Abstract
Percutaneous coronary intervention (PCI) addresses myocardial ischaemia, but a significant subset of patients encounter major adverse cardiovascular events (MACE) post‐treatment. This meta‐analysis investigated the relationship between the post‐PCI triglyceride‐glucose (TyG) index and MACE. Comprehensive searches of the Embase, PubMed, Cochrane Library, and Web of Science databases were conducted up to 3 March 2023, using relevant keywords. The effect size was determined based on I 2 statistic using random‐effects models. Cluster‐robust standard errors crafted the dose–response curve, and the GRADE Evaluation Scale was employed to rate the quality of evidence. The group with the highest TyG index had significantly higher post‐PCI MACE rates than the lowest index group, with hazard ratios (HRs) of 2.04 (95% CI 1.65–2.52; I 2 = 77%). Each unit increase in TyG index corresponded to HRs of 1.82 for MACE (95% CI 1.34–2.46; I 2 = 92%), 2.57 for non‐fatal MI (95% CI 1.49–4.41; I 2 = 63%), and 2.06 for revascularization (95% CI 1.23–3.50; I 2 = 90%). A linear relationship between TyG index and MACE risk was established (R 2 = 0.6114). For all‐cause mortality, the HR was 1.93 (95% CI 1.35–2.75; I 2 = 50%), indicating a higher mortality risk with elevated TyG index. The GRADE assessment yielded high certainty for non‐fatal MI but low certainty for all‐cause mortality, revascularization, and MACE. The TyG index may predict risks of post‐PCI MACE, all‐cause mortality, non‐fatal MI, and revascularization, with varied levels of certainty. A potential linear association between the TyG index and MACE post‐PCI was identified. Future research should validate these findings.
Keywords: Adverse cardiovascular events, Non‐fatal myocardial infarction, Percutaneous coronary intervention, Revascularization, Triglyceride‐glucose index
Introduction
Atherosclerosis cardiovascular disease (ASCVD), which primarily consists of ischaemic heart disease and ischaemic stroke, is the primary cause of death in China, and its prevalence and mortality rate are rapidly increasing. 1 For ASCVD, smoking, cholesterol, hypertension, abnormal coagulation, and diabetes are regarded as risk factors. 2 Pathologically, T2DM (diabetes mellitus type 2) is caused by insulin resistance. However, new research has shown that ASCVD risk factors such as dyslipidaemia, hypertension, and irregular blood clotting can develop in T2DM patients with insulin resistance. 3 , 4 , 5 Ty index is a novel index that is used to measure not only insulin resistance, but also vascular damage, such as subclinical atherosclerosis, 6 coronary artery disease severity, 7 subclinical cerebral small vessel disease, 8 and nephric microvascular damage. 9
The PCI is an essential treatment for myocardial ischaemia. Major adverse cardiovascular events (MACE) nevertheless occur in more than one‐fourth of those who receive PCI afterward. 10 According to studies, higher triglyceride‐glucose (TyG) index is associated with an increased risk of MACE following PCI, which comprises all‐cause mortality, revascularization, non‐fatal myocardial infarction (MI), and ischaemic stroke. 11 , 12 According to Guo et al., elevated TyG index increases the risk of revascularization and in‐stent restenosis following PCI. 13 Regarding arrhythmias, individuals with ST‐elevation myocardial infarction who undergo PCI have an elevated risk of developing new‐onset atrial fibrillation due to their high TyG index. 14 For individuals with secondary mitral regurgitation following PCI, Huang et al. found that high TyG index is an independent and significant risk factor for worsening heart failure. 15
This study summarized the relationship between the TyG index and MACE risk in post‐PCI population to provide the low‐cost, practicable, and reliable tool for predicting PCI prognosis so that high‐risk individuals can be managed and treated.
Methods
PROSPERO (International Prospective Register of Systematic Reviews) received the protocol with registration number CRD42023387411, available online at https://www.crd.york.ac.uk/prospero/. Without major changes, this investigation was carried out primarily under the registration plan. It was conducted in accordance with PRISMA 2020 statement (Table S1 ), an updated standard for reporting systematic reviews.
Literature search
This article searched the Cochrane Library, PubMed, Embase, and Web of Science databases until 3 March 2023, regardless of language restrictions. (1) Exposure factors and ‘triglyceride‐glucose index’ are current search queries. ‘TyG index’ ‘triglyceride and glucose index’ ‘triacylglycerol glucose index’; (2) population studied for ‘Coronary Intervention, Percutaneous’ ‘Coronary Percutaneous Interventions’ ‘Percutaneous Coronary Interventions’ ‘Percutaneous Coronary Revascularizations’ ‘Coronary Revascularization, Percutaneous’ ‘Coronary Revascularizations, Percutaneous’ ‘Intervention, Percutaneous Coronary’ ‘Revascularization, Percutaneous Coronary’ ‘Coronary Revascularizations Percutaneously’ ‘percutaneous coronary intervention’ ‘Coronary Percutaneous Revascula’‘Percutaneous Interventions in the Heart’. No restrictions were placed on endpoints or study methodologies in order to find all relevant studies. This investigation's precise search methodology is detailed in Table S2 .
Literature screening criteria
Using Endnote X9 (Thomson Reuters, New York, NY, USA), the literature was organized in accordance with our inclusion and exclusion criteria. Initially, duplicate literature was removed. Then, the literature germane to the topic of our research was screened based on the abstract and title, and a full‐text copy was obtained for a thorough analysis.
Population, intervention, comparison, outcome, and research strategy (PICOS) was used to determine inclusion and exclusion criteria for the current study. The inclusion criteria were as follows: (1) adults (18 years old) after PCI; (2) the study population is Chinese, or the research centre is located in China; (2) studies on TyG index as an exposure factor; (3) outcome indicators: MACE; (4) cohort study design; and (5) available adjusted hazard ratios (HR) from multivariable Cox regression models with 95% confidence intervals (CI).
Studies involving minors (under the age of 18) and those that met the inclusion criteria but posed a substantial risk of bias were excluded, respectively (e.g., follow‐up time 1 year and number of cases 500); three articles lacking study data (e.g., conference proceedings, reviews, and preclinical studies); four studies unrelated to the research topic, and if the same population was possibly involved in different research, we selected the study with the most data or the longest available time frame. 15
Literature screening, extraction of data, and assessment of quality
Two authors (C. X. Sun and J. Y. Zhang) independently conducted a literature review. For the purpose of reaching a consensus in the event of disagreements, a conversation was held. If no consensus could be reached, a third author (D. X. Li) made the decision.
