Abstract
Aims
Hypertonic saline solution (HSS) plus intravenous (IV) loop diuretic appears to enhance the diuretic response in patients hospitalized for heart failure (HF). The efficacy and safety of this therapy in the ambulatory setting have not been evaluated. We aimed to describe the design and baseline characteristics of the SALT‐HF trial participants.
Methods and results
‘Efficacy of Saline Hypertonic Therapy in Ambulatory Patients with HF’ (SALT‐HF) trial was a multicenter, double‐blinded, and randomized study involving ambulatory patients who experienced worsening heart failure (WHF) without criteria for hospitalization. Enrolled patients had to present at least two signs of volume overload, use ≥ 80 mg of oral furosemide daily, and have elevated natriuretic peptides. Patients were randomized 1:1 to treatment with a 1‐h infusion of IV furosemide plus HSS (2.6–3.4% NaCl depending on plasmatic sodium levels) versus a 1‐h infusion of IV furosemide at the same dose (125–250 mg, depending on basal loop diuretic dose). Clinical, laboratory, and imaging parameters were collected at baseline and after 7 days, and a telephone visit was planned after 30 days. The primary endpoint was 3‐h diuresis after treatment started. Secondary endpoints included (a) 7‐day changes in congestion data, (b) 7‐day changes in kidney function and electrolytes, (c) 30‐day clinical events (need of IV diuretic, HF hospitalization, cardiovascular mortality, all‐cause mortality or HF‐hospitalization).
Results
A total of 167 participants [median age, 81 years; interquartile range (IQR), 73–87, 30.5% females] were randomized across 13 sites between December 2020 and March 2023. Half of the participants (n = 82) had an ejection fraction >50%. Most patients showed a high burden of comorbidities, with a median Charlson index of 3 (IQR: 2–4). Common co‐morbidities included diabetes mellitus (41%, n = 69), atrial fibrillation (80%, n = 134), and chronic kidney disease (64%, n = 107).
Patients exhibited a poor functional NYHA class (69% presenting NYHA III) and several signs of congestion. The mean composite congestion score was 4.3 (standard deviation: 1.7). Ninety per cent of the patients (n = 151) presented oedema and jugular engorgement, and 71% (n = 118) showed lung B lines assessed by ultrasound. Median inferior vena cava diameter was 23 mm, (IQR: 21–25), and plasmatic levels of N‐terminal‐pro‐B‐type natriuretic peptide (NTproBNP) and antigen carbohydrate 125 (CA125) were increased (median NT‐proBNP 4969 pg/mL, IQR: 2508–9328; median CA125 46 U/L, IQR: 20–114).
Conclusions
SALT‐HF trial randomized 167 ambulatory patients with WHF and will determine whether an infusion of hypertonic saline therapy plus furosemide increases diuresis and improves decongestion compared to equivalent furosemide administration alone.
Keywords: Diuretic resistance, Hypertonic saline solution, Hypertonic therapy, Outpatient with heart failure
Introduction
The traditional model of managing worsening heart failure (WHF) in both inpatient and outpatient settings has several inherent challenges. Although essential for patients exhibiting severe symptoms such as respiratory failure and unstable arrhythmia, hospitalization is not always needed in patients in whom volume overload is the main driver of worsening symptoms. 1 In fact, the reliance on hospitalization is sometimes primarily due to the convenience of administering IV diuretic therapy and conducting close clinical and laboratory monitoring, highlighting a gap in outpatient care options for WHF patients. 2 This underscores the need to explore alternative, potentially more efficient, outpatient treatment modalities for managing WHF effectively.
Recognizing that diuresis is the primary intervention in patients hospitalized for HF decompensations and that some patients quickly improve with therapy (i.e. within hours), HF clinics have emerged as outpatient models aiming to provide comprehensive care where patients may obtain same‐day or walk‐in visits for worsening symptoms rather than a potential visit to the emergency department. 3 , 4 However, although some diuretic protocols have been proposed, 1 , 2 , 5 no randomized trials have evaluated different diuretic strategies in the outpatient setting.
Observational and randomized trials have evaluated IV furosemide and hypertonic saline solution (HSS) in hospitalized patients with acute HF. This therapy has been associated with increased diuretic efficiency, fluid and weight loss, and a decreased incidence of HF rehospitalizations. 6 , 7 , 8 , 9 , 10
However, the efficacy and safety of this approach in the ambulatory setting have not been evaluated. This study aims to bridge this gap by assessing both efficacy and safety, as well as feasibility, of this combined therapy (HSS plus IV furosemide) in ambulatory patients with WHF and systemic fluid overload.
Study design
The SALT‐HF trial was a multicenter, double‐blinded, and randomized trial involving ambulatory patients who presented an episode of WHF that required IV diuretics and without criteria for hospital admission at the treating physician's discretion. Patients were randomized to treatment with a 60‐min infusion of IV furosemide (125–250 mg) plus HSS (intervention group) versus an infusion of IV furosemide (125–250 mg) without HSS (control group), as is shown in Figure 1 .
Figure 1.
