Abstract
The orf162b sequence, the second open reading frame 3′ of the reaction center (RC) H protein gene puhA in the Rhodobacter capsulatus photosynthesis gene cluster, is shown to be transcribed from a promoter located 5′ of puhA. A nonpolar mutation of orf162b was generated by replacing most of the coding region with an antibiotic resistance cartridge. Although the mutant strain initiated rapid photosynthetic growth, growth slowed progressively and cultures often entered a pseudostationary phase. The amounts of the RC and light harvesting complex I (LHI) in cells obtained from such photosynthetic cultures were abnormally low, but these deficiencies were less severe when the mutant was grown to a pseudostationary phase induced by low aeration in the absence of illumination. The orf162b mutation did not significantly affect the expression of a pufB::lacZ translationally in-frame gene fusion under the control of the puf promoter, indicating normal transcription and translation of RC and LHI genes. Spontaneous secondary mutations in the strain with the orf162b disruption resulted in a bypass of the photosynthetic growth retardation and reduced the level of light harvesting complex II. These results and the presence of sequences similar to orf162b in other species indicate that the Orf162b protein is required for normal levels of the photosynthetic apparatus in purple photosynthetic bacteria.
Purple nonsulfur photosynthetic bacteria such as Rhodobacter capsulatus are capable of aerobic respiratory and anaerobic photosynthetic growth. The photosynthetic apparatus includes three membrane-bound pigment-protein complexes: the reaction center (RC), where light-dependent electron transfer is initiated; light harvesting (LH) complex I, which is adjacent to and perhaps forms a ring encircling the RC as part of the so-called core complex; and the LHII complex, which is thought to be present in multiple copies of a ring-shaped structure that interconnect core complexes (20). These complexes are located within differentiated invaginations of the cytoplasmic membrane called the intracytoplasmic membrane system (ICM), which is formed upon oxygen deprivation of cultures (12). The presence of the various photosynthetic complexes can be evaluated by their characteristic light absorption spectra, which reflect the protein environments around bacteriochlorophyll a (Bchl). For example, the Bchl's of the LHII complex of R. capsulatus absorb light of 800 and 850 nm, whereas the Bchl's of the less abundant LHI complex absorb approximately 870-nm light (13).
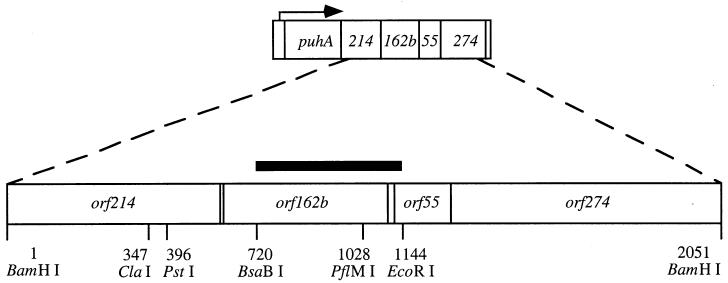
Two of the three protein subunits of the RC, designated RC L and RC M, and both protein subunits of LHI (LHI α and LHI β), are encoded by the puf operon (2). The third subunit of the RC, called RC H, is encoded by the puhA gene, which is transcribed as part of the bchFNBHLM-lhaA-puhA superoperon from two promoters, one 5′ of bchF and the other within the lhaA gene (3). As shown in Fig. 1, several open reading frames (ORFs) located 3′ of puhA and in the same transcriptional orientation have been identified on the basis of DNA sequence analysis (2). A previous publication reported that disruptions of orf214, located immediately 3′ of the puhA gene, resulted in reduced amounts of the RC and LHI complexes and abolished photosynthetic growth, and it was suggested that the Orf214 protein is an RC assembly factor (28).
FIG. 1.
The orf162b locus and flanking regions. The arrow indicates the direction of transcription from a promoter immediately 5′ of puhA. The 2-kbp BamHI fragment was cloned in pUC13, and the restriction sites shown were used to disrupt orf162b, construct a complementation plasmid, and produce a probe for RNA blot hybridization. The solid horizontal bar shows the extent of this BsaBI-to-EcoRI probe.
The orf162b sequence immediately follows orf214 as the second ORF 3′ of puhA. ORFs similar to orf162b have been found in four other species of purple photosynthetic bacteria: orf153 in Rhodobacter sphaeroides (M. Choudhary and S. Kaplan, personal communication), orf154 in Rubrivivax gelatinosus (K. Nagashima, personal communication), orfI3087 in Rhodospirillum rubrum (6), and orf168 in Rhodopseudomonas palustris (genome sequence made available by the Joint Genome Institute at http://spider.jgi-psf.org/JGI_microbial/html/rhodo_homepage.html). In all five species the ORF similar to orf162b is immediately 3′ of a homologue of orf214, which immediately follows the puhA gene, just as in R. capsulatus (2, 6, 9a; K. Nagashima, personal communication; M. Aklujkar, analysis of the R. palustris genome). The predicted protein sequences are only 43% (R. sphaeroides), 15% (R. gelatinosus), 14% (R. rubrum), and 17% (R. palustris) identical to Orf162b, but they have similar hydropathy profiles (using the Goldman-Engelman-Steitz algorithm and the TOPPRED program [10]) with a transmembrane segment near the amino terminus. None of these predicted proteins has significant sequence similarity to proteins of known function.
