Abstract
Aims
Patients with chronic kidney disease (CKD) or heart failure (HF) are disproportionally affected by frailty, an independent predictor of morbidity. The prevalence of frailty and its impact on quality of life (QoL) in a unique population of patients with both CKD and HF (CKD‐HF) is unclear. The aim of this study was to investigate the association between frailty and QoL in patients with CKD‐HF.
Methods and results
Patients were identified from a tertiary care cardiorenal clinic. Eligible patients had CKD‐HF with a stable estimated glomerular filtration rate of <60 mL/min/1.732. Data were collected from each participant at one point in time using surveys delivered by study personnel between 14 July 2022 and 31 March 2023. Frailty was defined as Modified Frailty Phenotype (MFP) score ≥3. The Medical Outcomes Study 36‐item Short Form Health Survey (SF‐36) was used to assess QoL. Demographic data were retrospectively collected from electronic patient records. Demographics and QoL were compared between frail and non‐frail cohorts using Pearson's R and Student's t‐test (two‐tailed, alpha‐priori = 0.05). One hundred five participants consented, and 103 completed the questionnaires in full. Amongst the 103 participants, 49.5% (n = 51) were frail. Frailty was related to sex (P = 0.021) and medication count (P = 0.007), however not to other clinical measures, including estimated glomerular filtration rate (P = 0.437) and ejection fraction (P = 0.911). Frail patients reported poorer QoL across physical functioning (P < 0.001), general health (P < 0.001), bodily pain (P = 0.004), social functioning (P < 0.001), and energy levels (P < 0.001), however not emotional wellbeing (P = 0.058); 51.5% cited ‘better quality of life’ as their healthcare priority, over longer survival (23.3%) or avoiding hospital admissions (22.3%). This was consistent across frail and non‐frail groups.
Conclusions
A large proportion of CKD‐HF patients are frail, regardless of disease severity, and more susceptible to significantly poorer QoL across physical and social domains. Improving QoL is the priority of patients across both frail and non‐frail cohorts, further emphasizing the need for prompt recognition of frailty as well as possible intervention and prevention.
Keywords: Chronic kidney failure, Frailty, Heart failure, Observational, Quality of life
Introduction
Both chronic kidney disease (CKD) and heart failure (HF) are lifelong, progressive conditions with a significant disease burden worldwide. 1 , 2 An increasing number of patients with CKD have concomitant HF (CKD‐HF), and vice versa. The physiological relationship between these two diseases is complex and bidirectional, with each increasing the risk of developing the other. 3 , 4 Furthermore, the management of patients with CKD‐HF is challenging as concerns due to worsening renal function and hyperkalaemia often preclude the use of evidence‐based HF medication. 5 In response to this, our tertiary centre has established a novel dedicated CKD‐HF inter‐disciplinary clinic with input from both cardiologists and nephrologists to manage these complex clinical situations. 5
Frailty is a term which describes cumulative decline in multiple physiological domains, causing a dynamic state of increased vulnerability to stressors (clinical or non‐clinical). 6 , 7 , 8 , 9 , 10 Individuals with frailty may experience a disproportionate reaction to a stressor, which may be insignificant in a healthy individual (e.g. increased risk of hospitalization or death, from a minor infection or introduction of a new medication). 10 Frailty has garnered increasing interest over recent years due its association with mortality, disability, falls and long‐term care. 6 Although not synonymous, the incidence of frailty is more common and seen at an earlier age in patients with multiple co‐morbidities. 8 , 11
Individuals with CKD or HF suffer frailty more frequently than both the general population and individuals with other chronic medical conditions. 12 , 13 , 14 Estimates of frailty amongst CKD patients vary depending on renal function; previous studies have found rates of 7% in a population with mean eGFR 49 mL/min/1.732, 15 43% with mean eGFR 26.8 mL/min/1.732, 16 and 60% in dialysis dependent populations. 13 , 14 Frailty amongst HF populations ranges from 15% to 74% depending on the study population and methodology used. 10 , 17 , 18 Comparatively, rates of frailty in community populations living without CKD or HF are lower at 11%. 19 To the authors knowledge, no previous study has investigated the prevalence of frailty in patients with both CKD‐HF.
Health‐related quality of life (HR‐QOL) is a widely used, validated measure of how an individual perceives their physical and mental health, and how their health impacts their quality of life. 11 , 20 In patients with CKD or HF, HR‐QOL scores tend to worsen with increasing disease severity 21 , 22 , 23 ; however, this has not previously been studied in patients with both CKD‐HF.
The association between frailty and HR‐QOL is complex and research investigating the relationship between them is limited. Previous studies have highlighted the importance of identifying frailty and poor HR‐QOL early, and the necessity for targeted interventions aimed at specific characteristics of frailty in order to optimize HR‐QOL. 16 , 24 , 25 , 26 , 27 However, these studies have been limited in small populations and have considered patients living with either CKD or HF, not both.
Furthermore, patients with CKD‐HF are often multimorbid and polypharmacy is common. 5 Previous studies have identified a positive relationship between polypharmacy and frailty 9 , 28 ; however, no previous study has considered this relationship within a sample of CKD‐HF patients. This is important as recent years have seen the expansion of evidence‐based therapies for HF with reduced ejection fraction (EF), and patients are now recommended to take at least four drugs. 29 The primary endpoint of these landmark trials on which these recommendations are based were predominantly mortality or hospital admissions. 29 However, the majority did not consider frailty status as an outcome. We hoped that this study would help us to improve our understanding of the relationship between frailty and polypharmacy in CKD‐HF patients. Furthermore, we wanted to learn about the healthcare priorities of patients with CKD‐HF, to better enable patient specific needs to be met in trials and clinical practice.
To our knowledge, no study has yet investigated the relationship between frailty and HR‐QOL in patients with CKD‐HF. Whilst evidence specific to this population is limited, there is unanimous agreement that both HR‐QOL and frailty are important outcomes in the management of both CKD and HF. Furthermore, their early assessment can provide important insights to guide the management for the unique challenges faced by this population.