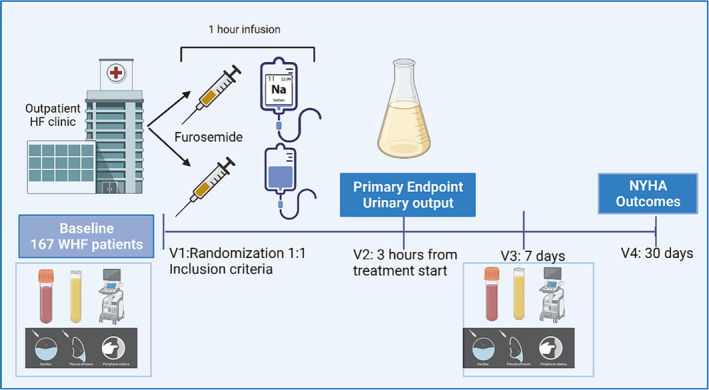
SALT‐HF trial design.
The research team conducted training sessions on the design and implementation of the protocol before and during the start of the study.
The local institutional ethics committees approved the trial, and it was conducted in accordance with the Declaration of Helsinki and the International Conference of Harmonization Guidelines for Good Clinical Practice. All participants provided written informed consent. The trial was registered at ClinicalTrials.gov (NCT04533997).
Eligibility
The study's inclusion and exclusion criteria are listed in Table 1 . Patients were eligible if they presented with WHF and at least two signs of volume overload (peripheral oedema, jugular enlargement, ascites, or pleural effusion) and had an N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) > 1000 pg/mL or a B‐type natriuretic peptide (BNP) > 250 ng/mL. In addition, patients should be treated with oral loop diuretics for ≥1 month before inclusion at a dose of ≥80 mg of furosemide or ≥40 mg of torsemide per day. The diagnosis of HF was assessed by the treating physician based on the current HF guidelines. 11 , 12 , 13 Key exclusion criteria included any of the following: cardiogenic shock, renal replacement therapy, severe metabolic derangements, or other high‐risk criteria that would require hospitalization.
Table 1.
Eligibility criteria of SALT‐HF trial
| Inclusion criteria |
|
| Exclusion criteria |
|
NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
The study did not include individuals with acute pulmonary oedema or basal oxygen saturation below 90%.
Objectives and endpoints
The primary objective of the SALT‐HF trial was to test whether the administration of HSS plus IV furosemide can improve decongestion over IV furosemide in WHF outpatients with predominant systemic volume overload. The hypothesis was that the combination therapy increases the diuresis volume 3 h after the start of treatment.
Primary endpoint
Diuresis after 3 h of treatment start was selected as the primary endpoint.
Secondary endpoints
Secondary endpoints included between‐treatment changes in (a) urinary sodium and body weight 3 h after treatment, (b) 7‐day changes in congestion parameters that included the composite congestion score, the body weight, the diameter of inferior vena cava, the presence of lung B‐lines by ultrasound, haemoconcentration parameters (haematocrit, albumin and proteins), and circulating biomarkers such as NT‐proBNP, antigen carbohydrate 125 (CA125), and urinary sodium, (c) 7‐day changes in NYHA and visual analogue scale (Table 2 ).
Table 2.
Study endpoints of SALT‐HF trial
| Primary endpoint |
| Total diuresis after 3 h of the start of treatment |
| Secondary endpoints |
|
| Safety endpoints |
|
CA125, antigen carbohydrate 125; CV, cardiovascular; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Association; WHF, worsening heart failure.
The decision to evaluate secondary clinical endpoints at 7 days was made to provide a pragmatic approach in line with routine clinical practice.
Safety endpoints
The safety endpoints included (a) 7‐day worsening of kidney function defined as an increase in serum creatinine ≥0.3 mg/dL, (b) electrolyte abnormalities defined as hypokalaemia (K+ < 3.5 mEq/L) or hyperkalaemia (K+ > 5.5 mEq/L), (c) WHF that required IV ambulatory diuretic, emergency department visit or HF rehospitalization at day 30, (d) CV mortality on day 30, and (e) all‐cause mortality and HF hospitalization at day 30 (Table 2 ).
Study intervention and procedures
The study flowchart is depicted in Figure 1 , and a summary of the procedures in each visit is presented in Table 3 .
Table 3.
Procedures of SALT‐HF trial
| Visits | Visit 1: Screening and randomization | Visit 2: 3‐h post‐treatment | Visit 3: 7 days | Visit 4: 30 days |
|---|---|---|---|---|
| Eligibility | x | |||
| Medical History | x | |||
| NYHA | x | x | x | |
| Visual analogue scale | x | x | ||
| ECG | x | x | ||
| Weight | x | x | x | |
| Blood pressure | x | x | x | |
| Diuresis | x | |||
| Congestion score | x | x | ||
| Local labs including haemoglobin, serum sodium, glucose, potassium, and kidney function measures | x | x | ||
| NT‐proBNP and CA125 | x | x | ||
| Urine sample | x | x | x | |
| Medication | x | X | x | |
| Randomization | x | |||
| Lung B‐lines (echo) | x | x | ||
| Inferior vena cava diameter | x | x | ||
| Therapy optimization | x | x | x | |
| Events including endpoints and adverse events | x | x |
CA125, antigen carbohydrate 125; ECG, electrocardiogram; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Association.
Visit 1: Screening and randomization
Patients meeting the inclusion criteria, with prior informed consent, were randomized 1:1 to treatment with IV furosemide plus HSS (intervention group) versus IV furosemide (control group) using a stratified block randomization method based on an automated online system, blinded to the physicians who evaluated the patient. Randomization was performed by a trained HF nurse in a separate room. The patient and the treating physician were blinded to the assigned treatment.
