Abstract
Symptoms of neurological diseases emerge through the dysfunction of neural circuits whose diffuse and intertwined architectures pose serious challenges for delivering therapies. Deep brain stimulation (DBS) improves Parkinson’s disease symptoms acutely but does not differentiate between neuronal circuits, and its effects decay rapidly if stimulation is discontinued. Recent findings suggest that optogenetic manipulation of distinct neuronal subpopulations in the external globus pallidus (GPe) provides long-lasting therapeutic effects in dopamine-depleted (DD) mice. We used synaptic differences to excite parvalbumin-expressing GPe neurons and inhibit lim-homeobox-6–expressing GPe neurons simultaneously using brief bursts of electrical stimulation. In DD mice, circuit-inspired DBS provided long-lasting therapeutic benefits that far exceeded those induced by conventional DBS, extending several hours after stimulation. These results establish the feasibility of transforming knowledge of circuit architecture into translatable therapeutic approaches.
Identification of distinct neuronal subpopulations has been essential for understanding brain function. Interventions that target specific neuronal subpopulations (e.g., optogenetics) can alleviate disease symptoms with few side effects in animal models. However, clinical approaches in humans are rarely tailored to achieve population-specific neuromodulation. Electrical deep brain stimulation (DBS) is increasingly used to treat an expanding list of conditions in humans. Although theoretical work has long hypothesized that patterned electrical stimulation can improve its precision in targeting specific neuronal subpopulations (1–5), these approaches have not yet achieved the desired cell type specificity. Motor symptoms of Parkinson’s disease (PD) are acutely responsive to DBS, but symptoms return quickly when stimulation is discontinued (6). The need for constant stimulation increases the risk of side effects and drains battery power. Using optogenetics in mice, we identified cellular nodes in the external globus pallidus (GPe) of the basal ganglia, where targeted interventions ameliorate circuit dysfunction and restore movement several hours after stimulation (7). This optogenetic approach requires genetically targeted tools to drive opposite firing responses of spatially intermixed GPe subpopulations: Parvalbumin-expressing (PV-GPe) neurons must be excited, whereas lim-homeobox-6–expressing (Lhx6-GPe) neurons must be suppressed. Refining DBS protocols to achieve the same degree of population-specific neuromodulation would provide a treatment approach translatable to humans that could extend the therapeutic duration of stimulation, representing a major therapeutic advance.
To gain insight into the cellular principles that dictate the responses of PV-GPe and Lhx6-GPe neurons to electrical stimulation, we recorded extracellular spiking activity from these populations in acute brain slices from Lhx6-GFP+/− and PV-tdTmt+/− mice (Fig. 1A and fig. S1A). Both populations exhibited similar excitement during continuous electrical stimulation (100 Hz, 1 s) (Fig. 1,B and C). However, synaptic recordings showed that Lhx6-GPe neurons received proportionately more inhibition than PV-GPe neurons (Fig. 1D and fig. S1, B and C). This synaptic asymmetry was even more pronounced at high stimulation frequencies, where rapid synaptic depression was observed (Fig. 1, E and F). Notably, Lhx6-GPe neurons experienced significantly greater inhibitory charge than PV-GPe neurons during the initial second of stimulation (Fig. 1G).
