Abstract
Aims
Heart failure (HF) with preserved ejection fraction (HFpEF) is a disease associated with high morbidity and mortality, for which it is difficult to identify patients with the poorest prognosis in routine clinical practice. Carbohydrate antigen 125 (CA 125) has been shown to be a potential marker of congestion and prognosis in HF. We sought to better characterize HFpEF patients with high CA 125 levels by using a multimodal approach.
Methods and results
We prospectively enrolled 139 HFpEF patients (78 ± 8 years; 60% females) and 25 controls matched for age and sex (77 ± 5 years; 60% females). They underwent two‐dimensional echocardiography, cardiac magnetic resonance with late gadolinium enhancement [including extracellular volume (ECV) measurement], and serum measurements of CA 125 level. The primary endpoint of the study was a composite of all‐cause mortality or first HF hospitalization. The prognostic impact of CA 125 was determined using Cox proportional hazard models. Median CA 125 levels were significantly higher in HFpEF patients compared with controls [CA 125: 23.5 (14.5–44.7) vs. 14.6 (10.3–21.0) U/mL, P = 0.004]. CA 125 levels were positively correlated with a congestion marker [N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) levels, Pearson's r = 0.37, P < 0.001] and markers of cardiac fibrosis estimated by both ECV (Pearson's r = 0.26, P = 0.003) and fibroblast growth factor 23 levels (Pearson's r = 0.50, P < 0.001). Over a median follow‐up of 49 (22–64) months, 97 HFpEF patients reached the composite endpoint. Even after adjustment for the Meta‐Analysis Global Group in Chronic risk score, a CA 125 level ≥35 U/mL was still a significant predictor of the composite endpoint [hazard ratio (HR): 1.58 (1.04–2.41), P = 0.032] and more particularly of HF hospitalization [HR: 1.81 (1.13–2.92), P = 0.014]. In contrast, NT‐proBNP levels were not an independent predictor.
Conclusions
CA 125 levels were significantly higher in HFpEF patients compared with controls matched for age and sex and were associated with markers of congestion and cardiac fibrosis. CA 125 levels were a strong and independent predictor of HF hospitalization in HFpEF patients. These data suggest a potential value of CA 125 as a biomarker for staging and risk prediction in HFpEF.
Keywords: Carbohydrate antigen 125, Heart failure, Cardiac fibrosis, Prognosis, Cardiac imaging, Biomarker, NT‐proBNP
Introduction
Heart failure (HF) with preserved ejection fraction (HFpEF) is associated with high morbidity and mortality. 1 The complex pathophysiology of HFpEF is related to the presence of numerous comorbidities resulting in systemic inflammation, microcirculation damage, increased cardiac stiffness due to hypertrophy, and myocardial fibrosis. 2 Despite numerous investigations, HFpEF remains a therapeutic challenge and few therapeutic classes have demonstrated an impact on prognosis. 3 , 4 In addition, there are also difficulties in identifying patients who require closer follow‐up and who are at greater risk of events. Several biomarkers have shown prognostic value, such as soluble ‘suppression of tumourigenicity 2’ (ST2) receptor and fibroblast growth factor 23 (FGF‐23), but only natriuretic peptides are readily available to clinicians. 5 , 6 However, carbohydrate antigen 125 (CA 125) is a globally accessible marker that has shown prognostic value in HF. 7
CA 125 is a repeat domain of a glycoprotein of the mucin family transcribed by the MUC16 gene. This glycoprotein is produced by mesothelial cells and has a lubricating and immunomodulatory role. MUC16 has an extracytosolic N‐terminal domain, a 60 tandem repeat domain of 156 amino acids, and an intracytosolic C‐terminal domain. 8 , 9 Cleavage of the extracellular site of MUC16 produces a molecule harbouring the CA 125 epitope in its repeat domains. The repeat domain corresponding to CA 125 is recognized by the clinical antibody ovarian carbohydrate 125 (OC 125), which provides the basis for CA 125‐detection by enzyme‐linked immunosorbent assay in clinical biology. 8 , 9 CA 125 production by mesothelial cells is induced by mechanical stress secondary to increased pleural space volume as seen in cardiac congestion as well as inflammation. 10 These stimuli result in activation of the c‐Jun N‐terminal kinase pathway, contributing to CA 125 synthesis and cleavage. Increased CA 125 levels in ascitic, pleural, or pericardial fluids are linked to cardiac failure, with serum levels reflecting its clinical stage. 11
It has been previously shown that CA 125 levels, mostly used in ovarian cancer screening and monitoring, are also increased in HF, including HFpEF. 12 , 13 Elevated levels are associated with congestion, right‐sided involvement, and poor prognosis. Thus, it could be a complementary tool to N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) level measurement. 14 However, very few studies have investigated this marker in chronic HFpEF patients. The objective of our study was to measure this marker in HFpEF population and age‐ and sex‐matched controls and to explore its association with congestion, cardiac fibrosis, and prognosis.
Methods
Population study
This study included patients from a Belgian HFpEF cohort described previously. 15 Briefly, consecutive patients with HFpEF were prospectively evaluated for inclusion in the study in a single centre between December 2015 and June 2017.
The following criteria had to be fulfilled for study inclusion: New York Heart Association (NYHA) Functional Class ≥II; typical symptoms and signs of HF; NT‐proBNP levels >350 pg/mL; and/or an HF hospitalization in the last 12 months, with preserved left ventricular (LV) ejection fraction (≥50%; HFpEF) and relevant structural heart disease [LV hypertrophy/left atrial (LA) enlargement], and/or diastolic dysfunction assessed by echocardiography for HFpEF, as previously described. 15 Among 183 patients included in the registry, 139 patients had a CA 125 level measurement in stable conditions and were included in this study. Of the 139 patients, 129 (96%) had an HFA‐PEFF score ≥5 points and therefore a definite diagnosis of HFpEF on the basis of morphological, functional, and biological criteria according to the diagnostic algorithm recommended by the European Society of Cardiology. 16 The remaining 10 patients had a score ranging from 4 to 5 points with a high likelihood of HFpEF; they have benefited from invasive haemodynamic measurements to confirm the diagnosis.
These patients were compared with controls without cardiovascular disease history, significant past medical history, or chronic disease. All controls were recruited by advertisement in the local community and underwent a full clinical examination, electrocardiogram, and exercise stress test, which all had to be normal prior to inclusion. A total of 89 controls with a CA 125 measurement were recruited; 25 controls were selected to be matched for age and sex with the HFpEF population.
Patients and controls underwent blood sampling, complete transthoracic echocardiography, and cardiac magnetic resonance (CMR) imaging (n = 116 HFpEF patients and n = 24 controls) in the absence of following contraindications: pacemaker, claustrophobia, or estimated glomerular filtration rate (eGFR < 30 mL/min/1.73 m2).
Echocardiography
All subjects underwent a complete two‐dimensional transthoracic echocardiography at inclusion (iE33 system, Philips Healthcare, Best, The Netherlands) to assess LV and right ventricular (RV) structure, systolic and diastolic function, and measurements of LA and right atrial volumes, in addition to a valvular evaluation. Pulmonary pressures were estimated using tricuspid regurgitation velocity. All measurements were averaged over three beats in patients suffering from atrial fibrillation.
Cardiac magnetic resonance
CMR imaging was performed using a 3T system (Ingenia, Philips Medical Systems, Best, The Netherlands). The different sequences have been described previously. 15 Pre‐ and post‐contrast modified look‐locker inversion recovery images were processed using the open‐source software MRmap Version 1.4 17 under Interactive Data Language® software. Pre‐ and post‐myocardial T1 times were measured in six myocardial regions of interest (anterior, anterolateral, inferolateral, inferior, inferoseptal, and anteroseptal). We calculated the average T1 time of the different regions of interest. Areas of ischaemic focal fibrosis identified by late gadolinium enhancement (LGE) were excluded from the analysis. Extracellular volume (ECV), a parameter reflecting myocardial interstitial fibrosis, was then computed according to the formula. 18 An ECV > 33% was used as a cut‐off to define significant diffuse myocardial fibrosis. 19
Biomarker measurement
Blood samples were obtained by venipuncture at inclusion. After centrifugation at 1.64 g for 10 min, serum aliquots were stored at −80°C. CA 125 levels were determined with a two‐site automated electrochemiluminescence assay on the Cobas® 8000 platform (Roche Diagnostics, Mannheim, Germany). The upper limit of the reference interval for the CA 125 assay was 35 U/mL. NT‐proBNP and high‐sensitivity troponin T (hsTnT) levels were also measured with automated electrochemiluminescence immunoassay on the Cobas® 8000 platform. Soluble ST2 and intact FGF‐23 levels were determined by immunoassays.
