Abstract
Introduction
Regdanvimab, a monoclonal antibody pharmaceutical, is the first Korean drug approved for treating coronavirus disease 2019 (COVID-19). We analyzed the therapeutic efficacy of regdanvimab in patients with the COVID-19 delta variant infection.
Methods
We retrospectively reviewed the electronic medical records of patients hospitalized at two Korean tertiary COVID-19 hospitals with COVID-19 delta variant infection between May 26, 2021, and January 30, 2022. To analyze the therapeutic efficacy of regdanvimab, the patients were divided into regdanvimab and non-regdanvimab groups and were 1:1 propensity-score (PS)-matched on age, severity at admission, and COVID-19 vaccination history.
Results
Of 492 patients, 262 (53.3%) and 230 (46.7%) were in the regdanvimab and non-regdanvimab groups, respectively. After PS matching the groups on age, severity at admission, and COVID-19 vaccination history, each group comprised 189 patients. The 30-day hospital mortality rates (0.0% vs. 1.6%, p = 0.030), proportions of patients with exacerbated conditions to severe/critical/died (9.5% vs. 16.4%, p = 0.047), proportions who received oxygen therapy because of pneumonia exacerbation (7.4% vs. 16.4%, p = 0.007), and proportions with a daily National Early Warning Score ≥ 5 from hospital day 2 were significantly lower in the regdanvimab group.
Conclusions
We showed that regdanvimab reduced the exacerbation rates of conditions and mortality in patients with the COVID-19 delta variant infection. Thus, it is recommended to streamline the drug approval system during epidemics of new variant viruses to improve the availability and usage of therapeutics for patients. To facilitate this, relevant institutional support is required.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-024-00971-w.
Keywords: Monoclonal antibody, COVID-19, Delta variant, Regdanvimab, Treatment outcome
Key Summary Points
| Why carry out the study? |
| Regdanvimab, a recombinant monoclonal antibody, was temporarily used as a treatment for COVID-19 during the delta variant pandemic wave |
| We assessed the therapeutic efficacy of regdanvimab in hospitalized patients with COVID-19 using propensity score matching |
| What was learned from the study? |
| Regdanvimab was associated with lower 30-day hospital mortality rates, proportions of patients with exacerbated conditions (severe/critical/death), and proportions of patients requiring oxygen therapy because of pneumonia exacerbation in hospitalized patients with COVID-19 |
| Regdanvimab was associated with rapid clinical improvement in hospitalized patients with COVID-19 |
Introduction
Monoclonal antibody therapeutics for coronavirus disease 2019 (COVID-19) are neutralizing antibodies developed by harvesting neutralizing antibody genes from the blood of patients who have recovered from COVID-19 and mass producing them using recombinant DNA technology [1]. Regdanvimab (Regkirona; Celltrion Inc., Incheon, South Korea), a monoclonal antibody drug developed in Korea, prevents intracellular penetration of severe acute respiratory coronavirus 2 (SARS-CoV-2) by blocking the binding site of the viral spike protein receptor-binding domain to the host’s cellular angiotensin-converting enzyme 2 receptor [2]. Following the conditional marketing authorization of the Korean Ministry of Food and Drug Safety (MFDS) in February 2021, regdanvimab usage commenced clinically in Korea's COVID-19-designated healthcare facilities [3]. Subsequently, a phase III clinical trial showed a significant reduction in the progression of infection severity and recovery time in patients with mild to moderate high-risk COVID-19. Accordingly, in Korea, on September 17, 2021, full regulatory approval with an expanded indication and reduced infusion duration was granted [3, 4].
SARS-CoV-2, the pathogen causing COVID-19, is a single-strain RNA virus, and most virus strains undergo mutations as part of their evolution during the process of transmission and proliferation to invade the immune system [5, 6]. If a variant provides the virus a selective advantage, it can spread more widely. Since the index case of SARS-CoV-2 infection in December 2019, several variants have emerged, including the beta (B.1.351) and Omicron (B.1.1.529) variants, alpha variant (B.1.1.7), delta variant (B.1.617.2), and gamma variant. Monoclonal antibody drugs such as regdanvimab were used during the delta variant outbreak, but their supply was halted because the Omicron variant, with twice as many spike protein mutations as the delta variant, became dominant, and the drug showed low to no neutralizing activity against the Omicron variant [7].
Regdanvimab has been shown to reduce the viral load and ameliorate clinical symptoms in gamma or delta variant-infected mice [8]. However, there are conflicting studies on the effectiveness [9, 10] and ineffectiveness of regdanvimab in patients with the delta variant in clinical practice [11]. Because the prognosis of COVID-19 varies depending on the vaccination status and type of variant in addition to the therapeutic agents used, it is challenging to examine the pure effects of a drug [12–14]. To conduct an accurate analysis of the efficacy of regdanvimab in clinical practice, we divided patients hospitalized because of the COVID-19 delta variant infection into groups treated with and without regdanvimab and propensity matched them by age, severity at admission, and COVID-19 vaccination history. In addition, we calculated and plotted the daily National Early Warning Score (NEWS), which includes the patients’ vital signs, to compare the clinical course of patients over time.