Before the start of treatment, data were collected on patient demographics, medical history, and medical and device therapy at baseline. Blood and urine tests were collected at baseline and analysed in the local laboratory at each centre.
A complete clinical evaluation that included vital signs, ECG, NYHA functional class, a visual analogue scale from 0 (worst state of health) to 100 (best state of health), 14 and a congestion multiparametric assessment was performed. 15
The multiparametric approach included
The composite congestion score [composed of the sum of orthopnea (0–3; 0: none, 1: seldom, 2: frequent, 3: continuous), leg oedema (0–3; 0: absent, 1: slight, 2: moderate, 3: marked), and jugular engorgement (cm H2O), (0–3; 0: ≤6, 1: 6–9, 2: 10–15, 3: ≥15)]. 16
Imaging parameters as the inferior vena cava diameter and a protocolized evaluation of lung B‐lines by ultrasound (Data S1). The presence of lung B‐lines was considered positive when two lung fields presented ≥3 B‐lines bilaterally. 17
Haematocrit, plasmatic albumin, and proteins as parameters of hemoconcentration.
Biomarkers that included natriuretic peptides and CA125 plasmatic levels 18 and urinary sodium.
Treatment preparation and administration
After randomization, the HF nurse prepared the treatment in a separate room. The infusion consisted of a fixed furosemide dose that depended on the previous patient's home dose, administered in 100 mL of 0.9% NaCl physiological solution for 1 h (Table 4 ).
Table 4.
Infusion preparation and placebo and treatment dose in SALT‐HF trial
| Patient oral daily dose | IV furosemide | 1‐h infusion | HSS group | Potassium supplements |
|---|---|---|---|---|
| Furosemide 80–160 mg | 125 mg | 100 ml of 0.9%NaCl physiological solution |
Na+: 125–135 mEq/L: 10 mL NaCl20% (2.6%) Na+: 135–145 mEq/L: 15 ml NaCl20% (3.4%) |
K+: 3.5–4 mEq/L: 16–20 mEq of oral potassium. |
|
Furosemide > 160 mg |
250 mg |
HSS, hypertonic saline solution; IV, intravenous; po, orally.
Patients with a home furosemide dose or equivalent equal to or inferior to 160 mg received 125 mg of furosemide. Patients with a home furosemide dose or equivalent superior to 160 mg received 250 mg of furosemide (Table 4 ). Torsemide was converted to the furosemide equivalent dose: 2 mg of oral furosemide was considered equivalent to 1 mg of oral torsemide.
In the absence of clear guidance from previous studies, or robust evidence supporting the use of double the home oral dose of loop diuretic, the SALT‐HF diuretic dose strategy was based on local protocols that had previously evaluated the safety of this diuretic approach. 19
In the group of patients randomized to HSS therapy, sodium chloride 200 mg/mL (10–15 mL) was added, depending on the patient's plasmatic sodium (2.6% HSS for patients with plasmatic sodium from 135 to 145 mEq/L, 3.4% HSS for patients with plasmatic sodium from 125 to 135 mEq/L).
Urine collection and sampling
Patients were asked to void empty before the administration of the infusion. From then on, the treatment, as well as the urine collection, started. The infusion was administered for 1 h, and the diuresis was collected for 3 h. Special care was taken to ensure that all urine was collected. The patient was advised to avoid food or liquid intake during this period.
The patients received the treatment and were monitored in a dedicated on‐site IV infusion space. All the participant centers (n = 13, Data S2) have well‐structured HF programmes led by specialized HF physicians and nurses.
Visit 2: 3‐h post‐treatment
Three hours after the start of the infusion, diuresis volume, blood pressure, and body weight were evaluated, and a new urine sample was collected.
To prevent heterogeneity in the treatment approach in the following days, we proposed a diuretic protocol adjustment at the time of discharge. Due to the potential risk of hypokalemia during diuretic treatment, the protocol also included recommendations about potassium supplements to mitigate the risk of hypokalaemia (Data S3).
Briefly, an increase in the diuretic treatment or combination therapy was recommended if no cause for decompensation was present. No other HF therapy modifications were allowed during the first 7 days.
Visit 3: 7‐day post‐treatment
Seven days after randomization, a new clinical and multiparametric evaluation that included all procedures of visit 1 was performed (Table 3 ). A 7‐day evaluation was set to offer a pragmatic approach similar to real‐life practice. Concomitant medication and adverse events, including any hospitalizations or deaths between treatment and day seven were recorded. Further therapy and changes in any medication at this stage were left to the treating physician's discretion.
Visit 4: 30‐day post‐treatment
Randomized patients were contacted by telephone 30 days following completion of the study treatment period to assess vital status, NYHA, the occurrence of adverse events, and current prescriptions for HF medications.
Statistical plan
Sample size and power calculation
The SALT‐HF trial was powered for its primary endpoint: diuresis after 3 h. Observational studies about diuretics in outpatients reported a 3‐h diuresis of 1100 mL. A similar diuresis after 3 h was considered in the standard of care group (IV furosemide) for sample size calculation. An increase in diuresis of 20% was deemed both achievable and clinically relevant. Assuming a two‐sided alpha of 0.05 and a statistical power of 80%, a sample size of 168 patients was calculated.