Follow‐up
Patients with HFpEF were prospectively followed up during outpatient visits and phone calls at 6 month intervals. Clinical and survival status were collected during follow‐up visits and by phone contact with the patients, their relatives, or their physician. The primary endpoint was a composite of all‐cause mortality or HF hospitalization, whichever came first. Hospitalization was defined as patients treated in the emergency room or admitted to a hospital, diagnosed with decompensated HF, and requiring intravenous diuretics. The secondary and tertiary endpoints were all‐cause mortality and HF hospitalization, respectively.
Statistical analyses
Statistical analyses were performed using SPSS Version 26 (SPSS Corp., Somers, NY) and R Version 4.1.2 software (http://www.r‐project.org). All tests were two‐sided, with statistical significance set at P < 0.05. Continuous variables were expressed as mean ± 1 standard deviation (SD), if normally distributed, or as median (25th and 75th percentiles), if not normally distributed. Normality of a continuous distribution was assessed using histograms, QQ plots, and skewness and kurtosis tests. Categorical variables were expressed as counts and percentages. Non‐normally distributed biomarker levels were log‐transformed (base 10) and assessed again for normality. The correlations between CA 125 levels and biomarkers were assessed using Pearson's correlation coefficient. Controls and HFpEF population were matched for age and sex using MatchIt package with the ‘nearest method’. 20 The cut‐off for abnormally high CA 125 levels was 35 U/mL, as reported in the literature. 21 , 22 The HFpEF patient population was divided into two groups according to this CA 125 level cut‐off, in line with previous studies. 23 , 24 , 25 Comparisons between groups were performed using an independent sample t‐test or χ 2 test, as appropriate. In cases where log‐transformation was applied and variables continued to exhibit non‐normal distribution, the non‐parametric Mann–Whitney U test was employed. A P value <0.05 was considered statistically significant. Univariate and multivariate linear regressions were performed to identify parameters associated with elevated CA 125 levels in HFpEF patients.
Primary, secondary, and tertiary endpoints were estimated using log‐rank tests and a Cox regression analysis. We tested the additional prognostic value of CA 125 over the Meta‐Analysis Global Group in Chronic (MAGGIC) score, a validated predictive risk tool for all‐cause mortality and HF hospitalization prediction in HFpEF patients, which incorporates multiple demographics, clinical data, and laboratory variables [age, gender, diabetes, chronic obstructive pulmonary disorder, HF diagnosed in the last 18 months, current smoker, NYHA class, beta‐blocker use, angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker, body mass index, systolic blood pressure, creatinine, and LV ejection fraction (LVEF)]. 26 These variables were available for all patients. We also tested the additional prognostic value of CA 125 over the haemoglobin level, a routine parameter not included in the MAGGIC risk score and associated with poor prognosis in HF. Kaplan–Meier curves based on the elevated CA 125 level (<35 or ≥35 U/mL) group were used to illustrate event‐free survival, overall survival, and hospitalization‐free survival of HFpEF patients.
Results
Baseline characteristics
The baseline and imaging characteristics of the study population (139 HFpEF patients and 25 age‐ and sex‐matched controls) are summarized in Table 1 . As expected, HFpEF patients displayed a higher incidence of cardiovascular risk factors and comorbidities compared with controls. These patients exhibited lower haemoglobin levels and eGFRs. Median NT‐proBNP, hsTnT, C‐reactive protein (CRP), FGF‐23, and ST2 levels were significantly higher in HFpEF patients. They also exhibited higher LA volumes, E‐wave velocities, E/e′ ratios, pulmonary pressures, and ECVs than controls. The slight disparity in NT‐proBNP levels between HFpEF patients in sinus rhythm and those in atrial fibrillation is likely attributable to the limited sample size. Nevertheless, within the subgroup of patients with a history of atrial fibrillation, those in atrial fibrillation at the time of inclusion exhibit a non‐significant trend towards higher NT‐proBNP levels compared with those in sinus rhythm. Median CA 125 levels were significantly higher in HFpEF patients compared with controls [CA 125: 23.5 (14.5–44.7) vs. 14.6 (10.3–21.0) U/mL, P = 0.004] (Figure 1 ). The abnormal value in our control population was 61.7 U/mL, which corresponds to the mean plus 2 SDs. CA 125 levels were positively correlated with NT‐proBNP levels (Pearson's r = 0.37, P < 0.001), hsTnT levels (Pearson's r = 0.34, P < 0.001), E/e′ ratios (Pearson's r = 0.26, P < 0.001), and ECVs (Pearson's r = 0.26, P = 0.003) and moderately correlated with FGF‐23 levels (Pearson's r = 0.50, P < 0.001) (Figure 2 ).
Table 1.
Baseline characteristics of HFpEF patients and controls matched for age and sex
| Control | HFpEF | P value | |
|---|---|---|---|
| n = 25 | n = 139 | ||
| Baseline characteristics | |||
| Age (years) | 77 ± 5 | 78 ± 8 | 0.157 |
| Female, n (%) | 15 (60) | 84 (60) | 1.000 |
| BMI (kg/m2) | 26 ± 4 | 28 ± 7 | 0.002 |
| Heart rate (b.p.m.) | 66 ± 9 | 72 ± 14 | 0.021 |
| Systolic blood pressure (mmHg) | 148 ± 20 | 138 ± 21 | 0.029 |
| Diastolic blood pressure (mmHg) | 83 ± 11 | 74 ± 13 | 0.001 |
| NYHA Class III or IV, n (%) | 0 (0) | 65 (47) | <0.001 |
| Medical history | |||
| Atrial fibrillation, n (%) | 1 (4) | 83 (60) | <0.001 |
| Coronary artery disease, n (%) | 0 (0) | 36 (33) | 0.012 |
| Prior myocardial infarction, n (%) | 0 (0) | 16 (12) | 0.118 |
| COPD, n (%) | 0 (0) | 13 (9) | 0.214 |
| Sleep apnoea, n (%) | 0 (0) | 18 (13) | 0.086 |
| Cardiovascular risk factors | |||
| Hypertension, n (%) | 17 (68) | 130 (93) | 0.002 |
| Diabetes, n (%) | 4 (16) | 52 (37) | 0.064 |
| Hypercholesterolaemia, n (%) | 21 (84) | 92 (67) | 0.135 |
| Smoking, n (%) | 6 (24) | 62 (45) | 0.006 |
| Family history of CV disease, n (%) | 5 (20) | 27 (20) | 1.000 |
| Medication | |||
| ACE inhibitor/ARB, n (%) | 11 (44) | 93 (67) | 0.050 |
| Beta‐blocker, n (%) | 3 (12) | 87 (63) | <0.001 |
