Abstract
Aims
The current literature provides limited guidance on the best diuretic strategy post‐hospitalization for acute heart failure (AHF). It is postulated that the efficacy and safety of the outpatient diuretic regimen may be significantly influenced by the degree of fluid overload (FO) encountered during hospitalization. We hypothesize that in patients with more pronounced FO, reducing their regular oral diuretic dosage might be associated with an elevated risk of unfavourable clinical outcomes.
Methods and results
It was a retrospective observational study of 410 patients hospitalized for AHF in which the dose of furosemide at admission and discharge was collected. Patients were categorized across diuretic dose status into two groups: (i) the down‐titration group and (ii) the stable/up‐titration group. FO status was evaluated by a clinical congestion score and circulating biomarkers. The endpoint of interest was the composite of time to all‐cause death and/or heart failure readmission. A multivariable Cox proportional hazard regression model was constructed to analyse the endpoints. The median age was 86 (78–92) years, 256 (62%) were women, and 80% had heart failure with preserved ejection fraction. After multivariate adjustment, the down‐titration furosemide equivalent dose remained not associated with the risk of the combined endpoint in the whole sample (hazard ratio 1.34, 95% confidence interval 0.86–2.06, P = 0.184). The risk of the combination of death and/or worsening heart failure associated with the diuretic strategy at discharge was significantly influenced by FO status, including clinical congestion scores and circulating proxies of FO like BNP and cancer antigen 125.
Conclusions
In patients hospitalized for AHF, furosemide down‐titration does not imply an increased risk of mortality and/or heart failure readmission. However, FO status modifies the effect of down‐titration on the outcome. In patients with severe congestion or residual congestion at discharge, down‐titration was associated with an increased risk of mortality and/or heart failure readmission.
Keywords: Acute heart failure, Loop diuretic, Down‐titration, Fluid overload, Prognosis
Introduction
Diuretics are the cornerstone of treatment in patients hospitalized for acute heart failure (AHF), and a maintenance dose of loop diuretics is prescribed in most of the patients at discharge. 1 This widespread prescription relies on the empirical observation that diuretics lead to rapid relief of symptoms. Chronic use of diuretics is not included in disease‐modifying treatment, and heterogeneous data are present in the literature on the ability of these drugs to reduce the risk of adverse clinical events. 2 , 3 , 4 , 5 Along this same line of thought, there is very limited evidence about the optimal diuretic strategy following hospitalization for AHF. Current guidelines suggest the lowest diuretic dose to achieve euvolaemia. 6 , 7 However, current evidence does not clarify the intensity of diuretic treatment at discharge following hospitalization from AHF. 8
An important proportion of patients admitted with AHF are on chronic diuretic treatment, and these numbers increase as the disease progresses. 9 Thus, there are an important number of patients receiving intensive diuretic treatment before decompensation, a profile with a higher risk of poor response to diuretics and adverse events. 10 In this scenario, there is no solid evidence about tailoring diuretic therapy at discharge. We speculate that the intensity of the outpatient diuretic regimen and the risk of adverse clinical events will be mainly influenced by the severity of fluid overload (FO) status during the hospitalization.
Thus, we hypothesize that a down‐titration of the prior oral maintenance dose may be associated with a higher risk of adverse events in those with greater FO.
Methods
Study design and patients
We performed a retrospective observational study of patients hospitalized for AHF, in which the dose of furosemide at admission and discharge was collected. All patients were admitted for hospitalization in the Internal Medicine department ward of a third‐level‐of‐care hospital in Spain from June 2020 and March 2022. The diagnosis of AHF was made by the attending physician according to current guidelines. 7
Eligibility criteria included patients who were on a previous oral maintenance dose of loop diuretics and survived until discharge at least 4 weeks before admission. Information related to demography, medical history, physical examination, 12‐lead electrocardiogram, standard laboratory tests, echocardiographic data, and pharmacological therapies during hospitalization and discharge was routinely recorded using pre‐established electronic questionnaires. A flow chart is presented in Figure 1 .
Figure 1.

Flow chart of the selection process for the study participants. ADHF, acute decompensated heart failure; AHF, acute heart failure.