Methods
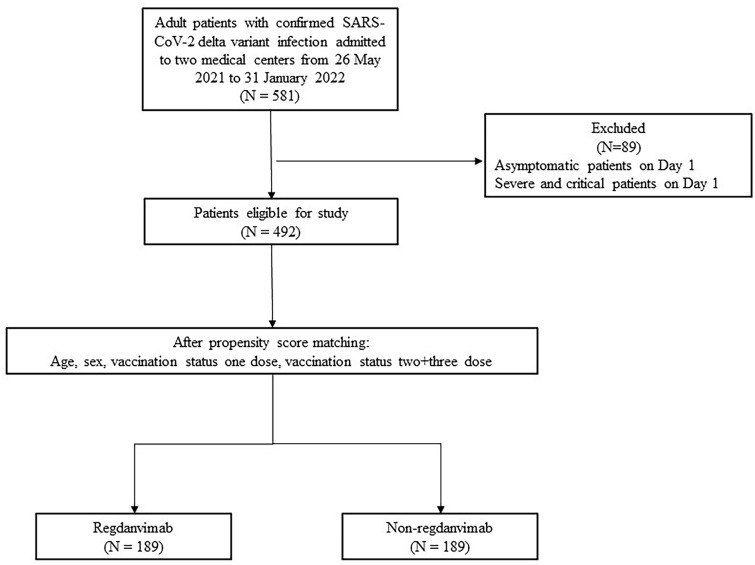
This retrospective study analyzed the electronic medical records (EMR) of patients with COVID-19 delta variant infection hospitalized in two COVID-19-designated tertiary hospitals in Korea (Kyunpook National University Chilgok Hospital and Kyunpook National University Hospital) between May 26, 2021, and January 30, 2022. This study enrolled patients (aged 19 years and older) with mild to moderate COVID-19 delta variant (B.1.617) infection severity at the time of admission (according to the National Institute of Health [NIH] severity classification system). COVID-19 was diagnosed using an MFDS-approved SARS-CoV-2 real-time polymerase chain reaction (RT-PCR) kit. The delta variant was tested for in residual samples using the PowerCheck SARS-CoV-2 S-gene mutation detection kit (Kogene Biotech Co., Ltd., Seoul, South Korea), spike protein sequencing, or whole genome sequencing to identify P681R and L452R mutations [15]. According to the NIH severity classification system, patients with mild to moderate conditions have > 94% oxygen saturation on room air, do not require oxygen supplementation, and have mild symptoms [16]. During the study period, regdanvimab was approved for use among patients with mild to moderate disease who had a high risk of progressing to a severe infection in Korea. High-risk groups were defined as (1) aged > 50 years, (2) having a pre-existing disease (one or more of the following: obesity [BMI > 30 kg/m2], cardiovascular disease [CVD], chronic lung disease, diabetes mellitus, chronic kidney disease, chronic liver disease, immunosuppressed state), or (3) pneumonia findings on chest x-ray or chest computed tomography (CT) and within 7 days since symptom onset [3]. To investigate the therapeutic efficacy of regdanvimab in clinical settings, we propensity score (PS) matched the regdanvimab and non-regdanvimab groups and compared their characteristics and treatment outcomes. Patients with mild to moderate conditions at the time of admission were 1:1 PS matched on age, severity at admission, and vaccination status (unvaccinated, first dose, second or third dose). As shown in Fig. 1, 189 patients each were included in the regdanvimab and non-regdanvimab groups, respectively, in this study. The clinical characteristics and treatment outcomes were compared between the two groups, and the daily NEWS plot was used to compare the clinical course between the two groups. Data were analyzed using the R software (version 4.1.1; The R Project for Statistical Computing, Vienna, Austria), and the modified Charlson Comorbidity Index (CCI) score was analyzed using the Kruskal-Wallis test and median values. The differences in the daily NEWS between the two groups were analyzed using an independent t-test, whereas other variables were analyzed using Pearson’s chi-squared test and Fisher’s exact test. To evaluate the PS-matched score balance, the cut-off score was set to an absolute value of 0.1 and analyzed using the standard mean difference (SMD) (Supplementary Table 3). This study was approved by the Daegu Joint Institutional Review Board, and the requirement for official written informed consent was waived (DGIRB 2021-10-002), given the retrospective nature of the study (using EMR and residual samples).
Fig. 1.
Patient disposition
Results
Of patients admitted to the two tertiary hospitals from May 26, 2021, to January 30, 2022, for COVID-19, 581 had the delta variant (523 and 58 in Kyungpook National University Chilgok Hospital and Kyungpook National University Hospital, respectively). After excluding 89 patients who were asymptomatic or had severe or critical conditions at the time of admission, 492 patients were enrolled in the study. Supplementary Table 1 shows the patients’ clinical characteristics and treatment outcomes before PS matching.
Tables 1 and 2 show the basic clinical characteristics and treatment outcomes of the regdanvimab and non-regdanvimab groups after PS matching on age, sex, vaccination status, and severity at the time of admission (proportion with mild and moderate condition). Of 378 PS-matched patients, 221 (58.5%) were male; 156 (41.3%) were aged < 50 years, and 12.2% (46/378) had a BMI ≥ 30 kg/m2, with a non-significantly higher mean BMI in the regdanvimab group (15.3% vs. 9.0%, p = 0.059). Of those with pre-existing conditions, 67 (17.7%) had diabetes mellitus, and of these, the regdanvimab group had a significantly higher proportion than the non-regdanvimab group (22.2% vs. 13.2%, p = 0.022). At the time of admission, the unvaccinated proportions in both groups (225 [59.5%]) were similar ([112/189, 59.3%] vs. 113/189, [59.8%]) (p = 0.917). The percentage of patients who received the first (12.2% vs. 12.2%, p = 1) and second doses of vaccine (27.0% vs. 28.0%, p = 0.818) did not significantly differ between the two groups. At admission, of 378 patients, 309 (81.7%) and 69 (18.3%) had mild and moderate conditions, respectively, and their proportions [(80.4% vs. 83.1%) and (19.6% vs. 16.9%)] did not differ between groups (p = 0.506). Also at admission, having a fever (≥ 37.5ºC), any symptoms (dyspnea, cough, sputum, diarrhea, sore throat, fatigue, and nausea) or pneumonia did not differ between the two groups (p > 0.05).