| Loop diuretics, n (%) | 0 (0) | 96 (70) | <0.001 |
| Thiazide, n (%) | 0 (0) | 28 (20) | 0.015 |
| MRA, n (%) | 1 (4) | 26 (19) | 0.094 |
| Anticoagulants, n (%) | 1 (4) | 75 (54) | <0.001 |
| Antiplatelet agents, n (%) | 8 (32) | 56 (40) | 0.576 |
| Statins, n (%) | 7 (28) | 59 (44) | 0.214 |
| Echocardiography study | |||
| LA volume index (mL/m2) | 19 ± 6 | 45 ± 19 | <0.001 |
| LV EDV index (mL/m2) | 61 ± 9 | 68 ± 18 | 0.010 |
| LV ejection fraction (%) | 65 ± 5 | 62 ± 8 | 0.065 |
| E/e′ septal ratio | 10 ± 3 | 19 ± 8 | <0.001 |
| eSPAP (mmHg) | 19 ± 5 | 33 ± 10 | <0.001 |
| RV fractional area change (%) | 61 ± 6 | 57 ± 8 | 0.021 |
| TAPSE (mm) | 23 ± 4 | 19 ± 5 | <0.001 |
| CMR study | |||
| LA volume index (mL/m2) | 55 ± 18 | 121 ± 57 | <0.001 |
| LV EDV index (mL/m2) | 61 ± 9 | 68 ± 18 | 0.010 |
| LV ejection fraction (%) | 32 ± 10 | 65 ± 29 | <0.001 |
| RV ejection fraction (%) | 66 ± 6 | 62 ± 8 | 0.021 |
| ECV (%) | 28 ± 2 | 33 ± 5 | <0.001 |
| Late gadolinium enhancement (%) | 0 (0) | 1.4 ± 2.6 | <0.001 |
| Biology | |||
| NT‐proBNP without AF history (pg/mL) | 106 (55–154) | 1860 (558–3923) | <0.001 |
| NT‐proBNP with AF history (pg/mL) | 143 (143–143) | 1927 (1163–3320) | 0.087 |
| Sinus rhythm | — | 1927 (823–3368) | — |
| AF | — | 2020 (1341–3280) | — |
| eGFR (mL/min/1.73 m2) by CK‐EPI | 70 ± 18 | 56 ± 24 | 0.001 |
| Haemoglobin (g/dL) | 13.9 ± 1.3 | 11.8 ± 1.9 | <0.001 |
| Total cholesterol (mg/dL) | 203 (184–226) | 146 (123–182) | <0.001 |
| LDL‐C (mg/dL) | 115 (94–143) | 72 (54–98) | <0.001 |
| HDL‐C (mg/dL) | 66 (58–74.0) | 51 (41–61) | <0.001 |
| Triglycerides (mg/dL) | 90 (75–117) | 94 (72–126) | 0.784 |
| CRP (mg/dL) | 0.20 (0.10–0.30) | 0.70 (0.20–2.45) | <0.001 |
| hsTnT (pg/mL) | 8.0 (6.0–12.0) | 27.0 (16.0–38.0) | <0.001 |
| FGF‐23 (RU/mL) | 62 (54–75) | 257 (107–565) | <0.001 |
| Soluble ST2 (ng/mL) | 1.4 (1.3–1.5) | 1.6 (1.5–1.8) | <0.001 |
| Albumin (g/L) | 44.0 (42.0–45.5) | 39.0 (35.0–42.0) | <0.001 |
| CA 125 (U/mL) | 14.6 (10.3–21.0) | 23.5 (14.5–44.7) | 0.004 |
ACE, angiotensin‐converting enzyme; AF, atrial fibrillation; ARB, angiotensin receptor blocker; b.p.m., beats per minute; BMI, body mass index; CA 125, carbohydrate antigen 125; CK‐EPI, Chronic Kidney Disease Epidemiology Collaboration; CMR, cardiac magnetic resonance; COPD, chronic obstructive pulmonary disease; CRP, C‐reactive protein; CV, cardiovascular; ECV, extracellular volume; EDV, end‐diastolic volume; eGFR, estimated glomerular filtration rate; eSPAP, estimated systolic pulmonary artery pressure; FGF‐23, fibroblast growth factor 23; HDL‐C, high‐density lipoprotein cholesterol; HFpEF, heart failure with preserved ejection fraction; hsTnT, high‐sensitivity troponin T; IQR, inter‐quartile range; LA, left atrial; LDL‐C, low‐density lipoprotein cholesterol; LV, left ventricular; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; RV, right ventricular; ST2, suppression of tumourigenicity 2; TAPSE, tricuspid annular plane systolic excursion.
Values are reported as means ± standard deviations or medians and IQRs (IQR 0.25–0.75). Categorical variables are expressed as counts and proportions. Differences between clinical characteristics were compared using an independent t‐test, Mann–Whitney U test, or χ 2 test where appropriate.
Figure 1.
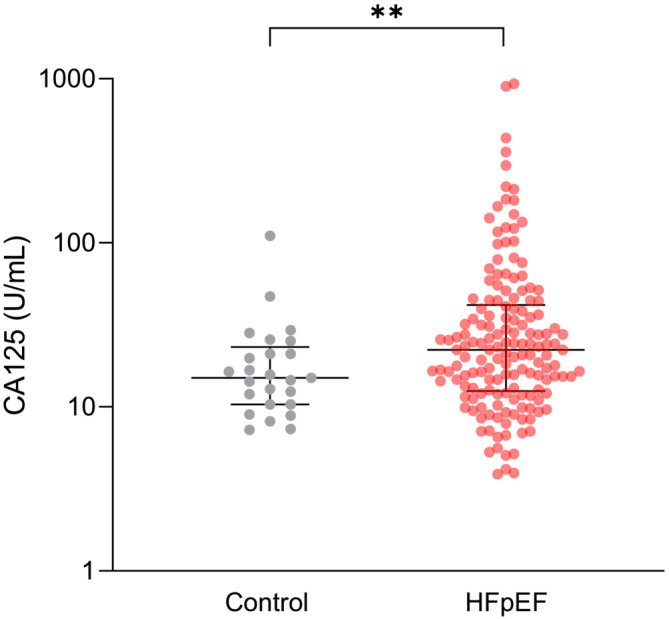
Box plot of carbohydrate antigen (CA 125) levels between heart failure with preserved ejection fraction (HFpEF) patients and controls matched for age and sex. Statistical analyses by independent t‐test, ** P < 0.01.
Figure 2.
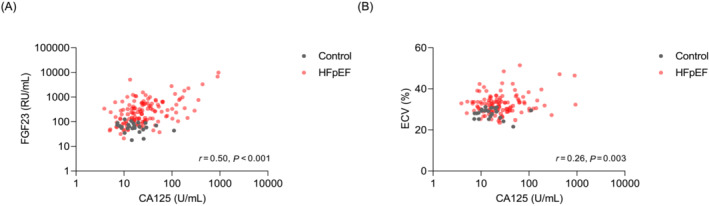
Correlation (A) between carbohydrate antigen 125 (CA 125) levels and fibroblast growth factor 23 (FGF‐23) levels and (B) between CA 125 levels and extracellular volumes (ECVs) in controls and heart failure with preserved ejection fraction (HFpEF) patients.
Clinical characteristics associated with elevated carbohydrate antigen 125 levels
Table 2 summarizes the clinical and imaging parameters of HFpEF patients stratified according to normal or high CA 125 (≥35 U/mL) levels. HFpEF patients with high CA 125 levels were more frequently classified as NYHA Class III/IV and had a lower body mass index. Median NT‐proBNP, FGF‐23, and hsTnT levels were significantly higher in patients with high CA 125 levels. This was also the case for the aspartate transaminase (AST)/alanine aminotransferase (ALT) ratio, a marker of hepatic venous congestion. Mean haemoglobin level was significantly lower in patients with high CA 125 levels. Regarding imaging parameters, HFpEF patients with high CA 125 levels displayed a trend for higher ECVs and septal E/e′ ratios, which are markers of cardiac fibrosis and diastolic dysfunction. Interestingly, HFpEF patients with high CA 125 levels had an increased inferior vena cava diameter and a decreased RV function (<45%) measured by CMR (26% vs. 6%, P = 0.006). In addition, there was no difference regarding renal function (<60 vs. ≥60 mL/min/1.73 m2) between patients with high and low CA 125 levels. If we categorize patients according to both their CA 125 levels (<35 or ≥35 U/mL) and NT‐proBNP levels (<1927 or ≥1927 pg/mL) corresponding to the median (Figure 3 ), patients with high CA 125 and NT‐proBNP levels were more symptomatic and had more events. Patients with high CA 125 levels regardless of NT‐proBNP levels presented more markers of RV dysfunction [tricuspid annular plane systolic excursion (TAPSE)] and congestion (tricuspid regurgitation and AST/ALT ratio). CA 125 levels were significantly higher in females compared with males [CA 125: 25.6 (14.4–47.8) vs. 19.6 (11.5–37.8), P = 0.042]. However, the proportion of females with a CA 125 ≥ 35 U/mL did not differ from males (35% vs. 27%, P = 0.48). As shown by the univariate linear regression analysis, higher CA 125 levels were associated with female sex, body mass index, NYHA Class III or IV, E/e′ ratio, RV ejection fraction assessed by CMR, ECV, eGFR, haemoglobin levels, FGF‐23 levels, and NT‐proBNP levels. The multivariate linear regression analysis revealed that only body mass index (β = −0.017, P = 0.007), NYHA Class III or IV (β = 0.201, P = 0.010), and FGF‐23 levels (β = 0.356, P < 0.001) were independently associated with elevated CA 125 levels (Table 3 ).
Table 2.