The study was conducted following the Declaration of Helsinki and was approved by Ramón y Cajal Hospital's ethics committee. All participants signed an informed consent form before participating in this study.
Patients were categorized across diuretic dose status in two groups: (i) the down‐titration group, whose discharge diuretic dose was lower than prior to hospitalization, and (ii) the stable/up‐titration group, in which patients received equal or higher doses at discharge. Furosemide equivalent doses (FEDs) were calculated using the following equivalence: 20 mg of oral torsemide = 40 mg of oral furosemide.
Fluid overload assessment
FO status was evaluated by a clinical congestion score and circulating biomarkers. A congestion score was calculated at discharge based on the sum of scores for the degree of oedema (0–3), pulmonary crackles (0–3), and dyspnoea (0–3). The main congestion markers derived from biochemical measurements were BNP at admission, cancer antigen 125 (CA125) during hospitalization, and estimated plasma volume status (ePVS) at discharge. Plasma volume state was estimated through instantaneous estimation of ePVS as described by Duarte et al. [(100 − haematocrit)/haemoglobin]. We chose the cut point of 5.5 mL/g for its utility in discriminating the prognosis of patients with heart failure with preserved ejection fraction (HFpEF). 11 , 12 BNP and CA125 levels were categorized in quartiles and compared Q4 vs. Q1–Q3 (985 pg/mL and 119 UI/mL, respectively).
Outcomes
The endpoint of interest was the composite of time to all‐cause death and/or heart failure (HF) readmission. The definition of readmission included any unplanned in‐hospital stay longer than 24 h requiring intravenous therapy.
Statistical analysis
Continuous variables were expressed as means and standard deviations, or median and interquartile ranges, as appropriate. Discrete variables were summarized as numbers and percentages. Baseline characteristics between groups (down‐titration or stable/up‐titration dose) were compared by the χ 2 test for categorical variables and the t‐test or Wilcoxon rank sum for continuous variables. Time‐to‐endpoint analysis was performed by Kaplan–Meier plots and a multivariable Cox proportional hazard regression model. Estimates of risk were presented as hazard ratios (HRs) with their respective 95% confidence intervals (95% CIs).
All covariates shown in Table 1 were evaluated for predictive purposes. Backward stepwise selection (removal/entry probabilities, 0.1/0.05) was used to derive a final model. In addition to our exposure (interaction of FO status with diuretic dose categories), the final multivariate model included the following covariates: age, sex, atrial fibrillation, chronic kidney disease, BNP, and length of stay (days). The proportional hazards assumption was tested on the basis of Schoenfeld residuals.
Table 1.
Baseline characteristics
| Variables | Total (n = 410) | Down‐titration (n = 52) | Stable dose (n = 358) | P‐value |
|---|---|---|---|---|
| Age, years | 86 (82–90) | 86.5 (82–88.5) | 86 (82–90) | 0.797 |
| Women, n (%) | 256 (62.4) | 33 (63.4) | 223 (62.2) | 0.871 |
| Medical history | ||||
| Arterial hypertension, n (%) | 363 (89) | 47 (92.1) | 316 (88.5) | 0.437 |
| Diabetes mellitus, n (%) | 179 (43.6) | 25 (48.1) | 154 (43.0) | 0.492 |
| Atrial fibrillation, n (%) | 241 (58.8) | 29 (55.7) | 212 (59.2) | 0.637 |
| HFpEF, n (%) | 327 (79.7) | 35 (67.3) | 292 (81.5) | 0.017 |
