Abstract
Aims
Inherited cardiomyopathies are relatively rare but carry a high risk of cardiac maternal morbidity and mortality during pregnancy and postpartum. However, data for risk stratification are scarce. The new CARPREG II score improves prediction of prognosis in pregnancies associated with heart disease, though its role in inherited cardiomyopathies is unclear. We aim to describe characteristics and cardiac maternal outcomes in patients with inherited cardiomyopathy during pregnancy, and to evaluate the interest of the CARPREG II risk score in this population.
Methods and results
In this retrospective single‐centre study, 90 consecutive pregnancies in 74 patients were included (mean age 32 ± 5 years), including 28 cases of dilated cardiomyopathy (DCM), 46 of hypertrophic cardiomyopathy, 11 of arrhythmogenic right ventricular cardiomyopathy and 5 of left ventricular noncompaction, excluding peripartum cardiomyopathy. The discriminatory power of several risk scores was assessed by the area under the receiver‐operating characteristic curve (AUC). Median CARPREG II score was 2 [0;3] and was higher in the DCM subgroup. A severe cardiac maternal complication was observed in 18 (20%) pregnancies, mainly driven by arrhythmia and heart failure (each event in 10 pregnancies), with 3 cardiovascular deaths. Forty‐three pregnancies (48%) presented foetal/neonatal complications (18 premature delivery, 3 foetal/neonatal death). CARPREG II was significantly associated with cardiac maternal complications (P < 0.05 for all) and showed a higher AUC (0.782) than CARPREG (0.755), mWHO (0.697) and ZAHARA (0.604).
Conclusions
Pregnancy in women with inherited cardiomyopathy carries a high risk of maternal cardiovascular complications. CARPREG II is the most efficient predictor of cardiovascular complications in this population.
Keywords: Cardiomyopathy, Pregnancy, Prognosis, Risk score
Introduction
Maternal heart disease affects between 1% and 4% of pregnancies and is the leading cause of maternal mortality in western countries, with a mortality rate around 1%. 1 , 2 , 3 The most frequently encountered diseases are congenital, hypertensive, and valvular heart disease. Conversely, cardiomyopathy (CMP) represents only 5% to 10% of registries, but appears to be at higher risk of cardiac complications. 2 , 3 , 4 The European ROPAC registry found a mortality rate of 2.4%, and 24% of heart failure in this subgroup, trends that were confirmed in the updated registry study. 2 , 3 However, data in CMP patients are scarce and are often recorded in heterogeneous populations including peripartum cardiomyopathies (PPCM) with different pathophysiology and prognosis. 6 , 7 , 8
Inherited CMP can be designated as all CMP of confirmed or presumed genetic origin, such as hypertrophic cardiomyopathy (HCM), dilated cardiomyopathy (DCM), arrhythmogenic right ventricular cardiomyopathy (ARVC) and left ventricular noncompaction (LVNC). Data about their cardiac risk during pregnancy are limited and are usually based on small observational studies, with heterogeneous results. Cardiac complications are reported in 23% to 48% of cases of HCM, 9 , 10 and similar disparities are found in registries of other CMP. 11 , 12 , 13 , 14 , 15 , 16
Risk stratification during pregnancy is important in patient management. Various scores have been proposed in women with cardiovascular disease to help predict cardiac complications during pregnancy and postpartum, such as the CARPREG score, ZAHARA, and the modified WHO classification. 1 , 5 , 17 These scores were developed mostly with data from patients with congenital heart disease and valvular heart disease, but data on CMP are scarce. 16 These scores thus have limitations in this population. 18
Recently, a new score emerged from a large prospective cohort led by Silversides et al. 19 The CARPREG II risk score integrates numerous predictors, general and lesion‐specific cardiac factors, as well as variables related to the process of care. 19 It performed better in risk stratification than previous scores. Again, CMP was poorly represented, limiting its application in this subgroup. The CARPREG II risk score has not yet been incorporated into the ESC Guidelines 1 and has never been specifically validated in a cohort of CMP. Our objective in this study was to describe characteristics and maternal outcomes in a cohort of pregnant women with inherited CMP, and to evaluate the value of the CARPREG II risk score in this population.
Methods
Study population
In this observational single‐centre retrospective study, we reviewed records of all consecutive pregnancies of patients with inherited CMP followed up or referred to the Pitié‐Salpêtrière hospital, AP‐HP, France, between March 1997 and May 2022. This hospital is a tertiary centre for genetic cardiomyopathies. Patients with DCM, defined according to the 2008 position statement, 20 HCM, according to ESC criteria, 21 ARVC, according to the task force criteria 22 and LVNC according to Jenni's criteria, 20 were included in the analysis. Exclusion criteria were as follows: significant valvular heart disease (VHD), congenital heart disease (CHD), or other condition explaining myocardial dysfunction (such as coronary artery disease, toxic cardiopathy, and severe hypertension). We also excluded patients with confirmed or suspected peripartum CMP, with different pathophysiology and prognosis. 6 , 7 , 23 Pregnancies in women who underwent termination or had a miscarriage before 20 weeks of gestation were excluded. The investigation conformed to the principles outlined in the Declaration of Helsinki. The study was performed in accordance with ethical standards, and all patient data were anonymized. According to French ethics and regulatory law, retrospective studies based on usual care data do not require prior submission to an ethics committee. This study complied with the reference methodology (MR004) of the French Data Protection Authority (CNIL).