Comparisons of HFpEF patients with CA 125 levels below or above 35 U/mL
| CA 125 < 35 U/mL | CA 125 ≥ 35 U/mL | P value | |
|---|---|---|---|
| n = 95 | n = 44 | ||
| Baseline characteristics | |||
| Age (years) | 78 ± 9 | 79 ± 8 | 0.492 |
| Female, n (%) | 55 (58) | 29 (66) | 0.476 |
| BMI (kg/m2) | 30 ± 7 | 26 ± 6. | <0.001 |
| Heart rate (b.p.m.) | 71 ± 13 | 73 ± 16 | 0.421 |
| Systolic blood pressure (mmHg) | 140 ± 21 | 134 ± 21 | 0.165 |
| Diastolic blood pressure (mmHg) | 75 ± 12 | 71 ± 14 | 0.108 |
| NYHA Class III or IV, n (%) | 38 (40) | 27 (61) | 0.030 |
| HFA‐PEFF score ≥5 | 86 (91) | 43 (98) | 0.169 |
| Medical history | |||
| Atrial fibrillation, n (%) | 56 (59) | 27 (61) | 0.933 |
| Coronary artery disease, n (%) | 23 (25) | 13 (30) | 0.671 |
| Prior myocardial infarction, n (%) | 13 (14) | 3 (7) | 0.371 |
| COPD, n (%) | 11 (12) | 2 (5) | 0.220 |
| Sleep apnoea, n (%) | 16 (17) | 2 (5) | 0.082 |
| Cardiovascular risk factors | |||
| Hypertension, n (%) | 87 (92) | 42 (96) | 0.117 |
| Diabetes, n (%) | 36 (38) | 16 (36) | 1.000 |
| Hypercholesterolaemia, n (%) | 64 (67) | 28 (65) | 0.948 |
| Smoking, n (%) | 45 (47) | 17 (40) | 0.502 |
| Family history of CV disease, n (%) | 23 (24) | 4 (9) | 0.070 |
| Medication | |||
| ACE inhibitor/ARB, n (%) | 62 (65) | 31 (71) | 0.681 |
| Beta‐blocker, n (%) | 63 (66) | 24 (55) | 0.252 |
| Loop diuretics, n (%) | 66 (70) | 30 (68) | 0.966 |
| Thiazide, n (%) | 21 (22) | 5 (12) | 0.212 |
| MRA, n (%) | 19 (20) | 9 (21) | 1.000 |
| Anticoagulants, n (%) | 53 (56) | 22 (50) | 0.650 |
| Antiplatelet agents, n (%) | 37 (39) | 19 (43) | 0.774 |
| Statins, n (%) | 45 (49) | 14 (33) | 0.110 |
| Echocardiography study | |||
| LA volume index (mL/m2) | 43 ± 19 | 48 ± 20 | 0.154 |
| LV EDV index (mL/m2) | 68 ± 18 | 67 ± 19 | 0.743 |
| LV ejection fraction (%) | 63 ± 8 | 62 ± 8 | 0.801 |
| E/e′ septal ratio | 18 ± 6 | 21 ± 11 | 0.047 |
| eSPAP (mmHg) | 33 ± 10 | 32 ± 10 | 0.674 |
| Severe tricuspid regurgitation | 8 (9) | 7 (16) | 0.238 |
| RV fractional area change (%) | 42 ± 8 | 40 ± 9 | 0.296 |
| TAPSE (mm) | 19 ± 5 | 18 ± 6 | 0.056 |
| Vena cava diameter (mm) | 15 ± 7 | 17 ± 5 | 0.045 |
| CMR study | |||
| LA volume index (mL/m2) | 65 ± 30 | 67 ± 27 | 0.659 |
| LV EDV index (mL/m2) | 68 ± 18 | 67 ± 19 | 0.743 |
| LV ejection fraction (%) | 62 ± 8 | 62 ± 8.3 | 0.912 |
| RV ejection fraction (%) | 58 ± 8 | 55 ± 10 | 0.198 |
| RV ejection fraction <45% | 5 (6) | 9 (26) | 0.006 |
| ECV (%) | 32 ± 4 | 34 ± 5.9 | 0.086 |
| Late gadolinium enhancement (%) | 1.4 ± 2.7 | 1.53 ± 2.22 | 0.782 |
| Biology | |||
| NT‐proBNP (pg/mL) without AF history | 1633 (502–2551) | 3667 (1871–7334) | 0.005 |
| NT‐proBNP (pg/mL) with AF history | 1580 (1054–2080) | 2401 (1464–3822) | 0.022 |
| eGFR (mL/min/1.73 m2) by CK‐EPI | 58 ± 25 | 51 ± 22 | 0.078 |
| eGFR < 60 mL/min/1.73 m2 | 52 (55) | 31 (30) | 0.116 |
| Haemoglobin (g/dL) | 12 ± 2 | 11 ± 2 | 0.006 |
| Total cholesterol (mg/dL) | 150 (124–188) | 142 (121–172) | 0.486 |
| LDL‐C (mg/dL) | 74 (54–100) | 71 (53–90) | 0.397 |
| HDL‐C (mg/dL) | 51 (41–60) | 50 (42–64) | 0.988 |
| Triglycerides (mg/dL) | 94 (74–124) | 89.5 (70.2–128) | 0.735 |
| CRP (mg/dL) | 0.60 (0.20–2.50) | 0.90 (0.50–2.03) | 0.331 |
| hsTnT (pg/mL) | 21.5 (14.0–34.0) | 33.5 (18.2–47.0) | 0.007 |
| FGF‐23 (RU/mL) | 199 (96.1–442) | 381 (189–761) | 0.001 |
| Soluble ST2 (ng/mL) | 42.5 (29.2–59.8) | 46 (33–62) | 0.481 |
| Albumin (g/L) | 39 ± 5 | 38 ± 5 | 0.180 |
| AST/ALT ratio a | 1.1 (0.9–1.3) | 1.4 (1.1–2.0) | 0.014 |
ACE, angiotensin‐converting enzyme; AF, atrial fibrillation; ALT, alanine aminotransferase; ARB, angiotensin receptor blocker; AST, aspartate transaminase; b.p.m., beats per minute; BMI, body mass index; CA 125, carbohydrate antigen 125; CK‐EPI, Chronic Kidney Disease Epidemiology Collaboration; CMR, cardiac magnetic resonance; COPD, chronic obstructive pulmonary disease; CRP, C‐reactive protein; CV, cardiovascular; ECV, extracellular volume; EDV, end‐diastolic volume; eGFR, estimated glomerular filtration rate; eSPAP, estimated systolic pulmonary artery pressure; FGF‐23, fibroblast growth factor 23; HDL‐C, high‐density lipoprotein cholesterol; HFpEF, heart failure with preserved ejection fraction; hsTnT, high‐sensitivity troponin T; IQR, inter‐quartile range; LA, left atrial; LDL‐C, low‐density lipoprotein cholesterol; LV, left ventricular; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; RV, right ventricular; ST2, suppression of tumourigenicity 2; TAPSE, tricuspid annular plane systolic excursion.
Values are reported as means ± standard deviations or medians and IQRs (IQR 0.25–0.75). Categorical variables are expressed as counts and proportions. Differences between clinical characteristics were compared using an independent t‐test, Mann–Whitney U test, or χ 2 test where appropriate.
Missing data (n = 53).
Figure 3.
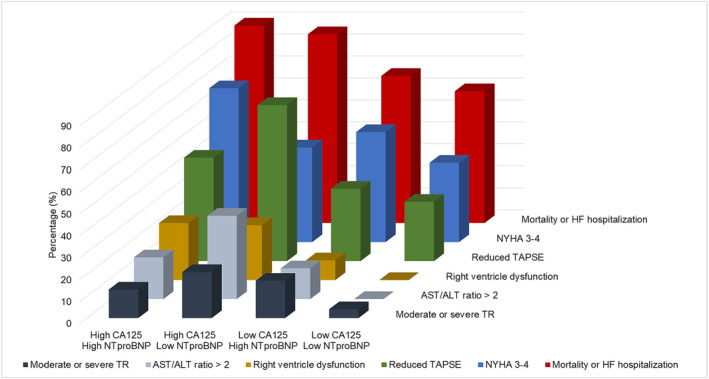
Three‐dimensional bar graph of clinical parameter repartition according to carbohydrate antigen 125 (CA 125) levels (<35 or ≥35 U/mL) and N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) levels (<1927 or ≥1927 pg/mL) in heart failure (HF) with preserved ejection fraction (HFpEF) patients. ALT, alanine aminotransferase; AST, aspartate transaminase; NYHA, New York Heart Association; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation.
Table 3.