Table 1.
Clinical characteristics in propensity score-matched group
| Variable | Total (N = 378) | Use of regdanvimab (N = 189) | No use of regdanvimab (N = 189) | p value |
|---|---|---|---|---|
| Male | 221 (58.5%) | 112 (59.3%) | 109 (57.7%) | 0.754 |
| Age | 0.918 | |||
| < 50 | 156 (41.3%) | 79 (41.8%) | 77 (40.7%) | |
| 50–59 | 65 (17.2%) | 34 (18.0%) | 31 (16.4%) | |
| 60–69 | 77 (20.4%) | 35 (18.5%) | 42 (22.2%) | |
| 70–79 | 52 (13.8%) | 26 (13.8%) | 26 (13.8%) | |
| ≥ 80 | 28 (7.4%) | 15 (7.9%) | 13 (6.9%) | |
| Body mass index ≥ 30 kg/m2 | 46 (12.2%) | 29 (15.3%) | 17 (9.0%) | 0.059 |
| Medical comorbidities | ||||
| Myocardial infarction | 11 (2.9%) | 6 (3.2%) | 5 (2.6%) | 0.760 |
| Heart failure | 7 (1.9%) | 2 (1.1%) | 5 (2.6%) | 0.449 |
| Cardiovascular disease | 3 (0.8%) | 3 (1.6%) | 0 (0.0%) | 0.248 |
| Neurovascular disease | 21 (5.6%) | 11 (5.8%) | 10 (5.3%) | 0.822 |
| Chronic obstructive pulmonary disease | 3 (0.8%) | 3 (1.6%) | 0 (0.0%) | 0.248 |
| Connective tissue disease | 11 (2.9%) | 7 (3.7%) | 4 (2.1%) | 0.359 |
| Chronic liver disease | 14 (3.7%) | 10 (5.3%) | 4 (2.1%) | 0.102 |
| Diabetes | 67 (17.7%) | 42 (22.2%) | 25 (13.2%) | 0.022 |
| Chronic kidney disease | 11 (2.9%) | 5 (2.6%) | 6 (3.2%) | 0.760 |
| Solid tumor | 0.458 | |||
| Localized | 34 (9.0%) | 16 (8.5%) | 18 (9.5%) | |
| Metastatic | 5 (1.3%) | 4 (2.1%) | 1 (0.5%) | |
| Vaccination status | ||||
| None | 225 (59.5%) | 112 (59.3%) | 113 (59.8%) | 0.917 |
| One dose | 46 (12.2%) | 23 (12.2%) | 23 (12.2%) | 1 |
| Two-dose | 104 (27.5%) | 51 (27.0%) | 53 (28.0%) | 0.818 |
| Three-dose | 3 (0.8%) | 3 (1.6%) | 0 (0.0%) | 0.248 |
| Severity on admission | 0.506 | |||
| Mild | 309 (81.7%) | 152 (80.4%) | 157 (83.1%) | |
| Moderate | 69 (18.3%) | 37 (19.6%) | 32 (16.9%) | |
| Fever (temperature ≥ 37.5 °C) | 178 (47.1%) | 95 (50.3%) | 83 (43.9%) | 0.216 |
| Symptoms | ||||
| Dyspnea | 58 (15.3%) | 28 (14.8%) | 30 (15.9%) | 0.775 |
| Cough | 179 (47.4%) | 92 (48.7%) | 87 (46.0%) | 0.607 |
| Diarrhea | 13 (3.4%) | 6 (3.2%) | 7 (3.7%) | 1 |
| Sputum | 75 (19.8%) | 33 (17.5%) | 42 (22.2%) | 0.246 |
| Sore throat | 97 (25.7%) | 41 (21.7%) | 56 (29.6%) | 0.077 |
| Fatigue | 7 (1.9%) | 4 (2.1%) | 3 (1.6%) | 1 |
| Myalgia | 80 (21.2%) | 39 (20.6%) | 41 (21.7%) | 0.801 |
| Nausea | 1 (0.3%) | 0 (0.0%) | 1 (0.5%) | 1 |
| Pneumonia at admission | 72 (19.0%) | 37 (19.6%) | 35 (18.5%) | 0.793 |
Table 2.