| CKD, n (%) | 207 (50.6) | 33 (63.5) | 174 (48.7) | 0.047 |
| Chronic treatment | ||||
| ACE‐Is, n (%) | 101 (24.8) | 11 (21.6) | 90 (25.3) | 0.566 |
| ARBs, n (%) | 115 (28.3) | 18 (34.6) | 97 (27.4) | 0.281 |
| Beta‐blockers, n (%) | 219 (53.7) | 28 (53.8) | 191 (53.6) | 0.979 |
| Thiazides, n (%) | 43 (10.5) | 7 (13.4) | 36 (10) | 0.454 |
| Espironolactone, n (%) | 61 (15) | 11 (22.4) | 50 (14.2) | 0.132 |
| Furosemide at admission, mg | 40 (20–80) | 100 (62.5–120) | 40 (10–60) | 0.001 |
| Furosemide at discharge, mg | 80 (40–80) | 50 (40–80) | 80 (40–80) | 0.037 |
| Vital signs | ||||
| Heart rate, b.p.m. | 82.5 (20.4) | 83.8 (23.6) | 82.3 (19.9) | 0.983 |
| SBP, mmHg | 136.2 (23) | 133.5 (23) | 136.5 (23.6) | 0.415 |
| Clinical presentation | ||||
| Pleural effusion, n (%) | 170 (41.4) | 17 (32.7) | 153 (42.7) | 0.169 |
| Oedema, n (%) | 275 (67.0) | 37 (71.1) | 238 (66.5) | 0.503 |
| Laboratory | ||||
| Haemoglobin, g/dL | 12 (10.6–13.2) | 11.5 (9.6–12.7) | 12 (10.7–13.3) | 0.055 |
| Haematocrit, % | 37.1 (6.3) | 35.9 (6.3) | 37.3 (6.3) | 0.149 |
| ePVS, mL/g | 5.3 (4.4–6.6) | 5.5 (4.6–7.1) | 5.3 (4.46.5) | 0.106 |
| Plasma sodium, mmol/L | 139 (5.8) | 139 (6.3) | 139 (5.7) | 0.966 |
| Plasma potassium, mmol/L | 4.1 (3.7–4.5) | 4.1 (3.8–4.6) | 4.1 (3.7–4.5) | 0.243 |
| Plasma chloride, mmol/L | 100 (96–102) | 99 (96–101.5) | 100 (96–102) | 0.372 |
| Creatinine, mg/dL | 1.26 (0.9–1.8) | 1.35 (1.0–2.0) | 1.25 (0.8–1.8) | 0.174 |
| eGFR, mL/min/1.73 m2 | 43.2 (31–62) | 39.8 (27.5–51.9) | 45 (31.4–62.7) | 0.049 |
| BNP, pg/mL | 548 (306–985) | 537 (315–886) | 548 (294–991) | 0.676 |
| CA125, U/mL | 58.2 (28–120) | 45.4 (22–102) | 59 (29–120.2) | 0.164 |
| Congestion at discharge | ||||
| Congestion score ≥ 1, n (%) | 212 (55.3) | 20 (42.5) | 192 (57.1) | 0.059 |
| Dyspnoea, n (%) | 54 (14.2) | 5 (10.6) | 49 (14.7) | 0.654 |
| Oedema, n (%) | 80 (20.9) | 8 (17) | 72 (21.3) | 0.569 |
| Crackles, n (%) | 163 (42.4) | 17 (36.2) | 146 (43.3) | 0.353 |
ACE‐Is, angiotensin‐converting enzyme inhibitors; ARBs, angiotensin receptor blockers; BNP, brain natriuretic peptide; CA125, cancer antigen 125; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; ePVS, estimated plasma volume status; HFpEF, heart failure with preserved ejection fraction; SBP, systolic blood pressure.
Additionally, subgroup analysis was conducted to address the effect modification of congestion status on the outcome. A two‐sided P‐value < 0.05 was considered statistically significant for all analyses. All analyses were performed using STATA 15.1.
Results
Baseline characteristics
A total of 410 patients were included in the study. A flow chart is presented in Figure 1 . The median age was 86 years (78–92), 256 (62%) were women, and 80% had HFpEF. The median oral FED prior to admission was 40 mg (20–80), and the median FED at discharge was 80 mg (40–80). Median doses of FED at discharge in the down‐titration and stable/up‐titration groups were 50 (40–80) vs. 80 (40–80) (P = 0.037). A down‐titration, maintenance, and up‐titration strategy was observed in 52 (13%), 134 (32%), and 224 (54%) patients, respectively. Patients in the down‐titration group had a higher prevalence of chronic kidney disease, a lower prevalence of HFpEF, and a higher FED prior to admission (Table 1 ).
The FO status differs between the two groups, with a higher prevalence of a congestion score ≥ 1 at discharge in the stable dose group/up‐titration. We did not find differences between groups in BNP, CA125, and ePVS values (Table 1 ).