The primary aim of this study was to investigate the impact of frailty on HR‐QOL in patients with both CKD‐HF. Objectives included (i) assessing the prevalence of frailty in this patient cohort of CKD‐HF; (ii) assessing the impact of frailty on HR‐QOL in CKD‐HF; (iii) assessing the relationship between frailty and HR‐QOL with clinical status, polypharmacy, demographics in patients with CKD‐HF; (iv) assessing patient priorities within healthcare planning.
Methods
Study design
This study used an observational cross‐sectional questionnaire, with a convenience sampling method. This was a single centre study that took place within a CKD‐HF inter‐disciplinary clinic in a tertiary university teaching hospital in London, England between 14 July 2022 and 31 March 2023. The study adhered to the Declaration of Helsinki and was approved by the Cambridge East Research Ethics Committee (REC) and approved by HRA (IRAS:294629).
All potentially eligible patients were informed about the study via a written participant information sheet (PIS) distributed via post, email or in person when they attended for a joint cardiorenal clinic appointment. This was followed up with a telephone call. If they wished to provide their informed consent following discussion, patients were offered to complete the questionnaire over the telephone, or to complete the questionnaire online using a secure online survey platform. 30
The inclusion and exclusion criteria for participants can be found in Table 1 .
Table 1.
Inclusion and exclusion criteria
Inclusion criteria:
|
Exclusion criteria:
|
CKD, chronic kidney disease; HF, heart failure; NICE, National Institute for Health and Care Excellence; SF‐36, 36‐item Short Form Survey Instrument.
Outcomes
Frailty instrument
Frailty was assessed and quantified using both the ‘Clinical Frailty Scale (CFS)’ and the ‘Modified Frailty Phenotype (MFP)’, which are both validated and used widely in clinical practice and research, including in HF and CKD, although are not specifically validated for these populations. 10 , 14 , 31 , 32 , 33 , 34 , 35 , 36 Both were deemed appropriate as they are simple and easy to use both over the telephone or within an online survey. The MFP constitutes four domains: slowness/weakness, exhaustion, physical inactivity and unintentional weight loss. Frailty is defined as a score equal to or greater than three. The CFS is a frailty tool ranging from 1 (very fit) to 9 (terminally ill), and constitutes assessment of several domains including cognition, function, and co‐morbidities. For the purposes of our analysis, MFP was deemed to be more appropriate and truly representative of frailty, as opposed to the CFS which also constitutes co‐morbidities which are common in patients with HF‐CKD. Thus, the analyses were conducted as patients that were deemed frail according to MFP, vs those that were not.
HR‐QOL instrument
The Medical Outcomes Study 36‐item Short Form Health Survey (SF‐36) tool was used to measure HR‐QOL. The SF‐36 is a publicly available, validated tool for assessing HR‐QOL and is used widely across research and clinical specialties. 37 , 38 , 39 , 40 The SF‐36 provides an objective assessment of the following eight domains: physical functioning, role limitation due to physical functioning, role limitations due to emotional functioning, energy/fatigue, emotional well‐being, social functioning, pain and general health. In SF‐36, scores range from zero (worst possible health state) to 100 (best possible health state). The SF‐36 was selected as the tool for this study due to the ease with which it can be carried out over the phone, the objective nature of the questions and low inter‐observer variability. 38 , 39 , 40
At the time that both questionnaires were completed, patients were asked to rank the following in terms of their healthcare priorities: (i) longer survival, (ii) better quality of life, or (iii) avoiding hospital admissions.
Demographic data
Participants' demographic data were collected retrospectively from electronic patient records including age, ethnicity, smoking history, BMI, number of regular medications, eGFR, EF, creatinine levels and albumin: creatinine ratio.
Statistical analysis
Sample size was calculated based on the prevalence of frailty. As patients with CKD have a frailty prevalence of approximately 40–60%, 12 , 13 , 14 , 16 and frailty in HF populations ranges from 15% to 74%, 17 , 18 a sample size of between 100 and 120 was considered appropriate to estimate a prevalence of 60–80% with a precision of 8–10%. Data were analysed and graphs were created using R 4.3.0 with ggplot2 and ggpubr 41 package. Descriptive statistics were used to describe baseline characteristics of participants.
Continuous variables were analysed with Shapiro–Wilk test to determine normality. Parametric variables were compared between frail and non‐frail patients using independent sample t‐test. Non‐parametric continuous variables were compared using Mann–Whitney U test, and categorical variables were compared using chi‐squared test. Bivariate correlations between continuous variables were calculated using Pearson's correlation coefficient (R). P‐values were two‐tailed and considered significant if <0.05.
Results
A total of 269 patients were identified as potentially eligible for the study and were approached. Thirteen were excluded as they did not satisfy the inclusion criteria (Figure 1 ). Forty‐two patients declined participation. Eight participants had unfortunately passed away by the time contact was attempted. One hundred five participants consented and were included in the study. There was no loss to follow‐up; however, two participants did not complete the survey in full and were excluded.
Figure 1.
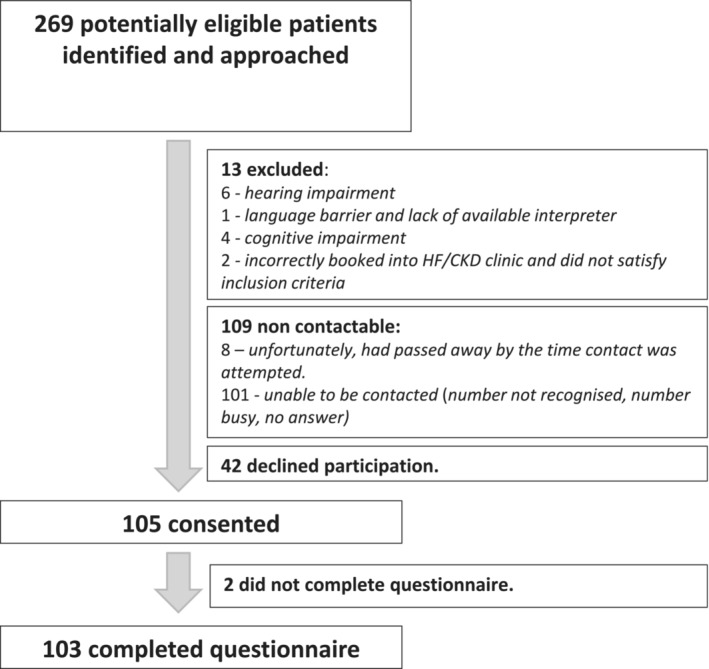
Recruitment flow chart.
