Abstract
Background
The giant roundworm Ascaris is an intestinal nematode, causing ascariasis by infecting humans and pigs worldwide. Recent estimates suggest that Ascaris infects over half a billion people, with chronic infections leading to reduced growth and cognitive ability. Ascariasis affects innumerable pigs worldwide and is known to reduce production yields via decreased growth and condemnation of livers. The predominant anthelminthic drugs used to treat ascariasis are the benzimidazoles. Benzimidazoles interact with β-tubulins and block their function, and several benzimidazole resistance-associated mutations have been described in the β-tubulins of ruminant nematodes. Recent research on ascarids has shown that these canonical benzimidazole resistance-associated mutations are likely not present in the β-tubulins of Ascaris, Ascaridia or Parascaris, even in phenotypically resistant populations.
Methods
To further determine the putative absence of key β-tubulin polymorphisms, we screened two β-tubulin isotypes of Ascaris, highly expressed in adult worms. Using adult and egg samples of Ascaris obtained from pigs and humans worldwide, we performed deep amplicon sequencing to look for canonical resistance-associated mutations in Ascaris β-tubulins. Subsequently, we examined these data in closer detail to study the population dynamics of Ascaris and genetic diversity within the two isotypes and tested whether genotypes appeared to partition across human and pig hosts.
Results
In the 187 isolates, 69 genotypes were found, made up of eight haplotypes of β-tubulin isotype A and 20 haplotypes of isotype B. Single nucleotide polymorphisms were seen at 14 and 37 positions for β-tubulin isotype A and isotype B, respectively. No evidence of any canonical benzimidazole resistance-associated mutations was found in either human- or pig-derived Ascaris isolates. There was, however, a difference in the genetic diversity of each isotype and distribution of β-tubulin genotypes between human- and pig-derived Ascaris. Statistical tests of population differentiation show significant differences (p < 0.001) between pig- and human-derived worms; however, more diversity was seen between worms from different populations than worms from different hosts.
Conclusions
Our work suggests an absence of canonical β-tubulin mutations within Ascaris, but alternative modes of anthelminthic resistance may emerge necessitating continued genetic scrutiny alongside monitoring of drug efficacy.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s13071-024-06306-5.
Keywords: Ascaris, Benzimidazole, Drug-resistance, β-Tubulin
Background
Over half a billion people are infected with the giant roundworm Ascaris, predominantly in low-middle-income countries [1, 2]. The WHO 2030 roadmap for action against neglected tropical diseases aims to eliminate Ascaris and other soil-transmitted helminths as a public health problem from 96 endemic countries by 2030 [3].
This will require several interventions including improved detection, and the uptake of a ‘One Health’ approach, in which hygiene and sanitation and environmental and animal reservoirs of disease are considered [4]. The incorporation of knowledge from mathematical models and the intensification of mass drug administration (MDA) in endemic regions are also key to reaching these targets [4, 5]. This increase in drug usage, in particular the increased use of benzimidazoles (BZ), could lead to the development of drug resistance, which is becoming widespread in many nematodes of veterinary importance [6–11]. Porcine ascariasis, mainly associated with Ascaris suum infection, is a global problem and can have economic effects by reducing meat yield [12, 13]. Benzimidazole drugs are also commonly used in pig production to control ascariasis. There are a few reports of potential BZ resistance in human Ascaris populations but none in pigs [14, 15]. Implementing surveillance is therefore paramount to prevent BZ resistance in Ascaris from becoming a problem for both human and animal health.
Most studies on BZ resistance in nematodes have focused on single nucleotide polymorphisms (SNPs) in the β-tubulin isotype-1 gene, which have been associated with phenotypic resistance in ruminant nematodes [16–18]. It is also possible for SNPs in other β-tubulin isotype genes to lead to resistance, as has been demonstrated for Haemonchus contortus [19]. Most work on Ascaris has focused on a single isotype; however, recent work has analysed multiple Ascaris isotypes to search for evidence of resistance, with none found [20, 21]. Two isotypes have been studied for Parascaris, with significant changes in the expression of isotype 1 in response to BZs observed but no evidence of resistance-associated SNPs [22–24]. There have so far only been a handful of phenotypically resistant isolates of ascarids identified that can be studied, with only two investigations screening for the common resistance-associated SNPs in resistant isolates [25–27]. Analysis of seven β-tubulin isotypes in BZ-resistant populations of Parascaris univalens found no evidence of resistance-associated SNPs in any isotype [26, 27].
The three common amino acid substitutions associated with resistance (F167Y, E198A and F200Y) are found close together in the β-tubulin protein; however, in the β-tubulin genes an intron separates the F167 codon from the E198 and F200 codons. Previous attempts to identify SNPs have therefore relied upon separate assays to capture the genetic material at both sites [14, 28–30]. Without the knowledge of which SNPs are most associated with resistance in Ascaris, it is vital that all three are analysed to ensure that no causative mutations are missed. The most efficient way to do this is to cover all three sites in one assay. Deep amplicon sequencing is more sensitive than traditional sequencing methods for the identification of alleles at low frequency. This methodology combines the high read depth of next-generation sequencing with the specificity of single marker sequencing and allows high sample throughput. Recent advances allow all three of these SNPs to be captured in a single assay and isolates can be screened for all three simultaneously [20, 31, 32]. In addition to enabling screening of all three SNPs at once, these newer sequencing methods allow researchers to perform population genetic analysis. The outcomes of such work can inform on the origins and spread of resistance and the diversity within and between populations [20, 33].
There has been much debate over the relationship between Ascaris lumbricoides (mainly infecting humans) and A. suum (mainly infecting pigs) with hybridization being possible and some suggesting that they may be separate lineages of a single species [34–37]. In industrialised countries where A. lumbricoides infection in humans is not endemic, Ascaris infections can often be attributed to A. suum and infection via contact with pigs [38–44]. Recent work in a region of Kenya that does not commonly practice pig farming found that most worms contained pig-associated mitochondrial haplotypes while showing signs of a mixed human/pig nuclear genome [45]. This supported the hypothesis that in the past there has been hybridization of A. suum and A. lumbricoides that gave rise to fertile offspring capable of being transmitted through humans. This study also calculated that there was not enough divergence between the mitochondrial cox1 gene to designate the pig- and human-associated clades as separate species [45]. The zoonotic capabilities of Ascaris mean that a One Health approach needs to be implemented to control these parasites. Determining the origin of infections will be a key aspect to monitor to prevent the spillback of infections.
In this paper, we build upon previous work to screen global Ascaris samples from both humans and pigs for the presence of canonical resistance-associated SNPs in the two most highly expressed β-tubulin isotypes (isotype A and isotype B). In addition, we assess the inter- and intra-farm diversity of A. suum isolates from UK pigs and analyse samples from a variety of locations worldwide from both humans and pigs to compare the diversity of these two isotypes between both region and host.
Methods
Egg isolation and larval culture
Ascaris suum eggs were isolated from faecal samples collected from four UK pig farms. For three of the farms, all eggs were isolated from a single faecal sample as these were the only faecal samples containing Ascaris eggs from that farm. For one farm (Farm B), eggs were collected from multiple faecal samples from a pen housing multiple piglets.
To isolate eggs, a previously described salt-sugar-flotation method was adapted with the sugar flotation step replaced by further salt flotation steps and the volumes scaled up to 25 g of faeces and 225 ml of saturated salt solution [46]. The pellet of eggs obtained using this method was suspended in 1000 μl PBS and stored at 5 °C until further use. The thick proteinaceous coat of Ascaris eggs can sometimes hinder nucleic acid extractions and the eggs were therefore de-coated. Egg suspensions were transferred to 50-ml Falcon tubes and incubated in 20 volumes of 3% sodium hypochlorite at 30 °C for 90 min in an incubated shaker at 100 rpm. Eggs were then washed three times by centrifugation at 1200 xg in PBS [47–49]. To acquire larval stages, A. suum decorticated eggs were incubated in 5 ml 0.1N (0.05 M) sulphuric acid at 28 °C for at least 21 days in 50-ml Falcon tubes. Samples were aerated by hand weekly by opening the lid and gently mixing tubes [50].
