Abstract
We have cloned a DNA fragment from a genomic library of Myxococcus xanthus using an oligonucleotide probe representing conserved regions of biotin carboxylase subunits of acetyl coenzyme A (acetyl-CoA) carboxylases. The fragment contained two open reading frames (ORF1 and ORF2), designated the accB and accA genes, capable of encoding a 538-amino-acid protein of 58.1 kDa and a 573-amino-acid protein of 61.5 kDa, respectively. The protein (AccA) encoded by the accA gene was strikingly similar to biotin carboxylase subunits of acetyl-CoA and propionyl-CoA carboxylases and of pyruvate carboxylase. The putative motifs for ATP binding, CO2 fixation, and biotin binding were found in AccA. The accB gene was located upstream of the accA gene, and they formed a two-gene operon. The protein (AccB) encoded by the accB gene showed high degrees of sequence similarity with carboxyltransferase subunits of acetyl-CoA and propionyl-CoA carboxylases and of methylmalonyl-CoA decarboxylase. Carboxybiotin-binding and acyl-CoA-binding domains, which are conserved in several carboxyltransferase subunits of acyl-CoA carboxylases, were found in AccB. An accA disruption mutant showed a reduced growth rate and reduced acetyl-CoA carboxylase activity compared with the wild-type strain. Western blot analysis indicated that the product of the accA gene was a biotinylated protein that was expressed during the exponential growth phase. Based on these results, we propose that this M. xanthus acetyl-CoA carboxylase consists of two subunits, which are encoded by the accB and accA genes, and occupies a position between prokaryotic and eukaryotic acetyl-CoA carboxylases in terms of evolution.
Acetyl coenzyme A (acetyl-CoA) carboxylase catalyzes the ATP-dependent carboxylation of acetyl-CoA to yield malonyl-CoA. The chain length of newly synthesized fatty acids appears to depend on the concentration of malonyl-CoA (17). In Escherichia coli, the rates of transcription of acetyl-CoA carboxylase genes are directly related to the rate of cell growth (27). E. coli and Pseudomonas citronellolis acetyl-CoA carboxylases consist of three functional units: carboxyltransferase, biotin carboxyl carrier protein, and biotin carboxylase (8, 13). The E. coli acetyl-CoA carboxylase is the only biotinylated protein in E. coli (9), and the enzyme does not catalyze a reaction analogous to that of propionyl-CoA carboxylase. In contrast to bacterial enzymes, eukaryotic acetyl-CoA carboxylases are unusually large enzymes and contain all components in a single protein.
Propionyl-CoA carboxylase forms methylmalonyl-CoA from propionyl-CoA by CO2 fixation. Methylmalonyl-CoA serves as a precursor for the synthesis of branched-chain fatty acids and polyketides. All propionyl-CoA carboxylases from prokaryotes and eukaryotes consist of two nonidentical subunits, biotin carboxylase and carboxyltransferase. The bacterial acyl-CoA carboxylases isolated from Mycobacterium pheli, Mycobacterium smegmatis, and Streptomyces erythreus show maximal rates of carboxylation with propionyl-CoA, but the enzymes are also able to carboxylate acetyl-CoA well (7, 16, 19). In several bacteria, a single enzyme with dual-substrate specificity catalyzes the carboxylation of both acetyl- and propionyl-CoA.
Myxococcus xanthus is a gram-shaped bacterium that displays cyclic and various social behaviors (6, 34). We reported previously that an M. xanthus propionyl-CoA carboxylase deletion mutant was unable to sporulate under conditions of nutrient starvation (20). The developing cells of the mutant also showed reduced levels of long-chain fatty acids compared to wild-type cells. Since the mutant grew as well as the wild type in growth medium, M. xanthus appears to contain acetyl-CoA carboxylase in addition to propionyl-CoA carboxylase. We attempted to clone the acetyl-CoA carboxylase gene from M. xanthus using appropriate oligonucleotide probes designed from the conserved sequences in the acetyl-CoA carboxylases.
Here, we describe the cloning and sequencing of the accA and accB genes encoding acetyl-CoA carboxylase from M. xanthus, and we discuss the structure and function of this enzyme.
MATERIALS AND METHODS
Strains, plasmids, and growth conditions.
The type strain of M. xanthus, IFO13542 (ATCC 25232), was grown in Casitone-yeast extract (CYE) medium at 28°C (2, 5). Fruiting body formation was assayed on clone fruiting (CF) medium containing 1.5% agar (14). Plasmids pBluescript II SK(−) (Stratagene, La Jolla, Calif.) and pT7 Blue-T (Novagen, Madison, Wis.) were used for cloning.
Cloning of the acetyl-CoA carboxylase gene.