The participants represented an older (median age 76 years), relatively diverse population (55% Caucasian, 23% Asian, 10% Black), representative of the diverse population served by the inner‐city tertiary hospital in which it was conducted. The full baseline demographics are presented in Table 2 .
Table 2.
Baseline characteristics of participants by frailty status
| Baseline characteristics | ||||
|---|---|---|---|---|
| MFP | Overall | No | Yes | |
| n | 103 | 52 | 51 | P‐value |
| Age, median [IQR] | 76.00 [68.50, 82.00] | 78.50 [69.75, 82.25] | 76.00 [67.50, 80.00] | 0.503 |
| Male, No. (%) | 64 (62.1) | 38 (73.1) | 26 (51.0) | 0.021 |
| Ethnicity, No. (%) | 0.079 | |||
| Asian | 24 (23.3) | 8 (15.4) | 16 (31.4) | |
| Black | 10 (9.7) | 3 (5.8) | 7 (13.7) | |
| Mixed | 2 (1.9) | 2 (3.8) | 0 (0.0) | |
| Other | 10 (9.7) | 5 (9.6) | 5 (9.8) | |
| White | 57 (55.3) | 34 (65.4) | 23 (45.1) | |
| BMI, mean (SD) | 28.65 (6.26) | 27.53 (5.68) | 29.76 (6.66) | 0.083 |
| Smoking, No. (%) | 0.749 | |||
| Ex | 53 (51.5) | 29 (55.8) | 24 (47.1) | |
| Never | 41 (39.8) | 18 (34.6) | 23 (45.1) | |
| Smoker | 7 (6.8) | 4 (7.7) | 3 (5.9) | |
| Unknown | 2 (1.9) | 1 (1.9) | 1 (2.0) | |
| CKD stage, No. (%) | 0.758 | |||
| CKD Stage 3 | 55 (53.4) | 29 (55.8) | 26 (25.2) | |
| CKD Stage 4 | 38 (36.9) | 19 (36.5) | 19 (37.3) | |
| CKD Stage 5 | 10 (9.7) | 4 (7.7) | 6 (11.8) | |
| Ejection fraction, median (IQR) | 42.50 [32.00, 52.00] | 38.50 [32.38, 49.00] | 42.50 [32.00, 53.50 | 0.911 |
| eGFR, mean (SD) | 29.34 (11.43) | 30.21 (11.30) | 28.45 (11.62) | 0.437 |
| Medication count, mean (SD) | 9.81 (3.4) | 8.92 (3.55) | 10.71 (3.0) | 0.007 |
CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; IQR, interquartile range; MFP, modified frailty phenotype; SD, standard deviation.
Fifty‐one (49.5%) participants were frail, and 52 (50.5%) were non‐frail according to the MFP. Frailty according to the CFS was significantly correlated with MFP (P‐value < 0.001), providing validation of these tools. Age and BMI were not significantly associated with frailty. The median (IQR) age of participants was frail 76 (12.5) and non‐frail 78.5 (12.5) (P‐value = 0.51). The mean (SD) BMI of participants was frail 29.76 (6.66) and non‐frail 27.53 (5.68) (P‐value = 0.083). A comparison between the baseline characteristics of frail and non‐frail study participants is presented in Table 2 .
Frail patients had significantly lower HR‐QOL scores in several domains. In SF‐36, scores range from zero (worst possible health state) to 100 (best possible health state). Frail patients had lower physical functioning scores (frail 20.0 [IQR 10.0–32.50], non‐frail 55.0 [IQR 35.0–75.0], P‐value < 0.001), general health scores (frail 30 [IQR 15.0–40.0], non‐frail 40 [IQR 30–60], P‐value < 0.001), and bodily pain scores (frail 32.50 [IQR 22.50–75.0], non‐frail 58.0 [IQR 37.50–100.0], P‐value = 0.008). Frail patients also had significantly lower social functioning scores (frail 50.0 [IQR 37.50–69.0], non‐frail 75.0 [IQR 62.5–100.0], P‐value = 0.001) and energy levels (frail 25.0 [IQR 10.0–40.0], non‐frail 50.0 [IQR 40.0–60.0], P‐value < 0.001). In keeping with the significantly lower physical functioning scores, frail patients had significantly worse role limitation due to physical health scores (frail 0.00 [IQR 0.0–25.0], non‐frail 25.0 [IQR 0.0–75.0], P‐value < 0.001) (Figure 2 ). A comparison between the baseline HR‐QOL scores in frail and non‐frail study participants is presented in Table 3 .
Figure 2.
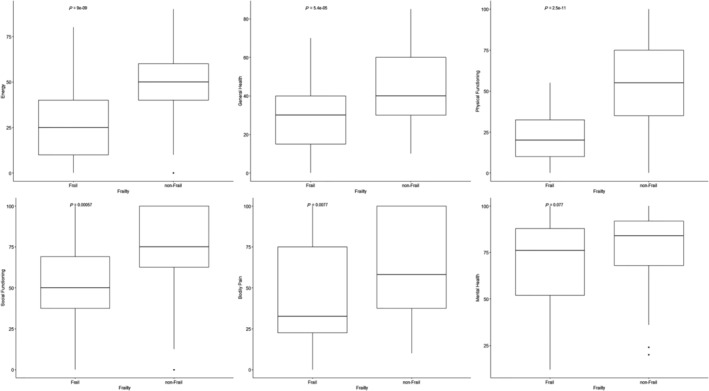
Graphs representing the relationship between frailty and several parameters of HR‐QOL: energy, general health, physical functioning, social functioning, bodily pain, and mental health.
