Abstract
Mycobacteroides (Mycobacterium) abscessus, which causes a variety of infectious diseases in humans, is becoming detected more frequently in clinical specimens as cases are spreading worldwide. Taxonomically, M. abscessus is composed of three subspecies of M. abscessus subsp. abscessus, M. abscessus subsp. bolletii, and M. abscessus subsp. massiliense, with different susceptibilities to macrolides. In order to identify rapidly these three subspecies, we determined useful biomarker proteins, including ribosomal protein L29, L30, and hemophore-related protein, for distinguishing the subspecies of M. abscessus using the matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) profiles. Thirty-three clinical strains of M. abscessus were correctly identified at the subspecies-level by the three biomarker protein peaks. This study ultimately demonstrates the potential of routine MALDI-MS-based laboratory methods for early identification and treatment for M. abscessus infections.
Subject terms: Microbiology, Clinical microbiology
Introduction
Members of the Mycobacteroides (Mycobacterium) abscessus cause various infectious diseases in humans that are spreading worldwide, including infections of the lungs, lymph nodes, skin, soft tissue, and bone1–3. However, treatment for M. abscessus infections are difficult due to their natural multidrug resistance4. The American Thoracic Society (ATS) and Infectious Disease Society of America (IDSA) recommended multidrug therapy based on macrolide in their 2007 guidelines5–7. Notably, the 2020 guideline from ATS, European Respiratory Society (ERS), European Society of Clinical Microbiology and Infectious Disease (ESCMID), and IDSA recommended the choice of antibiotics depending on the presence of the 23S rRNA methylase encoding gene, erm (41), and mutations in the 23S rRNA (rrl) gene8,9.
M. abscessus has been taxonomically reclassified many times and is now composed of three subspecies groups: M. abscessus subsp. abscessus, M. abscessus subsp. bolletii, and M. abscessus subsp. massiliense10,11. Members of the M. abscessus subsp. abscessus and M. abscessus subsp. bolletii are known to be mostly resistant to macrolide because these subspecies produce the 23S rRNA methylase Erm (41). On the other hand, M. abscessus subsp. massiliense is known to be relatively susceptible to macrolide because susceptible strains have a frameshift mutation in the 23S rRNA methylase Erm (41)12. Therefore, it is important to distinguish M. abscessus strains at the subspecies-level in treatment13,14.
In general, M. abscessus can be characterized at the subspecies-level by sequencing genes relevant to phylogeny and antibiotic resistance, such as 16S rRNA, erm (41), hsp65, and rpoB15,16. However, sequencing such genes is complicated and time-consuming. To solve these problems, GenoType NTM DR (Bruker Daltonik, Germany) was developed in 201617. The GenoType NTM DR is a PCR-based test capable of identifying M. abscessus subspecies and detecting clarithromycin or amikacin resistance without sequencing 5 h18.
Matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) can rapidly measure the mass of intact microbial constituents, such as proteins, with minimal sample pretreatment and can be used for the identification of microorganisms in the clinical laboratory on a routine basis19. The MALDI-MS-based method is considered rapid and highly accurate for the identification of species including rapidly growing mycobacterium (RGM) in clinical laboratories20. On the other hand, commercially available MALDI-MS microbial identification systems, such as Biotyper (Bruker Daltonik) and VITEK-MS (bioMerieux, France), have been exclusively designed for the identification of strains at the genus or species level, but it is difficult to distinguish the subspecies using these systems. Previous studies report that the three subspecies of M. abscessus can be distinguished by subspecies-specific MS peaks using the principal component analysis and machine learning of MALDI-MS20,21, but the biomarker peaks for some isolates of M. abscessus subsp. abscessus and M. abscessus subsp. massiliense are too similar and therefore hard to distinguish at the subspecies-level22–26.
In this study, we developed new criteria for the MALDI-MS proteotyping of M. abscessus, allowing for subspecies-level discrimination. Potential biomarker mass peaks were carefully selected in MALDI-MS measurements using cultures including all M. abscessus subspecies. Corresponding protein sequences of these peaks were inferred from the masses of the proteins theoretically encoded in the genome of each type strain. We newly detected some strong mass peaks that characterize the strains of M. abscessus at the subspecies-level. The detection of these peaks was then used as criteria for characterizing 33 clinical M. abscessus isolates at the subspecies-level. The present study contributes to the exact subspecies identification of M. abscessus strains in routine microbiological examinations and paves the way for early determination of treatment strategies.
Results
Clinical features and drug-resistant genes of M. abscessus
The 33 clinical strains of M. abscessus were isolated from 2013 to 2019 at Juntendo University Hospital. The whole-genomes of the 33 M. abscessus were sequenced using MiSeq. According to the latest method for distinguishing between different M. abscessus subspecies using average nucleotide identity (ANI), 16S rRNA, rpoB, hsp65, and erm genes15,27, we obtained clinical isolates of 22 M. abscessus subsp. massiliense and 11 M. abscessus subsp. abscessus. There were no clinical isolates of M. abscessus subsp. bolletii in this study. The detailed information of ANI, 16S rRNA, rpoB, hsp65, and erm genes are shown in Table S1. The results indicate that the M. abscessus subspecies cannot be distinguished using ANI or sequence of each gene.
The susceptibilities of these isolates to various antibiotics were tested using the microdilution method as described by the guidelines of the Clinical and Laboratory Standards Institute28. MICs of clarithromycin were determined at an early reading time (ERT) and a late reading time (LRT) for detecting the inducible macrolide resistance. Among the 11 M. abscessus subsp. abscessus isolates, one isolate was resistant to clarithromycin at the ERT, 8 were resistant to clarithromycin at the LRT, 3 were resistant to imipenem, and all 11 were susceptible to amikacin. The 9 clarithromycin-resistant isolates at the ERT and/or LRT had T28 erm (41) sequevar, whereas the remaining 2 clarithromycin-susceptible isolates had C28 erm (41) sequevar (Supplementary S2).
Among the 22 M. abscessus subsp. massiliense isolates, 2 isolates were resistant to clarithromycin at both the ERT and LRT, 8 were resistant to imipenem, and all 22 were susceptible to amikacin (Table 1). The 2 clarithromycin-resistant isolates at the ERT and/or LRT had a point mutation in rrl gene with an A2058C substitution in its 23S rRNA (Supplementary Table S2).
