Abstract
Rheumatoid meningitis (RM) is a rare but serious extra-articular manifestation of rheumatoid arthritis. Due to the absence of specific biomarkers, imaging findings, or guidelines for its detection, the diagnosis of RM is difficult. This report describes a patient of RM diagnosed with an open biopsy and discusses the utility of anticyclic citrullinated peptide antibodies (ACPA) levels in the serum and cerebrospinal fluid (CSF), and contrast-enhanced (CE) fluid-attenuated inversion recovery (FLAIR) images for screening and monitoring RM. A 65-year-old woman presented with a 2-month history of headaches. Imaging studies showed asymmetric meningeal and leptomeningeal involvement seen on brain magnetic resonance imaging (MRI). An open biopsy of the meninges and leptomeninges depicted palisaded and necrotizing granulomatous inflammation, which suggests rheumatoid nodules. Treatment with prednisolone and tocilizumab led to symptom improvement and reduced lesion intensity on follow-up MRI. Throughout the treatment, the ACPA index in her serum and CSF, and the findings of CE-FLAIR images, rather than the CE T1WI, reflected disease activity. For 6 months, the patient has been stable without symptom recurrence. The ACPA index and the CE-FLAIR images were useful for the diagnosis and monitoring of RM. To validate these findings, further studies are necessary.
Keywords: rheumatoid meningitis, anti-cyclic citrullinated peptide antibodies, enhanced fluid-attenuated inversion recovery images
Introduction
Rheumatoid meningitis (RM) is a rare but serious extra-articular manifestation of rheumatoid arthritis (RA). This manifestation primarily can not only occur in patients with long-standing RA1) but also independently of the presence or severity of RA. At the time of RM onset, more than 30% of cases are not diagnosed with RA.2) Nevertheless, RM can take place even in patients with well-controlled RA. A definitive diagnosis is more challenging when laboratory data or imaging findings suggest other diseases. Despite that asymmetric meningeal or leptomeningeal involvement seen on brain magnetic resonance imaging (MRI) is a characteristic feature of this disease, along with other diseases, such as malignancies and IgG4-related pathologies,3-5) gadolinium (Gd) enhancement can be blunted because it leaks into the adjacent cerebrospinal fluid (CSF) through damaged tissues. Hence, contrast-enhanced (CE) T1-weighted images (T1WI) may not accurately reflect the distribution of meningeal and leptomeningeal lesions. Anti-cyclic citrullinated peptide antibodies (ACPA) have been reported as a potential marker of RM.2,6) This antibody is an immune protein produced by autoreactive B cells and serves as a diagnostic biomarker in patients with RA. ACPA can also be found in the CSF because of passive diffusion from the serum due to the blood-CSF barrier dysfunction and has been shown to decrease in titer following treatment in several reports of RM.6) Herein, we report a patient with RM, and demonstrate the utility of ACPA levels in the serum, CSF, and enhanced fluid-attenuated inversion recovery (FLAIR) images for screening and monitoring RM is demonstrated. The patient involved in this case report provided informed consent.
Case Report
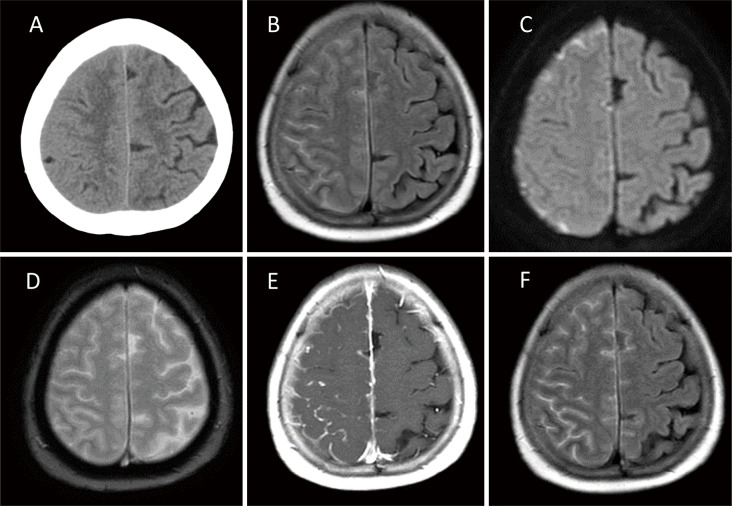
A 65-year-old woman presented with a 2-month history of headaches. She had a 26-year history of RA that was well-controlled with salazosulfapyridine, bucillamine, and iguratimod. Initial brain computed tomography (CT) showed less pronounced sulci on the right side than on the left (Fig. 1A). FLAIR and diffusion-weighted images (DWI) revealed hyperintensities in the right frontoparietal sulci (Fig. 1B, C). These lesions were negative for hemorrhage on T2* images (Fig. 1D). CE T1WI and CE FLAIR images showed meningeal and leptomeningeal enhancements on the same site (Fig. 1E, F), and the lesion distribution was clearer on CE FLAIR than on CE T1WI.
