Abstract
Aims
Predicting mortality in severe AL cardiac amyloidosis is challenging due to elevated biomarker levels and limited thresholds for stratifying severe cardiac damage.
Methods and results
This prospective, observational, cohort study included de novo, confirmed cardiac AL amyloidosis patients at the Henri Mondor National Reference Centre. The goal was to identify predictors of mortality to enhance prognostic stratification and improve informed decision‐making regarding therapy. Over the 12‐year study period, among the 233 patients included, 133 were NYHA III‐IV and 179 Mayo 2004 III. The independent predictors for mortality identified were hsTnT, NT‐proBNP, cardiac output, and conjugated bilirubin. A novel prognostic, conditional stratification, Mondor amyloidosis cardiac staging (MACS) was developed with biomarker cut‐off values for Stage 1: hsTnT ≤ 107 ng/L and NT‐proBNP ≤ 3867 ng/L (n = 77; 33%); for stage 2 NT‐proBNP > 3867 ng/L (n = 72; 30%). For stage 3, if troponin >107 ng/L, regardless of NT‐proBNP then CB 4 μmol/L, was added (n = 41; 17.5%) and stage 4: CB > 4 μmol/L (n = 43; 18.5%). The median overall survival was 8 months 95% CI [2–24]. At 1 year, 102 (44%) patients died and the Kaplan–Meier median survival with MACS Stage 1 was not reached, while stage 2 was 15.2 months (95% CI [11–18]) and stage 3, 6.6 months (95% CI [1–13]). Notably, among European stage II patients, 17.1%, n = 8 were MACS stage 3 and European stage IIIb 21.4% (n = 23) were MACS stage 4. Importantly, among European stage IIIb patients 42.2% (n = 29) were classified MACS stage 4 and 12.5% n = 9 were only MACS stage 2.
Conclusions
The Mondor prognostic staging system, including conjugate bilirubin may significantly improve prognostic stratification for patients with severe cardiac amyloidosis.
Keywords: AL amyloidosis, Mortality, Predictive biomarkers, Prognosis, Staging score
Introduction
In amyloidosis light chain involvement (AL), predicting prognosis is challenging. 1 Cardiac involvement 2 , 3 and tumour burden 4 , 5 are key factors that indicate a poor prognosis. Once heart failure symptoms appear, cardiac amyloidosis progresses rapidly and generally becomes incurable. 6 However, survival among AL patients is extremely heterogeneous, depending on the severity of cardiac damage and can range from several weeks after diagnosis up to 10 years. 6 , 7
Prognosis and treatment response is evaluated with current, validated staging systems. These systems measure circulating markers of cardiac (troponin and NT‐proBNP) 8 and renal involvement 9 or tumour burden with serum free light chain levels (dFLC). 5 Until recently, the Mayo Clinic 2004 was the most popular staging system, which has a NT‐proBNP threshold of 332 ng/L and a highly sensitive troponin T threshold of 50 ng/L (hsTnT > 0.035 μg/L or hs‐cTnI > 0.1 μg/L). Nowadays, the European staging modifications have been shown to better stratify very high‐risk stage III patients into IIIa and IIIb stages. 10 The BNP proved to be a reliable prognostic marker at a threshold of 81 pg/mL enabling a new stratification model when associated with troponin 8 Furthermore, the updated Mayo 2012 incorporated tumour burden markers (dFLC). Although these markers are independent predictors for survival at cut‐off levels for dFLC > 180 mg/L, hsTnT > 40 ng/L, and NT‐proBNP > 1800 ng/L, they were validated in a cohort with mild cardiac involvement. 11 , 12
Additionally, the Mayo 2012 score validated serum FLC cut‐off levels with the Freelite assay (The Binding Site, Birmingham UK) but not all treatment centres use this assay. However, a newer technique, the N‐Latex FLC assay (Siemens Healthcare Diagnostics), exists. This technique is a nephelometric technique, which uses polystyrene particles coated with monoclonal antibodies for human kappa and lambda immunoglobulins. However, this technique is inaccurate for prognostication and stratification.
Considering the emergence of new treatments, such as antibody therapy and immunotherapy for patients with severe cardiac involvement, there is a need to identify these patients to propose treatment.
Given the challenges identifying patients at very high mortality risk with the current prognostic scores and assay techniques, we sought to identify additional predictive factors for death or cardiac transplantation. We also aim to validate these factors in an independent AL cardiac amyloidosis (AL‐CA) cohort. If possible, cut‐off values to improve prognostic risk evaluation in high‐risk patients will be confirmed.
The objective of this study is to determine predictive mortality factors, their cut off value and to establish a mortality risk stratification adapted for patients with advanced cardiac amyloidosis and compare this prognostic value to established scores.
Methods
Ethics
All patients agreed to provide their health data for this study. This study was performed in accordance with the 1975 Helsinki Declaration. Data were recorded electronically in the Henri Mondor Amyloidosis Network registry approved by the local ethics committee (Creteil) and by the National Committee for Data Protection (CNIL no. 1431858).
Study population and setting
This observational, prospective, cohort study was conducted in the National Referral Centre for Cardiac Amyloidosis, Henri Mondor University Hospital, France, from September 2008 and May 2020. All existing patients with histologically confirmed, suspected or de novo AL amyloidosis with cardiac involvement or cardiogenic shock, referred to the centre were consecutively enrolled in the study. Those patients previously treated or had Waldenstrom's disease were excluded.
Data collection and measurement
Cardiac AL amyloidosis was confirmed, and all follow‐up visits were performed at the centre, according to usual clinical practice. At each clinical visit, clinical, laboratory, and imaging data were obtained and recorded into the Mondor Network database 13 from which data extraction and retrospective analysis were performed.
Diagnosis was confirmed on endomyocardial or extramyocardial biopsy with immunohisochemistry showing typical Congo red staining, and positive Kappa or Lambda antibody staining, 14 cardiac ultrasound, electrocardiogram, and magnetic resonance imaging (MRI). Also, any additional clinical signs and biological values (hsTnT ng/L and NT‐proBNP ng/L) were obtained from the medical history.
