Abstract
Aims
We aim to explore the associations between serum tyrosine (Tyr) to threonine (Thr) ratio and chronic heart failure (HF) with reduced or mildly reduced ejection fraction (EF) (HFrEF or HFmrEF).
Methods and results
The study recruited 418 subjects (77.5% males, mean age 65.2 ± 12.5 years), including 318 HF subjects (HFrEF or HFmrEF) and 100 cardiovascular subjects without acute or chronic HF [including heart failure with preserved ejection fraction (HFpEF)] as controls. Serum levels of 21 kinds of amino acids (AAs) were measured by mass spectrometry. Logistic regression analysis was conducted to measuring the association between the AAs levels and the presence of HF. Event‐free survival was determined by Kaplan–Meier curves and differences in survival were assessed using log‐rank tests. Cox regression analysis was used to assess the prognostic value of AAs in HF. Receiver‐operating characteristic (ROC) curve was performed to further confirm regression analysis. Along with the control, HFmrEF, and HFrEF subjects, serum tyrosine (Tyr) gradually increased (64.43 ± 15.28 μmol/L vs. 71.79 ± 18.74 μmol/L vs. 77.32 ± 25.90 μmol/L, P < 0.001) while serum threonine (Thr) decreased (165.21 ± 40.09 μmol/L vs. 144.93 ± 44.56 μmol/L vs. 135.25 ± 41.25 μmol/L, P < 0.001). Tyr/Thr ratio was the independent risk factor for the presence of HF in all subjects [odds ratio (OR), 3.510; 95% confidence interval (CI): 2.445–5.040; P < 0.001]. After following up for a mean year (11.10 ± 2.80 months) in 269 HF subjects (75.1% males, mean age 65.2 ± 12.8 years), the higher Tyr/Thr ratio was associated with a higher risk of HF endpoint events in HF subjects [hazard ratio (HR), 2.901; 95% CI: 1.228–6.851; P = 0.015]. By comparing the area under the receiver‐operating characteristic curve (AUC), Tyr/Thr ratio was superior to Fischer's ratio (FR) in predicting HF occurrence (0.767:0.573, P < 0.001) or cardiovascular (CV) death (0.715:0.550, P = 0.047).
Conclusions
Circulating elevated Tyr/Thr ratio confer an increased risk for the presence of HF and poor prognosis. Tyr/Thr index outweighs FR index in predicting HF occurrence or CV death.
Keywords: Aetiology, Amino acids, Chronic heart failure, Correlation, HFmrEF, HFrEF, Prognosis, Tyr/Thr ratio
Introduction
HF is a major public health problem worldwide with a high mortality and hospitalization. 1 , 2 It has a complex clinical syndrome with symptoms and signs that result from any structural or functional impairment of ventricular filling or ejection of blood. 3 , 4 Amino acids (AAs) play a crucial role as essential nutrient metabolites for cell development and survival. 5 Moreover, metabolomics demonstrated that AAs metabolism disorder was associated with pathological remodelling in hypertrophic and early‐stage failing mouse hearts. 6 , 7 These indicated that the AAs profile could reflect the state of HF.
However, nowadays, the exploration between AAs expression and HF disease mainly concentrated on branched‐chain amino acids (BCAAs) and aromatic amino acids (AAAs). Some studies found that the plasma BCAA concentrations could mediate the effects of protein‐energy malnutrition on cardiac functions and contribute to HF risk stratification. 8 , 9 , 10 Some studies showed that low perfusion and congestion may contribute to liver functional impairment in HF. 11 , 12 Then, the liver dysfunction led to reduced protein synthesis and amino acid metabolism, resulting in elevated levels of free AAAs in the blood. 13 Other studies pointed out that BCAA/AAA ratio (Fischer's ratio) could be used as a prognostic indicator for patients with HF. 9 , 14
In fact, a wide range of AAs are produced from protein metabolism and tissue degradation, and these AAs can be measured to inform the state of metabolism in HF and adverse events. 15 , 16 Nevertheless, little is known about the association between the ratio of tyrosine (Tyr) to threonine (Thr) and HF disease, especially with different HF aetiology. The purpose of our study was to examine the relationship between Tyr/Thr ratio and stable chronic HF (HFrEF or HFmrEF), meanwhile, comparing it with the FR index for better managing HF disease in the future.
Methods
Study design and population
Two separate sets of analyses were designed in this study. The cross‐sectional analysis was conducted on all 418 participants (77.5% males, mean age 65.2 ± 12.5 years) to examine the association of serum AAs levels and the presence of HF, whereas prospective analysis was performed on 269 stable chronic HF patients (75.1% males, mean age 65.2 ± 12.8 years) to assess the prognostic value of Tyr to Thr ratio in HF.
We continuously collected 348 HF (HFrEF or HFmrEF) subjects aged 18 years or older admitted to the Department of Cardiology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine from January 2021 to October 2022. For the purpose of this study, we excluded 13 subjects with acute myocardial infarction (n = 6) and acute heart failure (n = 7). Other non‐cardiogenic causes of HF such as myasthenia gravis (n = 3), sepsis (n = 1), malignancies tumours (n = 5), liver cirrhosis (n = 2), haematological diseases (n = 3), multiple myeloma (n = 1), and acute renal failure (n = 2) were also excluded. A total of 318 subjects with symptomatic and echocardiograph‐confirmed chronic HF (EF ≤ 50%) were finally recruited, containing the HFmrEF (EF > 40%, n = 116) and the HFrEF subjects (EF ≤ 40%, n = 202). Meanwhile, 100 cardiovascular subjects without acute or chronic HF (including HFpEF) were continuously enrolled as the controls and the above lesion exclusion standard was also applied to the controls. The mean age, proportion of sex, and HF‐related diseases were matched between the controls and HF subjects (P > 0.05). According to the aetiology of HF, 318 subjects were regrouped into dilated cardiomyopathy (DCM) (n = 131) and ischaemic heart disease (IHD) (n = 187) subgroups, which were further analysed with the controls (n = 100), respectively (Figure 1 ).
Figure 1.
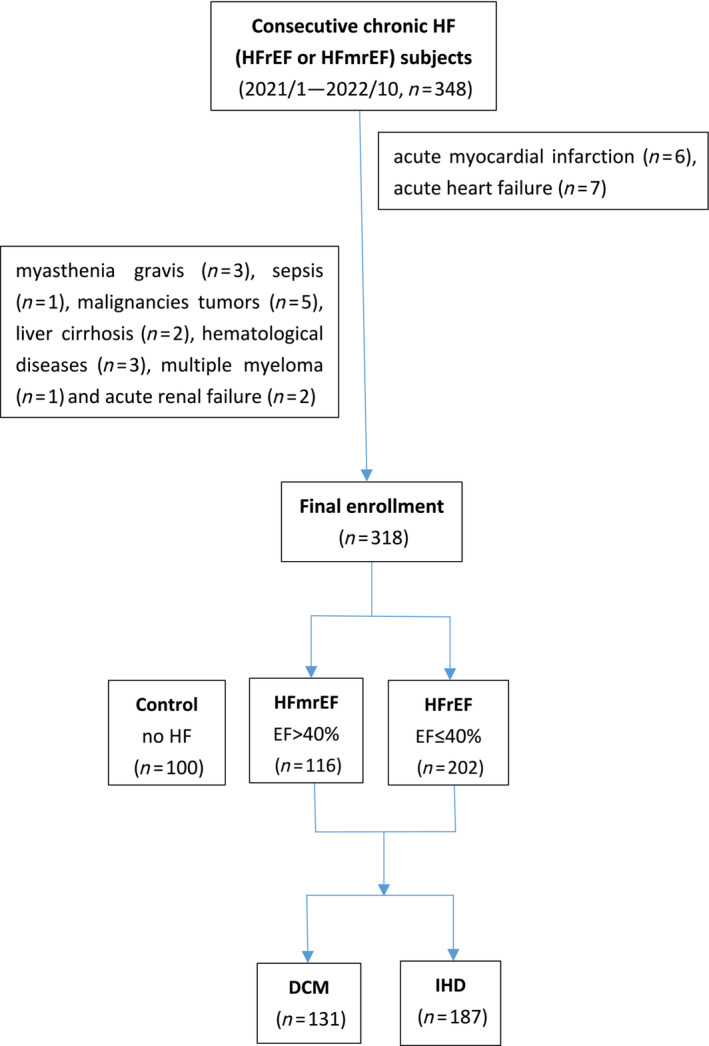
Flow chart of chronic HF patient enrollment analysis. DCM, dilated cardiomyopathy; EF, ejection fraction; HF, heart failure; HFmrEF, heart failure with mildly reduced ejection fraction; HFrEF, heart failure with reduced ejection fraction; IHD, ischaemic heart disease.