An M. xanthus genomic DNA library was prepared by partially digesting chromosomal DNA with Sau3AI, ligating the DNA with BamHI-cleaved λ EMBL3, and then packaging the DNA into phage particles. For the detection of the acetyl-CoA carboxylase gene of M. xanthus, oligonucleotides were designed as DNA probes for hybridization experiments. The oligonucleotides were labeled with digoxigenin (DIG)-11-dUTP by using an oligonucleotide tailing kit (Boehringer GmbH, Mannheim, Germany). One positive phage was cloned by hybridization with an oligonucleotide probe (ACC1). The sequence of ACC1 is 5′-(G/C)GCGATCTC(G/C)CC(G/C)CGGTTCG-3′ (oligo-1); it was designed on the basis of the consensus sequence (ANRGEIA) of acetyl-CoA carboxylases of E. coli and Anabaena sp. strain PCC 7120 (11). The 3.8-kb ApaI, 5.6-kb SacI, and 1.4-kb SmaI fragments of the clone hybridized with the probe and then were subcloned into pBluescript II SK(−).
DNA sequencing.
Nucleotide sequences were determined by the dideoxynucleotide chain termination method (31) using a model 4200 sequencer (Aloka, Tokyo, Japan). Both directional strands were completely analyzed by overlapping at every junction.
Insertional mutagenesis of the accA gene.
The 2.4-kb DNA fragment, which contains the accA gene and part of the accB gene, was amplified by PCR using two primers; 5′-CTTCAAGGACGAGTACGAC-3′ (oligo-2), which anneals at positions 1393 to 1411, and 5′-TCCGTCACGGCGTGCGCGCC-3′ (oligo-3), which anneals at positions 3777 to 3796 (Fig. 1). The 2.4-kb PCR product was ligated to the pT7 Blue-T vector. For deletion of an SmaI site of the pT7 Blue-T vector, the recombinant plasmid was digested with BamHI and EcoRI. The ends were blunted with T4 DNA polymerase and then ligated with T4 DNA ligase. This plasmid was designated pACC1. A kanamycin resistance (Kmr) gene of pTF1 was amplified by PCR using suitable primers, which contain SmaI sites (10). The resulting 1.2-kb fragment containing the Kmr gene was purified, digested with SmaI, and inserted into the SmaI site of plasmid pACC1. The accA gene with the Kmr gene inserted was amplified by PCR using the same primers. The resulting 3.6-kb fragment was purified and used for electroporation, which was performed as described by Plamann et al. (29). M. xanthus kanamycin-resistant colonies were grown in CYE medium containing 70 μg of kanamycin per ml, and chromosomal DNAs were prepared from the mutants. The chromosomal DNAs were digested with SacI and then analyzed by Southern hybridization using a 1.4-kb SmaI fragment as a probe. We confirmed that the SacI digested-chromosomal DNAs from the wild type and the mutants were hybridized at 5.6-kb and 6.8-kb fragments, respectively.
FIG. 1.
Nucleotide and deduced amino acid sequences of the accA and accB genes of M. xanthus. Putative ribosome-binding sites are double underlined. Arrows indicate the position of the palindrome sequence. The sequence corresponding to the probe is underlined. Boldfaced amino acids represent the putative biotin-binding site.
Gel electrophoresis and Western blot analysis.
M. xanthus wild-type and accA disruption mutant cells harvested in exponential growth phase, in stationary phase, and during development were used for Western blot analysis. Samples containing 250 μg of protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (8 or 12% polyacrylamide) and electroblotted onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad Laboratories, Ltd.) using a Trans Blot SD semidry transfer cell (Bio-Rad Laboratories, Ltd.) according to the manufacturer's instructions. The membranes were blocked with 3% bovine serum albumin in PBS-T buffer (10 mM sodium phosphate buffer [pH 7.2], 150 mM NaCl, and 0.1% Tween 20) and then incubated with streptavidin-linked horseradish peroxidase (Amersham Pharmacia Biotech) for 1 h. The membranes were washed with PBS-T buffer, and enzyme activities were detected by ECL Western blotting detection reagents (Amersham Pharmacia Biotech).
Enzyme assays.
M. xanthus wild-type and accA disruption strains were cultured in 200 ml of CYE medium at 28°C on a rotary shaker at 250 rpm. The cells were harvested at the mid-logarithmic phase of growth (optical density at 600 nm [OD600] 0.4 to 0.6) and washed with 20 mM sodium phosphate buffer (pH 7.2). The cells were suspended in the same buffer and disrupted by sonication with a Branson Sonifier (five 30-s bursts at a power setting of 1.5). The supernatant and cell debris were separated by centrifugation (12,000 × g for 10 min). Enzyme activity was determined from the increase in the product by high-performance liquid chromatography (HPLC) (21). For acetyl- and propionyl-CoA carboxylase assays, the reaction mixture contained 60 mM Tris-HCl (pH 7.2), 1.3 mM ATP, 1.8 mM MgCl2, 66 mM KHCO3, 0.4 mM acetyl-CoA or propionyl-CoA, and enzyme in a total volume of 0.1 ml. The mixtures were incubated at 30°C for 10 to 45 min. One unit of activity was defined as the amount of enzyme forming 1.0 μmol of malonyl-CoA or methylmalonyl-CoA per min at 30°C.