DNA isolation, preparation and sequencing
Genomic DNA was isolated from Ascaris via several methods. For larval DNA, pools of two to four eggs containing developed larvae from four populations across the UK were placed in 30 μl lysis buffer made up of a 20:1 ratio of DirectPCR Lysis reagent (Cell) (Viagen Biotech) and Proteinase K (New England Biolabs). For adult worms from Hungary and the Philippines, DNA was extracted from a 2 cm portion of the anterior end of the worm using the Qiagen DNeasy Blood and Tissue kit (Qiagen) following the manufacturer’s instructions. For all other samples, the DNA was supplied already purified and extraction has been previously described [40]. Samples came from nine countries with 151 and 39 originating from pigs and humans respectively (Table 1).
Table 1.
Characteristics of Ascaris samples included in this study
| Country | Region | Sample type | Host | Sample no. | References |
|---|---|---|---|---|---|
| Bangladesh | Unknown | Adult | Human | 11 | (Betson et al. 2014) |
| Belgium | Flanders | Adult | Pig | 109 | (Roose et al. 2021) |
| Denmark | Unknown | Adult | Pig | 7 | (Betson et al. 2014) |
| Ethiopia | Jimma | Adult | Human | 29 | (Roose et al. 2021) |
| Guatemala | Santa Rosa | Adult | Pig | 6 | (Anderson et al. 1993) |
| Guatemala | Santa Rosa | Adult | Human | 4 | (Anderson et al. 1993) |
| Hungary | Forráskút | Adult | Pig | 1 | This study |
| Hungary | Eger | Adult | Pig | 12 | This study |
| Nepal | Unknown | Adult | Human | 3 | (Nejsum et al. 2005b) |
| Philippines | Los Baños | Adult | Pig | 3 | (Anderson and Jaenike, 1997) |
| Philippines | Trento | Adult | Pig | 2 | This study |
| Philippines | Buwan | Adult | Pig | 2 | This study |
| Tanzania | Unguja | Adult | Human | 8 | (Betson et al. 2011) |
| Tanzania | Unknown | Adult | Pig | 8 | (Betson et al. 2014) |
| Tanzania | Pemba island | Adult | Human | 77 | (Roose et al. 2021) |
| Uganda | Kampala | Adult | Pig | 4 | (Betson et al. 2014) |
| Uganda | Kabale | Adult | Human | 8 | (Olsen et al. 2009) |
| Uganda | Kabale | Adult | Pig | 4 | (Nissen et al. 2011) |
| UK | Devon | Larvae | Pig | 20 | This study |
| UK | Clwyd | Larvae | Pig | 20 | This study |
| UK | Lincolnshire | Larvae | Pig | 19 | This study |
| UK | Derbyshire | Larvae | Pig | 20 | This study |
| UK | Cornwall | Adult | Human | 5 | (Bendall et al. 2011; Betson et al. 2014) |
| UK | Bedfordshire | Adult | Pig | 23 | (Betson et al. 2014) |
A first round of PCR was performed using BtA and BtB primers (BtA-For, BtA-Rev, BtB-For and BtB-Rev1) with added adapter sequences which amplify β-tubulin isotype A and B respectively, as previously described [20]. Four forward and four reverse primers were used, with each containing the specific primer and an Illumina adapter with between 0 and 3 random nucleotides separating them [20]. A primer mastermix was made for forward and reverse primers containing all four adapter primers at a final concentration of 10 μM. First round PCRs were carried out in 25-μl reactions containing 1 × KAPA HiFi Fidelity Buffer (KAPA Biosystems), 0.5U KAPA HiFi DNA Polymerase, 0.3 μM forward and reverse adapter primer mastermix, 0.75 mM KAPA dNTP Mix and 1 μl template DNA. Reactions were performed under the following conditions: initial denaturation at 95 °C for 3 min followed by 35 cycles of denaturation at 98 °C for 20 s, annealing at 61 °C for 15 s and extension at 72 °C for 30 s, and a final extension of 72 °C for 2 min. Equal volumes (20 μl) of the two separate PCR products, BtA and BtB, from the same samples were pooled prior to purification. PCR products were purified using NucleoMag NGS Clean-up and Size Select kit (Macherey-Nagel) following the manufacturer’s instructions.
A second round of PCR was performed to add Illumina Nextera XT P5/7 indices to each sample [32]. Sixteen forward and 24 reverse indexing primers were used to give 192 unique barcode combinations over two 96-well plates [32]. The second round of PCRs were carried out in 25 μl and contained 1 × KAPA HiFi Fidelity Buffer, 0.5U KAPA HiFi DNA Polymerase, 0.5 μM of each primer, 0.3 mM KAPA dNTP Mix and 2 μl of the purified product from the first round of PCR. Reactions were performed under the following conditions: initial denaturation at 98 °C for 45 s followed by seven cycles of denaturation at 98 °C for 20 s, annealing at 63 °C for 20 s and extension at 72 °C for 2 min.
Twenty μl from each well of both plates was pooled into a new plate (e.g. A1 of plate 1 and A1 of plate 2 into A1 of the pooled plate). Pooled products were again purified using NucleoMag NGS Clean-up and Size Select kit following the manufacturer’s instructions. All samples were pooled to make a master sequencing library. The master library was further purified using gel extraction using the GeneJET Gel Extraction Kit (Thermo Scientific) following the manufacturer’s instructions. All gel extraction solutions were run through a single spin column to maximise DNA yield and concentration was determined using a Collibri™ Library Quantification qPCR Kit (Invitrogen). The master sequencing library was run on an Illumina MiSeq sequencer using a 500-cycle paired-end reagent kit (MiSeq Reagent Kits v2,MS-103–2003, Illumina) at a concentration of 15 nM with the addition of 10–15% phiX Control v3 (FC-11–2003, Illumina). FASTQ files were split based on unique indexing barcodes.
Sequencing analysis
Raw Illumina sequencing data were analysed in R (v4.1.1.) [51] with the DADA2 package v1.20 [52] using the workflow described in nemabiome.ca (https://www.nemabiome.ca/dada2_workflow.html). Reads were trimmed of their primers using Cutadapt v3.4 [53] and any read that did not contain a sequence matching the primer was removed. The remaining reads were trimmed and filtered to leave reads with a minimum length of 200 bp, a maxEE of 6 and truncQ of 2, which then underwent an error learning process within DADA2. For BtA, overlapping forward and reverse reads were merged. The size of the BtB sequenced region was > 600 bp so overlaps were not seen. For BtB, the reads were trimmed to a uniform size of 268 bp and forward and reverse reads were concatenated using “justconcatenate = TRUE” command in DADA2 to merge reads. Finally, chimeras were removed, and the unique sequences were assigned to amplicon sequencing variants (ASVs). The R package phyloseq v1.36 [54] was then used to assign the read counts for each ASV back to the sample data in the form of an Operation Taxonomic Unit (OTU) table.
Genotyping and population genetics
The ASV sequences were aligned in BioEdit v7.2.5. [55] to check that all ASVs were β-tubulins and any sequences that did not align were removed. Data were screened to remove any read counts below 100 and any sample which had no reads from any ASV was removed. Samples which had reads for more than two ASVs were removed prior to any further analysis. The remaining data were used to screen the ASVs for the common resistance-associated SNPs. Sample information such as population of origin and host species was used to visualise allele frequency distributions in R using ggplot2 v3.3.5. [56]. The allelic richness was calculated for each isotype using the R package PopGenReport v3.0.4. [57].
Data from a recent study which sequenced the same gene regions of Ascaris samples from Belgium, Tanzania and Ethiopia were added to the dataset [20]. The DNASP v6.12.03. package [58] was used to create haplotype files. This file included the aligned variable nucleotides only and information on the frequencies of each sequence. PopART v1.7 [59] was used to visualise relationships between ASVs by means of a minimum spanning network. The networks show how each ASV is related to another with dashes representing a single nucleotide change. As haplotype networks can only be made with sequences without gaps, some alleles which differ by only a single insertion or deletion were merged. BtA ASVs 1, 3, 6, 8 and 14 form a single haplotype and in BtB ASVs 2 and 7, ASVs 18 and 24 and ASVs 21 and 29 were merged.
These sequences were further used to generate a maximum likelihood tree with MegaX using the T92 + G model with 1000 bootstraps [60]. Multiple correspondence analysis (MCA) was performed on the combined dataset to assess patterns in variation using the FactoMineR v2.4. [61] and factoextra v1.0.7. [62] packages in R.
Samples from regions with fewer than six isolates were removed from the datasets prior to further population genetics analysis using for Arlequin v3.5.2.2. [63], as these populations will be too small to give reliable results. This led to the removal of samples from Nepal and Guatemala and human samples from the UK. Variable nucleotide sequences for each isotype were combined to create a sequence representing individual genotypes. As not all samples had data for both isotypes, some samples were removed from the dataset for this analysis. Arlequin was used to calculate heterozygosity to test for deviation from Hardy-Weinberg equilibrium (HWE), analysis of molecular variance (AMOVA) and fixation indices. Separate analyses were run for each host and populations were grouped by country of origin.