In this paper we present evidence that orf162b encodes a protein that is required for optimal photosynthetic growth, and that a disruption of orf162b reduces the amounts of RC and LHI in oxygen-deprived and photosynthetically grown cells. This phenotype is complemented in trans by a plasmid-borne copy of orf162b. We also demonstrate that transcription of orf162b is abolished by insertion of a transcription termination sequence into the puhA gene, and that the orf162b mutant phenotype is suppressed by spontaneous mutations that reduce the amount of LHII.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The photosynthetically wild-type R. capsulatus strain SB1003 (25), the gene transfer agent (GTA) overproducer strain DE442 (29), and the puhA polar mutant DW1 (28) have been described previously. Escherichia coli C600 r− m+ (7) and DH5α (Life Technologies, GIBCO BRL) were used for the construction and maintenance of plasmids; strains HB101(pRK2013) and TEC5 (11, 26) were used in E. coli-to-R. capsulatus conjugations. Plasmids pXCA601::935, pUC13, and pRR5C have been described previously (1, 19, 30). Plasmid pUC4KIXX was from Pharmacia Biotech, Inc. Bacterial strains and plasmids produced in this study are described below.
Growth conditions and media.
E. coli strains were grown in Luria-Bertani (LB) medium (22) or on LB agar plates. Highly aerated and oxygen-deprived cultures of R. capsulatus were grown at 30°C without illumination in RCV medium (5), in Erlenmeyer flasks filled to 20 or 80% of nominal capacity, respectively, and shaken at 300 or 150 rpm, respectively. Highly aerated cultures did not enter the stationary phase until reaching about 300 Klett units (see below), whereas the growth of oxygen-deprived cultures began to slow at about 80 Klett units. Photosynthetic cultures were grown anaerobically in screw-cap tubes (20 ml) or Roux bottles (800 ml) inoculated from oxygen-deprived cultures and filled with RCV, or on RCV agar plates placed in BBL GasPak anaerobic jars (Becton Dickinson & Co.). Photosynthetic cultures were incubated at 30°C in an aquarium filled with water and illuminated by tungsten filament incandescent lamps at a light intensity of 150 microeinsteins/m2/s, measured with a photometer equipped with the LI-190SB quantum sensor (LI-COR Inc.). Culture turbidity was monitored with a Klett-Summerson photometer equipped with a red (no. 66) filter (100 Klett units = 3.3 × 108 CFU/ml). Antibiotic-resistant E. coli and R. capsulatus strains were selected with 25 and 10 μg of kanamycin/ml, 10 and 0.5 μg of tetracycline/ml, and 10 and 2 μg of gentamicin/ml, respectively.
DNA techniques.
Recombinant DNA procedures were carried out essentially as described previously (22). Plasmid DNA was isolated from cells and from agarose gels with kits from QIAGEN. Conjugative transfer of plasmids from E. coli to R. capsulatus was performed by mixing of overnight cultures of donor, helper, and recipient cells in a 1:1:1 ratio by volume; centrifugation at 15,000 × g for 2 min; resuspension in an equal volume of RCV; and incubation of a 10-μl aliquot adsorbed onto an RCV agar plate overnight at 30°C. Donor cells were absent from the negative controls. Cells from each spot were streaked onto RCV agar plates containing the appropriate antibiotics. Transconjugant colonies were streaked onto YPS agar (22) plates to ensure the absence of E. coli donors.
Construction of the nonpolar orf162b mutant SBK1.
A 2-kbp DNA fragment from BamHI digestion of the R′ plasmid pBLM2 (26) containing the orf162b gene and flanking sequences (Fig. 1) was inserted into the BamHI site of pUC13, and 63% of the coding sequence of orf162b was eliminated by digestion with BsaBI and PflMI. T4 DNA polymerase was used to resect single-stranded protruding 3′ ends from this deletion, and a NotI site in the linker (5′ AGCGGCCGCT 3′) was inserted by linker tailing (22); the resultant plasmid was cut at this NotI site, and the ends were filled in with the Klenow fragment. A 1.3-kbp SmaI fragment containing the kanamycin resistance gene and the first 153 bp of the bleomycin resistance gene from pUC4KIXX was ligated into the filled-in NotI site such that the transcriptional orientation of these genes is the same as in orf162b. This fragment does not usually terminate transcription (8), and its nonpolar effect was confirmed by genetic complementation (see Results). E. coli TEC5 was transformed with the plasmid carrying the deleted, kanamycin resistance-marked orf162b gene. Homologous recombination of this pUC-derived plasmid with the conjugative plasmid pDPT51 in E. coli TEC5 permitted conjugative transfer of kanamycin resistance from TEC5 to R. capsulatus strain DE442. DE442 overproduces phage-like GTA particles containing random 4.6-kbp linear fragments of genomic DNA (29), which were used to replace the wild-type orf162b gene in SB1003 by transduction, producing strain SBK1.
Construction of the complementation plasmid pAH8.
A pUC13 construct containing the ClaI-to-EcoRI segment including orf162b (Fig. 1) was digested with PstI, and an EcoRI site in the linker (5′ CCGAATTCGG 3′) was ligated into this site (made blunt with T4 DNA polymerase) by linker tailing. The 748-bp EcoRI fragment containing orf162b was excised from the resultant plasmid and ligated into the vector pRR5C (30). The resultant plasmid, called pAH8, which contains the orf162b gene transcribed from the puf promoter, was used to restore expression of orf162b in mutant backgrounds.
RNA blots.
