Abstract
Aims
Low‐density lipoprotein cholesterol (LDL‐C), anaemia and low platelets have been associated with worse clinical outcomes in heart failure patients. We investigated the relationship between the combination of these three components and clinical outcome in patients with heart failure with preserved ejection fraction (HFpEF).
Methods and results
We examined the data of 1021 patients with HFpEF hospitalized with acute decompensated heart failure (HF) from the PURSUIT‐HFpEF registry, a prospective, multicenter observational study. The enrolled patients were classified into four groups by an LEP (LDL‐C, Erythrocyte, and Platelet) score of 0 to 3 points, with 1 point each for LDL‐C, erythrocyte and platelet values less than the cut‐off values as calculated by receiver operating characteristic curve analysis. The endpoint, a composite of all‐cause death and HF readmission, was evaluated among the four groups. Median follow‐up duration was 579 [300, 978] days. Risk of the composite endpoint significantly differed among the four groups (P < 0.001). Kaplan–Meier analysis showed that the groups with an LEP score of 2 had higher risk of the composite endpoint than those with an LEP score of 0 or 1 (P < 0.001, and P = 0.013, respectively), while those with an LEP score of 3 had higher risk than those with an LEP score of 0, 1 or 2 (P < 0.001, P < 0.001 and P = 0.020, respectively). Cox proportional hazards analysis showed that an LEP score of 3 was significantly associated with the composite endpoint (P = 0.030). Kaplan–Meier analysis showed that risk of the composite of all‐cause death and HF readmission was significantly higher in low LDL values (less than the cut‐off values as calculated by receiver operating characteristic curve analysis) patients with statin use than in those without statin use (log rank P = 0.002).
Conclusions
LEP score, which comprehensively reflects extra‐cardiac co‐morbidities, is significantly associated with clinical outcomes in HFpEF patients.
Keywords: Erythrocyte, Heart failure with preserved ejection fraction, Low‐density lipoprotein cholesterol, Platelet, Prognosis
Introduction
Up to half of patients with heart failure (HF) experience preserved ejection fraction (HFpEF). 1 The proportion of HFpEF patients hospitalized with acute decompensated HF (ADHF) is increasing. 1 Treatment for HFpEF is required not only for cardiogenic abnormalities including ischaemia, arrhythmia, and cardiomyopathy but also for extra‐cardiac diseases including obesity, anaemia, nutritional status, infection, liver function, and renal function. 2 Therefore, few evidence‐based treatments have been found to reduce hospitalization and reduce mortality in patients with HFpEF. We previously demonstrated a relationship between clinical outcome and various markers associated with extra‐cardiac co‐morbidity, including uric acid, blood urea nitrogen, and inflammatory markers. 3 , 4 , 5 Considering the impact of these various extra‐cardiac co‐morbidities on the pathology of HFpEF, we considered that a combination of simple markers that could comprehensively cover various extra‐cardiac diseases would be useful for risk stratification in HFpEF. While the relationship between lipids and ischaemic heart disease is well acknowledged, low serum levels of total cholesterol (T‐Chol) and low‐density lipoprotein cholesterol (LDL‐C) have been paradoxically associated with worse clinical outcomes in HF patients. 6 Moreover, anaemia is present in approximately one‐half of HF patients and is associated with fatal clinical outcomes. 7 Several studies showed the relationship between iron deficiency and cardiotoxicity. 8 , 9 Anaemia can be caused by several factors including iron deficiency and inflammation, which are the most common extra‐cardiac diseases causing HFpEF and iron deficiency could potentially lead to myocarditis. 10 , 11 Low platelet count has been associated with risk of all‐cause death and HF readmission in patients with acute HF and haemodialysis patients. 12 , 13 Further, low LDL‐C reflects impaired immune function and cardiac cachexia. 14 , 15 Low erythrocytes have been associated with renal dysfunction, and low platelets have been related to impaired haemostasis function and liver dysfunction. Against this background, we hypothesized that the combination of LDL‐C, erythrocytes, and platelets was strongly associated with clinical outcomes in HFpEF patients.
Here, we aimed to elucidate the relationship between the combination of LDL‐C, erythrocytes, and platelets and clinical outcomes in HFpEF who were hospitalized with ADHF.
Methods
PURSUIT‐HFpEF registry
We enrolled patients from the PURSUIT‐HFpEF (Prospective, mUlticenteR, obServational stUdy of patIenTs with Heart Failure with Preserved Ejection Fraction) registry. The PURSUIT‐HFpEF is a prospective, multicentre (32 hospitals) observational study conducted by collaborating hospitals in the Osaka region of Japan. 3 , 4 , 5 , 16 The enrolled patients were hospitalized with ADHF based on the Framingham criteria 17 and had a left ventricular ejection fraction (LVEF) ≥ 50% by transthoracic echocardiography based on the comprehensive diagnostic algorithm for HFpEF from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). 18 Brain natriuretic peptide was ≥100 ng/L or N‐terminal pro brain natriuretic peptide (NT‐pro BNP) was ≥400 ng/L on admission. The exclusion criteria were: (i) severe aortic stenosis, aortic regurgitation, mitral stenosis, or mitral regurgitation due to structural changes of the valve detected by transthoracic echocardiography; (ii) age <20 years; (iii) acute coronary syndrome on admission; (iv) poor six‐month prognosis due to noncardiac diseases; and (v) status post heart transplantation. We followed each patient and collected outcome data of all‐cause death and HF readmission. The PURSUIT‐HFpEF Study was approved by the ethics committee of each participating facility and was conducted according to the Helsinki Declaration (UMIN‐CTR ID: UMIN000021831). All patients gave informed consent prior to participation. The protocol of the present study was approved by the Institutional Review Board of all participating facilities.
Echocardiography and laboratory measurement
Transthoracic echocardiography was performed on admission and at discharge. All echocardiographic measurements were consistent with recent echocardiography guidelines. 19 Laboratory measurements were also performed on emergency admission and at discharge by standard methods in the clinical laboratory of each participating hospital. FIB4 index was calculated as follows: [(age × aspartate aminotransferase (AST) (IU/L)]/[(platelet)(109/L) × alanine aminotransferase (ALT)1/2(IU/L)]. CONUT score was calculated by adding the scores of the following parameters: serum albumin concentration [≥3.5 g/dL (0 points), 3.0–3.4 g/dL (2 points), 2.5–2.9 g/dL (4 points), or <2.5 g/dL (6 points)], total lymphocyte count [≥1600 cells/mm3 (0 points), 1200–1599 cells/mm3 (1 point), 800–1199 cells/mm3 (2 points), or <800 cells/mm3 (3 points)], and total cholesterol level [≥180 mg/dL (0 point), 140–179 mg/dL (1 point), 100–139 mg/dL (2 points), or <100 mg/dL (3 points)].