At the first clinical evaluation visit, patients were classified according to Mayo 2004, 2012 and European staging systems and prospectively followed‐up until death or cardiac transplantation. At inclusion and each follow‐up visit, a clinical examination, cardiac ultrasound, and biological analyses were performed. Notable variables measured were cardiac function (LV longitudinal global strain, NT‐proBNP, and hsTnT), renal function (serum creatinine), hepatic markers [conjugated bilirubin (direct bilirubin), alkaline phosphatase and SGOT, SGPT], haematological evaluation, and circulating light chain measurement.
Amyloid cardiac involvement was defined by the presence of one or several criteria: elevations in cardiac biomarkers, left ventricular hypertrophy on echocardiogram or on MRI (in the absence of a history of hypertension or valvular disease), intraventricular septal thickness >12 mm, and/or by an endomyocardial biopsy specimen that prove amyloid fibril deposits. Concomitant medication was also recorded and if necessary, chemotherapy was started following blood count, myelogram, and FISH analysis.
Serum NT‐proBNP levels were measured with an automated double‐incubation sandwich assay (Roche Diagnostics; normal, 0.171 ng/L); hsTnT was measured with a sensitive fourth‐generation assay (Roche Diagnostics, Indianapolis, IN; normal <14 ng/L) and conjugate bilirubin (Roche Diagnostics, 8000, IN; normal <2.8 μmol/L). Transthoracic cardiac ultrasound was performed according to the American Society of Echocardiography criteria with commercially available GE Vivid 9 Ultrasounds (GE Healthcare, Little Chalfont, UK). 15 Wall thickness, chamber and left atrial (LA) dimension, interventricular septum (IVS) thickness, LV posterior wall (LVPW) thickness, LV end‐diastolic diameter (LVEDD), LV end‐systolic diameter (LVESD), and inferior vena cava were measured. LV fractional shortening was evaluated as the difference between the end‐diastolic and the end‐systolic diameters. LV ejection fraction (EF) was evaluated using the biplane Simpson's equation from 4‐chamber views and <50% was considered impaired. Conventional pulsed Doppler in the apical 4‐chamber view assessed early‐to‐atrial transmitral flow and velocity ratio (E/A ratio). The E to e′ ratio was the ratio between early diastolic transmitral flow velocity (E) and tissue Doppler derived early diastolic peak velocity at lateral mitral annulus. LV global longitudinal strain (GLS) was measured with a comprehensive 2D echocardiography and strain analysis to obtain 2D‐STE performed the echocardiographic studies using a Vivid E9 GE Medical System with a 2.5‐ to 4.0‐MHz transducer.
Serum FLC was determined using the N‐Latex technique 16
Serum kappa (κ) and lambda (λ) FLC concentrations were measured using N‐Latex FLC on a BN‐ProSpec nephelometer (Siemens Heatlhineers, Erlangen, Germany). 17 Normal ranges are: FLCκ, 6.7–22.4 mg/L; FLCλ, 8.3–27.0 mg/L; κ/λ ratio, 0.31 to 1.56. Monoclonal FLC concentration (dFLC) was an estimated difference between involved FLC (iFLC) and uninvolved FLC (uFLC).
Statistical analysis and ethics
Continuous variables were presented as median (interquartile range) if highly skewed, and categorical variables were summarized as counts (percentages). The χ 2 test or Fisher exact test (when call counts were small) was used to compare categorical variables and the Wilcoxon rank‐sum test was used to compare continuous variables, between different subgroup as previously described.
An overall cardiac survival curve (death or heart transplant) was produced using the Kaplan–Meier method. For each existing severity score (Mayo 2004, modified Mayo and European staging), Kaplan–Meier curves were created and stratified by group and these groups compared with univariate Cox models.
Univariate and multivariate Cox models were used to identify possible prognostic markers.
An additional regression analysis (conditional tree method) 18 was applied to the prognostic markers to identify their relative importance and cut‐off values.
Pearson correlation was used to compare principal prognostic cardiac amyloidosis parameters: conjugated bilirubin (CB) and cardiac markers (NT‐proBNP, troponin, C‐reactive protein and cardiac output).
Results
Study population
Among those registered in our centre during the study period, 395 had confirmed amyloidosis diagnosis (AL‐CA). Of these, 233 patients were de novo and included (Figure 1 ). Table 1 summarizes baseline clinical features, biological, and imaging data. The median patient age was 67 years, and the cohort was predominantly male (60%).
Figure 1.

Study cohort and flow chart. Strengthening the reporting of observational studies in epidemiology. STROBE diagram illustrates patient selection and study flow.
Table 1.