Statistical analysis
Continuous variables will be expressed as means (±1 standard deviation [SD]) or medians (interquartile range [IQR]), and discrete variables as percentages. At baseline, the means, medians, and frequencies between treatment groups will be compared using the t‐test, Wilcoxon test, and chi‐square test, respectively.
The primary endpoint (3‐h diuresis) between treatments will be analysed by linear regression analysis. Secondary endpoints (changes in congestion, changes in kidney function, and changes in electrolytes) will be evaluated by linear regression analysis, including the baseline value of the endpoint as a covariate (ANCOVA). For 30‐day adverse clinical events, a Cox regression analysis will be performed. Because of hierarchical levels of nesting (treatment sequence within patient ID and the latter among study centers), the models will include patient ID and study centre as random intercepts. All statistical comparisons will be performed under a modified intention‐to‐treat principle.
Current status
The SALT‐HF trial is complete and is currently in the analysis phase. One hundred sixty‐eight participants were randomized across 13 sites between December 4, 2020, and March 31, 2023. One patient had to be excluded due to screening failure (Figure 2 ). Baseline characteristics of the 167 patients did not present significant differences between the two groups across most parameters (Table 5 ). The SALT‐HF trial encompassed an elderly population [median age: 81 years (IQR: 73–87), 30.5% females] with a high burden of co‐morbidities such as diabetes, hypertension, atrial fibrillation, chronic obstructive pulmonary disease, and chronic kidney disease. Approximately half of the participants had an ejection fraction >50%. Most patients exhibited a poor functional NYHA class and several signs of congestion. Natriuretic peptides and CA125 were elevated at baseline. The chronic dose of diuretic was high (median furosemide dose: 120 mg), and the use of combination therapy was common (one‐third of the patients were on treatment with SGLT2i and/or thiazides and half of them received mineralocorticoid receptor antagonists).
Figure 2.
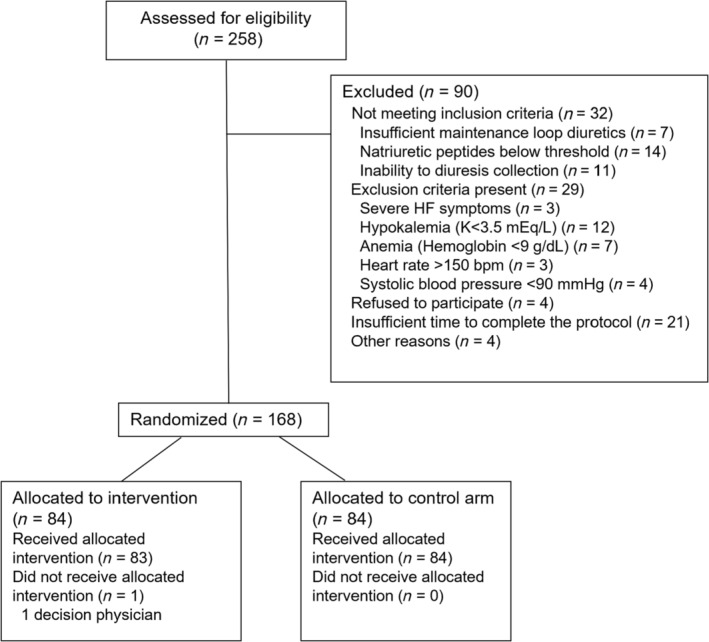
Flow diagram of patient inclusion.
Table 5.
Baseline demographics and clinical characteristics of the SALT‐HF trial population
| Parameter | Statistic | SALT‐HF trial (N = 167) | HSS + furosemide (n = 83) | IV furosemide (N = 84) | P‐value |
|---|---|---|---|---|---|
| Demographics and medical history | |||||
| Age (years) | Median (IQR) | 81 (73–87) | 83 (74–88) | 80 (73–86) | 0.072 |
| Female | n (%) | 51 (30.5) | 27 (32.5) | 24 (28.5) | 0.579 |
| Charlson index | Median (IQR) | 3 (2–4) | 3 (2–4) | 3 (2–4) | 0.677 |
| Diabetes mellitus | n (%) | 69 (41.4) | 32 (38.5) | 37 (44.0) | 0.398 |
| Hypertension | n (%) | 143 (85.6) | 68 (81.9) | 75 (89.3) | 0.175 |
| Hypercholesterolemia | n (%) | 93 (55.7) | 39 (46.9) | 54 (64.3) | 0.024 |
| Atrial fibrillation | n (%) | 134 (80.2) | 66 (9.5) | 68 (80.9) | 0.816 |
| COPD | n (%) | 49 (29.3) | 25 (30.1) | 24 (28.6) | 0.826 |
| CKD | n (%) | 107 (64.5) | 50 (60.1) | 57 (67.8) | 0.279 |
| Ischaemic cardiomyopathy | n (%) | 48 (28.7) | 21 (25.3) | 27 (32.1) | 0.928 |
| Valvular heart disease | n (%) | 34 (20.4) | 24 (28.9) | 10 (11.9) | 0.133 |
| Vital signs and basal assessment | |||||
| NYHA | n (%) | 0.907 | |||
| I | 3 (1.8) | 2 (2.4) | 1 (1.2) | ||
| II | 46 (27.5) | 24 (28.9) | 22 (26.2) | ||