Follow‐up and study endpoints
Investigational cardiologists and trained research nurses obtained patient data, including medical history, substance history, co‐morbidities, therapeutic procedures, and clinical events from the medical records and by direct interview of patients and family members during their hospital stay. They also obtained vital signs, body mass index, echocardiographic data, admission laboratory data, and medications at discharge. After discharge, all patients were followed by their treating hospital. Coordinators and investigators obtained clinical data by direct contact in an outpatient setting, telephone interview with patient families, or mail. Clinical endpoint was a composite of all‐cause death and HF readmission. A score of 1 point was assigned for each LDL‐C, erythrocyte, or platelet value that was less than the respective cut‐off value calculated by statistical methods as predictive of the composite of all‐cause death and HF readmission. Patients were then classified into 4 groups by the summed LEP score (LDL‐C, Erythrocyte and Platelet score) of 0, 1, 2, or 3 points. The study endpoint among the four groups was evaluated.
Statistical analysis
All statistical analyses were done using the JMP 17 statistical software (SAS Institute Inc., Cary, North Carolina, USA). Normality testing for continuous variables was done using the Shapiro–Wilk W test. Normal distribution was not confirmed for all variables. Continuous variables are expressed as median [interquartile range]. Two‐group comparisons were analysed by Mann–Whitney U test for continuous variables. The Kruskal–Wallis test was used for intergroup differences of continuous variables. The Bonferroni method was used to adjust P values in multiple testing. Categorical data are expressed as number (percentage) and were compared using the chi‐square test for categorical variables. Receiver operating characteristic curve (ROC) analysis of LDL‐C, erythrocyte and platelet levels predictive of the composite of all‐cause death and HF readmission was performed to determine cut‐off values. The composite of all‐cause death and HF readmission was estimated using the Kaplan–Meier method among the four groups in overall population, males, and females, with statistical significance determined using the log rank test. Multivariate analysis for the endpoint was conducted using Cox proportional hazards regression analysis, from which the following covariates of clinical importance were selected: age, gender, 20 body mass index, 21 history of hypertension, atrial fibrillation, 22 renal function, 23 albumin, 24 NT‐proBNP, 25 C reactive protein, 26 and medications. Hazard ratios (HR) and 95% confidence intervals were calculated for each endpoint. All statistical tests were two‐sided, and P < 0.05 was regarded as statistically significant.
Results
Study population
We used receiver operating characteristic curve analyses to determine the cut‐off value for LDL‐C, erythrocytes, and platelets predictive of the composite of all‐cause death and HF readmission. The optimally predictive cut‐off values were LDL‐C = 76 mg/dL (AUC = 0.54, sensitivity = 33.9%, specificity = 74.1%), erythrocytes = 362 × 104/μL (AUC = 0.58, sensitivity = 33.9%, specificity = 74.1%), and platelets = 24.0 × 104/μL (AUC = 0.53, sensitivity = 68.2%, specificity = 39.0%). This study population included a total of 1021 patients who were enrolled in the PURSUIT‐HFpEF registry between June 2016 and December 2021, and divided into four groups, according to LEP score (Figure 1 ). Baseline characteristics of the four groups are shown in Table 1 .
Figure 1.

Study flowchart. LDL‐C, low density lipoprotein cholesterol; ROC, receiver operating characteristics.
Table 1.
Baseline characteristics
| Overall (n = 1021) | LEP score = 0 (n = 193) | LEP score = 1 (n = 429) | LEP score = 2 (n = 287) | LEP score = 3 (n = 112) | P value | |
|---|---|---|---|---|---|---|
| Clinical data | ||||||
| Age, years | 83 [77, 87] | 81 [75, 86] | 82 [76, 86] | 84 [79, 89] | 83 [77, 88] | <0.001 |
| Male | 452 (44.3) | 78 (40.4) | 180 (42.0) | 133 (46.3) | 61 (54.5) | 0.063 |
| BMI | 24 [21, 27] | 25 [22, 28] | 24 [21, 27] | 24 [21, 27] | 23 [20, 26] | 0.012 |
| Hypertension | 867 (85.1) | 172 (89.6) | 351 (82.0) | 246 (85.7) | 98 (87.5) | 0.077 |
| Diabetes mellitus | 339 (33.2) | 59 (30.6) | 143 (33.3) | 96 (33.4) | 41 (36.6) | 0.759 |
| Sleep apnoea syndrome | 55 (5.4) | 6 (3.1) | 31 (7.2) | 13 (4.5) | 5 (4.5) | 0.153 |
| Ischaemic heart disease | 175 (17.1) | 25 (13.0) | 59 (13.8) | 60 (20.9) | 31 (27.7) | <0.001 |
| AF | 451 (44.2) | 86 (44.6) | 199 (46.4) | 127 (44.3) | 39 (34.8) | 0.184 |
| Echocardiographic parameters | ||||||
| LVDd, mm | 46 [41, 50] | 45 [40, 50] | 46 [41, 50] | 45 [41, 50] | 47 [43, 51] | 0.098 |