Fig. 1. Bursts of stimulation segregate the firing responses of PV-GPe and Lhx6-GPe neurons.
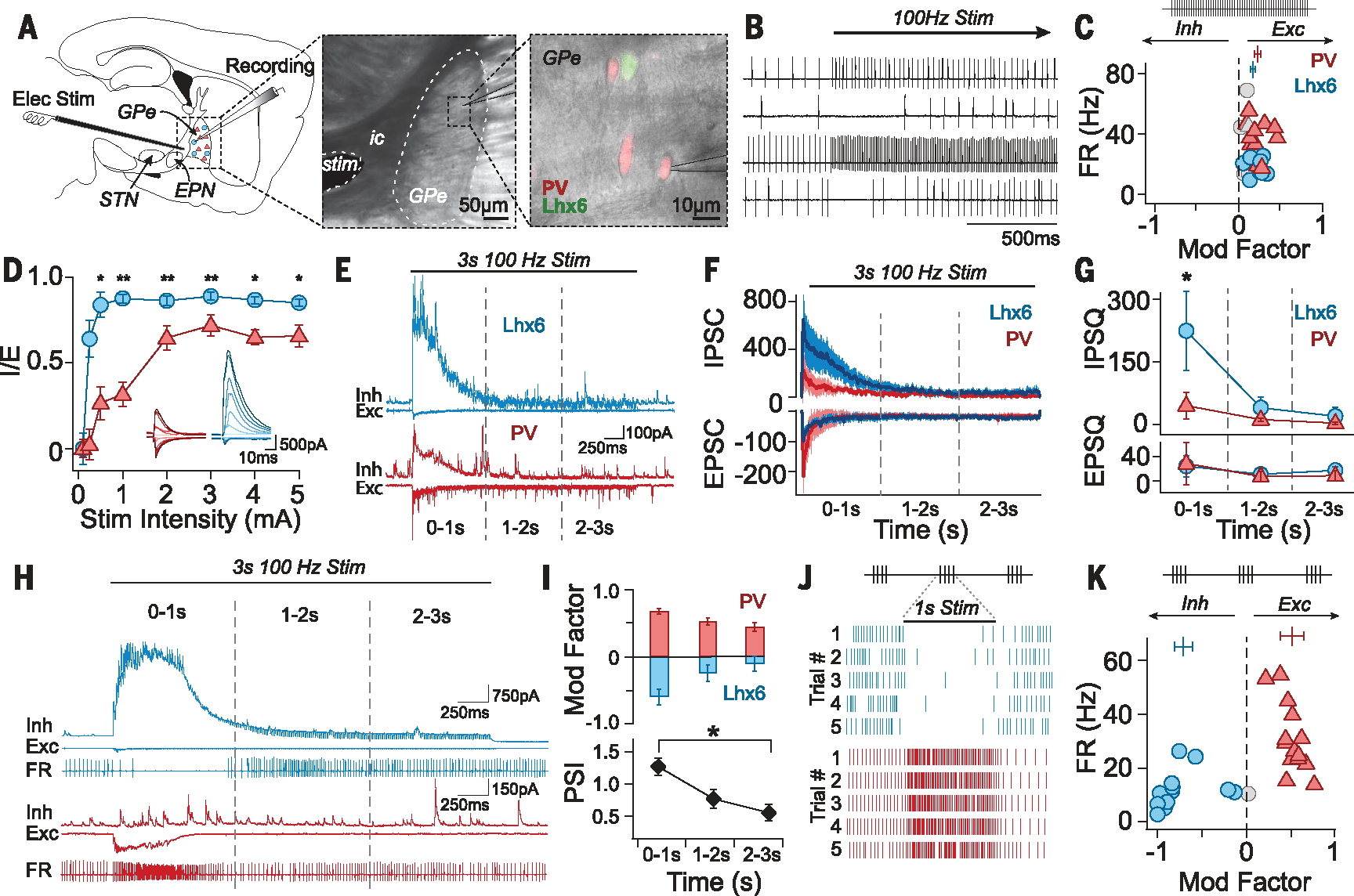
(A) Experimental configuration and fluorescent identification of GPe subpopulations (ic, internal capsule). (B) Extracellular traces during stimulation (100 Hz for 30 s) after artifact removal. (C) Stimulation at 100 Hz for 30 s modestly excited both populations; this was quantified as “modulation factor” (MF) of the firing rate (FR) for each neuron: (bars: MFLhx6 = 0.16 ± 0.11; MFPV = 0.22 ± 0.12; Mann-Whitney (MWU) P = 0.29; 12 pairs of neurons; six mice, error bars: SEM). Colored symbols indicate neurons significantly modulated during stimulation (paired t test, P < 0.05), and gray symbols indicate neurons not significantly modified. (D) Ratios of postsynaptic currents for inhibition (IPSC) and excitation (EPSC) were calculated with peak current amplitudes as [(IPSC)/(IPSC + EPSC)], showing that Lhx6-GPe neurons receive more inhibition than PV-GPe neurons [Kruskal-Wallis test (KW), χ2(7) = 57.4, P < 0.00001, post hoc pairwise MWU, *P < 0.05, **P < 0.001; 15 pairs; nine mice)]. Insets show currents from representative neurons. (E and F) Rapid depression of synaptic currents during 100-Hz stimulation (1 mA), from representative neurons (E) and across the population (F) (10 pairs; four mice; medians, error bars: SEM). (G) Synaptic charges [inhibitory postsynaptic charge (IPSQ) and excitatory postsynaptic charge (EPSQ)] transferred during the first, second, and third seconds of stimulation. IPSQ was larger in Lhx6-GPe neurons than in PV-GPe neurons during the initial second of stimulation (IPSQLhx6 = 284 ± 300 pC, IPSQPV = 98± 103 pC, MWU, *P = 0.023) (10 pairs; four mice). (H) Three-part recordings with 3 s of 100-Hz stimulation. (Bottom) Extracellular recording traces. (Middle and top) Whole-cell recordings of EPSCs (Exc, middle, Vhold = −70 mV), and IPSCs (Inh, top, Vhold =0 mV) (I). (Top) MF by cell type in 1 s time bins. (Bottom) Modulation was most segregated in the initial second of stimulation (PSI = MFPV − MFLhx6, see supplementary methods) [PSI0–1s = 1.27 ± 0.49; PSI1–2s = 0.77± 0.54; PSI2–3s = 0.55 ± 0.49; KW, χ2(2) = 12.0, *P = 0.003; 14 pairs; three mice]. (J) Raster plots of extracellular modulation by 1 s bursts of stimulation (100 Hz, 1 mA, 30 s intertrial interval). (K) PV-GPe neurons are excited whereas Lhx6-GPe neurons are inhibited (MFPV = 0.5 ± 0.1; MFLhx6 = −0.7 ± 0.3; 14 pairs; three mice).
To determine whether these synaptic asymmetries could be leveraged to drive population-specific neuromodulation, we took sequential extracellular and intracellular recordings. Firing responses of PV-GPe and Lhx6-GPe neurons were heavily influenced by the dynamics of their underlying synaptic inputs (Fig. 1H). Large inhibitory currents in Lhx6-GPe neurons were well correlated with transient suppressions in extracellular firing rates, which diminished as inhibitory synapses depressed. By contrast, PV-GPe neurons—which lacked large inhibitory currents—were instantly excited by stimulation.