Data collection and risk stratification
Cardiology and obstetrical records were retrospectively reviewed for each pregnancy to collect clinical and echocardiographic data. Specifically, the type of CMP, genetic status, previous cardiovascular events, treatments or interventions, and the presence of symptoms at baseline were collected. Echocardiography was often performed within the month before pregnancy for patients previously followed up in our centre, and at each trimester. Echocardiographic features, when available, were recorded as follows: left ventricular ejection fraction (LVEF), LV end‐diastolic diameter, significant left ventricle outflow tract obstruction (LVOTO) > 30 mmHg, right ventricle dilatation and/or dysfunction, and pulmonary artery hypertension.
The CARPREG II risk score was calculated for each pregnancy retrospectively based on Silversides et al. 19 It predicts the risk of cardiac maternal events during pregnancy or postpartum, according to 10 predictors: prior cardiac events or arrhythmias (3 points), baseline NYHA III‐IV or cyanosis (3 points), mechanical valve (3 points), ventricular dysfunction (2 points), high‐risk left‐sided valve disease/LVOTO (2 points), pulmonary hypertension (2 points), coronary artery disease (2 points), high‐risk aortopathy (2 points), no prior cardiac intervention (1 point), late pregnancy assessment >20 weeks of gestation (1 point). A CARPREG II score > 4 indicates a 41% risk of a cardiovascular event.
Other conventional risk scores (CARPREG, ZAHARA, and mWHO) were determined as published. 1 , 5 , 17
Cardiac and obstetrical outcomes
During follow‐up, clinical and echocardiographic data were collected on pregnancy, delivery, and during the 6 first months of postpartum, when available. Basic obstetrical and neonatal data were collected, including gestational age at delivery, mode of delivery, birth weight, and obstetrical and neonatal events.
Primary outcome was defined as in Silversides et al. 19 by the onset of any of the following cardiac maternal outcomes during pregnancy or within 6 months postpartum: maternal cardiac death; cardiac arrest, arrhythmia requiring treatment, heart failure, stroke or transient ischaemic attack, cardiac thromboembolism, myocardial infarction, and vascular dissection. Secondary obstetrical and neonatal outcomes were recorded when available. Foetal and neonatal events were defined as premature birth <37 weeks, low birth weight (below 10th centile of the neonatal weight curves), Apgar score <7, foetal or neonatal death (after 20 weeks of gestation, until 28 days from birth). 16 , 19
Statistical analyses
Continuous variables were expressed as mean and standard deviation if normally distributed, or median and interquartile ranges otherwise. Quantitative data were compared using Student's t test or Wilcoxon's test when appropriate, and proportions were compared using the chi‐square test or Fisher's exact test when appropriate. A P value <0.05 was considered significant. The discriminatory power of the CARPREG II risk score, as well as other scores, was assessed by calculating the area under the receiver‐operating characteristic curve (AUC). Statistical analyses were performed with MedCalc Statistical Software (Ostend, Belgium).
Results
Study population
We identified 107 pregnancies that were managed in our centre between March 1997 and June 2022. Seventeen pregnancies were excluded: two continuing pregnancies at the time of analysis; four patients with significant valvular heart disease; one with coronary artery disease; one with systolic dysfunction post‐anthracycline; three with post‐hypertensive heart disease; and six with peripartum cardiomyopathy.
A total of 90 pregnancies in 74 women were included in the final analysis: 46 HCM (51%), 28 DCM (31%), 11 ARVC (12%) and 5 LVNC (6%) (Figure 1 ).
Figure 1.
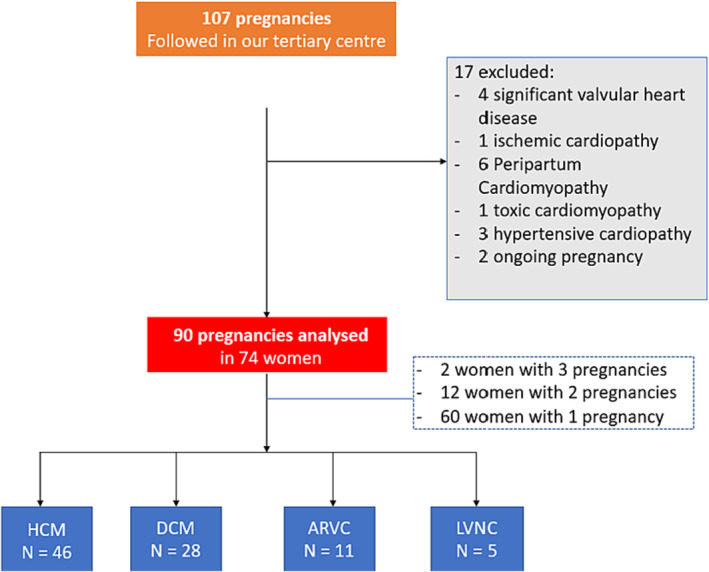
Flowchart of the cohort. ARVC, arrhythmogenic right ventricular cardiomyopathy; DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; LVNC, left ventricular noncompaction.
Baseline characteristics
Baseline characteristics are summarized in Table 1 , according to the type of CMP. Maternal mean age was 32 ± 5 years. In 44 pregnancies, mothers had a pathogenic mutation, most frequently in HCM and ARVC patients. In 30 pregnancies, (34%) there was history of cardiovascular events before pregnancy including 16 hospitalizations for heart failure. At baseline, one‐third of the cohort was symptomatic and only 5% with NYHA III/IV dyspnoea. Sixty‐eight patients were medically treated before pregnancy, a majority with beta‐blockers (73%). Regarding beta‐blockers, a great majority of patients continued the medical therapy through the pregnancy (61 over 65 pregnancies, 94%).
Table 1.