Observation of morphology, growth, and development.
Wild-type M. xanthus or the accA disruption mutant was grown in CYE medium at 28°C on a shaker. Cell numbers were estimated with a hemacytometer counting chamber. Generation times were calculated from the linear region of the growth curve by measuring the time needed for the cells of the culture to double. For spore formation, vegetative cells in TM buffer (10 mM Tris-HCl [pH 7.5]–8 mM MgSO4) were spotted onto CF agar plates. Cell morphologies of vegetative cells and spores were observed by light microscopy.
Nucleotide sequence accession number.
The sequences of the M. xanthus accA and accB genes have been deposited in the DDBJ sequence library under accession number AB039884.
RESULTS
Cloning of the structural genes for acetyl-CoA carboxylase.
One clone was isolated from an M. xanthus genomic DNA library by screening with an ACC1 oligonucleotide probe designed from the conserved sequences in the biotin carboxylase subunits of acetyl-CoA carboxylases. The 3.8-kb ApaI, 5.6-kb SacI, and 1.4-kb SmaI fragments of the clone DNA hybridized with the probe. Based on the restriction map derived from hybridization data, the 4-kb ApaLI-SmaI fragment was completely sequenced on both strands using synthetic oligonucleotide primers. Two open reading frames (ORFs) were identified in the 4-kb DNA fragment. The complete nucleotide sequence together with the deduced amino acid sequence is shown in Fig. 1. ORF1 and ORF2 have high percentages of G and C nucleotides (93.1 and 89.9%, respectively) in the third positions of the codons and exhibit a codon preference typical for M. xanthus. The putative initiation codons were preceded by purine-rich Shine-Dalgarno-like sequences (AGGAG at nucleotides 428 to 432 and 2058 to 2762).
ORF1, designated M. xanthus accB, started at position 440 with an ATG and ended at position 2056 with a TGA stop codon. The accB gene encodes a protein of 538 amino acid residues with a calculated Mr of 58,100. ORF2 was located immediately downstream of the accB gene. ORF2, designated M. xanthus accA, started at position 2071 with an ATG and ended at position 3792 with a TGA stop codon. ORF2 encodes a protein of 573 amino acid residues with a calculated Mr of 61,500. This downstream region contained an inverted repeat, CGGACGtAACGTTcCGTCCG, that could form a stem structure.
Deduced properties of AccA and AccB polypeptides.
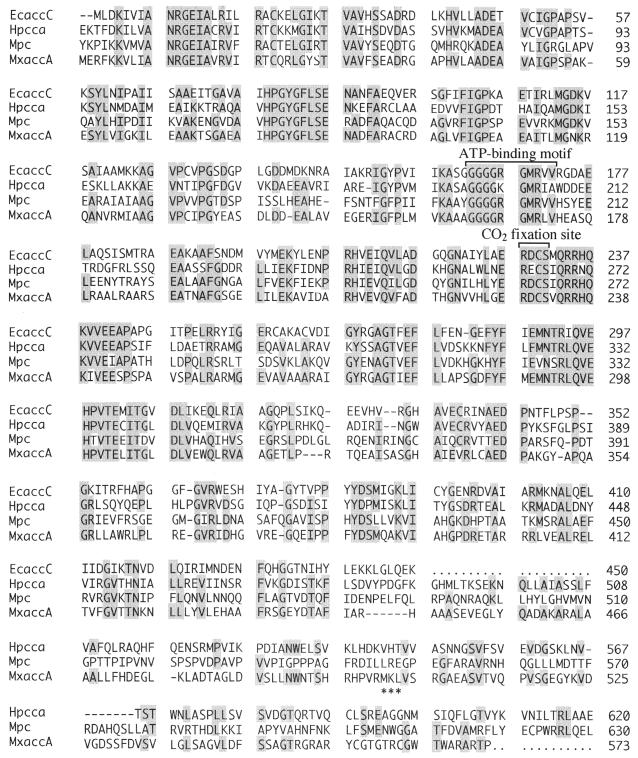
The predicted amino acid sequences of AccA and AccB were compared with those in the GenBank database using the PSI Blast program. AccA showed considerable sequence homology to the biotin carboxylase subunits of E. coli acetyl-CoA carboxylase (48% identity) (26) and human propionyl-CoA carboxylase (41% identity) (23) and to the N-terminal region of mouse pyruvate carboxylase (39% identity) (35) (Fig. 2). Multiple alignment of these sequences revealed that the ATP-binding motif and CO2 fixation site were present in AccA. The sequence Gly-Gly-Gly-Gly-Arg-Gly-Met-Arg-Leu-Val of AccA (residues 164 to 173) matched the consensus sequence of the Gly-rich motif that has been implicated in ATP binding by biotin carboxylases (30). The Cys residue of Arg-Asp-Cys-Ser (residues 229 to 232) was thought to be involved in CO2 fixation (26). The conserved biotin-binding site Met-Lys-Met, in which the lysine residue is biotinylated, was not found, but Met-Lys-Leu was present in the C-terminal region of AccA. Replacement of the methionine residue flanking the target lysine with leucine on the biotinylation domain of the biotin carboxylase subunit of human propionyl-CoA carboxylase demonstrated that the methionine residue is not essential for correct biotinylation of the protein (24). Met-Lys-Leu as a biotinylation site is also found in the biotin carboxyl carrier protein of acetyl-CoA carboxylase from Anabaena sp. strain PCC 7120 (11).