Extracellular recordings revealed additional differences between Lhx6-GPe and PV-GPe neurons after stimulation was initiated. Lhx6-GPe neurons were routinely inhibited during the initial second of stimulation, but less so in subsequent time bins. Notably, some neurons switched from inhibited to excited within 3 s of continuous stimulation (Fig. 1I and fig. S1D). By contrast, PV-GPe neurons were typically excited throughout (Fig. 1I and fig. S1D). The degree to which firing responses of PV-GPe and Lhx6-GPe neurons could be separated during stimulation was calculated as a population separation index (PSI) (Fig. 1I and supplementary methods). The PSI was maximal during the initial second of stimulation and decayed over time, largely a result of decreased suppression of Lhx6-GPe neurons (Fig. 1I).
These results led to the hypothesis that delivering stimulation in short bursts, rather than continuously, could achieve population-specific neuromodulation in the GPe. We therefore delivered repeated bursts (100 Hz, 1 s) to pairs of Lhx6-GPe and PV-GPe neurons. This stimulus pattern drove robust and reproducible suppression of Lhx6-GPe neurons and simultaneous excitation of PV-GPe neurons (Fig. 1, J and K).
To identify the range of parameter combinations that separate the responses of PV-GPe and Lhx6-GPe neurons, we used an iterative machine learning approach (see supplementary methods). Experimental data (Fig. 2A) were used to train models to predict potential nonlinear relationships between parameter combinations and firing responses of PV-GPe and Lhx6-GPe populations. In the first iteration, a dataset of 208 distinctive burst combinations (table S1), collected across 19 pairs of Lhx6-GPe and PV-GPe neurons, was used to train Gaussian process regressions with quadratic polynomial kernels for each population. Compared with other approaches, this approach yielded improved leave-one-out cross-validation performance (table S2). Parameters were then sampled (table S3) and evaluated to identify ranges of parameters predicted to yield a large PSI. This range was then used to collect a second iteration of data (table S4), which were used to further refine the models (fig. S3, A and B). Because there was strong convergence of predictions between the first and second iterations of the models, no additional iterations were run. Surface plots with modulation factor predictions for PV-GPe and Lhx6-GPe populations across many thousands of artificially generated parameters, as well as the predicted PSI across the parameter space, are shown in Fig. 2B and fig. S2A.
Fig. 2. Stimulus optimization with a machine learning approach accurately predicts burst designs that achieve population-specific neuromodulation in the GPe using electrical stimulation.
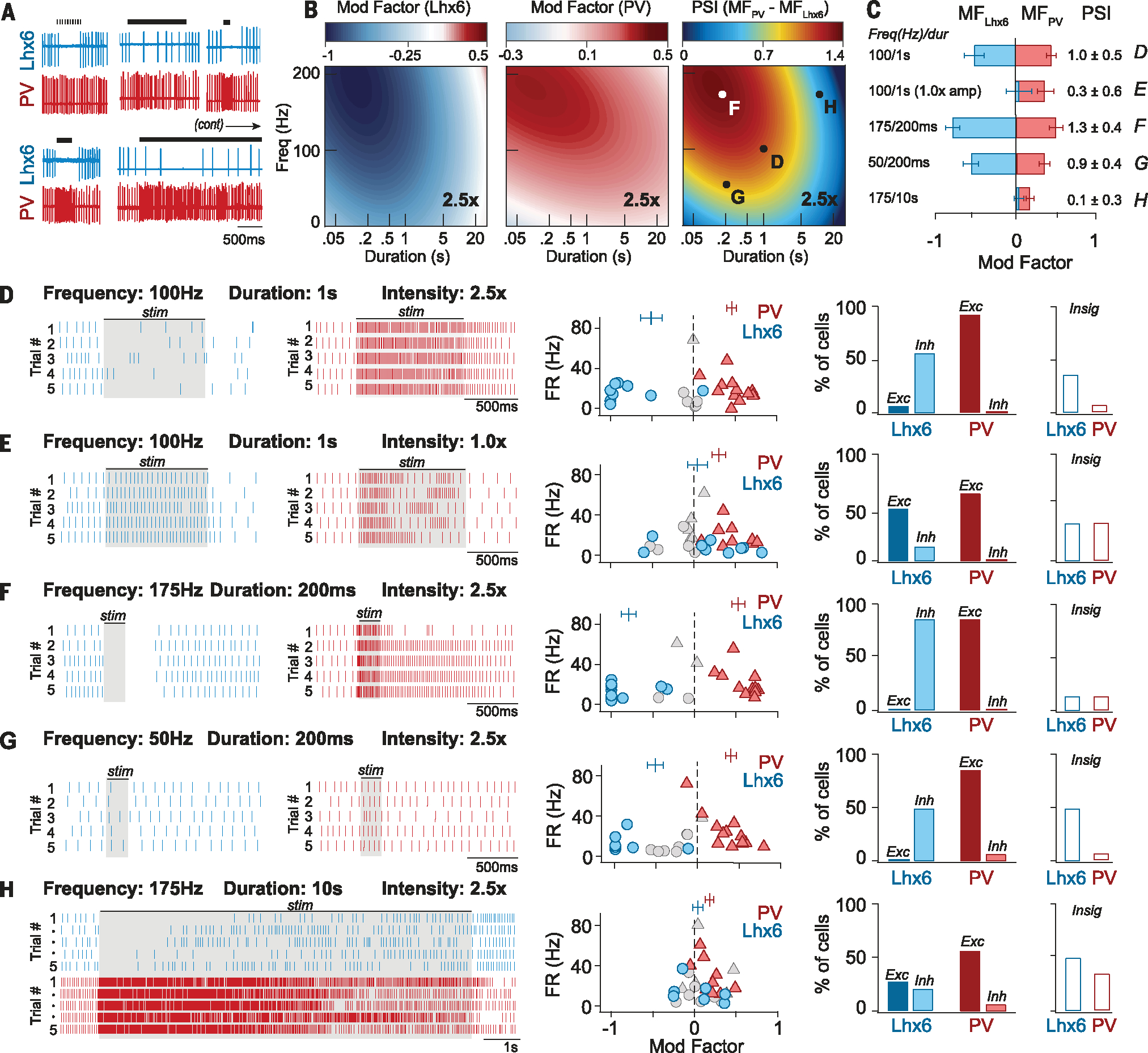