This study was approved by the institutional review committee of Ruijin Hospital affiliated to Shanghai Jiao Tong University School of Medicine (Shanghai, China) and was conducted in accordance with the principles of the 1964 Declaration of Helsinki and its later amendments. Written informed consent was obtained from all participants before enrolment.
Follow‐up and outcomes
The HF endpoints consisted of CV death and hospitalization due to HF. The follow‐up survey was conducted by hospital visits or through telephone. All endpoints were confirmed by independent cardiologists intensively. The recurrence of acute HF (acute HF re‐occurring in patients with chronic HF) was diagnosed on account of signs and symptoms, abnormal laboratory parameters, as well as imageological examination.
Clinical, biochemical assessments, and amino acids measurements
Detailed demographic data and clinical features were collected for each patient. Body mass index (BMI) was calculated as weight/height2 (kg/m2). Blood pressure (BP) was measured on the arm in a seated position after a 10‐min rest. Hypertension was diagnosed according to the seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure (JNC7). 17
For laboratory tests, blood samples were drawn after overnight fasting. Plasma creatinine, triglyceride, and total cholesterol were determined (HITACHI 912 Analyzer, Roche Diagnostics, Germany). Estimated glomerular filtration rate (eGFR) was computed using the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) equation. 18 Blood concentration of glycosylated haemoglobin (HbA1c) was measured using ion‐exchange high‐performance liquid chromatography (Bio‐Rad Laboratories, USA). Alanine transaminase (ALT) was detected by Beckmann AU5821 automatic biochemical analyser and matching reagents. N‐terminal brain natriuretic peptide (NT‐proBNP) was determined using a commercially available ELISA kit (Roche Diagnostics). Serum levels of high‐sensitivity C‐reactive protein (hsCRP) were tested by ELISA kit (Biocheck Laboratories, Toledo, OH, USA). Soluble suppression of tumourigenicity‐2 (sST2) values were detected by fluorescence immunochromatography (Jet‐star 3000 instruments and matching reagents).
Serum amino acids levels were measured by mass spectrometry using Recon amino acid kit (21 items) (Recon RZ500 instrument), 19 including lysine (Lys), tryptophan (Trp), phenylalanine (Phe), methionine (Met), threonine (Thr), leucine (Leu), isoleucine (Ile), valine (Val), arginine (Arg), tyrosine (Tyr), histidine (His), ornithine (Orn), alanine (Ala), proline (Pro), citrulline (Cit), glycine (Gly), serine (Ser), asparagine (Asn), aspartate (Asp), glutamate (Glu), and glutamine (Gln). Essential amino acid (EAA) was the sum of Val, Ile, Leu, Met, Lys, Phe, Trp, and Thr. BCAA was the sum of Val, Ile, and Leu. FR was calculated as the sum of Val, Ile, and Leu divided by the sum of Phe and Tyr.
RZ500 is a mass spectrometry developed by Shanghai Reigncom Biotechnology Co., Ltd (China), through collaboration with Thermo Fisher Scientific. The performance of RZ500 is on par with Thermo Fisher's top of the line Thermo Scientific™ TSQ Altis™ triple quadrupole mass spectrometer. The 21 amino acids assay kit is a medical device product registered in China by Reigncom in 2021 and received CE‐IVD mark in 2023.
Echocardiography
Two‐dimensional (2D) echocardiography and Doppler flow imaging were performed on the second day after admission. Using a commercially available system (Vivid‐I, GE Healthcare, Milwaukee, WI) with a 1.9‐ to 3.8‐MHz phased‐array transducer, images were recorded from standard parasternal and apical four‐chamber views with a frame rate of 60 to 100 frames/s. Data were stored digitally and analysed off‐line (EchoPac, version 7; GE Healthcare) by two independent cardiologists blinded to laboratory measurements. Left ventricular end‐diastolic and end‐systolic diameter (LVEDD and LVESD) were determined using modified Simpson's biplane technique and EF was calculated. The ratio of early diastolic velocity of the mitral inflow to mitral annulus (E/e′) was assessed using tissue Doppler and an average of septal and lateral E/e′ ratio was used for analysis.
Statistical analysis
Continuous variables were shown as mean ± standard deviation (SD). Log transformation was performed before analysis if it was not normally distributed. One‐way ANOVA was performed to determine the significance of trends among groups and further comparison between the two groups was done using the least significant difference procedure (LSD). Categorical variables are presented as counts (%) and significant differences between groups were determined using the chi‐square test or Fisher's exact test. Logistic or Cox regression models in univariate and multivariate analyses were performed to calculate HRs and 95% CIs. In regression analyses, results were presented according to the 3rd versus 1st tertile. The full adjustment contains sex, age, low systolic BP, BMI, heart rate, smoking, drinking, diabetes mellitus (DM), hyperlipidaemia, stroke, atrial fibrillation (AF), coronary disease, ALT, and low eGFR. The analysis of ROC curve uses the continuous variables carried out by logistic regression, and the best cut‐off value of the variables was determined, and the AUC was compared. Subjects who were lost during follow‐up were analysed according to the censored data. Free events was determined using Kaplan–Meier curves, and differences were assessed using the log‐rank test. All statistical analyses were made using the SPSS 22.0 software. A probability (P) level <0.05 was considered statistically significant.
Results
Clinical characteristics
Subjects in control, HFmrEF, and HFrEF groups did not significantly differ with respect to the serum Lys, Trp, Phe, Met, Leu, Ile, Orn, Ala, Pro, Cit, Gly, Gln, and BCAA levels (ANOVA, P > 0.05). Though significant differences were observed in the levels of Asn and Ser among the three participant groups in our study (ANOVA), there was no observed gradual increase or decrease in Asn and Ser levels across these groups. However, the values of serum Tyr, Asn, and Glu were gradually increased, while the values of serum Thr, Val, Arg, His, EAA, and FR were decreased step by step across the control, HFmrEF, and HFrEF groups (ANOVA, P < 0.05), in which, the values of serum Tyr and Thr were more meaningful (LSD, P < 0.05) (Table 1 ).
Table 1.
The level of serum amino acids in all subjects
| Amino acids | Control | HFmrEF | HFrEF | P‐value |
|---|---|---|---|---|
| (μmol/L) | n = 100 | n = 116 | n = 202 | |
| Lysine (Lys) | 196.09±37.19 | 186.82±43.00 | 187.13±48.33 | 0.206 |
| Tryptophan (Trp) | 55.55±11.84 | 53.82±14.40 | 55.72±16.30 | 0.520 |
| Phenylalanine (Phe) | 105.08±25.40 | 95.71±29.40* | 98.18±30.49 | 0.050 |
| Methionine (Met) | 28.04±6.78 | 28.46±8.15 | 30.70±17.89 | 0.185 |
| Threonine (Thr) | 165.21±40.09 | 144.93±44.56* | 135.25±41.25*# | <0.001 |
| Leucine (Leu) | 174.77±43.73 | 178.01±78.53 | 173.67±92.10 | 0.894 |
| Isoleucine (Ile) | 99.75±42.59 | 98.81±35.08 | 94.87±26.87 | 0.403 |
| Valine (Val) | 310.75±67.34 | 286.62±70.07* | 284.79±76.49* | 0.010 |
| Arginine (Arg) | 91.99±29.48 | 68.04±32.54* | 60.92±32.27* | <0.001 |
| Tyrosine (Tyr) | 64.43±15.28 | 71.79±18.74* | 77.32±25.90*, # | <0.001 |
| Histidine (His) | 88.82±14.77 | 82.19±17.96* | 81.11±20.42* | 0.003 |
| Ornithine (Orn) | 156.10±50.02 | 161.28± 68.37 | 167.44±70.44 | 0.348 |
| Alanine (Ala) | 505.77±109.79 | 470.03±170.60 | 465.31±150.86* | 0.073 |
| Proline (Pro) | 231.93±53.79 | 236.24±85.32 | 221.04±87.09 | 0.223 |
| Citrulline (Cit) | 38.08±14.33 | 40.86±17.56 | 40.85±18.21 | 0.371 |
| Glycine (Gly) | 317.75±89.29 | 311.52±119.13 | 322.10±92.44 | 0.661 |
| Serine (Ser) | 180.81±39.04 | 153.46±44.62* | 159.62±48.31* | <0.001 |
| Asparagine (Asn) | 42.59±8.33 | 45.40±11.25 | 47.04±14.57* | 0.014 |
| Aspartate (Asp) | 35.10±12.87 | 25.88±18.68* | 25.98±18.03* | <0.001 |
| Glutamate (Glu) | 175.78±64.76 | 178.48±80.57 | 212.81±106.04*, # | <0.001 |
| Glutamine (Gln) | 452.35±110.28 | 446.60±194.59 | 461.84±260.53 | 0.821 |
| EAA | 1135.25±176.98 | 1073.18±242.17 | 1060.32±252.16* | 0.029 |
| BCAA | 585.28±125.54 | 563.44±157.70 | 553.34±168.58 | 0.248 |
| Fischer's ratio | 3.58±1.09 | 3.41±0.76 | 3.28±0.97* | 0.030 |
BCAA, branched chain amino acid; EAA, essential amino acids; Fischer's ratio, the sum of valine, isoleucine and leucine divided by the sum of phenylalanine and tyrosine; HFmrEF, heart failure with mildly reduced ejection fraction; HFrEF, heart failure with reduced ejection fraction.