Fig. 1.
Brain computed tomography scan obtained on the day of admission showing effacement of the sulci at the right frontoparietal lobes (A). Magnetic resonance imaging (MRI), fluid-attenuated inversion recovery (FLAIR) (B), and diffusion-weighted images (DWI) (C) showing the hyperintensities on the right frontoparietal cortical sulci. These lesions demonstrate no evidence of hemorrhage on T2* images (D). Contrast-enhanced (CE) T1-weighted images (T1WI) (E) and CE-FLAIR images (F) showing the meningeal and leptomeningeal enhancement on the same site. Lesion distribution was more clearly depicted on CE FLAIR compared to CE T1WI.
On laboratory workup, serology exhibited elevated rheumatoid factor (RF) 33.5 IU/mL (0-15 IU/mL), ACPA 78.4 U/mL (0-4.5 U/mL), IgG 2,434 mg/dL (861-1,747 mg/dL), antinuclear antibody 80 times (0-39 times), soluble interleukin-2 receptor 490 U/mL (157-474 U/mL), and cancer antigen 15-3 (CA15-3) 25.3 U/mL (0-25.0 U/mL).
The other serology parameters were normal including beta2-microglobulin (β2MG), Aspergillus antigen, Cryptococcus neoformans antigen, cytoplasmic antineutrophil cytoplasmic antibodies (c-ANCA), perinuclear ANCA (p-ANCA), immunoglobulin G subclass IV (IgG4), angiotensin converting enzyme, anti-Sjogren's syndrome A (anti-SSA)/Ro antibody, anti-Sjogren's syndrome B (anti-SSB)/La antibody, anti-Sm antibody, and anti-ssDNA antibody, as well as tumor markers except CA15-3. Polymerase chain reaction (PCR) tests were negative for human immunodeficiency virus, Treponema pallidum, Epstein-Barr virus, varicella-zoster virus, herpes simplex virus, mumps virus, measles virus, and cytomegalovirus. QuantiFERON-TB and serum fungal testing were also negative.
CSF analysis revealed elevating opening pressure reaching 27 cm H2O, leukocyte count of 133/μL (~5/μL, 88% mononuclear cells and 12% polynuclear cells), protein level of 149 mg/dL (10-40 mg/dL), glucose level of 43 mg/dL (50-80 mg/dL), and interleukin-6 level of 349 pg/mL (0-4.0 pg/mL). The CSF PCR results for viral infections, as mentioned above, were negative. CSF cytology for malignancies and culture results were negative. CSF ACPA and CSF IgG were 12.1 U/mL (no reference values, higher than serum reference value) and 19.8 mg/dL (0.5-4.0 mg/dL), respectively. The ACPA index is obtained by dividing the CSF/serum ratio of ACPA by the CSF/serum ratio of IgG. A ratio of > 1.4 means that the ACPA is produced within the central nervous system.6) For antibodies other than ACPA, the antibody titer index using serum and CSF were calculated using the same formula, and an increase in this index indicated specific antibody production in the CSF.7) Based on this formula, the ACPA index was 19.25 in this case. Table 1 includes the time course of serological and CSF markers.
Table 1.
Time course of serological and cerebrospinal fluid markers
| RF (IU/mL) | Serum ACPA (U/mL) | CSF ACPA (U/mL) | Serum IgG (mg/dL) | CSF IgG (mg/dL) | ACPA index | |
|---|---|---|---|---|---|---|
| Pre-treatment | 33.5 | 78.4 | 12.1 | 2434 | 19.8 | 19.25 |
| Post-treatment | 20.7 | 22.4 | <0.6 | 699 | 3 | <6 |
RF, rheumatoid factor; ACPA, anti-cyclic citrullinated peptide antibodies; CSF, cerebrospinal fluid; IgG, immunoglobulin G
Whole-body CT revealed an enhanced lesion in the left breast. She had undergone surgery for left-sided breast cancer 2 years ago, and CT findings suggested breast cancer recurrence. As shown by breast ultrasound, the possibility of breast cancer recurrence cannot be ruled out.
