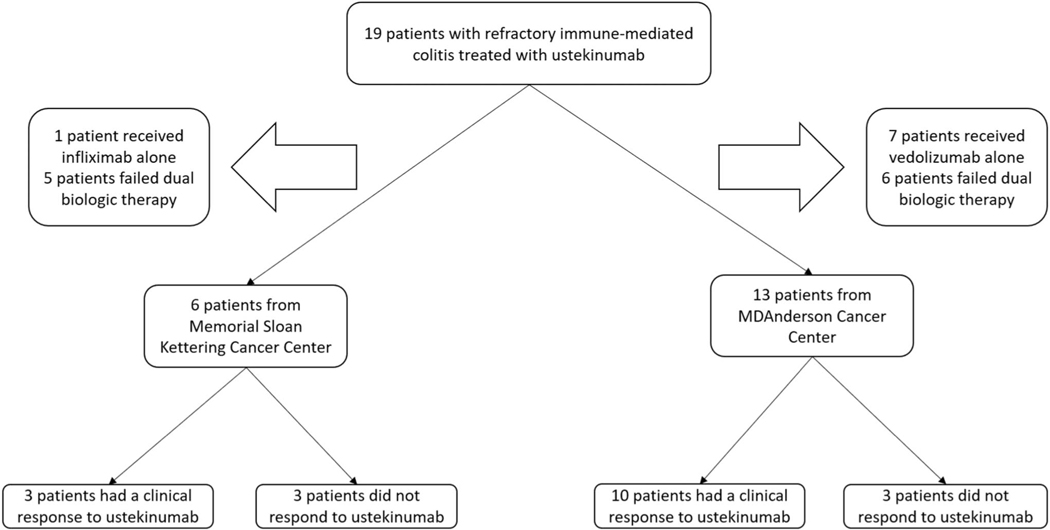
Details regarding the patient selection process from 2 tertiary cancer centers are shown in Figure 1. Table 1 highlights the demographic profile of our sample (n = 19) wherein most of them were White women who received PD 1/L1 monotherapy for stage IV cancer. Sixteen patients (84.2%) had CTCAE grade 3–4 diarrhea, and 14 patients (73.7%) required hospitalization for IMC. Eighteen (94.7%) patients were refractory to VDZ and 12 (63.1%) to IFX, with 11 (57.9%) patients failing both VDZ and IFX. Eight patients (42.1%) had high-risk endoscopic features of ulcerative colonic inflammation, which bears a poor prognosis (14).
Figure 1.
Patient selection flowchart.
Table 1.
Patients’ characteristics
| Characteristic | Cohort (N = 19) |
|---|---|
| Median age during IMC—yr (IQR) | 63 (58–72.5) |
| Male sex—n. (%) | 8 (42.1%) |
| White race—n. (%) | 17 (89.5%) |
| Cancer type—n. (%) | |
| Melanoma | 11 (57.9%) |
| GU | 1 (5.3%) |
| Lung | 2 (10.5%) |
| Breast | 1 (5.3%) |
| Head and neck/endocrine | 3 (15.8%) |
| Hematological cancer | 1 (5.3%) |
| Cancer stage IV | 11 (57.8%) |
| Immune checkpoint inhibitor type—n. (%) | |
| PD-1/L1 | 10 (52.6%) |
| Combination of CTLA-4 and PD-(L)1 | 9 (47.4%) |
| Median no. of ICI infusions before IMC (IQR) | 6 (2–9) |
| Immunotherapy was stopped because of IMC—n. (%) | 18 (94.7%) |
CTLA-4, cytotoxic T-lymphocyte associate protein-4; GU, genitourinary; IMC, immune-mediated colitis; IQR: interquartile range; PD-(L)1, programmed cell death protein (ligand) 1.
Clinical remission was achieved in 13 patients (68.4%) after treatment with UST, with 63.2% receiving more than 1 dose. We observed a striking improvement in fecal calprotectin post-UST therapy (Figure 2). Of the 11 patients who underwent an endoscopic follow-up, 64% had mucosal healing, similar to rates of healing seen in the UNIFI trial in ulcerative colitis (Table 2) (16; NCT02407236).
Figure 2.
Change in calprotectin levels before and after treatment with ustekinumab, with the black bar representing mean values.