| LVDs, mm | 29 [26, 33] | 29 [25, 33] | 29 [26, 33] | 29 [27, 33] | 30 [28, 33] | 0.196 |
| LVEF, % | 64 [58, 70] | 64 [59, 71] | 65 [59, 69] | 65 [57, 70] | 64 [58, 70] | 0.782 |
| LAD, mm | 44 [39, 49] | 43 [39, 48] | 45 [39, 49] | 44 [39, 50] | 44 [39, 50] | 0.320 |
| E/e′ | 13 [10, 17] | 12 [10, 16] | 12 [10, 17] | 13 [10, 17] | 14 [10, 18] | 0.133 |
| TAPSE, cm | 17 [15, 20] | 18 [15, 20] | 17 [15, 20] | 17 [15, 21] | 18 [15, 21] | 0.763 |
| IVCD, mm | 14 [11, 17] | 13 [10, 16] | 13 [11, 16] | 14 [11, 18] | 15 [12, 19] | <0.001 |
| Laboratory data | ||||||
| Leukocyte, ×103/μL | 5.4 [4.5, 6.7] | 6.3 [5.2, 7.5] | 5.5 [4.5, 6.6] | 5.0 [4.0, 6.3] | 5.0 [4.1, 5.9] | <0.001 |
| Erythrocytes, ×104/μL | 376 [334, 423] | 422 [388, 458] | 397 [366, 438] | 342 [312, 372] | 308 [275, 337] | <0.001 |
| Haemoglobin, g/dL | 11.3 [10.1, 12.7] | 12.2 [11.4, 13.4] | 11.8 [10.7, 13.3] | 10.5 [9.4, 11.8] | 9.7[8.7, 10.5] | <0.001 |
| Platelets, ×104/μL | 21 [17, 27] | 30 [26, 33] | 21 [17, 26] | 19 [15, 22] | 17 [14, 20] | <0.001 |
| Sodium, mEq/L | 140 [137, 141] | 140 [137, 141] | 140 [137, 141] | 140 [137, 142] | 139 [136, 141] | 0.482 |
| Potassium, mEq/L | 4.3 [3.9,4.6] | 4.3 [4.0, 4.6] | 4.3 [3.9, 4.6] | 4.2 [3.9, 4.6] | 4.2 [3.9, 4.6] | 0.264 |
| Albumin, g/dL | 3.4 [3.1, 3.7] | 3.5 [3.2, 3.7] | 3.5 [3.2, 3.7] | 3.3 [3.0, 3.6] | 3.2 [2.9, 3.5] | <0.001 |
| Creatinine, mg/dL | 1.1 [0.9, 1.5] | 1.0 [0.8, 1.2] | 1.0 [0.8, 1.4] | 1.2 [0.9, 1.7] | 1.6 [1.1, 2.1] | <0.001 |
| eGFR, mL/min/1.73 m2 | 41 [30, 54] | 47 [37, 60] | 44 [32, 56] | 38 [27, 52] | 32 [21, 41] | <0.001 |
| NT‐proBNP, pg/mL | 1059 [481, 2375] | 743 [337, 1652] | 954 [416, 1974] | 1315 [591, 2790] | 2041 [756, 3963] | <0.001 |
| Log NT‐pro BNP, pg/mL | 3.02 [2.68, 3.38] | 2.87 [2.53, 3.22] | 2.98 [2.62, 3.30] | 3.12 [2.77, 3.45] | 3.31 [2.88, 3.60] | <0.001 |
| CRP, mg/dL | 0.3 [0.1, 0.8] | 0.4 [0.2, 0.9] | 0.2 [0.1, 0.6] | 0.2 [0.1, 0.8] | 0.3 [0.1, 1.7] | 0.010 |
| T‐Chol, mg/dL | 158 [137, 181] | 170 [156, 190] | 167 [149, 190] | 146 [130, 170] | 125 [113, 133] | <0.001 |
| LDL‐C, mg/dL | 91 [73, 111] | 103 [91, 121] | 99 [84, 115] | 79 [66, 97] | 63 [56, 69] | <0.001 |
| HDL‐C, mg/dL | 43 [36, 52] | 42 [36, 50] | 45 [37, 53] | 44 [37, 52] | 40 [33, 53] | 0.050 |
| CONUT score | 3 [2, 5] | 2 [1, 4] | 3 [1, 5] | 4 [3, 6] | 5 [4, 7] | <0.001 |
| FIB4 index | 2.3 [1.6, 3.1] | 1.4 [1.1, 1.8] | 2.2 [1.6, 2.9] | 2.8 [2.1, 3.5] | 3.0 [2.1, 3.9] | <0.001 |
| Medications | ||||||
| ACEI/ARB | 546 (53.5) | 105 (54.4) | 230 (53.6) | 156 (54.4) | 55 (49.1) | 0.797 |
| Beta‐blocker | 553 (54.2) | 100 (51.8) | 230 (53.7) | 162 (56.5) | 61 (54.5) | 0.785 |
| MRA | 409 (40.1) | 80 (41.5) | 176 (41.0) | 112 (39.0) | 41 (36.6) | 0.800 |
| Statin | 343 (33.6) | 50 (25.9) | 135 (31.5) | 113 (39.4) | 45 (33.6) | 0.006 |
Continuous data are presented as the median (interquartile range). Categorical variables are presented as number (percentage).
ACEI, angiotensin converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; BMI, body mass index; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; HDL‐C, high‐density lipoprotein cholesterol; IVCD, inferior vena cava diameter; LAD, left atrial diameter; LDL‐C, low‐density lipoprotein cholesterol; LVDd, left ventricular end‐diastolic diameter left ventricular diameter; LVDs, left ventricular end‐systolic diameter; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonists; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide, TAPSE, tricuspid annular plane systolic excursion; T‐Chol, total cholesterol.
Endpoints
Median follow‐up duration was 579 [300, 978] days. All‐cause death occurred in 267 patients (26.2%) and the composite endpoint in 446 patients (43.7%). Details of cardiac and non‐cardiac death are shown in Table 2 . HF was the most common cause of cardiac death (55.8%), and infection was the most common cause of non‐cardiac death (31.3%). Kaplan–Meier analysis demonstrated that the composite of all‐cause death and HF readmission significantly differed among the four groups in overall population, males, and females (Figures 2 and S1 ). Patients with an LEP score of 2 had higher risk of the composite endpoint than those with an LEP score of 0 or 1, while those with an LEP score of 3 had higher risk than those with an LEP score of 0, 1, or 2. Multivariate Cox proportional hazards analysis indicated a significant association between an LEP score of 3 (using LEP score of 0 as reference), advanced age, male, higher creatinine levels, lower albumin serum concentrations, higher log NT‐proBNP levels, and the composite endpoint (HR = 1.54; P = 0.030, HR = 1.04; P < 0.001, HR = 1.31; P = 0.012, HR = 1.15; P = 0.004, HR = 0.75; P = 0.028, HR = 2.31; P < 0.001, respectively) (Table 3 ).
Table 2.