FIG. 2.
Amino acid sequence alignment of homologous regions in the E. coli acetyl-CoA carboxylase biotin carboxylase subunit (EcaccC), the human propionyl-CoA carboxylase α subunit (HpccA), the mouse pyruvate carboxylase (Mpc), and the M. xanthus acetyl-CoA carboxylase biotin carboxylase subunit (MxaccA). The putative biotin-binding site (MKL) of M. xanthus AccA is marked by asterisks.
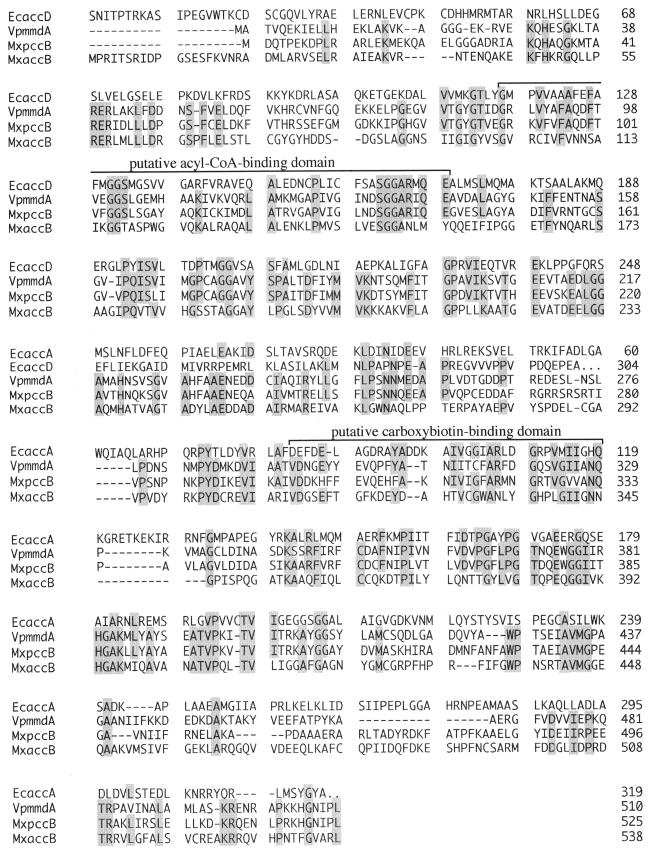
AccB showed high degrees of sequence similarities with the α subunit of Veillonella parvula methylmalonyl-CoA decarboxylase (27% identity) (18), the β subunit of M. xanthus propionyl-CoA carboxylase (28% identity) (20), and the carboxyltransferase α and β subunits of E. coli acetyl-CoA carboxylase (19 and 13% identity, respectively) (25) (Fig. 3). The putative acyl-CoA- and carboxybiotin-binding domains, which are conserved in several carboxyltransferase subunits of acetyl-CoA and propionyl-CoA carboxylases, were found in AccB at residues 102 to 154 and 311 to 345, respectively.
FIG. 3.
Amino acid sequence alignment of homologous regions in the E. coli acetyl-CoA carboxylase α subunit (EcaccA) and β subunit (EcaccD) of carboxyltransferase, the V. parvula methylmalonyl-CoA decarboxylase α subunit (VpmmdA), the M. xanthus propionyl-CoA carboxylase β subunit (MxpccB), and the M. xanthus acetyl-CoA carboxylase carboxyltransferase subunit (MxaccB).
Phenotypic characterization of the M. xanthus accA disruption mutant.
Using Southern hybridization and PCR analyses, we confirmed that the kanamycin resistance gene was inserted into the accA gene on the mutant chromosome. Insertional inactivation of the accA gene of the M. xanthus chromosome resulted in a marked change in the growth rate. In CYE liquid medium, the wild type exhibited a lag period of about 8 h and entered the stationary phase within 48 h. In contrast to the wild-type strain, the accA mutant started to grow after about 18 h of lag time and reached steady-state growth at 60 h. The generation times and final yields were 3.5 h and 3.0 × 109 cells/ml for the wild type and 5.0 h and 2.5 × 109 cells/ml for the accA mutant (data not shown). In M1 defined medium (4), the wild-type and accA mutant strains grew at similar generation times of approximately 10 h (data not shown). No significant differences in cell morphology or sporulation were observed between the wild-type and accA mutant strains. When accA mutant spores were cultured in CYE medium, they were able to germinate, although more slowly, approximately 24 h later, than wild-type spores.