Clinical characteristics of patients with AL cardiac amyloidosis
| Variables | Overall (N = 233) |
|---|---|
| Time of symptom onset | |
| Delay 1st CV symptom – diagnostic, days | 191 (84–459) |
| Delay 1st extra CV symptom – diagnostic, days | 217 (72–608) |
| Clinical characteristics | |
| Age, years | 67 (60–75) |
| Male, n (%) | 139 (60) |
| Body mass index, kg/m2 | 24 (21–27) |
| CV characteristics | |
| NYHA III–IV, n (%) | 133 (58) |
| Heart rate, b.p.m. | 82 (73–94) |
| Systolic blood pressure, mmHg | 110 (100–126) |
| Atrial fibrillation, n (%) | 22 (11) |
| QRS duration, ms | 94 (80–115) |
| Pacemaker or ICD, n (%) | 123 (53) |
| Amyloid characteristics | |
| Free kappa light chain, mg/L | 19 (12–40) |
| Free lambda light chain, mg/L | 155 (49–338) |
| Free light chain kappa/lambda ratio | 0.12 (0.04–0.47) |
| dFLC, mg/L | 176 (89–418) |
| Medullar plasmocytes | |
| Plasmocyte >10%, n (%) | 41 (38), 12 (11) |
| FISH: t (11–14) or t (4–14), n (%): (n = 109) | |
| Organ involved | |
| Kidney involvement, n (%) | 82 (44) |
| Polyneuropathy, n (%) | 51 (22) |
| Gastrointestinal tract involvement, n (%) | 21 (9) |
| Automic nervous system, n (%) | 81 (43) |
| Hepatic involvement, n (%) | 14 (6) |
| Soft tissues involvement, n (%) | 82 (44) |
| Biology variables | |
| NT‐proBNP, ng/L | 5270 (226–11 020) |
| hsTnT, ng/L | 81 (51–138) |
| Haemoglobin, g/mL | 12.7 (11.2–13.9) |
| C‐reactive protein, mg/L | 3.5 (1.5–8.8) |
| Proteinuria, g/L | 0.23 (0.08–1.45) |
| Serum creatinine, μmol/L | 98 (79–127) |
| SGP, UI | 25 (17–36) |
| SGOT, UI | 31 (22–40) |
| Alkaline phosphatase, U/L | 84 (67–122) |
| Total bilirubin, μmol/L | 9 (6.0–15.0) |
| Conjugated bilirubin, μmol/L | 3 (2–6) |
| Serum albumin, g/L | 34 (28–38) |
| Echocardiographic characteristics | |
| LVEF, % | 53 (45–61) |
| Interventricular septum, mm | 16 (13–17) |
| Posterior wall thickness, mm | 15 (13–17) |
| h/r ratio relative wall thickness | 0.74 (0.60–0.88) |
| GL strain, % | –10 (−7 to –13) |
| LVEDD, mm | 41 (37–46) |
| LVESD, mm | 29 (25–33) |
| LVmass index, g/m2 | 140 (117–172) |
| LVEDV, mL | 71 (53–91) |
| LVESV, mL | 32 (25–44) |
| Mitral flow velocity E/A | 1.6 (1.1–2.9) |
| E/Ea | 18 (12–25) |
| Cardiac flow index, mL/mn/m2 | 2.4 (2.0–2.9) |
| Bone scintigraphy and cardiac MRI | |
| Cardiac fixation + bone scintigraphy, n (%): (n = 170) | 26 (15) |
| LGE + cardiac MRI, n (%): (n = 182) | 119 (64) |
Clinical characteristics: Laboratory and echographic and imaging parameters at the first evaluation on the cardiac amyloidosis centre. Variables are expressed as median and interquartile and numbers and per cent for categorical variables.
A, peak A‐wave velocity cm/s; CV, cardiovascular; DFLC, free light chain difference; E, peak E‐wave velocity 5 cm/s; GL strain, global longitudinal strain; ICD, implantable cardioverter–defibrillator; LGE, late gadolinium enhancement; LVEDD, left ventricular end diastolic diameter; LVEDV, left ventricular end systolic volume; LVEF, left ventricular ejection fraction; LVESD, left ventricular end systolic diameter; LVESV, left ventricular end systolic diameter volume; mitral E/A ratio, MV E velocity divided by A‐wave velocity–pulsed–wave TDI E′ velocity; mitral E/E′, MV E velocity divided by mitral annular E′ velocity; NT‐proBNP: N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; SGOT–SGPT, serum glutamic pyruvic transaminase–serum glutamic oxaloacetic transaminase.
Concerning baseline cardiac severity, most patients were NYHA classes III or IV and among those with autonomic involvement, 26% had symptomatic hypotension. LV systolic function was moderately preserved, but global longitudinal strain (−10%) and indexed cardiac output were reduced (2.4 mL/mn/m2 [2–2.9]). Although the kidney, autonomic nervous system, and soft tissue were involved, hepatic involvement occurred least often.
Among those with confirmed AL amyloidosis, most had Lambda type and 16% had plasma cell infiltration greater than 10%. FISH analysis was available for only 109 patients and revealed that among these, 38% had translocation t (11; 14) and 11% had t (4; 14). All patients had cardiac involvement. Cardiac severity differed according to staging system used: Mayo 2004 staging identified (77%) Stage III, Mayo 2012 staging identified 40.2% Stage III and 40% Stage IV, whereas European Staging identified 46.7% IIIa and 30.2% stage IIIb (Table 2 ). Almost all patients received Chemotherapy (CyBorD) within our centre according to the following protocol: cyclophosphamide (days 1, 8, 15), bortezomib (days 8, 15, 22), and dexamethasone (days 15, 22, 23) sequentially depending on cardiac severity. Four patients also received daratumumab first‐line. 13
Table 2.
Definition and prevalence of patients of the cohort in each Mayo Clinic 2004 stages, Mayo Clinic 2012 stages European 2015 stages
| Mayo Clinic 2004 Staging | Mayo Clinic 2012 Staging | European 2015 Staging | |||
|---|---|---|---|---|---|
| Definition of each stages | |||||
|
I: Troponin hs < 50 ng/L and Nt‐ProBNP <332 ng/L II: Troponin hs ≥ 50 ng/L or Nt‐ProBNP ≥332 ng/L III: Troponin hs ≥ 50 ng/L and Nt‐ProBNP ≥332 ng/L |
I: Troponin hs < 40 ng/L and NT‐proBNP <1800 ng/L and dFLC<180 mg/L II: Troponin hs ≥ 40 ng/L or NT‐proBNP ≥1800 ng/L or dFLC ≥180 mg/L (one condition) III: Presence of Two conditions IV: Presence of Three conditions |
I: Troponin hs < 50 ng/L and NT‐ProBNP <332 ng/L. II: Troponin hs ≥ 50 ng/L or NT‐ProBNP ≥332 ng/L. IIIa: Troponin hs ≥ 50 ng/L and NT‐PproBNP ≥332 ng/L and <8500 ng/L IIIb: Troponin hs ≥ 50 ng/L and NT6proBNP ≥ 8500 ng/L |
|||
| Number and prevalence of the patients depending on each stages, n (%) | |||||
| I | 9 (3.8) | I | 15 (6.4) | I | 9 (3.8) |
| II | 45 (19.2) | II | 26 (11.2) | II | 45 (19.3) |
| III | 179 (77) | III | 98 (42.1) | IIIa | 109 (46.7) |
| IV | 94 (40.3) | IIIb | 70 (30.2) | ||
dFLC, free light chain difference; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Survival
Variables independently associated with mortality
Following univariate and multivariate analysis of plasma cell markers, clone‐related characteristics, and cardiac and liver involvement, we identified independent variables for patient prognosis (Table 3 ). Unsurprisingly, NT‐proBNP, hsTnT, and cardiac output significantly predicted mortality on univariate analysis (Table 3 ). Interestingly, CB and cardiac output were also found to be independent predictors of mortality on both univariate and multivariate analyses. Furthermore, conditional tree analysis identified mortality cut‐off ranges in order of relative importance: hsTnT (107 ng/L), equivalent to 7.6 times higher than the normal range, NT‐proBNP (3867 ng/L), equivalent to 19 times the normal range and 4 μmol/L for CB, equivalent to 1.5 times the normal range (Figure 2 ). Neither serum creatinine, dFLC or GLS predicted mortality.