Table 3.
Patient priorities and Quality of Life parameter results by frailty status
| MFP | Overall | No | Yes | |
|---|---|---|---|---|
| N | 103 | 52 | 51 | P‐value |
| First priority (%) | 0.208 | |||
| Longer survival | 24 (23.3) | 15 (28.8) | 9 (17.6) | |
| Better quality of life | 53 (51.5) | 26 (50.0) | 27 (52.9) | |
| Reduced hospital admission | 23 (22.3) | 11 (21.2) | 12 (23.5) | |
| Not sure | 3 (2.9) | 0 (0.0) | 3 (5.9) | |
| Second priority (%) | 0.637 | |||
| Longer survival | 39 (37.9) | 22 (42.3) | 17 (33.3) | |
| Better quality of life | 33 (32.0) | 16 (30.8) | 17 (33.3) | |
| Reduced hospital admission | 27 (26.2) | 13 (25.0) | 14 (27.5) | |
| Not sure | 4 (3.9) | 1 (1.9) | 3 (5.9) | |
| Third priority (%) | 0.119 | |||
| Longer survival | 36 (35.0) | 14 (26.9) | 22 (43.1) | |
| Better quality of life | 14 (13.6) | 10 (19.2) | 4 (7.8) | |
| Reduced hospital admission | 49 (47.6) | 27 (51.9) | 22 (43.1) | |
| Not sure | 4 (3.9) | 1 (1.9) | 3 (5.9) | |
| Clinical Frailty Scale (CFS), mean (SD) | 4.79 (1.54) | 3.83 (1.32) | 5.76 (1.07) | <0.001 |
| Frailty CFS (%) | 81 (78.6) | 30 (57.7) | 51 (100.0) | <0.001 |
| Moderate or severe frailty on CFS (%) | 39 (37.9) | 6 (11.5) | 33 (64.7) | <0.001 |
| Physical functioning, median [IQR] | 35.00 [15.00, 55.00] | 55.00 [35.00, 75.00] | 20.00 [10.00, 32.50] | <0.001 |
| Role limitations due to physical health, median [IQR] | 0.00 [0.00, 50.00] | 25.00 [0.00, 75.00] | 0.00 [0.00, 25.00] | <0.001 |
| Emotional well‐being, median [IQR] | 80.00 [60.00, 92.00] | 84.00 [68.00, 92.00] | 76.00 [52.00, 88.00] | 0.121 |
| Role limitations due to emotional problems, median [IQR] | 100.00 [33.33, 100.00] | 100.00 [66.67, 100.00] | 66.67 [16.50, 100.00] | 0.058 |
| Energy/fatigue, median [IQR] | 40.00 [20.00, 50.00] | 50.00 [40.00, 60.00] | 25.00 [10.00, 40.00] | <0.001 |
| Social functioning, median [IQR] | 62.50 [37.50, 100.00] | 75.00 [62.50, 100.00] | 50.00 [37.50, 69.00] | <0.001 |
| Pain, median [IQR] | 47.50 [23.00, 90.00] | 58.00 [37.50, 100.00] | 32.50 [22.50, 75.00] | 0.004 |
| General health, median [IQR] | 35.00 [20.00, 50.00] | 40.00 [30.00, 60.00] | 30.00 [15.00, 40.00] | <0.001 |
IQR, interquartile range; MFP, Modified Frailty Phenotype; SD, standard deviation.
Interestingly, there was no significant difference in emotional wellbeing (frail 76.0 [IQR 52.0–88.0], non‐frail 84.0 [IQR 68.0–92.0], P‐value = 0.121) or role limitation due to emotional health (frail 66.67 [IQR 16.50–100.0], non‐frail 100.00 [IQR 66.67–100.0], P‐value = 0.058).
Furthermore, there was no relationship between stages 3, 4, and 5 CKD and any HR‐QOL parameter: physical functioning (P = 0.705), role limitation due to physical health (P = 0.305), emotional wellbeing (P = 0.396), role limitation due to emotional problems (P = 0.401), energy (P = 0.094), social functioning (P = 0.692), pain (P = 0.707), and general health (P = 0.748) (Figure 3 ).
Figure 3.
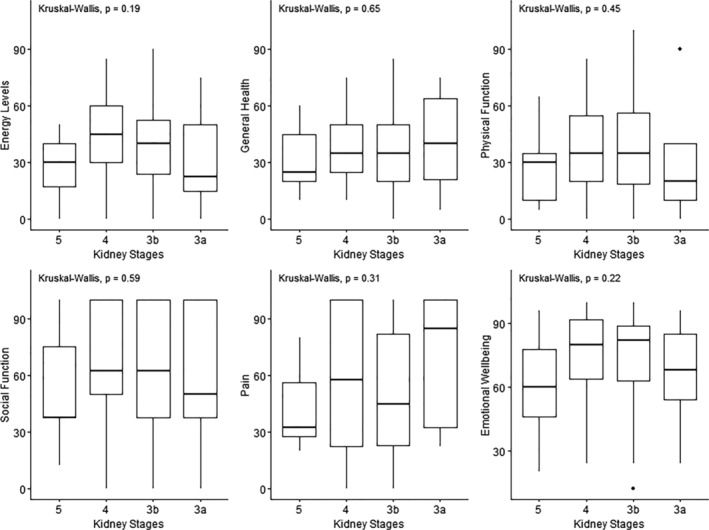
Graphs representing the relationship between chronic kidney disease stages and several parameters of HR‐QOL: general health, energy, physical functioning, social functioning, bodily pain, and emotional wellbeing.
Similarly, EF was not correlated with frailty or quality of life scores. There was also no relationship between EF and frailty (P = 0.911). Nor was there a relationship between EF and any of the HR‐QOL domains; energy (= 0.07, P = 0.48), general health (R = −0.0, P = 0.87), physical functioning (R = −1.6, P = 0.99), social functioning (R = 0.15, P = 0.14), bodily pain (R = 0.05, P = 0.59), mental health (R = −0.0, P = −0.52), role limitation due to emotional health (R = −7.0, P = 0.94), and role limitation due to physical health (R = 0.02, P = 0.85).