P < 0.05 HFmrEF group, HFrEF versus control group.
P < 0.05 HFmrEF group versus HFrEF group.
Totally, 418 subjects were enrolled and divided into roughly equal three groups, low (Tyr/Thr < 0.441), median (0.441 ≤ Tyr/Thr < 0.585), and high (Tyr/Thr ≥ 0.585) levels, according to the tertiles of serum Tyr/Thr levels. The baseline data of the subjects are shown in Table 2 . Subjects in the three groups did not significantly differ with respect to sex, age, BMI, diastolic BP, smoking, drinking, coronary disease with aspirin therapy, AF, hypertension, hyperlipidaemia with serum triglyceride and total cholesterol, and statin therapy, stroke, DM with abnormal glucose control and insulin therapy, and chronic kidney disease with serum eGFR (P > 0.05). As the Tyr/Thr tertiles increased, serum ALT value, the incidence of NYHA II‐IV and the morbidity of DCM or IHD, the left atrial and left ventricular sizes, E/e′ ratio, serum NT‐proBNP level, as well as proportion of subjects requiring aggressive anti‐HF therapy increased (beta‐blocker, digoxin, and diuretics therapy) and left ventricular EF decreased (P < 0.05). Subjects in lowest tertile of Tyr/Thr levels tend to have the highest systolic BP with the highest number of calcium antagonist therapy, while subjects in highest tertiles tend to have highest heart rate (P < 0.05). Due to the common used for anti‐HF or anti‐BP therapy, the utilization rate of ACEI/ARB/ARNI drugs has no difference among the three groups (P > 0.05).
Table 2.
Baseline characteristics of all subjects according to Tyr/Thr tertiles
| Tyr/Thr < 0.441 (n = 139) | 0.441 ≤ Tyr/Thr < 0.585 (n = 139) | Tyr/Thr ≥ 0.585 (n = 140) | P value | |
|---|---|---|---|---|
| Demographic and clinical features | ||||
| Male, n (%) | 110 (79.1) | 106 (76.3) | 108 (77.1) | 0.841 |
| Age (years) | 64.72 ± 12.55 | 66.01 ± 11.80 | 64.86 ± 13.14 | 0.064 |
| BMI (kg/m2) | 24.35 ± 3.05 | 24.33 ± 3.41 | 24.61 ± 4.91 | 0.060 |
| Systolic BP (mmHg) | 133.19 ± 23.62 | 126.19 ± 20.54* | 126.67 ± 22.80* | <0.001 |
| Diastolic BP (mmHg) | 75.96 ± 16.06 | 75.13 ± 13.39 | 76.12 ± 15.22 | 0.854 |
| Heart rate (b.p.m.) | 80.37 ± 13.07 | 80.08 ± 17.76 | 85.28 ± 18.27* , # | 0.027 |
| NYHA class II, n (%) | 35 (25.2) | 46 (33.1) | 43 (30.7) | <0.001 |
| III, n (%) | 32 (23.0) | 57 (41.0)* | 64 (45.7)* | |
| IV, n (%) | 9 (6.5) | 10 (7.2) | 22 (15.7)* , # | |
| Medical history | ||||
| Smoking, n (%) | 51 (36.7) | 39 (28.1) | 46 (32.9) | 0.306 |
| Drinking, n (%) | 29 (20.9) | 22 (15.8) | 37 (26.4) # | 0.094 |
| DCM, n (%) | 26 (18.7) | 46 (33.1)* | 59 (42.1)* | <0.001 |
| IHD, n (%) | 50 (36.0) | 67 (48.2)* | 70 (50.0)* | 0.038 |
| Coronary disease, n (%) | 92 (66.2) | 75 (54.0)* | 77 (55.0) | 0.072 |
| AF, n (%) | 30 (21.6) | 42 (30.2) | 40 (28.6) | 0.225 |
| Hypertension, n (%) | 94 (67.6) | 87 (62.6) | 83 (59.3) | 0.348 |
| Hyperlipidaemia, n (%) | 12 (8.6) | 8 (5.8) | 11 (7.9) | 0.638 |
| Stroke, n (%) | 11 (7.9) | 18 (12.9) | 14 (10.0) | 0.381 |
| DM, n (%) | 50 (36.0) | 42 (30.2) | 47 (33.6) | 0.593 |
| Chronic kidney disease, n (%) | 20 (14.4) | 11 (7.9) | 10 (7.1) | 0.083 |
| Echocardiography and laboratory values | ||||
| Left atrial diameter (mm) | 43.63 ± 5.69 | 43.69 ± 6.96 | 45.94 ± 6.83* , # | 0.004 |
| Left ventricular: EDD (mm) | 56.75 ± 7.63 | 58.96 ± 8.71* | 61.68 ± 9.37* , # | <0.001 |
| ESD (mm) | 42.28 ± 9.70 | 46.16 ± 9.97* | 50.21 ± 10.44* , # | <0.001 |
| EF (%) | 48.94 ± 13.86 | 42.17 ± 11.28* | 36.04 ± 10.75* , # | <0.001 |
| E/e′ | 12.12 ± 5.34 | 13.54 ± 5.64 | 15.98 ± 7.32* , # | <0.001 |
| Log NT‐proBNP | 2.75 ± 0.88 | 2.99 ± 0.70* | 3.36 ± 0.70* , # | <0.001 |
| Triglyceride (mmol/L) | 1.43 ± 0.73 | 1.43 ± 0.82 | 1.34 ± 0.75 | 0.506 |
| Total cholesterol (mmol/L) | 3.91 ± 1.16 | 3.87 ± 1.02 | 4.10 ± 1.23 | 0.207 |
| HbA1c (%) | 6.44 ± 1.29 | 6.41 ± 1.34 | 6.23 ± 1.43 | 0.374 |
| ALT (IU/L) | 22.88 ± 18.52 | 30.19 ± 48.00 | 46.26 ± 117.05* | 0.027 |
| eGFR (mL/min/1.73 m2) | 68.84 ± 27.92 | 69.66 ± 22.94 | 69.60 ± 25.02 | 0.957 |
| Medications | ||||
| ACEI/ARB/ARNI (%) | 92 (66.2) | 98 (70.5) | 106 (75.7) | 0.215 |
| Beta‐blocker (%) | 98 (70.5) | 114 (82.0)* | 119 (85.0)* | 0.007 |
| Calcium antagonist (%) | 30 (21.6) | 14 (10.1)* | 13 (9.3)* | 0.004 |
| Digoxin (%) | 3 (2.2) | 6 (4.3) | 12 (8.6)* | 0.044 |
| Diuretics (%) | 41 (29.5) | 71 (51.1)* | 79 (56.4)* | <0.001 |
| Statin (%) | 109 (78.4) | 96 (69.1) | 98 (70.0) | 0.157 |
| Aspirin (%) | 62 (44.6) | 64 (46.0) | 51 (36.4) | 0.215 |
| Insulin (%) | 13 (9.4) | 9 (6.5) | 10 (7.1) | 0.640 |
ACEI, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ALT, alanine transaminase; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; BMI, body mass index; BP, blood pressure; DCM, dilated cardiomyopathy; DM, diabetes mellitus; E/e′, the ratio of early diastolic mitral inflow to mitral annular tissue velocities; EDD, end‐diastolic diameter; EF, ejection fraction; eGFR, estimated glomerular filtration rate; ESD, end‐systolic diameter; HbA1c, glycated haemoglobin A1c; IHD, ischaemic heart disease; NT‐proBNP, N‐terminal brain natriuretic peptide; NYHA, New York Heart Association; Thr, threonine; Tyr, tyrosine.