Baseline characteristics of the cohort, according to the type of cardiomyopathy, before pregnancy
| Overall | DCM | HCM | ARVC | LVNC | ||
|---|---|---|---|---|---|---|
| N | ||||||
| Number of pregnancies, n | 90 | 90 | 28 | 46 | 11 | 5 |
| Number of women, n (%) | 74 (100) | 26 (35) | 34 (46) | 10 (14) | 4 (5.4) | |
| Age (years) | 90 | 32 (5.0) | 32.3 (4.1) | 32.6 (5.2) | 31.5 (4.2) | 26.0 (6.3) |
| Body mass index (kg/m2) | 81 | 24.8 (6.8) | 27.4 (9.0) | 23.9 (5.5) | 23.6 (4.9) | 21.8 (1.1) |
| Late pregnancy assessment (>20 weeks of gestation) | 87 | 13 (15) | 4 (14) | 6 (14) | 1 (9.1) | 2 (50) |
| Cardiovascular history | ||||||
| Mutation, n (%) | 72 | 44 (61) | 8 (33) | 26 (76) | 7 (64) | 3 (100) |
| Cardiopathy diagnosed before pregnancy, n (%) | 90 | 85 (94) | 26 (93) | 44 (96) | 10 (91) | 5 (100) |
| Prior cardiac event, n (%) | 88 | 30 (34) | 13 (46) | 13 (30) | 2 (18) | 2 (40) |
| Prior urgent hospitalization for heart failure, n (%) | 88 | 14 (16) | 8 (29) | 5 (11) | 0 (0) | 1 (20) |
| History of stroke, n (%) | 88 | 3 (3) | 1 (3.6) | 2 (4.5) | 0 (0) | 0 (0) |
| History of sustained arrhythmia, n (%) | 88 | 5 (6) | 4 (14) | 1 (2.3) | 0 (0) | 0 (0) |
| Pacemaker, n (%) | 88 | 3 (3) | 3 (11) | 0 (0) | 0 (0) | 0 (0) |
| Implantable automatic defibrillator, n (%) | 88 | 12 (14) | 4 (14) | 6 (14) | 2 (18) | 0 (0) |
| Prior cardiac intervention or surgery a , n (%) | 88 | 6 (7) | 1 (3.6) | 3 (6.8) | 2 (18) | 0 (0) |
| Arterial hypertension n (%) | 89 | 4 (5) | 3 (11) | 1 (2.2) | 0 (0) | 0 (0) |
| Diabetes, n (%) | 89 | 1 (1) | 1 (3.6) | 0 (0) | 0 (0) | 0 (0) |
| Symptoms before pregnancy, n (%) | 88 | 31 (35) | 10 (36) | 16 (36) | 2 (18) | 3 (60) |
| Dyspnoea, n (%) | 89 | 21 (24) | 6 (21) | 13 (30) | 0 (0) | 2 (40) |
| NYHA > II, n (%) | 89 | 4 (5) | 1 (3.6) | 3 (6.8) | 0 (0) | 0 (0) |
| Palpitations, n (%) | 87 | 14 (16) | 5 (18) | 6 (14) | 1 (9.1) | 2 (50) |
| Medical treatment, n (%) | 89 | 68 (76) | 21 (75) | 34 (76) | 8 (73) | 5 (100) |
| At least two treatments, n (%) | 88 | 24 (27) | 14 (50) | 5 (11) | 4 (36) | 1 (20) |
| Beta‐blocker, n (%) | 89 | 65 (73) | 21 (75) | 33 (73) | 7 (64) | 4 (80) |
| Other anti‐arrhythmic drug, n (%) | 88 | 13 (15) | 6 (22) | 2 (4.4) | 4 (36) | 1 (20) |
| Renin‐angiotensin‐aldosterone system inhibitor, n (%) | 89 | 13 (15) | 10 (36) | 1 (2) | 1 (9) | 1 (20) |
| Diuretics, n (%) | 89 | 8 (9) | 6 (21) | 2 (4) | 0 (0) | 0 (0) |
| Mineralocorticoid receptor antagonist, n (%) | 89 | 4 (5) | 4 (14) | 0 (0) | 0 (0) | 0 (0) |
| Anticoagulation, n (%) | 89 | 5 (6) | 3 (11) | 1 (2) | 0 (0) | 1 (20) |
| Echocardiographic features | ||||||
| LVEF (%) | 90 | 57 (12) | 46 (10) | 66 (7) | 60 (5) | 39 (8) |
| LVEF < 50%, n (%) | 90 | 24 (27) | 19 (68) | 0 (0) | 0 (0) | 5 (100) |
| LVEF < 40%, n (%) | 90 | 8 (7%) | 7 (25) | 0 (0) | 0 (0) | 1 (20) |
| LV end‐diastolic diameter (mm) | 86 | 48 (10) | 56 (7) | 42 (7) | 51 (4) | 59 (1) |
| LVOTO > 30 mmHg, n (%) | 90 | 11 (12) | 0 (0) | 11 (24) | 0 (0) | 0 (0) |
| Pulmonary hypertension, n (%) | 90 | 5 (7) | 0 (0) | 3 (7.9) | 1 (10) | 1 (20) |
| Right ventricle dilatation, n (%) | 90 | 3 (5) | 1 (5) | 0 (0%) | 2 (20%) | 0 (0%) |
| Right ventricle dysfunction, n (%) | 90 | 4 (5) | 1 (4.2) | 0 (0%) | 3 (30%) | 0 (0%) |
Values are mean ± standard deviation or n (%). Column N indicates number of patients with available data.
ARVC, arrhythmogenic right ventricular cardiomyopathy; DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; LV, left ventricle; LVEF, left ventricular ejection fraction; LVOTO, left ventricle outflow tract obstruction; LVNC, left ventricular noncompaction; PVC, premature ventricular complex; VT, ventricular tachycardia.