Additionally, two authors (C. X. Sun and J. Y. Zhang) independently extracted the data. The title of the first author, the year of publication, the journal, the location of the research, the number of participants, the length of follow‐up, the main clinical characteristics of each patient, the adjusted variables, the TyG index, and the 95% confidence intervals for HR and MACE were all noted. Two authors (C. X. Sun and D. X. Li) evaluated the standard of their writing using the Newcastle‐Ottawa Scale (NOS), with higher scores indicating a higher quality study. The range of the score is 0 to 9. Those with a NOS score of 6 were considered to be of high quality. 16
Statistical analysis
The statistical analysis was conducted with RevMan software, version 5.4 from Cochrane Collaboration 2014, Nordic Cochrane Centre Copenhagen, Denmark. As reference metrics, HR and their associated 95% CI were utilized to ascertain the relationship between TyG index and MACE in post‐PCI group. 17
We compared the greatest and lowest categories using TyG index after categorizing the data into various groups. In addition, we analysed TyG index as the continuous variable and normalized it by its unit (per 1 unit). Using Greenland S.’s method, we generated linear trends with 95% CI and evaluated the impact magnitude and 95% CI using the natural logarithm of the TyG index category. 18 For nonlinear dose–response study reported by Xu and Doi, we analysed data with I 2 > 50% using a robust error meta‐regression approach and a random‐effects model to increase reliability. 19 , 20 This procedure interprets each study as the subset of total sample and employs a ‘one‐stage approach.’ Cluster‐Robust Standard Errors are utilized to analyse intercorrelation. These methods are only pertinent to quantitative exposure factor research and require at least two data sets containing TyG index levels, HR, and HR variance. For research lacking TyG index figures, we used the median or mean of the cohort. In cases where neither the median nor the mean TyG index for a group is available, we determined the midpoint of each group by averaging the upper and lower bounds. For open groups, we assume that the length of the open interval is equivalent to the length of the adjacent interval. However, if there are only two open groups, this method cannot be used in the literature review. We use I 2 testing to assess heterogeneity, where an I 2 value of less than 25% indicates no heterogeneity and I 2 values of 26–50%, 51–75%, and over 75%, respectively, represent low, medium, and high heterogeneity. The sensitivity analysis involves systematically omitting each study. This article defines a statistically significant difference as P < 0.05.
Evidence quality evaluation
We grade each result according to the Grading of Recommended Suggestion, Evaluation, Development, and Evaluation (GRADE) method, 21 which determines the level of quality and intensity of every result. For every result, C.X. Sun and X.Y. Li independently rated the strength of the supporting evidence. Have applied the GRADE profiler software to generate an evidence analysis table. Our report includes the measure of each outcome and footnotes supporting any decisions that impacted the quality of evidence positively or negatively.
Results
Literature screening
Figure 1 illustrates our method for filtering search results. In total, 133 publications were found using our search method (PubMed = 31, Embase = 34, Cochrane Library = 24, and Web of Science = 44), 60 of which were duplicates. We reviewed the remaining 73 articles after deleting the duplicates. We determined that 49 of them were unrelated to our study based on their titles and abstracts. The remaining 24 papers were reviewed in full text after that. Fifteen of them were eliminated for the ensuing causes: article types with no study data (n = 3), inappropriate study design (n = 2), studies without appropriate outcome measures (n = 2), and overlapping or duplicate studies with those already included (n = 8). In the end, a total of nine cohort studies 11 , 12 , 15 , 22 , 23 , 24 , 25 , 26 , 27 were included, and no randomized controlled trials were included. Table S3 presents the justifications for leaving out the research.
Figure 1.
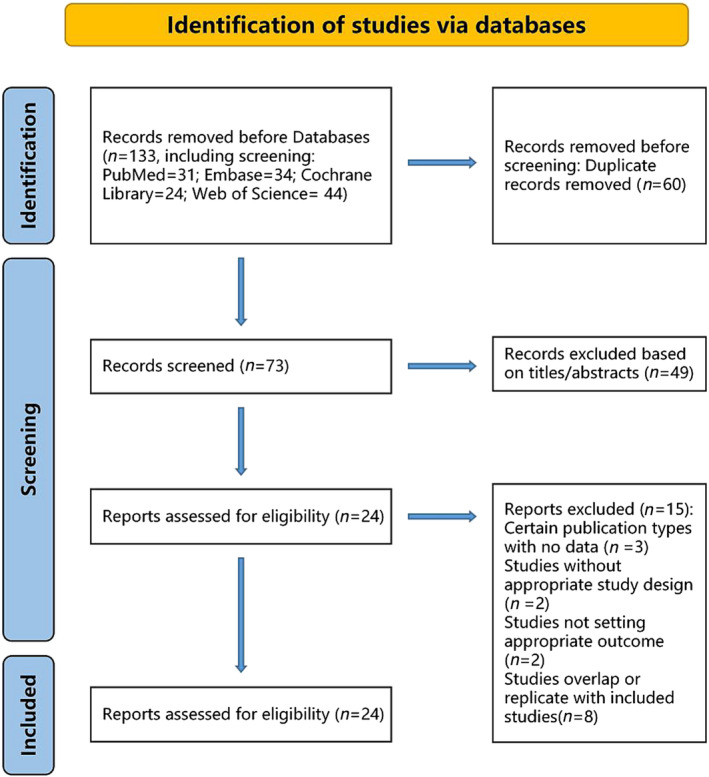
A flowchart for the link among the TyG index and risk of MACE following PCI in a meta‐analysis research. MACE, major adverse cardiovascular events; PCI, percutaneous coronary intervention; TyG, triglyceride‐glucose.
Study characteristics and quality assessment
All of the articles featured are listed in Table S1 . With sample sizes ranging from 515 to 2055 and follow‐up periods longer than 12 months, the 10 164 patients in these nine papers, which were published between 2018 and 2023, were all included. There were four research centers located in Beijing, two in Zhengzhou, one in Nanjing, one in Guangzhou, and one in Chengdu. Each of the nine publications discussed MACE, with three of them focusing independently on all‐cause mortality, non‐fatal MI, and revascularization and five articles including stroke in their definition of MACE. The definition of MACE varied slightly among the articles, as detailed in Table S4 . As all research centers were in China, we assumed that all included patients were Chinese.
All the articles had scores greater than or equal to 6, indicating that they were of high quality. All the articles adjusted for HR, but the specific methods varied among the articles, as detailed in Table S5 . All studies have made adjustments for various confounding variables. The correlation between TyG index and post‐PCI MACE incidence.