Table 2.
Characteristics of gastrointestinal adverse events
| Characteristic | Cohort (N = 19) |
|---|---|
| Time from ICI to immune-related adverse events, days, median (IQR) | 98 (37–180) |
| Peak fecal calprotectin before UST, mean ± SEM | 629.8 ± 101.5 |
| Highest grade of diarrhea (3–4)—n (%) | 16 (84.2) |
| Highest grade of colitis—n (%) | |
| 1–2 | 17 (89.5%) |
| 3–4 | 2 (10.5%) |
| Initial endoscopic findings—n (%) | |
| Ulcers | 8(42.1%) |
| Nonulcer inflammation | 6(31.6%) |
| Normal | 5 (26.3%) |
| Hospitalizations—n (%) | 14 (73.7%) |
| Other treatment of GI adverse event—n (%) | |
| Steroid | 19 (100%) |
| Infliximab | 12 (63.2%) |
| Vedolizumab | 18 (94.7%) |
| FMTa | 8(42.1%) |
| Resumed cancer treatment after Rx—n (%) | 8(42.1%) |
| Resumed ICI—n (%)b | 6(31.6%) |
| >1 dose of ustekinumab | 12 (63.2%) |
| Clinical remission after ustekinumab treatmentc—n (%) | 13 (68.4%) |
| Endoscopic remission at the last follow up—n = 7(%) | 5 (26.3%) |
| Fecal calprotectin after UST, mean ± SEM | 92.0 ± 21.7 |
| Cancer status at the last follow up—n (%) | |
| Remission | 5 (26.4%) |
| Stable disease | 6(31.6%) |
| Progression | 8(42.1%) |
ICI, immune checkpoint inhibitor; UST: ustekinumab.
8 patients received FMT: 4 before ustekinumab, 4 after ustekinumab. Of the 4 after ustekinumab, 2 did not respond to ustekinumab. 2 discontinued the drug because of allergic reactions and loss of insurance coverage.
4 of these patients (66.7%) were ustekinumab responders, 2 were nonresponders.
1 patient had a good response to ustekinumab after 1 dose initially but then developed severe side effects that led to its discontinuation. Another patient also had a good initial response to ustekinumab but discontinued the drug because of loss of insurance coverage. Finally, 1 patient received 1 dose of ustekinumab with persistent symptoms initially and then lost insurance coverage and responded to FMT afterward.
We found no significant differences for clinical/endoscopic presentation of IMC or prior exposure to immunosuppression among UST responders versus nonresponders (Table 3). Numerically, more nonresponders had cancer progression compared with responders (83% vs 31%, P = 0.057). We noted a numeric difference in prior biologic exposure between the groups, with UST response rates of 87.5% after a single prior biologic versus 54.5% after 2 prior biologics (Table 3) (P = 0.18). This mirrors poorer IBD response rates in patients with prior exposure to anti-TNF (16) and highlights an important need for additional data to guide biologic sequencing in IMC.
Table 3.
Characteristics of UST responders and nonresponders
| N (%) |
|||
|---|---|---|---|
| Characteristics | Responders N = 13 | Nonresponders N = 6 | P value |
| History of autoimmune disease, n = 13 | 3(30%) | 2(66.7%) | 0.252 |
| Cancer status before IMC, n = 13 | 0.079 | ||
| Stable disease | 6(60%) | 0 | |
| Progression | 4(40%) | 3(100%) | |
| Median days from IMC to UST, (IQR) | 389(287–583) | 345.5(161.25–757.75) | 0.898 |
| Peak calprotectin before UST | 627.8 ± 119 | 635.8 ± 223.6 | 0.976 |
| Drop in calprotectin after treatment, mean ± SEM | 563 ± 140.4 | 635 ± 161.3 | 0.758 |
| Colitis grade≥2 | 8(61.5) | 5(83.3) | 0.605 |
| Diarrhea grade≥2 | 10(76.9) | 6(100) | 0.517 |
| Endoscopic findings | 1.000 | ||
| Normal | 3(23.2) | 2(33.3) | |
| Nonulcerative | 5(38.4) | 1(16.7) | |
| Ulcerative | 5(38.4) | 3(50) | |
| Histologic findings | 1.000 | ||
| Acute inflammation | 5(38.4) | 2(33.3) | |
| Chronic inflammation | 5(38.4) | 3(50) | |
| Microscopic colitis | 3(23.2) | 1(16.7) | |
| Steroid duration, days, median (IQR) | 34(20–57.5) | 48.5(33–62.5) | 0.412 |
| Previous biologic treatment | 0.177 | ||
| Single biologic agent | 7(53.8) | 1(16.7) | |
| Two biologic agents | 6(46.2) | 5(83.3) | |
| Doses of SIT, median (IQR) | 6(2.5–9.5) | 6.5(4.5–10) | 0.701 |
| Median days from last biologic to UST, (IQR) | 52(26–153) | 68.5(21–129.25) | 0.831 |
ICI, immune checkpoint inhibitor; IMC, inhibitor-mediated colitis; IQR: interquartile range; SIT, selective immunosuppressive therapy; UST: ustekinumab.