Results
No evidence of resistance associated SNPs in Ascaris
The sequencing results yielded data from 190 samples with 187 usable results from β-tubulin isotype A (BtA) and 164 from β-tubulin isotype B (BtB). None of the previously described BZ resistance-associated SNPs were identified in the sequence analysis (Additional file 1: Fig. S1 and Fig. S2).
Genetic variation in Ascaris β-tubulin isotypes
After quality checks and filtering of reads, 8 ASVs were identified for BtA and 20 ASVs were found for BtB, indicating a greater genetic diversity in BtB compared to BtA. For both isotypes, most of the sequenced DNA formed part of an intron, with only a small portion at the end of exon 4 and the beginning of exon 5 needed to capture the resistance-associated SNPs. Most variation in BtA was found in the intron and hence was not translated to any changes in the protein sequence. No variation was noted for any BtA ASV in the exon 4 region of the sequence and only one synonymous change was seen in the exon 5 region for ASV 5 (Additional file 1: Fig. S1). In BtB, most variation is also in the intron although there is some variation in both exons for this isotype. There were two synonymous SNPs in exon 4 and four synonymous SNPs in exon 5. ASVs 8, 11 and 14 all had the same four non-synonymous SNPs, with one in exon 4 and three in exon 5. In addition to this, ASV 14 also had two non-synonymous SNPS in exon 4. In total, SNPS were seen in 14 positions in BtA and 37 positions in BtB (Additional file 1: Fig. S2). A total of 69 genotypes were found in 161 samples. The addition of samples from Roose et al. [20] resulted in 14 BtA ASVs, 25 BtB ASVs and 131 genotypes in 370 samples (Additional file 1: Table S1).
For BtA, most groups contained only ASVs 1 and 2 when separated by country. Most genetic diversity was seen in the UK samples because of the high proportion of samples originating from this population compared to samples from some countries containing low numbers of ASVs (Fig. 1). For BtB, ASVs 1 to 3 were the most frequently seen across the samples. However, in BtB, ASVs were only found in samples from one or two countries at low frequencies (Fig. 2). The UK pig farms each had their own unique composition of alleles for both β-tubulin isotypes. All the larvae from the UK pig farms A, C and D samples were cultured from eggs isolated from a single faecal sample. Farm B larvae were isolated from multiple faecal samples from a pen housing many piglets. In these samples, BtB was also more diverse than BtA (see Additional file 1: Table S1 for full allele composition).
Fig. 1.
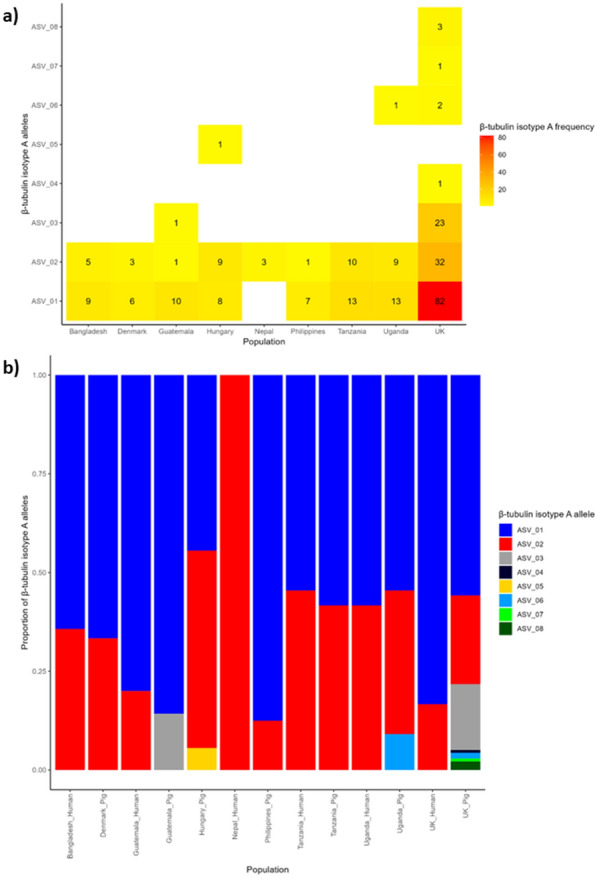
β-tubulin isotype A (BtA) allele frequencies and distribution. a Numerical distribution of amplicon sequencing variants (ASVs) among countries; the number of individuals containing each ASV is indicated by the number within tile and tile colour. b Representative density of each ASV in the populations separated by country and host species. The 187 samples with data for BtA were used to calculate allele frequencies and distribution
Fig. 2.
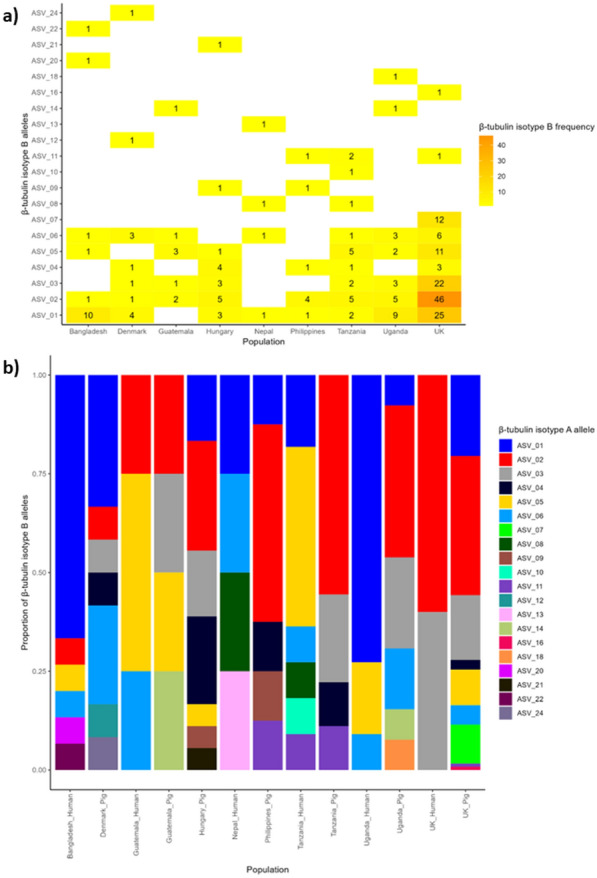
β-Tubulin isotype B (BtB) allele frequencies and distribution. a Numerical distribution of amplicon sequencing variants (ASVs) among countries; the number of individuals containing each ASV is indicated by the number within each tile and tile colour. b Representative density of each ASV in the populations separated by both country and host species. The 164 samples with data for BtB were used to calculate alleles frequencies and distribution
The minimum spanning networks showed that BtA ASV1 (ASV_1_3_6_8_14) was the central ASV from which others have diverged (Fig. 3a). For BtA, all ASVs were very similar with only one difference between ASV1 and most of the other ASVs, with the exception of ASV11 with two differences and ASV5 which had nine differences from the central ASV1. Most samples had ASVs 1 and 2 and are comprised of samples from both human and pig hosts (Fig. 3a). For BtB, ASV2 (ASV_2_7) appeared to be the central ASV. However, for BtB, much more divergence was seen between the ASVs, with up to 19 changes seen between the central ASV2 and the most divergent ASVs. Most ASVs contained samples derived from both human and pig hosts in multiple regions so there seems to be no clear structuring; however, ASV4 contains samples from multiple locations that are all of pig origin (Fig. 3b).
Fig. 3.