Major adverse cardiovascular events
The study incorporated nine observational studies with a total of 10 164 participants to determine the relationship between MACE and the TyG index. Compared with individuals in the group with the lowest TyG index, those in the group with the highest TyG index demonstrated a significantly higher risk of post‐PCI MACE as illustrated in Figure 2 A . Subgroup analysis outcomes based on varying definitions of MACE are presented in Figure S1 . Although two studies acknowledged baseline differences concerning smoking habits and BMI/obesity, these factors were not controlled for in their respective analyses. However, the omission of studies that did not account for smoking and BMI/obesity did not yield a notable difference. Among the five studies with the collective participant count of 7025, effect of the 1 standard unit increment in TyG index on MACE risk was assessed. The comprehensive dose–response analysis indicated that each unit rise in the TyG index augments the likelihood of post‐PCI MACE by 82%, as depicted in Figure 2 B . A strong positive linear correlation was found between TyG index and risk of MACE, with an R 2 value of 0.6114 (see Figure 2 C ). The HR values derived from the linear exposure analysis for specific TyG index measurements are detailed in Table S6 .’
Figure 2.
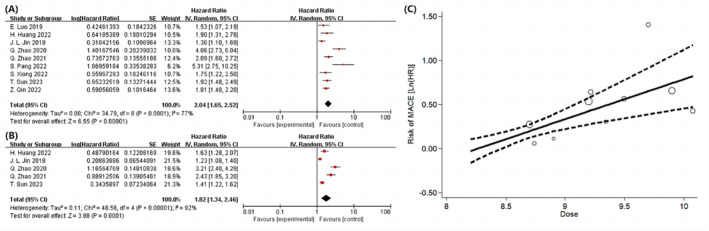
Following PCI, the association among the TyG index and MACE in the population was analysed using a forest plot, a dose–response curve, and category variables for the greatest and lowest values (A) and per 1 standard unit (B). MACE, major adverse cardiovascular events; PCI, percutaneous coronary intervention; TyG, triglyceride‐glucose.
Patients without/with diabetes
(P < 0.0001; HR = 2.28; 95% CI 1.58–3.03; I 2 = 78%), based on a meta‐analysis of 4 cohort studies involving 3122 individuals 22 , 24 , 25 (Figure 3 A ).
Figure 3.
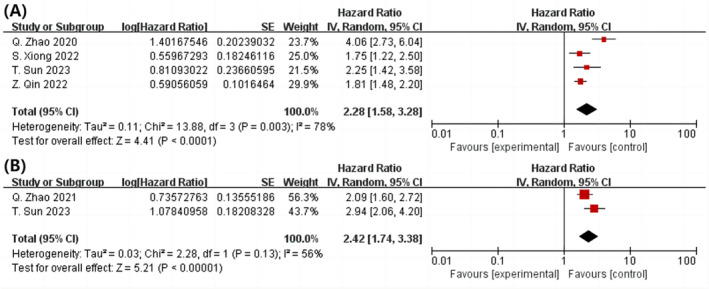
In the post‐PCI population with diabetes, a forest plot illustrating the connection between the TyG index and MACE is shown. With (A) or without diabetes (B) (highest vs. lowest) were examined as category variables. MACE, major adverse cardiovascular events; PCI, percutaneous coronary intervention; TyG, triglyceride‐glucose.
(P < 0.00001; HR = 2.42; 95% CI 1.74–3.03; I 2 = 56%), based on data from two cohort studies and 2773 patients 11 , 25 (Figure 3 B ).
Triglyceride‐glucose index and the frequency of a single adverse event are correlated
All‐cause death
In the three studies, examining the highest and lowest TyG index, there were a total of 4363 participants 11 , 24 , 25 (P = 0.0003, HR = 1.93; 95% CI = 1.35–2.75; I 2 = 50%). A greater TyG index was associated with increased mortality risk from all causes following PCI, as shown in Figure 4 A . Despite this, the total dose–response analysis (P = 0.92) did not reveal the statistically significant correlation between increase of 1 unit in TyG index and risk of mortality from all causes following PCI.
Figure 4.
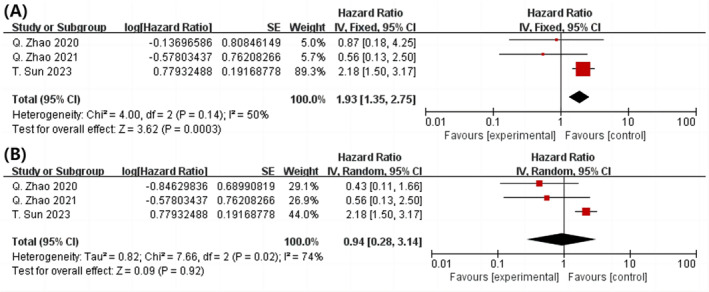
Following PCI, a forest plot shows the connection between the TyG index and all‐cause mortality in the population, analysed as highest and lowest category variables (A) and per 1 standard unit (B). PCI, percutaneous coronary intervention; TyG, triglyceride‐glucose.
Non‐fatal MI
Three cohort studies were analysed, and results revealed that those with the highest TyG index had a significantly greater risk of nonfatal MI than those with the lowest TyG index (P = 0.001, HR = 2.02, 95% CI: 1.33–3.10, I 2 = 0%) (Figure 5 A ). 11 , 24 , 25 The complete dose–response analysis revealed that 1‐unit increase in TyG index was associated with 157% increase in the incidence of nonfatal MI after PCI (P = 0.0007; HR = 2.57; 95% CI 1.49–4.41; I 2 = 63%) (Figure 5 B ). As shown in Figure S3A , the TyG index and risk of nonfatal MI were also found to have a significant positive and linear relationship (R 2 = 0.6986). The linear exposure impact of the selected TyG index values is evaluated, and the associated HR estimates are tabulated. The data for this investigation came from the linear indicator shown in Table S6 .
Figure 5.
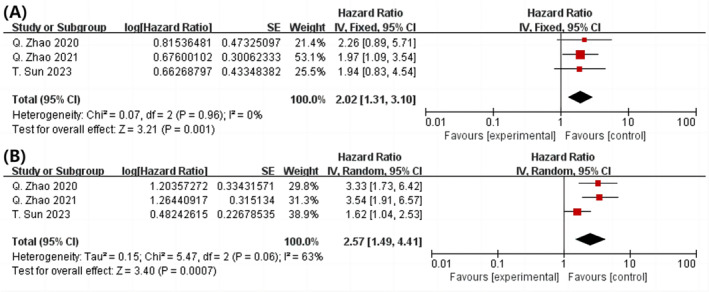
After PCI, a forest plot shows the connection among the TyG index and non‐fatal MI in the population, analysed as highest and lowest category variables (A) and per 1 standard unit (B). Myocardial infarction (MI), percutaneous coronary intervention (PCI), and triglyceride‐glucose (TyG) are all abbreviations.