Table 3.
Prognostic factors determined by univariate and multivariate Cox analysis in AL cardiac amyloidosis
| Univariate Cox model | Multivariate Cox model | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age, years | 0.999 (0.999–1.000) | 0.018 | 0.9997 (0.9992–1.0002) | 0.238 |
| Log‐NT‐proBNP, ng/L | 1.36 (1.24–1.51) | <0.001 | 1.11 1.003–1.29) | 0.005 |
| Conjugated Bilirubin, μmol/L | 1.09 (1.05–1.12) | <0.001 | 1.07 (1.04–1.12) | <0.001 |
| Alkaline phosphatase, U/L | 1.0019 (1.0007–1.0031) | 0.002 | 1.0019 (0.9996–1.0041) | 0.101 |
| hsTnT, ng/L | 1.43 (1.29–1.52) | <0.001 | 1.27 (1.09–1.48) | 0.001 |
| dFLC, mg/L | 1.0001 (1.0000–1.0003) | 0.142 | 1.0001 (0.9999–1.0003) | 0.376 |
| CRP hs, mg/L | 1.1001 (1.0001–1.0030) | 0.086 | 1.003 (0.9866–1.120) | 0.121 |
| Serum creatinine, μmol/L | 1.0001 (1.0001–1.0012) | 0.045 | 1.0001 (0.789–1.004) | 0.343 |
| Cardiac flow index, mL/mn/m2 | 1.1200 (1.002–1.2004) | 0.0001 | 0.696 (0.529–0.916) | 0.008 |
| GLS % | 1.00001 (0.967–1.0001) | 0.234 | 0.967 (0.8670–1.003) | 0.453 |
GLS, global longitudinal strain; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Figure 2.
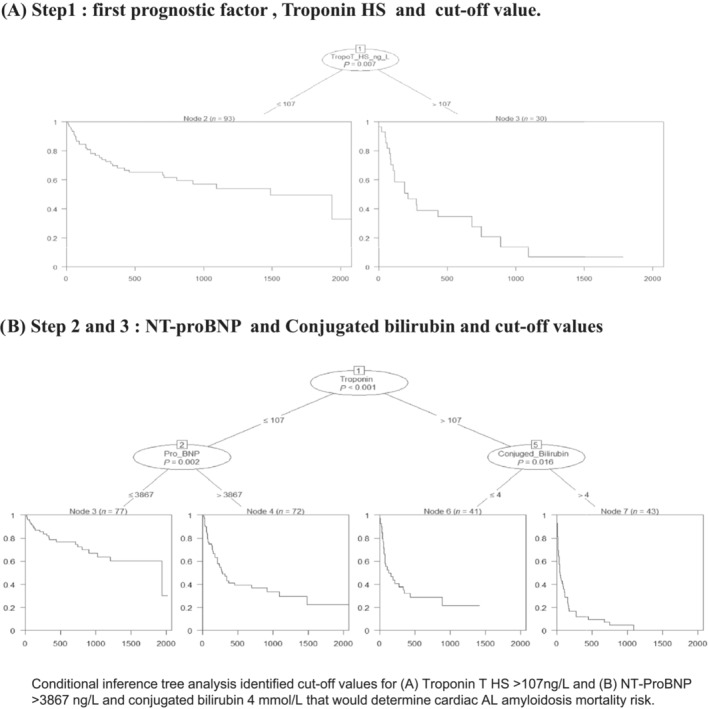
Order of prognostic biomarkers and cut‐off values for survival and cardiac transplantation. Conditional inference tree analysis identified cut off values for (A) troponin T HS > 107 ng/L and (B) NT‐proBNP > 3867 ng/L and conjugated bilirubin 4 μmol/L that would determine cardiac AL amyloidosis mortality risk.
Interestingly, a third of the total population had CB > 4 μmol/L. Among those, most (75%) had NYHA stage III‐IV heart failure and significantly higher cardiac biomarker levels (hsTnT and NT‐proBNP), CRP level, and lower cardiac function compared with those with CB ≤ 4 μmol/L (Table S1 ) Furthermore, the Pearson correlation between principal prognostic factors revealed that increased CB was associated with reduced cardiac output (−0.283, P = 0.0001). Also, hsTnT was significantly correlated with NT‐proBNP and CRP and inversely correlated with indexed cardiac output.
However, no correlation was observed between CB and hsTnT, NT‐proBNP or cardiac output or dFLC and cardiac or hepatic biological markers or cardiac output (Table S2 ).
Overall mortality and survival according to Mayo 2004, European and Mayo 2012 prognostic staging
The median overall survival in our cohort was 8 months (95% CI [2–24]). During follow‐up, 126 (53.2%) patients died and 9 (3.8%) received a heart transplant and the median overall time to death was 3 months (95% CI [1–9]) Among the survivors, the median survival was 20 months (95% CI [5–35] (Figure 3 A ).
Figure 3.

All‐cause mortality and cardiac transplant in the whole cohort, Mayo Clinic 2004 and European staging. Kaplan–Meier estimated survival curves among (A) the total cohort, n = 233; (B) patients with Mayo 2004 Stage 1 (n = 9), Stage 2 (n = 45), Stage 3 (n = 175) with a log rank test = 0.001; and (C) patients with European staging Stage 1 (n = 9), Stage 2 (n = 45), Stage 3a (n = 109), Stage 3b (n = 69) with a log rank test P = 0.012 that showed statistical significance between stages.
Concerning mortality risk according to staging system, multivariate Cox analysis revealed a significantly higher mortality risk among patients at 2004 Mayo Clinic stage 3 was at HR: 2.55 (1.56–4.15) P < 0.001 compared with stages 1 and 2 (Figure 4 A ). Also, survival was shorter for patients with Mayo 2004 stage 2 or 3. Mayo stage 2 (n = 48) patients survived a median of 45 months (95% CI [7–40]), and the 1 year survival rate was estimated at 69% (Figure 3 B ).
Figure 4.