There was a significant relationship between polypharmacy and frailty, with frail patients taking significantly more regular medications (10.71 ± 3.00), compared with non‐frail patients (8.92 ± 3.55), (P = 0.007). We divided patients into groups based on their number of regular medications (0–5, 6–10, 11–15, and 16–20). There was a statistically significant difference in median physical functioning, general health, and bodily pain scores between these groups (P‐values = 0.018, 0.042, and 0.004, respectively) (Figure 4 ). There was no difference between groups in social functioning (P = 0.088), emotional wellbeing (P = 0.303), energy (0.176), role limitation due to emotional health (0.718), or role limitation due to physical health (0.296).
Figure 4.
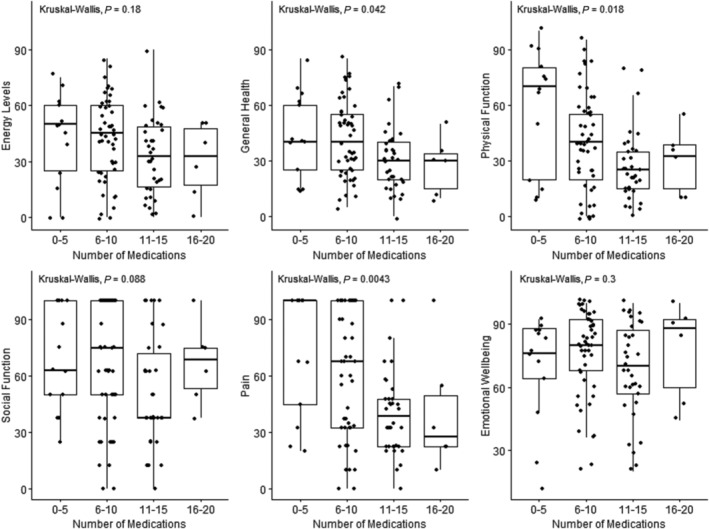
Graphs representing the relationship between numbers of medications and several parameters of HR‐QOL: general health, energy, physical functioning, social functioning, bodily pain, and emotional wellbeing.
When asked to select their priority when it came to planning their healthcare, most respondents (52%) opted for ‘Better Quality of Life’, whilst 23% chose ‘Longer Survival’, and 22% selected ‘Reduced Hospital Admissions’ (Figure 5 ). There was no significant difference between frail and non‐frail patients' priorities (P‐values = 0.495, 0.734, and 0.092 for priority one, two, and three, respectively) (Table 2 ).
Figure 5.
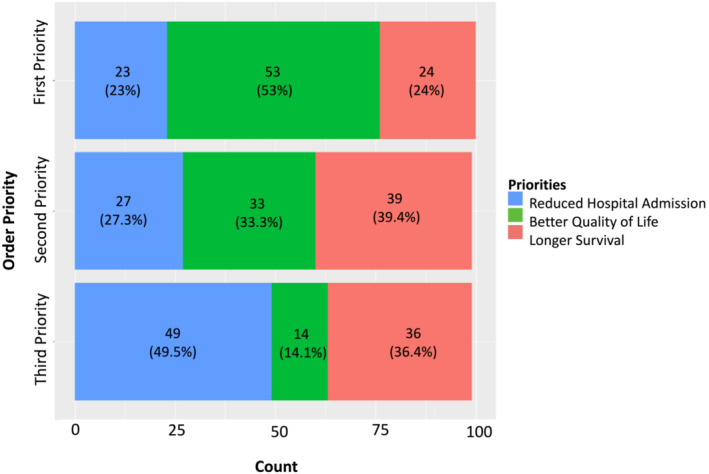
Participants order of priority when asked to prioritize ‘Longer Survival’, ‘Better Quality of Life’ or ‘Reduced Hospital Admissions’. The majority of respondents (53%) cited ‘Better Quality of Life’ as their first priority, when it came to planning their healthcare.
Discussion
This cross‐sectional study in patients with CKD‐HF managed at a tertiary centre demonstrated nearly half of the cohort was frail, and that frailty was negatively associated with health‐related quality of life, which patients prioritized over increased survival or avoiding hospital admissions. To our knowledge, this is the first study to evaluate this within this complex cohort of patients.
This study found high levels of frailty, consistent with other studies in CKD or HF populations. 16 , 24 , 25 , 26 , 27 Our frailty prevalence of 49.5% with a mean eGFR of 29.6 mL/min/1.732 was higher than other estimates in pre‐dialysis CKD 15 , 16 and HF populations, 19 suggesting that the combination of these conditions may independently increase risk of frailty. Frailty levels observed were, however, lower than we expected when we powered the study. This study also replicated the finding from other studies that female sex is significantly associated with increased frailty. 16
The finding of patients prioritization was recently observed in an international survey of both patients with HF and healthcare professionals who work in HF. Patients chose ‘Improving overall quality of life’ and ‘being able to live a normal life’ amongst their priority treatment objectives, in contrary to healthcare professionals who prioritized ‘prolonging life’ and ‘reducing the number of hospitalizations’. 42 The authors recommend increased measurement and reporting of quality of life within clinical trials in order to evaluate the effect of interventions on outcomes identified as important by patients. There may also be a need to shift focus to fostering more discussions about patient priorities in clinical settings to ensure we meet patient's goal.
Frailty was significantly associated with reduced HR‐QOL in several domains: physical functioning, general health, bodily pain, social functioning, energy levels, and role limitation due to physical health. This corroborates previous studies that have found a negative correlation between frailty and quality of life. 16 , 27 Proposed mechanisms for this relationship include that frailty is associated with weight loss, increased fatigue, weakness, mild cognitive impairment, and social exclusion, which in turn all affect an individual's functional capacity, and, consequently, quality of life. 16
Thus, early recognition of frailty and appropriate intervention should be a priority of healthcare professionals. The stepwise cumulative deterioration of frailty should enable physicians to detect patients at risk and to deliver evidence‐based interventions in order to prevent progression and to protect quality of life. 43 These interventions should encompass the following domains: physical activity, strength, balance, mobility, endurance, motor processing, nutrition, and cognition. 43 , 44 , 45
Multiple studies have been done in older adults living with frailty regarding the impact of interventions to identify and improve frailty using physical exercise and nutritional inputs. 46 Interventions have been shown to be effective for frail patients on dialysis. 47 There may be similar scope to incorporate these interventions (e.g. prescribe exercise) as part of a holistic management plan for cardiorenal patients living with frailty.