| III | 116 (69.5) | 56 (67.4) | 60 (71.4) | ||
| IV | 2 (1.2) | 1 (1.2) | 1 (1.2) | ||
| Analogue visual scale | Mean (SD) | 54.6 (18.7) | 55.4 (19.0) | 53.8 (18.4) | 0.695 |
| Composite congestion score | Mean (SD) | 4.3 (1.7) | 4.2 (1.7) | 4.3 (1.6) | 0.651 |
| Jugular engorgement | n (%) | 151(91.4) | 74 (89.2) | 77 (91.2) | 0.582 |
| Orthopnoea | n (%) | 113 (67.7) | 53 (63.9) | 60 (71.4) | 0.296 |
| Lower limb oedema | n (%) | 151 (90.4) | 78 (90.4) | 73 (86.9) | 0.121 |
| Systolic blood pressure (mmHg) | Median (IQR) | 118 (107–131) | 116 (105–128) | 122 (110–133) | 0.022 |
| Heart rate (b.p.m.) | Median (IQR) | 70 (65–81) | 71 (65–82) | 70 (63–80) | 0.540 |
| Weight (kg) | Median (IQR) | 76.3 (66.5–86) | 74.9 (65.0–84.8) | 78.0 (68.5–88.0) | 0.236 |
| Echocardiography | |||||
| LVEF (%) | Median (IQR) | 50 (38–60) | 50 (40–60) | 50 (35–60) | 0.453 |
| HFrEF | n (%) | 44 (26.3) | 20 (24.1) | 24 (28.5) | 0.602 |
| HFmEF | n (%) | 41 (24.6) | 23 (27.7) | 18 (21.4) | |
| HFpEF | n (%) | 82 (49.1) | 40 (48.1) | 42 (50.0) | |
| Inferior vena cava (mm) | Median (IQR) | 23 (21–25) | 23 (21–26) | 23 (21–25) | 0.457 |
| Presence of lung B lines a | n (%) | 118 (71.0) | 59 (71.0) | 59 (70.2) | 1.000 |
| Laboratory data | |||||
| Haemoglobin (g/dL) | Median (IQR) | 12.0 (10.8–13.4) | 11.7 (10.8–13.0) | 12.1 (10.8–13.7) | 0.560 |
| Haematocrit (%) | Median (IQR) | 36.9 (33.2–42.0) | 36.9 (34–41.9) | 37.0 (33.0–43.0) | 0.941 |
| eGFR (mL/min/1.73m2) | Median (IQR) | 40.2 (29.3–53.1) | 40.3 (29.4–54.6) | 39.1 (28.7–52.2) | 0.354 |
| Creatinine (mg/dL) | Median (IQR) | 1.5 (1.1–1.9) | 1.5 (1.1–1.8) | 1.5 (1.2–2.0) | 0.160 |
| Sodium (mEq/L) | Median (IQR) | 140 (137–141) | 140 (137–142) | 139 (137–141) | 0.803 |
| Potassium (mEq/L) | Median (IQR) | 4.2 (3.9–4.5) | 4.2 (3.9–4.5) | 4.1 (3.7–4.4) | 0.220 |
| Chloride (mEq/L) | Median (IQR) | 101 (99–104) | 101 (99–104) | 100 (98–104) | 0.239 |
| NT‐proBNP (pg/mL) | Median (IQR) | 4969 (2508–9328) | 5302 (2467–9790) | 4851 (2546–8770) | 0.875 |
| CA125 (U/mL) | Median (IQR) | 46 (20–114) | 40 (20–94) | 56 (19–123) | 0.630 |
| Urinary sodium (mEq/L) | Median (IQR) | 67 (43–88) | 70 (48–89) | 65 (36–88) | 0.379 |
| Treatment | |||||
| Furosemide (mg) | Median (IQR) | 120 (80–160) | 120 (80–160) | 120 (80–160) | 0.117 |
| Thiazides | n (%) | 47 (28.1) | 25 (30.1) | 22 (26.2) | 0.572 |
| Mineral receptor antagonists | n (%) | 81 (48.5) | 36 (43.4) | 45 (53.6) | 0.187 |
| Acetazolamide | n (%) | 8 (4.8) | 5 (6.2) | 3 (3.6) | 0.469 |
| Beta‐blockers | n (%) | 116 (69.5) | 60 (72.3) | 56 (66.7) | 0.430 |
| RAASi | n (%) | 91 (54.5) | 47 (56.6) | 44 (52.3) | 0.582 |
| SGLT2i | n (%) | 61 (36.5) | 25 (30.1) | 36 (42.9) | 0.087 |
CA125, antigen carbohydrate 125; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; HFmEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; RAASi, renin‐angiotensin‐aldosterone system inhibitors; SGLT2i, sodium‐glucose co‐transporter‐2 inhibitors.
Considered positive when ≥3 B‐lines were bilaterally observed in ≥2 lung fields.
Discussion
The SALT‐HF trial will evaluate whether HSS plus IV furosemide therapy is safe and more effective in improving diuretic response than IV furosemide in ambulatory patients suffering WHF, a subgroup frequent in clinical practice but underrepresented in clinical trials. The ultimate goal is to provide novel insights into diuretic strategies that may help relieve congestion and prevent HF hospitalizations.