Mortality and cardiac transplant risk varies according to Mayo 2004, European Staging and Mayo 2012 classifications. The results of the cox regression analysis comparing prognosis between staging systems, illustrated in forest plot (A) indicate a significantly higher mortality risk among patients with Mayo 2004 Stage 3 compared to Mayo 2004 Stage 1‐2. (P = 0.04) Similarly, forest plot (B) illustrates the mortality risk is three times higher among patients with European Stage 3b compared to stages 1‐2 (P < 0.001). Forest plot (C) indicates mortality and cardiac transplant.
Importantly, among those patients at European stage 3b, the mortality risk was three times higher HR: 5.12 (3.02–8.69) P < 0.001 than those with European stage 3a HR: 1.75 (1.04–2.94) P = 0.04 (Figure 4 B ). Among patients classified European stage 3a, the 1 year survival was markedly lower (59%) with a median survival of 14 months (95% CI [4–30]). Lastly, among those classified European stage 3b, the median survival was only 3 months (95% CI [0–5]), and only 18% survived 1 year (Figure 3 C ).
According to the 2012 modified Mayo stratification, the mortality risk was 1.5 times higher among those patients at stage 4 than 3 (Figure 4 C ). Concerning survival, those patients in Stages 1 and 2 (n = 38) had a positive prognosis with a median survival of 30 months, but the 95% confidence interval was very broad (5 to 54 months), the 1 year survival was 71%. Among patients in Stage 3, the 1 year survival was 54%. Lastly, among patients in Stage 4, the median survival was as low as 5 months (95% CI: 2–7) (Figure S2 ).
Developing a new staging system adapted to severe cardiac amyloidosis
Having identified CB > 4 μmol/L as an independent predictor of heart failure (NYHA III‐IV), mortality and lower cardiac output with marked remodelling in the AL‐CA cohort, a new risk stratification strategy was developed, the Mondor amyloidosis cardiac staging (MACS). This proposed risk strategy combined known prognostic variables hsTnT, NT‐proBNP with the newly identified CB. Each prognostic stage was allocated a step using decisional tree variables to stratify mortality stages as follows:
Stage 1: hsTnT≤107 ng/L and NT‐proBNP value ≤3867 ng/L (n = 77; 33%).
Stage 2: hsTnT≤107 ng/L and NT‐proBNP is >3867 ng/L (n = 72; 30%). If troponin was above 107 ng/L then CB is considered. Stage 3 was defined as CB ≤ 4 μmol/L (n = 41; 17.5%). Stage 4 was defined as CB > 4 μmol/L (n = 43; 18.5%) (Central Illustration).
When applied to the cohort, the number of patients identified with MACS stage 1 was 77 (33%), stage 2 was 72 (31%), stage 3 was 41 (18%), and stage 4 was 43 (18%) (Table S3 ).
Importantly, the MACS staging model identified a significantly higher mortality risk compared with either the European or Mayo scores. The hazard risk ratio at MACS stage 2 was 2.59 (1.53–4.37), P = 0.001 and increased to 4.48 (2.53–7.91) P = 0.001 in stage 3, and 8.46 (4.98–14.36), P = 0.0001 at Stage 4 (Figure 5 A ).
Figure 5.

All‐cause mortality and cardiac transplantation risk when reclassified using Mondor amyloidosis cardiac staging (MACS). The Cox regression analysis comparing prognosis between MACS stages, illustrated in forest plot (A) indicate a significantly higher mortality risk among patients with Stage 4 compared with Stage 3, Stage 2, and Stage 1. (B) The Kaplan–Meier estimated survival curves according to MACS stage with an overall log rank test of P = 0.001 shows statistical significance in mortality between all stages. (C) Among patients classified according to European and MACS stages, Mondor Stage 2 was found among severer European Stage IIIa and IIIb and MACS Stage 4 was found among less severe European Stage IIIa.
Concerning survival using MACS staging model and the Kaplan–Meier approach, median patient survival was not reached for stage 1, was 15.2 months for stage 2 (95% CI [11–18]), 6.6 months for stage 3 (95% CI [1–13]) and 2 months for stage 4 (95% CI [1–4]) (Figure 5 B ). At 1 year, estimated survival was 75% for stage 1, 52%, for stage 2, 39% for stage 3 and 12% for stage 4 (P = 0.002) (Figure 5 B ).
Comparison between Mondor amyloidosis cardiac staging and European stages and Mayo 2012
When patients were stratified with the European and the new MACS classification, all patients classified as European stage 1 were also classified as MACS 1 (Figure 5 C ). However, among those classified into European stage 2 and 3, we found patients with MACS severity ranging from 2 to 4. Particularly, among those with European stage 3a and 3b (Figure 5 C ).
Similarly, patients in stage 4 of the Mayo Clinic 2012 were distributed in different MACS stages: 15.8% in 1, 28.4% in 2, 26.1% in 3 and 29.5% in 4 (Figure S3 ).
Discussion
Using extensive data from the French national reference centre for amyloidosis, we identified an additional predictive biomarker for mortality or transplantation in patients with AL‐CA and new thresholds for current cardiac factors. Importantly, using these prognostic factors, we also developed a novel staging approach that stratifies AL‐CA patients with severe cardiac involvement more clearly. The predictive biomarkers identified were CB > 4 μmol/L, troponin and NT‐proBNP at thresholds higher than current prognostic models. However, dFLC and GLS were not predictive factors of mortality and cardiac transplant as previously described. 19 , 20
This is important for these high mortality risk patients who often die before starting therapy, excluding them from clinical trials thus making survival and therapy data rare or unavailable.
Conjugated bilirubin as prognostic factor
To our knowledge, this is the first study to report CB as an independent prognostic factor in AL‐CA. More importantly, CB > 4 μmol/L identified high‐risk AL‐CA patients and was positively correlated with troponin, NT‐proBNP, and negatively with cardiac output. This suggests CB increase may reflect the degree of cardiac failure. It is conceivable that CB reflects hepatic infiltration and amyloid right ventricular involvement, which is underestimated by the NT‐proBNP and troponin measures. Although total bilirubin has been studied in heart failure, little research is available concerning the prognostic value of total bilirubin in AL‐CA. Yet total bilirubin was not identified as a predictive factor in this study. Also, altered hepatic function in amyloidosis is multifactorial and complex but is principally caused by cardiac failure and hepatic amyloid infiltration. However, it is difficult to differentiate between these two pathophysiological mechanisms. 21 Additionally, secondary hepatic lesions occur from monoclonal light chain or associated fragment deposits and this may best be reflected by ALP levels. 22
This concurs with recent Chinese studies that found total bilirubin was independently associated with mortality. 23
A new staging system to improve prognostic stratification for patients with severe cardiac involvement
Having identified CB at a threshold of 4 μmol/L as a new prognostic factor, as well as new cardiac biomarker thresholds, we developed a new cardiac stratification model adapted to patients with moderate to severe AL‐CA (MACS).