Adverse clinical events
During a median follow‐up of 187 days (72–346), we registered 196 events (47%), 146 (35.6%) deaths and 183 (44.7%) HF readmissions. As a main term, Kaplan–Meier curves showed no significant differences between FED groups, as is shown in Figure 2 . After multivariate adjustment, down‐titration FED was not associated with the risk of the combined endpoint in the whole sample (HR 1.34, 95% CI 0.86–2.06, P = 0.184).
Figure 2.

Kaplan–Meier plots by diuretic strategy at discharge.
Diuretic down‐titration and adverse clinical events across surrogates of fluid overload status
The risk of the combination of death and/or worsening HF associated with the diuretic strategy at discharge was significantly influenced by FO status (Table 2 ), including clinical congestion scores and circulating proxies of FO like BNP, CA125, and ePVS.
Table 2.
Combined outcome grouped by fluid overload status and diuretic strategy at discharge
| Total patients (N = 410) | No down‐titration | Down‐titration | Readmission or death | ||
|---|---|---|---|---|---|
| Event/total (%) | Event/total (%) | HR (95% CI) | P for effect | P for interaction | |
| Congestion score | |||||
| ≥1 | 95/192 (49%) | 13/20 (65%) | 2.11 (1.14–3.9) | 0.017 | 0.016 |
| <1 | 60/144 (41%) | 13/27 (48%) | 1.40 (0.73–2.67) | 0.304 | |
| BNP | |||||
| Above upper quartile | 52/85 (61%) | 7/9 (77%) | 2.38 (1.06–5.33) | 0.027 | 0.032 |
| Below upper quartile | 97/237 (41%) | 20/38 (52%) | 1.19 (0.72–1.98) | 0.485 | |
| CA125 | |||||
| Above upper quartile | 32/76 (42%) | 8/8 (100%) | 5.03 (2.03–12.42) | 0.001 | 0.005 |
| Below upper quartile | 97/217 (44%) | 14/34 (41%) | 0.86 (0.46–1.59) | 0.644 | |
| Plasma volume (ePVS) | |||||
| ≥5.5 mL/g | 70/144 (48%) | 17/26 (65%) | 1.64 (0.92–2.91) | 0.09 | 0.260 |
| <5.5 mL/g | 97/214 (45%) | 12/26 (46%) | 1.05 (0.54–2.06) | 0.866 | |
BNP, brain natriuretic peptide; CA125, cancer antigen 125; CI, confidence interval; ePVS, estimated plasma volume status; HR, hazard ratio.
Clinical congestion scores
The association between diuretic dose (down‐titration vs. stable/up‐titration) was heterogeneous across the clinical congestion score (P‐value for interaction = 0.016). In patients with a clinical congestion score ≥ 1, down‐titration at discharge was associated with an increased risk of death or rehospitalization when compared with patients in whom a stable/up‐titration strategy was prescribed (HR 2.11, 95% CI 1.14–3.90, P = 0.017). On the contrary, in the subgroup of patients with a congestion score < 1, we found no difference in terms of mortality and rehospitalization between the two diuretic strategies (HR 1.40, 95% CI 0.73–2.67, P = 0.304) (Figure 3 ).
Figure 3.
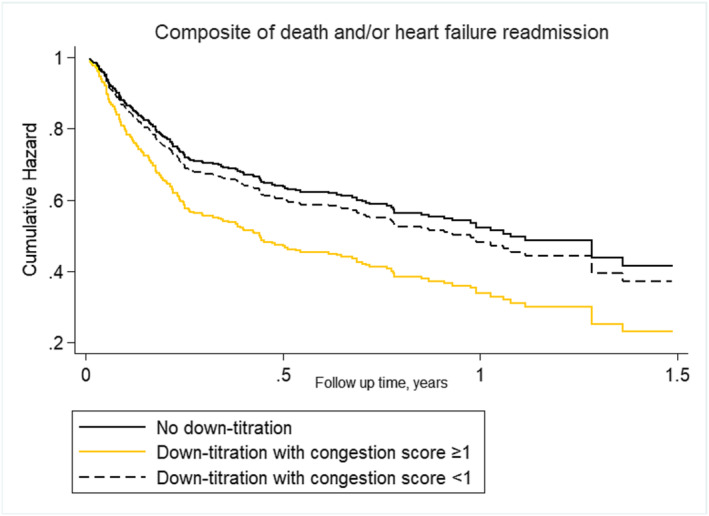
Cox multivariable proportional hazard model curves by diuretic strategy/congestion score categories for the combined outcome.