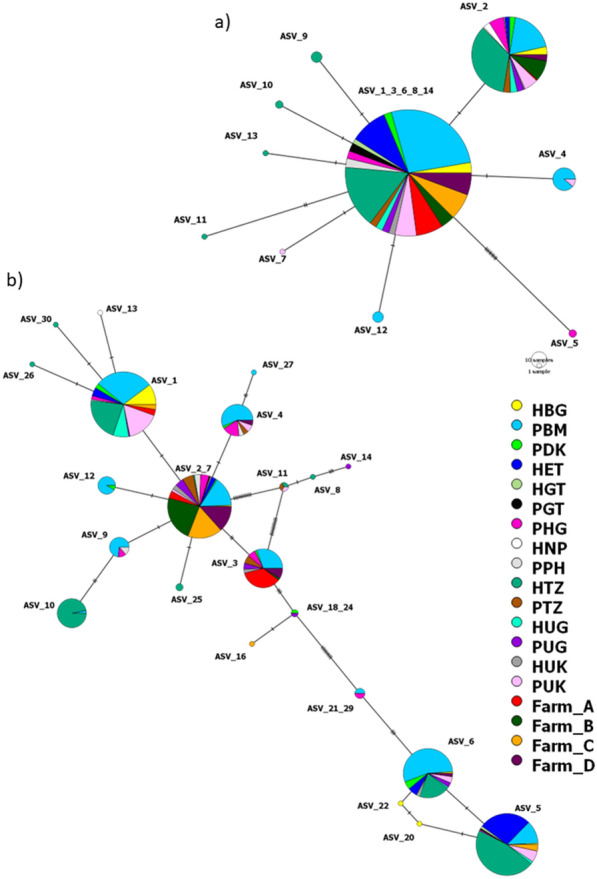
Haplotype distribution. Minimum spanning network showing relationship between amplicon sequencing variant (ASV) with notches between haplotypes representing a sequences change. Each country and host grouping is represented by a unique colour and size of the circle indicates the frequency of the haplotype. There are some regions with numerous changes between two ASVs which is likely due to missing haplotypes that are extinct or yet to be sampled. H humans, P pig, BG Bangladesh, BM Belgium, DK Denmark, ET Ethiopia, GT Guatemala, HG Hungary, NP Nepal, PH Philippines, TZ Tanzania, UG Uganda, UK United Kingdom. Farms A, B, C and D indicate the separate pig farms from the UK
The phylogenetic tree created for the combined dataset showed that sequences clustered into three groups (Additional file 1: Fig. S3). Each group contained a mix of samples from both human and pig hosts from different regions.
Population genetics shows differences between Ascaris samples from humans and pigs
The molecular diversity indices showed that the samples from pigs in Belgium and Hungary show significant deviation from HWE for both alleles. Results showed deviation from HWE for BtA in worm samples from humans in Tanzania and pigs in the UK and larval samples from Farm A and Farm B. For BtB, worms from the Philippines and Tanzania and larvae from UK pigs (Farm C and Farm D) deviated from HWE.
A degree of inbreeding within the sample groups was indicated by positive and statistically significant values for the inbreeding coefficient (FIS) in the samples from humans in Tanzania and in the pig samples from Belgium, the UK and Farm A (Table 2). AMOVA analysis showed significant variation between different samples within a country and between individuals within a country for each host. Most variation in this analysis was attributed to differences between individuals (Table 3).
Table 2.
Molecular diversity indices from the combined β-tubulin genotype dataset stratified by host and country
| Molecular diversity indices | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Molecular diversity | Hardy-Weinberg equilibrium | Population-specific FIS indices (1023 permutations) | ||||||
| Sample size | No. of haplotypes | Average gene diversity over loci | Locus | Obs. Het | Exp. Het | P-value | FIS | P(Rand FIS ≥ Obs FIS) | |
| HBG | 22 | 7 | 0.433 | A | 0.273 | 0.455 | 0.232 | 0.412 | 0.226 |
| B | 0.364 | 0.411 | 0.451 | ||||||
| HET | 58 | 7 | 0.305 | A | 0.069 | 0.131 | 0.105 | 0.477 | 0.111 |
| B | 0.483 | 0.479 | 0.671 | ||||||
| HTZ | 184 | 22 | 0.626 | A | 0.250 | 0.526 | < 0.001a | 0.526 | < 0.001a |
| B | 0.696 | 0.726 | 0.375 | ||||||
| HUG | 16 | 4 | 0.421 | A | 0.500 | 0.500 | 1 | < 0.001a | 0.778 |
| B | 0.375 | 0.342 | 1 | ||||||
| PBM | 208 | 32 | 0.652 | A | 0.269 | 0.456 | < 0.001a | 0.411 | < 0.001a |
| B | 0.750 | 0.847 | 0.044a | ||||||
| PDK | 14 | 9 | 0.632 | A | 0.286 | 0.440 | 0.440 | 0.368 | 0.436 |
| B | 0.714 | 0.824 | 0.195 | ||||||
| PHG | 26 | 11 | 0.712 | A | 0.385 | 0.588 | 0.048a | 0.355 | 0.096a |
| B | 0.385 | 0.837 | 0.000a | ||||||
| PPH | 10 | 4 | 0.367 | A | N/A | N/A | N/A | N/A | N/A |
| B | 0.200 | 0.733 | 0.016a | ||||||
| PTZ | 16 | 6 | 0.571 | A | 0.500 | 0.500 | 1 | < 0.001a | 0.789 |
| B | 0.125 | 0.642 | 0.001a | ||||||
| PUG | 16 | 9 | 0.675 | A | 0.375 | 0.575 | 0.439 | 0.364 | 0.182 |
| B | 0.625 | 0.775 | 0.168 | ||||||
| PUK | 46 | 11 | 0.552 | A | 0.304 | 0.486 | 0.014a | 0.379 | 0.025a |
| B | 0.478 | 0.617 | 0.107 | ||||||
| Farm A | 32 | 6 | 0.353 | A | 0.063 | 0.179 | 0.032a | 0.659 | 0.029a |
| B | 0.563 | 0.526 | 0.580 | ||||||
| Farm B | 36 | 3 | 0.282 | A | 0.889 | 0.508 | 0.002a | -0.167 | 1 |
| B | 0.056 | 0.056 | 1 | ||||||
| Farm C | 34 | 11 | 0.528 | A | 0.412 | 0.355 | 1 | 0.252 | 0.163 |
| B | 0.294 | 0.701 | < 0.001a | ||||||
| Farm D | 28 | 9 | 0.566 | A | 0.286 | 0.378 | 0.316 | -0.789 | 1 |
| B | 0.357 | 0.754 | 0.001a | ||||||
aIndicates a p-value < 0.05. Abbreviations: Obs.Het. = observed heterozygosity, Exp.Het. = expected heterozygosity, H = humans, P = pig, BG = Bangladesh, BM = Belgium, DK = Denmark, ET = Ethiopia, GT = Guatemala, HG = Hungary, NP = Nepal, PH = Philippines, TZ = Tanzania, UG = Uganda, UK = United Kingdom. Farms A, B, C and D indicate the separate pig farms from the UK. Hardy-Weinberg equilibrium is calculated for each isotype over 1,000,000 Markov chain steps, and the p-value is based on the deviation between the observed and expected values. Population-specific inbreeding coefficient (FIS) calculated over 1023 permutations is shown for each population with p-values of divergence between random FIS vs observed FIS
Table 3.
Analysis of MOlecular VAriance (AMOVA) to identify sources of variation and calculate fixation indices
| Amova analysis | ||||||||
|---|---|---|---|---|---|---|---|---|
| Host | Source of variation | d.f | Sum of squares | Variance components | Percentage of variation | Fixation indices | P-value | |
| Human | Among countries | 3 | 4.989 | N/A Va | N/A | FCT | 0.000 | 0.000 |
| Among individuals within populations | 136 | 44.339 | 0.10587 Vc | N/A | FIS | 0.48088 | < 0.001a | |
| Within individuals | 140 | 16 | 0.11429 Vd | N/A | FIT | 0.000 | 0.000 | |
| Total | 279 | 65.329 | N/A | |||||
| Pig | Among countries | 6 | 6.156 | − 0.06328 Va | -23.85 | FCT | − 0.23854 | 0.69306 |
| Among individuals within populations | 222 | 60.542 | 0.05374 Vc | 20.26 | FIS | 0.24541 | < 0.001a | |
| Within individuals | 233 | 38.5 | 0.16524 Vd | 62.29 | FIT | 0.37711 | < 0.001a | |
| Total | 465 | 121.605 | 0.26527 | |||||
Ascaris samples (individuals) were grouped by country with analysis run separately for worms derived from different hosts. For the UK pig farm data, each farm was designated as a separate population. Results show the degrees of freedom (d.f.) sum of squares and variance components which are used to calculate the percentage of variation contained within the sample divisions. Fixation indices are shown for FCT, FIS and FIT along with p-values from 1023 permutations
When grouping by worm host, molecular diversity indices showed deviation from HWE and significant FIS in each host (Table 4). AMOVA analysis showed no significant variation between hosts, with low Fct scores indicating low divergence between groups and most variation occurring between individuals or between populations (Table 4). Low Fct scores compared to other F-statistics showed that there was more diversity between worms from different countries and regions than there was between worms from different hosts (Table 4). However, both the global and population exact tests of sample differentiation showed that there is a significant difference between the hosts (p < 0.001) for both tests.