Table 1.
Drug susceptibility profiles in clinical isolates of Mycobacterium abscessus (N = 33).
| Subspecies | Antibiotics reagent | Breakpoint for resistance (μg/ml) | No. of resistant isolates (%) | MIC (μg/ml) | ||
|---|---|---|---|---|---|---|
| Range | MIC50 | MIC90 | ||||
| M. abscessus subsp. abscessus (N = 11) | Clarithromycin ERT* | ≥ 8 | 9.1 | 0.0625–128 | 0.25 | 1 |
| Clarithromycin LRT** | ≥ 8 | 72.7 | 0.0625–128 | 128 | > 128 | |
| Amikacin | ≥ 64 | 0 | 4–16 | 8 | 16 | |
| Imipenem | ≥ 16 | 27.3 | 4–16 | 8 | 16 | |
| M. abscessus subsp. massiliense (N = 22) | Clarithromycin ERT* | ≥ 8 | 9.1 | 0.0625–128 | 0.0625 | 0.0625 |
| Clarithromycin LRT** | ≥ 8 | 9.1 | 0.0625–128 | 0.0625 | 0.25 | |
| Amikacin | ≥ 64 | 0 | 0.125–16 | 8 | 16 | |
| Imipenem | ≥ 16 | 36.4 | 0.0625–16 | 8 | 16 | |
Breakpoints for antimicrobial resistance were determined according to CLSI guidelines.
*ERT: early reading time (reading on the 5th day).
**LRT: late reading time (reading on the 14th day).
Our study revealed that 72.7% of M. abscessus subsp. abscessus isolates were resistant to clarithromycin whereas 9.1% of M. abscessus subsp. massiliense isolates were resistant to clarithromycin.
MALDI-MS from type strains of M. abscessus
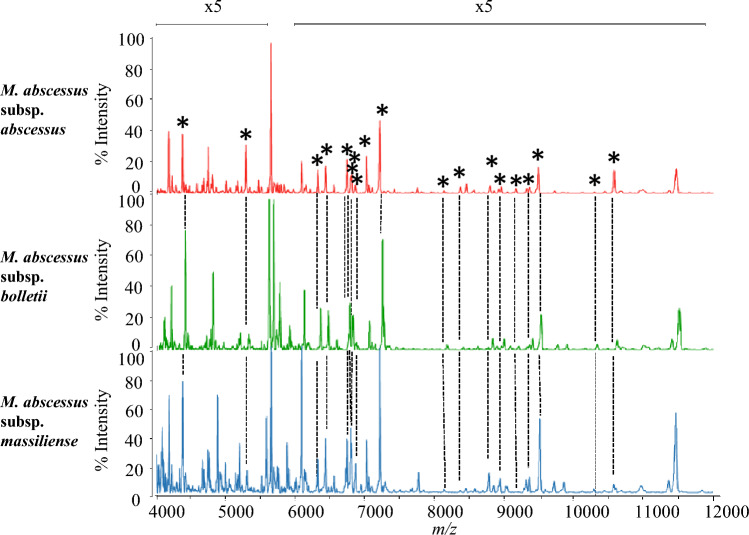
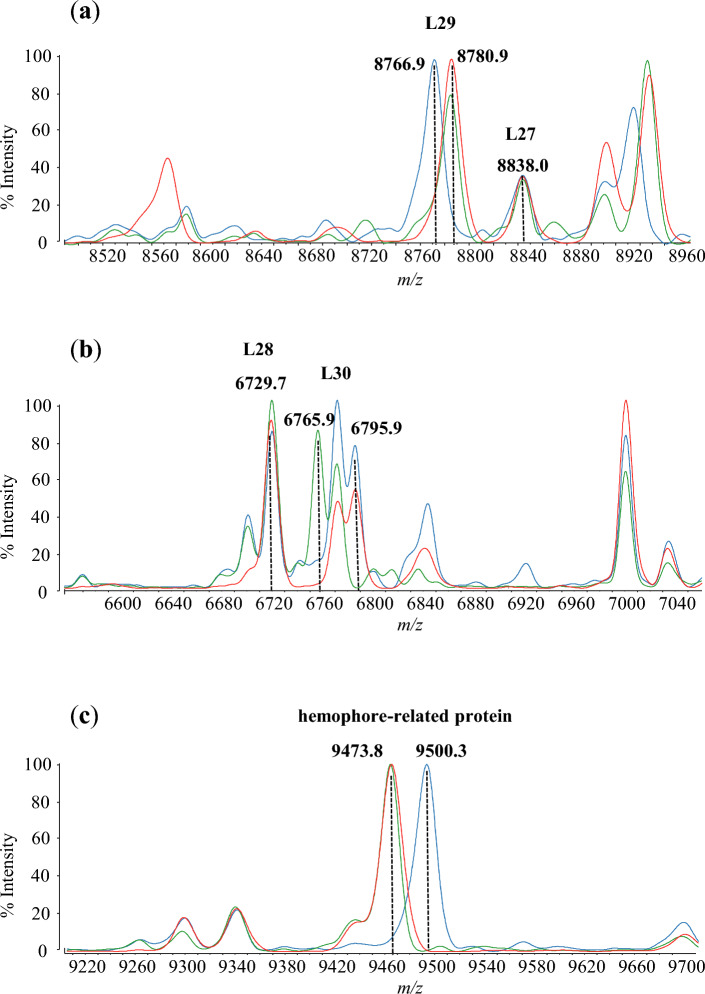
The observed peaks of the three M. abscessus subspecies using MALDI-MS are shown in Fig. 1. Table 2 summarizes the assigned proteins by MALDI-MS, together with the calculated and observed masses. Nineteen detected peaks were assigned with annotated proteins and reproducibility (Fig. 1; Table 2). Of these 19 annotated proteins, 14 proteins were ribosomal subunit proteins. The three biomarkers, including L29, L30 and hemophore-related protein, are able to distinguish the three subspecies of M. abscessus from the 19 annotated proteins. The peaks of M. abscessus subsp. abscessus and M. abscessus subsp. bolletii isolates were detected at m/z 8780.9 as ribosomal protein L29, whereas M. abscessus subsp. massiliense isolates were detected at m/z 8766.9. Moreover, the peaks of M. abscessus subsp. abscessus and M. abscessus subsp. massiliense isolates were detected at m/z 6795.9 as ribosomal protein L30, whereas M. abscessus subsp. bolletii isolates were detected at m/z 6765.9. Finally, the peaks of M. abscessus subsp. abscessus and M. abscessus subsp. bolletii isolates were detected at m/z 9473.8 as hemophore-related protein, whereas M. abscessus subsp. massiliense isolates were detected at m/z 9500.3 (Fig. 2). Analysis by MALDI-8020 and Microflex LT/SH revealed that the peaks of ribosomal protein L29, L30, and hemophore-related protein from the three subspecies cultured in 5% sheep blood agar (Becton, Dickinson-Diagnostic Systems, Sparks, MD, USA) were identical to those cultured in Middlebrook 7H11 Agar plates (Becton, Dickinson-Diagnostic Systems) (Supplementary Tables S3 and S4).