Outpatient management of worsening heart failure
A substantial rise in HF burden in the Western population is projected for the next decades. 20 We observed that patients included in the SALT‐HF trial were significantly older and had more co‐morbidities than previously reported series of ambulatory HF patients. 10 Beyond the significant burden on healthcare costs, HF hospitalizations are associated with a further increased risk of death and worsening quality of life. 21 , 22 , 23 Therefore, a shift from the classic hospital‐centric model to ambulatory WHF management strategies is of growing interest to both patients and healthcare providers.
Multidisciplinary HF management programmes are recommended (class IA) in HF guidelines to reduce hospitalizations and mortality. 11 , 12 Even though guidelines describe the characteristics and components of HF programmes, they do not provide any recommendations about diuretic approaches for ambulatory worsening HF, and the management of these patients remains empirical. To address this gap, the Heart Failure Working Group of the French Society of Cardiology has recently published a document about the practical outpatient management of WHF. 2 The document defines ‘outpatient HF’ as the worsening of HF signs and symptoms in a patient with chronic HF that requires escalation of therapy without an urgent need for hospitalization. The stratification of patients who will not require hospital admission in the first instance is one of the key elements for a successful ambulatory approach. Determinant clinical scenarios, HF profiles, co‐morbidities, and social criteria should be considered to determine the feasibility and safety of outpatient management. SALT‐HF inclusion and exclusion criteria define the clinical profile most likely to fit an ambulatory IV diuretic programme.
Diuretic approach in the outpatient setting
Unfortunately, limited data exist regarding IV diuretic strategies and outcomes in ambulatory WHF. The document about the practical outpatient management of WHF proposes a standardized diuretic protocol based on data from the largest study that has evaluated an outpatient IV diuretic approach. 2 Briefly, Buckley et al. assessed the diuretic response and outcomes in 283 patients with WHF. 1 The diuretic protocol consisted of a 3‐h IV diuretic infusion based on the furosemide equivalent of patient's home oral diuretic total daily dose. This strategy was associated with significant urine output and weight loss. This and other observational studies suggest that an IV‐diuretic ambulatory approach may provide an alternative to hospitalization for the management of selected patients with HF. 3 , 4
On the other hand, diuresis after 3 h of treatment was selected as the primary endpoint of our study because (i) urinary output is commonly used as a metric of loop diuretic efficacy, 24 (ii) the direct effect of loop diuretics is increasing diuresis, 25 (iii) urinary output is an objective and reproducible endpoint, not open for bias, (iv) 3‐h diuresis has been evaluated in observational studies assessing ambulatory diuretic treatment. 1 , 5
Hypertonic saline therapy in worsening heart failure
Observational studies, randomized trials, and metanalysis have shown the potential benefits of HSS plus IV loop diuretic in improving diuretic response, kidney function, and outcomes in patients hospitalized with WHF. 10 , 26 , 27
However, the differences in the population included in the studies and the heterogeneity in the infusion preparation or the diuretic dose (Data S4) have limited the adoption of this therapy in clinical practice. In addition, many physicians often struggle with administering sodium in patients who present with fluid overload. We specifically excluded patients with pulmonary oedema or low oxygen saturation.
Therefore, in this trial, we will assess the efficacy and safety of this therapeutical approach in patients with predominant tissue systemic volume overload, which includes patients with lower limb oedema, ascites, and/or pleural effusion. We hypothesize that the administration of HSS may improve the diuretic effectiveness of furosemide in patients with predominant extravascular and systemic volume overload. The rationale of this approach is the osmotic capacity of HSS, which leads to fluid mobilization from the interstitial space into the intravascular compartment, increasing intravascular volume and renal blood flow and facilitating the delivery of the diuretic agents to the nephron. 28 Although some research suggests that the blunted diuretic response observed in chronic furosemide users is primarily due to decreased tubular responsiveness rather than insufficient furosemide tubular delivery, 29 we speculate that the volume expansion and the action of IV furosemide will lead to a more efficient diuretic response in a cohort of patients with data of diuretic resistance.
Notably, the administration of chloride together with sodium may also play a role in the potential benefits of this therapy in HF patients. Several observational studies have shown the association of low chloride levels with poor diuretic response, increased neurohormonal activation, and a worse prognosis. 30 The cardiorenal effects of sodium‐free chloride supplementation are currently being tested in patients with ADHF. (Mechanism and Effects of Manipulating Chloride Homeostasis in Stable Heart Failure; NCT03440970).
The hypothesis that will be tested in the SALT‐HF trial is important in several aspects. First, there is a growing need for strategies that prevent HF hospitalizations. Second, to our knowledge, no randomized trials evaluating diuretic strategies in the outpatient setting have been performed, and treatment remains empirical. Finally, although HSS therapy appears to be a promising strategy to overcome diuretic resistance, a growing body of evidence supporting the beneficial effects may promote implementing this approach in outpatient WHF patients.
Conclusions
The SALT‐HF trial will investigate whether a combined therapy of IV furosemide with HSS can increase diuresis after 3 h compared with IV furosemide in ambulatory patients with WHF and systemic fluid overload.
Funding
This work was partially supported by grants from the Instituto de Salud Carlos III (PI20/00689). (Co‐funded by European Regional Development Fund/European Social Fund ‘A way to make Europe’/‘Investing in your future’).