In detail, there was four PVC/VT ablation (three ARVC and one DCM), and two septal reductions in obstructive HCM.
In this cohort, CMP was diagnosed before pregnancy in most cases, but 13 pregnancies were referred late for medical follow‐up.
On echocardiography, mean LVEF was 57 ± 12%, 24 patients presented LVEF < 50%, and 8 with LVEF < 40%, 11 HCM had LVOTO > 30 mmHg (Table 1 ).
Out of the 4 women with LVNC (five pregnancies), one patient presented severe LV dysfunction (LVEF 25%), the three others presented mildly reduced ejection fraction (between 40% and 45%), two presented a pathogenic variant, and none reached criteria for DCM (especially no dilatation).
Cardiovascular and maternal outcomes
Maternal primary cardiac outcome occurred in 18 pregnancies (20%), nine in DCM, seven in HCM, and only one in ARVC and LVNC. Details are reported in Table 2 . Maternal cardiac death occurred in three pregnancies (3%, including one woman with DCM, one with LVNC, and one with HCM). One woman with DCM died suddenly at day 5 postpartum, in a context of late pregnancy assessment and interruption of treatments. One woman with undiagnosed HCM presented sudden cardiac death at 36 weeks of gestation, and one woman with LVNC and severe LV dysfunction died suddenly following massive pulmonary embolism at 33 weeks of gestation, in a context of pregnancy denial with late pregnancy assessment. The most frequent complications were related to heart failure or arrhythmia (10 events each) (Table 2 ).
Table 2.
Incidence of maternal cardiac complications, according to the type of cardiomyopathy
| Overall (n = 90) | DCM (n = 28) | HCM (n = 46) | ARVC (n = 11) | LVNC (n = 5) | |
|---|---|---|---|---|---|
| Maternal cardiac event, n (%) | 18 (20) | 9 (32) | 7 (15) | 1 (9.1) | 1 (20) |
| Maternal cardiac death, n (%) | 3 (3.3) | 1 (3.6) | 1 (2.2) | 0 (0) | 1 (20) |
| Maternal cardiac arrest, n (%) | 4 (4.4) | 1 (3.6) | 2 (4.3) | 0 (0) | 1 (20) |
| Significant arrhythmia, n (%) | 10 (11) | 3 (11) | 6 (13) | 1 (9) | 0 (0) |
| Heart failure, n (%) | 10 (11) | 7 (25) | 3 (6.5) | 0 (0) | 0 (0) |
| Stroke, n (%) | 2 (2.2) | 1 (3.6) | 1 (2.2) | 0 (0) | 0 (0) |
| Myocardial infarction or any cardiac thromboembolism, n (%) | 1 (1.1) | 0 (0) | 0 (0) | 0 (0) | 1 (20) |
Values are mean ± standard deviation or n (%).
ARVC, arrhythmogenic right ventricular cardiomyopathy; DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; LVNC, left ventricular noncompaction.
Out of 14 women with repeated pregnancy, only three women experienced cardiovascular complications. For two of them, the second pregnancy was associated with a worse evolution than the first one. The last one, a woman with non‐obstructive HCM, presented an episode of atrial fibrillation during the first pregnancy but did not present any complications during the second pregnancy. The other 11 women did not develop cardiovascular complications, neither during first nor following pregnancy.
Secondary obstetrical and foetal/neonatal outcomes are summarized in Table S1 . Caesarean section was undertaken in 41 pregnancies. Four adverse foetal/neonatal outcomes occurred, including three foetal/neonatal deaths, premature birth occurred in 20% of cases, mostly induced for maternal reasons, and low birth weight was recorded in 18% of cases.
Risk stratification
Median CARPREG II risk score in our cohort was 2 (IC [0; 3]) and was higher in the DCM and LVNC subgroups (Table 3 ). Other risk scores are described in Table 3 . Notably, one patient had CARPREG score of 3, and 12 were in class IV of the mWHO classification.
Table 3.
Maternal cardiac risk scores, according to the type of cardiomyopathy
| Overall (n = 90) | DCM (n = 28) | HCM (n = 46) | ARVC (n = 11) | LVNC (n = 5) | |
|---|---|---|---|---|---|
| CARPREG II | 2 [0; 3] | 3 [2; 5] | 1 [0; 3] | 0 [0; 2.5] | 2 [1.75; 4.25] |
| CARPREG | 1 [0; 1] | 1 [0.75; 1] | 0 [0; 1] | 0 [0; 0.75] | 0 [0; 1.25] |
| CARPREG: 0, n (%) | 40 (44) | 6 (21) | 23 (50) | 8 (73) | 3 (60) |
| CARPREG: 1, n (%) | 36 (40) | 15 (54) | 17 (37) | 3 (27) | 1 (20) |
| CARPREG: 2, n (%) | 7 (7,8) | 4 (14) | 2 (4,3) | 0 (0) | 1 (20) |
| CARPREG: 3, n (%) | 1 (1,1) | 0 (0) | 1 (2,1) | 0 (0) | 0 |
| ZAHARA sore | 1.5 [0.0; 3.0] | 1.5 [0.94; 3.0] | 1.5 [0.0; 3.0] | 1.5 [0.0; 1.5] | 1.5 [1.5; 1.88] |
| mWHO, n (%) | |||||
| I | 4 (4.4) | 1 (3.6) | 0 (0) | 3 (27) | 0 (0) |
| II | 12 (13) | 4 (15) | 1 (2.2) | 7 (64) | 0 (0) |
| II–III | 56 (62) | 16 (57) | 36 (78) | 1 (9.1) | 3 (60) |
| III | 3 (3.4) | 2 (7.1) | 1 (2.2) | 0 (0) | 0 (0) |
| IV | 12 (13) | 4 (14) | 6 (13) | 0 (0) | 2 (40) |
Values are median [interquartile interval] or n (%).
mWHO, modified WHO score.