One patient developed severe side effects of sinus congestion/infection attributed to UST, which resolved after discontinuing the medication and treatment with antibiotics. While larger studies are necessary to determine the safety profile of IL-12/23 blockade in an immunocompromised cancer population, our findings suggest preliminary safety of UST in this group. That being said, the implications of opposing roles of IL-12 and IL-23 in maintaining dormancy and outgrowth of tumors in a cancer patient population is yet to be determined (17). In fact, preclinical mouse models have demonstrated that titrating this balance in combination with ICI can promote tumor suppression (18–20).
Last, 2 patients responded to FMT post-UST. FMT for refractory IMC represents a novel approach wherein the gut microbial composition is targeted to confer a therapeutic benefit. While little is known about the effect of IL-12/23 blockade on the gut microbiome, the question of a synergistic effect of such blockade with prior selective immunosuppressive therapy needs to be considered (Table 4). Our study is limited by its retrospective nature, small sample size, and the lack of a control arm to appropriately measure the impact of UST on IMC and cancer.
Table 4.
Multivariate logistic regression of factors related to ustekinumab treatment response
| Characteristic | Odds ratio (CI) | P value |
|---|---|---|
| Ustekinumab doses | 0.4 (0.2–1.2) | 0.122 |
| Failure of single or dual SIT agents | 0.05 (0.01–2.69) | 0.143 |
| Total doses of SIT | 0.9 (0.6–1.3) | 0.576 |
| Male sex | 0.22 (0.01–3.42) | 0.277 |
SIT, selective immunosuppressive therapy.
CONCLUSIONS
Blockade of IL-12/23 with ustekinumab is a promising therapy for the management of refractory IMC. Larger studies are needed to guide sequencing of biologics in IMC and explore their potential impact on cancer outcomes.
Footnotes
Guarantor of the article: Yinghong Wang.
Ethics approval and consent to participation: Ethics approval for this study was granted by the MD Anderson and Memorial Sloan Kettering Cancer Center Institutional Review Boards. Patient informed consent was waived for this study.
Data Sharing Statement: All data, analytic methods, and study material is available on request by contacting the corresponding author.
CONFLICTS OF INTEREST
Potential competing interests: Y.W. served as consultant for Sorriso Pharma, MabQuest, AzurRx, Sanarentero, and Ilya Pharma. D.F. has received consulting fees from Kaleido Biosciences, AzurRx, Mallinckrodt Pharmaceuticals, and Equillium. N.P. spoke for Allergan, Bristol Myers Squibb, Falk, Ferring, Janssen, Pfizer, Tillotts, and Takeda and served as a consultant and/or an advisory board member for AbbVie, Allergan, Celgene, Bristol Myers Squibb, Ferring, and Vifor Pharma. E.D.T. served as a consultant for AstraZeneca, Bayer, BMS, EISAI, Eli Lilly & Co, Pfizer, IPSEN, Terumo, and Roche. He has received third-party funding for scientific research from Arqule, Astra-Zeneca, BMS, Bayer, Eli Lilly, and IPSEN and Roche. He also received reimbursement of meeting attendance fees and travel expenses from Arqule, Astrazeneca, BMS, Bayer, Celsion, and Roche and lecture honoraria from BMS and Falk. R.W. spoke for Immunocore and Castle Biosciences and served as a consultant for Immunocore, Regeneron, Novartis, Pfizer, ACCC, and Castle Biosciences. He receives patent royalties from Reagents of University of Missouri. M.S., N.B.K., H.P.T., S.E.L., and A.T. have no conflict of interest.