Clinical outcomes in propensity score matched group
| Variable | Total (N = 378) | Use of regdanvimab (N = 189) | No use of regdanvimab (N = 189) | p value |
|---|---|---|---|---|
| Use of oxygen therapies | 45 (11.9%) | 14 (7.4%) | 31 (16.4%) | 0.007 |
| Use of high flow nasal cannula | 19 (5.0%) | 5 (2.6%) | 14 (7.4%) | 0.034 |
| Use of mechanical ventilation | 2 (0.5%) | 1 (0.5%) | 1 (0.5%) | 1 |
| Use of intensive care units | 20 (5.3%) | 8 (4.2%) | 12 (6.3%) | 0.358 |
| Worst severity during hospitalization | < 0.001 | |||
| Asymptomatic | 2 (0.5%) | 2 (1.1%) | 0 (0.0%) | |
| Mild | 211 (55.8%) | 88 (46.6%) | 123 (65.1%) | |
| Moderate | 116 (30.7%) | 81 (42.9%) | 35 (18.5%) | |
| Severe | 41 (10.8%) | 17 (9.0%) | 24 (12.7%) | |
| Critical | 8 (2.1%) | 1 (0.5%) | 7 (3.7%) | |
| Mortality | 7 (1.9%) | 1 (0.5%) | 6 (3.2%) | 0.122 |
| 30-Day mortality | 6 (1.6%) | 0 (0.0%) | 6 (3.2%) | 0.030 |
| Severe/critical or mortality | 49 (13.0%) | 18 (9.5%) | 31 (16.4%) | 0.047 |
| Use of additional therapeutic agents | ||||
| Remdesivir | 34 (9.0%) | 13 (6.9%) | 21 (11.1%) | 0.150 |
| Dexamethasone | 42 (11.1%) | 16 (8.5%) | 26 (13.8%) | 0.102 |
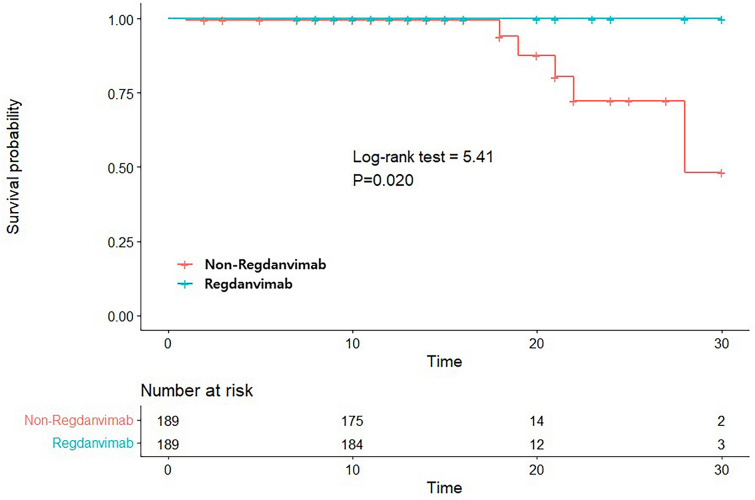
In Table 2, the percentages of patients who required supplemental oxygen therapy because of exacerbation of pneumonia during hospital stay (7.4% vs. 16.4%, p = 0.007), those who were placed on high-flow nasal cannula (HFNC) (2.6% vs. 7.4%, p = 0.034), 30-day mortality (0.0% vs. 3.2%, p = 0.030, Kaplan-Meier survival curve in Fig. 2), and those progressing to severe or critical condition or died during hospital stay (9.5% vs. 16.4%, p < 0.001) were significantly lower in the regdanvimab group. Furthermore, those who were mechanically ventilated (0.5% vs. 0.5%, p = 1) or admitted to the intensive care unit (ICU) (4.2% vs. 6.3%, p = 0.358) did not significantly differ between groups. In terms of the worst level of severity identified after admission, the numbers of those progressing to severe versus critical levels in the regdanvimab group (9.9% [17/189] vs. 0.5% [1/189]) were significantly lower than for those in the non-regdanvimab group (12.7% [24/189] vs. 3.7% [7/189]), respectively (p < 0.001). All-cause mortality rates did not differ between regdanvimab and non-regdanvimab groups (0.5% [1/189] vs. 3.2% [6/189], p = 0.122).
Fig. 2.
Kaplan-Meier survival curves for 30-day survival according to use of regdanvimab
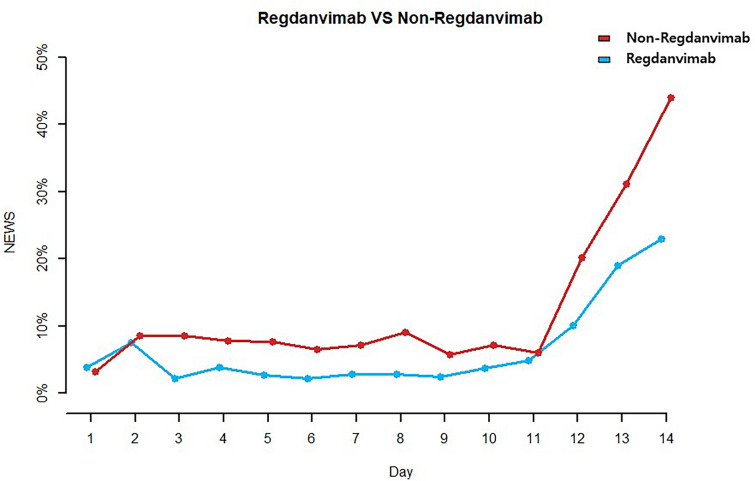
Table 3 and Fig. 3 show the percentages of patients with a daily NEWS ≥ 5 (calculated by daily heart rate, respiration rate, systolic blood pressure, oxygen saturation, and body temperature) during hospital stay in both groups. On hospital days (HD) 3 (9/188, 4.8% vs. 18/187, 9.6%; p = 0.011) and 8 (7/186, 3.8% vs. 17/168, 10.1%; p = 0.020), the percentages of patients with a NEWS ≥ 5 were significantly lower in the regdanvimab group.
Table 3.