Table 4.
Molecular diversity indices based on multiple tests of molecular diversity on the combined β-tubulin genotype dataset
| (a) Molecular diversity indices | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Molecular diversity indices | Hardy-Weinberg equilibrium | Population-specific FIS indices (1023 permutations) | |||||||
| Group | Sample size | No. of haplotypes | Average gene diversity over loci | Locus | Obs. Het | Exp. Het | p-value | FIS | p-value |
| Human | 286 | 26 | 0.591 | A | 0.224 | 0.468 | < 0.001a | 0.523 | < 0.001a |
| B | 0.615 | 0.714 | 0.009a | ||||||
| Pig | 474 | 50 | 0.681 | A | 0.325 | 0.517 | < 0.001a | 0.372 | < 0.001a |
| B | 0.544 | 0.845 | < 0.001a | ||||||
| (b) Analysis of MOlecular VAriance (AMOVA) | |||||||
|---|---|---|---|---|---|---|---|
| Source of variation | d.f | Sum of squares | Variance components | Percentage of variation | Fixation indices | p-value | |
| Among groups | 1 | 1.587 | − 0.0128 | − 5.07 | FCT | − 0.051 | 0.853 |
| Among populations within groups | 17 | 29.553 | 0.047 | 18.84 | FSC | 0.179 | < 0.001a |
| Among individuals within populations | 361 | 104.882 | 0.074 | 29.23 | FIS | 0.339 | < 0.001a |
| Within individuals | 380 | 54.5 | 0.143 | 57 | FIT | 0.43 | < 0.001a |
aIndicates a p-value < 0.05. Abbreviations: Obs.Het. = observed heterozygosity, Exp.Het. = expected heterozygosity. (a) Hardy-Weinberg equilibrium was calculated for each isotype over 1,000,000 Markov chain steps and the p-value is based on the deviation between the observed and expected values. Population-specific inbreeding coefficient (FIS) calculated over 1023 permutations is shown for each population with p-values of divergence between random FIS vs observed FIS. (b) Analysis of MOlecular VAriance tests to identify sources of variation and calculate fixation indices. Samples (individuals) were grouped by host species and then populations were separated by country, for the UK pig farm data each farm was designated as a separate population. Samples from humans in the UK were grouped with the pigs as they have been previously shown to be of porcine origin. Results show the degrees of freedom (d.f.) sum of squares and variance components which are used to calculate the percentage of variation contained within the sample divisions. Fixation indices are shown for FCT, FSC, FIS and FIT along with p-values from 1023 permutations. Most variation occurs between individuals within the entire population. Fixation indices show that there is little differentiation between groups but significant differentiation between individuals and populations
Using initial multiple correspondence analysis (MCA), there was clear separation between the sample hosts, with exception of the UK human samples that clustered closely with the pig samples (Fig. 4a). The MCA showed that there was a greater dispersal of samples of pig origin than there was for the human samples (Fig. 4a). Most samples clustered closely within their country of origin, although for the countries that contained samples from both human and pig hosts, the host does play a role in variation as the samples from different hosts are spread out as seen with the Ugandan and Tanzanian populations (Fig. 4b). Within the pig samples, the continental European samples (Belgium, Denmark and Hungary) clustered close to each other, whereas the Philippine samples were found between the European and UK samples (Fig. 4b).
Fig. 4.
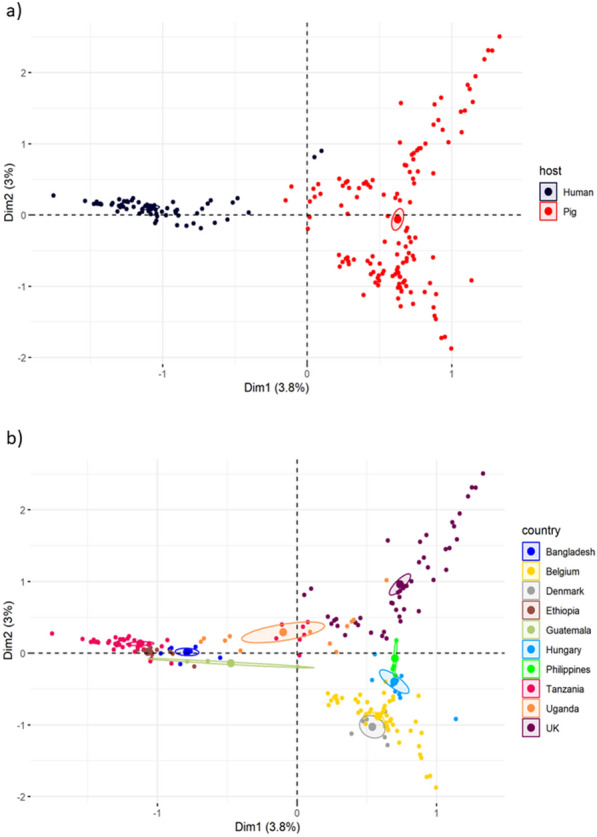
Multiple component analysis (MCA) of Ascaris β-tubulins. a MCA variance based on host species. b Distribution of MCA variance based on country of origin. H humans, P pig, BG Bangladesh, BM Belgium, DK Denmark, ET Ethiopia, GT Guatemala, HG Hungary, NP Nepal, PH Philippines, TZ Tanzania, UG Uganda, UK United Kingdom. Farms A, B, C and D indicate the separate pig farms from the UK. Numbers within the population name represent the genotype number
Discussion
In this study, an extensive screen of Ascaris samples across the world was performed, and we have assessed in detail sequence allelic variation within two β-tubulin isotype genes, showing an absence of canonical resistance-associated SNPs. The inclusion of data from previous studies allowed us to analyse the diversity of these genes in a much larger group of geographically widespread samples, inclusive of human- and pig-derived samples. We found differentiation between Ascaris derived from pig and humans, which we consider to indicate a low risk of transfer of resistance genes might they ever arise.
A small number of studies have previously found resistance-associated mutations in Ascaris. The F200Y substitution was found at low frequency (0.5%) in Brazil, although, unfortunately, no host treatment history was available so it remains unknown whether these samples were phenotypically resistant to BZs [14]. There were also reports of a high proportion (77–100%) of F167Y substitutions in samples as geographically disparate as Kenya, Haiti and Panama [28]. Even when the F167Y substitutions were present, these populations appeared to have no change in drug susceptibility, and the proportion of these substitutions did not change after treatment, suggesting this mutation alone does not lead to BZ resistance in Ascaris [28]. Recent studies looking for evidence of resistance in ascarids have not detected canonical resistance markers, not even in phenotypically resistant parasites [26, 27]. Taken together, the results presented here and in previous studies suggest that BZ resistance via the classical amino acid substitutions in β-tubulins is unlikely to be present in Ascaris and closely related nematodes. Whilst not direct orthologues, based on the expression profiles, BtA and BtB in Ascaris are analogous to β-tubulin isotypes 1 and 2 in strongyles, respectively [20, 64]. It is important to analyse both of these isotypes when screening for resistance as studies in H. contortus have shown that mutations in β-tubulin-2 are also linked to BZ resistance [19].
Our analysis based on variation in BtA and BtB indicated significant differences between Ascaris from pigs and Ascaris from human hosts, apart from worm samples from humans in the UK, which are likely infections from close contact with pigs and would therefore likely be A. suum [39]. This aligns with previous studies [40, 45, 65–67], which found a genetic separation based on host species, but with clear evidence of cross-transmission and recombination due to some shared haplotypes. This indicates that there is a divide between the species, with introgression keeping the diversity low. Thus, if resistance were to arise in human or pig Ascaris it might not be able to cross host species barriers.