Aerobic and oxygen-limited cultures were grown to a density of 100 Klett units, and RNA was isolated from 25 ml of each culture using the RNeasy kit (QIAGEN). Samples were treated with 30 U of DNase I in 100 mM sodium acetate–5 mM MgSO4 (pH 5.0) for 30 min at room temperature, followed by phenol-chloroform extraction and ethanol precipitation. Seven micrograms of each RNA sample was used for formaldehyde gel electrophoresis (16) and electroblotted onto a nylon membrane (ICN) for 2 h at 80 V in 0.5× Tris-borate-EDTA (TBE). The membrane was baked at 80°C for 2 h, placed on a nylon mesh, and prehybridized in 10 ml of 50% formamide–10% dextran sulfate–5.8% NaCl–1% sodium dodecyl sulfate (SDS)–0.2% bovine serum albumin (BSA)–0.2% Ficoll–0.2% polyvinylpyrrolidone–0.1 sodium pyrophosphate–50 mM Tris HCl (pH 7.5) with 0.1 mg of sheared salmon sperm DNA (Sigma)/ml at 42°C for 3 h with rotation (in an oven from BIO/CAN Scientific). The probe was a gel-purified DNA fragment extending from the BsaBI site in orf162b to the EcoRI site in orf55 (Fig. 1), labeled with 32P by using the Redi-Prime kit (Amersham Pharmacia Biotech) for 2 h. After 16 h of hybridization, the membrane was washed with agitation twice with 100 ml of 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) for 10 min at room temperature, twice with 100 ml of 2× SSC containing 1% SDS for 15 min at 60°C, and once with 100 ml of 0.1× SSC for 15 min at room temperature. Hybridization signals were detected with film (Kodak).
β-Galactosidase assays.
Cells from oxygen-deprived R. capsulatus cultures at 80 or 165 Klett units, and photosynthetic cultures at 125 Klett units, were harvested by centrifugation. Pellets from 40 ml of culture were stored at −80°C before assay as previously described (18).
Chromatophore isolation.
Oxygen-deprived and photosynthetic cultures grown to 150 Klett units were centrifuged, and the cell pellets were stored at −80°C. The cells were resuspended in 20 ml of chromatophore buffer [20 mM 3-(N-morpholino)propanesulfonate (MOPS), 100 mM KCl, 1 mM MgCl2 (pH 7.2)] and passed through a French press three times. Cell debris was removed by centrifugation at 12,000 × g for 15 min, and the supernatant was centrifuged at 412,000 × g for 14 min to pellet membrane vesicles (chromatophores), which were resuspended in chromatophore buffer. An aliquot stored at 4°C was used for flash spectroscopy, and the remainder was stored at −80°C and used for protein electrophoresis.
Spectroscopy.
Absorption spectroscopy of intact cells was performed as described previously (17), and data were collected with the Spectra Calc software package (Galactic Industries Corporation). Light scattering at 650 nm was used to normalize the spectra. Low-temperature absorption spectroscopy used a Hitachi 557 double-beam spectrophotometer. Chromatophores in chromatophore buffer (see above) were mixed with an equal volume of anhydrous glycerol and frozen in liquid nitrogen. Spectra were obtained with the samples chilled by, but not immersed in, liquid nitrogen. Flash spectroscopy was carried out as previously described (17).
SDS-PAGE.
The amount of protein in each chromatophore preparation was determined by a modified Lowry method, with bovine serum albumin as the standard (21). Samples containing 40 μg of protein were mixed with loading buffer, heated at 50°C for 10 min, and used in a Tricine-SDS-polyacrylamide gel electrophoresis (PAGE) system (23). Gels were stained in a solution of 0.025% Coomassie brilliant blue G-250 in 40% methanol and 10% acetic acid and were destained in the same solution lacking the dye.
RESULTS
orf162b is transcribed as part of the puhA superoperon.
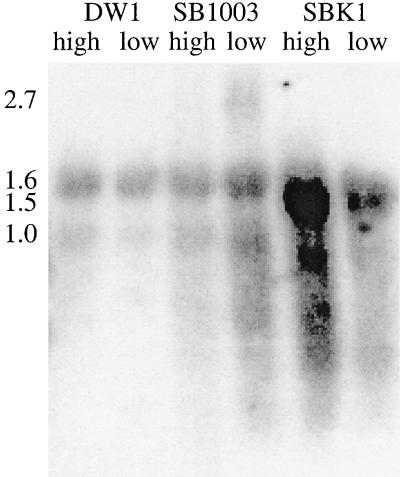
Hybridization of RNA blots with an orf162b-specific probe (Fig. 1) showed that orf162b is transcribed to produce messages at higher levels in oxygen-deprived than in highly aerated SB1003 cells (Fig. 2); transcripts were also detected in photosynthetically grown cultures (data not shown). The largest RNA species detected was approximately 2.7 kb in size and was distinct from a smear of less than 1.0 kb, indicating that degradation of the primary transcript is rapid. The probe also bound to molecules of 1.5 kb from SBK1 cultures (attributed to transcription originating at the kanamycin resistance cartridge promoter, which would be complementary to the 3′ end of the probe [Fig. 1]). There was nonspecific hybridization of the probe to rRNA in all six samples (sizes of 1.6 and 1.0 kb) (31). However, no 2.7-kb species or low-molecular-weight smears were detected in lanes containing RNA from strain DW1, in which transcription is terminated at the Ω cartridge inserted into puhA (28). Therefore, orf162b is cotranscribed with the puhA and orf214 genes and is part of a bchFNBHLM-lhaA-puhA-orf214-orf162b superoperon (4).
FIG. 2.
Blot of R. capsulatus RNA hybridized with the orf162b probe (Fig. 1). RNA was isolated from highly aerated and oxygen-deprived cultures of DW1, SB1003, and SBK1. The approximate sizes of relevant signals (in kilobases) are given on the left.
Photosynthetic growth kinetics of the orf162b mutant and complemented strains.