In univariate analysis, CARPREG II was significantly higher in patients with, versus without, maternal cardiac outcome (3 [2.0; 5.0] vs. 2 [0; 3], P < 0.01), similarly to other scores, except for ZAHARA (no difference).
Thirty‐six pregnancies were at low risk according to CARPREG II (score 0 or 1) (Figure 2 ). This subgroup presented only two cardiac events (one heart failure and one short ventricular tachycardia leading to introduction of beta‐blockers), all in the first week postpartum. Conversely, in the 16 high‐risk pregnancies (CARPREG II score ≥4), 50% presented a primary cardiac outcome. All three maternal deaths occurred in patients with a CARPREG score > 4 (Figure 2 ).
Figure 2.
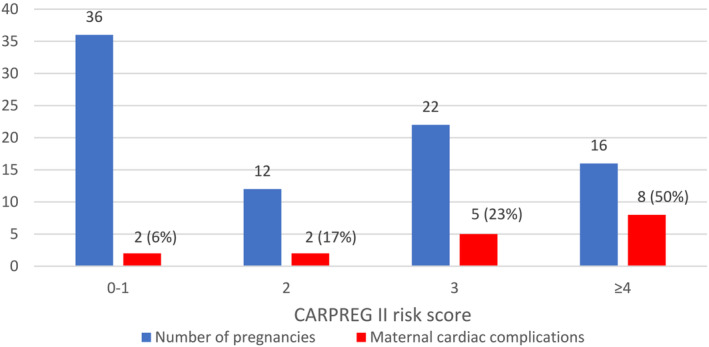
Incidence of cardiac maternal outcomes stratified according to CARPREG II risk score. Blue bars represent number of pregnancies according to CARPREG II risk score (categorized into four groups: in 0–1, 2, 3, and ≥4). Red bars represent number of cardiac maternal complications.
To evaluate the prediction efficiency of CARPREG II score in our CMP cohort, we built a ROC curve using sensitivity and specificity. CARPREG II showed good discriminatory power for risk stratification, with an area under the ROC curve (AUC) of 0.782 [0.697–0.904] (P < 0.05). CAPREG II had a higher AUC than other scores (CARPREG AUC 0.7555 [0.628–0.883]; mWHO AUC 0.697 [0.554–0.836], ZAHARA AUC 0.604 [0.452–0.756]), Figure 3 ).
Figure 3.
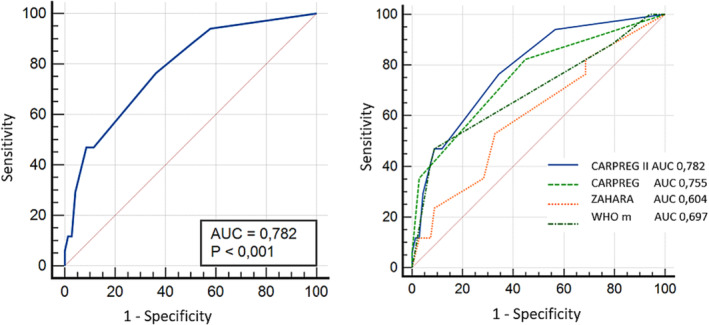
Performance of CARPREG II and other risk scores in prediction of cardiac maternal outcomes. Left figure: ROC curve analysis of CARPREG II risk score for cardiac maternal outcomes. Right figure: ROC curves of CARPREG II and previous scores CARPREG, ZAHARA, and mWHO, for cardiac maternal outcomes. *Cardiac maternal outcome is defined as the onset of any of the following cardiac maternal outcomes during pregnancy or within 6 months postpartum: maternal cardiac death, cardiac arrest, arrhythmia requiring treatment, heart failure, stroke or transient ischaemic attack, cardiac thromboembolism, myocardial infarction, and vascular dissection.
Discussion
In this retrospective single‐centre study conducted in a referral centre for inherited CMP, we found that maternal cardiovascular events were relatively frequent in this population, as there were cardiac complications in 20% of cases, including death in 3%. We also studied the prediction efficiency of several risk scores during pregnancy, including the recent CARPREG II score. 19 We observed that the CARPREG II risk score was the most efficient score in women with CMP in predicting cardiovascular risk during pregnancy and postpartum (AUC of ROC curve 0.782). To our knowledge, this is the first study to evaluate this score in this specific subgroup of women with cardiac disease.
Inherited cardiomyopathy: A population at high risk during pregnancy
We report a higher overall cardiac complication rate than in previous cohorts of pregnant women with cardiac disease in general. 2 , 3 , 19 In the CARPREG II study, Silversides et al. found 16% of cardiac adverse events, with the same primary endpoint, and a lower mortality rate of 0.6%. 19 Similarly, in the ROPAC registry, mortality was around 1% for overall heart disease. 2 These cohorts mostly included CHD and VHD, which are known to be associated with lower cardiovascular risk than CMP. Indeed, mortality reached 2.4% in the subgroup of CMP, 2 close to the result of our study, highlighting that CMP represents a rare subgroup at higher risk and therefore requires careful attention and proactive management during pregnancy and the postpartum period. 3
Only one previous study focused on inherited CMP. Billebeau et al., in 2017, in a retrospective cohort of 43 cases of inherited CMP, observed cardiac adverse events in 35% of cases, a higher rate than in our study. This difference could be explained by a less restrictive primary outcome in Billebeau et al., including asymptomatic alteration of LVEF or LVOTO. 16 Also, our patients were less severely ill at baseline than in the cohort of Billebeau et al. In particular, we had a lower proportion of cases of HCM with LVOTO or DCM with severe LV dysfunction, which are known to be major determinants of maternal cardiac outcome. 16 In addition, our inclusion period was from 1997 to 2022; thus, as reported by Roos‐Hesslink et al., there may be a period effect, with a trend to reduction of cardiac complications and mortality in the last decade. 3 This trend is observed in our cohort. Indeed, only 5 of the 18 pregnancies with cardiac outcomes occurred after 2013, and no maternal death. This underlines the improvement of the process of care of pregnant women with cardiac disease.