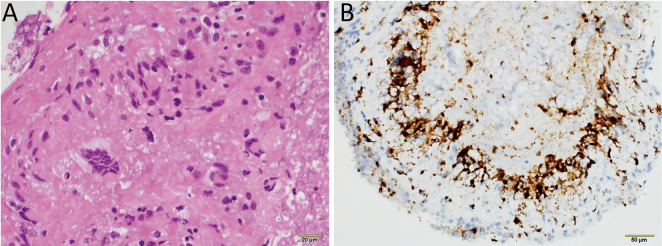
We performed an open craniotomy and biopsy of the meninges and leptomeninges of the frontal convexity, which was enhanced on MRI, and a needle biopsy of the breast lesion. The meninges were thickened but did not adhere to the leptomeninges. In the leptomeninges, yellow white caseous necrosis was observed. These biopsies showed palisaded and necrotizing granulomatous inflammation, which indicates the presence of rheumatoid nodules (Fig. 2A, B). Tests for bacterial, fungal, Mycobacterium tuberculosis, and viral tissue infections were negative. A needle biopsy of the left breast exhibited no evidence of malignancy, and the enhanced lesion on body CT was considered a postoperative change. These findings showed that the patient was diagnosed with RM. Oral prednisolone was initiated at a dosage of 60 mg/day with gradual tapering. Her symptoms gradually improved, and she was discharged 1 month later.
Fig. 2.
(A) Meningeal and leptomeningeal biopsy demonstrating palisaded and necrotizing granulomatous inflammation. (B) Immunostaining revealing CD68-positive perivascular histiocytes. No evidence of bacterial, fungal, Mycobacterium tuberculosis, or viral infections was found, indicating the rheumatoid nodules.
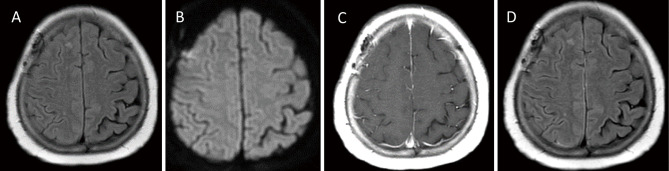
Follow-up MRI 4 months later showed attenuation of the hyperintensities in the sulci on FLAIR and DWI (Fig. 3A, B). Enhancement on the T1WI and FLAIR images was also attenuated (Fig. 3C, D). Follow-up laboratory workup revealed decreasing RF 20.7 IU/mL, ACPA 22.4 U/mL, and IgG 699.0 mg/dL (Table 1). CSF analysis revealed opening pressure reaching 26 cmH2O, leukocyte count of 2/μL, protein level of 87 mg/dL, and glucose level of 115 mg/dL. CSF ACPA and CSF IgG were <0.6 U/mL and 3.0 mg/dL, respectively (Table 1). The ACPA index decreased to <6 with the actual value possibly being lower because of the CSF ACPA level being below the detection limit (Table 1). She stopped taking salazosulfapyridine, bucillamine, and iguratimod 10 days after the biopsy when the pathological diagnosis results arrived. She started taking prednisolone orally at 60 mg/day, tapered it down by 5 mg/week to 20 mg/day, and is now managed with subcutaneous injections of tocilizumab at a dose of 162 mg every 2 weeks, along with an oral intake of prednisolone at a dose of 20 mg/day, without symptom recurrence.
Fig. 3.
Follow-up MRI conducted four months later (A, B, C, and D). FLAIR (A) and DWI (B) demonstrating the regression of the leptomeningeal involvement. CE-T1WI (C) and CE-FLAIR (D) demonstrating the attenuating of the enhancement.
Discussion
The incidence of RA is estimated to be 1% of the population. RM is rarer in clinical practice, and its true prevalence is unknown.8,9) No specific biomarkers or guidelines for detecting RM are available; hence, the diagnosis is based on a combination of clinical presentation, hematological CSF parameters, imaging findings, and the exclusion of other etiologies.8)
RM exhibits diverse MRI features, with asymmetric meningeal or leptomeningeal involvement being relatively common;4,10) nevertheless, MRI findings alone cannot reliably differentiate between the RM and meningeal metastasis because some RM cases present with diffuse or bilateral lesions on MRI.11) In meningeal and leptomeningeal lesions, the concentration of Gd is lower than that in intra-axial brain lesions considering that Gd concentration is reduced as it leaks into the adjacent CSF through damaged vessels.12,13) CE FLAIR is more sensitive to low concentrations of Gd than CE T1WI, and this sequence is suitable for the diagnosis of inflammatory meningeal and leptomeningeal disease.12,14-16) Conversely, the diagnostic accuracy of meningitis with FLAIR is highly likely to depend on the amount of CSF protein concentration.15) Even in case reports wherein imaging findings improved after treatment17-19) including the present case, the narrowness of the cerebral sulcus remains and has not completely disappeared. These imaging findings may be attributed to a combination of inflammatory exudates with necrotizing granulomatous inflammation and leakage of Gd from the capillaries to the surrounding area. In the present case, CE FLAIR is the most accurate sequence for depicting the distribution of lesions, and it should be considered for detecting the meningeal and leptomeningeal lesions.