Figure 1.
Representative mass spectra of M. abscessus. Red, green, and blue spectra are M. abscessus subsp. abscessus (ATCC 19977T), M. abscessus subsp. bolletii (JCM 15297T) and M. abscessus subsp. massiliense (JCM 15300T), respectively. Mass spectra and observed proteins from m/z 4000 to 12,000 were merged. The peaks marked with asterisks indicate the assigned peaks based on calculated masses within the tolerance at 500 ppm.
Table 2.
Annotated peaks of type strains of Mycobacterium abscessus.
| Biomarker proteins | Subspecies | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mycobacterium abscessus subsp. abscessus ATCC 19977T | Mycobacterium abscessus subsp. bolletii JCM 15297T | Mycobacterium abscessus subsp. massiliense JCM 15300T | ||||||||||
| Calculated masses (m/z) | Average | SE | Peak numbers (n = 5) | Calculated masses (m/z) | Average | SE | Peak numbers (n = 5) | Calculated masses (m/z) | Average | SE | Peak numbers (n = 5) | |
| L36 | 4371.3 | 4372.0 | 0.18 | 5 | 4371.3 | 4369.8 | 0.37 | 5 | 4371.3 | 4373.0 | 0.36 | 5 |
| L34 | 5460.4 | 5461.0 | 0.36 | 3 | 5460.4 | 5460.6 | 0.44 | 5 | 5460.4 | 5462.2 | 0.37 | 4 |
| L33 2 | 6310.2 | 6310.8 | 0.24 | 5 | 6310.2 | 6310.7 | 0.43 | 5 | 6310.2 | 6311.1 | 0.40 | 5 |
| L32 | 6423.5 | 6423.7 | 0.23 | 5 | 6423.5 | 6423.7 | 0.44 | 5 | 6423.5 | 6424.7 | 0.37 | 5 |
| L28 | 6729.7 | 6729.6 | 0.16 | 5 | 6729.7 | 6730.6 | 0.39 | 5 | 6729.7 | 6731.2 | 0.42 | 5 |
| L30 | 6795.9 | 6796.5 | 0.18 | 5 | 6765.9 | 6767.4 | 0.43 | 5 | 6795.9 | 6797.3 | 0.38 | 5 |
| S14 type Z | 6781.1 | 6782.3 | 0.17 | 5 | 6781.1 | 6782.3 | 0.40 | 5 | 6781.1 | 6782.9 | 0.25 | 5 |
| Pup | 6852.2 | 6853.6 | 0.24 | 5 | 6824.1 | 6825.8 | 0.38 | 5 | 6852.2 | 6854.9 | 0.33 | 5 |
| L35 | 7092.2 | 7092.1 | 0.39 | 2 | 7092.2 | 7093.2 | 0.44 | 5 | 7092.2 | 7092.9 | 0.57 | 5 |
| Probable cold shock protein A | 7199.9 | 7201.0 | 0.17 | 5 | 7199.9 | 7201.8 | 0.42 | 5 | 7199.9 | 7202.1 | 0.33 | 5 |
| L31 | 8123.2 | 8122.3 | 0.29 | 5 | 8123.2 | 8125.3 | 0.37 | 5 | 8123.2 | 8122.1 | 0.64 | 3 |
| Translation initiation factor IF-1 | 8358.7 | 8358.4 | 0.28 | 5 | 8358.7 | 8361.9 | 0.48 | 5 | 8358.7 | 8359.0 | 0.86 | 2 |
| L29 | 8780.9 | 8781.0 | 0.21 | 5 | 8780.9 | 8784.5 | 0.43 | 5 | 8766.9 | 8768.1 | 0.24 | 5 |
| L27 | 8838.0 | 8838.7 | NA* | 1 | 8838.0 | 8842.6 | 0.77 | 5 | 8838.0 | 8840.1 | 0.48 | 5 |
| S18 2 | 9153.7 | 9152.6 | 0.19 | 5 | 9153.7 | 9152.8 | 0.43 | 5 | 9153.7 | NA | NA | 0 |
| S20 | 9348.8 | 9348.9 | 0.08 | 5 | 9348.8 | 9353.0 | 0.52 | 5 | 9348.8 | 9350.7 | 0.28 | 1 |
| Hemophore-related protein | 9473.8 | 9473.2 | 0.22 | 5 | 9473.8 | 9477.3 | 0.44 | 5 | 9500.3 | 9502.4 | 0.35 | 5 |
| S15 | 10,279.0 | NA | NA | 0 | 10,279.0 | 10,283.9 | 0.58 | 5 | 10,279.0 | 10,281.2 | 0.47 | 5 |
| 10 kDa chaperonin | 10,559.9 | 10,561.3 | 0.29 | 5 | 10,559.9 | NA | NA | 5 | 10,559.9 | 10,564.6 | 0.31 | 5 |
*Not assigned.
Figure 2.