P < 0.05 intermediate‐tertile (0.441 ≤ Tyr/Thr < 0.585), high‐tertile (Tyr/Thr ≥ 0.585) vs. low tertile (Tyr/Thr < 0.441).
P < 0.05 intermediate‐tertile (0.441 ≤ Tyr/Thr < 0.585) vs. High‐tertile (Tyr/Thr ≥ 0.585).
The tyrosine/threonine index was associated with chronic heart failure by logistic analysis
In all subjects, the univariate and multivariate regression analyses showed that low systolic BP, heart rate, and low eGFR were associated with HF presence (all P < 0.05). The tertiles of Tyr/Thr were correlated with HF presence by the univariate analysis (P < 0.001). After adjustment for low systolic BP, heart rate and low eGFR, the tertiles of Tyr/Thr remained an independent risk factor for the presence of HF (OR, 3.510; 95% CI: 2.445–5.040; P < 0.001) (Table 3 ).
Table 3.
Univariate and multivariate regression analysis for HF in all subjects
| Univariate | Multivariate model 1 | Multivariate model 2 | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Sex (male) | 0.964 (0.561–1.656) | 0.893 | 0.950 (0.490–1.843) | 0.879 | — | — |
| Age | 1.199 (0.762–1.886) | 0.433 | 0.862 (0.487–1.526) | 0.611 | — | — |
| BMI | 0.769 (0.547–1.080) | 0.130 | 0.762 (0.511–1.135) | 0.181 | — | — |
| Low systolic BP | 1.810 (1.128–2.906) | 0.014 | 1.915 (1.128–3.250) | 0.016 | 1.875 (1.076–3.268) | 0.027 |
| Heart rate | 3.165 (1.558–6.430) | 0.001 | 3.158 (1.440–6.924) | 0.004 | 3.077 (1.351–7.010) | 0.007 |
| Smoking | 1.033 (0.638–1.671) | 0.896 | 0.883 (0.449–1.736) | 0.719 | — | — |
| Drinking | 1.688 (0.919–3.101) | 0.091 | 2.456 (1.097–5.501) | 0.029 | — | — |
| Diabetes | 1.794 (1.074–2.996) | 0.025 | 1.699 (0.946–3.051) | 0.076 | — | — |
| Hyperlipidaemia | 0.466 (0.218–0.997) | 0.049 | 0.498 (0.207–1.199) | 0.120 | — | — |
| Stroke | 0.792 (0.390–1.608) | 0.519 | 0.808 (0.364–1.796) | 0.602 | — | — |
| AF | 1.055 (0.633–1.758) | 0.837 | 0.740 (0.412–1.330) | 0.314 | — | — |
| Coronary disease | 0.734 (0.461–1.168) | 0.191 | 0.648 (0.364–1.153) | 0.140 | — | — |
| ALT | 1.054 (0.672–1.652) | 0.819 | 1.025 (0.614–1.713) | 0.924 | — | — |
| Low eGFR | 2.239 (1.655–3.029) | <0.001 | 2.469 (1.731–3.522) | <0.001 | 2.520 (1.806–3.517) | <0.001 |
| Tyr/Thr tertiles | 3.225 (2.307–4.507) | <0.001 | — | — | 3.510 (2.445–5.040) | <0.001 |
Model 1, adjusted for conventional HF risk factors; Model 2, adjusted for low systolic BP, heart rate, low eGFR and with the addition of the tertiles of Tyr/Thr.
95% CI, 95% confidence interval; AF, atrial fibrillation; ALT, alanine transaminase; BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; HF, heart failure; OR, odds ratio; Tyr/Thr, tyrosine/threonine.
After full adjustment (sex, age, BMI, low systolic BP, heart rate, smoking, drinking, DM, hyperlipidaemia, stroke, AF, coronary disease, ALT, and low eGFR), the tertiles of Tyr/Thr were also associated with the presence of HF (OR, 3.928; 95% CI: 2.649–5.825; P < 0.001). Compared with the lowest tertile, the risk of HF presence in the highest tertile increased by 13.02 times (OR, 14.02; 95% CI: 6.332–31.043; P < 0.001). Moreover, the Tyr/Thr index was still related to the occurrence of HF not only in DCM and control subjects but also in IHD and control subjects (all P < 0.001) (Table 4 ).
Table 4.
Tyr/Thr was associated with the presence of HF in all subjects
| Unadjusted OR | P value | Adjusted OR | P value | |
|---|---|---|---|---|
| HF (n = 318) | ||||
| Tyr/Thr tertiles | 3.225 (2.307–4.507) | <0.001 | 3.928 (2.649–5.825) | <0.001 |
| Tertile 1 | 1 (ref) | 1 (ref) | ||
| Tertile 2 | 3.603 (2.096–6.192) | <0.001 | 4.856 (2.567–9.185) | <0.001 |
| Tertile 3 | 9.721 (4.826–19.584) | <0.001 | 14.020 (6.332–31.043) | <0.001 |
| HF with DCM (n = 131) | ||||
| Tyr/Thr tertiles | 3.696 (2.500–5.463) | <0.001 | 3.491 (1.752–6.953) | <0.001 |
| Tertile 1 | 1 (ref) | 1 (ref) | ||
| Tertile 2 | 4.287 (2.208–8.322) | <0.001 | 2.966 (0.993–8.857) | 0.051 |
| Tertile 3 | 12.997 (5.902–28.618) | <0.001 | 13.186 (3.013–57.708) | 0.001 |
| HF with IHD (n = 187) | ||||
| Tyr/Thr tertiles | 2.912 (2.042–4.154) | <0.001 | 3.826 (2.349–6.232) | <0.001 |
| Tertile 1 | 1 (ref) | 1 (ref) | ||
| Tertile 2 | 3.247 (1.808–5.832) | <0.001 | 7.327 (3.171–16.928) | <0.001 |
| Tertile 3 | 8.018 (3.840–16.741) | <0.001 | 11.686 (4.514–30.252) | <0.001 |
The presence risk of HF (n = 318), HF with DCM (n = 131), or HF with IHD (n = 187) was analysed in combination with controls (n = 100), respectively. Adjusted for sex, age, BMI, low systolic BP, heart rate, smoking, drinking, DM, hyperlipidaemia, stroke, AF, coronary disease, ALT, and low eGFR.
DCM, dilated cardiomyopathy; HF, heart failure; IHD, ischaemic heart disease; OR, odds ratio.
Receiver‐operating characteristic curve analysis of tyrosine/threonine or Fischer's ratio index for chronic heart failure
In all subjects, the optimal value of the cut‐off point for the index of Tyr/Thr to predict the presence of HF was 0.420, and the AUC was 0.767 (95% CI: 0.714–0.820; sensitivity 65.72%, specificity 76.00%; P < 0.001). Meanwhile, the optimal value of the cut‐off point for the index of FR to predict the presence of HF was 0.182, and the AUC was 0.573 (95% CI: 0.512–0.634; sensitivity 35.22%, specificity 83.00%; P = 0.028). Tyr/Thr ratio was significantly superior to FR in predicting HF occurrence by the AUC difference analysis (0.767:0.573, P < 0.001) (Figure 2A ).
Figure 2.