As noted in our study, inherited CMP is a heterogeneous subgroup of cardiac diseases, with severity and cardiac outcomes that depend on the CMP subtype. Few previous studies have focused on these different inherited CMP subtypes, mostly in small cohorts, with various and limited data. HCM, the most frequent inherited CMP, is the most described. Most studies found a relatively low risk of major cardiac events and low mortality. 9 , 24 , 25 , 26 Indeed, Autore et al. reported cardiac events in 15% of cases in an Italian cohort and two cardiac deaths. Thaman et al. reported cardiac events in 27% of cases, but no death. In contrast, Tanaka et al. reported, in a small cohort of 27 patients, a high cardiac complication rate of 48%, including non‐sustained arrhythmia on systematic Holter‐ECG. 10 We found cardiac adverse events in 15% of our HCM patients, a value close to those of previous studies. Women with inherited DCM are less frequent and appear at higher risk during pregnancy, as suggested by Billebeau et al., but the data are very limited, and inconsistent. Grewal et al., in 2009, found in a cohort of 36 cases of DCM a complication rate of 39%. 11 More recently, in contrast, Boyle et al., in an Australian cohort of 14 cases of DCM, found no cardiovascular event, but a high premature birth rate. 12 In DCM, Billebeau et al. reported a complication rate of 60% versus 32% in our cohort. Overall, these data suggest a high risk of cardiac complications in DCM, but also the need for larger registries. In ARVC, cardiac complications seem to be relatively infrequent during pregnancy, as suggested in previous large registries on ARVC, and confirmed in our study. 13 , 14 , 27
Few studies have focused on obstetrical and neonatal outcomes in women with heart disease, especially in inherited CMP. In our study, we reported a high rate of complications including 20% premature births (vs. up to 30% in the ROPAC registry), 2 18% of cases of low birth weight, and three foetal/neonatal deaths. These studies highlight the need for close multidisciplinary collaboration with obstetricians and paediatricians in the management of pregnant women with CMP.
Risk stratification in inherited cardiomyopathy
Several risk scores have emerged in the past decades to help predict cardiovascular outcomes in pregnant women with heart disease, such as the CARPREG score, ZAHARA, and the mWHO classification. Although these scores are currently used and suggested in the latest guidelines, mostly the mWHO, they all have limitations, and their validation in the different subgroups of heart disease remains uncertain. 1 , 18 Two studies found that mWHO performed better that the CARPREG score, one in a specific cohort of CHD, 28 the other in a large cohort of 179 patients with various heart diseases, although CMP was poorly represented at only 8% of the cohort. 29 Conversely, Billebeau et al. reported better performances of CARPREG in their cohort of inherited CMP women, compared with the mWHO classification. 16 CARPREG showed, however, limitations in the classification of women at low risk, as 23% of women with a score of 0 presented complications. In this context, the CARPREG II risk score, built on a large prospective cohort of pregnant women, and based on 10 variables, including general cardiac as well as specific cardiac factors, but also considering the process of care, appears promising for better risk stratification. Its efficiency has already proven better than those of the previous CARPREG and mWHO scores, in the first study, with a c‐statistic of 0.78. 19 No study before ours has evaluated the performance of CAPREG II in specific subgroups. In our inherited CMP cohort, CAPREG II performed well in predicting cardiac complications, with an AUC of the ROC curve of 0.782, a value similar to that reported by Silversides et al. 19 In addition, we observed that the efficiency of the CARPREG II score was better, with a higher AUC, than that of previous scores, including the mWHO, although our study was underpowered to statistically demonstrate it. Moreover, CARPREG II efficiently identified women at low risk of complications, in line with the results reported by Silversides et al., and in contrast to the poor discrimination of CARPREG in CMP patients previously pointed out by Billebeau et al. 16 , 19
Several predictors included in the CARPREG II risk score have already been identified as risk factors in inherited cardiomyopathies, specifically features focusing on the initial severity of the cardiopathy. Indeed, left ventricle dysfunction is frequently reported as a major risk factor in DCM 11 and ARVC. 14 In our study, 4 cardiovascular events occurred in patients with mild left ventricular dysfunction (LVEF 40–50%). This could explain why CARPREG II is more sensitive than CARPREG or mWHO, focusing on severe left ventricular dysfunction. Conversely, in HCM patients, the role of LVOTO in the onset of cardiac events is uncertain, although it appears in the CARPREG II risk score. 9 , 10 We found no difference of LVOTO according to the onset of cardiac outcome, although our study was not designed for that purpose. One advantage of the CARPREG II risk score, reported by Silversides et al., was that it includes lesion‐specific variables, such as coronary artery disease, mechanical prosthesis, high‐risk aortopathy or pulmonary hypertension. However, these features do not concern inherited CMP. For the first time, this score includes a variable related to the process of care. Late pregnancy assessment was indeed found to be associated with cardiac outcomes (odds ratio 1.6). 19 In our study, cardiac events were more frequent in patients with late maternal assessment, and all three cardiac deaths occurred in patients without regular follow‐up, underlining the accuracy of CARPREG II in this population.