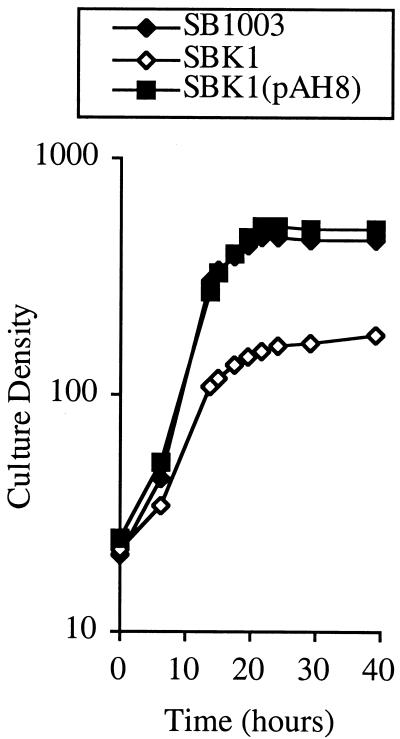
The partial deletion and KIXX disruption of orf162b in strain SBK1 impaired photosynthetic growth, as evidenced by a progressive slowing of growth and often a premature stationary phase compared to the growth of SB1003 (Fig. 3). Complementation of strain SBK1 with the orf162b gene borne on plasmid pAH8 resulted in a photosynthetic growth pattern similar to that of the parental strain. Thus it seems that orf162b transcripts are translated, the resultant Orf162b protein is required to sustain rapid photosynthetic growth, and the wild-type allele is dominant over the disrupted orf162b allele.
FIG. 3.
Effect of orf162b mutation and trans complementation with pAH8 on the photosynthetic growth of R. capsulatus strains.
Evaluation of the presence and function of RC and LH complexes by SDS-PAGE and spectroscopy.
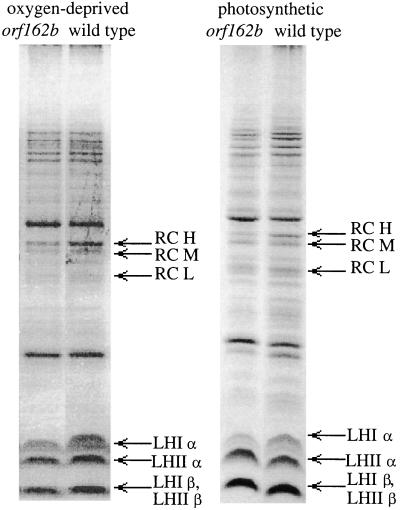
The levels of RC and LH proteins in chromatophores isolated from oxygen-deprived (dark) and anaerobic photosynthetic (illuminated) cultures of strains SBK1 and SB1003 were compared by SDS-PAGE (Fig. 4). In both modes of growth, the orf162b mutant strains had lower levels of the RC proteins H, M and L, and was deficient in the LHI α protein. The level of the LHI β protein could not be ascertained due to its comigration with the LHII β protein. The levels of the LHII α and β proteins were not greatly affected by the orf162b mutation.
FIG. 4.
Effects of the orf162b mutation on RC and LHI protein levels in chromatophores isolated from oxygen-deprived or photosynthetic cultures of SBK1 (orf162b mutant) compared to SB1003 (wild type), as revealed by SDS-PAGE.
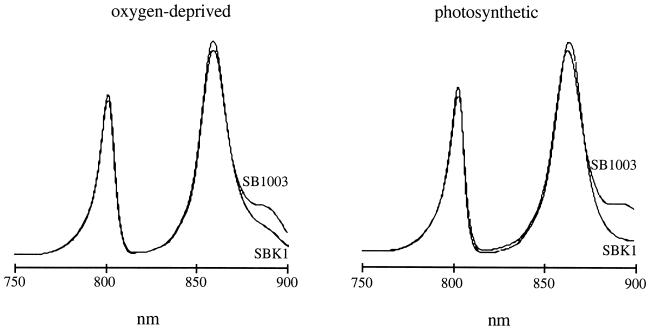
Room temperature absorption spectra of SBK1 intact cells indicated a decrease in the 870-nm LHI shoulder on the 850-nm LHII peak (not shown). Absorption spectra at 77 K (liquid N2) of chromatophores isolated from oxygen-deprived and photosynthetic cultures of SBK1 and SB1003 clearly revealed a significant decrease in the LHI 870-nm absorption (shoulder of the LHII 850-nm peak in Fig. 5). These spectra show that the level of the LHI complex is lower in the orf162b mutant than in the parental strain SB1003, and that the difference is more pronounced in photosynthetically grown cells. The LHII peaks at 800 and 850 nm were marginally increased by the orf162b mutation in these samples, which were normalized on the basis of Bchl content.
FIG. 5.
Low-temperature absorption spectra of chromatophores from SB1003 and SBK1 cultures grown under either oxygen-deprived or photosynthetic conditions.