(A) Samples of neural response data used to train models (supplementary methods). (B) Surface plots (2.5× intensity) showing predicted FR responses of Lhx6-GPe (MFLhx6, left) and PV-GPe (MFPV, middle) populations across a range of stimulus parameters. Predicted increases in FR are shown in red, and predicted decreases in FR are shown in blue. (Right) Surface plot showing the degree to which stimulus parameters are predicted to segregate the responses of PV-GPe and Lhx6-GPe populations (PSI: red, more segregation; blue, less segregation). Lettered points indicate burst combinations selected for experimental validation [(D) to (G)]. (C) Experimentally measured responses of PV-GPe and Lhx6-GPe populations to burst protocols developed using our model (B). The degree to which burst designs segregated the responses of PV-GPe and Lhx6-GPe neurons (PSI) is shown to the right, with letters on right referencing matching panel letters. Error bars, SEM. (D to H) Experimental validation of model predictions. (Left) Rasters of extracellularly recorded FR from Lhx6-GPe (blue) and PV-GPe (red) neurons responding to burst designs drawn from regions of parameter space indicated in (B). Shaded boxes denote the time bin used to calculate modulation factors. (Middle) Modulation factors for each neuron (averaged across five trials), plotted against its baseline firing rate (14 pairs of neurons; four mice). Gray symbols, neurons in which firing rate was not significantly modified from baseline (P > 0.05, paired t test, FRbaseline versus FRstim, trials 1 to 5). Vertical markers show average MFLhx6 and MFPV, error bars: SEM. (Right) Bar graphs indicating the percentage of Lhx6-GPe and PV-GPe neurons that were excited (Exc), inhibited (Inh), or had no significant change in firing rate (Insig) during stimulation.
As predicted by our model, 100 Hz, 1-s bursts, delivered in brain slices, were highly effective at segregating the responses of PV-GPe and Lhx6-GPe neurons at a stimulus intensity of 2.5×, but less effective at weaker stimulus intensities (Fig. 2, C to E). Furthermore, we improved our experimentally measured PSI by using shorter, higher-frequency bursts (175 Hz, 200 ms; Fig. 2, B, C, and F). The model also accurately predicted the boundaries of parameter combinations that could dissociate the responses of PV-GPe and Lhx6-GPe neurons. For example, reducing intraburst frequency to 50 Hz while keeping a 200-ms duration (50 Hz, 200 ms) weakened the PSI but did not eliminate it entirely (Fig. 2, B, C, and G), whereas increasing the burst duration (175 Hz, 10 s) prevented stimulation from driving population-specific responses (Fig. 2, B, C, and H). Deviations from model predictions for experimental outcomes were quantified with root mean squared error (fig. S3, C and D, and table S5).
Given the critical role of inhibitory synaptic transmissions in differentiating the responses of PV-GPe and Lhx6-GPe neurons, we sought to identify the mechanism underlying its cell type specificity. To test the hypothesis that Lhx6-GPe inhibition was driven by the local GPe collateral network, engaged by excitation from the subthalamic nucleus (STN) (8, 9), we used optogenetics to selectively stimulate STN afferents. The fast channelrhodopsin variant Chronos enabled stimulation of STN fibers at high frequencies (10). However, 1-s bursts of STN stimulation (100 Hz) excited both populations of GPe neurons to a similar degree (Fig. 3, A and E).
Fig. 3. Population-specific neuromodulation in the GPe is driven by convergent excitation from the STN and inhibition from D1-SPNs.