The peaks of ribosomal protein L27, L28, L29, L30, and hemophore-related protein are indicated in red for M. abscessus subsp. abscessus (ATCC 19977T), green for M. abscessus subsp. bolletii (JCM 15297T), and blue for M. abscessus subsp. massiliense (JCM 15300T). (a) The peaks of m/z 8766.9, m/z 8780.9, and m/z 8780.9 are represented as ribosomal protein L29 of M. abscessus subsp. massiliense (JCM 15300T) (blue), M. abscessus subsp. abscessus (ATCC 19977T) (red), and M. abscessus subsp. bolletii (JCM 15297T) (green), respectively. (b) The peaks m/z 6765.9, and m/z 6795.9 are represented as ribosomal protein L30 of M. abscessus subsp. bolletii (JCM 15297T) (green), M. abscessus subsp. abscessus (ATCC 19977T) (red), and M. abscessus subsp. massiliense (JCM 15300T) (blue), respectively. (c) The peaks m/z 9500.3, m/z 9473.8, and m/z 9473.8 are represented as hemophore-related protein of M. abscessus subsp. massiliense (JCM 15300T) (blue), M. abscessus subsp. abscessus (ATCC 19977T) (red), and M. abscessus subsp. bolletii (JCM 15297T) (green), respectively.
When compared with the amino acid sequences of these three proteins in M. abscessus subsp. abscessus, an amino acid substitution was observed at position 5 of L29 from Ile to Val (Ile5Val) in M. abscessus subsp. massiliense, at position 12 of L30 from Thr to Ala (Thr12Ala) in M. abscessus subsp. bolletii, and at position 90 of hemophore-related protein from Lys to Arg (Lys90Arg) in M. abscessus subsp. massiliense (Fig. 3). The amino acid substitutions of Ile5Val, Thr12Ala, and Lys90Arg were unique in M. abscessus subsp. massiliense, M. abscessus subsp. bolletii, and M. abscessus subsp. massiliense, respectively.
Figure 3.
Amino acid sequence alignment of ribosomal protein L29, L30, and hemophore-related protein of M. abscessus, M. chelonae, M. franklinii, and M. salmoniphilum. Amino acid substitutions are shaded in black. The arrows indicate the start of amino acid residues after post-transcriptional modifications.
These results indicate that L29, L30, and hemophore-related protein can be biomarkers to distinguish the three subspecies of M. abscessus.
The peaks of L29, L30, and hemophore-related protein in clinical isolates
The evaluation of L29, L30, and hemophore-related protein using 33 clinical strains of M. abscessus shows the peaks of 11 M. abscessus subsp. abscessus isolates at m/z 8780.9 as L29 and at m/z 9473.8 as hemophore-related protein, and the peaks of 20 M. abscessus subsp. massiliense isolates were detected at m/z 8766.9 as L29 and at m/z 9500.3 as hemophore-related protein. The remaining 2 isolates of M. abscessus subsp. massiliense were detected at m/z 8766.9 as L29 and at m/z 9473.8 as hemophore-related protein (Table 3). The condition of these evaluations was conducted in Middlebrook 7H11 Agar plates by MALDI-8020.
Table 3.
The peaks of biokarker proteins in clinical isolates of Mycobacterium abscessus.
| Subspecies | Strains | Average of L29 | SE | Average of hemophore-related protein | SE |
|---|---|---|---|---|---|
| M. abscessus subsp. abscessus* | type strain | 8781.0 | 0.21 | 9473.2 | 0.22 |
| M3 | 8780.9 | 0.16 | 9472.8 | 0.11 | |
| M4 | 8780.2 | 0.19 | 9472.6 | 0.28 | |
| M7 | 8781.2 | 0.23 | 9473.2 | 0.26 | |
| M10 | 8780.8 | 0.33 | 9472.8 | 0.34 | |
| M14 | 8780.3 | 0.30 | 9472.5 | 0.33 | |
| M16 | 8780.9 | 0.28 | 9472.9 | 0.36 | |
| M19 | 8780.7 | 0.37 | 9473.0 | 0.31 | |
| M20 | 8780.3 | 0.25 | 9472.1 | 0.36 | |
| M23 | 8780.6 | 0.28 | 9472.9 | 0.28 | |
| M33 | 8779.5 | 0.22 | 9471.9 | 0.30 | |
| M34 | 8780.5 | 0.20 | 9472.9 | 0.37 | |
| M. abscessus subsp. massiliense** | Type strain | 8768.1 | 0.24 | 9502.4 | 0.35 |
| M5 | 8766.1 | 0.31 | 9500.2 | 0.30 | |
| M6 | 8766.8 | 0.26 | 9501.3 | 0.39 | |
| M8 | 8766.1 | 0.15 | 9500.3 | 0.09 | |
| M11 | 8765.6 | 0.35 | 9500.3 | 0.35 | |
| M13 | 8765.9 | 0.15 | 9500.2 | 0.12 | |
| M15 | 8766.1 | 0.31 | 9500.3 | 0.33 | |
| M26 | 8765.7 | 0.25 | 9499.7 | 0.04 | |
| M27 | 8766.7 | 0.34 | 9501.5 | 0.27 | |
| M28 | 8766.5 | 0.23 | 9500.8 | 0.37 | |
| M29 | 8765.6 | 0.34 | 9500.3 | 0.37 | |
| M30 | 8766.0 | 0.36 | 9500.3 | 0.34 | |
| M32 | 8765.6 | 0.13 | 9499.8 | 0.23 | |
| M36 | 8766.1 | 0.23 | 9500.4 | 0.20 | |
| M37 | 8765.6 | 0.39 | 9499.8 | 0.30 | |
| M38 | 8766.2 | 0.34 | 9500.4 | 0.35 | |
| M39 | 8765.9 | 0.38 | 9500.2 | 0.26 | |
| M40 | 8766.1 | 0.28 | 9500.4 | 0.40 | |
| M43 | 8767.0 | 0.19 | 9501.4 | 0.31 | |
| M44 | 8765.4 | 0.25 | 9499.7 | 0.29 | |
| M45 | 8767.0 | 0.38 | 9501.2 | 0.37 | |
| M41 | 8766.1 | 0.20 | 9472.6 | 0.21 | |
| M42 | 8766.2 | 0.10 | 9472.8 | 0.08 | |
| M. abscessus subsp. bolletii* | Type strain | 8784.5 | 0.43 | 9477.3 | 0.44 |
*The theoretical masses of L29 and hemophore-related protein are m/z 8780.9 and m/z 9473.8.