Circulating proxies of fluid overload
We also found a differential association between diuretic strategy across BNP and CA125 (P‐value for interactions < 0.05 for both comparisons). In those patients in the upper quartile of BNP, diuretic down‐titration was associated with a higher risk of adverse events (HR 2.38, 95% CI 1.06–5.33, P = 0.027). Conversely, in those in the three lower quartiles, diuretic down‐titration was not related to clinical outcomes (HR 1.19, 95% CI 0.72–1.98, P = 0.485) (Figure 4 ). Similarly, diuretic down‐titration was associated with an increased risk of the composite endpoint in patients in the upper quartile of CA125 (HR 5.03, 95% CI 2.03–12.42, P = 0.001) but not in the rest of patients (HR 0.86, 95% CI 0.46–1.59, P = 0.644) (Figure 5 ). The ePVS status was not differentially associated with the association between diuretic down‐titration and prognosis (P for interaction = 0.260).
Figure 4.
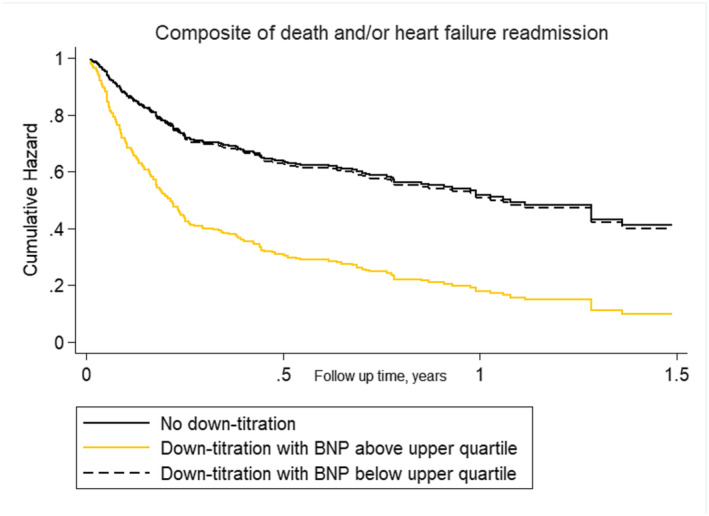
Cox multivariable proportional hazard model curves by diuretic strategy/brain natriuretic peptide (BNP) categories for the combined outcome.
Figure 5.

Cox multivariable proportional hazard model curve by diuretic strategy/cancer antigen 125 (CA125) categories for the combined outcome.
Discussion
In elderly patients hospitalized for AHF, predominantly with HFpEF, down‐titration of oral diuretics at discharge (compared with baseline prior to diuretic treatment) does not have a negative effect on mortality or HF hospitalization in the whole sample. However, the risk associated with the diuretic strategy at discharge was significantly influenced by the FO status assessed during hospitalization. In those with overt signs of FO at admission or residual congestion at discharge, FED down‐titration was related to a higher risk of adverse clinical events.
Down‐titration and prognosis
In the current analysis, loop diuretic oral maintenance dose down‐titration at discharge is not an independent predictor of mortality and/or HF readmission in the whole population. These findings are consistent with previous studies. DeVore and colleagues 13 analysed the impact on the prognosis of loop diuretic dose adjustments after hospitalization, showing no changes in the composite outcome of 30 day mortality or hospitalization. A recent Japanese study conducted by Seko et al. 14 confirmed no difference in terms of mortality when comparing patients in whom a decreased dose of loop diuretics was prescribed at discharge with patients in whom the same dose was maintained.