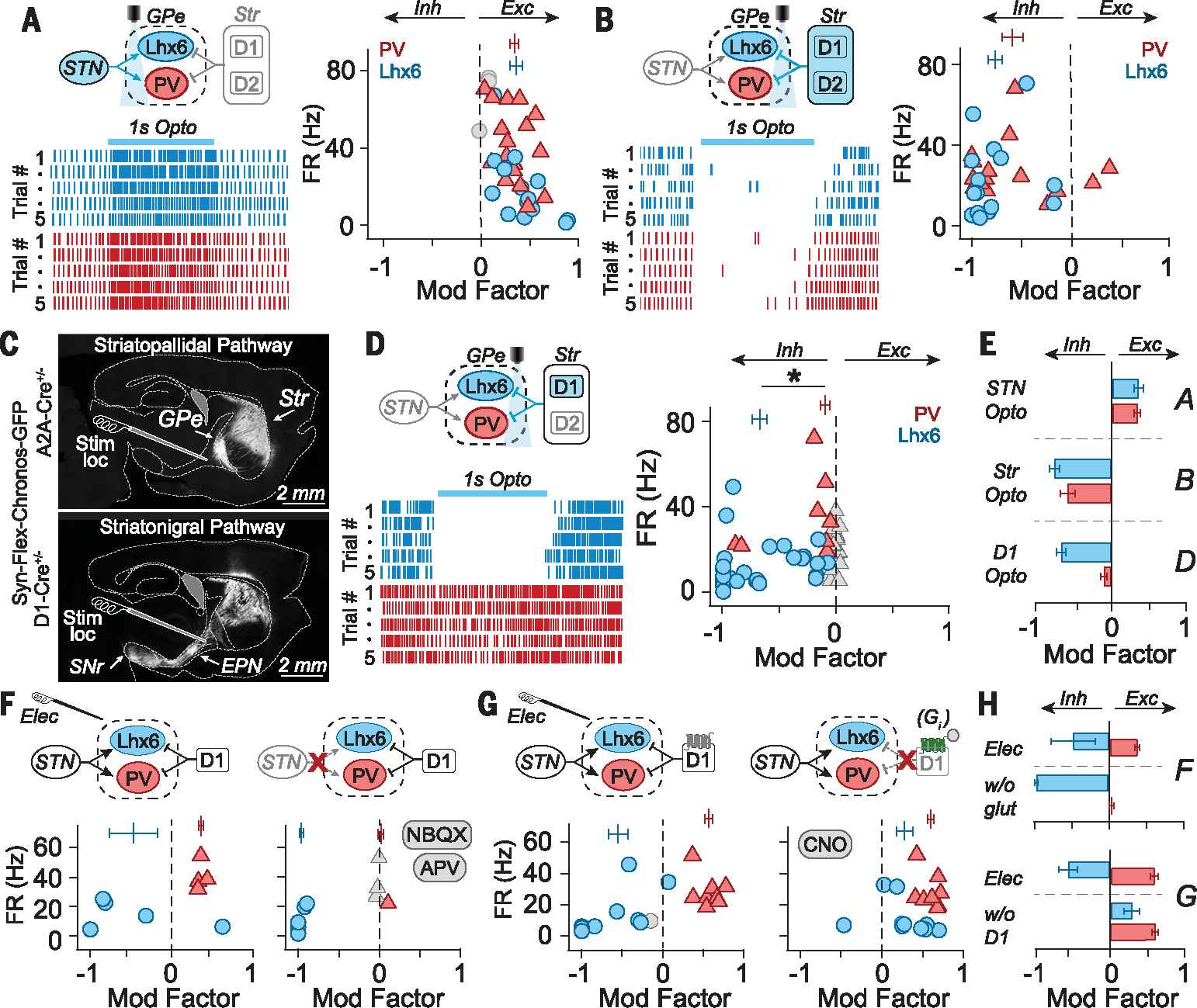
(A) (Left) Schematic and rasters from two representative GPe neurons during optogenetic stimulation of STN fibers (100 Hz for 1 s). (Right) Modulation factors (MFs) for individual neurons (symbols) and populations (vertical bars), showing that PV-GPe and Lhx6-GPe neurons are similarly excited (MFLhx6 = 0.36 ± 0.27, MFPV = 0.34 ± 0.17; MWU P = 0.976; 17 pairs of neurons; three mice). (B) (Left) Schematic and rasters from two neurons during striatal fiber stimulation (100 Hz for 1 s). (Right) MFs show that both PV-GPe and Lhx6-GPe neurons are similarly inhibited by striatal fiber stimulation (MFLhx6 = −0.77 ± 0.29, MFPV: −0.59 ± 0.41; MWU P = 0.25; 16 pairs; two mice). (C) (Top) Fluorescent image of striatopallidal pathway. (Bottom) Fluorescent image of striatonigral pathway. Typical placement of the stimulating electrode is shown for reference. (D) (Left) Schematic and rasters from two neurons during D1-SPN striatal fiber stimulation. (Right) MFs show that Lhx6-GPe neurons are preferentially inhibited (MFLhx6 = −0.68 ± 0.34; MFPV = −0.1 ± 0.23; MWU *P < 0.00001; 27 pairs; four mice). (E) Summary of MFLhx6 (blue) and MFPV (red) for experiments A to D; error bars: SEM. (F) MFs in response to electrical stimulation before (left) and after (right) application of 10 mM NBQX/50 mM APV. Excitation of PV-GPe neurons was blocked (MFCtrl: 0.36 ± 0.05, MFNBQX/APV: 0.01 ± 0.07, paired t test, P = 0.0001), but not Lhx6-GPe inhibition (MFCtrl: −0.47 ± 0.66, MFNBQX/APV: −0.96 ± 0.05, paired t test, P = 0.18) (5 Lhx6-GPe neurons, 4 PV-GPe neurons; two mice). (G) MFs in response to electrical stimulation before (left) and after (right) chemogenetic inhibition of D1-SPN fibers [AAV2-hsyn-DIO-hM4D(Gi)-mCherry + CNO, see methods]. Inhibition of Lhx6-GPe neurons was blocked (MFpre = −0.54 ± 0.39, MFCNO = 0.28 ± 0.33, paired t test, P = 0.003), but excitation of PV-GPe neurons was not (MFpre = 0.57 ± 0.15, MFCNO = 0.59 ± 0.13, paired t test, P = 0.7) (n = 10 Lhx6-GPe neurons, n= 8 PV-GPe neurons; n = five mice). (H) Summary of MFLhx6 (blue) and MFPV (red) for experiments F to G. Error bars: SEM.