Our results provide some insights into potential spread of resistance genes between countries and farms. In the MCA analysis, all human-derived samples cluster tightly together. Conversely the pig-derived samples are much more spread with much clearer boundaries based on geographical origin, indicating that there may be greater potential for resistance gene flow between Ascaris populations infecting humans than those infecting pigs. At a local level, Ascaris samples from all four UK pig farms have unique ASVs which would imply limited population mixing between sites. It should be noted that due to the pooling of larvae prior to extraction, ASVs cannot be assigned to individual larvae in each sample. However, these methods do allow a general overview of the diversity within each farm. With this being said, there is limited diversity within each farm, suggesting that infections may come from the farm environment rather than multiple introductions with stock being brought in. This aligns with the fact that UK farms sampled were all small-scale farms housing various pig breeds from different sources. Studies in Brazil, China, Denmark and the USA found no clear genetic structuring between locations [66–69]. Similarly, a study on Ascaridia galli from 10 farms in Denmark and Sweden showed high levels of gene flow between sites [70]. Most farms in Denmark source their pigs from a relatively small number of large-scale breeders resulting in populations of Ascaris from those few breeders being spread widely across the country [67]. Thus, the potential for spread of genes associated with anthelmintic resistance between farms depends on the setting and farming practices.
When assessing the relative genetic diversity of human- and pig-derived Ascaris, it should be noted that sample sets from each country contained unequal data for each host species, so it is hard to make direct comparisons. The human-derived samples mainly come from regions that have been the target of MDA, which could lead to genetic bottlenecking and a reduced genetic diversity due to the reduced effective population size [71, 72]. Limited information is available about the anthelmintic treatment history of the pig hosts, but two of the UK pig farms where samples were obtained treated pigs with macrocyclic lactones whereas the other two used macrocyclic lactones and BZ.
One limitation of this study is that some of the Ascaris samples from humans were obtained after treatment with mebendazole, meaning that they are susceptible to BZs and making it unlikely that they would carry any resistance-associated genes. This contrasts to Ascaris samples from pigs which were obtained at slaughter or from faeces. A second limitation is that only two markers (BtA and BtB) were used for population genetic analysis. Use of multiple markers or whole genome comparisons would allow more confident inferences to be made in terms of population genetics. Finally, sample numbers varied substantially between host and location and for samples taken from pigs in UK farms, three out of four sites were composed of samples isolated from a single host meaning that we may not be representing the full diversity of each site.
Conclusions
Our study adds to the growing evidence that the canonical BZ resistance-associated SNPs seen in other nematodes are not present in Ascaris or in other ascarids. This indicates that resistance to BZs in ascarids may depend on an as yet unknown mechanism(s). Future work will need to focus on identifying the possible mechanisms of resistance in the phenotypically resistant populations of ascarids that have been identified and continue to monitor BZ efficacy in ascarids. Meanwhile, the genetic diversity seen in Ascaris populations has shown that the parasites infecting pigs and humans represent different populations, suggesting limited potential for flow of resistance genes between Ascaris infecting different host species.
Supplementary Information
Additional file 1: Fig. S1. β-Tubulin isotype A (BtA) amplicon sequencing variants (ASVs) sequence alignment. Fig. S2. β-Tubulin isotype B (BtB) amplicon sequencing variants (ASVs) sequence alignment. Fig. S3. Maximum likelihood phylogeny of Ascaris β-tubulin genotypes. Table S1. Sample information and β-tubulin isotype alleles.
Acknowledgements
We thank all the farmers and pig owners that participated in this study. We also gratefully acknowledge everyone who donated Ascaris samples or DNA used in this study, including Richard Bendall (Royal Cornwall Hospital, UK), Helena Ngowi (Sokoine University of Agriculture, Tanzania), Ida-Hella Poulsen and Sofie Nissen (University of Copenhagen, Denmark), Jaap Boes (Danish Cattle Federation, Denmark), Harriet Namwanje (Ministry of Health, Uganda), Rashidul Haque (International Center for Diarrheal Diseases Research, Bangladesh) and Tim J. C. Anderson (Southwest Foundation for Biomedical Research, Texas, USA).
Author contributions
BPJ, MB, AHMV, and EJL designed the study with advice from SR and PG. BPJ, AJ, AJIA, KRKL, VYB and VGVP collected samples. BPJ, AJIA, KRKL and KK conducted laboratory work. BPJ analysed the data with support from UC. BJP drafted the manuscript. MB, AHMV, JRS, PN and VGVP substantially revised the manuscript. All authors approved the submitted version of the manuscript.
Funding
This study was funded by the University of Surrey Kenneth Longhurst Trust PhD studentship. Funding for the ZooTRIP study was provided by the Newton Fund awarded through the Medical Research Council (MRC) (grant number MR/R025592/1) and the Philippine Council for Health Research and Development [fund code number N9A6823]. For the purpose of open access, the author has applied a Creative Commons Attribution (CC BY) licence to any Author Accepted Manuscript version arising.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Declarations
Ethics approval and consent to participate
Collection of pig faecal samples from which Ascaris suum eggs were harvested and cultured was carried out as part of the project “The role of Ascaris β-tubulin isotypes in the emergence of anthelmintic resistance” approved by the University of Surrey Ethics Committee (UEC 2019 084). Written informed consent was obtained from farmers providing faeces from their pigs. Adult Ascaris from Trento and Bunawan in the Philippines were collected from the intestines of pigs post-slaughter as part of the “Zoonotic transmission of intestinal parasites: Implications for control and elimination” study [73]. Ethical approval for this study was given by the University of Surrey Ethics Committee (UEC 2019 049), University of Philippines Manila Research Ethics Board (UPMREB 2019–084–01) and the Animal Care and Use Committee of University of Philippines Los Baños (CAS-2018–020). The study also conformed with the ethics and good practice of the Animal Welfare and Ethical Review Board of the University of Surrey (OUT036). Ascaris samples from Hungary were obtained from the intestines of pigs by an official veterinarian from abattoirs in Hungary post-slaughter. Information on ethical considerations for other Ascaris DNA samples used can be found in the original published studies (see references in Table 1).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Holland C, Sepidarkish M, Deslyper G, Abdollahi A, Valizadeh S, Mollalo A, et al. Global prevalence of Ascaris infection in humans (2010–2021): a systematic review and meta-analysis. Infect Dis Poverty. 2022;11:113. doi: 10.1186/s40249-022-01038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Institute for Health Metrics and Evaluation. Ascariasis — Level 4 cause [Internet]. Inst. Heal. Metrics Eval. 2020. http://www.healthdata.org/results/gbd_summaries/2019/ascariasis-level-4-cause
- 3.World Health Organization . Ending the neglect to attain the sustainable development goals: a road map for neglected tropical diseases 2021–2030 World Health Organization. Geneva: WHO Press; 2020. [Google Scholar]
- 4.Forbes K, Basáñez M-G, Hollingsworth TD, Anderson RM. Introduction to the special issue: challenges and opportunities in the fight against neglected tropical diseases: a decade from the London declaration on NTDs. Philos Trans R Soc B Biol Sci. 2023 doi: 10.1098/rstb.2022.0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collyer BS, Truscott JE, Mwandawiro CS, Njenga SM, Anderson RM. How important is the spatial movement of people in attempts to eliminate the transmission of human helminth infections by mass drug administration? Philos Trans R Soc B Biol Sci. 2023;378:20220273. doi: 10.1098/rstb.2022.0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaudhry U, Miller M, Yazwinski T, Kaplan R, Gilleard J. The presence of benzimidazole resistance mutations in Haemonchus placei from US cattle. Vet Parasitol. 2014;204:411–415. doi: 10.1016/j.vetpar.2014.05.025. [DOI] [PubMed] [Google Scholar]