Data availability statement:
The data sets used and analyzed in this study are available from the corresponding author on reasonable request.
REFERENCES
- 1.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33(17):1974–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brahmer JR, Abu-Sbeih H, Ascierto PA, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cance.r 2021;9(6): e002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grover S, Rahma OE, Hashemi N, et al. Gastrointestinal and hepatic toxicities of checkpoint inhibitors: Algorithms for management. Am Soc Clin Oncol Educ Book. 2018;38:13–9. [DOI] [PubMed] [Google Scholar]
- 4.Zou F, Faleck D, Thomas A, et al. Efficacy and safety of vedolizumab and infliximab treatment for immune-mediated diarrhea and colitis in patients with cancer: A two-center observational study. J Immunother Cancer. 2021;9(11):e003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Wiesnoski DH, Helmink BA, et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis [published correction appears in Nat Med. 2018 Nov 27. Nat Med. 2018;24(12):1804–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perez Del Nogal G, Patel N. Refractory checkpoint inhibitor colitis responsive to ustekinumab. ACG Case Rep J. 2022;9(12): e00946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmstroem RB, Dahl EK, Helms M, et al. Tofacitinib and faecal microbiota transplantation in treating checkpoint inhibitor-induced enterocolitis: Case report. BMJ Open Gastroenterol. 2022;9(1): e000989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sasson SC, Slevin SM, Cheung VTF, et al. Oxford Inflammatory Bowel Disease Cohort Investigators. Interferon-Gamma-producing CD8+ tissue resident memory T cells are a targetable hallmark of immune checkpoint inhibitor-colitis. Gastroenterology. 2021;161(4): 1229–44.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zellweger M, Rogler G, Komminoth P, et al. Management of persistent colitis after successful immunotherapy for non-small cell carcinoma of the lung. Z Gastroenterol. 2022;60:1124–30. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Ma W, Abu-Sbeih H, et al. Fecal microbiota transplantation(FMT) for immune checkpoint inhibitor induced–colitis (IMC) refractory to immunosuppressive therapy. J Clin Onc. 2020;38(15): 3067. [Google Scholar]
- 11.Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016;375(20): 1946–60. [DOI] [PubMed] [Google Scholar]
- 12.Thomas AS, Ma W, Wang Y. Ustekinumab for refractory colitis associated with immune checkpoint inhibitors. N Engl J Med. 2021; 384(6):581–3. [DOI] [PubMed] [Google Scholar]
- 13.Fernández-GordÓn Sánchez FM, Gόmez-Domínguez E, Paredes Ruiz D, et al. Ustekinumab for corticodependent immune-mediated colitis by pembrolizumab, an alternative for patients with concomitant liver injury. Rev Esp Enferm Dig. 2022;114(6):356–7. [DOI] [PubMed] [Google Scholar]
- 14.Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J. 1955;2(4947):1041–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019; 381(13):1201–14. [DOI] [PubMed] [Google Scholar]
- 16.Abu-Sbeih H, Ali FS, Luo W, et al. Importance of endoscopic and histological evaluation in the management of immune checkpoint inhibitor-induced colitis. J Immunother Cancer. 2018;6(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atreya R, Neurath MF. IL-23 blockade in anti-TNF refractory IBD: From mechanisms to clinical reality. J Crohns Colitis 2022;16(Supplement_2): ii54–ii63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teng MW, Vesely MD, Duret H, et al. Opposing roles for IL-23 and IL-12 in maintaining occult cancer in an equilibrium state. Cancer Res. 2012; 72(16):3987–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vom Berg J, Vrohlings M, Haller S, et al. Intratumoral IL-12 combined with CTLA-4 blockade elicits T cell-mediated glioma rejection. J Exp Med. 2013;210(13):2803–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Scheidt B, Leung PS, Yong MC, et al. Combined anti-CD40 and anti-IL-23 monoclonal antibody therapy effectively suppresses tumor growth and metastases. Cancer Res. 2014;74(9):2412–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets used and analyzed in this study are available from the corresponding author on reasonable request.