Revascularization
According to the three cohort studies, we examined ta individuals with the highest TyG index were at a greater risk for revascularization than those with the lowest TyG index 11 , 24 , 25 (P = 0.001, HR = 2.61, 95% CI = 1.47–4.66, I 2 = 84%) (P = 0.001; HR = 2.61; 95% CI = 1.47 to 4.66; I 2 = 84%) (Figure 6 A ). According to our overall dose–response analysis, the risk of post‐PCI revascularization increased by 106% for each 1 unit increase in TyG index (P = 0.008; HR = 2.06; 95% CI: 1.23–3.50, I 2 = 90%) (Figure 6 B ). The correlation between the TyG index and the probability of revascularization was positive and linear (R 2 = 0.4927), as shown in Figure S3B . Table S6 lists selected TyG index values and the dose–response curve HR estimate values corresponding to those values.
Figure 6.
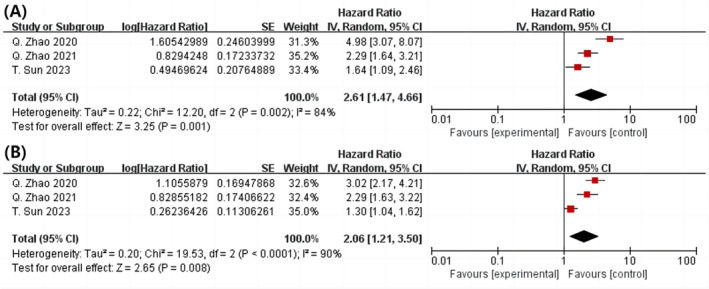
Analysed as category variables greatest and lowest, a forest plot showing the connection among the TyG index and revascularization in the population following PCI (A) and per 1 standard unit (B). PCI, percutaneous coronary intervention; TyG, triglyceride‐glucose.
Subgroup analysis sensitivity analysis and publication bias
The results of the conducted sensitivity analysis, in which each study was systematically eliminated, were uniformly consistent (Figure S4 ). Due to the small number of included studies (n < 10), this investigation was unable to conduct publication bias and subgroup analyses according to established guidelines.
Assessment of quality
The GRADE system was used to evaluate the validity of evidence. Due to their dose–response relationship, the outcomes of MACE and individual maladies are anticipated to improve. Due to the greater heterogeneity observed in MACE (I 2 = 77%) and revascularization (I 2 = 84%), their respective ratings have been downgraded. In conclusion, among the four outcomes considered, only nonfatal MI is supported by strong evidence, whereas all‐cause mortality and revascularization have less certainty. On the other hand, MACE has been determined to have a low likelihood (Tables S7 and S8 ).
Discussion
TyG index, which is essentially a composite statistic that takes into account both triglycerides and fasting glucose. The index of TyG has received considerable consideration as a biomarker of insulin resistance in diverse ethnic communities due to its simplicity of use, rapidity, and non‐invasiveness. 17 , 28 As insulin resistance can substantially increase the risk of MACE, TyG index is widely acknowledged as a reliable measure of it. 29 The specificity and sensitivity of the TyG score for the diagnosis of impaired fasting glucose in non‐obese populations are 66.2% and 69.1%, respectively. 30 In a study involving Chinese participants and a mean follow‐up period of 9 years, the TyG index predicted the incidence of T2DM better than the homeostasis model evaluation of insulin resistance (HOMA‐IR). 31 The Developing Factors in Risk Collaboration study, published in ‘The Lancet’, found minor correlation between fasting blood glucose levels and the risk of MACE, and diabetes has been shown to double this risk. 32
According to Sajdeya et al., TyG index can serve as an independent predictor of subclinical atherosclerosis and arterial stiffness risk. 33 Several meta‐analyses that have been conducted to date indicate that the TyG index is a predictor of the likelihood of developing coronary artery disease. 34 , 35 A higher TyG index is independently associated with the development of heart failure in the general population, according to reports based on Mendelian randomization analysis and meta‐analysis. 36 Yang et al. included a total of 592 616 individuals in their meta‐analysis and discovered the correlation between TyG index and ischaemic stroke in the general population. 37 In numerous large‐scale clinical observational studies, it has been shown that the incidence of post‐PCI MACE and TyG index are positively correlated. 38 , 39 , 40
There is a correlation between TyG index and prevalence of MACE in post‐PCI populations; however, results of these studies have been inconsistent. After adjusting for age, sex, previous stroke, hypertension, previous PCI, previous MI, and previous CABG, Hu et al. found a significant relationship between the TyG index and post‐PCI MACE incidence. 39 Yang et al. did not find a correlation between TyG index and incidence of post‐PCI MACE (P < 0.05), 41 despite employing the same definition of MACE as Hu et al. and making the same significant modifications. It is essential to interpret the variations in these results with caution. In addition, the TyG index can be utilized in investigations of either acute or chronic coronary artery disease to predict the frequency of post‐PCI MACE. According to research by Shao et al., the TyG index outperformed other indices in predicting the risk of post‐PCI MACE in patients with acute coronary syndrome. 42 According to Lin et al.'s study, the TyG index may be a predictor of post‐PCI MACE risk in T2DM patients with chronic complete coronary artery occlusion, whereas the TyG index had a multiplicative effect on the baseline risk model's capacity to predict MACE. 40 Several studies have revealed that the TyG index and PCI prognosis have a poor correlation. For example, after the implantation of drug‐eluting stents in patients with acute coronary syndrome, an increase in the TyG index was independently and positively associated with in‐stent restenosis. 43
BMI, which represents levels of obesity, is a significant influencing factor. The TyG index is calculated using fasting blood sugar and triglycerides and is affected by body mass index and smoking. According to Forouhi et al., weight reduction can delay or prevent cardiovascular events. 44 T2DM is closely associated with overweight or obesity and insulin resistance. Considering that over 80% of obese or overweight individuals have hypertriglyceridaemia, the journal recommends that obesity is one of the most significant risk factors for secondary hypertriglyceridemia. 45 Nearly all MACE risk variables are positively influenced by adiposity, which also has negative effects on cardiovascular structure and function. Nearly all overweight or obese individuals have a higher risk of cardiovascular disease compared with normal‐weight individuals. 46 Consequently, the relationship between BMI and obesity substantially influences both the TyG index and the incidence of MACE. In addition, we found that two of the studies showed statistically significant differences in BMI and obesity between groups, but neglected to account for these variables in their multivariate analyses. 23 , 47 Nevertheless, our findings indicate that even after excluding studies that did not compensate for this factor, the risk of developing post‐PCI MACE is still associated with TyG index. There was no evaluation of weight‐specific relationships because so few papers were included, which calls for additional research.