- 7.Demeler J, Krüger N, Krücken J, von der Heyden VC, Ramünke S, Küttler U, et al. Phylogenetic characterization of β-tubulins and development of pyrosequencing assays for benzimidazole resistance in cattle nematodes. PLoS ONE. 2013;8:e70212. doi: 10.1371/journal.pone.0070212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furtado LFV, de Paiva Bello ACP, Rabelo ÉML. Benzimidazole resistance in helminths: from problem to diagnosis. Acta Trop. 2016;162:95–102. doi: 10.1016/j.actatropica.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 9.Melville LA, Redman E, Morrison AA, Rebecca Chen PC, Avramenko R, Mitchell S, et al. Large scale screening for benzimidazole resistance mutations in Nematodirus battus, using both pyrosequence genotyping and deep amplicon sequencing, indicates the early emergence of resistance on UK sheep farms. Int J Parasitol Drugs Drug Resist. 2020;12:68–76. doi: 10.1016/j.ijpddr.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Redman E, Whitelaw F, Tait A, Burgess C, Bartley Y, Skuce PJ, et al. The emergence of resistance to the benzimidazole anthlemintics in parasitic nematodes of livestock is characterised by multiple independent hard and soft selective sweeps. PLoS Negl Trop Dis. 2015;9:1–24. doi: 10.1371/journal.pntd.0003494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Von Samson-Himmelstjerna G, Blackhall WJ, McCarthy JS, Skuce PJ. Single nucleotide polymorphism (SNP) markers for benzimidazole resistance in veterinary nematodes. Parasitology. 2007;134:1077–1086. doi: 10.1017/S0031182007000054. [DOI] [PubMed] [Google Scholar]
- 12.Massaglia S, Merlino VM, Borra D, Verduna T, Renna M, Rambozzi L. Impact of swine ascariasis on feeding costs and revenues in farms associated with the Italian PDOS dry-cured hams industry. Qual - Access to Success. 2018;19:146–154. [Google Scholar]
- 13.Whitehead B, Thamsborg SM, Denwood MJ, Nejsum P. Assessing the impact of Ascariasis and Trichuriasis on weight gain using a porcine model. PLoS Negl Trop Dis. 2022 doi: 10.1371/journal.pntd.0010709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furtado LFV, Medeiros CDS, Zuccherato LW, Alves WP, De Oliveira VNGM, Da Silva VJ, et al. First identification of the benzimidazole resistance-associated F200Y SNP in the betatubulin gene in Ascaris lumbricoides. PLoS ONE. 2019;14:1–11. doi: 10.1371/journal.pone.0224108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krücken J, Fraundorfer K, Mugisha JC, Ramünke S, Sifft KC, Geus D, et al. Reduced efficacy of albendazole against Ascaris lumbricoides in Rwandan schoolchildren. Int J Parasitol Drugs Drug Resist. 2017;7:262–271. doi: 10.1016/j.ijpddr.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghisi M, Kaminsky R, Mäser P. Phenotyping and genotyping of Haemonchus contortus isolates reveals a new putative candidate mutation for benzimidazole resistance in nematodes. Vet Parasitol. 2007;144:313–320. doi: 10.1016/j.vetpar.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Kwa MSG, Veenstra JG, Roos MH. Benzimidazole resistance in Haemonchus contortus is correlated with a conserved mutation at amino acid 200 in β-tubulin isotype 1. Mol Biochem Parasitol. 1994;63:299–303. doi: 10.1016/0166-6851(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 18.Silvestre A, Cabaret J. Mutation in position 167 of isotype 1 β-tubulin gene of Trichostrongylid nematodes: role in benzimidazole resistance? Mol Biochem Parasitol. 2002;120:297–300. doi: 10.1016/S0166-6851(01)00455-8. [DOI] [PubMed] [Google Scholar]
- 19.Rufener L, Kaminsky R, Mäser P. In vitro selection of Haemonchus contortus for benzimidazole resistance reveals a mutation at amino acid 198 of β-tubulin. Mol Biochem Parasitol. 2009;168:120–122. doi: 10.1016/j.molbiopara.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Roose S, Avramenko RW, Pollo SMJ, Wasmuth JD, Ame S, Ayana M, et al. Characterization of the β-tubulin gene family in Ascaris lumbricoides and Ascaris suum and its implication for the molecular detection of benzimidazole resistance. PLoS Negl Trop Dis. 2021;15:0009777. doi: 10.1371/journal.pntd.0009777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones BP, van Vliet AHM, LaCourse EJ, Betson M. Identification of key interactions of benzimidazole resistance-associated amino acid mutations in Ascaris β-tubulins by molecular docking simulations. Sci Rep. 2022;12:1–13. doi: 10.1038/s41598-022-16765-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tydén E, Dahlberg J, Karlberg O, Höglund J. Deep amplicon sequencing of preselected isolates of Parascaris equorum in β-tubulin codons associated with benzimidazole resistance in other nematodes. Parasit Vectors. 2014;7:1–8. doi: 10.1186/1756-3305-7-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tydén E, Skarin M, Andersson-Franko M, Sjöblom M, Höglund J. Differential expression of β-tubulin isotypes in different life stages of Parascaris spp after exposure to thiabendazole. Mol Biochem Parasitol. 2016;205:22–28. doi: 10.1016/j.molbiopara.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Jones BP, van Vliet AHM, LaCourse EJ, Betson M. In silico docking of nematode β-tubulins with benzimidazoles points to gene expression and orthologue variation as factors in anthelmintic resistance. Front Trop Dis. 2022;3:54. doi: 10.3389/fitd.2022.898814. [DOI] [Google Scholar]
- 25.Collins JB, Jordan B, Baldwin L, Hebron C, Paras K, Vidyashankar AN, et al. Resistance to fenbendazole in Ascaridia dissimilis, an important nematode parasite of turkeys. Poult Sci. 2019;98:5412–5415. doi: 10.3382/ps/pez379. [DOI] [PubMed] [Google Scholar]
- 26.Martin F, Halvarsson P, Delhomme N, Höglund J, Tydén E. Exploring the β-tubulin gene family in a benzimidazole-resistant Parascaris univalens population. Int J Parasitol Drugs Drug Resist. 2021;17:84–91. doi: 10.1016/j.ijpddr.2021.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Özben M, Von Samson-Himmelstjerna G, Freiin Von Streit MKB, Wilkes EJA, Hughes KJ, Krücken J. Absence of polymorphisms in codons 167, 198 and 200 of all seven β-tubulin isotypes of benzimidazole susceptible and resistant Parascaris spp Specimens from Australia. Pathog. 2022;11:490. doi: 10.3390/pathogens11050490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diawara A, Halpenny CM, Churcher TS, Mwandawiro C, Kihara J, Kaplan RM, et al. Association between response to albendazole treatment and β-tubulin genotype frequencies in soil-transmitted helminths. PLoS Negl Trop Dis. 2013;7:e2247. doi: 10.1371/journal.pntd.0002247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palma A, Matamoros G, Escobar D, Sánchez AL, Fontecha G. Absence of mutations associated with resistance to benzimidazole in the beta-tubulin gene of Ascaris suum. Rev Soc Bras Med Trop. 2020;53:2–4. doi: 10.1590/0037-8682-0155-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zuccherato LW, Furtado LF, da Medeiros C. screening of polymorphisms associated with benzimidazole resistance in Necator americanus and Ascaris lumbricoides from different geographical regions in Brazil. PLoS Negl Trop Dis. 2018;12:1–13. doi: 10.1371/journal.pntd.0006766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Avramenko RW, Redman EM, Melville L, Bartley Y, Wit J, Queiroz C, et al. Deep amplicon sequencing as a powerful new tool to screen for sequence polymorphisms associated with anthelmintic resistance in parasitic nematode populations. Int J Parasitol. 2019;49:13–26. doi: 10.1016/j.ijpara.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Sargison ND, MacLeay M, Morrison AA, Bartley DJ, Evans M, Chaudhry U. Development of amplicon sequencing for the analysis of benzimidazole resistance allele frequencies in field populations of gastrointestinal nematodes. Int J Parasitol Drugs Drug Resist. 2019;10:92–100. doi: 10.1016/j.ijpddr.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaudhry U, Redman EM, Raman M, Gilleard JS. Genetic evidence for the spread of a benzimidazole resistance mutation across southern India from a single origin in the parasitic nematode Haemonchus contortus. Int J Parasitol. 2015;45:721–728. doi: 10.1016/j.ijpara.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Betson M, Nejsum P, Stothard JR. From the twig tips to the deeper branches: new insights into evolutionary history and phylogeography of Ascaris. In: Holland CV, editor. Ascaris: The Neglected Parasite. Amsterdam: Elsevier; 2013. [Google Scholar]
- 35.Betson M, Stothard JR. Ascaris lumbricoides or Ascaris suum: What’s in a name? J Infect Dis. 2016;213:1355–1356. doi: 10.1093/infdis/jiw037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.da Silva Alves EB, Conceição MJ, Leles D. Ascaris lumbricoides, Ascaris suum, or “Ascaris lumbrisuum”. J Infect Dis. 2016;213:1355. doi: 10.1093/infdis/jiw027. [DOI] [PubMed] [Google Scholar]