Some factors seem to influence the decision of the physicians on the choice of the diuretic strategy at discharge. In accordance with a previous study, 1 in our research, baseline characteristics analysis showed that a stable dose strategy has been especially prescribed in patients with residual congestion. On the contrary, the factors associated with a down‐titration strategy seem to be high loop diuretic dose at admission and the presence of chronic kidney disease, suggesting that clinicians relate worse kidney function with a more intensive diuretic strategy, ignoring most of the patients with HF and kidney dysfunction who may show congestive nephropathy and probably a more intensive requirement of diuretics. 15 , 16 , 17
Concurrent medical therapies, such as sacubitril/valsartan or sodium‐glucose co‐transporter 2 inhibitor (SGLT2i), may also influence the decision on down‐titration. Sacubitril/valsartan shows a diuretic sparing effect in patients with HF with reduced ejection fraction, 18 and guidelines suggest diuretic dose reduction when introducing this drug. In a similar way, a reduction in new initiations of loop diuretics and a tendency to dose decrease were observed in patients treated with SGLT2i 19 , 20 compared with placebo.
In our cohort, only a very small percentage of patients were on sacubitril/valsartan before hospitalization, and we did not recollect data about SGLT2i treatment because the vast majority of patients were recruited before the publication of EMPEROR‐Preserved 21 and DELIVER. 22
The modulating role of fluid overload status: a time for precision medicine
The main learning from our study is that, regarding diuretic therapy, one size does not fit all. It probably explains why old and recent clinical trials have failed to show a reduction in adverse clinical events. 23 , 24 , 25 , 26
Indeed, we found that diuretic down‐titration may be related to higher risks and then be inappropriate in patients with overt signs of FO during admission. Specifically, we found that down‐titration in patients with severe congestion at admission or residual clinical congestion at discharge (congestion score ≥ 1) led to a worse prognosis. Interestingly, beyond clinical evaluation, we also found that in‐hospital assessment of CA125 and BNP may also be useful for guiding diuretic therapy at discharge. Indeed, the CHANCE‐HF randomized clinical trial showed that CA125‐guided diuretic therapy after an episode of AHF translated into a significant reduction of 1 year adverse clinical events. 27 A similar utility in guiding treatment has not been previously demonstrated for BNP. 28 , 29 We believe further diuretic trials should focus on an appropriate selection of patients using multiparametric but also widely available tools. 15
Clinical implications
Our research underscores the critical need for an in‐depth evaluation of FO in patients hospitalized for AHF before they are discharged. This could help us to guide the intensity of the diuretic approach the first week‐months after discharge. 8
In patients without residual congestion (congestion score < 1) or lower congestive status (BNP and CA125 below the upper quartile), down‐titration did not modify prognosis, and an attempt to diminish the intensity of diuretic therapy seems reasonable. This speculation is consistent with results from previous studies in ambulatory patients followed for chronic HF. 30 On the contrary, patients with overt FO may require more extended, intensive diuretic therapy after discharge.
Limitations
Several limitations need to be acknowledged. First, this is an observational study conducted at a single centre. This implies that some confounders may not have been considered, and some negative results could be explained by the insufficient statistical power. Thus, the results should be interpreted as hypothesis generating. Second, baseline differences between intervention groups were found, although multivariate analysis was conducted to try to mitigate their effect. Third, BNP and CA125 were not collected in 12% and 20% of patients, respectively. Fourth, intravenous FEDs during hospitalization were not registered. Fifth, changes in diuretic doses after discharge were not assessed. Sixth, we did not collect information on the prevalence of patients on oxygen therapy during hospitalization, an important parameter that could have influenced the clinician's decision on down‐titration.
Conclusions
In patients hospitalized for AHF, furosemide down‐titration does not imply an increased risk of mortality and/or HF readmission. However, FO status modifies the effect of down‐titration on the outcome. In patients with severe congestion or residual congestion at discharge, down‐titration was associated with an increased risk of mortality and/or HF readmission.
Conflict of interest
None declared.
Funding
This study has no funding.
Croset, F. , Llàcer, P. , Núñez, J. , Campos, J. , García, M. , Pérez, A. , Fernández, C. , Fabregate, M. , López, G. , Tello, S. , Fernández, J. M. , Ruiz, R. , and Manzano, L. (2024) Loop diuretic down‐titration at discharge in patients hospitalized for acute heart failure. ESC Heart Failure, 11: 1739–1747. 10.1002/ehf2.14749.