Next, we tested the hypothesis that inhibition from the striatum (11) was weighted more heavily toward Lhx6-GPe neurons than PV-GPe neurons. Chronos was globally expressed in the striatum (Fig. 3, B and C). Optogenetic stimulation (100 Hz, 1 s) of striatal afferents in the GPe similarly inhibited both PV-GPe and Lhx6-GPe neurons (12–14) (Fig. 3, B and E).
These results seem to suggest that striatal inputs lack the cell type specificity needed for population-specific neuromodulation. However, considering the placement of our stimulating electrode (Fig. 3C), electrical stimulation was unlikely to engage all striatal cell types equally. Instead, electrical stimulation was likely biased toward antidromic activation of D1-type dopamine receptor SPNs (D1-SPNs), which are not a canonical source of inhibitory input to the GPe, although they do make some synaptic contacts (12, 15–18). To test the hypothesis that striatal afferents from D1-SPNs preferentially inhibit Lhx6-GPe neurons, Chronos expression was restricted to D1-SPNs (Fig. 3D) in the striatum. This time, optogenetic stimulation of D1-SPN afferents differentiated the responses of GPe neurons, driving robust inhibition of Lhx6-GPe but not PV-GPe neurons (Fig. 3, D and E).
These results suggest that the circuit mechanism by which electrical stimulation drives population-specific neuromodulation was through the converging effects of excitatory inputs from the STN and inhibitory inputs from D1-SPNs. We therefore assessed the effects of systematically blocking each input during electrical stimulation. Blocking excitatory inputs eliminated the excitation of PV-GPe neurons during stimulation and unmasked even greater inhibition of Lhx6-GPe neurons (Fig. 3, F and H). Conversely, chemogenetic suppression of D1-SPN fibers during electrical stimulation blocked the inhibitory response of Lhx6-GPe neurons, unmasking an excitatory effect (Fig. 3, G and H).
Finally, to test whether an electrical DBS protocol tailored to drive population-specific neuromodulation would mimic the persistent therapeutic effects induced by cell-type–specific optogenetic manipulations (7), we compared the therapeutic efficacy of conventional versus burst DBS (supplementary methods) in mice rendered parkinsonian by bilateral injections of the toxin 6-hydroxydopamine (6-OHDA) into the medial forebrain bundle (MFB) (Fig. 4A, supplementary methods). Burst DBS consisted of short bursts delivered once a second with a duration of 200 ms and an intraburst frequency of 175 Hz. Recordings in brain slices confirmed that the population specificity of this protocol was retained when delivered with a biphasic stimulating electrode, as required in vivo, both for naïve tissue and in tissue from dopamine-depleted (DD) mice (fig. S4, A and B).
Fig. 4. Burst stimulation restores movement persistently in parkinsonian mice.