Daily National Early Warning Score ≥ 5 ratio
| Use of regdanvimab | No use of regdanvimab | p value | |
|---|---|---|---|
|
Day 1 (n = 378) Use of regdanvimab: 189 No use of regdanvimab: 189 |
9 (4.8%) | 8 (4.2%) | 0.804 |
|
Day 2 (n = 378) Use of regdanvimab: 189 No use of regdanvimab: 189 |
16 (8.5%) | 18 (9.5%) | 0.719 |
|
Day 3 (n = 376) Use of regdanvimab: 189 No use of regdanvimab: 187 |
6 (3.2%) | 18 (9.6%) | 0.011 |
|
Day 4 (n = 370) Use of regdanvimab: 189 No use of regdanvimab: 181 |
9 (4.8%) | 16 (8.8%) | 0.118 |
|
Day 5 (n = 363) Use of regdanvimab: 188 No use of regdanvimab: 175 |
7 (3.7%) | 15 (8.6%) | 0.053 |
|
Day 6 (n = 360) Use of regdanvimab: 187 No use of regdanvimab: 173 |
6 (3.2%) | 13 (7.5%) | 0.068 |
|
Day 7 (n = 358) Use of regdanvimab: 186 No use of regdanvimab: 172 |
7 (3.8%) | 14 (8.1%) | 0.078 |
|
Day 8 (n = 350) Use of regdanvimab: 182 No use of regdanvimab: 168 |
7 (3.8%) | 17 (10.1%) | 0.020 |
|
Day 9 (n = 340) Use of regdanvimab: 177 No use of regdanvimab: 163 |
6 (3.4%) | 11 (6.7%) | 0.156 |
|
Day 10 (n = 315) Use of regdanvimab: 169 No use of regdanvimab: 146 |
8 (4.7%) | 12 (8.2%) | 0.206 |
|
Day 11 (n = 250) Use of regdanvimab: 135 No use of regdanvimab: 115 |
8 (5.9%) | 8 (7.0%) | 0.740 |
|
Day 12 (n = 83) Use of regdanvimab: 45 No use of regdanvimab: 38 |
5 (11.1%) | 8 (21.1%) | 0.214 |
|
Day 13 (n = 58) Use of regdanvimab: 30 No use of regdanvimab: 28 |
6 (20.0%) | 9 (32.1%) | 0.291 |
|
Day 14 (n = 46) Use of regdanvimab: 25 No use of regdanvimab: 21 |
6 (24.0%) | 9 (45.0%) | 0.138 |
Fig. 3.
Ratio of Daily National Early Warning Score ≥ 5 in the regdanvimab group and non-regdanvimab group
Discussion
In this retrospective observational study, we found that regdanvimab treatment was significantly associated with reduced 30-day hospital mortality and the proportions who received oxygen therapy because of pneumonia exacerbation in COVID-19 delta variant patients. In a previous study on the efficacy of regdanvimab, the percentage of patients placed on supplemental oxygen because of exacerbation of pneumonia was higher among 66 patients treated with regdanvimab during the delta variant-dominant period than 66 patients treated with regdanvimab during the pre-delta variant-dominant period, and the above study reported that the therapeutic efficacy of regdanvimab is unclear [17]. However, the above study did not confirm delta variant and only compared the before and during delta variant dominance and also did not adjust for vaccination status, which limits an accurate determination of the therapeutic effects of regdanvimab. Another recent study on patients with confirmed delta variant COVID-19 infection also reported that the percentages of patients with < 90% SpO2 on room air (1.0% vs. 3.5%, p = 0.0987), those placed on HFNC or higher oxygen supplementation (2.0% vs. 5.2%, p = 0.1029), and those who died (0.0% vs. 0.9%, p = 0.2791) were non-significantly lower among the 297 patients treated with regdanvimab compared with the 115 patients not treated with regdanvimab [9]. However, in their study, when analyzing all COVID-19 patients, the percentage of patients who deteriorated to < 90% SpO2 on room air was significantly lower (1.2% vs. 7.9%, p < 0.0001) among the 418 patients treated with regdanvimab compared with the 304 patients not treated with regdanvimab [9]. The therapeutic effects of regdanvimab in their study were consistent with the results of our study, but when comparing only the delta variant group, there was no significant difference with a p-value > 0.05. This is because their study did not consider the patient age, vaccination status, and severity. In their study, among all subgroups, the delta variant subgroup treated with regdanvimab exhibited the highest severity and the lowest vaccination rate, potentially leading to an underestimation of the effectiveness of regdanvimab. In our study, we included only patients confirmed to have delta variant infection and PS matched the regdanvimab and non-regdanvimab groups on age, sex, COVID-19 vaccination history, and severity at the time of admission (percentage of patients with mild and moderate conditions). These measures allowed our study to more accurately assess the therapeutic efficacy of regdanvimab against the COVID-19 delta variant than other previous studies.
Diabetes mellitus and obesity are major risk factors for COVID-19 [18]. In our study, there were no differences between groups in the percentage of patients with other pre-existing conditions; however, that with DM was significantly higher in the regdanvimab group, both before and after PS matching. Moreover, there were also significantly more patients with a BMI ≥ 30 kg/m2 in the regdanvimab group before PS matching. This is presumably because only patients at high risk for exacerbation of the condition, which includes those with diabetes mellitus and BMI ≥ 30 kg/m2, were indicated to receive regdanvimab. Despite the higher percentage of patients with obesity and diabetes mellitus, two major risk factors of COVID-19, in the regdanvimab group before PS matching, those who required supplemental oxygen because of exacerbation of pneumonia during hospital stay, those placed on HFNC, those progressing to a severe condition, and those who died were significantly fewer in the regdanvimab group than in the non-regdanvimab group.