Biotinylated proteins in wild-type and accA disruption mutant cells.
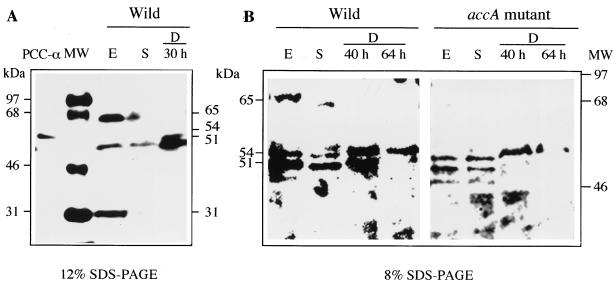
Biotinylated proteins are rare in bacteria, but four proteins in M. xanthus wild-type protein extracts reacted with streptavidin in Western blot analysis (Fig. 4A). The sizes of the four proteins were 65, 54, 51, and 31 kDa. The 65-, 51-, and 31-kDa biotin-containing proteins were mainly detected in the exponential-phase wild-type protein extract. The expression of the three proteins dropped off during the stationary phase and development. In the accA disruption mutant, only the 65-kDa protein was absent in the exponential-phase cells (Fig. 4B). The value of 65 kDa obtained by SDS-PAGE corresponded well with the molecular mass (61.5 kDa) of the M. xanthus accA gene calculated from the predicted amino acid sequence. When the AccA protein was overexpressed in E. coli, its molecular size in SDS-PAGE was 66 kDa (data not shown). The results indicated that the product of the accA gene was a biotinylated protein that was expressed mainly in the exponential phase. The 54-kDa protein, which is expressed mainly during development, is thought to be the α subunit of propionyl-CoA carboxylase, because the purified propionyl-CoA carboxylase of M. xanthus contains a 53-kDa biotinylated protein (α subunit) (21).
FIG. 4.
Detection of biotinylated polypeptides in the protein extracts of wild-type and accA mutant strains. Proteins, biotinylated molecular mass standards (MW), and purified M. xanthus propionyl-CoA carboxylase (PCC-α) were separated by SDS–12% PAGE (A) or SDS–8% PAGE (B) and probed with streptavidin-linked horseradish peroxidase. The protein extracts were prepared from cultures at the exponential growth phase (E), at stationary phase (S), and during development (D).
Acetyl-CoA carboxylase assay.
DNA sequence analysis suggested that the accA and accB genes may encode two subunits of propionyl-CoA carboxylase or acetyl-CoA carboxylase. To test this hypothesis, the enzyme activities of the wild type and the accA disruption mutant were assayed in crude cell extracts. Western blot analysis indicated that AccA protein was expressed during the exponential growth phase. Therefore, the enzyme activities were assayed in extracts from exponential-phase cells. Acetyl-CoA carboxylase activities were found in both wild-type and accA mutant cell extracts (Table 1). However, the specific activity in accA mutant cells was decreased by approximately 40% compared to that in wild-type cells. In the propionyl-CoA carboxylase assay, the difference between the specific activities from wild-type and accA mutant extracts was not significant.
TABLE 1.
Acetyl-CoA and propionyl-CoA carboxylase activitiesa in wild-type and accA mutant strains
| Fraction | Acetyl-CoA carboxylase
|
Propionyl-CoA carboxylase
|
||
|---|---|---|---|---|
| Total activity (mU) | Sp act (mU/mg of protein) | Total activity (mU) | Sp act (mU/mg of protein) | |
| Wild type | 22.3 | 1.0 | 69.0 | 3.1 |
| accA mutant | 12.3 | 0.6 | 60.9 | 3.0 |
The acetyl-CoA and propionyl-CoA carboxylase activities were determined with acetyl-CoA and propionyl-CoA as substrates from the rate of malonyl-CoA and methylmalonyl-CoA formations, respectively. The total and specific activities were expressed as means of triplicate enzyme assays.