**The theoretical masses of L29 and hemophore-related protein are m/z 8766.9 and m/z 9500.3.
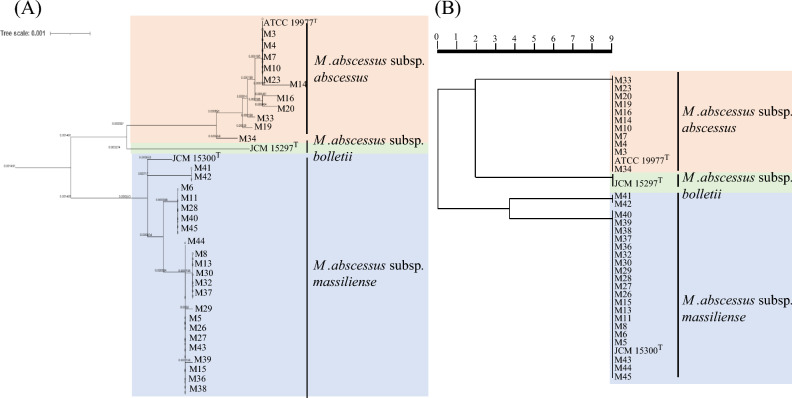
Phylogenetic analysis and cluster analysis
A genome-based phylogenetic tree was classified into the three groups of M. abscessus subsp. abscessus, M. abscessus subsp. bolletii, and M. abscessus subsp. massiliense. Furthermore, 2 isolates with peaks at m/z 9473.8 as hemophore-related protein shown in Table 3 were subclassified in the M. abscessus subsp. massiliense group (Fig. 4A).
Figure 4.
Phylogenetic tree of 33 clinical isolates of M. abscessus and three type strains of M. abscessus, including M. abscessus subsp. abscessus, M. abscessus subsp. bolletii, and M. abscessus subsp. massiliense. (A) The tree was constructed based on concatenated single-copy marker protein sequences predicted from genomes. (B) The tree was constructed by Strain Solution based on the biomarker proteins from the MALDI-MS data.
A cluster analysis using the MALDI-MS data reveals that M. abscessus subsp. abscessus, M. abscessus subsp. bolletii, and M. abscessus subsp. massiliense are classified into the three groups (Fig. 4B). The phylogenic trees based on whole-genome and cluster analysis are identical.
Discussion
MALDI-MS proteotyping is useful for accurate and rapid identification of M. abscessus subspecies, compared to sequencing using ANI, 16S rRNA, rpoB, hsp65, and erm genes or GenoType NTM-DR. While GenoType NTM-DR was developed for identifying M. abscessus subspecies and for determining resistance against clarithromycin and amikacin in 201618, this method does not seem to be prevalent in clinical laboratories.
The prediction of the theoretical protein masses based on genomes of M. abscessus is useful for detecting the biomarker peaks using MALDI-MS. The theoretical protein mass database will accurately identify bacterial strains at the species to subspecies levels based in which correct identification for > 90% of measured spectra using MALDI-MS29. This approach can easily predict MALDI-MS spectra based on genome sequences from cultured and uncultured strains rather than experimentally acquired spectra. In this study, 19 detected peaks could be assigned with annotated proteins and 3 of 19 biomarker peaks, including ribosomal L29, L30 and hemophore-related protein, were screened as biomarkers for detecting subspecies of M. abscessus. Although we need to validate the identification for the other Mycobacterium in future, the closely related Mycobacterium species will not affect the identification of M. abscessus subspecies, because the amino acids of L29, L30 and hemophore-related protein are different.
Ribosomal protein L29, L30, and hemophore-related protein will be useful candidates as biomarkers for detecting subspecies of M. abscessus. Many of the peaks of MALDI- MS are derived from the ribosomal proteins, but it is difficult to extract and detect the protein fragments that require additional sample preparation for some microorganisms such as Mycobacterium spp.30. In the previous reports, the specific peaks of M. abscessus are distinct due to differences in sample preparation, mediums, and the instruments used25. In this study, all samples were crushed by a high-speed homogenizer and frozen according to the recommended methods in previous reports22,25. It has been reported that freezing the samples prior to MALDI-MS analysis effectively damages the bacterial cells for detecting MS peaks31. The peaks of L29, L30, and hemophore-related protein can be detected regardless of the mediums and the instruments.
In previous reports, four to seven peaks were used for distinguishing M. abscessus subspecies20,22–26, but we recommend a combination of three peaks: L29, L30, and hemophore-related protein. The three candidate proteins were screened by genome annotations and theoretical-protein-mass predictions. Suzuki et al.25 reported the peaks of m/z 8780.9 and m/z 9473.8 of M. abscessus subsp. abscessus and M. abscessus subsp. bolletii and the peaks of m/z 8766.9 and m/z 9500.3 of M. abscessus subsp. massiliense. The report also describes the peaks of m/z 4391.24 and m/z 4385.05, which are the divalent ion of the peaks of m/z 8780.9 and m/z 8766.9, respectively, for detecting M. abscessus. We newly developed the target peaks of L30, m/z 6765.9 and m/z 6795.9, for detecting M. abscessus subsp. bolletii.
Hemophore-related protein will be essential as a target peak. Previously, it has been reported that some isolates of M. abscessus subsp. massiliense has peaks of m/z 9473.8225 and m/z 9473.3124, which are similar to our finding for hemophore-related protein in M. abscessus subsp. abscessus. Using hemophore-related protein as a biomarker peak for distinguishing the subspecies will prevent misidentification.
For the early determination of effective therapy, it is necessary to distinguish the three M.abscessus subspecies’ susceptibilities to macrolides in routine testing. However, several isolates of clarithromycin-sensitive M. abscessus subsp. abscessus and clarithromycin-resistant M. abscessus subsp. massiliense with specific gene mutations were detected in a previous study32 as well as this study. Thus, the bacterial identification tests including MALDI-MS have a limitation in that they cannot accurately estimate drug susceptibility. On the other hand, drug susceptibility testing is not enough for the classification of strains harboring resistant genes. Therefore, the combination of MALDI-MS and drug susceptibility testing is important for the identification of M. abscessus subspecies in clinical laboratories.