Clinical, biological, and echocardiographic factors associated with elevated CA 125 levels in HFpEF patients by linear regression analysis
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| β | P value | β | P value | |
| Age (years) | 0.003 | 0.55 | ||
| Female | 0.160 | 0.039 | 0.062 | 0.44 |
| BMI (kg/m2) | −0.020 | <0.001 | −0.017 | 0.007 |
| NYHA Class III or IV | 0.275 | <0.001 | 0.201 | 0.010 |
| Atrial fibrillation | −0.030 | 0.70 | ||
| Diabetes | 0.044 | 0.58 | ||
| Loop diuretics | 0.021 | 0.80 | ||
| Left ventricular EF Simpson (%) | −0.004 | 0.46 | ||
| E/e′ septal ratio | 0.012 | 0.014 | 0.009 | 0.13 |
| eSPAP (mmHg) | 0.002 | 0.68 | ||
| Mean TAPSE (mm) | −0.011 | 0.133 | ||
| Extracellular volume CMR (%) | 0.020 | 0.015 | −0.002 | 0.75 |
| Right ventricular EF CMR (%) | −0.010 | 0.042 | −0.002 | 0.76 |
| eGFR (mL/min/1.73 m2) by CK‐EPI | −0.004 | 0.022 | −0.001 | 0.50 |
| Haemoglobin (g/dL) | −0.041 | 0.033 | 0.018 | 0.39 |
| FGF‐23 (log RU/mL) | 0.468 | <0.001 | 0.356 | <0.001 |
| NT‐proBNP (log pg/mL) | 0.362 | <0.001 | 0.115 | 0.25 |
BMI, body mass index; CA 125, carbohydrate antigen 125; CK‐EPI, Chronic Kidney Disease Epidemiology Collaboration; CMR, cardiac magnetic resonance; EF, ejection fraction; eGFR, estimated glomerular filtration rate; eSPAP, estimated systolic pulmonary artery pressure; FGF‐23, fibroblast growth factor 23; HFpEF, heart failure with preserved ejection fraction; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; TAPSE, tricuspid annular plane systolic excursion.
Carbohydrate antigen 125 levels and clinical outcome
Over a median follow‐up of 49 (22–64) months, 63 patients (45%) died, and 76 patients (55%) were hospitalized for HF. One patient was lost to follow‐up. Overall, 97 patients (70%) reached the primary composite endpoint of all‐cause mortality or HF hospitalization, whichever came first. Kaplan–Meier curves for the composite endpoint (Figure 4 A ), for all‐cause mortality (Figure 4 B ), and for HF hospitalization (Figure 4 C ) according to the CA 125 cut‐off showed that patients with elevated CA 125 levels displayed the worst prognosis.
Figure 4.
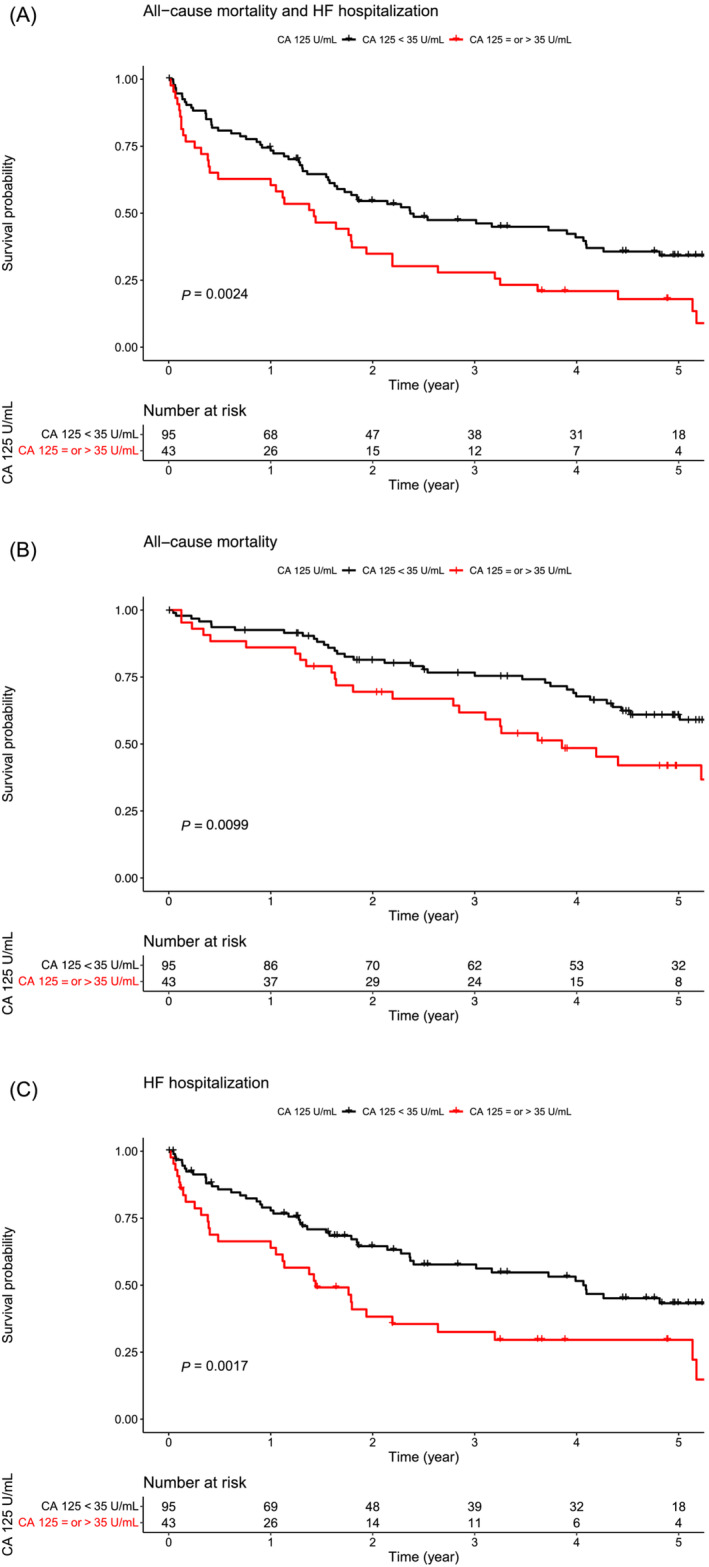
Kaplan–Meier curves for (A) the composite endpoint [all‐cause mortality and heart failure (HF) hospitalization], (B) all‐cause mortality, or (C) HF hospitalization according to the carbohydrate antigen 125 (CA 125) level cut‐off (<35 or ≥35 U/mL) in HF with preserved ejection fraction (HFpEF) patients.
In multivariate Cox regression analysis, only CA 125 levels ≥35 U/mL [hazard ratio (HR): 1.58 (1.04–2.41), P = 0.032] provided an additional prognostic value over the MAGGIC risk score [HR: 1.06 (1.02–1.10), P = 0.002] or haemoglobin level [HR: 1.73 (1.14–2.61), P = 0.010] in predicting the composite endpoint (all‐cause mortality or HF hospitalization). CA 125 levels ≥35 U/mL and continuous CA 125 levels adjusted for the MAGGIC risk score [CA 125 levels ≥35 U/mL, HR: 1.50 (1.81–2.92), P = 0.014; continuous CA 125 levels, HR: 1.65 (1.02–2.59), P = 0.045] or the haemoglobin level [CA 125 levels ≥35 U/mL, HR: 1.88 (1.18–2.99), P = 0.008; continuous CA 125 levels, HR: 1.79 (1.14–2.81), P = 0.012] were independent predictors of HF hospitalization. In contrast, NT‐proBNP levels did not provide an additional prognostic value over the MAGGIC risk score in predicting all clinical outcomes (Table 4 ).
Table 4.