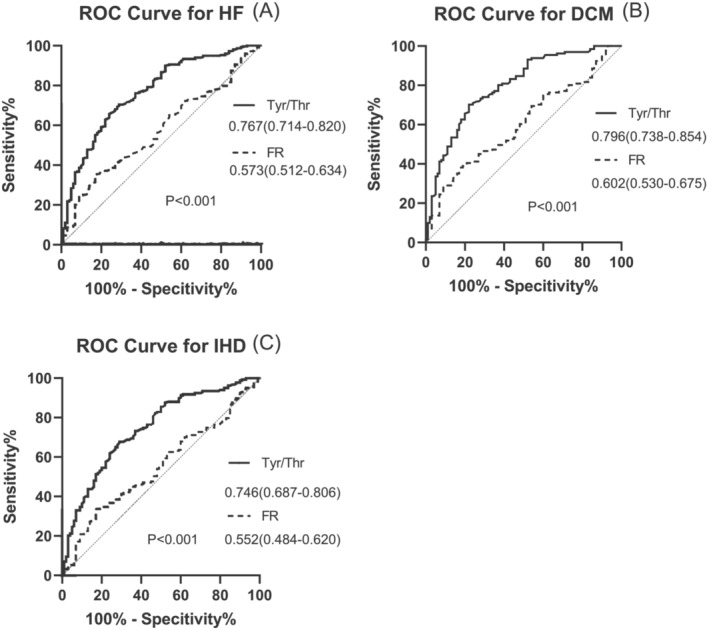
ROC curve for the presence of chronic HF. (A) ROC curve for the presence of HF in all subjects; (B) ROC curve for the presence of DCM in control and DCM subjects; (C) ROC curve for the presence of IHD in control and IHD subjects. DCM, dilated cardiomyopathy; FR, Fischer's ratio; HF, heart failure; IHD, ischaemic heart disease; ROC curve, receiver‐operating‐characteristic curve; Tyr/Thr, tyrosine/threonine.
In DCM and control subjects, the AUC of Tyr/Thr for predicting DCM was higher than that of FR (0.796:0.602; P < 0.001) (Figure 2B ). In IHD and control subjects, the AUC of Tyr/Thr for predicting IHD was also higher than that of FR (0.746:0.552; P < 0.001) (Figure 2C ).
Correlation between tyrosine/threonine ratio and soluble suppression of tumourigenicity‐2, high‐sensitivity C‐reactive protein, or N‐terminal brain natriuretic peptide
In chronic HF, Tyr/Thr ratio was correlated with sST2 or hsCRP by univariate analysis (all P < 0.05). After full adjustment, Tyr/Thr ratio was associated with sST2 (OR, 2.755; 95% CI: 1.105–6.873; P = 0.030), hsCRP (OR, 1.728; 95% CI: 1.212–2.464; P = 0.003), or NT‐proBNP (OR, 1.737; 95% CI: 1.187–2.544; P = 0.005), respectively (Table 5 ).
Table 5.
Correlation between Tyr/Thr and sST2, hsCRP, or NT‐proBNP in HF subjects
| n | Unadjusted OR | P value | Adjusted OR | P value | |
|---|---|---|---|---|---|
| sST2 | |||||
| Tyr/Thr | 66 | 1.946 (1.013–3.737) | 0.046 | 2.755 (1.105–6.873) | 0.030 |
| hsCRP | |||||
| Tyr/Thr | 270 | 1.421 (1.044–1.934) | 0.026 | 1.728 (1.212–2.464) | 0.003 |
| Log NT‐proBNP | |||||
| Tyr/Thr | 318 | 1.319 (0.994–1.750) | 0.055 | 1.737 (1.187–2.544) | 0.005 |
Adjusted for sex, age, low systolic BP, BMI, heart rate, smoking, drinking, DM, hyperlipidaemia, stroke, AF, coronary disease, ALT, and low eGFR.
HF, heart failure; hsCRP, high‐sensitivity C‐reactive protein; NT‐proBNP, N‐terminal brain natriuretic peptide; OR, odds ratio; sST2, the soluble form of suppression of tumourigenicity 2 protein; Tyr/Thr, tyrosine/threonine.
Prognosis study in chronic heart failure
To explore the prognostic value of Tyr/Thr ratio in chronic HF, 269 subjects were followed up for a mean year (11.10 ± 2.80 months) after their first discharges and 49 subjects were lost, containing 8 who died in the initial hospitalization and 41 who could not be contacted after their first discharge. Considering that lost cases can affect our understanding of prognosis, we reclassified 269 subjects into three rough groups, low (Tyr/Thr < 0.472), median (0.472 ≤ Tyr/Thr < 0.614), and high (Tyr/Thr ≥ 0.614) levels, according to the tertiles of serum Tyr/Thr levels. The CV death and the first readmission due to HF were chosen as HF endpoint. Of 269 HF subjects, 60 (22.30%) met the HF endpoint, including 26 (9.67%) who had CV death and 60 (22.30%) who had HF readmission. The HF subjects who met the HF endpoint or only met CV death or HF readmission had significantly higher Tyr/Thr ratio at baseline than those who did not (P < 0.01) (Figure 3A ). The Kaplan–Meier survival curves to visualize the relationship between Tyr/Thr ratio and outcomes. When grouped by the Tyr/Thr ratio tertiles, subjects in the highest tertile were more likely to meet the HF endpoint, CV death or HF readmission (all Log‐rank P < 0.05) (Figure 3B ). Following multivariable Cox regression analyses adjusting for conventional risk factors including sex, age, BMI, systolic BP, heart rate, smoking, drinking, DM, hyperlipidaemia, stroke, AF, coronary disease, ALT, low eGFR, hsCRP, and NT‐proBNP, the elevated (tertile 3 vs. tertile 1) serum Tyr/Thr remained correlated to increased risk of the endpoint (HR: 2.901, 95% CI: 1.228–6.851, P = 0.015). Furthermore, subjects in the highest tertile of Tyr/Thr values demonstrated a significantly elevated risk of CV death (HR: 8.908, 95% CI: 1.703–46.590, P = 0.010) and HF rehospitalization (HR: 2.982, 95% CI: 1.262–7.048, P = 0.013) compared with those in the lowest tertile after adjustment (Table 6 ).
Figure 3.
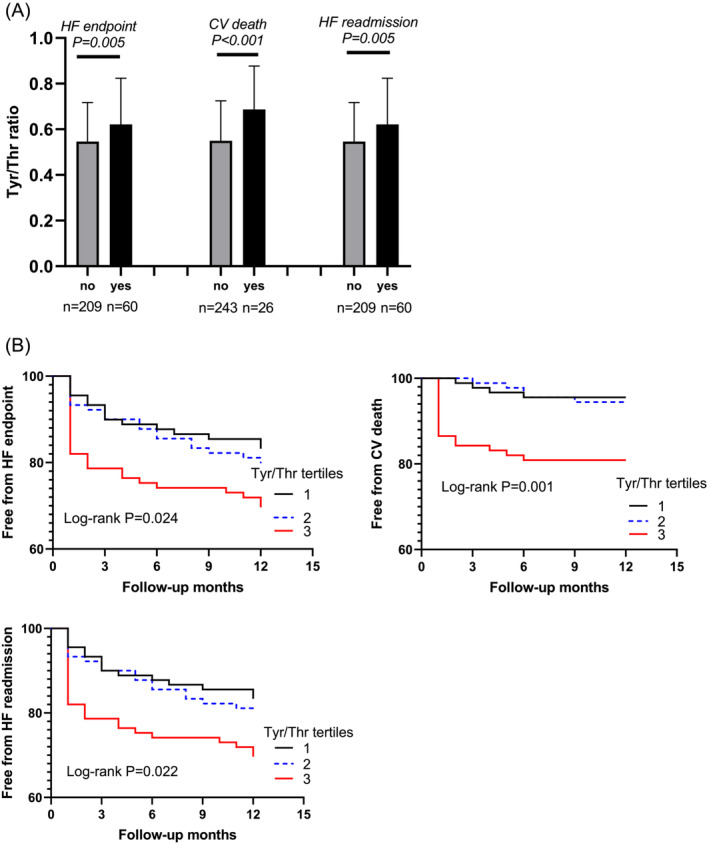
Follow‐up data of HF subjects and prognosis analysis. Tyr/Thr, Tyrosine/Threonine; HF, heart failure; CV, cardiovascular. (A) HF patients who met the HF endpoint, CV death or HF readmission had higher levels of Tyr/Thr ratio at baseline. (B) Kaplan–Meier curves and log‐rank analysis for the HF endpoint, CV death or HF readmission according to Tyr/Thr tertiles, respectively.
Table 6.