Conflict of interest
None declared. All the authors have approved the manuscript.
Supporting information
Data S1: Assessment of B‐lines with lung ultrasound.
Data S2: Participant Centers of SALT‐HF trial.
Data S3: Diuretic protocol at discharge.
Data S4: Hypertonic saline solution and diuretic dose in different trials.
Cobo Marcos, M. , Comín‐Colet, J. , de la Espriella, R. , Rubio Gracia, J. , Morales‐Rull, J. L. , Zegrí, I. , Llacer, P. , Diez‐Villanueva, P. , Jiménez‐Marrero, S. , de Juan Bagudá, J. , Ortiz Cortés, C. , Goirigolzarri‐Artaza, J. , García‐Pinilla, J. M. , Barrios, E. , del Prado Díaz, S. , Montero Hernández, E. , Sanchez‐Marteles, M. , and Nuñez, J. (2024) Design and baseline characteristics of SALT‐HF trial: hypertonic saline therapy in ambulatory heart failure. ESC Heart Failure, 11: 1767–1776. 10.1002/ehf2.14720.
References
- 1. Buckley LF, Stevenson LW, Cooper IM, Knowles DM, Matta L, Molway DW, et al. Ambulatory treatment of worsening heart failure with intravenous loop diuretics: A four‐year experience. J Card Fail 2020;26:798‐799. doi: 10.1016/j.cardfail.2019.10.015 [DOI] [PubMed] [Google Scholar]
- 2. Girerd N, Mewton N, Tartière JM, Guijarro D, Jourdain P, Damy T, et al. Practical outpatient management of worsening chronic heart failure. Eur J Heart Fail 2022;24:750‐761. doi: 10.1002/ejhf.2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hebert K, Dias A, Franco E, Tamariz L, Steen D, Arcement LM. Open access to an outpatient intravenous diuresis program in a systolic heart failure disease management program. Congest Heart Fail 2011;17:309‐313. doi: 10.1111/j.1751-7133.2011.00224.x [DOI] [PubMed] [Google Scholar]
- 4. Ryder M, Murphy NF, McCaffrey D, O'Loughlin C, Ledwidge M, McDonald K. Outpatient intravenous diuretic therapy; potential for marked reduction in hospitalisations for acute decompensated heart failure. Eur J Heart Fail 2008;10:267‐272. doi: 10.1016/j.ejheart.2008.01.003 [DOI] [PubMed] [Google Scholar]
- 5. Buckley LF, Carter DM, Matta L, Cheng JW, Stevens C, Belenkiy RM, et al. Intravenous diuretic therapy for the management of heart failure and volume overload in a multidisciplinary outpatient unit. JACC Heart Fail 2016;4:‐8. doi: 10.1016/j.jchf.2015.06.017 [DOI] [PubMed] [Google Scholar]
- 6. Griffin M, Soufer A, Goljo E, Colna M, Rao VS, Jeon S, et al. Real world use of hypertonic saline in refractory acute decompensated heart failure: A U.S. center's experience. JACC Heart Fail 2020;8:199‐208. doi: 10.1016/j.jchf.2019.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. De Vecchis R, Esposito C, Ariano C, Cantatrione S. Hypertonic saline plus i.v. furosemide improve renal safety profile and clinical outcomes in acute decompensated heart failure: A meta‐analysis of the literature. Herz 2015;40:423‐435. doi: 10.1007/s00059-013-4041-6 [DOI] [PubMed] [Google Scholar]
- 8. Gandhi S, Mosleh W, Myers RBH. Hypertonic saline with furosemide for the treatment of acute congestive heart failure: A systematic review and meta‐analysis. Int J Cardiol 2014;173:139‐145. doi: 10.1016/j.ijcard.2014.03.020 [DOI] [PubMed] [Google Scholar]
- 9. Licata G, Di Pasquale P, Parrinello G, Cardinale A, Scandurra A, Follone G, et al. Effects of high‐dose furosemide and small‐volume hypertonic saline solution infusion in comparison with a high dose of furosemide as bolus in refractory congestive heart failure: long‐term effects. Am Heart J 2003;145:459‐466. doi: 10.1067/mhj.2003.166 [DOI] [PubMed] [Google Scholar]
- 10. Paterna S, Fasullo S, Parrinello G, Cannizzaro S, Basile I, Vitrano G, et al. Short‐term effects of hypertonic saline solution in acute heart failure and long‐term effects of a moderate sodium restriction in patients with compensated heart failure with New York heart association class III (class C) (SMAC‐HF study). Am J Med Sci 2011;342:27‐37. [DOI] [PubMed] [Google Scholar]
- 11. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599‐3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 12. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol [Internet] 2022;79:e263‐e421. [DOI] [PubMed] [Google Scholar]
- 13. Bozkurt B, Coats AJ, Tsutsui H, Abdelhamid M, Adamopoulos S, Albert N, et al. Universal Definition and Classification of Heart Failure: A report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail [Internet] 2021;27:387‐413. doi: 10.1002/ejhf.2115 [DOI] [PubMed] [Google Scholar]
- 14. Measurement of feelings using visual analogue scales ‐ PubMed. [DOI] [PMC free article] [PubMed]