References
- 1. Faselis C, Arundel C, Patel S, Lam PH, Gottlieb SS, Zile MR, et al. Loop diuretic prescription and 30‐day outcomes in older patients with heart failure. J Am Coll Cardiol 2020;76:669‐679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eshaghian S, Horwich TB, Fonarow GC. Relation of loop diuretic dose to mortality in advanced heart failure. American Journal of Cardiology 2006;97:1759‐1764. doi: 10.1016/j.amjcard.2005.12.072 [DOI] [PubMed] [Google Scholar]
- 3. Dini FL, Ghio S, Klersy C, Rossi A, Simioniuc A, Scelsi L, et al. Effects on survival of loop diuretic dosing in ambulatory patients with chronic heart failure using a propensity score analysis. Int J Clin Pract 2013;67:656‐664. doi: 10.1111/ijcp.12144 [DOI] [PubMed] [Google Scholar]
- 4. Faris R, Flather M, Purcell H, Henein M, Poole‐Wilson P, Coats A. Current evidence supporting the role of diuretics in heart failure: A meta analysis of randomised controlled trials. Int J Cardiol 2002;82:149‐158. doi: 10.1016/S0167-5273(01)00600-3 [DOI] [PubMed] [Google Scholar]
- 5. Testani JM, Chen J, McCauley BD, et al. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation 2010;122:265‐272. doi: 10.1161/CIRCULATIONAHA.109.933275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022;145:895‐1032. doi: 10.1161/CIR.0000000000001063 [DOI] [PubMed] [Google Scholar]
- 7. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599‐3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 8. Mullens W, Damman K, Harjola VP, Mebazaa A, Brunner‐la Rocca HP, Martens P, et al. The use of diuretics in heart failure with congestion—A position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019;21:137‐155. doi: 10.1002/ejhf.1369 [DOI] [PubMed] [Google Scholar]
- 9. Metra M, Tomasoni D, Adamo M, Bayes‐Genis A, Filippatos G, Abdelhamid M, et al. Worsening of chronic heart failure: Definition, epidemiology, management and prevention. A clinical consensus statement by the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2023;25:776‐791. doi: 10.1002/ejhf.2874 [DOI] [PubMed] [Google Scholar]
- 10. Damman K, Kjekshus J, Wikstrand J, et al. Loop diuretics, renal function and clinical outcome in patients with heart failure and reduced ejection fraction. Eur J Heart Fail 2016;18:328‐336. doi: 10.1002/ejhf.462 [DOI] [PubMed] [Google Scholar]
- 11. Duarte K, Monnez JM, Albuisson E, et al. Prognostic value of estimated plasma volume in heart failure. JACC Heart Fail 2015;3:886‐893. doi: 10.1016/j.jchf.2015.06.014 [DOI] [PubMed] [Google Scholar]
- 12. Huang CY, Lin TT, Wu YF, et al. Long‐term prognostic value of estimated plasma volume in heart failure with preserved ejection fraction. Sci Rep 2019;9:14369. doi: 10.1038/s41598-019-50427-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. DeVore AD, Hasselblad V, Mentz RJ, et al. Loop diuretic dose adjustments after a hospitalization for heart failure: Insights from ASCEND‐HF. Eur J Heart Fail 2015;17:340‐346. doi: 10.1002/ejhf.235 [DOI] [PubMed] [Google Scholar]
- 14. Seko Y, Kato T, Morimoto T, et al. Association between changes in loop diuretic dose and outcomes in acute heart failure. ESC Heart Fail 2023;10:1757‐1770. doi: 10.1002/ehf2.14338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de la Espriella R, Cobo M, Santas E, Verbrugge FH, Fudim M, Girerd N, et al. Assessment of filling pressures and fluid overload in heart failure: An updated perspective. Revista Española de Cardiología (English Edition) 2023;76:47‐57. doi: 10.1016/j.rec.2022.07.009 [DOI] [PubMed] [Google Scholar]
- 16. Lorenzo M, Núñez G, Fuertes‐Kenneally L, de la Espriella R, Villar S, Miró O, et al. Early glomerular filtration rates changes and risk of mortality in acute heart failure. The modifying role of admission renal function and decongestion. Eur J Intern Med 2023;115:96‐103. doi: 10.1016/j.ejim.2023.05.037 [DOI] [PubMed] [Google Scholar]