Cox regression analysis of CA 125 levels ≥35 U/mL, continuous CA 125 levels, or continuous NT‐proBNP levels for the composite endpoint (all‐cause mortality and HF hospitalization), all‐cause mortality, or HF hospitalization, unadjusted, adjusted for the MAGGIC risk score, and adjusted for the haemoglobin level
| Endpoints | Unadjusted | Adjusted for the MAGGIC risk score | Adjusted for the haemoglobin level | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| All‐cause mortality and HF hospitalization | ||||||
| CA 125 ≥ 35 U/mL | 1.89 (1.24–2.81) | 0.003 | 1.58 (1.04–2.41) | 0.032 | 1.73 (1.14–2.61) | 0.010 |
| CA 125 (log U/mL) | 1.85 (1.24–2.77) | 0.002 | 1.44 (0.92–2.23) | 0.11 | 1.70 (1.13–2.56) | 0.011 |
| NT‐proBNP (log U/mL) | 1.51 (0.98–2.34) | 0.06 | 1.14 (0.72–1.81) | 0.59 | 1.43 (0.92–2.22) | 0.11 |
| All‐cause mortality | ||||||
| CA 125 ≥ 35 U/mL | 1.92 (1.16–3.18) | 0.013 | 1.50 (1.02–1.12) | 0.13 | 1.81 (1.08–3.06) | 0.026 |
| CA 125 (log U/mL) | 1.74 (1.05–2.91) | 0.033 | 1.24 (0.70–2.19) | 0.46 | 1.64 (0.96–2.78) | 0.068 |
| NT‐proBNP (log U/mL) | 2.10 (1.20–3.65) | 0.009 | 1.07 (0.88–2.79) | 0.12 | 2.06 (0.83–1.08) | 0.012 |
| HF hospitalization | ||||||
| CA 125 ≥ 35 U/mL | 2.05 (1.30–3.26) | 0.002 | 1.81 (1.13–2.92) | 0.014 | 1.88 (1.18–2.99) | 0.008 |
| CA 125 (log U/mL) | 1.96 (1.26–3.07) | 0.003 | 1.65 (1.02–2.59) | 0.045 | 1.79 (1.14–2.81) | 0.012 |
| NT‐proBNP (log U/mL) | 1.31 (0.79–2.15) | 0.30 | 1.02 (1.01–1.10) | 0.93 | 1.22 (0.74–2.01) | 0.45 |
CA 125, carbohydrate antigen 125; CI, confidence interval; HF, heart failure; HR, hazard ratio; MAGGIC, Meta‐Analysis Global Group in Chronic; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide.
Discussion
Carbohydrate antigen 125 level elevation as a marker of congestion and cardiac fibrosis in heart failure with preserved ejection fraction patients
Since the first description of the relationship between CA 125 levels and cardiac function in 1999 by Nägele et al., 27 elevated circulating CA 125 levels in HF have been proposed as a diagnostic or prognostic marker by numerous studies involving large cohorts of HF with reduced ejection fraction (HFrEF) and HFpEF patients. 7 , 13 , 28 Although the role of this biomarker in the pathophysiology of HF still remains poorly understood, it is known to positively correlate with parameters associated with right‐sided HF, such as pleural effusion, peripheral oedema, inferior vena cava diameter, and tricuspid regurgitation severity. 14 , 29 , 30 These findings were confirmed in our cohort, in which RV dysfunction markers were significantly associated with elevated CA 125 levels. Indeed, elevated CA 125 levels in congestive HF with significant fluid retention could arise from mechanical stress on mesothelial cells due to fluid buildup. Another theory suggests that HF‐related inflammatory cytokine (interleukin‐1β, interleukin‐6, and tumour necrosis factor‐α) activation could also drive heightened CA 125 production in these cells. 23 , 31 This inflammatory and congestive environment may also explain the lower haemoglobin levels in patients with high CA 125 levels. Certainly, anaemia is due to multiple factors in HFpEF, which encompass conditions related to iron deficiency, dependency on erythropoietin with chronic kidney disease, inflammation, and volume overload, probably explaining the elevated CA 125 levels. 32
Additionally, our study demonstrated for the first time in a chronic HFpEF patient population a positive correlation between CA 125 and FGF‐23 levels, a biomarker of cardiac fibrosis, 6 as well as between CA 125 levels and ECV, a CMR parameter of myocardial interstitial fibrosis. 15 We also observed a positive correlation between CA 125, hsTnT, and NT‐proBNP levels, which are associated with myocardial injury and cardiac congestion, respectively. Interestingly, very few studies have explored the link between myocardial fibrosis and increased CA 125 levels. Núñez et al. found that CA 125 interacts with galectin‐1 and galectin‐3, suggesting its role as a lectin counter‐receptor. 33 It is estimated that CA 125 might contribute to cardiac remodelling by regulating galectin activity, and potentially by affecting the intercellular matrix composition, particularly in patients with elevated CA 125 levels in decompensated HF. Our group also demonstrated that CA 125 levels were correlated to galectin‐3 and FGF‐23 in HFrEF supporting the link between CA 125 to fibrosis and cardiovascular remodelling. 34
Carbohydrate antigen 125 levels predict HF hospitalization
Our study revealed that CA 125 levels provided additional prognostic value over the MAGGIC risk score in predicting our composite endpoint of all‐cause mortality and HF hospitalization, whereas NT‐proBNP levels did not provide any additional value. Studies focusing on chronic HFpEF patients are scarce. Among these studies, Hung et al. 35 examined a small cohort of 35 women with stable HFpEF enrolled in an outpatient cardiovascular clinic. They reported that elevated CA 125 and NT‐proBNP levels were independent predictors of the risk of HF hospitalization and that the association of these markers increased their prognostic value. In a more recent study by Miñana et al., 13 a large cohort of 2369 HFpEF patients with recent admissions for acute HF was studied. The study highlighted that CA 125 and NT‐proBNP levels were independently associated with long‐term all‐cause mortality, but that only CA 125 levels were associated with the risk of cumulative readmission for acute HF. They also showed that CA 125 adjusted for haemoglobin remained a better predictor than NT‐proBNP for several clinical outcomes. This superior specificity of CA 125 levels in predicting HF hospitalization rather than all‐cause mortality in HFpEF patients could be explained by the close link between CA 125 levels and congestion. 23
In addition to the data supporting the potential benefits of CA 125 measurement in the follow‐up of stable HFpEF patients, it is noteworthy that CA 125 levels are minimally influenced by impaired renal function and are not associated with patient age, unlike NT‐proBNP and FGF‐23 levels. 36 This feature strongly argues in favour of its use, especially considering that demographics indicate that patients with HFpEF are relatively old and are therefore more likely to suffer from chronic kidney disease. Furthermore, the proportion of females is usually high in the HFpEF population, and we can expect an effect of sex on the levels of CA 125. In premenopausal females, CA 125 levels can vary according to their ovarian cycle. However, in postmenopausal females, the abnormal threshold value is set at 35 U/mL, and CA 125 may be increased in the context of mesothelial pathology. 37 We found that there was no disparity in abnormal CA 125 values between males and females with HFpEF, indicating that sex was not an independent factor contributing to elevated CA 125 levels. Because female HFpEF patients are usually elderly and postmenopausal, we can assume that higher CA 125 levels are due to HF in the absence of other pathological conditions (e.g. ovarian tumour). It is interesting to note that our data revealed an inverse association between body mass index and CA 125 levels, a finding already reported in patients with metabolic syndrome. 38 The specific explanation for this inverse correlation remains unknown, but the most convincing hypothesis suggests a volumetric dilution due to the greater volume of distribution in subjects with a high body mass index. However, the obesity paradox is well described in HF and lower body mass index is associated with a poor prognosis, perhaps due to sarcopenic obesity in advanced HFpEF. It has been demonstrated in several cohorts, including our own. 39 , 40 , 41 The increase in CA 125, an indicator of poor prognosis, is probably linked to metabolic changes in advanced disease reflected by a reduced body mass index. Thus, it is important to highlight this confounding factor and draw attention to the cautious interpretation of CA 125 levels in this subpopulation.
Carbohydrate antigen 125 in clinical practice and perspectives
Another advantage of CA 125 as a monitoring marker for patients with stable HFpEF is its significantly lower interindividual biological variability compared with natriuretic peptides. This could explain its superiority to reflect the congestive status of HF patients. Indeed, natriuretic peptides can vary greatly from one patient to another and do not vary much over time for the same patient, despite the evolution of the congestive state. In contrast, the intraindividual variability of CA 125 is very sensitive to the congestive state, making it an interesting asset for monitoring HF patients. 42 In addition, contrary to other existing tools such as FGF‐23 measurement, which is not routinely available for clinicians, or ECV measurement, which requires costly CMR, CA 125 assays are low‐cost and widely available in almost all clinical laboratories worldwide. They offer robust analytical performance, are standardized, and have been used for many years in the management of ovarian cancer. Furthermore, CA 125 exhibits remarkable stability and can be assayed in either plasma or serum, which could be coupled with natriuretic peptide measurement. In practical terms, it would be interesting to perform a combined NT‐proBNP–CA 125 assay both at the time of diagnosis and during follow‐up in HFpEF patients. Our suggestion is to focus on patients exhibiting unusually high CA 125 levels (CA 125 ≥ 35 U/mL) as they face a heightened risk of HF hospitalization. These patients could undergo more frequent monitoring. This intensified monitoring could also involve a more aggressive decongestive therapy and haemodynamic follow‐up with repeated measurements of CA 125. 42 Finally, sodium–glucose co‐transporter‐2 (SGLT2) inhibitors are now recommended in HFpEF. 3 , 4 They have demonstrated efficacy in reducing HF‐related hospital admissions and lowering CA 125 levels in HFrEF patients, likely by addressing congestion‐related issues. 21 , 43 , 44 However, to the best of our knowledge, no study evaluating the impact of SGLT2 inhibitors on CA 125 levels in HFpEF has been published yet. Additional prospective longitudinal studies are required to validate our findings and examine the impact of SGLT2 inhibitors on CA 125 levels.