- 15. de la Espriella R, Cobo M, Santas E, Verbrugge FH, Fudim M, Girerd N, et al. Assessment of filling pressures and fluid overload in heart failure: An updated perspective. Rev Esp Cardiol (Engl Ed) 2023;76:47‐57. doi: 10.1016/j.rec.2022.07.009 [DOI] [PubMed] [Google Scholar]
- 16. Ambrosy AP, Pang PS, Khan S, Konstam MA, Fonarow GC, Traver B, et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: Findings from the EVEREST trial †. Eur Heart J 2013;34:835‐843. doi: 10.1093/eurheartj/ehs444 [DOI] [PubMed] [Google Scholar]
- 17. Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, et al. International evidence‐based recommendations for point‐of‐care lung ultrasound. Intensive Care Med 2012;38:577‐591. doi: 10.1007/s00134-012-2513-4 [DOI] [PubMed] [Google Scholar]
- 18. Núñez J, de la Espriella R, Miñana G, Santas E, Llácer P, Núñez E, et al. Antigen carbohydrate 125 as a biomarker in heart failure: a narrative review. Eur J Heart Fail 2021;23:1445‐1457. doi: 10.1002/ejhf.2295 [DOI] [PubMed] [Google Scholar]
- 19. Portolés A, Cobo M, Zegrí, Vázquez López‐Ibor J, Soria Gómez T, Escobar López LE, et al. Eficacia y seguridad de la furosemida intravenosa junto con suero salino hipertónico en el paciente ambulatorio con insuficiencia cardiaca descompensada. Rev Esp Cardiol 2020;73:434.32198006 [Google Scholar]
- 20. Roger VL. Epidemiology of heart failure: a contemporary perspective. Circ Res 2021;128:1421‐1434. doi: 10.1161/CIRCRESAHA.121.318172 [DOI] [PubMed] [Google Scholar]
- 21. Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J 2007;154:260‐266. doi: 10.1016/j.ahj.2007.01.041 [DOI] [PubMed] [Google Scholar]
- 22. Nieminen MS, Dickstein K, Fonseca C, Serrano JM, Parissis J, Fedele F, et al. The patient perspective: Quality of life in advanced heart failure with frequent hospitalisations. Int J Cardiol 2015;191:256‐264. doi: 10.1016/j.ijcard.2015.04.235 [DOI] [PubMed] [Google Scholar]
- 23. Ambrosy AP, Khan H, Udelson JE, Mentz RJ, Chioncel O, Greene SJ, et al. Changes in dyspnea status during hospitalization and postdischarge health‐related quality of life in patients hospitalized for heart failure: findings from the EVEREST trial. Circ Heart Fail 2016;9: doi: 10.1161/CIRCHEARTFAILURE.115.002458 [DOI] [PubMed] [Google Scholar]
- 24. Testani JM, Brisco MA, Turner JM, Spatz ES, Bellumkonda L, Parikh CR, et al. Loop diuretic efficiency: A metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure. Circ Heart Fail 2014;7:261‐270. doi: 10.1161/CIRCHEARTFAILURE.113.000895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Felker GM, Ellison DH, Mullens W, Cox ZL, Testani JM. Diuretic therapy for patients with heart failure: JACC state‐of‐the‐art review. J Am Coll Cardiol 2020;75:1178‐1195. doi: 10.1016/j.jacc.2019.12.059 [DOI] [PubMed] [Google Scholar]
- 26. Lafrenière G, Béliveau P, Bégin JY, Simonyan D, Côté S, Gaudreault V, et al. Effects of hypertonic saline solution on body weight and serum creatinine in patients with acute decompensated heart failure. World J Cardiol 2017;9:685‐692. doi: 10.4330/wjc.v9.i8.685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yayla Ç, Akyel A, Canpolat U, Gayretli Yayla K, Eyiol A, Akboğa MK, et al. Comparison of three diuretic treatment strategies for patients with acute decompensated heart failure. Herz 2015;40:1115‐1120. doi: 10.1007/s00059-015-4327-y [DOI] [PubMed] [Google Scholar]
- 28. Mazzoni MC, Borgstrom P, Arfors KE, Intaglietta M. Dynamic fluid redistribution in hyperosmotic resuscitation of hypovolemic hemorrhage. Am J Physiol 1988;255:H629‐H637. doi: 10.1152/ajpheart.1988.255.3.H629 [DOI] [PubMed] [Google Scholar]
- 29. Biegus J, Zymliński R, Testani J, Fudim M, Cox ZL, Guzik M, et al. The blunted loop diuretic response in acute heart failure is driven by reduced tubular responsiveness rather than insufficient tubular delivery. The role of furosemide urine excretion on diuretic and natriuretic response in acute heart failure. Eur J Heart Fail 2023;25:1323‐1333. doi: 10.1002/ejhf.2852 [DOI] [PubMed] [Google Scholar]
- 30. Ter Maaten JM, Damman K, Hanberg JS, Givertz MM, Metra M, O'Connor CM, et al. Hypochloremia, diuretic resistance, and outcome in patients with acute heart failure. Circ Heart Fail 2016;9: doi: 10.1161/CIRCHEARTFAILURE.116.003109 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1: Assessment of B‐lines with lung ultrasound.
Data S2: Participant Centers of SALT‐HF trial.
Data S3: Diuretic protocol at discharge.
Data S4: Hypertonic saline solution and diuretic dose in different trials.