Furthermore, in this study a relationship was observed between polypharmacy and frailty, with frail patients taking significantly more regular medications than non‐frail patients. We analysed whether frail patients were more or less likely to be established on the guideline‐directed medical therapies for HF (i.e. renin‐angiotensin aldosterone inhibitors, beta‐blockers, sodium‐glucose co‐transporter 2 inhibitors, and mineralocorticoid receptor blockers); however, there was no statistically significant relationship observed (Table 4 ). Interestingly, in our cohort, non‐frail patients were statistically significantly more likely to be taking diuretics regularly, compared with frail patients (Table 4 ). For this study, we only collected information on HF medications, so it remains unclear which classes of medication constituted the additional prescriptions taken by frail patients. It is plausible that these patients had more co‐morbidities for which they were prescribed medications.
Table 4.
Use of guideline‐directed medical therapies and diuretics in patients in our study, by frailty status
| MFP | Overall | No | Yes | |
|---|---|---|---|---|
| n | 103 | 52 | 51 | P‐value |
| RAASi, No. (%) | 29 (28.2) | 18 (34.6) | 11 (21.6) | 0.210 |
| MRA, No. (%) | 67 (65.0) | 33 (63.5) | 34 (66.7) | 0.893 |
| Beta‐blocker, No. (%) | 47 (45.6) | 24 (46.2) | 23 (45.1) | 1.000 |
| SGLT2i, No. (%) | 47 (45.6) | 24 (46.2) | 23 (45.1) | 1.000 |
| Diuretic, No. (%) | 37 (35.9) | 25 (48.1) | 12 (23.5) | 0.017 |
MFP, Modified Frailty Phenotype; MRA, mineralocorticoid receptor antagonist; RAASi, renin‐angiotensin‐aldosterone system inhibitor; SGLT2i, sodium/glucose cotransporter 2 inhibitor.
Polypharmacy has been associated with increased frailty via increased drug interactions and side‐effects, increased falls, cognitive impairment, and reduced compliance with intended medication regimes. 9 However, there is also good evidence for medication optimization within frail patients, and a pre‐specified post‐hoc analysis from the DELIVER trial showed patients living with frailty benefited the most from treatment with dapagliflozin. 29 , 48
Further incorporation of QOL/frailty outcomes in longitudinal studies will help to investigate the relationship between polypharmacy and frailty. Our study supports a person‐centred approach to prescribing in this population and for physicians to consider when the addition of a new medication may, instead of resulting in the intended beneficial effect, cause a deterioration in a patient's physical and mental health.
Limitations
There are several limitations of this study. Firstly, the cross‐sectional design limits inference of temporal relationships between CKD‐HF, frailty and HR‐QOL. Furthermore, we have evaluated a sample of patients recruited from a single centre, albeit a specialized tertiary centre interdisciplinary cardiorenal clinic. The association between worsening renal function and increasing frailty was not replicated in this study, and this may be due to the limited sample size of 103 patients with a mean eGFR of 30 mL/min/1.732, with <10% of participants with stage 5 CKD. Importantly, by employing the exclusion criterion of communication barriers and cognitive impairment, we have excluded some of the frailest patients in this population. Furthermore, several patients who declined participation cited poor health as their reason for doing so, meaning that the respondents to this survey are likely healthier than the overall cohort of patients with CKD‐HF.
Conclusions
To our knowledge, this is the first study that has evaluated the relationship between frailty and HR‐QOL within CKD‐HF.
Frailty is significantly associated with reduced HR‐QOL in several domains in patients with CKD‐HF. HR‐QOL is a priority for patients. Frailty should be considered as an important and modifiable factor in maintaining HR‐QOL, and thus, healthcare professionals should recognize the importance of early detection and intervention of frailty. Longitudinal studies are needed to evaluate interventions to reduce frailty and consequently improve quality of life in patients with CKD‐HF.
Conflict of interest
The funding source had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Acknowledgements
This study received £1000 from St George's University Academic Training (GAT) Faculty ‘Small Grant Fund’ – St George's University, GAT Office, Crammer Terrace, London SW17 0RE, UK.
McNally, T. , Tumelty, E. , Chung, I. , Hussain, S. , Mookerjee, S. , Ali, M. A. , Anderson, L. , Rosano, G. , and Banerjee, D. (2024) Investigating the relationship between FRailty And Quality of LIfe in patients with heart faiLure and CKD (FRAIL study). ESC Heart Failure, 11: 1411–1421. 10.1002/ehf2.14693.
Thomas McNally and Ella Tumelty are joint first authors.
Contributor Information
Ella Tumelty, Email: ella.tumelty@stgeorges.nhs.uk.
Debasish Banerjee, Email: debasish.banerjee@stgeorges.nhs.uk.