Limitations
Our study may have some limitations. First, this is an observational retrospective study, with some missing data, thus limiting statistical analysis and conclusions. Although this cohort is larger than previous inherited CMP cohorts, it is relatively small, with a lack of power to compare the risk scores statistically or to evaluate risk factors in multivariate analysis. However, our objective was to describe the real‐life experience of a reference centre for inherited CMP. Finally, because of the possible selection bias of a tertiary centre, it is difficult to generalize the results to other centres. Data reported in our study are, however, consistent with the previous results of Silversides et al. in cardiac diseases in general, thus supporting our conclusions.
Conclusion
Pregnancies in women with inherited cardiomyopathies are at high risk of maternal cardiac adverse events and therefore require careful management. We observed that the recent CARPREG II risk score was the most efficient score in this specific population in discriminating and stratifying maternal cardiac risk during pregnancy and postpartum. This may be relevant for clinical practice and further recommendations in this field.
Funding
None.
Conflict of interest
None declared in relation to this work. Estelle Gandjbakhch reported consulting/educational and presentation fees, not related to the present work, from medtronic/boston: abbott/zoll.
Supporting information
Table S1. Obstetrical and foetal/neonatal outcomes.
Acknowledgements
We thank Stéphanie Rouanet and ACTION for fruitful statistical discussion.
Wallet, T. , Legrand, L. , Isnard, R. , Gandjbakhch, E. , Pousset, F. , Proukhnitzky, J. , Dommergues, M. , Nizard, J. , and Charron, P. (2024) Pregnancy and cardiac maternal outcomes in women with inherited cardiomyopathy: interest of the CARPREG II risk score. ESC Heart Failure, 11: 1506–1514. 10.1002/ehf2.14694.
Contributor Information
Thomas Wallet, Email: thomas.wallet@aphp.fr, Email: thomas.walletd@aphp.fr.
Philippe Charron, Email: philippe.charron@aphp.fr.
References
- 1. Regitz‐Zagrosek V, Roos‐Hesselink JW, Bauersachs J, Blomström‐Lundqvist C, Cífková R, De Bonis M, et al. 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J 2018;39:3165‐3241. doi: 10.1093/eurheartj/ehy340 [DOI] [PubMed] [Google Scholar]
- 2. Roos‐Hesselink JW, Ruys TPE, Stein JI, Thilén U, Webb GD, Niwa K, et al. Outcome of pregnancy in patients with structural or ischaemic heart disease: Results of a registry of the European Society of Cardiology. Eur Heart J 2013;34:657‐665. doi: 10.1093/eurheartj/ehs270 [DOI] [PubMed] [Google Scholar]
- 3. Roos‐Hesselink J, Baris L, Johnson M, De Backer J, Otto C, Marelli A, et al. Pregnancy outcomes in women with cardiovascular disease: Evolving trends over 10 years in the ESC Registry Of Pregnancy And Cardiac disease (ROPAC). Eur Heart J 2019;40:3848‐3855. doi: 10.1093/eurheartj/ehz136 [DOI] [PubMed] [Google Scholar]
- 4. Siu SC, Lee DS, Rashid M, Fang J, Austin PC, Silversides CK. Long‐term cardiovascular outcomes after pregnancy in women with heart disease. J Am Heart Assoc 2021;10:e020584. doi: 10.1161/JAHA.120.020584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Siu SC, Sermer M, Colman JM, Alvarez AN, Mercier LA, Morton BC, et al. Prospective multicenter study of pregnancy outcomes in women with heart disease. Circulation 2001;104:515‐521. doi: 10.1161/hc3001.093437 [DOI] [PubMed] [Google Scholar]
- 6. Sliwa K, Hilfiker‐Kleiner D, Petrie MC, Mebazaa A, Pieske B, Buchmann E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail 2010;12:767‐778. doi: 10.1093/eurjhf/hfq120 [DOI] [PubMed] [Google Scholar]
- 7. Sliwa K, Petrie MC, van der Meer P, Mebazaa A, Hilfiker‐Kleiner D, Jackson AM, et al. Clinical presentation, management, and 6‐month outcomes in women with peripartum cardiomyopathy: An ESC EORP registry. Eur Heart J 2020;41:3787‐3797. doi: 10.1093/eurheartj/ehaa455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lu CH, Lee WC, Wu M, Chen SW, Yeh JK, Cheng CW, et al. Comparison of clinical outcomes in peripartum cardiomyopathy and age‐matched dilated cardiomyopathy. Medicine (Baltimore) 2017;96:e6898. doi: 10.1097/MD.0000000000006898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goland S, van Hagen IM, Elbaz‐Greener G, Elkayam U, Shotan A, Merz WM, et al. Pregnancy in women with hypertrophic cardiomyopathy: data from the European Society of Cardiology initiated Registry of Pregnancy and Cardiac disease (ROPAC). Eur Heart J 2017;38:2683‐2690. doi: 10.1093/eurheartj/ehx189 [DOI] [PubMed] [Google Scholar]
- 10. Tanaka H, Kamiya C, Katsuragi S, Tanaka K, Miyoshi T, Tsuritani M, et al. Cardiovascular events in pregnancy with hypertrophic cardiomyopathy. Circ J 2014;78:2501‐2506. doi: 10.1253/circj.CJ-14-0541 [DOI] [PubMed] [Google Scholar]