- 37.Leles D, Gardner SL, Reinhard K, Iñiguez A, Araujo A. Are Ascaris lumbricoides and Ascaris suum a single species? Parasit Vectors. 2012;5:42. doi: 10.1186/1756-3305-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson TJC. Ascaris infections in humans from North America: molecular evidence for cross-infection. Parasitology. 1995;110:215–219. doi: 10.1017/S0031182000063988. [DOI] [PubMed] [Google Scholar]
- 39.Bendall RP, Barlow M, Betson M, Stothard JR, Nejsum P. Zoonotic Ascariasis, United Kingdom. Emerg Infect Dis. 2011;17:1964–1966. doi: 10.3201/eid1710.101826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Betson M, Nejsum P, Bendall RP, Deb RM, Stothard JR. Molecular epidemiology of ascariasis: a global perspective on the transmission dynamics of Ascaris in people and pigs. J Infect Dis. 2014;210:932–941. doi: 10.1093/infdis/jiu193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nejsum P, Betson M, Bendall RP, Thamsborg SM, Stothard JR. Assessing the zoonotic potential of Ascaris suum and Trichuris suis: looking to the future from an analysis of the past. J Helminthol. 2012;86:148–155. doi: 10.1017/S0022149X12000193. [DOI] [PubMed] [Google Scholar]
- 42.Nejsum P, Parker ED, Frydenberg J, Roepstorff A, Boes J, Haque R, et al. Ascariasis is a zoonosis in Denmark. J Clin Microbiol. 2005;43:1142–1148. doi: 10.1128/JCM.43.3.1142-1148.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou C, Jian S, Peng W, Li M. Genetic diversity of Ascaris in China assessed using simple sequence repeat markers. Korean J Parasitol. 2018;56:175–181. doi: 10.3347/kjp.2018.56.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou C, Li M, Yuan K, Deng S, Peng W. Pig Ascaris: An important source of human ascariasis in China. Infect Genet Evol. 2012;12:1172–1177. doi: 10.1016/j.meegid.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 45.Easton A, Gao S, Lawton SP, Bennuru S, Khan A, Dahlstrom E, et al. Molecular evidence of hybridization between pig and human Ascaris indicates an interbred species complex infecting humans. Elife. 2020;9:1–26. doi: 10.7554/eLife.61562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mes THM, Ploeger HW, Terlou M, Kooyman FNJ, van der Ploeg MPJ, Eysker M. A novel method for the isolation of gastro-intestinal nematode eggs that allows automated analysis of digital images of egg preparations and high throughput screening. Parasitology. 2001;123:309–14. doi: 10.1017/S0031182001008496. [DOI] [PubMed] [Google Scholar]
- 47.Kang Y, Wang J, Davis R. Nuclei isolation from nematode ascaris. Bio-Protocol. 2017;7:1–10. doi: 10.21769/BioProtoc.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao J, Han Q, Liao C, Wang J, Wu L, Liu Q, et al. Effects of in vivo and in vitro treatment of Ascaris suum eggs with anthelmintic agents on embryonation and infectivity for mice. J Parasitol. 2017;103:598–601. doi: 10.1645/17-21. [DOI] [PubMed] [Google Scholar]
- 49.Zhao J, Williams AR, Hansen TVA, Thamsborg SM, Cai J, Song S, et al. An in vitro larval migration assay for assessing anthelmintic activity of different drug classes against Ascaris suum. Vet Parasitol. 2017;238:43–48. doi: 10.1016/j.vetpar.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 50.Cruz LM, Allanson M, Kwa B, Azizan A, Izurieta R. Morphological changes of Ascaris spp eggs during their development outside the host. J Parasitol. 2012;98:63–8. doi: 10.1645/GE-2821.1. [DOI] [PubMed] [Google Scholar]
- 51.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria; 2021. https://www.r-project.org/
- 52.Callahan BJ, Mcmurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. dada2: high-resolution sample inference from illumina amplicon data. 2016. 13. [DOI] [PMC free article] [PubMed]
- 53.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011;17:10. doi: 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- 54.McMurdie PJ, Holmes S. phyloseq: An r package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013;8:e61217. doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- 56.Wickham H. Ggplot2: elegant graphics for data analysis. 2. Cham, Switzerland: Springer International Publishing; 2016. [Google Scholar]
- 57.Adamack AT, Gruber B. PopGenReport: simplifying basic population genetic analyses in R. Methods Ecol Evol. 2014;5:384–387. doi: 10.1111/2041-210X.12158. [DOI] [Google Scholar]
- 58.Rozas J, Ferrer-Mata A, Sanchez-DelBarrio JC, Guirao-Rico S, Librado P, Ramos-Onsins SE, et al. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol Biol Evol. 2017;34:3299–3302. doi: 10.1093/molbev/msx248. [DOI] [PubMed] [Google Scholar]
- 59.Leigh JW, Bryant D. POPART: Full-feature software for haplotype network construction. Methods Ecol Evol. 2015;6:1110–1116. doi: 10.1111/2041-210X.12410. [DOI] [Google Scholar]
- 60.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–9. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lê S, Josse J, Husson F. FactoMineR: An R Package for Multivariate Analysis. J Stat Softw. 2008;25:1–18. doi: 10.18637/jss.v025.i01. [DOI] [Google Scholar]
- 62.Kassambara A, Mundt F. Extract and visualize the results of multivariate data analyses. 2020.
- 63.Excoffier L, Lischer HEL. Arlequin suite ver 35: a new series of programs to perform population genetics analyses under linux and windows. Mol Ecol Resour. 2010;10:564–7. doi: 10.1111/j.1755-0998.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- 64.Saunders GI, Wasmuth JD, Beech R, Laing R, Hunt M, Naghra H, et al. Characterization and comparative analysis of the complete Haemonchus contortus β-tubulin gene family and implications for benzimidazole resistance in strongylid nematodes. Int J Parasitol. 2013;43:465–475. doi: 10.1016/j.ijpara.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 65.Anderson TJC, Jaenike J. Host specificity, evolutionary relationships and macrogeographic differentiation among Ascaris populations from humans and pigs. Parasitology. 1997;115:325–342. doi: 10.1017/S0031182097001339. [DOI] [PubMed] [Google Scholar]
- 66.Monteiro KJL, Calegar DA, Santos JP, Bacelar PAA, Coronato-Nunes B, Reis ERC, et al. Genetic diversity of Ascaris spp infecting humans and pigs in distinct Brazilian regions, as revealed by mitochondrial DNA. PLoS One. 2019;14:0218867. doi: 10.1371/journal.pone.0218867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nejsum P, Frydenberg J, Roepstorff A, Parker ED. Population structure in Ascaris suum (Nematoda) among domestic swine in Denmark as measured by whole genome DNA fingerprinting. Hereditas. 2005;142:7–14. doi: 10.1111/j.1601-5223.2005.01864.x. [DOI] [PubMed] [Google Scholar]
- 68.Nadler SA, Lindquist RL, Near TJ. Genetic Structure of Midwestern Ascaris suum Populations: A Comparison of Isoenzyme and RAPD Markers. J Parasitol. 1995;81:385. doi: 10.2307/3283820. [DOI] [PubMed] [Google Scholar]
- 69.Zou Y, Wu F, Guo Y-X, Wang H-B, Fang Y-Q, Kang M, et al. Determining geographical variations in Ascaris suum isolated from different regions in northwest China through sequences of three mitochondrial genes. Mitochondrial DNA Part A. 2017;28:411–415. doi: 10.3109/19401736.2015.1129404. [DOI] [PubMed] [Google Scholar]
- 70.Höglund J, Morrison DA, Engström A, Nejsum P, Jansson DS. Population genetic structure of Ascaridia galli re-emerging in non-caged laying hens. Parasit Vectors. 2012;5:97. doi: 10.1186/1756-3305-5-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Coeli R, Baba EH, Araujo N, Coelho PMZ, Oliveira G. Praziquantel treatment decreases Schistosoma mansoni genetic diversity in experimental infections. PLoS Negl Trop Dis. 2013;7:2596. doi: 10.1371/journal.pntd.0002596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Daniels RF, Schaffner SF, Bennett A, Porter TR, Yukich JO, Mulube C, et al. Evidence for reduced malaria parasite population after application of population-level antimalarial drug strategies in Southern Province. Zambia Am J Trop Med Hyg. 2020;103:66–73. doi: 10.4269/ajtmh.19-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kajero OT, Janoušková E, Bakare EA, Belizario V, Divina B, Alonte AJ, et al. Co-infection of intestinal helminths in humans and animals in the Philippines. Trans R Soc Trop Med Hyg. 2022;116:727–735. doi: 10.1093/trstmh/trac002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1. β-Tubulin isotype A (BtA) amplicon sequencing variants (ASVs) sequence alignment. Fig. S2. β-Tubulin isotype B (BtB) amplicon sequencing variants (ASVs) sequence alignment. Fig. S3. Maximum likelihood phylogeny of Ascaris β-tubulin genotypes. Table S1. Sample information and β-tubulin isotype alleles.
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files.