References
- 1. Eriksson D, Goldsmith D, Teitsson S, Jackson J, Nooten F. Cross‐sectional survey in CKD patients across Europe describing the association between quality of life and anaemia. BMC Nephrol 2016;17:97. doi: 10.1186/s12882-016-0312-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lesyuk W, Kriza C, Kolominsky‐Rabas P. Cost‐of‐illness studies in heart failure: A systematic review 2004‐2016. BMC Cardiovasc Disord 2018;18: doi: 10.1186/s12872-018-0815-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. House AA, Wanner C, Sarnak MJ, Piña IL, McIntyre CW, Komenda P. Heart failure in chronic kidney disease: conclusions from a kidney disease: Improving global outcomes (KDIGO) controversies conference. Kidney Int 2019;95:1304‐1317. doi: 10.1016/j.kint.2019.02.022 [DOI] [PubMed] [Google Scholar]
- 4. Kottgen A, Russell SD, Loehr LR, Crainiceanu CM, Rosamond WD, Chang PP. Reduced kidney function as a risk factor for incident heart failure: the atherosclerosis risk in communities (ARIC) study. J Am Soc Nephrol 2007;18:1307‐1315. doi: 10.1681/ASN.2006101159 [DOI] [PubMed] [Google Scholar]
- 5. Nguyen M, Rumjaun S, Lowe‐Jones R, Ster IC, Rosano G, Anderson L, et al. Management and outcomes of heart failure patients with CKD: Experience from an inter‐disciplinary clinic. ESC Heart Fail 2020;7:3225‐3230. doi: 10.1002/ehf2.12796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet Elsevier BV 2013;381:752‐762. doi: 10.1016/S0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Campbell AJ, Buchner DM. Unstable disability and the fluctuations of frailty. Age Ageing 1997;26:315‐318. doi: 10.1093/ageing/26.4.315 [DOI] [PubMed] [Google Scholar]
- 8. Roshanravan B, Khatri M, Robinson‐Cohen C, Levin G, Patel KV, De Boer IH, et al. A prospective study of frailty in nephrology‐referred patients with CKD. Am J Kidney Dis 2012;60:912‐921. doi: 10.1053/j.ajkd.2012.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nwadiugwu M. Frailty and the risk of polypharmacy in the older person: Enabling and preventative approaches. J Aging Res doi: 10.1053/j.ajkd.2012.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vitale C, Jankowska E, Hill L, Piepoli M, Doehner W, Anker SD. Heart failure Association of the European Society of cardiology position paper on frailty in patients with heart failure. Eur J Heart Fail 2019;21:1299‐1305. doi: 10.1002/ejhf.1611 [DOI] [PubMed] [Google Scholar]
- 11. Soni RK, Weisbord SD, Unruh ML. Health‐related quality of life outcomes in chronic kidney disease. Curr Opin Nephrol Hypertens 2010;19:153‐159. doi: 10.1097/MNH.0b013e328335f939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shlipak MG, Stehman‐Breen C, Fried LF, Song X, Siscovick D, Fried LP. The presence of frailty in elderly persons with chronic renal insufficiency. Am J Kidney Dis 2004;43:861‐867. doi: 10.1053/j.ajkd.2003.12.049 [DOI] [PubMed] [Google Scholar]
- 13. Bao Y, Dalrymple L, Chertow GM, Kaysen GA, Johansen KL. Frailty, dialysis initiation, and mortality in end‐stage renal disease. Arch Intern Med 2012;172:1071‐1077. doi: 10.1001/archinternmed.2012.3020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johansen KL, Chertow GM, Jin C, Kutner NG. Significance of frailty among dialysis patients. J Am Soc Nephrol 2007;18:2960‐2967. doi: 10.1681/ASN.2007020221 [DOI] [PubMed] [Google Scholar]
- 15. Reese PP, Cappola AR, Shults J, Townsend RR, Gadegbeku CA, Anderson C, et al. Physical performance and frailty in chronic kidney disease. Am J Nephrol 2013;38:307‐315. doi: 10.1159/000355568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mansur HN, Colugnati FA, Grincenkov FRDS, Bastos MG. Frailty and quality of life: A cross‐sectional study of Brazilian patients with pre‐dialysis chronic kidney disease. Health Qual Life Outcomes 2014;12:27. doi: 10.1186/1477-7525-12-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cacciatore F, Abete P, Mazzella F, Viati L, Della Morte D, D'Ambrosio D. Frailty predicts long‐term mortality in elderly subjects with chronic heart failure. Eur J Clin Invest 2005;35:723‐730. doi: 10.1111/j.1365-2362.2005.01572.x [DOI] [PubMed] [Google Scholar]
- 18. McNallan SM, Chamberlain AM, Gerber Y, Singh M, Kane RL, Weston SA. Measuring frailty in heart failure: A community perspective. Am Heart J 2013;166:768‐774. doi: 10.1016/j.ahj.2013.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community‐dwelling older persons: A systematic review. J Am Geriatr Soc 2012;60:1487‐1492. doi: 10.1111/j.1532-5415.2012.04054.x [DOI] [PubMed] [Google Scholar]
- 20. Yin S, Njai R, Barker L, Siegel PZ, Liao Y. Summarizing health‐related quality of life (HRQOL): Development and testing of a one‐factor model. Popul Health Metr 2016;14:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Avramovic M, Stefanovic V. Health‐related quality of life in different stages of renal failure. Artif Organs 2012;36:581‐589. doi: 10.1111/j.1525-1594.2011.01429.x [DOI] [PubMed] [Google Scholar]
- 22. Lee H, Oh YJ, Kim M, Kim H, Lee JP, Kim S. The association of moderate renal dysfunction with impaired preference‐based health‐related quality of life: Third Korean national health and nutritional examination survey. BMC Nephrol 2012;13:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gallagher AM, Lucas R, Cowie MR. Assessing health‐related quality of life in heart failure patients attending an outpatient clinic: A pragmatic approach. ESC Heart Fail 2019;6:3‐9. doi: 10.1002/ehf2.12363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee SJ, Son H, Shin SK. Influence of frailty on health‐related quality of life in pre‐dialysis patients with chronic kidney disease in Korea: A cross‐sectional study. Health Qual Life Outcomes 2015;13: doi: 10.1186/s12955-015-0270-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Buck HG, Riegel B. The impact of frailty on health related quality of life in heart failure. Eur J Cardiovasc Nurs 2011;10:159‐166. doi: 10.1016/j.ejcnurse.2010.06.001 [DOI] [PubMed] [Google Scholar]