Details of cardiac death and non‐cardiac death
| LEP score | ||||||
|---|---|---|---|---|---|---|
| Cardiac death | All (n = 120) | Score = 0 (n = 19) | Score = 1 (n = 36) | Score = 2 (n = 39) | score = 3 (n = 26) | P value |
| Heart failure | 67 (55.8) | 10 (52.6) | 19 (52.8) | 22 (56.4) | 16 (61.5) | 0.904 |
| Arrhythmia / sudden cardiac death | 12 (10.0) | 1 (5.3) | 3 (8.3) | 5 (12.8) | 3 (11.5) | 0.802 |
| Myocardial infarction | 3 (2.5) | 1 (5.3) | 1 (2.8) | 1 (2.6) | 0 (0) | 0.735 |
| Others | 38 (31.7) | 7 (36.8) | 13 (36.1) | 11 (28.2) | 7 (26.9) | 0.789 |
| Non‐cardiac death | All (n = 147) | Score = 0 (n = 17) | Score = 1 (n = 63) | Score = 2 (n = 44) | Score = 3 (n = 23) | P value |
|---|---|---|---|---|---|---|
| Infection | 46 (31.3) | 5 (29.4) | 21 (33.3) | 12 (27.3) | 8 (34.8) | 0.894 |
| Malignant disease | 24 (16.3) | 1 (5.9) | 13 (20.6) | 9 (20.5) | 1 (4.3) | 0.159 |
| Renal failure | 11 (7.5) | 0 (0) | 4 (6.3) | 6 (13.6) | 1 (4.3) | 0.238 |
| Stroke | 12 (8.2) | 1 (5.9) | 3 (4.8) | 5 (11.4) | 3 (13.0) | 0.490 |
| Others | 54 (36.7) | 10 (58.8) | 22 (34.9) | 12 (27.3) | 10 (43.5) | 0.122 |
Categorical variables are presented as number (percentage).
Figure 2.
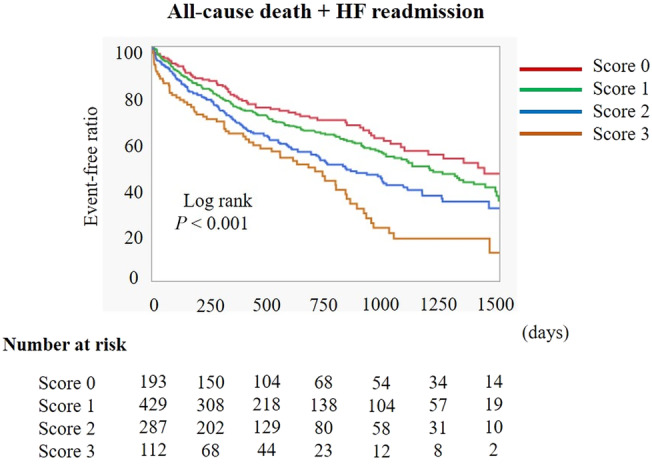
Kaplan–Meier analysis of the composite of all‐cause death and HF readmission among the four groups, according to LEP score. HF, heart failure.
Table 3.
Cox proportional hazard analysis for the composite of all‐cause death and HF readmission
| HR | 95% CI | P value | |
|---|---|---|---|
| LEP score | |||
| Score = 0 (reference) | 1.00 | ||
| Score = 1 | 1.13 | 0.83–1.53 | 0.432 |
| Score = 2 | 1.17 | 0.85–1.61 | 0.349 |
| Score = 3 | 1.54 | 1.04–2.27 | 0.030 |
| Age | 1.04 | 1.02–1.05 | <0.001 |
| Gender (male) | 1.31 | 1.06–1.62 | 0.012 |
| BMI | 1.01 | 0.98–1.03 | 0.579 |
| Hypertension | 0.86 | 0.65–1.14 | 0.296 |
| AF | 0.91 | 0.74–1.12 | 0.359 |
| Ischaemic heart disease | 1.30 | 0.98–1.72 | 0.071 |
| Creatinine | 1.15 | 1.05–1.28 | 0.004 |
| Albumin | 0.75 | 0.59–0.97 | 0.028 |
| Log NT‐proBNP | 2.31 | 1.80–2.99 | <0.001 |
| CRP | 0.95 | 0.88–1.02 | 0.192 |
| ACEI/ARB | 0.91 | 0.74–1.12 | 0.372 |
| Beta‐blocker | 1.03 | 0.84–1.27 | 0.755 |
| MRA | 0.94 | 0.76–1.20 | 0.560 |
| Statin | 0.96 | 0.76–1.20 | 0.702 |
ACEI, angiotensin converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; BMI, body mass index; CI, confidence interval; CRP, C‐reactive protein; HR, hazard ratio; MRA, mineralocorticoid receptor antagonists; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide.
Low low‐density lipoprotein cholesterol level and statin
Among patients with LDL‐C < 76 mg/dL (less than the cut‐off value for predicting the composite endpoint), those taking statins accounted for 156 patients (55.9%). To elucidate the effect of statin use on clinical outcomes in patients with LDL‐C < 76 mg/dL, we divided the patients with LDL‐C < 76 mg/dL into statin users and non‐users, and evaluated the difference in the endpoint. On Kaplan–Meier analysis, risk of the composite of all‐cause death and HF readmission was significantly higher in the LDL‐C < 76 mg/dL patients without statin use than in those with statin use (log rank P = 0.002) (Figure 3 ).
Figure 3.

Kaplan–Meier analysis of the composite of all‐cause death and HF readmission in patients with LDL‐C < 76 mg/dL with and without statin use. HF, heart failure; LDL‐C, low density lipoprotein cholesterol.
Discussion
Main findings
The main finding of this study is that LEP score, which reflects the decrease of LDL‐C, erythrocyte and platelet, was significantly associated with the composite of all‐cause death and HF readmission. This finding suggests that LEP score, which reflects extra‐cardiac co‐morbidity, including immune function, nutritional status, anaemia, renal function, haemostasis function, and liver function, is significantly associated with high risk of the composite of all‐cause death and HF readmission in HFpEF patients. In addition, in HFpEF patients with a low LDL‐C level, risk of the composite of all‐cause death and HF readmission was significantly higher in those with statin medication than in those without statin use.