This study has several limitations: first, this is a single-center study with a small number of samples. Second, the data from the clinical isolates of M. abscessus subsp. bolletii is missed in this study. It is necessary to confirm that the three biomarkers, including L29, L30 and hemophore-related protein, are useful for the identification of M. abscessus subspecies using more isolates obtained in the other hospital laboratories from different countries in future. Third, although the amino acid sequences of L29, L30 and hemophore-related protein were unique in M. abscessus subspecies, it is necessary to confirm the spectra of the other RGMs with different amino acid sequences and the same theoretical protein mass.
In conclusion, the detection of the peaks of L29, L30, and hemophore-related protein by MALDI-MS proteotyping will be useful for accurate and rapid identification of M. abscessus, compared to traditional methods of sequencing. The identification of M. abscessus by MALDI-MS combined with drug susceptibility testing will be the best way for an early decision on a course of treatment.
Materials and methods
Bacterial strains
The type strains of each subspecies were used to select biomarker candidates to distinguish the three subspecies of M. abscessus using MALDI-MS. The type strain of M. abscessus subsp. abscessus GTC 15115T (= ATCC 19977T) was obtained from Gifu University Center for Conservation of Microbial Genetic Resource and the type strains of M. abscessus subsp. bolletii JCM 15297T and M. abscessus subsp. massiliense JCM 15300T were both obtained from the RIKEN BRC (Tsukuba, Ibaraki, Japan). Thirty-three clinical isolates of M. abscessus were obtained between June 2013 and October 2019 from 33 patients treated at Juntendo University Hospital in Japan. The isolates were cultured in Middlebrook 7H11 Agar plates (Becton, Dickinson-Diagnostic Systems) or 5% sheep blood agar (Becton, Dickinson-Diagnostic Systems) under aerobic conditions at 35 °C for 3 days.
Drug susceptibility testing
Drug susceptibility was tested as described by the Clinical and Laboratory Standard Institute (CLSI) guidelines28. The antibiotic concentrations of clarithromycin, amikacin, and imipenem ranged from 0.063 to 128 μg/mL. The minimum inhibitory concentrations (MICs) of each antimicrobial agent were determined by broth microdilution methods using Muller Hinton broth and 96-well microtiter plates (Kohjin Bio, Co., Ltd. Saitama, Japan). The MICs of clarithromycin, amikacin, and imipenem were determined on the 5th day at an early reading time (ERT) and on the 14th day at a delayed reading time (LRT).
Whole-genome sequencing
Genomic DNA of the 33 clinical M. abscessus isolates were extracted using DNeasy blood and tissue kits (Qiagen, Tokyo, Japan) and DNA libraries were prepared using Nextera XT DNA Library Prep Kit (Illumina, San Diego, CA). Their genomes were sequenced by Illumina MiSeq platform using v3 chemistry (600 cycles) and the summary of the assembly is shown in Supplementary Table S5. Raw reads of each isolate were trimmed and assembled using CLC Genomic Workbench version 10.0.1 using quality control and assembly tools with default settings (CLC bio, Aarhus, Denmark). Species identities of these isolates were determined using an ANI calculator33 and the sequences of 16S rRNA, rpoB, hsp65, and erm genes12,15. The ANI values and sequence identities of 16S rRNA, rpoB, hsp65, and erm genes were calculated and adopt the closest of the reference genomes of M. abscessus subsp. abscessus (ATCC 19977T, genome accession number GCF_000069185.1), M. abscessus subsp. bolletii (JCM 15297T, GCF_003609715.1), and M. abscessus subsp. massiliense (JCM 15300T, GCF_000497265.2)27. The mutations of erm and rrl genes were detected in silico using CLC Genomics Workbench34.
Phylogenetic analysis
The genome completeness and contamination were assessed using CheckM2 v1.0.1 with lineage wf and default settings35. Phylogenetic trees were constructed based on concatenated single-copy marker protein sequences predicted from genomes using GTDB-Tk v2.2.6 software36 and visualized using iTol ver.6 (https://itol.embl.de/). The type strains of M. abscessus subsp. abscessus (ATCC 19977T, genome accession number GCF_000069185.1), M. abscessus subsp. bolletii (JCM 15297T, GCF_003609715.1), and M. abscessus subsp. massiliense (JCM 15300T, GCF_000497265.2) were used as the reference strains.
Accession numbers
The whole-genome sequences of all 33 isolates have been deposited in the GenBank as accession number PRJDB15290.
Calculation of the theoretical mass of M. abscessus
Theoretical masses of proteins encoded in genomes of M. abscessus were calculated for the following genomes as part of the development of a genomically predicted protein mass database of Bacteria and Archaea: M. abscessus subsp. abscessus (ATCC 19977T, GCF_000069185.1), M. abscessus subsp. bolletii (JCM 15297T, GCF_003609715.1), and M. abscessus subsp. massiliense (JCM 15300T, GCF_000497265.2)29. The genome sequences were obtained from the NCBI database (https://www.ncbi.nlm.nih.gov/). Gene prediction from the genomes was performed using Prodigal v2.6.337. The calculation of the theoretical mass of individual gene products was performed by python scripts with consideration of the average [M + H]+ of all the gene products. For all amino-acid sequences, methionine loss was considered if the first amino acid at the N-terminal was “M” and the second amino acid was either “G”, “A”, “S”, “P”, “V”, “T”, or “C”29. Mature protein sequences were inferred using SignalP v5.038 with command line flags “-org gram + ” for genomes.
Bacterial sample preparation for MALDI-MS
Alpha-cyano-4-hydroxycinnamic acid (CHCA) was used as a matrix. To prepare this matrix solution, 10 mg of 4-CHCA was dissolved in 1 mL of the solvent consisting of 1% (v/v) trifluoroacetic acid, 35% (v/v) ethanol, 15% (v/v) acetonitrile, and milliQ water. A full loop of bacterial cells was dispersed in 200 μL of distilled water in a microtube and mixed with 800 μL of ethanol with zirconia beads. The suspensions were vortexed briefly and centrifuged at 15,000 g for 2 min. The pellets were then dried for 5 min. After freezing the tubes at − 80 °C at least 1 h, the pellets were resuspended in MilliQ water, crushed using a Fast Prep 24 apparatus (Funakoshi Co., Ltd.) for a total of 3 min (9 times for 20 s), and centrifuged at 15,000 g for 2 min. Supernatants were analyzed by MALDI-MS according to the manufacturer’s instruction.