Cox regression analysis for endpoint in HF subjects
| Unadjusted HR | P value | Adjusted HR | P value | |
|---|---|---|---|---|
| HF endpoint | ||||
| Tyr/Thr tertiles | 1.428 (1.038–1.966) | 0.029 | 1.634 (1.099–2.430) | 0.015 |
| Tertile 1 | 1 (ref) | 1 (ref) | ||
| Tertile 2 | 1.208 (0.609–2.396) | 0.589 | 2.146 (0.867–5.312) | 0.099 |
| Tertile 3 | 1.980 (1.053–3.723) | 0.034 | 2.901 (1.228–6.851) | 0.015 |
| CV death | ||||
| Tyr/Thr tertiles | 2.452 (1.402–4.287) | 0.002 | 2.887 (1.417–5.884) | 0.004 |
| Tertile 1 | 1 (ref) | 1 (ref) | ||
| Tertile 2 | 1.243 (0.334–4.627) | 0.746 | 3.320 (0.546–20.200) | 0.193 |
| Tertile 3 | 4.675 (1.572–13.899) | 0.006 | 8.908 (1.703–46.590) | 0.010 |
| HF readmission | ||||
| Tyr/Thr tertiles | 1.432 (1.041–1.971) | 0.027 | 1.653 (1.112–2.457) | 0.013 |
| Tertile 1 | 1 (ref) | 1 (ref) | ||
| Tertile 2 | 1.215 (0.612–2.411) | 0.577 | 2.211 (0.894–5.470) | 0.086 |
| Tertile 3 | 1.992 (1.059–3.746) | 0.032 | 2.982 (1.262–7.048) | 0.013 |
Adjusted for sex, age, BMI, low systolic BP, heart rate, smoking, drinking, DM, hyperlipidaemia, stroke, AF, coronary disease, ALT, low eGFR, hsCRP, and NT‐proBNP.
CV, cardiovascular; HF, heart failure; HR, hazard ratio; hsCRP, high‐sensitivity C‐reactive protein; Tyr/Thr, tyrosine/threonine.
In 269 HF subjects, the optimal value of the cut‐off point for the index of Tyr/Thr to predict the CV death was 0.414 and the AUC was 0.715 (CI: 0.600–0.830; sensitivity 73.08%, specificity 68.31%; P < 0.001). Meanwhile, the optimal value of the cut‐off point for the index of FR to predict the presence of HF was 0.160 and the AUC was 0.550 (CI: 0.435–0.665; sensitivity 65.38%, specificity 50.62%; P = 0.403). Tyr/Thr ratio was superior to FR in predicting CV death by the AUC difference analysis (0.715:0.550, P = 0.047) (Figure 4 ).
Figure 4.
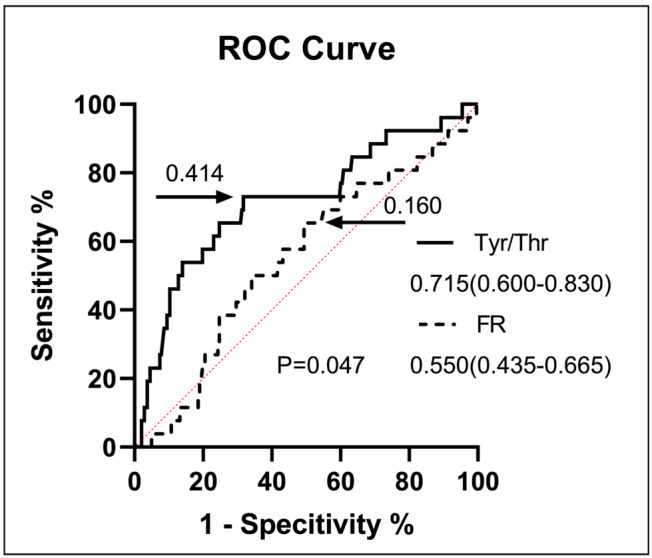
ROC curve for CV death in subjects with HF. CV, cardiovascular; FR, Fischer's ratio; HF, heart failure; ROC curve, receiver‐operating‐characteristic curve; Tyr/Thr, tyrosine/threonine.
In HF subjects with HF endpoint (or HF readmission), Tyr/Thr ratio was gradually increased along with the elevation of hsCRP (n = 60, r = 0.337, P = 0.017). Moreover, Tyr/Thr ratio was significantly positively correlated with the value of hsCRP in subjects with CV death (n = 26, r = 0.674, P = 0.001) (Figure 5 ). However, Tyr/Thr ratio has no significant correlation with log NT‐proBNP or sST2 in HF endpoint. Interestingly, in non‐HF endpoint or non‐CV death or non‐HF readmission subjects, Tyr/Thr ratio was positively associated with log NT‐proBNP or sST2 (P < 0.05) (Figure 5 ).
Figure 5.
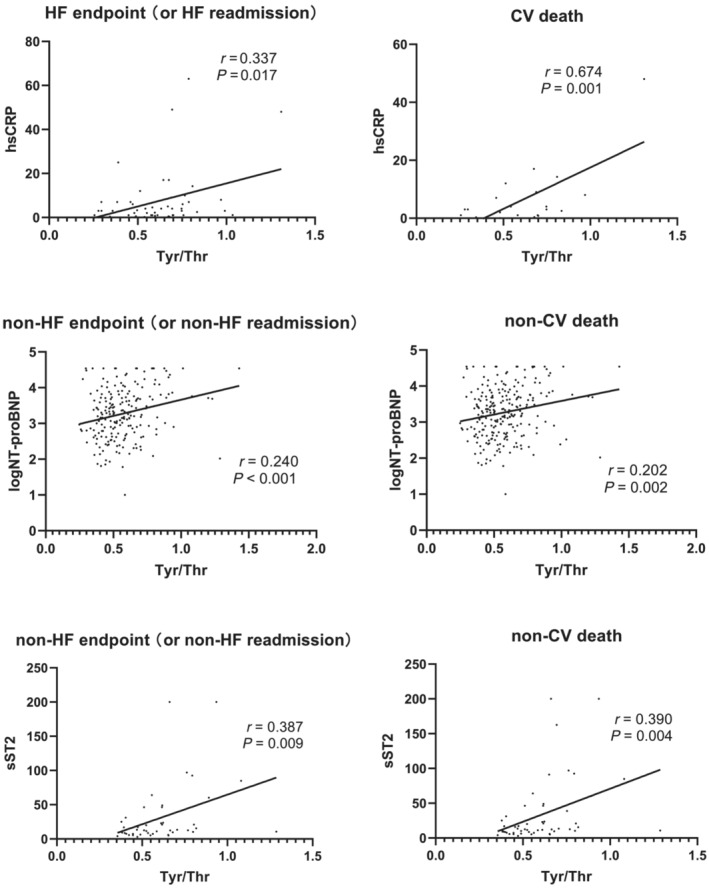
Correlation between Tyr/Thr ratio and sST2, hsCRP or logNT‐proBNP in HF endpoint analysis. CV, cardiovascular; HF, heart failure; hsCRP, high‐sensitivity C‐reactive protein; NT‐proBNP, N‐terminal brain natriuretic peptide; sST2, the soluble form of suppression of tumourigenicity 2 protein; Tyr/Thr, tyrosine/threonine.
Discussion
In the present study, three major findings were reported. First, with the presence of HF (HFrEF or HFmrEF), serum Tyr levels increased and Thr levels decreased. Second, circulating Tyr/Thr ratio was an independent risk factor for predicting HF presence in all subjects. Last, high Tyr/Thr levels were associated with a higher risk of HF endpoint events in chronic HF subjects. Though it is not the first evidence to prove the associations between AAs and HF, the present study still has its unique value: On the one hand, it is the comprehensive AAs detection in Chinese HF subjects for clinic and further comparing with the FR index. On the other hand, it is the first proof to find the relationship between Tyr/Thr index with the risk of HF presence in various HF aetiology.