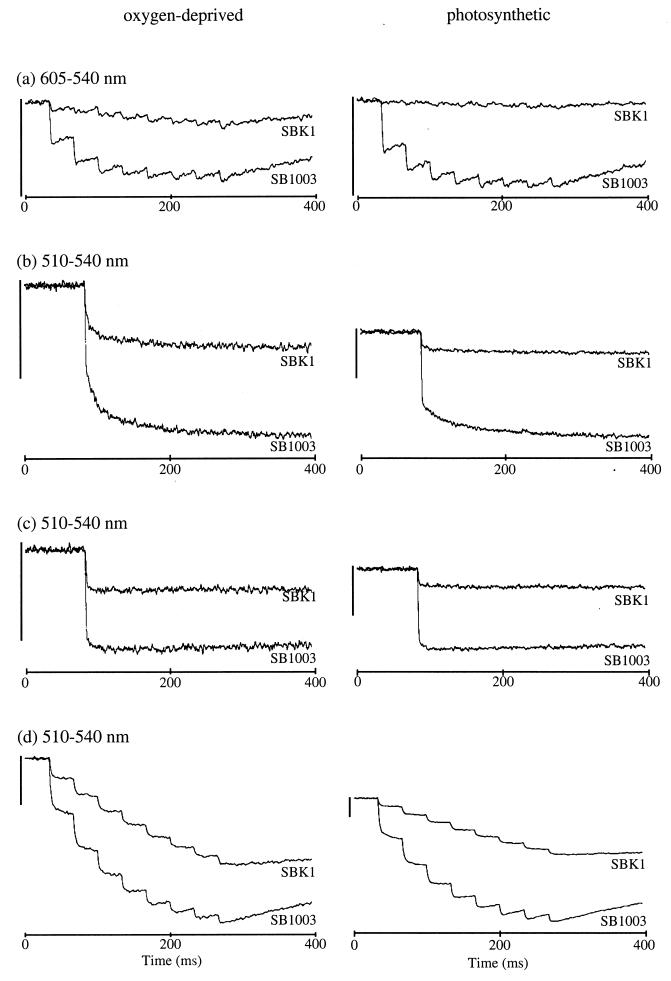
The magnitude of the SBK1 RC Bchl special pair bleaching in response to a train of eight flashes of actinic light was greatly reduced in this orf162b mutant relative to SB1003 (Fig. 6a). These and other experiments indicate that strain SBK1 contains approximately 33% (in oxygen-deprived cells) or 3% (in cells from photosynthetically grown cultures in premature stationary phase) of the level of RCs in SB1003 cells grown to the same cell density under identical conditions, based on the extent of RC bleaching after the eighth flash.
FIG. 6.
Flash spectroscopy analysis of chromatophores from SB1003 and SBK1 strains. (a) The amount of functional RC. (b) Single-flash carotenoid bandshift without antimycin. (c) Single-flash carotenoid bandshift with antimycin. (d) Carotenoid bandshift over eight flashes without antimycin. The vertical bars on the left represent 0.00435 absorbance units at the wavelength pairs indicated.
The carotenoid bandshift of R. capsulatus, like that of R. sphaeroides, is an intrinsic monitor of transmembrane potentials that change in response to light-driven electron and proton translocation (15). In concert with the measurements of the RC, the carotenoid bandshift after a single flash was smaller in the orf162b-disrupted strain SBK1 than in SB1003, both in the absence (Fig. 6b) and in the presence (Fig. 6c) of antimycin, which inhibits the cytochrome b/c1 complex (27). All three phases of the bandshift were apparent in the single-flash absorbance changes of the deletion strain in the absence of antimycin (Fig. 6b), indicating that functional RCs were connected appropriately to the cytochrome b/c1 complex. Multiple flashes resulted in successive turnovers of the RC and progressively greater transmembrane potentials, but to a lesser extent in SBK1 than in SB1003, and the difference was more pronounced in chromatophores from cells grown photosynthetically (Fig. 6d).
In summary, the flash-induced spectral changes indicate that the RC of SBK1 cells is present in only small amounts, but that neither the catalytic activity of the RC nor the quinone transfer from the RC to the b/c1 complex is impaired. The spectroscopic data, like the SDS-PAGE data, show that this mutation reduces the amounts of the RC and LHI complexes, and reveal a more severe effect of the orf162b disruption after an initial period of rapid growth under photosynthetic conditions.
pufB (LHI β) gene fusion expression in the orf162b mutant strain SBK1.
To evaluate whether the reduction in LHI and RC levels in SBK1 might be due to decreased expression of the puf genes that encode LHI and two of the three RC proteins, the β-galactosidase activities expressed from a translationally in-frame pufB::lacZ gene fusion, which is transcribed from the puf promoter in plasmid pXCA601::935, in strains SBK1(pXCA601::935) and SB1003(pXCA601::935) were determined. During oxygen-deprived growth, the SBK1(pXCA601::935) β-galactosidase activities ranged from 84 to 95% of the SB1003(pXCA601::935) values, and during photosynthetic growth the relative activity was 155%. We conclude that neither initiation of transcription from the puf promoter nor initiation of translation of the LHI β protein is significantly decreased by the orf162b mutation, and that the Orf162b protein functions posttranslationally to yield appropriately large amounts of RC and LHI complexes.
Suppression of the orf162b phenotype.