These findings confirm the difficulty to define prognosis with the current prognostic scores and stratify patients with severe cardiac involvement with troponin, BNP/NT‐proBNP, and/or dFLC values (Mayo Clinic Staging III or European Staging IIIa and IIIb). 8 Moreover, the proposed MACS staging system identified severe patients who had been underestimated using the Mayo 2004 and European staging systems.
These observations concur with Ditrich, who found that the European classification predicted mortality more accurately than Mayo 2004 and Mayo 2012 in AL patients with functional renal failure and frequent cardiac complications. 24 , 25
New cardiac biomarker thresholds
Troponin T (hs‐cTnT) is a specific and sensitive cardiac toxicity marker, which reflects light chain injury on myocytes rather than amyloid infiltrate deposits and indicates progressive damage and survival impact. It is the strongest prognostic factor of mortality and cardiac transplantation in the AL‐CA population. 26 , 27 , 28 We found troponin was correlated to NT‐proBNP, cardiac output, and C‐reactive protein, confirming the hypothesis of myocyte and interstitial extracellular matrix reactivity, which increases static amyloid infiltration.
Although NT‐proBNP clearly reflects the degree of amyloid infiltration, increased filling pressure typical of classic heart failure, and may stratify at‐risk patients with AL‐CA, it also reflects the parietal stress and the direct effect of amyloid deposits in pericardiomyocytes. However, elevated NT‐proBNP is insufficient to evaluate heart failure severity alone, which is triggered by FLCs. 29
Additionally, we identified higher thresholds for troponin and NT‐proBNP biomarkers. For the NT‐proBNP, we found a threshold of 3867 ng/L, compared with other scores except for European stage IIIb (8500 ng/L). Also, troponin threshold was found to be higher compared to other risk stratification studies (107 ng/L vs. 50, 54, 77 ng/L). 26 , 27 , 30 Although these differences may be due to the severe cardiac characteristics in our population. More importantly, these thresholds were statistically determined using the conditional tree analysis, as opposed to cohort medians as other risk stratification studies have commonly used.
Direct free light chain involvement in severe light chain cardiac amyloidosis population
At a very advanced disease stage and especially once cardiac tissue is involved, FLCs are directly toxic for myocytes. Initially, FLCs cause oxidative stress and free radical release that dysregulate calcium homeostasis, causing cell lysis demonstrated by elevated troponin levels.
Intriguingly, we found dFLC did not statistically predict death in univariate or multivariate analysis. Considering our particularly severe cohort, possibly FLC levels may not be directly related to cardiac severity, but instead the light chain type, which causes cardiac toxicity and tropism. This could make FLC a secondary mortality factor. This hypothesis is supported by a prognostic study conducted by Usman et al. in 2019 on a severe AL‐CA cohort. 31
Measuring direct free light chains with N‐Latex technique may be useful for prognosis
We supposed that the non‐significant, dFLC predictive result may be due to different assay measurements to validate dFLC thresholds in previous scores. The original Mayo scores and other validation studies used the Freelite technique. However, we used a more recent, reproducible Freelite N‐Latex assay. 32 Although the Freelite method is used to measure dFLC in AL amyloidosis, to our knowledge, this is the first study to use N‐Latex technique to evaluate dFLC prognostic value. 33 Although both FLC assays are well correlated to detect abnormal light chain subtypes, they differ in absolute values and thus cannot be interchangeable. Therefore, Freelite cut‐off values are not equivalent to N‐Latex assay values for AL staging. So clinicians should consider using the same assay for follow‐up. 34
Although both testing systems are now used in diagnostic laboratories, the reference values and clinical cut‐offs are specific, 17 , 35 but clinical cut‐offs for N‐Latex are still needed for AL‐CA prognostic testing.
Study limits
This monocentric, retrospective study reflects the centre's specialized activity in cardiac amyloidosis. Although our cohort is relatively large, only 233 patients had N‐Latex assay results available for analysis. Also, we focused on current data without considering the follow‐up or a hematologic response to treatment.
We recognize that these results are based on retrospective data and therefore subject to inherent selection bias, lack of information and a centre effect. Therefore, before this model can be incorporated into clinical decision‐making particularly for Stage III patients, future work should confirm the predictive value of CB in the MACS stratification model with a prospective, replication cohort. Although we contacted our colleagues in similar reference centres, to replicate this work, unfortunately, conjugated bilirubin is not routinely collected in their centres.
Also, an additional right ventricular function study, anatomical liver pathology (biopsy) would be necessary to show if bilirubin reflects more a lower cardiac and hepatic output than amyloid hepatic involvement (or both). Histological liver injury has only been confirmed for 6% of the patients.
Conclusion
The new MACS prognostic staging system including hs‐troponin, NT‐proBNP, and conjugated bilirubin, with modified thresholds may improve prognostic staging for patients with AL‐CA and severe cardiac involvement. This model may be useful to adapt patient treatment and management according to disease and prognosis.
Conflict of interest
The authors have not conflict of interest or funding to declare.
Supporting information
Table S1: Pearson coefficient correlation between principal prognostic parameters in cardiac amyloidosis.
Table S2: Pearson coefficient correlation between principal prognostic parameters in cardiac amyloidosis.
Table S3: Comparison of clinical characteristics according to Mondor Amyloidosis Cardiac Staging
Acknowledgements
We thank all patients and physicians who are involved in the Mondor Amyloidosis Network for their continued dedication to caring for people living with amyloidosis. We also thank Amanda Whereat B.Sc. UNSW, Australia (Speak the Speech Consulting) for her assistance writing the manuscript.