Low‐density lipoprotein cholesterol
Previous studies showed a significant association between low LDL‐C and worse clinical outcomes in HF patients. 6 Cholesterol levels reflect nutritional status, which also has a prognostic impact, but other mechanisms of the relationship between LDL‐C and clinical outcomes in HF patients have been proposed. In vitro studies have suggested that circulating cytokines may decrease lipoprotein levels by decreasing hepatic lipoprotein production and increasing LDL receptor activity. 27 There is a contradiction between the poor prognosis associated with low LDL‐C levels and the improved prognosis of statins in the low LDL‐C group. Low LDL‐C level may reflect impaired immune function and vulnerability to infection that triggers HF readmission. 14 Statin use causes decrease in LDL‐C levels, but it may have a prognostic effect on cardiovascular disease, cancer, neurodegenerative diseases, and non‐alcoholic fatty liver disease, which has been linked to increased cardiac disease morbidity and mortality due to its pleiotropic effects. 28
Erythrocytes and platelets
A low erythrocyte count can be caused by any of several mechanisms, including renal dysfunction and inflammatory status. HF patients have hematinic deficiencies, particularly iron deficiency, which is present in approximately one‐half of patients. 7 The presence of chronic inflammation in HF patients is an important cause of iron deficiency and of erythropoietin resistance. 29 Anaemia is known to increase venous return and cardiac workload (oxygen consumption) via the stimulation of sympathetic tone in response to a reduced oxygen supply, which in turn result in the development and progression of left atrial dysfunction, left ventricular hypertrophy, left ventricular remodelling, and myocardial ischaemia, finally leading to fatal clinical outcomes. 30
Low numbers of circulating platelets and platelet dysfunction increase the risk of bleeding. In one study, low platelet counts predicted non‐cardiovascular mortality in a cohort of elderly individuals. 31 Low platelet counts were associated with risk for all‐cause death and HF readmission in patients with acute HF. 6 That study speculated a relationship between low platelets and long‐standing congestion and coexisting congestive hepatopathy. Inflammation and renin angiotensin aldosterone system activation induce megakaryocytopoiesis, and this condition may be accompanied by an increase in mean platelet volume and low platelets. 32 In our study, patients with a high LEP score (=2 and 3) showed a higher incidence of renal failure in non‐cardiac death, and a higher creatinine level than those with a low LEP score (=0 and 1) (Table 1 ). Anaemia induces structural changes in HFpEF patients and exacerbates renal dysfunction. The reverse of these relationships is also seen. In addition, the patients with a high LEP score (=2 and 3) showed a higher NT‐proBNP level and larger inferior vena cava diameter than those with a low LEP score (=0 and 1) (Table 1 ). These results suggest coexisting congestive hepatopathy, which can cause a low platelet count.
Relationship between the three components and clinical outcome
The three LEP components comprehensively reflect the involvement of multiple extra‐cardiac diseases. CONUT score was significantly higher in patients with LDL‐C < 76 mg/dL than those with LDL‐C ≥ 76 mg/dL (Figure S2 ). Estimated glomerular filtration rate was significantly lower in patients with erythrocyte <362 × 104/μL than those with erythrocyte ≥362 × 104/μL (Figure S2 ). FIB4 index was significantly higher in patients with platelet <24.0 × 104/μL than those with platelet ≥24.0 × 104/μL (Figure S2 ). Low LDL‐C suggests cardiac cachexia, which correlates with neurohormonal derangements, proinflammatory immune activation, anorexia, malabsorbtion, and anabolic hormone resistance and deteriorate HF. 33 It also suggests immune dysfunction and vulnerability to infection that triggers HF readmission. Low erythrocyte suggests coexisting iron deficiency, and the impacts on myocardial abnormal changes and cardiotoxicity were reported. 8 , 9 , 30 Low erythrocyte was associated with poor clinical outcomes in HF patients. Low platelet is associated with renin angiotensin aldosterone system activation due to the influence of its recruitment, aggregation, and consumption. 34 Therefore, LEP score, which comprehensively reflect extra‐cardiac diseases, can be associated with the value of natriuretic peptide. A possible mechanism for the impact of these three components on HFpEF is shown in Figure 4 .
Figure 4.

Possible mechanism of the impact of the three components on HFpEF. HF, heart failure; HFpEF, heart failure with preserved ejection fraction; LDL‐C, low density lipoprotein cholesterol; RAAS, renin‐angiotensin‐aldosterone system.
Clinical implications
Iron deficiency has emerged as one of the most important causes of anaemia in patients with HF. The current ESC guidelines report the effectiveness of intravenous iron supplementation in HF with reduced ejection fraction (HFrEF) patients with iron deficiency. 35 Future research to elucidate the impact of iron supplementation on clinical outcomes in HFpEF patients appears mandatory. A previous study showed that statin use has a beneficial effect on mortality in HFpEF without coronary artery disease. 36 Paradoxically, in contrast, low LDL‐C levels were associated with worse clinical outcomes in HF patients. 6 In the present study, patients with low LDL‐C in the absence of statin use were at higher risk of the composite of all‐cause death and HF readmission than those with low LDL‐C but with statin use. Considering our present and these previous results, we speculate that statins may reduce the risk of poor prognosis not only in HFpEF patients with high LDL‐C levels but also in those with low LDL‐C levels. A recent study suggested a relationship between low platelet count and congestive hepatopathy. 12 Optimal decongestion may lead to restoration of platelet count. On the other hand, platelet abnormalities in chronic HF have been well described and include increased whole blood aggregation and platelet‐derived adhesion molecules. 37 Beta‐blockers reduce platelet aggregation induced by epinephrine and adenosine diphosphate in vitro. 38 ACE inhibitors probably inhibit platelet activation induced by angiotensin II. 39 Spironolactone inhibits platelet activation in congestive heart failure induced by aldosterone in animal models. 34 In conventional HF management, maintenance of platelet count and function may be particularly important in HFpEF patients with a high LEP score.
Study limitations
Several limitations of this study should be acknowledged. First, it was conducted in a small number of patients enrolled in a multicentre prospective HFpEF registry. Second, the HFpEF registry population is East Asian, which would limit the generalizability of our current findings to other races. The cut‐off value for LDL‐C likely differs to those in other countries. Third, <76 mg/dL of LDL‐C, <362 × 104/μL of erythrocytes, and <24.0 × 104/μL of platelet do not mean below the normal value, and we evaluated the relationship between the number of low values in the three items of LDL‐C, erythrocytes, and platelet and the prognosis of HFpEF. However, <362 × 104/μL of erythrocytes and <24.0 × 104/μL of platelet include all patients with anaemia and thrombocytopenia. Therefore, we believe that <362 × 104/μL of erythrocytes and <24.0 × 104/μL of platelet are strongly correlated with anaemia and thrombocytopenia. Fourth, the normal range of erythrocyte is different between male and female. However, no significant difference in the percentage of male/female was observed among the four groups (Table 1 ), and there was no significant difference in erythrocyte between male and female in the enrolled patients (383 × 104/μL vs. 378 × 104/μL, P = 0.263). In addition, Kaplan–Meier analysis demonstrated that the composite of all‐cause death and HF readmission significantly differed among the four groups in males and females (Figure S1 ). We believe that gender difference in erythrocyte dose not significantly affect the present results. Fifth, we showed the prognostic value of LDL‐C stratified with the use of statin therapy, but the result was based on observational data and included potential biases. Lastly, there are concerns about the impact of differences in NT‐proBNP measurement methods between facilities on measured values, but the previous report demonstrated that multicentre analytical performance showed a good correlation among several analysers and similar cut‐points may be used to exclude both heart failure patients. 40
Conclusions
LEP score, derived from a combination of LDL‐C level, erythrocyte, and platelet counts was significantly associated with the composite of all‐cause death and HF readmission in HFpEF patients.