Limitations
Our study has the advantage of presenting original results on a cohort of HFpEF patients and controls matched for age and sex. The multimodal approach (echocardiography and CMR) confers interest to the measurement of CA 125 in this population. However, our study has some limitations. It is a monocentric study with a Western European cohort that is moderately older and with more females than other cohorts. 39 In addition, our HFpEF cohort includes over 63% of patients on beta‐blockers, and recent data have shown that beta‐blocker use is associated with higher risk of HF hospitalization in HFpEF population. 45 However, we did not observe a prognostic interaction between beta‐blocker use and CA 125 levels in our cohort. Still, our findings are consistent with the data available in the literature, and for this reason, we have chosen to use an arbitrary threshold for CA 125 levels (35 U/mL), in line with other HF studies. Méndez et al. suggested that CA 125 levels >60 U/mL in a stable HF population were associated with a poor prognosis, which corresponds to the abnormal value calculated in our control population. 46 However, it would have been interesting to propose a CA 125 threshold associated with the risk of hospitalization and explore CA 125 cut‐offs in other large acute and stable HFpEF cohorts. In addition, patients of our cohort were enrolled before 2021 and we could not evaluate the impact of SGLT2 inhibitors therapeutic class on CA 125 levels. It would be interesting in the future to reproduce these analyses by evaluating the impact of SGLT2 inhibitors on CA 125 levels with longitudinal follow‐up.
Conclusions
Our study showed that CA 125 levels are elevated in patients with chronic HFpEF compared with age‐ and sex‐matched healthy controls. Unlike NT‐proBNP, our data demonstrated that its measurement could independently determine the risk of HF hospitalization and thus help identify patients in need of closer follow‐up. Our data support that CA 125 measurement can be of great interest in combination with traditional natriuretic peptide assays in the follow‐up of HFpEF patients.
Conflict of interest
None declared.
Funding
This work was supported by a grant from the Fonds National de la Recherche Scientifique of the Belgian government (FRS‐FNRS, Wallonia‐Brussels Federation). A.‐C.P. is a clinical master specialist at FNRS. N.M. is supported by Fondation Saint Luc for her fellowship.
Menghoum, N. , Badii, M. C. , Deltombe, M. , Lejeune, S. , Roy, C. , Vancraeynest, D. , Pasquet, A. , Gerber, B. L. , Horman, S. , Gruson, D. , Beauloye, C. , and Pouleur, A.‐C. (2024) Carbohydrate antigen 125: a useful marker of congestion, fibrosis, and prognosis in heart failure with preserved ejection fraction. ESC Heart Failure, 11: 1493–1505. 10.1002/ehf2.14699.
References
- 1. Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev 2017;3:7‐11. doi: 10.15420/cfr.2016:25:2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Paulus WJ, Zile MR. From systemic inflammation to myocardial fibrosis: The heart failure with preserved ejection fraction paradigm revisited. Circ Res 2021;128:1451‐1467. doi: 10.1161/CIRCRESAHA.121.318159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451‐1461. doi: 10.1056/NEJMoa2107038 [DOI] [PubMed] [Google Scholar]
- 4. Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med 2022;387:1089‐1098. doi: 10.1056/NEJMoa2206286 [DOI] [PubMed] [Google Scholar]
- 5. Zile MR, Jhund PS, Baicu CF, Claggett BL, Pieske B, Voors AA, et al. Plasma biomarkers reflecting profibrotic processes in heart failure with a preserved ejection fraction: Data from the Prospective Comparison of ARNI With ARB on Management of Heart Failure With Preserved Ejection Fraction Study. Circ Heart Fail 2016;9:e002551. doi: 10.1161/CIRCHEARTFAILURE.115.002551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roy C, Lejeune S, Slimani A, de Meester C, Ahn As SA, Rousseau MF, et al. Fibroblast growth factor 23: A biomarker of fibrosis and prognosis in heart failure with preserved ejection fraction. ESC Heart Fail 2020;7:2494‐2507. doi: 10.1002/ehf2.12816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Núñez J, de la Espriella R, Miñana G, Santas E, Llácer P, Núñez E, et al. Antigen carbohydrate 125 as a biomarker in heart failure: A narrative review. Eur J Heart Fail 2021;23:1445‐1457. doi: 10.1002/ejhf.2295 [DOI] [PubMed] [Google Scholar]
- 8. Scholler N, Urban N. CA125 in ovarian cancer. Biomark Med 2007;1:513‐523. doi: 10.2217/17520363.1.4.513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lloyd KO, Yin BW. Synthesis and secretion of the ovarian cancer antigen CA 125 by the human cancer cell line NIH:OVCAR‐3. Tumour Biol 2001;22:77‐82. doi: 10.1159/000050600 [DOI] [PubMed] [Google Scholar]
- 10. Kumric M, Kurir TT, Bozic J, Glavas D, Saric T, Marcelius B, et al. Carbohydrate antigen 125: A biomarker at the crossroads of congestion and inflammation in heart failure. Card Fail Rev 2021;7:e19. doi: 10.15420/cfr.2021.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sikaris KA. CA125—A test with a change of heart. Heart Lung Circ 2011;20:634‐640. doi: 10.1016/j.hlc.2010.08.001 [DOI] [PubMed] [Google Scholar]
- 12. Zhang M, Cheng S, Jin Y, Zhao Y, Wang Y. Roles of CA125 in diagnosis, prediction, and oncogenesis of ovarian cancer. Biochim Biophys Acta Rev Cancer 2021;1875:188503. doi: 10.1016/j.bbcan.2021.188503 [DOI] [PubMed] [Google Scholar]
- 13. Miñana G, de la Espriella R, Palau P, Llácer P, Núñez E, Santas E, et al. Carbohydrate antigen 125 and risk of heart failure readmissions in patients with heart failure and preserved ejection fraction. Sci Rep 2022;12:1344. doi: 10.1038/s41598-022-05328-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Soler M, Miñana G, Santas E, Núñez E, de la Espriella R, Valero E, et al. CA125 outperforms NT‐proBNP in acute heart failure with severe tricuspid regurgitation. Int J Cardiol 2020;308:54‐59. doi: 10.1016/j.ijcard.2020.03.027 [DOI] [PubMed] [Google Scholar]
- 15. Roy C, Slimani A, de Meester C, Amzulescu M, Pasquet A, Vancraeynest D, et al. Associations and prognostic significance of diffuse myocardial fibrosis by cardiovascular magnetic resonance in heart failure with preserved ejection fraction. J Cardiovasc Magn Reson 2018;20:55. doi: 10.1186/s12968-018-0477-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pieske B, Tschöpe C, de Boer RA, Fraser AG, Anker SD, Donal E, et al. How to diagnose heart failure with preserved ejection fraction: The HFA‐PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J 2019;40:3297‐3317. doi: 10.1093/eurheartj/ehz641 [DOI] [PubMed] [Google Scholar]
- 17. Messroghli DR, Rudolph A, Abdel‐Aty H, Wassmuth R, Kühne T, Dietz R, et al. An open‐source software tool for the generation of relaxation time maps in magnetic resonance imaging. BMC Med Imaging 2010;10:16. doi: 10.1186/1471-2342-10-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kellman P, Wilson JR, Xue H, Ugander M, Arai AE. Extracellular volume fraction mapping in the myocardium, part 1: Evaluation of an automated method. J Cardiovasc Magn Reson 2012;14:63. doi: 10.1186/1532-429X-14-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roy C, Slimani A, de Meester C, Amzulescu M, Pasquet A, Vancraeynest D, et al. Age and sex corrected normal reference values of T1, T2 T2* and ECV in healthy subjects at 3T CMR. J Cardiovasc Magn Reson 2017;19:72. doi: 10.1186/s12968-017-0371-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao QY, Luo JC, Su Y, Zhang YJ, Tu GW, Luo Z. Propensity score matching with R: Conventional methods and new features. Ann Transl Med 2021;9:812. doi: 10.21037/atm-20-3998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Docherty KF, McDowell K, Welsh P, Osmanska J, Anand I, de Boer RA, et al. Association of carbohydrate antigen 125 on the response to dapagliflozin in patients with heart failure. J Am Coll Cardiol 2023;82:142‐157. doi: 10.1016/j.jacc.2023.05.011 [DOI] [PubMed] [Google Scholar]