- 17. Mullens W, Damman K, Testani JM, Martens P, Mueller C, Lassus J, et al. Evaluation of kidney function throughout the heart failure trajectory—A position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2020;22:584‐603. doi: 10.1002/ejhf.1697 [DOI] [PubMed] [Google Scholar]
- 18. Vardeny O, Claggett B, Kachadourian J, Desai AS, Packer M, Rouleau J, et al. Reduced loop diuretic use in patients taking sacubitril/valsartan compared with enalapril: The PARADIGM‐HF trial. Eur J Heart Fail 2019;21:337‐341. doi: 10.1002/ejhf.1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chatur S, Vaduganathan M, Claggett B, Vardeny O, Desai AS, Jhund PS, et al. Dapagliflozin and diuretic utilization in heart failure with mildly reduced or preserved ejection fraction: The DELIVER trial. Eur Heart J 2023;44:2930‐2943. doi: 10.1093/eurheartj/ehad283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Packer M, Butler J, Zannad F, Filippatos G, Ferreira JP, Pocock SJ, et al. Effect of empagliflozin on worsening heart failure events in patients with heart failure and preserved ejection fraction: EMPEROR‐Preserved trial. Circulation 2021;144:1284‐1294. doi: 10.1161/CIRCULATIONAHA.121.056824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. New England Journal of Medicine 2021;385:1451‐1461. doi: 10.1056/NEJMoa2107038 [DOI] [PubMed] [Google Scholar]
- 22. Solomon SD, McMurray JJV, Claggett B, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. New England Journal of Medicine 2022;387:1089‐1098. doi: 10.1056/NEJMoa2206286 [DOI] [PubMed] [Google Scholar]
- 23. Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, et al. Diuretic strategies in patients with acute decompensated heart failure. New England Journal of Medicine 2011;364:797‐805. doi: 10.1056/NEJMoa1005419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mullens W, Dauw J, Martens P, Verbrugge FH, Nijst P, Meekers E, et al. Acetazolamide in acute decompensated heart failure with volume overload. New England Journal of Medicine 2022;387:1185‐1195. doi: 10.1056/NEJMoa2203094 [DOI] [PubMed] [Google Scholar]
- 25. Trullàs JC, Morales‐Rull JL, Casado J, Carrera‐Izquierdo M, Sánchez‐Marteles M, Conde‐Martel A, et al. Combining loop with thiazide diuretics for decompensated heart failure: The CLOROTIC trial. Eur Heart J 2023;44:411‐421. doi: 10.1093/eurheartj/ehac689 [DOI] [PubMed] [Google Scholar]
- 26. Mentz RJ, Anstrom KJ, Eisenstein EL, et al. Effect of torsemide vs furosemide after discharge on all‐cause mortality in patients hospitalized with heart failure: The TRANSFORM‐HF randomized clinical trial. JAMA 2023;329: doi: 10.1001/jama.2022.23924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Núñez J, Llàcer P, Bertomeu‐González V, Bosch MJ, Merlos P, García‐Blas S, et al. Carbohydrate antigen‐125–guided therapy in acute heart failure: CHANCE‐HF: A randomized study. JACC Heart Fail 2016;4:833‐843. doi: 10.1016/j.jchf.2016.06.007 [DOI] [PubMed] [Google Scholar]
- 28. Desai AS. Are serial BNP measurements useful in heart failure management? Circulation 2013;127:509‐516. doi: 10.1161/CIRCULATIONAHA.112.120493 [DOI] [PubMed] [Google Scholar]
- 29. Pfisterer M, Buser P, Rickli H, Gutmann M, Erne P, Rickenbacher P, et al. BNP‐guided vs symptom‐guided heart failure therapy. JAMA 2009;301:383‐392. doi: 10.1001/jama.2009.2 [DOI] [PubMed] [Google Scholar]
- 30. Martens P, Verbrugge FH, Nijst P, Dupont M, Mullens W. Changes in loop diuretic dose and outcome after cardiac resynchronization therapy in patients with heart failure and reduced left ventricular ejection fractions. American Journal of Cardiology 2017;120:267‐273. doi: 10.1016/j.amjcard.2017.04.021 [DOI] [PubMed] [Google Scholar]