Cigarette smoking is a major confounding factor. In China, 68% of men and 3% of women are regular users, respectively. 48 Variations in fasting blood glucose and triglyceride levels cause fluctuations in the TyG index. Smokers have a higher risk of developing T2DM than non‐smokers, according to a meta‐analysis involving more than 6 million individuals. 49 Moreover, smoking is significant lifestyle risk associated with hypertriglyceridemia, 45 whose mechanism is characterized by its complexity and potential association with lipid‐related genetic locus alterations due to smoking. 50 Additional research has demonstrated that smoking is positively associated with an increase in the sovereign risk index TyG. 51 In addition, it is well‐known that smoking is a major risk factor for MACE, leading to its increased incidence and long‐lasting effects. Using Mendelian randomization, Larsson et al. established a strong causal relationship between smoking and various MACEs, including coronary artery disease. 52 Subsequent research has shown that quitting smoking can reduce the incidence of stroke. 53 As a separate risk factor for both TyG index and MACE, cigarette consumption poses a double threat. Interestingly, many studies suggest that the TyG index can also reflect MACE risk beyond smoking, whether in the general population or patients undergoing PCI. 42 , 54 The incidence of TyG index and post‐PCI MACE is affected by smoking. Two of the included studies failed to account for statistical differences in smoking between groups. 22 , 25 Excluding these two trials revealed a significant correlation between the TyG index and post‐PCI MACE risk. Due to the limited purview of the research, smoking‐specific correlations were not evaluated in this study; therefore, future research is necessary.
As the study population consisted of individuals who had undergone PCI, it was inevitable that those with a history of MACE, particularly those with coronary artery disease, were included. According to previous meta‐analyses, the TyG index and the incidence of MACE in the general population are related. 35 , 36 , 37 In addition, our findings demonstrate a positive correlation between the TyG index and MACE. In addition to BMI and smoking, diabetes is a significant factor in determining the risk of MACE. According to the results of our meta‐analysis, TyG index is associated with a statistically significant increase in the risk of post‐PCI MACE in both diabetic and non‐diabetic populations. A more thorough examination is required through more research involving non‐diabetic groups to evaluate the TyG index's prognostic value.
Previous researches
In contrast to the previous TyG index meta‐analysis, which was primarily based on the general population, this study focused predominantly on the population following PCI. The research on the influence of the TyG index on the risk of MACE was discussed in the section preceding this one, although the study population consisted primarily of the general population. 34 , 35 , 36 , 37 In addition, our study included a wider variety of MACE categories. To increase the precision of our findings, we conducted separate correlation analyses between the TyG index and multiple MACE types.
Potential mechanisms
TyG index is a tool for identifying insulin resistance and impaired fasting glucose as well as predicting the onset of diabetes in the future, all of which are associated with an increased risk of MACE. 29 , 32 The TyG index can be used to predict the probability of MACE following PCI procedures. Specific processes are implicated in the formation of ASCVD, which is characterized by low‐grade inflammation that contributes to the impairment of adipose function, oxidative stress, and endothelial dysfunction. Particularly elevated concentrations of small dense LDL‐C and low‐density lipoprotein cholesterol (LDL‐C), dyslipidaemia caused by insulin resistance, and triglycerides are risk factors for ASCVD. 55 Even when LDL‐C levels are normal, small dense LDL‐C, which tends to be smaller in size and more prone to deposition, can increase the risk of ASCVD. 56 Interestingly, LDL‐C levels in diabetic patients often do not increase considerably, but this is because a greater proportion of LDL‐C is small dense LDL‐C. 57 In addition, diabetes can increase triglyceride‐rich lipoproteins. According to the research conducted by Duran et al., tiny dense LDL‐C and triglyceride‐rich lipoproteins increase the risk of coronary and cerebrovascular diseases by approximately twofold. 58 Diabetes has no effect on the morphological characteristics of atherosclerotic lesions, but it accelerates lesion progression and retards regression. 57
Some risk factors associated with the development of MACE following PCI are also closely related to the TyG index. According to an article published in ‘The Lancet’ by the Unindustrialized Risk Factors Association, diabetics are more likely than nondiabetics to develop MACE, such as coronary heart disease. 32 This study did not detect a significant difference in the TyG index's ability to predict post‐PCI MACE risk between the non‐diabetic and diabetic populations. We believe that TyG index can predict post‐PCI MACE risk regardless of whether or not the patient has diabetes, assuming that the variance caused by the limited sample size can be ruled out. The TyG index also affects coronary artery calcification (CAC), but this variable was omitted from our meta‐analysis. The TyG index may completely replace the CAC score and predict the long‐term prognosis for individuals with multi‐vessel CAD in acute coronary syndrome, according to a study involving 935 Chinese participants conducted by Wang et al. 59 , 60 , 61 , 62
Limitations
Our meta‐analysis has several limitations. First, we exclusively incorporated cohort studies, with no other eligible study types discovered, potentially leaving room for residual confounding despite adjusting for multiple factors. The nine studies, encompassing just over 10 000 post‐PCI participants, limited the robustness of detailed subgroup discussions due to insufficient research support. Although these studies spanned five distinct cities (Beijing, Zhengzhou, Nanjing, Guangzhou, and Chengdu), the limited number of papers prevented an examination of regional disparities.
Clinical implications
The TyG index, a marker of insulin resistance, blood lipid, and sugar levels, shows a significant correlation with MACE and PCI. Although not a part of standard cardiovascular risk assessments, TyG index's potential to predict MACE, all‐cause mortality, non‐fatal myocardial infarction, and revascularization is recognized. Its calculation, using routine hospital measurements of blood lipids and glucose, offered cost‐effective tool for improving patient prognosis and tailoring treatment post‐PCI. However, further research is needed to establish its clinical utility and optimal cut‐off values, especially in conjunction with tools like GRACE score to enhance MACE risk prediction.
Conclusions
Observational studies conducted to date indicate a strong correlation between a high TyG index and post‐PCI MACE, non‐fatal MI, all‐cause mortality, and revascularization. In addition, there may be a positive linear correlation between the incidence of MACE, non‐fatal MI, and revascularization and each standard unit of TyG index. Notably, the effect of the TyG index on post‐PCI MACE appears to be somewhat less pronounced in individuals with diabetes (HR = 2.28), compared with those without diabetes (HR = 2.42). GRADE evaluation reveals a high value for non‐fatal MI, a low value for all‐cause mortality and revascularization, and a very low value for MACE, which requires additional research to support.