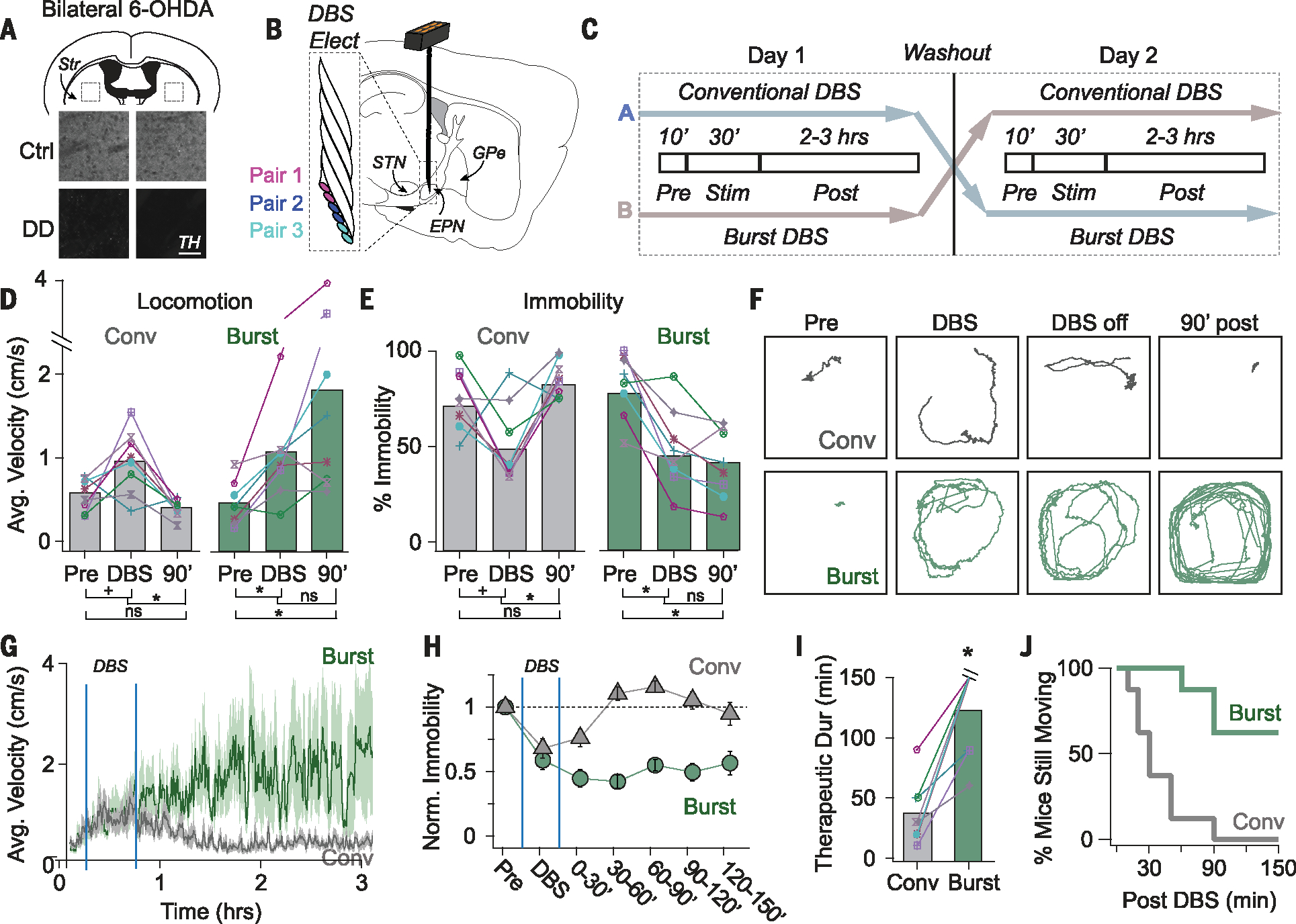
(A) Schematic showing bilateral dopamine depletion (6.43 ± 0.09% tyrosine hydroxylase (TH) remaining compared with littermate controls). (B) Schematic showing DBS electrode placement near the EPN. (C) Cross-over design illustrating a pseudorandom experimental protocol in which DD mice receive conventional or burst DBS on alternate days. (D) Average velocity of DD mice increased during conventional DBS (gray) but did not persist after stimulation (Pre: 0.54 ± 0.07, Stim: 0.94 ± 0.13, Post: 0.40 ± 0.06 cm/s) [Wilcoxon signed rank (WSR): Pre versus Stim, +P = 0.055; Stim versus 90 min (90’) *P = 0.008; Pre versus 90’. P = 0.195, n = eight mice]. Average velocity increased during burst DBS (green) and was still elevated 90 min after stimulation (Pre: 0.47 ± 0.09, Stim: 1.03 ± 0.19, Post: 1.77 ± 0.54 cm/s) (WSR: Pre versus Stim *P = 0.016; Stim versus 90’ P = 0.195; Pre versus. 90’ *P = 0.008). Data from individual mice are shown, and colors and symbols are consistent throughout (ns, not significant). (E) Immobility of DD mice decreased during conventional DBS (gray) but did not persist (Pre: 75 ± 6 Stim: 51 ± 7, Post: 87 ± 4% time immobile) (WSR: Pre versus Stim, +P = 0.056 Stim versus 90’ *P = 0.008, Pre versus 90’ = 0.195). Immobility decreased during burst DBS (green) and was still low 90 min after stimulation (Pre: 82 ± 6, Stim: 48 ± 7, Post: 45 ± 9% time immobile) (WSR: Pre versus Stim, *P = 0.016; Stim versus 90’; P = 0.641; Pre versus. 90’ *P = 0.016). (F) Movement paths over 8-min intervals throughout one trial. Data are from the same mouse treated with conventional DBS (gray) or burst DBS (green). (G to H) Average movement velocities (G) and immobility (H) are plotted for the duration of behavioral trials. Movement velocities were averaged over 30-s time bins and percent immobility was averaged over 30-min time bins. Gray, conventional DBS; green, burst DBS (n = eight mice). (I to J) Amount of time after DBS before mice returned to pre-stim levels of immobility (“therapeutic duration”). Therapeutic duration was significantly longer after burst DBS (123.8 ± 37.4 min, green) than after conventional DBS (37.5 ± 25.5 min, gray) (WSR, *P = 0.012, n = eight mice), shown for individual mice (I) and cumulatively across the population (J).
The therapeutic effects of burst versus conventional DBS were then compared using a cross-over study design with a 1-day washout period between treatments in DD mice (Fig. 4C), using an approach shown to recapitulate a number of features of DBS in humans (19). Six-lead DBS electrodes were implanted bilaterally near the entopeduncular nucleus (EPN) (Fig. 4B and fig. S5), an electrode placement often used in human patients receiving DBS in the internal globus pallidus (GPi) (20, 21) and an area where fibers from the STN and D1-SPNs can be coactivated.