Tyr is a substrate for various bio‐molecular biosynthesis, including thyroid hormone, catecholamine and neurotransmitter. When the uptake and use of Tyr decrease, these biosynthesis could be influenced which is identified as a cause of HF. 20 In addition, in HF, inflammation‐induced production of reactive oxygen species may consume a significant portion of tetrahydrobiopterin (BH4), a co‐factor of Phe and Tyr hydroxylases, leaving Phe and Tyr unmetabolized. 21 , 22 Moreover, the pathogenesis of HF could also influence Phe and Tyr hydroxylases. 23 Thus, the accumulation of Tyr in the serum was inclined to the worse cardiac function. It should be noted that hypothyroidism could reduce liver's clearance of Tyr and also lead to the increased tyrosinemia. 24 Though the thyroid function is frequently impaired during HF, hypothyroidism has been identified more often as a cause of HF. Moreover, the isolated finding of the increased signals of these amino acids excludes the possibility that it may derive from a hypercatabolic status, prompting the search for HF mechanism. 20
Thr, as one of EAAs, plays a pivotal role as cardiac nutrition. EAAs are fundamental for the synthesis of proteins, many metabolic and signalling intermediates and many cofactors. 25 AAs could enter the Krebs cycle, 26 promoting anabolism, favouring mitochondrial biogenesis and reducing hyperautophagy. 27 Otherwise, malnutrition (low EAAs) and autophagy are closely related, especially in hypercatabolic conditions, to the heart. Massive activation of autophagy may be detrimental for the heart in certain stress conditions. 28 , 29 Therefore, low Thr levels may be detrimental to cardiac function. Furthermore, HF patients may suffer from severe nausea or vomiting and hiccup due to the high concentration of natriuretic peptide in the blood anterior atrium, as well as reduced intestinal blood flow, leading to further impaired nutrient absorption in the body. 30 Prior studies also found that dietary AAs and especially EAAs, was a potential adjuvant therapeutic strategy to treat systolic dysfunction HF. 31
Then, our research showed that the elevated Tyr/Thr ratio was positively correlated with sST2 or hsCRP level, but was weakly correlated with log NT‐proBNP value in HF subjects. The sST2 responds mainly to cardiac fibrosis, and remodelling while hsCRP indicates inflammatory response, and NT‐proBNP is a marker of volume overload and congestion, which reflect diverse pathophysiological pathways implicated in highly complex HF pathology. 32 Meanwhile, in the present research, the relationship between Tyr/Thr index and HF still exists in various HF aetiology (DCM or IHD). These observations substantiate a notion that the presence of HF disease due to Tyr and Thr metabolic abnormalities may be primarily caused by the aggravation of myocardial fibrosis since C‐reactive protein (CRP) principally participating in the cause of ischaemic HF and atherosclerosis disease. 33 However, a research reported that CRP could also cause myocardial damage in DCM through activation of the complement system and chemotaxis of macrophages. 34 Therefore, the main mechanism of AAs to HF needs our further exploration in fundamental research.
Next, we observed that the close relationship between elevated Tyr/Thr ratio and poor prognosis accompanied with aggravating inflammation reaction. The explanations were as follows, first, due to the decreased uptake and use of Tyr as a substrate for the biosynthesis of various biological molecules, higher Tyr levels were prone to be found in the HF serum with poor prognosis of CV death. 35 Second, individualized nutritional support could reduce the risk of major CV events and mortality in chronic HF. 36 Thr, as one of the EAAs was vital to reduce the risk of poor prognosis and lack of it certainly increase HF adverse endpoints. Third, numerous researches have shown that overactive or prolonged inflammatory response can lead to further cardiac damage, so high plasma hsCRP levels portend poor outcomes, particularly in those with severe concomitant systolic dysfunctions. 37 , 38 , 39 So it is conceivable that elevated Tyr/Thr level leads to worse prognosis with inflammatory response.
Last, we compared the index of Tyr/Thr ratio with the FR index which was often used for the valuation of the metabolism of HF or other diseases. 10 , 40 In this research, the Tyr/Thr ratio was superior to FR in predicting HF occurrence (with different aetiology) or CV death. These gave us a hint that Tyr/Thr ratio has not only a unique value in understanding the risk of HF presence but also a prognostic value for HF.
Study limitations
We recognize certain limitations of this study. First, our study was a single center study, but all data collection and echocardiography were performed in a standard form to ensure the consistency in each case. Second, some unidentified factors (e.g. thyroid disease and dietary intake) might not have been evaluated which could potentially be confounders for modified metabolite levels. Third, sST2 entered the clinic at a later stage, so only little number of HF subjects were tested. The study on its correlation with AAs needs to be further verified by more samples. Last, our HF subjects enrolled were those with HFrEF or HFmrEF. Thus, whether the indicative value of AAs still exist in HFpEF remains unknown. Further studies should focus on combining these HF‐associated indicators to build a more comprehensive assessment tool to manage HF patients.
Conclusions
Circulating increased Tyr to Thr value provide independent clinical value for the presence of chronic HF and was also correlated with HF of DCM or IHD. Moreover, elevated Tyr to Thr ratio was positively correlated with poor prognosis. Compared with FR index, Tyr to Thr ratio was more valuable for predicting HF occurrence or CV death.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Funding
None.
Acknowledgements
We wish to thank all participants enrolled in this study for their patience and understanding.
Zhou Q. F., Yang F., Dai Y., Chen S., Zhang F. R., Lu L., and Lu Q. Y. (2024) Tyrosine to threonine ratio was related to heart failure with reduced or mildly reduced ejection fraction, ESC Heart Failure, doi: 10.1002/ehf2.14700
References
- 1. Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, et al. The global health and economic burden of hospitalizations for heart failure: Lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 2014;63:1123–1133. doi: 10.1016/j.jacc.2013.11.053 [DOI] [PubMed] [Google Scholar]
- 2. Solomon SD, Anavekar N, Skali H, McMurray JJ, Swedberg K, Yusuf S, et al. Candesartan in Heart Failure Reduction in Mortality (CHARM) Investigators. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation 2005;112:3738–3744. [DOI] [PubMed] [Google Scholar]
- 3. Mentz RJ, O'Connor CM. Pathophysiology and clinical evaluation of acute heart failure. Nat Rev Cardiol 2016;13:28–35. doi: 10.1038/nrcardio.2015.134 [DOI] [PubMed] [Google Scholar]
- 4. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022;145:e895–e1032. doi: 10.1016/j.jacc.2021.12.012 [DOI] [PubMed] [Google Scholar]
- 5. Posey EA, Bazer FW, Wu G. Amino acids and their metabolites for improving human exercising performance. Adv Exp Med Biol 2021;1332:151–166. doi: 10.1007/978-3-030-74180-8_9 [DOI] [PubMed] [Google Scholar]
- 6. Lai L, Leone TC, Keller MP, Martin OJ, Broman AT, Nigro J, et al. Energy metabolic reprogramming in the hypertrophied and early stage failing heart: A multisystems approach. Circ Heart Fail 2014;7:1022–1031. doi: 10.1161/CIRCHEARTFAILURE.114.001469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sansbury BE, DeMartino AM, Xie Z, Brooks AC, Brainard RE, Watson LJ, et al. Metabolomic analysis of pressure‐overloaded and infarcted mouse hearts. Circ Heart Fail 2014;7:634–642. doi: 10.1161/CIRCHEARTFAILURE.114.001151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aquilani R, La Rovere MT, Corbellini D, Pasini E, Verri M, Barbieri A, et al. Plasma amino acid abnormalities in chronic heart failure. Mechanisms, potential risks and targets in human myocardium metabolism. Nutrients 2017;9:1251. doi: 10.3390/nu9111251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hiraiwa H, Okumura T, Kondo T, Kato T, Kazama S, Ishihara T, et al. Usefulness of the plasma branched‐chain amino acid/aromatic amino acid ratio for predicting future cardiac events in patients with heart failure. J Cardiol 2020;75:689–696. doi: 10.1016/j.jjcc.2019.12.016 [DOI] [PubMed] [Google Scholar]