Conflict of interests
The authors declare no conflict of interest.
Conflict of interest
None declared.
Supporting information
Table S1. PRISMA 2020 Checklist.
Table S2. Detailed description of the search strategy.
Table S3. Studies excluded (n = 15) with reasons.
Table S4. The definition of MACE.
Table S5. Quality assessment of included studies.
Table S6. Tables of HRs and 95% CIs from non‐linear dose–response analysis of TyG index.
Table S7. Summary of findings for the MACE.
Table S8. GRADE evidence profile for the MACE.
Figure S1. Forest plot for MACE with different definitions.
Figure S2. Adjusted forest plot (BMI/obesity or smoking).
Figure S3. Dose–response between TyG index and non‐fatal MI/revascularization.
Figure S4. Sensitivity analyses of association between TyG and MACE after PCI (categorical variables, highest vs. lowest). A. MACE; B. all‐cause death, non‐fatal MI and revascularization.
Acknowledgements
This research was supported by grants from the Capital Health Research and Development of Special Fund (2022‐2‐4172), the National Natural Science Foundation of China (No. 81973689) and the Natural Science Foundation of Beijing Municipality (No. 7202176). [Correction added on 20 February 2024, after first online publication: The acknowledgement statement has been corrected in this version.]
Sun, C. , Hu, L. , Li, X. , Zhang, X. , Chen, J. , Li, D. , Zhang, J. , Liu, L. , and Wu, M. (2024) Triglyceride‐glucose index's link to cardiovascular outcomes post‐percutaneous coronary intervention in China: a meta‐analysis. ESC Heart Failure, 11: 1317–1328. 10.1002/ehf2.14679.
Contributor Information
LongTao Liu, Email: sci987654321@outlook.com, Email: liulongtao1976@126.com.
Min Wu, Email: wumin19762000@126.com.
References
- 1. Zhao D, Liu J, Wang M, Zhang X, Zhou M. Epidemiology of cardiovascular disease in China: Current features and implications. Nat Rev Cardiol 2019;16:203‐212. doi: 10.1038/s41569-018-0119-4 [DOI] [PubMed] [Google Scholar]
- 2. DeFilippis AP, Trainor PJ, Thanassoulis G, Brumback LC, Post WS, Tsai MY, et al. Atherothrombotic factors and atherosclerotic cardiovascular events: The multi‐ethnic study of atherosclerosis. Eur Heart J 2022;43:971‐981. doi: 10.1093/eurheartj/ehab600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beverly JK, Budoff MJ. Atherosclerosis: Pathophysiology of insulin resistance, hyperglycemia, hyperlipidemia, and inflammation. J Diabetes 2020;12:102‐104. doi: 10.1111/1753-0407.12970 [DOI] [PubMed] [Google Scholar]
- 4. Alizargar J, Bai CH, Hsieh NC, Wu SFV. Use of the triglyceride‐glucose index (TyG) in cardiovascular disease patients. Cardiovasc Diabetol 2020;19:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang L, Cong HL, Zhang JX, Hu YC, Wei A, Zhang YY, et al. The triglyceride‐glucose index predicts adverse cardiovascular events in patients with diabetes and acute coronary syndrome. Cardiovasc Diabetol 2020;19:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lu YW, Chang CC, Chou RH, Tsai YL, Liu LK, Chen LK, et al. Gender difference in the association between TyG index and subclinical atherosclerosis: Results from the I‐Lan longitudinal aging study. Cardiovasc Diabetol 2021;20:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Su J, Li Z, Huang M, Wang Y, Yang T, Ma M, et al. Triglyceride glucose index for the detection of the severity of coronary artery disease in different glucose metabolic states in patients with coronary heart disease: A RCSCD‐TCM study in China. Cardiovasc Diabetol 2022;21:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nam KW, Kwon HM, Jeong HY, Park JH, Kwon H, Jeong SM. High triglyceride‐glucose index is associated with subclinical cerebral small vessel disease in a healthy population: a cross‐sectional study. Cardiovasc Diabetol 2020;19:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhao S, Yu S, Chi C, Fan X, Tang J, Ji H, et al. Association between macro‐ and microvascular damage and the triglyceride glucose index in community‐dwelling elderly individuals: The northern Shanghai study. Cardiovasc Diabetol 2019;18:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ding D, Huang J, Westra J, Cohen DJ, Chen Y, Andersen BK, et al. Immediate post‐procedural functional assessment of percutaneous coronary intervention: Current evidence and future directions. Eur Heart J 2021;42:2695‐2707. doi: 10.1093/eurheartj/ehab186 [DOI] [PubMed] [Google Scholar]
- 11. Zhao Q, Zhang TY, Cheng YJ, Ma Y, Xu YK, Yang JQ, et al. Triglyceride‐glucose index as a surrogate marker of insulin resistance for predicting cardiovascular outcomes in Nondiabetic patients with non‐ST‐segment elevation acute coronary syndrome undergoing percutaneous coronary intervention. J Atheroscler Thromb 2021;28:1175‐1194. doi: 10.5551/jat.59840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pang S, Miao G, Zhou Y, du Y, Rui Z, Zhao X. Addition of TyG index to the GRACE score improves prediction of adverse cardiovascular outcomes in patients with non‐ST‐segment elevation acute coronary syndrome undergoing percutaneous coronary intervention: A retrospective study. Front Cardiovasc Med 2022;9:957626. doi: 10.3389/fcvm.2022.1060542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guo X, Shen R, Yan S, Su Y, Ma L. Triglyceride‐glucose index for predicting repeat revascularization and in‐stent restenosis in patients with chronic coronary syndrome undergoing percutaneous coronary intervention. Cardiovasc Diabetol 2023;22:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ling Y, Fu C, Fan Q, Liu J, Jiang L, Tang S. Triglyceride‐glucose index and new‐onset atrial fibrillation in ST‐segment elevation myocardial infarction patients after percutaneous coronary intervention. Front Cardiovasc Med. 2022;9:838761. doi: 10.3389/fcvm.2022.838761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang H, Li Q, Liu J, Qiao L, Chen S, Lai W, et al. Association between triglyceride glucose index and worsening heart failure in significant secondary mitral regurgitation following percutaneous coronary intervention. Cardiovasc Diabetol 2022;21:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lo CK, Mertz D, Loeb M. Newcastle‐Ottawa scale: Comparing reviewers' to authors' assessments. BMC Med Res Methodol 2014;14:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guerrero‐Romero F, Simental‐Mendía LE, González‐Ortiz M, Martínez‐Abundis E, Ramos‐Zavala MG, Hernández‐González SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic‐hyperinsulinemic clamp. J Clin Endocrinol Metab 2010;95:3347‐3351. doi: 10.1210/jc.2010-0288 [DOI] [PubMed] [Google Scholar]