- 22. Lorenzo M, Palau P, Llàcer P, Domínguez E, Ventura B, Núñez G, et al. Clinical utility of antigen carbohydrate 125 for planning the optimal length of stay in acute heart failure. Eur J Intern Med 2021;92:94‐99. doi: 10.1016/j.ejim.2021.05.037 [DOI] [PubMed] [Google Scholar]
- 23. Huang F, Chen J, Liu Y, Zhang K, Wang J, Huang H. New mechanism of elevated CA125 in heart failure: The mechanical stress and inflammatory stimuli initiate CA125 synthesis. Med Hypotheses 2012;79:381‐383. doi: 10.1016/j.mehy.2012.05.042 [DOI] [PubMed] [Google Scholar]
- 24. Duman D, Palit F, Simsek E, Bilgehan K. Serum carbohydrate antigen 125 levels in advanced heart failure: Relation to B‐type natriuretic peptide and left atrial volume. Eur J Heart Fail 2008;10:556‐559. doi: 10.1016/j.ejheart.2008.04.012 [DOI] [PubMed] [Google Scholar]
- 25. D'Aloia A, Faggiano P, Aurigemma G, Bontempi L, Ruggeri G, Metra M, et al. Serum levels of carbohydrate antigen 125 in patients with chronic heart failure: Relation to clinical severity, hemodynamic and Doppler echocardiographic abnormalities, and short‐term prognosis. J Am Coll Cardiol 2003;41:1805‐1811. doi: 10.1016/s0735-1097(03)00311-5 [DOI] [PubMed] [Google Scholar]
- 26. Rich JD, Burns J, Freed BH, Maurer MS, Burkhoff D, Shah SJ. Meta‐Analysis Global Group in Chronic (MAGGIC) heart failure risk score: Validation of a simple tool for the prediction of morbidity and mortality in heart failure with preserved ejection fraction. J Am Heart Assoc 2018;7:e009594. doi: 10.1161/JAHA.118.009594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nägele H, Bahlo M, Klapdor R, Schaeperkoetter D, Rödiger W. CA 125 and its relation to cardiac function. Am Heart J 1999;137:1044‐1049. doi: 10.1016/S0002-8703(99)70360-1 [DOI] [PubMed] [Google Scholar]
- 28. Núñez J, Bayés‐Genís A, Revuelta‐López E, Ter Maaten JM, Miñana G, Barallat J, et al. Clinical role of CA125 in worsening heart failure: A BIOSTAT‐CHF study subanalysis. JACC Heart Fail 2020;8:386‐397. doi: 10.1016/j.jchf.2019.12.005 [DOI] [PubMed] [Google Scholar]
- 29. Yilmaz MB, Zorlu A, Tandogan I. Plasma CA‐125 level is related to both sides of the heart: A retrospective analysis. Int J Cardiol 2011;149:80‐82. doi: 10.1016/j.ijcard.2009.12.003 [DOI] [PubMed] [Google Scholar]
- 30. Sahin A, Kaya H, Avci O. Cancer antigen‐125 is a predictor of mortality in patients with pulmonary arterial hypertension. Clin Biochem 2021;89:58‐62. doi: 10.1016/j.clinbiochem.2020.12.010 [DOI] [PubMed] [Google Scholar]
- 31. Miñana G, Núñez J, Sanchis J, Bodí V, Núñez E, Llàcer A. CA125 and immunoinflammatory activity in acute heart failure. Int J Cardiol 2010;145:547‐548. doi: 10.1016/j.ijcard.2010.04.081 [DOI] [PubMed] [Google Scholar]
- 32. Pintér A, Behon A, Veres B, Merkel ED, Schwertner WR, Kuthi LK, et al. The prognostic value of anemia in patients with preserved, mildly reduced and recovered ejection fraction. Diagnostics (Basel, Switzerland) 2022;12:517. doi: 10.3390/diagnostics12020517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Núñez J, Rabinovich GA, Sandino J, Mainar L, Palau P, Santas E, et al. Prognostic value of the interaction between galectin‐3 and antigen carbohydrate 125 in acute heart failure. PLoS ONE 2015;10:e0122360. doi: 10.1371/journal.pone.0122360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gruson D, Maisin D, Pouleur AC, Ann SA, Rousseau MF. CA125, Galectin‐3 and FGF‐23 are interrelated in heart failure with reduced ejection fraction. Ejifcc 2023;34:103‐109. [PMC free article] [PubMed] [Google Scholar]
- 35. Hung CL, Hung TC, Liu CC, Wu YJ, Kuo JY, Hou CJ, et al. Relation of carbohydrate antigen‐125 to left atrial remodeling and its prognostic usefulness in patients with heart failure and preserved left ventricular ejection fraction in women. Am J Cardiol 2012;110:993‐1000. doi: 10.1016/j.amjcard.2012.05.030 [DOI] [PubMed] [Google Scholar]
- 36. Menzin AW, Kobrin S, Pollak E, Goodman DB, Rubin SC. The effect of renal function on serum levels of CA 125. Gynecol Oncol 1995;58:375‐377. doi: 10.1006/gyno.1995.1245 [DOI] [PubMed] [Google Scholar]
- 37. Nguyen HN, Jacobson A, Patino‐Paul R. New reference levels for CA125 in pre‐ and postmenopausal women. Prim Care Update Ob Gyns 1998;5:157. doi: 10.1016/S1068-607X(98)00046-8 [DOI] [PubMed] [Google Scholar]
- 38. Kim JH, Park BR, Yang WJ. Dilution effect of serum CA125 and CA19‐9 over a cutoff value, according to obesity. Int J Biol Markers 2015;30:e122‐e126. doi: 10.5301/jbm.5000110 [DOI] [PubMed] [Google Scholar]
- 39. Lejeune S, Roy C, Slimani A, Pasquet A, Vancraeynest D, Beauloye C, et al. Heart failure with preserved ejection fraction in Belgium: Characteristics and outcome of a real‐life cohort. Acta Cardiol 2021;76:697‐706. doi: 10.1080/00015385.2020.1770460 [DOI] [PubMed] [Google Scholar]
- 40. Carbone S, Lavie CJ. Disparate effects of obesity on survival and hospitalizations in heart failure with preserved ejection fraction. Int J Obes (Lond) 2020;44:1543‐1545. doi: 10.1038/s41366-020-0579-6 [DOI] [PubMed] [Google Scholar]
- 41. Kirkman DL, Bohmke N, Billingsley HE, Carbone S. Sarcopenic obesity in heart failure with preserved ejection fraction. Front Endocrinol 2020;11:558271. doi: 10.3389/fendo.2020.558271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Núñez J, Núñez E, Bayés‐Genís A, Fonarow GC, Miñana G, Bodí V, et al. Long‐term serial kinetics of N‐terminal pro B‐type natriuretic peptide and carbohydrate antigen 125 for mortality risk prediction following acute heart failure. Eur Heart J Acute Cardiovasc Care 2017;6:685‐696. doi: 10.1177/2048872616649757 [DOI] [PubMed] [Google Scholar]
- 43. de la Espriella R, Miñana G, Santas E, Núñez G, Lorenzo M, Núñez E, et al. Effects of empagliflozin on CA125 trajectory in patients with chronic congestive heart failure. Int J Cardiol 2021;339:102‐105. doi: 10.1016/j.ijcard.2021.06.045 [DOI] [PubMed] [Google Scholar]
- 44. Amiguet M, Palau P, Domínguez E, Seller J, Pinilla JMG, de la Espriella R, et al. Dapagliflozin and short‐term changes on circulating antigen carbohydrate 125 in heart failure with reduced ejection fraction. Sci Rep 2023;13:10591. doi: 10.1038/s41598-023-37491-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Arnold SV, Silverman DN, Gosch K, Nassif ME, Infeld M, Litwin S, et al. Beta‐blocker use and heart failure outcomes in mildly reduced and preserved ejection fraction. JACC Heart Fail 2023;11:893‐900. doi: 10.1016/j.jchf.2023.03.017 [DOI] [PubMed] [Google Scholar]
- 46. Méndez AB, Ordoñez‐Llanos J, Ferrero A, Noguero M, Mir T, Mora J, et al. Prognostic value of increased carbohydrate antigen in patients with heart failure. World J Cardiol 2014;6:205‐212. doi: 10.4330/wjc.v6.i4.205 [DOI] [PMC free article] [PubMed] [Google Scholar]