In our study, the percentage of unvaccinated patients was significantly higher in the regdanvimab group (179/262, 68.3%) than in the non-regdanvimab group (113/179, 49.1%) before PS matching. Moreover, the percentage of patients with a moderate condition at the time of admission was higher in the regdanvimab group (25.6%) than in the non-regdanvimab group (14.8%). This may be attributable to the complex system when regdanvimab was first approved with emergency authorization for use on patients. Then, the healthcare providers had to obtain informed consent from the patient, submit a drug request via fax for approval, and receive the drug via parcel services. Therefore, regdanvimab was not used in vaccinated patients or those with mild conditions. Furthermore, patients with mild symptoms sometimes refused the drug because of reluctance to use a new drug. In addition, even after official authorization, the supply of regdanvimab was halted for a time because of its rapidly increased usage as well as when unvaccinated patients or those with severe conditions (those at a higher risk of clinical deterioration) were prioritized for the available drugs because the drug was insufficient for all patients who meet the indications. These reasons might have contributed to the higher percentage in the regdanvimab group.
To evaluate how quickly regdanvimab stabilizes patients’ vital signs, we calculated daily NEWS based on the respiration rate, oxygen saturations, any supplemental oxygen, temperature, systolic blood pressure, heart rate, and level of consciousness from the day of admission to discharge. NEWS has been used to assess patient prognosis in several studies. A systematic review of 121 articles showed that the most frequently used cut-off value reported in the literature was ≥ 5 points [19]. Furthermore, a study on NEWS and quick Sequential Organ Failure Assessment (qSOFA) in 1713 patients admitted to five acute care hospitals in the UK reported that NEWS is a better risk assessment tool than qSOFA and that the risk for ICU admission and death significantly increased among patients with a NEWS ≥ 5 [20]. Regarding these studies, we considered patients with a NEWS ≥ 5 as at high clinical risk group and compared the percentage between the two groups. From HD 2, there were fewer patients with a NEWS ≥ 5 in the regdanvimab group and significantly fewer on HD 3 and 8. Thus, regdanvimab is believed to have helped stabilize the vital signs of patients with COVID-19 delta variant infection.
First discovered in India in October 2020, the COVID-19 delta variant is reported to have a higher transmissibility and to spread more quickly than the original and alpha variants [21], with a higher risk for progression to severe infection and mortality compared to the alpha variant [22–24]. However, regdanvimab was available only to a limited number of patients because of the conditional approval for emergency use during the delta variant wave, and even patients indicated for regdanvimab could not receive the drug because of the complex supply process. Since 2020, monoclonal antibody COVID-19 drugs have been developed by several pharmaceutical companies at an unprecedented rate, and many countries granted an emergency-use authorization to promptly roll them out to COVID-19 patients [25]. Since its official authorization in September 2021 in Korea, regdanvimab has been supplied to many COVID-19 treatment facilities, including residential treatment centers and long-term care facilities. Nevertheless, its supply was suspended in February 2022—5 months after the official approval—because the Omicron variant became dominant. The Omicron variant has more than 30 spike protein mutations, and 15 of them are on the receptor-binding domain. Neutralizing antibodies such as regdanvimab act by binding to the receptor-binding domain of SARS-CoV 2 and prevent viral attachment to the host’s ACE2 receptor. However, several mutations on the receptor-binding domain, the binding site for neutralizing antibodies, in the Omicron variant reduced the sensitivity to neutralization by most monoclonal antibodies [26–28].
Our study provides important implications by confirming that regdanvimab was effective in reducing the rate of severity progression, lowering the 30-day mortality, and more quickly stabilizing vital signs in high-risk patients with the delta variant, which had higher severity and mortality rates compared to the Omicron variant. Although regdanvimab is not currently in use, the efficacy of monoclonal antibodies like regdanvimab remains contentious, and continued research on them holds promise for informing future treatments of viral infectious diseases.
The development of new drugs typically takes several years. However, Celltrion Inc., the manufacturers of regdanvimab, significantly shortened the period from drug development to completion of phase 2 clinical trials to 10 months [3]. One key strategy for rapid development was obtaining blood samples from the Korea Disease Control and Prevention Agency (KDCA) during the antibody discovery phase to construct a library of candidate antibodies. Furthermore, the time taken to select the final antibody was shortened through real-time virus neutralization tests conducted by the KDCA. Through pre-submission discussions with regulatory authorities, the company and regulatory agencies narrowed their differences in opinions, and they could swiftly review and supplement clinical trial protocols, chemistry, manufacturing, and control (CMC) as well as good manufacturing practice (GMP) by utilizing the pre-review system of the MFDS. Through these rapid preliminary discussions and rolling reviews in Korea, regdanvimab obtained conditional approvals for emergency use unusually quickly, just 1 month after submitting the final phase 2 clinical trial report. However, it is unfortunate that the process of applying for and receiving regdanvimab spanned from 1 to 4 days. According to the guidelines for using conditional approval drugs, physicians need to obtain explicit consent from the patient, submit the completed supply request form to the company via fax or email, and then receive the drug by delivery. Monoclonal antibodies such as regdanvimab are most effective when administered early in the course of illness. However, this process led to delayed administration of regdanvimab to patients, and in some cases, administration was not possible via fax or email delivery delays. In the future, it would be beneficial to implement real-time checking of consent forms and supply requests through internet computing. Additionally, preemptive distribution of drugs to local hospitals could enable same-day delivery, streamlining the process and ensuring timely access to medications.