Zaroui, A. , Kharoubi, M. , Gounot, R. , Oghina, S. , Degoutte, C. , Bezard, M. , Galat, A. , Guendouz, S. , Roulin, L. , Audard, V. , Leroy, V. , Teiger, E. , Poullot, E. , Molinier‐Frenkel, V. , Le Bras, F. , Belhadj, K. , Bastard, J.‐P. , Fellahi, S. , Shourick, J. , Lemonier, F. , and Damy, T. (2024) Prognostic mortality factors in advanced light chain cardiac amyloidosis: A prospective cohort study. ESC Heart Failure, 11: 1707–1719. 10.1002/ehf2.14671.
References
- 1. Kyle RA, Gertz MA. Primary systemic amyloidosis: Clinical and laboratory features in 474 cases. Semin Hematol 1995;32:45‐59. [PubMed] [Google Scholar]
- 2. Aurich M, Bucur J, Vey JA, Greiner S, Aus dem Siepen F, Hegenbart U, et al. Prognosis of light chain amyloidosis: A multivariable analysis for survival prediction in patients with cardiac involvement proven by endomyocardial biopsy. Open. Heart 2023;10:e002310. doi: 10.1136/openhrt-2023-002310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brenner DA, Jain M, Pimentel DR, Wang B, Connors LH, Skinner M, et al. Human amyloidogenic light chains directly impair cardiomyocyte function through an increase in cellular oxidant stress. Circ Res 2004;94:1008‐1010. doi: 10.1161/01.RES.0000126569.75419.74 [DOI] [PubMed] [Google Scholar]
- 4. Kumar S, Dispenzieri A, Katzmann JA, Larson DR, Colby CL, Lacy MQ, et al. Serum immunoglobulin free light‐chain measurement in primary amyloidosis: Prognostic value and correlations with clinical features. Blood 2010;116:5126‐5129. doi: 10.1182/blood-2010-06-290668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Milani P, Basset M, Russo F, Foli A, Merlini G, Palladini G. Patients with light‐chain amyloidosis and low free light‐chain burden have distinct clinical features and outcome. Blood 2017;130:625‐631. doi: 10.1182/blood-2017-02-767467 [DOI] [PubMed] [Google Scholar]
- 6. Staron A, Zheng L, Doros G, Connors LH, Mendelson LM, Joshi T, et al. Marked progress in AL amyloidosis survival: A 40‐year longitudinal natural history study. Blood Cancer J 2021;11:139. doi: 10.1038/s41408-021-00529-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Muchtar E, Gertz MA, Lacy MQ, Go RS, Buadi FK, Dingli D, et al. Ten‐year survivors in AL amyloidosis: Characteristics and treatment pattern. Br J Haematol 2019;187:588‐594. doi: 10.1111/bjh.16096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lilleness B, Ruberg FL, Mussinelli R, Doros G, Sanchorawala V. Development and validation of a survival staging system incorporating BNP in patients with light chain amyloidosis. Blood 2019;133:215‐223. doi: 10.1182/blood-2018-06-858951 [DOI] [PubMed] [Google Scholar]
- 9. Palladini G, Hegenbart U, Milani P, Kimmich C, Foli A, Ho AD, et al. A staging system for renal outcome and early markers of renal response to chemotherapy in AL amyloidosis. Blood 2014;124:2325‐2332. doi: 10.1182/blood-2014-04-570010 [DOI] [PubMed] [Google Scholar]
- 10. Wechalekar AD, Schonland SO, Kastritis E, Gillmore JD, Dimopoulos MA, Lane T, et al. A European collaborative study of treatment outcomes in 346 patients with cardiac stage III AL amyloidosis. Blood 2013;121:3420‐3427. doi: 10.1182/blood-2012-12-473066 [DOI] [PubMed] [Google Scholar]
- 11. Kourelis TV, Kumar SK, Gertz MA, Lacy MQ, Buadi FK, Hayman SR, et al. Coexistent multiple myeloma or increased bone marrow plasma cells define equally high‐risk populations in patients with immunoglobulin light chain amyloidosis. J Clin Oncol 2013;31:4319‐4324. doi: 10.1200/JCO.2013.50.8499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Colby C, et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol 2012;30:989‐995. doi: 10.1200/JCO.2011.38.5724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bezard M, Oghina S, Vitiello D, Kharoubi M, Kordeli E, Galat A, et al. Dexamethasone is associated with early deaths in light chain amyloidosis patients with severe cardiac involvement. PLoS ONE 2021;16:e0257189. doi: 10.1371/journal.pone.0257189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Béquignon E, Guellich A, Bartier S, Raynal M, Prulière‐Escabasse V, Canouï‐Poitrine F, et al. How your ears can tell what is hidden in your heart: Wild‐type transthyretin amyloidosis as potential cause of sensorineural hearing loss inelderly‐AmyloDEAFNESS pilot study. Amyloid 2017;24:96‐100. doi: 10.1080/13506129.2017.1330744 [DOI] [PubMed] [Google Scholar]
- 15. Zoghbi WA, Adams D, Bonow RO, Enriquez‐Sarano M, Foster E, Grayburn PA, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: A report from the american society of echocardiography developed in collaboration with the society for cardiovascular magnetic resonance. J Am Soc Echocardiogr 2017;30:303‐371. doi: 10.1016/j.echo.2017.01.007 [DOI] [PubMed] [Google Scholar]
- 16. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233‐271. doi: 10.1093/ehjci/jev014 [DOI] [PubMed] [Google Scholar]
- 17. Palladini G, Jaccard A, Milani P, Lavergne D, Foli A, Bender S, et al. Circulating free light chain measurement in the diagnosis, prognostic assessment and evaluation of response of AL amyloidosis: Comparison of Freelite and N Latex FLC assays. Clin Chem Lab Med 2017;55:1734‐1743. doi: 10.1515/cclm-2016-1024 [DOI] [PubMed] [Google Scholar]
- 18. Hothorn T, Hornik K, Zeileis A. Unbiased recursive partitioning: A conditional inference framework. J Comput Graph Stat 2006;15:651‐674. doi: 10.1198/106186006X133933 [DOI] [Google Scholar]