- 26. Uchmanowicz I, Gobbens RJJ. The relationship between frailty, anxiety and depression, and health‐related quality of life in elderly patients with heart failure. Clin Interv Aging 2015;10:1595‐1600. doi: 10.2147/CIA.S90077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Warraich HJ, Kitzman DW, Whellan DJ, Duncan PW, Mentz RJ, Pastva AM. Physical function, frailty, cognition, depression, and quality of life in hospitalized adults ≥60 years with acute decompensated heart failure with preserved versus reduced ejection fraction. Circ Heart Fail 2018;11:e005254. doi: 10.1161/CIRCHEARTFAILURE.118.005254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lien CTC, Gillespie ND, Struthers AD, McMurdo MET. Heart failure in frail elderly patients: Diagnostic difficulties, co‐morbidities, polypharmacy and treatment dilemmas. Eur J Heart Fail 2002;4:91‐98. doi: 10.1016/S1388-9842(01)00200-8 [DOI] [PubMed] [Google Scholar]
- 29. Authors/Task Force Members , McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail 2022;24:4‐131. doi: 10.1002/ejhf.2333 [DOI] [PubMed] [Google Scholar]
- 30. Jisc Online Surveys (Formerly BOS). In 2023. Accessed 10 October 2023. https://www.onlinesurveys.ac.uk, doi: 10.3233/JPD-230168. [DOI]
- 31. Romero‐Ortuno R, Forsyth DR, Wilson KJ, Cameron E, Wallis S, Biram R. The association of geriatric syndromes with hospital outcomes. J Hosp Med 2017;12:83‐89. doi: 10.12788/jhm.2685 [DOI] [PubMed] [Google Scholar]
- 32. Singh M, Stewart R, White H. Importance of frailty in patients with cardiovascular disease. Eur Heart J 2014;35:1726‐1731. doi: 10.1093/eurheartj/ehu197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Church S, Rogers E, Rockwood K, Theou O. A scoping review of the clinical frailty scale. BMC Geriatr 2020;20:393. doi: 10.1186/s12877-020-01801-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Woods NF, LaCroix AZ, Gray SL, Aragaki A, Cochrane BB, Brunner RL. Frailty: Emergence and consequences in women aged 65 and older in the Women's Health Initiative observational study. J Am Geriatr Soc 2005;53:1321‐1330. doi: 10.1111/j.1532-5415.2005.53405.x [DOI] [PubMed] [Google Scholar]
- 35. Li CY, Al Snih S, Chen NW, Markides KS, Sodhi J, Ottenbacher KJ. Validation of the modified frailty phenotype measure in older Mexican Americans. J Am Geriatr Soc 2019;67:2393‐2397. doi: 10.1111/jgs.16104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Denfeld QE, Winters‐Stone K, Mudd JO, Gelow JM, Kurdi S, Lee CS. The prevalence of frailty in heart failure: A systematic review and meta‐analysis. Int J Cardiol 2017;236:283‐289. doi: 10.1016/j.ijcard.2017.01.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. DeOreo PB. Hemodialysis patient‐assessed functional health status predicts continued survival, hospitalization, and dialysis‐attendance compliance. Am J Kidney Dis 1997;30:204‐212. doi: 10.1016/S0272-6386(97)90053-6 [DOI] [PubMed] [Google Scholar]
- 38. Carmichael P, Popoola J, John I, Stevens PE, Carmichael AR. Assessment of quality of life in a single Centre dialysis population using the KDQOL‐SF (tm) questionnaire. Qual Life Res 2000;9:195‐205. doi: 10.1023/A:1008933621829 [DOI] [PubMed] [Google Scholar]
- 39. Ware JEJ, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Med Care 1992;30:473‐483. doi: 10.1097/00005650-199206000-00002 [DOI] [PubMed] [Google Scholar]
- 40. Bunevicius A. Reliability and validity of the SF‐36 health survey questionnaire in patients with brain tumors: A cross‐sectional study. Health Qual Life Outcomes 2017;15: doi: 10.1186/s12955-017-0665-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. ggplot2 Based Publication Ready Plots. Accessed May 21, 2023. https://rpkgs.datanovia.com/ggpubr/
- 42. Jankowska EA, Liu PP, Cowie MR, Groenhart M, Cobey KD, Howlett J, et al. Personalized care of patients with heart failure: are we ready for a REWOLUTION? Insights from two international surveys on healthcare professionals' needs and patients' perceptions. Eur J Heart Fail 2023;25:364‐372. doi: 10.1002/ejhf.2798 [DOI] [PubMed] [Google Scholar]
- 43. Vitale C, Spoletini I, Rosano GM. Frailty in heart failure: Implications for management. Card Fail Rev 2018;4:104‐106. doi: 10.15420/cfr.2018.22.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tan EJ, Rebok GW, Yu Q, Frangakis CE, Carlson MC, Wang T, et al. The long‐term relationship between high‐intensity volunteering and physical activity in older African American women. J Gerontol B Psychol Sci Soc Sci 2009;64:304‐311. doi: 10.1093/geronb/gbn023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fried LP, Carlson MC, Freedman M, Frick KD, Glass TA, Hill J, et al. A social model for health promotion for an aging population: Initial evidence on the experience corps model. J Urban Health Bull N Y Acad Med 2004;81:64‐78. doi: 10.1093/jurban/jth094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dent E, Martin FC, Bergman H, Woo J, Romero‐Ortuno R, Walston JD. Management of frailty: Opportunities, challenges, and future directions. The Lancet 2019;394:1376‐1386. doi: 10.1016/S0140-6736(19)31785-4 [DOI] [PubMed] [Google Scholar]
- 47. Clarkson MJ, Bennett PN, Fraser SF, Warmington SA. Exercise interventions for improving objective physical function in patients with end‐stage kidney disease on dialysis: A systematic review and meta‐analysis. Am J Physiol Renal Physiol 2019;316:F856‐F872. doi: 10.1152/ajprenal.00317.2018 [DOI] [PubMed] [Google Scholar]
- 48. Butt JH, Jhund PS, Belohlávek J, De Boer RA, Chiang CE, Desai AS, et al. Efficacy and safety of dapagliflozin according to frailty in patients with heart failure: A prespecified analysis of the DELIVER trial. Circulation 2022;146:1210‐1224. doi: 10.1161/CIRCULATIONAHA.122.061754 [DOI] [PMC free article] [PubMed] [Google Scholar]