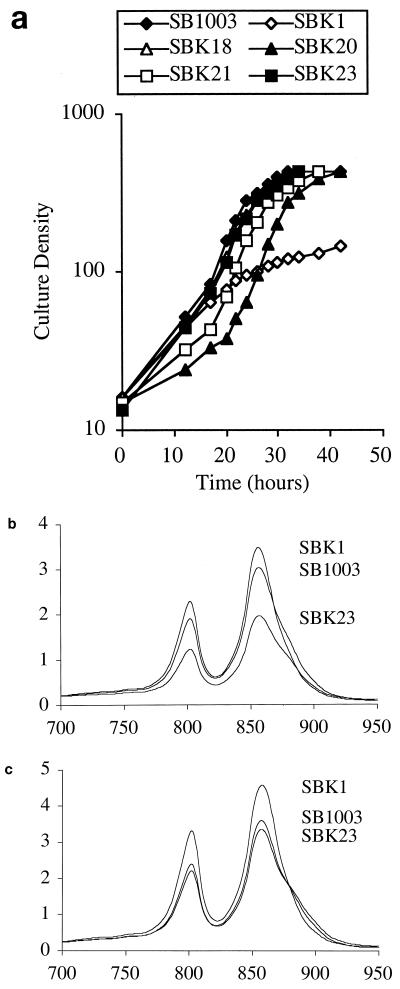
An agar plate spread with about 106 SBK1 cells that had reached premature stationary phase during photosynthetic growth was incubated under photosynthetic growth conditions, and about 150 colonies formed within 2 to 4 days. Four strains in which the orf162b photosynthetic growth phenotype was thus suppressed (designated SBK18, SBK20, SBK21, and SBK23) were isolated from these colonies. When grown photosynthetically in liquid culture, these suppressor strains did not exhibit a premature slowing of growth, in contrast to the SBK1 parental strain (Fig. 7a). During growth under oxygen-deprived conditions, all four suppressor strains were found to contain less of the LHII complex than did SBK1 and the parental strain SB1003, as indicated by decreases in the 800- and 850-nm peaks of room temperature absorption spectra of intact cells (Fig. 7b; for brevity, only SBK23 data are shown). When grown photosynthetically to the density at which SBK1 enters the characteristic premature stationary phase, SBK18 and SBK21 contained less LHII than SBK1 and SB1003; the level of LHII in SBK20 and SBK23 was comparable to that in SB1003 but less than that in SBK1 (Fig. 7c). In the stationary phase of photosynthetic growth, all four suppressor strains had approximately the same level of LHII as SB1003 (not shown). The spectra in Fig. 7b and c also show that SBK1 contains more LHII than SB1003 when samples are normalized to equivalent cell numbers. The frequency of suppressor mutations (ca. 1.5 × 10−4) and the different effects on LHII levels indicate that the orf162b mutant phenotype is suppressed by several types of second-site mutations.
FIG. 7.
(a) Photosynthetic growth of suppressor strains SBK18, SBK20, SBK21, and SBK23, compared to growth of SB1003 and SBK1. (b and c) Absorption spectra (normalized to light scattering at 650 nm) of intact cells from oxygen-deprived (b) and photosynthetic (c) cultures of the representative suppressor strain SBK23 against those of the wild-type SB1003 and orf162b mutant SBK1 strains. The vertical axes give absorbance units, and the horizontal axes give wavelengths (in nanometers).
DISCUSSION
As noted above, in the five species of purple photosynthetic bacteria for which DNA sequence data are available, the puhA gene is followed by orf214-like and orf162b-like ORFs in the same transcriptional orientation as puhA. In R. capsulatus, genetic experiments indicated that puhA and orf214 are cotranscribed with at least one 3′ gene (28), and similar conclusions were reached as a result of puhA disruption experiments on R. sphaeroides (9, 24). Our RNA blots provide biochemical evidence that synthesis of orf162b messages depends on transcription that initiates 5′ of puhA from either or both of the promoters located within the lhaA gene and 5′ of the bchF gene. Such a long transcript was not detected (Fig. 2), and so we suggest that degradation of segments of these primary transcripts is so rapid that such long molecules are undetectable in blot hybridizations, by analogy to the R. capsulatus crtEF-bchXYZ-pufQBALMX superoperon (1, 4). This rapid degradation may explain the extremely small amounts of the orf162b 2.7-kb RNA segment detected with the orf162b probe, and why Bauer et al. (3) did not detect an orf162b precursor RNA molecule in blots probed with a puhA-specific probe. This interpretation implies that the bchFNBHLM-lhaA-puhA-orf214 superoperon of R. capsulatus (28) includes orf162b, which suggests a physiological connection among the puhA, orf214, and orf162b genes.
Hybridization of the orf162b probe to a 1.5-kb transcript in SBK1 suggests that the 3′ region of the disrupted orf162b gene is transcribed from the kanamycin resistance cartridge promoter. The reason why this transcript was more abundant in highly aerated SBK1 cultures is not known. This transcription may or may not cause overexpression of downstream ORFs, which have no known function at present. However, such hypothetical overexpression could not be responsible for the impaired photosynthetic growth of strain SBK1, because plasmid pAH8 (which expresses orf162b from the puf promoter) restores normal growth, indicating that the Orf162b protein is produced in wild-type R. capsulatus and is required for optimal photosynthetic growth.
Disruption of orf162b leads to impaired photosynthetic growth, typically exhibited as a premature stationary phase. Our spectroscopic and SDS-PAGE data demonstrate that SBK1 is deficient in the RC and LHI proteins. These findings indicate that the function of Orf162b is to promote the expression, assembly, or stabilization of these pigment-protein complexes. The presence or absence of the Orf162b protein did not significantly reduce initiation of transcription from the puf promoter or translation of a pufB::lacZ fusion, and so the effect of Orf162b on the LHI and the RC complexes appears to be manifested posttranslationally. We propose that Orf162b enhances the assembly or stability of RC-LHI core complexes.
The impaired photosynthetic growth of SBK1 was overcome by frequent spontaneous secondary mutations that led to a decrease in the amount of LHII. This might indicate that the premature slowing of growth is due to lower levels of the RC and LHI complexes caused by an increase in the ratio of LHII to LHI-RC core complexes, rather than a simple decrease in the amounts of the core complex. It is conceivable that the Orf162b protein provides an optimal arrangement of complexes within the ICM of R. capsulatus such that, in the absence of Orf162b, LHII mixes with the core complex and interferes with assembly. Alternatively, Orf162B may function to remove a growth-inhibiting factor associated with excess LHII. Regardless, the orf162b and suppressor mutant phenotypes suggest that the function of Orf162b relates to all three complexes of the photosynthetic apparatus. Because an orf162b-like ORF is present in R. rubrum, which lacks LHII, and because we have observed an orf162b mutant phenotype in an LHII− R. capsulatus background (14a), we suggest that the effects of orf162b disruption on LHII are secondary to a primary effect on the RC and LHI.