This study had a few limitations. First, it was conducted in two centers and was a retrospective study using EMR. It would have been beneficial to involve additional facilities and a larger study population. However, the specific SARS-CoV-2 gene and mutations are not identified for most inpatients. Thus, since this study only included patients diagnosed with the delta variant, it was difficult to enroll patients from other facilities. However, the study hospitals were tertiary hospitals designated as COVID-19 hospitals in 2021; thus, they had a high number of high-risk inpatients, and the inpatients were representative of the COVID-19 inpatient population in Korea. Second, although we adjusted for age, sex, vaccination status, and severity at the time of admission through PS matching, we could not adjust for all confounders, such as pre-existing conditions and antibiotics used. However, that diabetes mellitus and obesity, which are key risk factors, were more prevalent in the regdanvimab group suggests that the therapeutic efficacy of regdanvimab could have actually been underestimated in our study. In addition, the use of antibiotics is helpful only in cases of co- or secondary infections, and there were no significant differences in the use of steroids and remdesivir, which have been confirmed to be helpful in treating patient outcomes of COVID-19 between the two groups. Thus, we believe that there is little bias from unmeasured confounding factors. Third, in this study, we were unable to determine the side effects of regdanvimab. However, there were no reported cases of side effects, including serious allergic reactions such as anaphylaxis, and there were no cases of discontinuation of administration due to decreased liver or kidney function following the administration of regdanvimab.
Conclusions
In this study, regdanvimab lowered the percentage of patients progressing to severe or critical infection or dying, the 30-day mortality rate, and the percentage of patients with a NEWS ≥ 5 from HD 2 among patients with mild and moderate COVID-19 delta variant infection at high risk for clinical deterioration. Despite such efficacy, regdanvimab was only given emergency-use authorization at the time of the delta wave and thus was unavailable for use in all healthcare facilities. Furthermore, the process to request and obtain the drug for administration was too complicated, hindering its administration to all patients who meet the indications. As viruses, such as SARS-CoV2, continually mutate, developing therapeutic drugs and vaccines is challenging, as are conducting clinical trials and examining treatment outcomes. Thus, the administration process should be streamlined, and institutional support is required to enable prompt administration of available therapeutic drugs for patients upon the spread of a new variant virus.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all patients and investigators involved in the study.
Author Contributions
Study concept and design: Soyoon Hwang, Nan Young Lee, Ki Tae Kwon, and Changhee Lee. Acquisition of data: Eunkyung Nam, Shin-Woo Kim, and Yu Kyung Kim. Analysis and interpretation of data: Hyun-Ha Chang, Yoonjung Kim, Sohyun Bae, and Juhwan Jeong. Drafting of the manuscript: Soyoon Hwang, Nan Young Lee, and Ki Tae Kwon. Critical revision of the manuscript for important intellectual content: Ki Tae Kwon and Changhee Lee.
Funding
This study was funded by Celltrion, Inc. Celltrion, Inc. reviewed the manuscript throughout development for scientific accuracy and funded the journal’s Rapid Service Fee.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
Declarations
Conflict of Interest
Soyoon Hwang, Nan Young Lee, Eunkyung Nam, Yu Kyung Kim, Shin-Woo Kim, Hyun-Ha Chang, Yoonjung Kim, Sohyun Bae, Juhwan Jeong, Jae-Ho Shin, Guehwan Jang, Changhee Lee, and Ki Tae Kwon declare no conflicts of interest.
Ethical Approval and Informed Consent
This study was approved by the Daegu Joint Institutional Review Board, and the requirement for official written informed consent was waived (DGIRB 2021-10-002), given the retrospective nature of the study (using EMR and residual samples).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Soyoon Hwang and Nan Young Lee contributed equally to this manuscript as co-first authors.
Contributor Information
Changhee Lee, Email: changhee@gnu.ac.kr.
Ki Tae Kwon, Email: ktkwon@knu.ac.kr.
References
- 1.Deb P, Molla MMA, Saif-Ur-Rahman K. An update to monoclonal antibody as therapeutic option against COVID-19. Biosaf Health. 2021;3(2):87–91. doi: 10.1016/j.bsheal.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim C, Ryu D-K, Lee J, et al. A therapeutic neutralizing antibody targeting receptor binding domain of SARS-CoV-2 spike protein. Nat Commun. 2021;12(1):288. doi: 10.1038/s41467-020-20602-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Syed YY. Regdanvimab: First approval. Drugs. 2021;81(18):2133–2137. doi: 10.1007/s40265-021-01626-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Streinu-Cercel A, Săndulescu O, Preotescu L-L, et al. Efficacy and safety of regdanvimab (CT-P59): a phase 2/3 randomized, double-blind, placebo-controlled trial in outpatients with mild-to-moderate coronavirus disease 2019. Open Forum Infect Dis. 2022;9:ofac053. doi: 10.1093/ofid/ofac053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao Y, Huang J, Zhang L, Chen S, Gao J, Jiao H. The global transmission of new coronavirus variants. Environ Res. 2022;206:112240. doi: 10.1016/j.envres.2021.112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobs JL, Haidar G, Mellors JW. COVID-19: challenges of viral variants. Ann Rev Med. 2023;74:31–53. doi: 10.1146/annurev-med-042921-020956. [DOI] [PubMed] [Google Scholar]