Conflict of interest
Y. Sotomi has received grants from Roche Diagnostics, FUJIFILM Toyama Chemical, TOA EIYO, Bristol‐Myers Squibb, Biosense Webster, Abbott Medical Japan, and NIPRO, and personal fees from Abiomed, AstraZeneca, Amgen Astellas BioPharma, Biosensors, Boehringer Ingelheim, Bristole‐Myers Squibb, Abbott Medical Japan, Boston Scientific Japan, Bayer, Daiichi Sankyo, Novartis, TERUMO, Medtronic, and Pfizer Pharmaceuticals.
Daisaku Nakatani has received honoraria from Roche Diagnostics. Daisaku Nakatani has received honoraria from Roche Diagnostics. Shungo Hikoso has received personal fees from Daiichi Sankyo Company, Bayer, Astellas Pharma, Pfizer Pharmaceuticals and Boehringer Ingelheim Japan, and grants from Roche Diagnostics, FUJIFILM Toyama Chemical and Actelion Pharmaceuticals. Yasushi Sakata has received personal fees from Otsuka Pharmaceutical, Ono Pharmaceutical, Daiichi Sankyo Company, Mitsubishi Tanabe Pharma Corporation and Actelion Pharmaceuticals, and grants from Roche Diagnostic, FUJIFILM Toyama Chemical, Abbott Medical, Japan, Otsuka Pharmaceutical, Daiichi Sankyo Company, Mitsubishi Tanabe Pharma Corporation and Biotronik. The other authors have no conflicts of interest to disclose.
Funding
This work was funded by Roche Diagnostics K.K. and Fuji Film Toyama Chemical Co. Ltd.
Supporting information
Figure S1. Kaplan–Meier analysis of the composite of all‐cause death and HF readmission among the four groups, according to LEP score in males and females. HF, heart failure.
Figure S2. (A) CONUT score between the patients with LDL‐C < 76 mg/dL and LDL ≥ 76 mg/dL. (B) eGFR between the patients with erythrocyte < 362 × 104/μl and erythrocyte ≥ 362 × 104/μl. (C) FIB4 index between the patients with platelet < 24.0 × 104/μl and platelet ≥ 24.0 × 104/μl. eGFR, estimated glomerular filtration rate.
Acknowledgements
The authors thank Nagisa Yoshioka, Kyoko Tatsumi, Satomi Kishimoto, Noriko Murakami, and Sugako Mitsuoka for their excellent assistance with data collection.
Yano, M. , Nishino, M. , Kawanami, S. , Ukita, K. , Kawamura, A. , Yasumoto, K. , Tsuda, M. , Okamoto, N. , Matsunaga‐Lee, Y. , Egami, Y. , Yamada, T. , Yasumura, Y. , Seo, M. , Hayashi, T. , Nakagawa, A. , Nakagawa, Y. , Tamaki, S. , Sotomi, Y. , Nakatani, D. , Hikoso, S. , Sakata, Y. , and the Osaka CardioVascular Conference (OCVC)‐Heart Failure Investigators (2024) Low‐density lipoprotein cholesterol, erythrocyte, and platelet in heart failure with preserved ejection fraction. ESC Heart Failure, 11: 1758–1766. 10.1002/ehf2.14734.
The OCVC‐Heart Failure Investigators: Masahiro Seo, Tetsuya Watanabe, and Takahisa Yamada, Osaka General Medical Center, Osaka, Japan; Takaharu Hayashi and Yoshiharu Higuchi, Osaka Police Hospital, Osaka, Japan; Masaharu Masuda, Mitsutoshi Asai, and Toshiaki Mano, Kansai Rosai Hospital, Amagasaki, Japan; Hisakazu Fuji, Kobe Ekisaikai Hospital, Kobe, Japan; Daisaku Masuda, Shunsuke Tamaki, Ryu Shutta, and Shizuya Yamashita, Rinku General Medical Center, Izumisano, Japan; Masami Sairyo and Yusuke Nakagawa, Kawanishi City Hospital, Kawanishi, Japan; Haruhiko Abe, Yasunori Ueda, and Yasushi Matsumura, National Hospital Organization Osaka National Hospital, Osaka, Japan; Kunihiko Nagai, Ikeda Municipal Hospital, Ikeda, Japan; Masamichi Yano, Masami Nishino, and Jun Tanouchi, Osaka Rosai Hospital, Sakai, Japan; Yoh Arita and, Nobuyuki Ogasawara, Japan Community Health Care Organization Osaka Hospital, Osaka, Japan; Takamaru Ishizu, Minoru Ichikawa and Yuzuru Takano, Higashiosaka City Medical Center, Higashiosaka, Japan; Eisai Rin, Kawachi General Hospital, Higashiosaka, Japan; Yukinori Shinoda, Koichi Tachibana and Shiro Hoshida, Yao Municipal Hospital, Yao, Japan; Masahiro Izumi, Kinki Central Hospital, Itami, Japan; Hiroyoshi Yamamoto and Hiroyasu Kato, Japan Community Health Care Organization, Osaka Minato Central Hospital, Osaka, Japan; Kazuhiro Nakatani and Yuji Yasuga, Sumitomo Hospital, Osaka, Japan; Mayu Nishio and Keiji Hirooka, Saiseikai Senri Hospital, Suita, Japan; Takahiro Yoshimura and Yoshinori Yasuoka, National Hospital Organization Osaka Minami Medical Center, Kawachinagano, Japan; Akihiro Tani, Kano General Hospital, Osaka, Japan; Yasushi Okumoto, Kinan Hospital, Tanabe, Japan; Yasunaka Makino, Hyogo Prefectural Nishinomiya Hospital, Nishinomiya, Japan; Toshinari Onishi and Katsuomi Iwakura, Sakurabashi Watanabe Hospital, Osaka, Japan; Yoshiyuki Kijima, Japan Community Health Care Organization, Hoshigaoka Medical Center, Hirakata, Japan; Takashi Kitao, Minoh City Hospital, Minoh, Japan; Masashi Fujita, Osaka International Cancer Institute, Osaka, Japan; Koichiro Harada, Suita Municipal Hospital, Suita, Japan; Masahiro Kumada and Osamu Nakagawa, Toyonaka Municipal Hospital, Toyonaka, Japan; Ryo Araki and Takayuki Yamada, Otemae Hospital, Osaka, Japan; Akito Nakagawa and Yoshio Yasumura, Amagasaki Chuo Hospital, Amagasaki, Japan; and Taiki Sato, Akihiro Sunaga, Bolrathanak Oeun, Hirota Kida, Yohei Sotomi, Tomoharu Dohi, Kei Nakamoto, Katsuki Okada, Fusako Sera, Hidetaka Kioka, Tomohito Ohtani, Toshihiro Takeda, Daisaku Nakatani, Hiroya Mizuno, Shungo Hikoso, and Yasushi Sakata, Osaka University Graduate School of Medicine, Suita, Japan.