- 18. Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 1987;9:1‐30. doi: 10.1093/oxfordjournals.epirev.a036298 [DOI] [PubMed] [Google Scholar]
- 19. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986;7:177‐188. doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 20. Xu C, Doi SAR. The robust error meta‐regression method for dose‐response meta‐analysis. Int J Evid Based Healthc 2018;16:138‐144. doi: 10.1097/XEB.0000000000000132 [DOI] [PubMed] [Google Scholar]
- 21. Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490. doi: 10.1136/bmj.328.7454.1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Qin Z, Xu S, Yuan R, Wang Z, Lu Y, Xu Y, et al. Combination of TyG index and GRACE risk score as long‐term prognostic marker in patients with ACS complicated with T2DM undergoing PCI. Diabetes Metab Syndr Obes 2022;15:3015‐3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Luo E, Wang D, Yan G, Qiao Y, Liu B, Hou J, et al. High triglyceride‐glucose index is associated with poor prognosis in patients with acute ST‐elevation myocardial infarction after percutaneous coronary intervention. Cardiovasc Diabetol 2019;18:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhao Q, Zhang TY, Cheng YJ, Ma Y, Xu YK, Yang JQ, et al. Impacts of triglyceride‐glucose index on prognosis of patients with type 2 diabetes mellitus and non‐ST‐segment elevation acute coronary syndrome: Results from an observational cohort study in China. Cardiovasc Diabetol 2020;19:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sun T, Huang X, Zhang B, Ma M, Chen Z, Zhao Z, et al. Prognostic significance of the triglyceride‐glucose index for patients with ischemic heart failure after percutaneous coronary intervention. Front Endocrinol (Lausanne) 2023;14:1100399. doi: 10.3389/fendo.2023.1100399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xiong S, Chen Q, Zhang Z, Chen Y, Hou J, Cui C, et al. A synergistic effect of the triglyceride‐glucose index and the residual SYNTAX score on the prediction of intermediate‐term major adverse cardiac events in patients with type 2 diabetes mellitus undergoing percutaneous coronary intervention. Cardiovasc Diabetol 2022;21:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jin JL, Cao YX, Wu LG, You XD, Guo YL, Wu NQ, et al. Triglyceride glucose index for predicting cardiovascular outcomes in patients with coronary artery disease. J Thorac Dis 2018;10:6137‐6146. doi: 10.21037/jtd.2018.10.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ramdas Nayak VK, Satheesh P, Shenoy MT, Kalra S. Triglyceride glucose (TyG) index: A surrogate biomarker of insulin resistance. J Pak Med Assoc 2022;72:986‐988. doi: 10.47391/JPMA.22-63 [DOI] [PubMed] [Google Scholar]
- 29. Li M, Chi X, Wang Y, Setrerrahmane S, Xie W, Xu H. Trends in insulin resistance: insights into mechanisms and therapeutic strategy. Signal Transduct Target Ther 2022;7:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Park B, Lee HS, Lee YJ. Triglyceride glucose (TyG) index as a predictor of incident type 2 diabetes among nonobese adults: A 12‐year longitudinal study of the Korean genome and epidemiology study cohort. Transl Res 2021;228:42‐51. doi: 10.1016/j.trsl.2020.08.003 [DOI] [PubMed] [Google Scholar]
- 31. Park HM, Lee HS, Lee YJ, Lee JH. The triglyceride‐glucose index is a more powerful surrogate marker for predicting the prevalence and incidence of type 2 diabetes mellitus than the homeostatic model assessment of insulin resistance. Diabetes Res Clin Pract 2021;180:109042. doi: 10.1016/j.diabres.2021.109042 [DOI] [PubMed] [Google Scholar]
- 32. Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta‐analysis of 102 prospective studies. Lancet 2010;375:2215‐2222. doi: 10.1016/S0140-6736(10)60484-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sajdeya O, Beran A, Mhanna M, Alharbi A, Burmeister C, Abuhelwa Z, et al. Triglyceride glucose index for the prediction of subclinical atherosclerosis and arterial stiffness: A meta‐analysis of 37,780 individuals. Curr Probl Cardiol 2022;47:101390. doi: 10.1016/j.cpcardiol.2022.101390 [DOI] [PubMed] [Google Scholar]
- 34. Liu X, Tan Z, Huang Y, Zhao H, Liu M, Yu P, et al. Relationship between the triglyceride‐glucose index and risk of cardiovascular diseases and mortality in the general population: A systematic review and meta‐analysis. Cardiovasc Diabetol 2022;21:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ding X, Wang X, Wu J, Zhang M, Cui M. Triglyceride‐glucose index and the incidence of atherosclerotic cardiovascular diseases: A meta‐analysis of cohort studies. Cardiovasc Diabetol 2021;20:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li X, Chan JSK, Guan B, Peng S, Wu X, Lu X, et al. Triglyceride‐glucose index and the risk of heart failure: Evidence from two large cohorts and a mendelian randomization analysis. Cardiovasc Diabetol 2022;21:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang Y, Huang X, Wang Y, Leng L, Xu J, Feng L, et al. The impact of triglyceride‐glucose index on ischemic stroke: A systematic review and meta‐analysis. Cardiovasc Diabetol 2023;22:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ma X, Dong L, Shao Q, Cheng Y, Lv S, Sun Y, et al. Triglyceride glucose index for predicting cardiovascular outcomes after percutaneous coronary intervention in patients with type 2 diabetes mellitus and acute coronary syndrome. Cardiovasc Diabetol 2020;19:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hu C, Zhang J, Liu J, Liu Y, Gao A, Zhu Y, et al. Discordance between the triglyceride glucose index and fasting plasma glucose or HbA1C in patients with acute coronary syndrome undergoing percutaneous coronary intervention predicts cardiovascular events: A cohort study from China. Cardiovasc Diabetol 2020;19:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lin XL, Li QY, Zhao DH, Liu JH, Fan Q. A high triglyceride‐glucose index associated with adverse cardiovascular events in patients with type 2 diabetes mellitus and chronic total Occlusion after percutaneous coronary intervention. J Invest Med 2023;71:471‐481. doi: 10.1177/10815589231152823 [DOI] [PubMed] [Google Scholar]