The core complex deficiencies of SBK1 were more pronounced when photosynthetic growth slowed after a brief period of rapid cell division than when growth of this mutant had slowed due to oxygen deprivation, and so this low-oxygen growth condition appears to compensate in some measure for the loss of Orf162b function. We entertained the possibility that the presence of oxygen allows the oxidation of a cofactor required for photosynthesis and that Orf162b catalyzes this oxidation under anaerobic conditions; however, inclusion of the alternative electron acceptor dimethyl sulfoxide (14) in the medium of photosynthetic cultures did not compensate for orf162b disruption (unpublished data). We favor an alternative hypothesis, that the low rate of cell division (and ICM synthesis) in the pseudostationary phase of oxygen-limited cultures allows the inefficient accumulation of core complexes in the ICM, whereas cell division upon transfer to anaerobic, illuminated conditions of growth is too rapid for commensurate accumulation of core complexes, leading to a gradual decrease in the number of core complexes per cell so that rapid growth cannot be sustained. This model will be investigated in future experiments.
We suggest that the ORFs in R. sphaeroides, R. gelatinosus, R. rubrum, and R. palustris that resemble orf162b also encode proteins which optimize the levels of RC-LHI core complexes and that these ORFs are genes that are cotranscribed with puhA. If these Orf162b-like sequences found in different species are indeed homologous, they exhibit remarkable evolutionary divergence. A predicted transmembrane domain common to the N-terminal regions of all five sequences suggests that membrane attachment and hence the proximity of Orf162b to the membrane-bound photosynthetic apparatus may be important for the accumulation of RC-LHI core complexes in the ICM. This function may require Orf162b and related proteins to associate intimately with other proteins that have evolved divergently in different species, but in parallel with them within each species. Further studies of Orf162b function will be accompanied by analyses of the orf153 locus in R. sphaeroides, the orf154 locus in R. gelatinosus, the orfI3087 locus in R. rubrum, and the orf168 locus in R. palustris to assess whether these ORFs are functionally equivalent to the R. capsulatus orf162b gene.
In conclusion, our analyses of the orf162b locus revealed that it encodes a protein that affects the relative levels of the RC, LHI, and LHII complexes and is required for optimal photosynthetic growth of R. capsulatus. The transcriptional coregulation of orf162b with the puhA gene in R. capsulatus and the presence of ORFs similar to orf162b at the same chromosomal location relative to puhA in other species suggest that Orf162b-like proteins perform a similar function in a variety of purple photosynthetic bacteria.
ACKNOWLEDGMENTS
We thank W. Collins for construction of the pUC13::BamHI plasmid and C. Harwood, S. Kaplan, and K. Nagashima for provision of unpublished data.
M.A. was supported in part by a fellowship from UBC, and this research was funded by NSERC (Canada) grant 5-82796 to J.T.B.
REFERENCES
- 1.Adams C W, Forrest M E, Cohen S N, Beatty J T. Structural and functional analysis of transcriptional control of the Rhodobacter capsulatus puf operon. J Bacteriol. 1989;171:473–482. doi: 10.1128/jb.171.1.473-482.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alberti M, Burke D E, Hearst J E. Structure and sequence of the photosynthetic gene cluster. In: Blankenship R E, Madigan M T, Bauer C E, editors. Anoxygenic photosynthetic bacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 1083–1106. [Google Scholar]
- 3.Bauer C E, Buggy J, Yang Z, Marrs B L. The superoperonal organization of genes for pigment biosynthesis and reaction center proteins is a conserved feature in R. capsulatus: analysis of overlapping bchB and puhA transcripts. Mol Gen Genet. 1991;228:438–444. doi: 10.1007/BF00260637. [DOI] [PubMed] [Google Scholar]
- 4.Beatty J T. Organization of photosynthesis gene transcripts. In: Blankenship R E, Madigan M T, Bauer C E, editors. Anoxygenic photosynthetic bacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 1209–1219. [Google Scholar]
- 5.Beatty J T, Gest H. Generation of succinyl-coenzyme A in photosynthetic bacteria. Arch Microbiol. 1981;129:335–340. [Google Scholar]
- 6.Bérard J, Gingras G. The puh structural gene coding for the H subunit of the Rhodospirillum rubrum photoreaction center. Biochem Cell Biol. 1991;69:122–131. doi: 10.1139/o91-019. [DOI] [PubMed] [Google Scholar]
- 7.Bibb M J, Cohen S N. Gene expression in Streptomyces: construction and application of promoter-probe plasmid vectors in Streptomyces lividans. Mol Gen Genet. 1982;187:265–277. doi: 10.1007/BF00331128. [DOI] [PubMed] [Google Scholar]
- 8.Bollivar D W, Suzuki J Y, Beatty J T, Dobrowski J M, Bauer C E. Directed mutational analysis of bacteriochlorophyll a biosynthesis in Rhodobacter capsulatus. J Mol Biol. 1994;237:622–640. doi: 10.1006/jmbi.1994.1260. [DOI] [PubMed] [Google Scholar]
- 9.Chen X-Y, Yurkov V, Paddock M L, Okamura M Y, Beatty J T. A puhA gene deletion and plasmid complementation system for facile site directed mutagenesis of the reaction center H protein of Rhodobacter sphaeroides. Photosyn Res. 1998;55:369–373. [Google Scholar]