Before stimulation, DD mice were highly immobile. Both conventional and burst DBS reduced this immobility, enabling mice to move around the arena during stimulation (Pre and DBS in Fig. 4, D to F, and movies S1 and S2). After receiving conventional DBS, mice quickly returned to the immobile state (Fig. 4, D to H). Conversely, after receiving burst DBS, mice continued to move around the arena (Fig. 4, D to H), with five out of the eight mice still moving at the end of the trial, ~2.5 hours after stimulation (Fig. 4, I and J, 90’+, movies S1 and S2). On average, the therapeutic effects of burst DBS persisted >4.5 times as long as those of conventional DBS (Fig. 4I), a conservative estimate as only 37% of mice had returned to the immobile state at the end of the trial (Fig. 4J). DBS protocols predicted to be less effective at dissociating the responses of PV-GPe, and Lhx6-GPe neurons were less effective at inducing persistent rescue (fig. S5).
These results demonstrate how fundamental knowledge about the organization and function of basal ganglia circuitry can be used to refine the population specificity of electrical stimulation, ultimately prolonging the therapeutic benefits of DBS beyond those achieved with conventional methods. Other alternative forms of DBS, including adaptive and coordinated reset DBS (22–25), can induce persistent therapeutic effects with the use of specialized implants, and it is plausible that the long-lasting effect observed in our study shares a common underlying mechanism with these approaches. However, our burst DBS protocol can be delivered through commonly used DBS implants and falls within US Food and Drug Administration–approved stimulus frequencies, enabling immediate testing in PD models across species, including human patients. This work joins the growing field of opto-inspired DBS (26–28), in which fundamental discoveries about the organizing principles of neural circuits gained through the use of optogenetics guide the development of more robust electrical DBS approaches that can be rapidly translated to humans.
Supplementary Material
ACKNOWLEDGMENTS
We thank J. Schwenk and C. Snyder for expert animal care and technical assistance, J. Schorr for DBS training and guidance, and R. Gerkin for specialized IGOR analysis and acquisition routines.
Funding:
This work was supported by the Richard King Mellon Foundation Presidential Fellowship in the Life Sciences (T.A.S.), Lane Fellowship in Computational Biology (I.M.K.), Michael J. Fox Foundation (A.H.G.), National Institutes of Health grant T32GM008208 (S.N.), and National Institutes of Health grants R01NS101016, R01NS104835, and R01NS117058 (A.H.G.)
Footnotes
Competing interests: The authors declare that they have no competing interests.
Data and materials availability:
The processed data used to generate the figure panels (29) and the code used for Gaussian regression modeling (30) can be found at Zenodo.
REFERENCES AND NOTES
- 1.Guo T et al. , Front. Neurosci. 13, 413 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anastassiou CA, Montgomery SM, Barahona M, Buzsáki G, Koch C, J. Neurosci. 30, 1925–1936 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McIntyre CC, Grill WM, Ann. Biomed. Eng. 28, 219–233 (2000). [DOI] [PubMed] [Google Scholar]
- 4.Radman T, Ramos RL, Brumberg JC, Bikson M, Brain Stimul. 2, 215–228.e3 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McIntyre CC, Grill WM, J. Neurophysiol. 88, 1592–1604 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Temperli P et al. , Neurology 60, 78–81 (2003). [DOI] [PubMed] [Google Scholar]
- 7.Mastro KJ et al. , Nat. Neurosci. 20, 815–823 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miguelez C et al. , J. Physiol. 590, 5861–5875 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kita H, Tachibana Y, Nambu A, Chiken S, J. Neurosci. 25, 8611–8619 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klapoetke NC et al. , Nat. Methods 11, 338–346 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith DR et al. , Neuroscience 86, 135–146 (1998). [DOI] [PubMed] [Google Scholar]
- 12.Cazorla M et al. , Neuron 81, 153–164 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan X-S et al. , eLife 6, e29055 (2017).29022877 [Google Scholar]
- 14.Kovaleski RF et al. , J. Physiol. 598, 1897–1927 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawaguchi Y, Wilson CJ, Emson PC, J. Neurosci. 10, 3421–3438 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ketzef M, Silberberg G, Neuron 109, 516–529.e4 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Cui Q et al. , J. Neurosci. 41, 3966–3987 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Y, Richard S, Parent A, Neurosci. Res. 38, 49–62 (2000). [DOI] [PubMed] [Google Scholar]
- 19.Schor JS, Nelson AB, J. Clin. Invest. 129, 3833–3838 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Au KLK et al. , Neurol. Ther. 10, 7–30 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vitek JL, Hashimoto T, Peoples J, DeLong MR, Bakay RA, Mov. Disord. 19, 907–915 (2004). [DOI] [PubMed] [Google Scholar]
- 22.Adamchic I et al. , Mov. Disord. 29, 1679–1684 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tass PA et al. , Ann. Neurol. 72, 816–820 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Wang J et al. , Brain Stimul. 9, 609–617 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosin B et al. , Neuron 72, 370–384 (2011). [DOI] [PubMed] [Google Scholar]
- 26.Creed M, Pascoli VJ, Lüscher C, Science 347, 659–664 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Gittis AH, Yttri EA, Curr. Opin. Biomed. Eng. 8, 14–19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valverde S et al. , Nat. Commun. 11, 2388 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spix T et al. , Zenodo (2021); doi: 10.5281/zenodo.5211556. [DOI] [Google Scholar]
- 30.Spix T et al. , Zenodo (2021); doi: 10.5281/zenodo.5218517. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The processed data used to generate the figure panels (29) and the code used for Gaussian regression modeling (30) can be found at Zenodo.