DISCUSSION
Acetyl-CoA carboxylase catalyzes the synthesis of malonyl-CoA, the first committed step in the biosynthesis of fatty acids (1). In E. coli, there is a direct correlation between the levels of transcription of the acetyl-CoA carboxylase genes and the rate of cell growth (27). A Saccharomyces cerevisiae acetyl-CoA carboxylase mutant showed inhibition of the synthesis of very-long-chain fatty acids, and the reduction in levels of these very-long-chain fatty acids resulted in marked alterations of the nuclear envelope (33). In the M. xanthus accA mutant, the total amounts of long-chain fatty acids (C16 to C18) in vegetative and developing cells were decreased by about 4 and 6%, respectively, compared to those in wild-type cells (data not shown), but this reduction was not as marked as those observed previously in a propionyl-CoA carboxylase (dcm-1)-deficient mutant. E. coli and S. cerevisiae acetyl-CoA carboxylases are essential for growth (12, 28). In M. xanthus, disruption of the accA gene was not lethal, and the mutant was not completely deficient in acetyl-CoA carboxylase activity. Western blot analysis revealed the presence of 31- and 51-kDa biotinylated proteins during the exponential phase. The molecular masses on SDS-PAGE of biotin carboxyl carrier proteins of acetyl-CoA carboxylases from E. coli, Anabaena sp. strain PCC 7120, and Pseudomonas aeruginosa are 22 to 25 kDa (3, 11, 26). Since the 31-kDa protein was similar to these proteins in size, M. xanthus may have another acetyl-CoA carboxylase, like the enzyme from E. coli. Most higher plants have two types of acetyl-CoA carboxylases, the E. coli-like and eukaryotic acetyl-CoA carboxylases, which exist in plastids and the cytosol, respectively (22). The molecular mass of the biotinylated proteins of E. coli-like acetyl-CoA carboxylases in plants was 35 kDa on SDS-PAGE (22, 32).
The acetyl-CoA carboxylase activity was detected in growing M. xanthus cells. In previous studies, M. xanthus cells harvested in the stationary phase and during development showed very low acetyl-CoA carboxylase activity, but propionyl-CoA carboxylase activity was detected both in the stationary phase and during development and reached a maximum during the sporulation phase (21). The results of enzyme assays corresponded well to the expression of biotinylated subunits of acetyl-CoA and propionyl-CoA carboxylases as determined by Western blot analysis in this study. The M. xanthus accA mutant showed a decreased growth rate, but no significant differences in cell morphology or sporulation were observed. The presence of substantial acetyl-CoA carboxylase activity in the accA mutant cells may account for the absence of any difference in cell morphology or sporulation in the mutant.
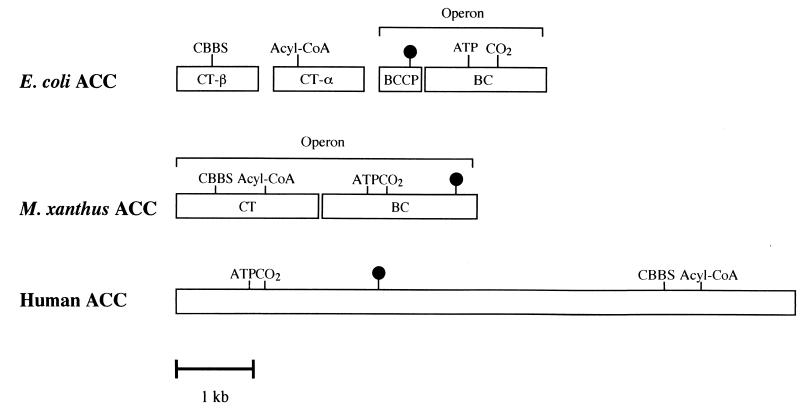
The acetyl-CoA carboxylases can be divided into two basic types, a bacterial type and a eukaryotic type, by their structure. The bacterial type contains four dissociated proteins, the biotin carboxylase, the biotin carboxyl carrier protein, and two carboxyltransferase subunits (α and β), organized into three functional domains (Fig. 5). The α and β subunits of the carboxyltransferase component of E. coli have acyl-CoA-binding and carboxybiotin-binding domains, respectively. The genes (accA and accD) encoding the two carboxyltransferase subunits are located almost directly opposite each other in the E. coli chromosome. The biotin carboxyl carrier protein gene (accB) and biotin carboxylase gene (accC) from E. coli and P. aeruginosa form a two-gene operon, and the two genes are cotranscribed (3, 26). In the eukaryotic type, these proteins are part of a single multifunctional polypeptide derived from the expression of a single gene. From the amino terminus, the biotin carboxylase component, biotin-binding site, carboxybiotin-binding site, and acyl-CoA-binding domain are distributed in this order along the eukaryotic acetyl-CoA carboxylase (Fig. 5). The results of this study indicated that M. xanthus AccA is a biotinylated biotin carboxylase subunit of acetyl-CoA carboxylase. The accB gene encoding the putative carboxyltransferase subunit was located upstream of the biotin carboxylase gene (accA). We did not confirm whether AccB is a carboxyltransferase subunit of acetyl-CoA carboxylase. However, since the genes encoding AccA and AccB formed a two-gene operon, AccB was thought to function as a carboxyltransferase subunit of the enzyme. We propose that the M. xanthus accA and accB genes may have been constructed by the fusion of E. coli accB- and accC-like genes and of E. coli accA- and accD-like genes, respectively, and may have integrated to form a single gene, such as a eukaryotic acetyl-CoA carboxylase gene.
FIG. 5.