RM exhibits diverse laboratory findings and may mimic several neurological diseases, infections, and malignancies.6) ACPA is an autoantibody directed against cyclic citrullinated peptides found in the joints and blood of patients with RA and is useful for early diagnosis of RA because it presents prior to the appearance of the symptoms of joint inflammation.20) The ACPA index, which is calculated by dividing the ACPA CSF/serum ratio by the IgG CSF/serum ratio, is considered to be more useful than serum ACPA alone6) for diagnosing and monitoring RM. This may be because the underlying disease results in higher baseline inflammatory cytokine levels.21) Screening for serum ACPA in patients with asymmetric meningeal or leptomeningeal involvement seen on brain MRI is acceptable. If serum ACPA levels are increased, testing for CSF ACPA and CSF IgG may reduce repeat testing and minimize a missed diagnosis.
In this case, the patient developed RM while receiving salazosulfapyridine, bucillamine, and iguratimod. If RM develops while taking medications for RA, there is no consensus on the next treatment option. Although a certain number of RM case reports are believed to include drug-induced meningitis,18) its diagnosis is difficult in cases wherein the same medication has been employed for a long period, and the pathogenesis of which is not well understood.22) Considering that she had previously developed interstitial pneumonia, she was unable to use methotrexate (MTX), which is the first choice for RA. She also had to discontinue anti-tumor necrosis factor (TNF) alpha agents, which were previously utilized for cases where MTX could not be employed or was not sufficiently effective, due to liver dysfunction. Rituximab, a monoclonal antibody against the CD20 surface marker expressed on B cells, is often utilized in patients who are resistant to treatment with the two drugs mentioned above; nevertheless, rituximab is not covered by health insurance in Japan and cannot be utilized. She is now receiving tocilizumab subcutaneously and prednisolone orally. Tocilizumab is a recombinant humanized anti-interleukin-6 receptor monoclonal antibody and treats multiple autoimmune diseases effectively. It is effective for RA and is especially more potent than anti-TNF alpha agents in the absence of MTX.23) Reportedly, tocilizumab is more effective than rituximab in RA patients with low or absent B-cell lineage expression signatures in synovial tissue.24) Nevertheless, tocilizumab is not believed to cross the blood-brain barrier because of its high molecular weight.25) Hence, these findings cannot be directly applied to CNS diseases. The immunohistochemical findings obtained by biopsy may be reflected in future treatments.
In conclusion, even in patients with well-controlled RA, RM is a rare but must be critically considered. Despite that biopsy is still the gold standard for definitive diagnosis, MRI findings on CE-FLAIR images and ACPA titers may help screen and monitor treatment responses in patients with RM. However, to validate these findings, larger studies are required.
Conflicts of Interest Disclosure
All authors declare that there are no conflicts of interest (COIs) concerning this article according to the criteria of The Japan Neurosurgical Society (JNS). The authors (TS, YA, SS, and SO) who are members of JNS have registered online self-reported COI disclosure statement forms through the website for JNS members.