- 10. Saleem TH, Algowhary M, Kamel FEM, El‐Mahdy RI. Plasma amino acid metabolomic pattern in heart failure patients with either preserved or reduced ejection fraction: the relation to established risk variables and prognosis. Biomed Chromatogr 2021;35:e5012. doi: 10.1002/bmc.5012 [DOI] [PubMed] [Google Scholar]
- 11. Verbrugge FH, Dupont M, Steels P, Grieten L, Malbrain M, Tang WH, et al. Abdominal contributions to cardiorenal dysfunction in congestive heart failure. J Am Coll Cardiol 2013;62:485–495. doi: 10.1016/j.jacc.2013.04.070 [DOI] [PubMed] [Google Scholar]
- 12. Samsky MD, Patel CB, DeWald TA, Smith AD, Felker GM, Rogers JG, et al. Cardiohepatic interactions in heart failure: An overview and clinical implications. J Am Coll Cardiol 2013;61:2397–2405. doi: 10.1016/j.jacc.2013.03.042 [DOI] [PubMed] [Google Scholar]
- 13. Dejong CH, van de Poll MC, Soeters PB, Jalan R, Olde Damink SW. Aromatic amino acid metabolism during liver failure. J Nutr 2007;137:1579S–1585S; discussion 1597S–1598S. doi: 10.1093/jn/137.6.1579S [DOI] [PubMed] [Google Scholar]
- 14. Kimura Y, Okumura T, Kazama S, Shibata N, Oishi H, Arao Y, et al. Usefulness of plasma branched‐chain amino acid analysis in predicting outcomes of patients with nonischemic dilated cardiomyopathy. Int Heart J 2020;61:739–747. doi: 10.1536/ihj.20-010 [DOI] [PubMed] [Google Scholar]
- 15. Hiraiwa H, Okumura T, Murohara T. Amino acid profiling to predict prognosis in patients with heart failure: An expert review. ESC Heart Fail 2023;10:32–43. doi: 10.1002/ehf2.14222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kouzu H, Katano S, Yano T, Ohori K, Nagaoka R, Inoue T, et al. Plasma amino acid profiling improves predictive accuracy of adverse events in patients with heart failure. ESC Heart Fail 2021;8:5045–5056. doi: 10.1002/ehf2.13572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2 [DOI] [PubMed] [Google Scholar]
- 18. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. CKD‐EPI (chronic kidney disease epidemiology collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smon A, Cuk V, Brecelj J, Murko S, Groselj U, Zerjav Tansek M, et al. Comparison of liquid chromatography with tandem mass spectrometry and ion‐exchange chromatography by post‐column ninhydrin derivatization for amino acid monitoring. Clin Chim Acta 2019;495:446–450. doi: 10.1016/j.cca.2019.05.007 [DOI] [PubMed] [Google Scholar]
- 20. Tenori L, Hu X, Pantaleo P, Alterini B, Castelli G, Olivotto I, et al. Metabolomic fingerprint of heart failure in humans: A nuclear magnetic resonance spectroscopy analysis. Int J Cardiol 2013;168:e113–e115. doi: 10.1016/j.ijcard.2013.08.042 [DOI] [PubMed] [Google Scholar]
- 21. Bendall JK, Douglas G, McNeill E, Channon KM, Crabtree MJ. Tetrahydrobiopterin in cardiovascular health and disease. Antioxid Redox Signal 2014;20:3040–3077. doi: 10.1089/ars.2013.5566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nishijima Y, Sridhar A, Bonilla I, Velayutham M, Khan M, Terentyeva R, et al. Tetrahydrobiopterin depletion and NOS2 uncoupling contribute to heart failure‐induced alterations in atrial electrophysiology. Cardiovasc Res 2011;91:71–79. doi: 10.1093/cvr/cvr087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cheng CW, Liu MH, Tang HY, Cheng ML, Wang CH. Factors associated with elevated plasma phenylalanine in patients with heart failure. Amino Acids 2021;53:149–157. doi: 10.1007/s00726-020-02933-1 [DOI] [PubMed] [Google Scholar]
- 24. Bélanger R, Chandramohan N, Misbin R, Rivlin RS. Tyrosine and glutamic acid in plasma and urine of patients with altered thyroid function. Metabolism 1972;21:855–865. doi: 10.1016/0026-0495(72)90009-1 [DOI] [PubMed] [Google Scholar]
- 25. Corsetti G, Pasini E, Romano C, Chen‐Scarabelli C, Scarabelli TM, Flati V, et al. How can malnutrition affect autophagy in chronic heart failure? Focus and perspectives. Int J Mol Sci 2021;22:3332. doi: 10.3390/ijms22073332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pasini E, Corsetti G, Aquilani R, Romano C, Picca A, Calvani R, et al. Protein‐amino acid metabolism disarrangements: The hidden enemy of chronic age‐related conditions. Nutrients 2018;10:391. doi: 10.3390/nu10040391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. D'Antona G, Ragni M, Cardile A, Tedesco L, Dossena M, Bruttini F, et al. Branched‐chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle‐aged mice. Cell Metab 2010;12:362–372. doi: 10.1016/j.cmet.2010.08.016 [DOI] [PubMed] [Google Scholar]
- 28. Gundewar S, Calvert JW, Jha S, Toedt‐Pingel I, Ji SY, Nunez D, et al. Activation of AMP‐activated protein kinase by metformin improves left ventricular function and survival in heart failure. Circ Res 2009;104:403–411. doi: 10.1161/CIRCRESAHA.108.190918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sciarretta S, Maejima Y, Zablocki D, Sadoshima J. The role of autophagy in the heart. Annu Rev Physiol 2018;80:1–26. doi: 10.1146/annurev-physiol-021317-121427 [DOI] [PubMed] [Google Scholar]
- 30. Tsuji S, Koyama S, Taniguchi R, Fujiwara T, Fujiwara H, Sato Y. Nutritional status of outpatients with chronic stable heart failure based on serum amino acid concentration. J Cardiol 2018;72:458–465. doi: 10.1016/j.jjcc.2018.05.007 [DOI] [PubMed] [Google Scholar]
- 31. Ragni M, Greco CM, Felicetta A, Ren SV, Kunderfranco P, Ruocco C, et al. Dietary essential amino acids for the treatment of heart failure with reduced ejection fraction. Cardiovasc Res 2023;119:982–997. doi: 10.1093/cvr/cvad005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lotierzo M, Dupuy AM, Kalmanovich E, Roubille F, Cristol JP. sST2 as a value‐added biomarker in heart failure. Clin Chim Acta 2020;501:120–130. doi: 10.1016/j.cca.2019.10.029 [DOI] [PubMed] [Google Scholar]
- 33. Shabani M, Bakhshi H, Ostovaneh MR, Ma X, Wu CO, Ambale‐Venkatesh B, et al. Temporal change in inflammatory biomarkers and risk of cardiovascular events: the multi‐ethnic study of atherosclerosis. ESC Heart Fail 2021;8:3769–3782. doi: 10.1002/ehf2.13445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zimmermann O, Bienek‐Ziolkowski M, Wolf B, Vetter M, Baur R, Mailänder V, et al. Myocardial inflammation and non‐ischaemic heart failure: Is there a role for C‐reactive protein? Basic Res Cardiol 2009;104:591–599. doi: 10.1007/s00395-009-0026-2 [DOI] [PubMed] [Google Scholar]
- 35. Stryeck S, Gastrager M, Degoricija V, Trbušić M, Potočnjak I, Radulović B, et al. Serum concentrations of citrate, tyrosine, 2‐ and 3‐hydroxybutyrate are associated with increased 3‐month mortality in acute heart failure patients. Sci Rep 2019;9:6743. doi: 10.1038/s41598-019-42937-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hersberger L, Dietz A, Bürgler H, Bargetzi A, Bargetzi L, Kägi‐Braun N, et al. Individualized nutritional support for hospitalized patients with chronic heart failure. J Am Coll Cardiol 2021;77:2307–2319. doi: 10.1016/j.jacc.2021.03.232 [DOI] [PubMed] [Google Scholar]
- 37. Zhang X, Wang S, Fang S, Yu B. Prognostic role of high sensitivity C‐reactive protein in patients with acute myocardial infarction. Front Cardiovasc Med 2021;8:659446. doi: 10.3389/fcvm.2021.659446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lourenço P, Paulo Araújo J, Paulo C, Mascarenhas J, Friões F, Azevedo A, et al. Higher C‐reactive protein predicts worse prognosis in acute heart failure only in noninfected patients. Clin Cardiol 2010;33:708–714. doi: 10.1002/clc.20812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tang WH, Shrestha K, Van Lente F, Troughton RW, Martin MG, Borowski AG, et al. Usefulness of C‐reactive protein and left ventricular diastolic performance for prognosis in patients with left ventricular systolic heart failure. Am J Cardiol 2008;101:370–373. doi: 10.1016/j.amjcard.2007.08.038 [DOI] [PubMed] [Google Scholar]
- 40. Yanagisawa R, Kataoka M, Inami T, Momose Y, Kawakami T, Takei M, et al. Usefulness of circulating amino acid profile and Fischer ratio to predict severity of pulmonary hypertension. Am J Cardiol 2015;115:831–836. doi: 10.1016/j.amjcard.2014.12.048 [DOI] [PubMed] [Google Scholar]