- 19. Bak M, Kim D, Choi JO, Kim K, Kim SJ, Jeon ES. Prognostic implication of longitudinal changes of left ventricular global strain after chemotherapy in cardiac light chain amyloidosis. Front Cardiovasc Med 2022;9:904878. doi: 10.3389/fcvm.2022.1001753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barros‐Gomes S, Williams B, Nhola LF, Grogan M, Maalouf JF, Dispenzieri A, et al. Prognosis of light chain amyloidosis with preserved LVEF: Added value of 2D speckle‐tracking echocardiography to the current prognostic staging system. JACC Cardiovasc Imaging 2017;10:398‐407. doi: 10.1016/j.jcmg.2016.04.008 [DOI] [PubMed] [Google Scholar]
- 21. Slivnick J, Zareba KM, Varghese J, Truong V, Wallner AL, Tong MS, et al. Prevalence and haemodynamic profiles of pulmonary hypertension in cardiac amyloidosis. Open Heart 2022;9:e001808. doi: 10.1136/openhrt-2021-001808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xu J, Qiu Z, Yan M, Wang B, Song Z, Liu H, et al. Prognostic factors of AL‐PCMM and AL‐MM: A single‐center retrospective study. Int J Med Sci 2022;19:588‐595. doi: 10.7150/ijms.61712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang L‐l, Shen K‐n, Zhang C‐l, Qiu Y, Miao H‐l, Feng J, et al. Clinical presentation and prognostic analysis of Chinese patients with systemic light chain amyloidosis with liver involvement. Leuk Res 2019;86:106226. doi: 10.1016/j.leukres.2019.106226 [DOI] [PubMed] [Google Scholar]
- 24. Dittrich T, Kimmich C, Hegenbart U, Schonland SO. Prognosis and staging of AL amyloidosis. Acta Haematol 2020;143:388‐400. doi: 10.1159/000508287 [DOI] [PubMed] [Google Scholar]
- 25. Al Saleh AS, Parmar HV, Vaxman I, Visram A, Hasib Sidiqi M, Muchtar E, et al. Prognostic value of NT‐proBNP and troponin T in patients with light chain amyloidosis and kidney dysfunction undergoing autologous stem cell transplantation. Bone Marrow Transplant 2021;56:274‐277. doi: 10.1038/s41409-020-0990-6 [DOI] [PubMed] [Google Scholar]
- 26. Dispenzieri A, Gertz MA, Kumar SK, Lacy MQ, Kyle RA, Saenger AK, et al. High sensitivity cardiac troponin T in patients with immunoglobulin light chain amyloidosis. Heart 2014;100:383‐388. doi: 10.1136/heartjnl-2013-304957 [DOI] [PubMed] [Google Scholar]
- 27. Kristen AV, Giannitsis E, Lehrke S, Hegenbart U, Konstandin M, Lindenmaier D, et al. Assessment of disease severity and outcome in patients with systemic light‐chain amyloidosis by the high‐sensitivity troponin T assay. Blood 2010;116:2455‐2461. doi: 10.1182/blood-2010-02-267708 [DOI] [PubMed] [Google Scholar]
- 28. Apridonidze T, Steingart RM, Comenzo RL, Hoffman J, Goldsmith Y, Bella JN, et al. Clinical and echocardiographic correlates of elevated troponin in amyloid light‐chain cardiac amyloidosis. Am J Cardiol 2012;110:1180‐1184. doi: 10.1016/j.amjcard.2012.05.061 [DOI] [PubMed] [Google Scholar]
- 29. Nordlinger M, Magnani B, Skinner M, Falk RH. Is elevated plasma B‐natriuretic peptide in amyloidosis simply a function of the presence of heart failure? Am J Cardiol 2005;96:982‐984. doi: 10.1016/j.amjcard.2005.05.057 [DOI] [PubMed] [Google Scholar]
- 30. Palladini G, Barassi A, Klersy C, Pacciolla R, Milani P, Sarais G, et al. The combination of high‐sensitivity cardiac troponin T (hs‐cTnT) at presentation and changes in N‐terminal natriuretic peptide type B (NT‐proBNP) after chemotherapy best predicts survival in AL amyloidosis. Blood 2010;116:3426‐3430. doi: 10.1182/blood-2010-05-286567 [DOI] [PubMed] [Google Scholar]
- 31. Tahir UA, Doros G, Kim JS, Connors LH, Seldin DC, Sam F. Predictors of mortality in light chain cardiac amyloidosis with heart failure. Sci Rep 2019;9:8552. doi: 10.1038/s41598-019-44912-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Velthuis HT, Knop I, Stam P, van den Broek M, Bos HK, Hol S, et al. N Latex FLC ‐ New monoclonal high‐performance assays for the determination of free light chain kappa and lambda. Clin Chem Lab Med 2011;49:1323‐1332. doi: 10.1515/CCLM.2011.624 [DOI] [PubMed] [Google Scholar]
- 33. Bochtler T, Hegenbart U, Cremer FW, Heiss C, Benner A, Hose D, et al. Evaluation of the cytogenetic aberration pattern in amyloid light chain amyloidosis as compared with monoclonal gammopathy of undetermined significance reveals common pathways of karyotypic instability. Blood 2008;111:4700‐4705. doi: 10.1182/blood-2007-11-122101 [DOI] [PubMed] [Google Scholar]
- 34. Mahmood S, Wassef NL, Salter SJ, Sachchithanantham S, Lane T, Foard D, et al. Comparison of free light chain assays: Freelite and N Latex in diagnosis, monitoring, and predicting survival in light chain amyloidosis. Am J Clin Pathol 2016;146:78‐85. doi: 10.1093/ajcp/aqw079 [DOI] [PubMed] [Google Scholar]
- 35. Henriot B, Rouger E, Rousseau C, Escoffre M, Sébillot M, Bendavid C, et al. Prognostic value of involved/uninvolved free light chain ratio determined by Freelite and N Latex FLC assays for identification of high‐risk smoldering myeloma patients. Clin Chem Lab Med 2019;57:1397‐1405. doi:10.1515/cclm‐2018‐1369 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Pearson coefficient correlation between principal prognostic parameters in cardiac amyloidosis.
Table S2: Pearson coefficient correlation between principal prognostic parameters in cardiac amyloidosis.
Table S3: Comparison of clinical characteristics according to Mondor Amyloidosis Cardiac Staging