MALDI-MS measurement
MALDI-MS measurements were performed in positive linear mode using MALDI-8020 RUO (Shimadzu Corporation, Japan) and Microflex LT/SH (Bruker Daltonik) equipped with a 200 Hz Nd: YAG laser (355 nm) and 60 Hz nitrogen laser (337 nm), respectively.
Before the sample analysis, the MALDI-MS instrument was mass-calibrated externally using 6 peaks with m/z 4365.4, 5381.4, 6411.6, 7274.0, 8369.8, and 10,300.1 from Escherichia coli DH5α. More than five individual mass spectra were acquired for each bacterial extract in the range of m/z 2000–20,000 and self-calibrated using three ribosomal protein peaks with m/z 4371.3, 6310.2, and 9348.8, which were commonly detected from corresponding type strains of the three subspecies of M. abscessus. Peak assignment was carried out using eMSTAT Solution™ software (Shimadzu Corp.). The peaks were assigned using a comparison with the calculated masses of genome sequenced type strains of M. abscessus subsp. abscessus, M. abscessus subsp. bolletii, and M. abscessus subsp. massiliense.
Cluster analysis
For biomarker validation, 36 M. abscessus isolates including three subspecies were analyzed by MALDI-MS. Four mass spectra were acquired for each strain, and peak lists were extracted from those mass spectra, considering the peak intensity and reproducibility. Biomarker analysis software Strain Solution™ was used to prepare a binary matrix.
Ethical statement
This study was approved by the Ethical Committee of Juntendo University (approval number: E21-0232).
Supplementary Information
Acknowledgements
The authors would like to thank the staff of Juntendo University Hospital for their contribution in collecting data.
Author contributions
ST, KTe and YT designed the study. ST, KTe, YS and AK carried out data collection and analysis. ST, KTe, TT, YT, SI and KTa performed data analysis and wrote the manuscript. TT contributed to writing and preparing the manuscript. HI, MT, YH, SM, TN and TK discussed experiments and contributed to data interpretation; all authors critically reviewed and revised the manuscript for content and approved the manuscript for publication.
Funding
This study was supported by grants from the Japan Society for the Promotion of Science (Grant number: 21K07031 to TT and 22K15675 to ST).
Data availability
All data generated or analyzed during this study are included in this published article. The sequence data generated in this study have been submitted to the DDBJ database (http://getentry.ddbj.nig.ac.jp/) under the accession numbers PRJDB15290.
Competing interests
The Department of MALDI-TOF MS at Juntendo University Graduate School of Medicine has been endowed partially by Shimadzu Corp. (Kyoto, Japan) to develop and validate new diagnostic technology and to conduct academic research through collaborations. ST, KTe, HI, SM, TN, TK and YT belong to the Department of MALDI-TOF MS. Shimadzu Corp. provided MALDI-8020 and reagents for MALDI-MS analysis free of cost to YT. All other authors declare no conflict of interest. The study was performed by scientifically proper methods without any bias.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-61549-7.
References
- 1.Petrini B. Mycobacterium abscessus: An emerging rapid-growing potential pathogen. Apmis. 2006;114:319–328. doi: 10.1111/j.1600-0463.2006.apm_390.x. [DOI] [PubMed] [Google Scholar]
- 2.Namkoong H, et al. Epidemiology of pulmonary nontuberculous mycobacterial disease Japan. Emerg. Infect. Dis. 2016;22:1116–1117. doi: 10.3201/eid2206.151086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai CC, Wang HC. Clinical significance of Mycobacterium abscessus isolates at a medical center in Northern Taiwan. J. Microbiol. Immunol. Infect. 2011;44:488–489. doi: 10.1016/j.jmii.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Bryant JM, et al. Whole-genome sequencing to identify transmission of Mycobacterium abscessus between patients with cystic fibrosis: A retrospective cohort study. Lancet. 2013;381:1551–1560. doi: 10.1016/s0140-6736(13)60632-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takei S, et al. The synergetic effect of Imipenem-clarithromycin combination in the Mycobacteroides abscessus complex. BMC Microbiol. 2020;20:316. doi: 10.1186/s12866-020-02000-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyasaka T, et al. In vitro efficacy of imipenem in combination with six antimicrobial agents against Mycobacterium abscessus. Int. J. Antimicrob. Agents. 2007;30:255–258. doi: 10.1016/j.ijantimicag.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Griffith DE, et al. An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 8.Daley CL, et al. Treatment of nontuberculous mycobacterial pulmonary disease: An official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur. Respir. J. 2020;56:1. doi: 10.1183/13993003.00535-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bastian S, et al. Assessment of clarithromycin susceptibility in strains belonging to the Mycobacterium abscessus group by erm(41) and rrl sequencing. Antimicrob. Agents Chemother. 2011;55:775–781. doi: 10.1128/aac.00861-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan JL, Khang TF, Ngeow YF, Choo SW. A phylogenomic approach to bacterial subspecies classification: proof of concept in Mycobacterium abscessus. BMC Genomics. 2013;14:879. doi: 10.1186/1471-2164-14-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tortoli, E. et al. Emended description of Mycobacterium abscessus, Mycobacterium abscessus subsp. abscessus and Mycobacteriumabscessus subsp. bolletii and designation of Mycobacteriumabscessus subsp. massiliense comb. nov. Int. J. Syst. Evol. Microbiol.66, 4471–4479. 10.1099/ijsem.0.001376 (2016). [DOI] [PubMed]
- 12.Kim HY, et al. Mycobacterium massiliense is differentiated from Mycobacterium abscessus and Mycobacterium bolletii by erythromycin ribosome methyltransferase gene (erm) and clarithromycin susceptibility patterns. Microbiol. Immunol. 2010;54:347–353. doi: 10.1111/j.1348-0421.2010.00221.x. [DOI] [PubMed] [Google Scholar]
- 13.Koh WJ, et al. Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am. J. Respir. Crit. Care Med. 2011;183:405–410. doi: 10.1164/rccm.201003-0395OC. [DOI] [PubMed] [Google Scholar]