- 41. Yang J, Tang YD, Zheng Y, Li C, Zhou Q, Gao J, et al. The impact of the triglyceride‐glucose index on poor prognosis in nondiabetic patients undergoing percutaneous coronary intervention. Front Endocrinol (Lausanne). 2021;12:710240. doi: 10.3389/fendo.2021.710240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shao QY, Ma XT, Yang ZQ, Li QX, Wang YF, Liang J, et al. Prognostic significance of multiple triglycerides‐derived metabolic indices in patients with acute coronary syndrome. J Geriatr Cardiol 2022;19:456‐468. doi: 10.11909/j.issn.1671-5411.2022.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhu Y, Liu K, Chen M, Liu Y, Gao A, Hu C, et al. Triglyceride‐glucose index is associated with in‐stent restenosis in patients with acute coronary syndrome after percutaneous coronary intervention with drug‐eluting stents. Cardiovasc Diabetol 2021;20:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Forouhi NG, Misra A, Mohan V, Taylor R, Yancy W. Dietary and nutritional approaches for prevention and management of type 2 diabetes. BMJ 2018;361:k2234. doi: 10.1136/bmj.k2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Simha V. Management of hypertriglyceridemia. Bmj 2020;371:m3109. doi: 10.1136/bmj.m3109 [DOI] [PubMed] [Google Scholar]
- 46. Lavie CJ, Arena R, Alpert MA, Milani RV, Ventura HO. Management of cardiovascular diseases in patients with obesity. Nat Rev Cardiol 2018;15:45‐56. doi: 10.1038/nrcardio.2017.108 [DOI] [PubMed] [Google Scholar]
- 47. Zhao Q, Cheng YJ, Xu YK, Zhao ZW, Liu C, Sun TN, et al. Comparison of various insulin resistance surrogates on prognostic prediction and stratification following percutaneous coronary intervention in patients with and without type 2 diabetes mellitus. Cardiovasc Diabetol 2021;20:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liu X, Bragg F, Yang L, Kartsonaki C, Guo Y, du H, et al. Smoking and smoking cessation in relation to risk of diabetes in Chinese men and women: A 9‐year prospective study of 0·5 million people. Lancet Public Health 2018;3:e167‐e176. doi: 10.1016/S2468-2667(18)30026-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pan A, Wang Y, Talaei M, Hu FB, Wu T. Relation of active, passive, and quitting smoking with incident type 2 diabetes: A systematic review and meta‐analysis. Lancet Diabetes Endocrinol 2015;3:958‐967. doi: 10.1016/S2213-8587(15)00316-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bentley AR, Sung YJ, Brown MR, Winkler TW, Kraja AT, Ntalla I, et al. Multi‐ancestry genome‐wide gene‐smoking interaction study of 387,272 individuals identifies new loci associated with serum lipids. Nat Genet 2019;51:636‐648. doi: 10.1038/s41588-019-0378-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Baek W, Lee JW, Lee HS, Han D, Choi SY, Chun EJ, et al. Concurrent smoking and alcohol consumers had higher triglyceride glucose indices than either only smokers or alcohol consumers: A cross‐sectional study in Korea. Lipids Health Dis 2021;20:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Larsson SC, Mason AM, Bäck M, Klarin D, Damrauer SM, Million Veteran Program , et al. Genetic predisposition to smoking in relation to 14 cardiovascular diseases. Eur Heart J 2020;41:3304‐3310. doi: 10.1093/eurheartj/ehaa193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jeong SM, Jeon KH, Shin DW, Han K, Kim D, Park SH, et al. Smoking cessation, but not reduction, reduces cardiovascular disease incidence. Eur Heart J 2021;42:4141‐4153. doi: 10.1093/eurheartj/ehab578 [DOI] [PubMed] [Google Scholar]
- 54. Hong S, Han K, Park CY. The triglyceride glucose index is a simple and low‐cost marker associated with atherosclerotic cardiovascular disease: A population‐based study. BMC Med 2020;18:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kosmas CE, Bousvarou MD, Kostara CE, Papakonstantinou EJ, Salamou E, Guzman E. Insulin resistance and cardiovascular disease. J Int Med Res 2023;51: doi: 10.1177/03000605231164548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Superko H, Garrett B. Small dense LDL: Scientific background, clinical relevance, and recent evidence still a risk even with ‘normal’ LDL‐C levels. Biomedicine 2022;10:829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Eckel RH, Bornfeldt KE, Goldberg IJ. Cardiovascular disease in diabetes, beyond glucose. Cell Metab 2021;33:1519‐1545. doi: 10.1016/j.cmet.2021.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Duran EK, Aday AW, Cook NR, Buring JE, Ridker PM, Pradhan AD. Triglyceride‐rich lipoprotein cholesterol, small dense LDL cholesterol, and incident cardiovascular disease. J Am Coll Cardiol 2020;75:2122‐2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang C, Jia P, Yu L, Xu C. Introduction to methodology of dose‐response meta‐analysis for binary outcome: With application on software. J Evid Based Med 2018;11:125‐129. doi: 10.1111/jebm.12267 [DOI] [PubMed] [Google Scholar]
- 60. Wang J, Huang X, Fu C, Sheng Q, Liu P. Association between triglyceride glucose index, coronary artery calcification and multivessel coronary disease in Chinese patients with acute coronary syndrome. Cardiovasc Diabetol 2022;21:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111‐188. doi: 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 62. Xiong S, Chen Q, Chen X, Hou J, Chen Y, Long Y, et al. Adjustment of the GRACE score by the triglyceride glucose index improves the prediction of clinical outcomes in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Cardiovasc Diabetol 2022;21:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. PRISMA 2020 Checklist.
Table S2. Detailed description of the search strategy.
Table S3. Studies excluded (n = 15) with reasons.
Table S4. The definition of MACE.
Table S5. Quality assessment of included studies.
Table S6. Tables of HRs and 95% CIs from non‐linear dose–response analysis of TyG index.
Table S7. Summary of findings for the MACE.
Table S8. GRADE evidence profile for the MACE.
Figure S1. Forest plot for MACE with different definitions.
Figure S2. Adjusted forest plot (BMI/obesity or smoking).
Figure S3. Dose–response between TyG index and non‐fatal MI/revascularization.
Figure S4. Sensitivity analyses of association between TyG and MACE after PCI (categorical variables, highest vs. lowest). A. MACE; B. all‐cause death, non‐fatal MI and revascularization.