Schematic diagram for the subunit structures of acetyl-CoA carboxylases. Abbreviations and symbols: CBBS, carboxybiotin-binding site; Acyl-CoA, acyl-CoA-binding site; ATP, ATP-binding site; CO2, CO2 fixation site; ●, biotin-binding site; ACC, acetyl-CoA carboxylase; CT, carboxyltransferase; BCCP, biotin carboxyl carrier protein; BC, biotin carboxylase.
REFERENCES
- 1.Alberts A, Vagelos P R. Acyl-CoA carboxylase. In: Boyer P D, editor. The enzymes. 3rd ed. Vol. 6. New York, N.Y: Academic Press, Inc.; 1972. pp. 37–82. [Google Scholar]
- 2.Avery L, Kaiser D. In situ transposon replacement and isolation of a spontaneous tandem duplication. Mol Gen Genet. 1983;191:99–109. doi: 10.1007/BF00330896. [DOI] [PubMed] [Google Scholar]
- 3.Best E A, Knauf V C. Organization and nucleotide sequences of the genes encoding the biotin carboxyl carrier protein and biotin carboxylase protein of Pseudomonas aeruginosa acetyl coenzyme A carboxylase. J Bacteriol. 1993;175:6881–6889. doi: 10.1128/jb.175.21.6881-6889.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bretscher A P, Kaiser D. Nutrition of Myxococcus xanthus, a fruiting myxobacterium. J Bacteriol. 1978;133:763–768. doi: 10.1128/jb.133.2.763-768.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campos M J, Geisselesoder J, Zusman R D. Isolation of bacteriophage MX4, a generalized transducing phage for Myxococcus xanthus. J Mol Biol. 1978;119:167–178. doi: 10.1016/0022-2836(78)90431-x. [DOI] [PubMed] [Google Scholar]
- 6.Dworkin M, Kaiser D. Cell interactions in myxobacterial growth and development. Science. 1985;230:18–24. doi: 10.1126/science.3929384. [DOI] [PubMed] [Google Scholar]
- 7.Erfle J D. Acetyl-CoA and propionyl-CoA carboxylation by Mycobacterium pheli: partial purification and some properties of the enzyme. Biochim Biophys Acta. 1973;316:143–155. doi: 10.1016/0005-2760(73)90004-0. [DOI] [PubMed] [Google Scholar]
- 8.Fall R R. Stabilization of an acetyl-CoA carboxylase complex from Pseudomonas citronellolis. Biochim Biophys Acta. 1976;450:175–480. doi: 10.1016/0005-2760(76)90022-9. [DOI] [PubMed] [Google Scholar]
- 9.Fall R R, Alberts A W, Vagelos P R. Analysis of bacterial biotin proteins. Biochim Biophys Acta. 1975;379:496–503. doi: 10.1016/0005-2795(75)90156-7. [DOI] [PubMed] [Google Scholar]
- 10.Furuichi T, Inoue M, Inoue S. Novel one-step cloning vector with a transposable element: application to the Myxococcus xanthus genome. J Bacteriol. 1985;164:270–275. doi: 10.1128/jb.164.1.270-275.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gornicki P, Scappino L, Haselkorn R. Genes for two subunits of acetyl-CoA carboxylase of Anabaena sp. strain PCC 7120: biotin carboxylase and biotin carboxyl carrier protein. J Bacteriol. 1993;175:5268–5272. doi: 10.1128/jb.175.16.5268-5272.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan X, Wurtele E S. Reduction of growth and acetyl-CoA carboxylase activity by expression of a chimeric streptavidin gene in Escherichia coli. Appl Microbiol Biotechnol. 1996;44:753–758. doi: 10.1007/BF00178614. [DOI] [PubMed] [Google Scholar]
- 13.Guchhait R B, Polakis S E, Dimroth P, Stoll E, Moss J, Lane M D. Acetyl-coenzyme A carboxylase system of Escherichia coli. J Biol Chem. 1974;249:6633–6645. [PubMed] [Google Scholar]
- 14.Hagen C D, Bretscher P A, Kaiser D. Synergism between morphogenic mutants of Myxococcus xanthus. Dev Biol. 1979;64:284–296. doi: 10.1016/0012-1606(78)90079-9. [DOI] [PubMed] [Google Scholar]
- 15.Hasslacher M, Ivessa A, Paltauf F, Kohlwein S D. Acetyl-CoA carboxylase from yeast is an essential enzyme and is regulated by factors that control phospholipid metabolism. J Biol Chem. 1993;268:10946–10952. [PubMed] [Google Scholar]
- 16.Henrikson K, Allen S H G. Purification and subunit structure of propionyl coenzyme A carboxylase of Mycobacterium smegmatis. J Biol Chem. 1979;254:5888–5891. [PubMed] [Google Scholar]
- 17.Hori T, Nakamura N, Okuyama H. Possible involvement of acetyl coenzyme A carboxylase as well as fatty acid synthetase in the temperature-controlled synthesis of fatty acids in Saccharomyces cerevisiae. J Biochem. 1987;101:949–956. doi: 10.1093/oxfordjournals.jbchem.a121964. [DOI] [PubMed] [Google Scholar]