Clinical Trial Registration: UMIN000021831<https://center6.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000024414>; PURSUIT‐HFpEF.
References
- 1. Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, et al. Outcome of heart failure with preserved ejection fraction in a population‐based study. N Engl J Med 2006;355:260–269. doi: 10.1056/NEJMoa051530 [DOI] [PubMed] [Google Scholar]
- 2. Wintrich J, Kindermann I, Ukena C, Selejan S, Werner C, Maack C, et al. Therapeutic approaches in heart failure with preserved ejection fraction: Past, present, and future. Clin Res Cardiol 2020;109:1079–1098. doi: 10.1007/s00392-020-01633-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nishino M, Egami Y, Kawanami S, Sugae H, Ukita K, Kawamura A, et al. Lowering uric acid may improve prognosis in patients with hyperuricemia and heart failure with preserved ejection fraction. J Am Heart Assoc 2022;11:e026301. doi: 10.1161/JAHA.122.026301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yano M, Nishino M, Ukita K, Kawamura A, Nakamura H, Matsuhiro Y, et al. Clinical impact of blood urea nitrogen, regardless of renal function, in heart failure with preserved ejection fraction. Int J Cardiol 2022;363:94–101. doi: 10.1016/j.ijcard.2022.06.061 [DOI] [PubMed] [Google Scholar]
- 5. Yano M, Nishino M, Ukita K, Kawamura A, Nakamura H, Matsuhiro Y, et al. High density lipoprotein cholesterol/C reactive protein ratio in heart failure with preserved ejection fraction. ESC Heart Fail 2021;8:2791–2801. doi: 10.1002/ehf2.13350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rauchhaus M, Clark AL, Doehner W, Davos C, Bolger A, Sharma R, et al. The relationship between cholesterol and survival in patients with chronic heart failure. J Am Coll Cardiol 2003;42:1933–1940. doi: 10.1016/j.jacc.2003.07.016 [DOI] [PubMed] [Google Scholar]
- 7. Okonko DO, Mandal AK, Missouris CG, Poole‐Wilson PA. Disordered iron homeostasis in chronic heart failure: prevalence, predictors, and relation to anemia, exercise capacity, and survival. J Am Coll Cardiol 2011;58:1241–1251. doi: 10.1016/j.jacc.2011.04.040 [DOI] [PubMed] [Google Scholar]
- 8. Cirovic A, Cirovic A. Iron deficiency as a promoter of cadmium‐induced cardiotoxicity. Eur Heart J 2023;44:2639–2640. doi: 10.1093/eurheartj/ehad296 [DOI] [PubMed] [Google Scholar]
- 9. Savarese G, Anker MS, Anker SD. Iron deficiency as a promoter of cardiotoxicity: not only cadmium‐induced. Eur Heart J 2023;44:2641. doi: 10.1093/eurheartj/ehad297 [DOI] [PubMed] [Google Scholar]
- 10. Nairz M, Weiss G. Iron in infection and immunity. Mol Aspects Med 2020;75:100864. doi: 10.1016/j.mam.2020.100864 [DOI] [PubMed] [Google Scholar]
- 11. Cirovic A, Cirovic A. Could iron deficiency potentiate the appearance of complications in influenza a virus infection? Proc Natl Acad Sci U S A 2023;120:e2311262120. doi: 10.1073/pnas.2311262120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yamaguchi S, Abe M, Arakaki T, Arasaki O, Shimabukuro M. Incremental prognostic value of platelet count in patients with acute heart failure ‐ A retrospective observational study. Circ J 2019;83:576–583. doi: 10.1253/circj.CJ-18-0961 [DOI] [PubMed] [Google Scholar]
- 13. Zhao X, Niu Q, Gan L, Hou FF, Liang X, Ni Z, et al. Thrombocytopenia predicts mortality in Chinese hemodialysis patients ‐ An analysis of the China DOPPS. BMC Nephrol 2022;23:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb‐Peploe KM, et al. Wasting as independent risk factor for mortality in chronic heart failure. Lancet 1997;349:1050–1053. doi: 10.1016/S0140-6736(96)07015-8 [DOI] [PubMed] [Google Scholar]
- 15. Rauchhaus M, Coats AJ, Anker SD. The endotoxin‐lipoprotein hypothesis. Lancet 2000;356:930–933. doi: 10.1016/S0140-6736(00)02690-8 [DOI] [PubMed] [Google Scholar]
- 16. Suna S, Hikoso S, Yamada T, Uematsu M, Yasumura Y, Nakagawa A, et al. Study protocol for the PURSUIT‐HFpEF study: A prospective, multicenter, observational study of patients with heart failure with preserved ejection fraction. BMJ Open 2020;10:e038294. doi: 10.1136/bmjopen-2020-038294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: The Framingham study. N Engl J Med 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601 [DOI] [PubMed] [Google Scholar]
- 18. Pieske B, Tschöpe C, de Boer RA, Fraser AG, Anker SD, Donal E, et al. How to diagnose heart failure with preserved ejection fraction: the HFA‐PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail 2020;22:391–412. doi: 10.1002/ejhf.1741 [DOI] [PubMed] [Google Scholar]
- 19. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 20. Lam CSP, Arnott C, Beale AL, Chandramouli C, Hilfiker‐Kleiner D, Kaye DM, et al. Sex differences in heart failure. Eur Heart J 2019;40:3859–3868c. doi: 10.1093/eurheartj/ehz835 [DOI] [PubMed] [Google Scholar]
- 21. Adamson C, Kondo T, Jhund PS, de Boer RA, Cabrera Honorio JW, Claggett B, et al. Dapagliflozin for heart failure according to body mass index: The DELIVER trial. Eur Heart J 2022;43:4406–4417. doi: 10.1093/eurheartj/ehac481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kotecha D, Lam CS, Van Veldhuisen DJ, Van Gelder IC, Voors AA, Rienstra M. Heart failure with preserved ejection fraction and atrial fibrillation: Vicious twins. J Am Coll Cardiol 2016;68:2217–2228. doi: 10.1016/j.jacc.2016.08.048 [DOI] [PubMed] [Google Scholar]