- 14.Harada T, et al. Clinical and microbiological differences between Mycobacterium abscessus and Mycobacterium massiliense lung diseases. J. Clin. Microbiol. 2012;50:3556–3561. doi: 10.1128/jcm.01175-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakanaga K, et al. Discrimination of Mycobacterium abscessus subsp. massiliense from Mycobacterium abscessus subsp. abscessus in clinical isolates by multiplex PCR. J. Clin. Microbiol. 2014;52:251–259. doi: 10.1128/jcm.01327-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adékambi T, Drancourt M. Dissection of phylogenetic relationships among 19 rapidly growing Mycobacterium species by 16S rRNA, hsp65, sodA, recA and rpoB gene sequencing. Int. J. Syst. Evol. Microbiol. 2004;54:2095–2105. doi: 10.1099/ijs.0.63094-0. [DOI] [PubMed] [Google Scholar]
- 17.Kehrmann J, Kurt N, Rueger K, Bange FC, Buer J. GenoType NTM-DR for identifying Mycobacterium abscessus subspecies and determining molecular resistance. J. Clin. Microbiol. 2016;54:1653–1655. doi: 10.1128/jcm.00147-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huh HJ, et al. Genotype NTM-DR performance evaluation for identification of Mycobacterium avium complex and Mycobacterium abscessus and determination of clarithromycin and amikacin resistance. J. Clin. Microbiol. 2019;57:1. doi: 10.1128/jcm.00516-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eigner U, et al. Performance of a matrix-assisted laser desorption ionization-time-of-flight mass spectrometry system for the identification of bacterial isolates in the clinical routine laboratory. Clin. Lab. 2009;55:289–296. [PubMed] [Google Scholar]
- 20.Rodríguez-Temporal D, et al. Identification of Mycobacterium abscessus subspecies by MALDI-TOF mass spectrometry and machine learning. J. Clin. Microbiol. 2023;61:e0111022. doi: 10.1128/jcm.01110-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo L, et al. Evaluation of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of Mycobacterium abscessus subspecies according to whole-genome sequencing. J. Clin. Microbiol. 2016;54:2982–2989. doi: 10.1128/jcm.01151-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teng SH, et al. Matrix-assisted laser desorption ionization-time of flight mass spectrometry can accurately differentiate between Mycobacterium masilliense (M. abscessus subspecies bolletti) and M. abscessus (sensu stricto) J. Clin. Microbiol. 2013;51:3113–3116. doi: 10.1128/jcm.01239-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fangous MS, et al. Classification algorithm for subspecies identification within the Mycobacterium abscessus species, based on matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 2014;52:3362–3369. doi: 10.1128/jcm.00788-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panagea T, et al. Mycobacterium abscessus complex identification with matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 2015;53:2355–2358. doi: 10.1128/jcm.00494-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki H, et al. A novel cluster of Mycobacterium abscessus complex revealed by matrix-assisted laser desorption ionization-time-of-flight mass spectrometry (MALDI-TOF MS) Diagn. Microbiol. Infect. Dis. 2015;83:365–370. doi: 10.1016/j.diagmicrobio.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Tseng SP, et al. Rapid identification of M. abscessus and M. massiliense by MALDI-TOF mass spectrometry with a comparison to sequencing methods and antimicrobial susceptibility patterns. Future Microbiol. 2013;8:1381–1389. doi: 10.2217/fmb.13.115. [DOI] [PubMed] [Google Scholar]
- 27.Adekambi T, Sassi M, van Ingen J, Drancourt M. Reinstating Mycobacterium massiliense and Mycobacterium bolletii as species of the Mycobacterium abscessus complex. Int. J. Syst. Evol. Microbiol. 2017;67:2726–2730. doi: 10.1099/ijsem.0.002011. [DOI] [PubMed] [Google Scholar]
- 28.Woods, G. L., Lin, G., & Turnidge, J. D. Clinical and Laboratory Standards Institute, Wayne (PA) (2018).
- 29.Sekiguchi Y, et al. A large-scale genomically predicted protein mass database enables rapid and broad-spectrum identification of bacterial and archaeal isolates by mass spectrometry. Genome Biol. 2023;24:257. doi: 10.1186/s13059-023-03096-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freiwald A, Sauer S. Phylogenetic classification and identification of bacteria by mass spectrometry. Nat. Protoc. 2009;4:732–742. doi: 10.1038/nprot.2009.37. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez-Temporal D, Perez-Risco D, Struzka EA, Mas M, Alcaide F. Evaluation of two protein extraction protocols based on freezing and mechanical disruption for identifying nontuberculous mycobacteria by matrix-assisted laser desorption ionization-time of flight mass spectrometry from liquid and solid cultures. J. Clin. Microbiol. 2018;56:1. doi: 10.1128/jcm.01548-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jong BE, et al. Impact on macrolide resistance of genetic diversity of mycobacterium abscessus species. Microb. Spectr. 2022;10:e0274922. doi: 10.1128/spectrum.02749-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoon SH, Ha SM, Lim J, Kwon S, Chun J. A large-scale evaluation of algorithms to calculate average nucleotide identity. Antonie Van Leeuwenhoek. 2017;110:1281–1286. doi: 10.1007/s10482-017-0844-4. [DOI] [PubMed] [Google Scholar]
- 34.Lipworth S, et al. Whole-genome sequencing for predicting clarithromycin resistance in mycobacterium abscessus. Antimicrob. Agents Chemother. 2019;63:1. doi: 10.1128/aac.01204-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parks DH, Imelfort M, Skennerton CT, Hugenholtz P, Tyson GW. CheckM: Assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015;25:1043–1055. doi: 10.1101/gr.186072.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaumeil PA, Mussig AJ, Hugenholtz P, Parks DH. GTDB-Tk v2: Memory friendly classification with the genome taxonomy database. Bioinformatics. 2022;38:5315–5316. doi: 10.1093/bioinformatics/btac672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hyatt D, et al. Prodigal: Prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics. 2010;11:119. doi: 10.1186/1471-2105-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Almagro Armenteros JJ, et al. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019;37:420–423. doi: 10.1038/s41587-019-0036-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article. The sequence data generated in this study have been submitted to the DDBJ database (http://getentry.ddbj.nig.ac.jp/) under the accession numbers PRJDB15290.