- 9a.Choudhary M, Kaplan S. DNA sequence analysis of the photosynthesis region of Rhodobacter sphaeroides 2.4.1T. Nucleic Acids Res. 2000;28:862–867. doi: 10.1093/nar/28.4.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Claros M G, von Heijne G. TopPred II, version 1.1: prediction of transmembrane segments in integral membrane proteins, and the putative topologies. CABIOS. 1995;10:685–686. [Google Scholar]
- 11.Ditta G, Schmidhauser T, Yakobsen E, Lu P, Liang X-W, Finlay D R, Guiney D, Helinski D R. Plasmids related to the broad host range vector, pRK290, useful for gene cloning and for monitoring gene expression. Plasmid. 1985;13:149–153. doi: 10.1016/0147-619x(85)90068-x. [DOI] [PubMed] [Google Scholar]
- 12.Drews G, Golecki J R. Structure, molecular organization, and biosynthesis of membranes of purple bacteria. In: Blankenship R E, Madigan M T, Bauer C E, editors. Anoxygenic photosynthetic bacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 231–257. [Google Scholar]
- 13.Feick R, van Grondelle R, Rijgersberg C P, Drews G. Fluorescence emission by wild-type and mutant strains of Rhodopseudomonas capsulata. Biochim Biophys Acta. 1980;593:241–253. doi: 10.1016/0005-2728(80)90062-6. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson S J, Jackson J B, McEwan A G. Anaerobic respiration in the Rhodospirillaceae: characterization of pathways and evaluation of roles in redox balancing during photosynthesis. FEMS Microbiol Rev. 1987;46:117–143. [Google Scholar]
- 14a.Harmer A L. University of British Columbia, Vancouver, British Columbia, Canada. 1998. M. Sc. thesis. [Google Scholar]
- 15.Jackson J B, Crofts A R. The high energy state in chromatophores from Rhodopseudomonas sphaeroides. FEBS Lett. 1969;4:185–189. doi: 10.1016/0014-5793(69)80230-9. [DOI] [PubMed] [Google Scholar]
- 16.LeBlanc H, Lang A S, Beatty J T. Transcript cleavage, attenuation, and an internal promoter in the Rhodobacter capsulatus puc operon. J Bacteriol. 1999;181:4955–4960. doi: 10.1128/jb.181.16.4955-4960.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lilburn T G, Haith C E, Prince R C, Beatty J T. Pleiotropic effects of pufX gene deletion on the structure and function of the photosynthetic apparatus of Rhodobacter capsulatus. Biochim Biophys Acta. 1992;1100:160–170. doi: 10.1016/0005-2728(92)90077-f. [DOI] [PubMed] [Google Scholar]
- 18.Manoil C. Analysis of membrane protein topology using alkaline phosphatase and β-galactosidase. Methods Cell Biol. 1991;34:61–75. doi: 10.1016/s0091-679x(08)61676-3. [DOI] [PubMed] [Google Scholar]
- 19.Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- 20.Papiz M Z, Prince S M, Hawthornthwaite-Lawless A M, McDermott G, Freer A A, Isaacs N W, Cogdell R J. A model for the photosynthetic apparatus of purple bacteria. Trends Plant Sci. 1996;1:198–206. [Google Scholar]
- 21.Peterson G. Determination of total protein. Methods Enzymol. 1983;91:95–119. doi: 10.1016/s0076-6879(83)91014-5. [DOI] [PubMed] [Google Scholar]
- 22.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 23.Schägger H, von Jagow H. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 24.Sockett R E, Donohue T J, Varga A R, Kaplan S. Control of photosynthetic membrane assembly in Rhodobacter sphaeroides mediated by puhA and flanking sequences. J Bacteriol. 1989;171:436–446. doi: 10.1128/jb.171.1.436-446.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solioz M, Marrs B. The gene transfer agent of Rhodopseudomonas capsulata. Arch Biochem Biophys. 1977;181:300–307. doi: 10.1016/0003-9861(77)90508-2. [DOI] [PubMed] [Google Scholar]
- 26.Taylor D P, Cohen S N, Clark W G, Marrs B L. Alignment of genetic and restriction maps of the photosynthesis region of the Rhodopseudomonas capsulata chromosome by a conjugation-mediated marker rescue technique. J Bacteriol. 1983;154:580–590. doi: 10.1128/jb.154.2.580-590.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van den Berg W H, Prince R C, Bashford C L, Takamiya K, Bonner W D, Dutton P L. Electron and proton transport in the ubiquinone-cytochrome b-c2 oxidoreductase of Rhodopseudomonas sphaeroides: patterns of binding and inhibition by antimycin. J Biol Chem. 1979;254:8594–8604. [PubMed] [Google Scholar]
- 28.Wong D K-H, Collins W J, Harmer A, Lilburn T G, Beatty J T. Directed mutational analysis of the Rhodobacter capsulatus puhA gene and orf214: pleiotropic effects on photosynthetic reaction center and light-harvesting I complexes. J Bacteriol. 1996;178:2334–2342. doi: 10.1128/jb.178.8.2334-2342.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yen H C, Hu N T, Marrs B L. Characterization of the gene transfer agent made by an overproducer mutant of Rhodopseudomonas capsulata. J Mol Biol. 1979;131:157–168. doi: 10.1016/0022-2836(79)90071-8. [DOI] [PubMed] [Google Scholar]
- 30.Young C S, Reyes R C, Beatty J T. Genetic complementation and kinetic analysis of Rhodobacter capsulatus ORF1696 mutants indicate that the ORF1696 protein enhances assembly of the light-harvesting I complex. J Bacteriol. 1998;180:1759–1765. doi: 10.1128/jb.180.7.1759-1765.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu P-L, Hohn B, Falk H, Drews G. Molecular cloning of the ribosomal RNA genes of the photosynthetic bacterium Rhodopseudomonas capsulata. Mol Gen Genet. 1982;188:392–398. [Google Scholar]