References
- 1). Parsons AM, Aslam F, Grill MF, Aksamit A, Goodman BP: Rheumatoid meningitis: clinical characteristics, diagnostic evaluation, and treatment. Neurohospitalist 10: 88-94, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Nissen MS, Nilsson AC, Forsberg J, et al. : Use of cerebrospinal fluid biomarkers in diagnosis and monitoring of rheumatoid meningitis. Front Neurol 10: 666, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Mahendru G, Chong V: Meninges in cancer imaging. Cancer Imaging 9: S14-S21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Choi SJ, Park YO, Kim JA, Han JH, Choe G, Kim SY: Pearls & Oy-sters: asymmetric meningeal involvement is a common feature of rheumatoid meningitis. Neurology 88: e108-e110, 2017 [DOI] [PubMed] [Google Scholar]
- 5). Mehta SH, Switzer JA, Biddinger P, Rojiani AM: IgG4-related leptomeningitis: a reversible cause of rapidly progressive cognitive decline. Neurology 82: 540-542, 2014 [DOI] [PubMed] [Google Scholar]
- 6). Caputi L, Boncoraglio GB, Bernardi G, et al. : Anti-cyclic citrullinated peptide antibody index in the cerebrospinal fluid for the diagnosis and monitoring of rheumatoid meningitis. Biomedicines 10: 2401, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Reiber H, Peter JB: Cerebrospinal fluid analysis: disease-related data patterns and evaluation programs. J Neurol Sci 184: 101-122, 2001 [DOI] [PubMed] [Google Scholar]
- 8). Joshi S, Masiak A, Zdrojewski Z: Rheumatoid arthritis with pachymeningitis - a case presentation and review of the literature. Reumatologia 58: 116-122, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Manolios E, Manolios N, Spencer D: Leptomeningitis in rheumatoid arthritis. Eur J Rheumatol 8: 48-50, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Matsuura D, Ohshita T, Nagano Y, Ohtsuki T, Kohriyama T, Matsumoto M: [Case of rheumatoid meningitis: findings on diffusion-weighted image versus FLAIR image]. Rinsho Shinkeigaku 48: 191-195, 2008. (Japanese) [DOI] [PubMed] [Google Scholar]
- 11). Shimada K, Matsui T, Kawakami M, et al. : Diffuse chronic leptomeningitis with seropositive rheumatoid arthritis: report of a case successfully treated as rheumatoid leptomeningitis. Mod Rheumatol 19: 556-562, 2009 [DOI] [PubMed] [Google Scholar]
- 12). Lee EK, Lee EJ, Kim S, Lee YS: Importance of contrast-enhanced fluid-attenuated inversion recovery magnetic resonance imaging in various intracranial pathologic conditions. Korean J Radiol 17: 127-141, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Jin T, Ge M, Huang R, et al. : Utility of contrast-enhanced T2 FLAIR for imaging brain metastases using a half-dose high-relaxivity contrast agent. AJNR Am J Neuroradiol 42: 457-463, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Roozpeykar S, Azizian M, Zamani Z, et al. : Contrast-enhanced weighted-T1 and FLAIR sequences in MRI of meningeal lesions. Am J Nucl Med Mol Imaging 12: 63-70, 2022 [PMC free article] [PubMed] [Google Scholar]
- 15). Vaswani AK, Nizamani WM, Ali M, Aneel G, Shahari BK, Hussain S: Diagnostic accuracy of contrast-enhanced FLAIR magnetic resonance imaging in diagnosis of meningitis correlated with CSF analysis. ISRN Radiol 2014: 578986, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Fukuoka H, Hirai T, Okuda T, et al. : Comparison of the added value of contrast-enhanced 3D fluid-attenuated inversion recovery and magnetization-prepared rapid acquisition of gradient echo sequences in relation to conventional postcontrast T1-weighted images for the evaluation of leptomeningeal diseases at 3T. AJNR Am J Neuroradiol 31: 868-873, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Fan S, Zhao J, Hou B, et al. : Rheumatoid meningitis: a rare neurological complication of rheumatoid arthritis. Front Immunol 14: 1065650, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Qin Z, Kim J, Valencia D, et al. : Rheumatoid meningitis: a case report and review of the literature. Neurol Clin Pract 10: 73-83, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Ikeda K, Takazawa T, Ito H, et al. : Rheumatoid leptomeningitis: radiological alteration of cerebral hypoperfusion and subarachnoid lesions. Intern Med 49: 1911-1916, 2010 [DOI] [PubMed] [Google Scholar]
- 20). Mekic M, Hadzigrahic E: Anti-cyclic citrullinated peptide antibody as a predictor of rheumathoid arthritis complications. Med Arch 74: 183-186, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Lee DW, Gardner R, Porter DL, et al. : Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124: 188-195, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Bihan K, Weiss N, Theophile H, Funck-Brentano C, Lebrun-Vignes B: Drug-induced aseptic meningitis: 329 cases from the French pharmacovigilance database analysis. Br J Clin Pharmacol 85: 2540-2546, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Dougados M, Kissel K, Sheeran T, et al. : Adding tocilizumab or switching to tocilizumab monotherapy in methotrexate inadequate responders: 24-week symptomatic and structural results of a 2-year randomized controlled strategy trial in rheumatoid arthritis (ACT-RAY). Ann Rheum Dis 72: 43-50, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24). Humby F, Durez P, Buch MH, et al. : Rituximab versus tocilizumab in anti-TNF inadequate responder patients with rheumatoid arthritis (R4RA): 16-week outcomes of a stratified, biopsy-driven, multicentre, open-label, phase 4 randomised controlled trial. Lancet 397: 305-317, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Prince EW, Hoffman LM, Vijmasi T, et al. : Adamantinomatous craniopharyngioma associated with a compromised blood-brain barrier: patient series. J Neurosurg Case Lessons 1: CASE2150, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]