- 23. Komajda M, Carson PE, Hetzel S, McKelvie R, McMurray J, Ptaszynska A, et al. Factors associated with outcome in heart failure with preserved ejection fraction: Findings from the irbesartan in heart failure with preserved ejection fraction study (I‐PRESERVE). Circ Heart Fail 2011;4:27–35. doi: 10.1161/CIRCHEARTFAILURE.109.932996 [DOI] [PubMed] [Google Scholar]
- 24. Prenner SB, Pillutla R, Yenigalla S, Gaddam S, Lee J, Obeid MJ, et al. Serum albumin is a marker of myocardial fibrosis, adverse pulsatile aortic hemodynamics, and prognosis in heart failure with preserved ejection fraction. J Am Heart Assoc 2020;9:e014716. doi: 10.1161/JAHA.119.014716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hamatani Y, Nagai T, Shiraishi Y, Kohsaka S, Nakai M, Nishimura K, et al. Long‐term prognostic significance of plasma B‐type natriuretic peptide level in patients with acute heart failure with reduced, mid‐range, and preserved ejection fractions. Am J Cardiol 2018;121:731–738. doi: 10.1016/j.amjcard.2017.12.012 [DOI] [PubMed] [Google Scholar]
- 26. DuBrock HM, AbouEzzeddine OF, Redfield MM. High‐sensitivity C‐reactive protein in heart failure with preserved ejection fraction. PLoS ONE 2018;13:e0201836. doi: 10.1371/journal.pone.0201836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fraunberger P, Schaefer S, Werdan K, Walli AK, Seidel D. Reduction of circulating cholesterol and apolipoprotein levels during sepsis. Clin Chem Lab Med 1999;37:357–362. [DOI] [PubMed] [Google Scholar]
- 28. Sahebkar A, Foroutan Z, Katsiki N, Jamialahmadi T, Mantzoros CS. Ferroptosis, a new pathogenetic mechanism in cardiometabolic diseases and cancer: Is there a role for statin therapy? Metabolism 2023;146:155659. doi: 10.1016/j.metabol.2023.155659 [DOI] [PubMed] [Google Scholar]
- 29. van der Meer P, Lok DJ, Januzzi JL, de la Porte PW, Lipsic E, van Wijngaarden J, et al. Adequacy of endogenous erythropoietin levels and mortality in anaemic heart failure patients. Eur Heart J 2008;29:1510–1515. doi: 10.1093/eurheartj/ehn205 [DOI] [PubMed] [Google Scholar]
- 30. Doi T, Nakata T, Hashimoto A, Yuda S, Wakabayashi T, Kouzu H, et al. Cardiac mortality assessment improved by evaluation of cardiac sympathetic nerve activity in combination with hemoglobin and kidney function in chronic heart failure patients. J Nucl Med 2012;53:731–740. doi: 10.2967/jnumed.111.095786 [DOI] [PubMed] [Google Scholar]
- 31. Wang MC, Huang CE, Lin MH, Yang YH, Lu CH, Chen PT, et al. Impacts of demographic and laboratory parameters on key hematological indices in an adult population of southern Taiwan: A cohort study. PLoS ONE 2018;13:e0201708. doi: 10.1371/journal.pone.0210200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kuter DJ. The physiology of platelet production. Stem Cells 1996;14:88–101. [DOI] [PubMed] [Google Scholar]
- 33. Valentova M, Anker SD, von Haehling S. Cardiac cachexia revisited: The role of wasting in heart failure. Cardiol Clin 2022;40:199–207. doi: 10.1016/j.ccl.2021.12.008 [DOI] [PubMed] [Google Scholar]
- 34. Schäfer A, Fraccarollo D, Hildemann S, Christ M, Eigenthaler M, Kobsar A, et al. Inhibition of platelet activation in congestive heart failure by aldosterone receptor antagonism and ACE inhibition. Thromb Haemost 2003;89:1024–1030. [PubMed] [Google Scholar]
- 35. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128 [DOI] [PubMed] [Google Scholar]
- 36. Marume K, Takashio S, Nagai T, Tsujita K, Saito Y, Yoshikawa T, et al. Effect of statins on mortality in heart failure with preserved ejection fraction without coronary artery disease ‐ Report from the JASPER study. Circ J 2019;83:357–367. doi: 10.1253/circj.CJ-18-0639 [DOI] [PubMed] [Google Scholar]
- 37. Serebruany V, McKenzie M, Meister A, Fuzaylov S, Gurbel P, Atar D, et al. Whole blood impedance aggregometry for the assessment of platelet function in patients with congestive heart failure (EPCOT trial). Eur J Heart Fail 2002;4:461–467. doi: 10.1016/S1388-9842(02)00026-0 [DOI] [PubMed] [Google Scholar]
- 38. Gasser JA, Betterridge DJ. Comparison of the effects of carvedilol, propranolol, and verapamil on in vitro platelet function in healthy volunteers. J Cardiovasc Pharmacol 1991;18:S29–S34. doi: 10.1097/00005344-199118041-00007 [DOI] [PubMed] [Google Scholar]
- 39. Schwemmer M, Sommer O, Bassenge E. Angiotensin receptor blocker losartan suppresses platelet activity by interfering with thromboxane signaling. Cardiovasc Drugs Ther 2001;15:301–307. doi: 10.1023/A:1012750430056 [DOI] [PubMed] [Google Scholar]
- 40. Cauliez B, Boucher S, Duflo‐Leroy A, Lavoinne A. NT‐proBNP measurement on Immulite 2500 (DPC): Analytical performance and comparison with Roche diagnostics and Dade‐Behring NT‐proBNP immunoassays. Ann Biol Clin (Paris) 2007;65:93–98. doi: 10.1684/abc.2007.0030 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Kaplan–Meier analysis of the composite of all‐cause death and HF readmission among the four groups, according to LEP score in males and females. HF, heart failure.
Figure S2. (A) CONUT score between the patients with LDL‐C < 76 mg/dL and LDL ≥ 76 mg/dL. (B) eGFR between the patients with erythrocyte < 362 × 104/μl and erythrocyte ≥ 362 × 104/μl. (C) FIB4 index between the patients with platelet < 24.0 × 104/μl and platelet ≥ 24.0 × 104/μl. eGFR, estimated glomerular filtration rate.