- 7.Planas D, Saunders N, Maes P, et al. Considerable escape of SARS-CoV-2 omicron to antibody neutralization. Nature. 2022;602(7898):671–675. doi: 10.1038/s41586-021-04389-z. [DOI] [PubMed] [Google Scholar]
- 8.Ryu D-K, Kang B, Noh H, et al. The in vitro and in vivo efficacy of CT-P59 against gamma, delta and its associated variants of SARS-CoV-2. Biochem Biophys Res Commun. 2021;578:91–96. doi: 10.1016/j.bbrc.2021.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jang YR, Oh YJ, Kim JY. Regdanvimab for patients with mild-to-moderate COVID-19: a retrospective cohort study and subgroup analysis of patients with the delta variant. Int J Infect Dis. 2023;130:94–100. doi: 10.1016/j.ijid.2022.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwak YG, Song JE, Kang J, et al. Use of the monoclonal antibody regdanvimab to treat patients hospitalized with COVID-19: real-world data during the delta variant predominance. Infect Chemother. 2022;54(4):781. doi: 10.3947/ic.2022.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim H, Jang YR, Lee JY, et al. Effectiveness of regdanvimab treatment for SARS-CoV-2 delta variant, which exhibited decreased in vitro activity: a nationwide real-world multicenter cohort study. Front Cell Infect Microbiol. 2023;13:1192512. doi: 10.3389/fcimb.2023.1192512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tenforde MW, Self WH, Gaglani M, et al. Effectiveness of mRNA vaccination in preventing COVID-19-associated invasive mechanical ventilation and death—United States, March 2021–January 2022. Morb Mortal Wkly Rep. 2022;71(12):459. doi: 10.15585/mmwr.mm7112e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kodera S, Rashed EA, Hirata A. Estimation of real-world vaccination effectiveness of mRNA COVID-19 vaccines against delta and omicron variants in Japan. Vaccines. 2022;10(3):430. doi: 10.3390/vaccines10030430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Horo JC, Challener DW, Speicher L, et al. Effectiveness of monoclonal antibodies in preventing severe COVID-19 with emergence of the delta variant. Mayo Clin Proc. 2022;97:327–332. doi: 10.1016/j.mayocp.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park S, Lim SY, Kim JY, et al. Clinical and virological characteristics of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) B.1.617.2 (delta) variant: a prospective cohort study. Clin Infect Dis. 2022;75(1):e27–e34. doi: 10.1093/cid/ciac239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Institutes of Health. National Institutes of Health COVID-19 treatment guidelines. Therapeutic management of nonhospitalized adults with COVID. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/nonhospitalized-adults--therapeutic-management/. Accessed 2 Nov 2023.
- 17.Lee CM, Park S-W, Lee E. Early oxygen requirement in patients with wild-to-moderate COVID-19 who received regdanvimab after delta-variant outbreak. Infect Chemother. 2022;54(2):258. doi: 10.3947/ic.2022.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tchang BG, Askin G, Sahagun A, et al. The independent risk of obesity and diabetes and their interaction in COVID-19: a retrospective cohort study. Obesity. 2021;29(6):971–975. doi: 10.1002/oby.23172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holland M, Kellett J. A systematic review of the discrimination and absolute mortality predicted by the National Early Warning Scores according to different cut-off values and prediction windows. Eur J Intern Med. 2022;98:15–26. doi: 10.1016/j.ejim.2021.12.024. [DOI] [PubMed] [Google Scholar]
- 20.Silcock DJ, Corfield AR, Staines H, Rooney KD. Superior performance of National Early Warning Score compared with quick Sepsis-related Organ Failure Assessment Score in predicting adverse outcomes: a retrospective observational study of patients in the prehospital setting. Eur J Emerg Med. 2019;26(6):433–439. doi: 10.1097/MEJ.0000000000000589. [DOI] [PubMed] [Google Scholar]
- 21.Alexandar S, Ravisankar M, Kumar RS, Jakkan K. A comprehensive review on Covid-19 delta variant. Int J Pharmacol Clin Res (IJPCR) 2021;5(83–85):7. [Google Scholar]
- 22.Twohig KA, Nyberg T, Zaidi A, et al. Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B. 1.617. 2) compared with alpha (B. 1.1. 7) variants of concern: a cohort study. Lancet Infect Dis. 2022;22(1):35–42. doi: 10.1016/S1473-3099(21)00475-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khedar RS, Mittal K, Ambaliya HC, et al. Greater Covid-19 severity and mortality in hospitalized patients in second (delta variant) wave compared to the first: single centre prospective study in India. medRxiv. 2021:2021.09. 03.21263091.
- 24.Zali A, Khodadoost M, Gholamzadeh S, et al. Mortality among hospitalized COVID-19 patients during surges of SARS-CoV-2 alpha (B. 1.1. 7) and delta (B. 1. 617. 2) variants. Sci Rep. 2022;12(1):18918. doi: 10.1038/s41598-022-23312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hurt AC, Wheatley AK. Neutralizing antibody therapeutics for COVID-19. Viruses. 2021;13(4):628. doi: 10.3390/v13040628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Touret F, Baronti C, Bouzidi HS, de Lamballerie X. In vitro evaluation of therapeutic antibodies against a SARS-CoV-2 Omicron B. 1.1. 529 529 isolate. Sci Rep. 2022;12(1):4683. doi: 10.1038/s41598-022-08559-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greaney AJ, Starr TN, Gilchuk P, et al. Complete mapping of mutations to the SARS-CoV-2 spike receptor-binding domain that escape antibody recognition. Cell Host Microbe. 2021;29(1):44–57.e9. doi: 10.1016/j.chom.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aggarwal A, Stella AO, Walker G, et al. SARS-CoV-2 Omicron: evasion of potent humoral responses and resistance to clinical immunotherapeutics relative to viral variants of concern. medRxiv: 2021-12 (2021) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article/as supplementary information files.