- 11. Grewal J, Siu SC, Ross HJ, Mason J, Balint OH, Sermer M, et al. Pregnancy outcomes in women with dilated cardiomyopathy. J Am Coll Cardiol 2009;55:45‐52. doi: 10.1016/j.jacc.2009.08.036 [DOI] [PubMed] [Google Scholar]
- 12. Boyle S, Nicolae M, Kostner K, Davies K, Cukovski I, Cunliffe A, et al. Dilated cardiomyopathy in pregnancy: Outcomes from an Australian tertiary centre for maternal medicine and review of the current literature. Heart Lung Circ 2019;28:591‐597. doi: 10.1016/j.hlc.2018.02.017 [DOI] [PubMed] [Google Scholar]
- 13. Gandjbakhch E, Varlet E, Duthoit G, Fressart V, Charron P, Himbert C, et al. Pregnancy and newborn outcomes in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Int J Cardiol 2018;258:172‐178. doi: 10.1016/j.ijcard.2017.11.067 [DOI] [PubMed] [Google Scholar]
- 14. Wu L, Liang E, Fan S, Zheng L, Hu F, Liu S, et al. Effect of pregnancy in arrhythmogenic right ventricular cardiomyopathy. Am J Cardiol 2020;125:613‐617. doi: 10.1016/j.amjcard.2019.11.008 [DOI] [PubMed] [Google Scholar]
- 15. Ueda Y, Kamiya CA, Nakanishi A, Horiuchi C, Miyoshi T, Hazama R, et al. Cardiomyopathy phenotypes and pregnancy outcomes with left ventricular noncompaction cardiomyopathy. Int Heart J 2018;59:862‐867. doi: 10.1536/ihj.17-336 [DOI] [PubMed] [Google Scholar]
- 16. Billebeau G, Etienne M, Cheikh‐Khelifa R, Vauthier‐Brouzes D, Gandjbakhch E, Isnard R, et al. Pregnancy in women with a cardiomyopathy: Outcomes and predictors from a retrospective cohort. Arch Cardiovasc Dis 2018;111:199‐209. doi: 10.1016/j.acvd.2017.05.010 [DOI] [PubMed] [Google Scholar]
- 17. Drenthen W, Boersma E, Balci A, Moons P, Roos‐Hesselink JW, Mulder BJM, et al. Predictors of pregnancy complications in women with congenital heart disease. Eur Heart J 2010;31:2124‐2132. doi: 10.1093/eurheartj/ehq200 [DOI] [PubMed] [Google Scholar]
- 18. Davis MB, Arendt K, Bello NA, Brown H, Briller J, Epps K, et al. Team‐based care of women with cardiovascular disease from pre‐conception through pregnancy and postpartum: JACC focus seminar 1/5. J Am Coll Cardiol 2021;77:1763‐1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Silversides CK, Grewal J, Mason J, Sermer M, Kiess M, Rychel V, et al. Pregnancy outcomes in women with heart disease: The CARPREG II study. J Am Coll Cardiol 2018;71:2419‐2430. doi: 10.1016/j.jacc.2018.02.076 [DOI] [PubMed] [Google Scholar]
- 20. Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, et al. Classification of the cardiomyopathies: A position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2008;29:270‐276. doi: 10.1093/eurheartj/ehm342 [DOI] [PubMed] [Google Scholar]
- 21. Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: The Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2733‐2779. doi: 10.1093/eurheartj/ehu284 [DOI] [PubMed] [Google Scholar]
- 22. Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D). Circulation 2010;121:1533‐1541. doi: 10.1161/CIRCULATIONAHA.108.840827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bauersachs J, König T, van der Meer P, Petrie MC, Hilfiker‐Kleiner D, Mbakwem A, et al. Pathophysiology, diagnosis and management of peripartum cardiomyopathy: A position statement from the Heart Failure Association of the European Society of Cardiology Study Group on peripartum cardiomyopathy. Eur J Heart Fail 2019;21:827‐843. doi: 10.1002/ejhf.1493 [DOI] [PubMed] [Google Scholar]
- 24. Probst V, Langlard JM, Desnos M, Komajda M, Bouhour JB. Familial hypertrophic cardiomyopathy. French study of the duration and outcome of pregnancy. Arch Mal Coeur Vaiss 2002;95:81‐86. [PubMed] [Google Scholar]
- 25. Thaman R, Varnava A, Hamid MS, Firoozi S, Sachdev B, Condon M, et al. Pregnancy related complications in women with hypertrophic cardiomyopathy. Heart 2003;89:752‐756. doi: 10.1136/heart.89.7.752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Autore C, Conte MR, Piccininno M, Bernabò P, Bonfiglio G, Bruzzi P, et al. Risk associated with pregnancy in hypertrophic cardiomyopathy. J Am Coll Cardiol 2002;40:1864‐1869. doi: 10.1016/S0735-1097(02)02495-6 [DOI] [PubMed] [Google Scholar]
- 27. Bauce B, Daliento L, Frigo G, Russo G, Nava A. Pregnancy in women with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Eur J Obstet Gynecol Reprod Biol 2006;127:186‐189. doi: 10.1016/j.ejogrb.2005.10.011 [DOI] [PubMed] [Google Scholar]
- 28. Lu CW, Shih JC, Chen SY, Chiu HH, Wang JK, Chen CA, et al. Comparison of 3 risk estimation methods for predicting cardiac outcomes in pregnant women with congenital heart disease. Circ J 2015;79:1609‐1617. doi: 10.1253/circj.CJ-14-1368 [DOI] [PubMed] [Google Scholar]
- 29. Pijuan‐Domènech A, Galian L, Goya M, Casellas M, Merced C, Ferreira‐Gonzalez I, et al. Cardiac complications during pregnancy are better predicted with the modified WHO risk score. Int J Cardiol 2015;195:149‐154. doi: 10.1016/j.ijcard.2015.05.076 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Obstetrical and foetal/neonatal outcomes.