- 18.Huder J B, Dimroth P. Sequence of the sodium ion pump methylmalonyl-CoA decarboxylase from Veillonella parvula. J Biol Chem. 1993;268:24564–24571. [PubMed] [Google Scholar]
- 19.Hunaiti A, Kolattukudy P E. Isolation and characterization of an acyl-coenzyme A carboxylase from an erythromycin-producing Streptomyces erythreus. Arch Biochem Biophys. 1982;216:362–371. doi: 10.1016/0003-9861(82)90222-3. [DOI] [PubMed] [Google Scholar]
- 20.Kimura Y, Sato R, Mimura K, Sato M. Propionyl coenzyme A carboxylase is required for development of Myxococcus xanthus. J Bacteriol. 1997;179:7098–7102. doi: 10.1128/jb.179.22.7098-7102.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimura Y, Kojo T, Kimura I, Sato M. Propionyl coenzyme A carboxylase of Myxococcus xanthus: catalytic properties and function in developing cells. Arch Microbiol. 1998;170:179–184. doi: 10.1007/s002030050631. [DOI] [PubMed] [Google Scholar]
- 22.Konishi T, Shinohara K, Yamada K, Sasaki Y. Acetyl-CoA carboxylase in higher plants: most plants other than Gramineae have both the prokaryotic and the eukaryotic forms of this enzyme. Plant Cell Physiol. 1996;37:117–122. doi: 10.1093/oxfordjournals.pcp.a028920. [DOI] [PubMed] [Google Scholar]
- 23.Lamhonwah M A, LeClerc D, Loyer M, Clarizio R, Gravel A R. Correction of the metabolic defect in propionic acidemia fibroblasts by microinjection of a full-length cDNA or RNA transcript encoding the propionyl-CoA carboxylase beta subunit. Genomics. 1994;19:500–505. doi: 10.1006/geno.1994.1099. [DOI] [PubMed] [Google Scholar]
- 24.Leon-del-Rio A, Gravel R A. Sequence requirements for the biotinylation of carboxyl-terminal fragments of human propionyl-CoA carboxylase α subunit expressed in Escherichia coli. J Biol Chem. 1994;269:22964–22968. [PubMed] [Google Scholar]
- 25.Li S-J, Cronan J E., Jr The genes encoding the two carboxyltransferase subunits of Escherichia coli acetyl-CoA carboxylase. J Biol Chem. 1992;267:855–863. [PubMed] [Google Scholar]
- 26.Li S-J, Cronan J E., Jr The genes encoding the biotin carboxylase subunits of Escherichia coli acetyl-CoA carboxylase. J Biol Chem. 1992;267:16841–16847. [PubMed] [Google Scholar]
- 27.Li S-J, Cronan J E., Jr Growth rate regulation of Escherichia coli acetyl coenzyme A carboxylase, which catalyzes the first committed step of lipid biosynthesis. J Bacteriol. 1993;175:323–340. doi: 10.1128/jb.175.2.332-340.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagano Y, Matsuno R, Sasaki Y. An essential gene of Escherichia coli that has sequence similarity to a chloroplast gene of unknown function. Mol Gen Genet. 1991;228:62–64. doi: 10.1007/BF00282448. [DOI] [PubMed] [Google Scholar]
- 29.Plamann L, Kuspa A, Kaiser D. Proteins that rescue A-signal-defective mutants of Myxococcus xanthus. J Bacteriol. 1992;174:3311–3318. doi: 10.1128/jb.174.10.3311-3318.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Post L E, Post D J, Raushel F M. Dissection of the functional domains of Escherichia coli carbamoyl phosphate synthetase by site-directed mutagenesis. J Biol Chem. 1990;265:7742–7747. [PubMed] [Google Scholar]
- 31.Sanger F, Nicklen S, Coulson A. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sasaki Y, Hakamada K, Suama Y, Nagano Y, Furusawa I, Matsuno R. Chloroplast-encoded protein as a subunit of acetyl-CoA carboxylase in pea plants. J Biol Chem. 1993;268:25118–25123. [PubMed] [Google Scholar]
- 33.Schneiter R, Hitomi M, Ivessa A S, Fasch E-V, Kohlwein S D, Tartakoff A M. A yeast acetyl coenzyme A carboxylase mutant links very-long-chain fatty acid synthesis to the structure and function of the nuclear membrane-pore complex. Mol Cell Biol. 1996;16:7161–7172. doi: 10.1128/mcb.16.12.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimkets L J. Social and developmental biology of the Myxobacteria. Microbiol Rev. 1990;54:473–501. doi: 10.1128/mr.54.4.473-501.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J, Xia W L, Brew K, Ahmad F. Adipose pyruvate carboxylase: amino acid sequence and domain structure deduced from cDNA sequencing. Proc Natl Acad Sci USA. 1993;90:1766–1770. doi: 10.1073/pnas.90.5.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]