Abstract
Circadian clocks temporally coordinate daily organismal biology over the 24-h cycle. Their molecular design, preserved between fungi and animals, is based on a core-oscillator composed of a one-step transcriptional-translational-negative-feedback-loop (TTFL). To test whether this evolutionarily conserved TTFL architecture is the only plausible way for achieving a functional circadian clock, we adopted a transcriptional rewiring approach, artificially co-opting regulators of the circadian output pathways into the core-oscillator. Herein we describe one of these semi-synthetic clocks which maintains all basic circadian features but, notably, it also exhibits new attributes such as a “lights-on timer” logic, where clock phase is fixed at the end of the night. Our findings indicate that fundamental circadian properties such as period, phase and temperature compensation are differentially regulated by transcriptional and posttranslational aspects of the clockworks.
Keywords: Circadian Rhythms, Transcriptional Rewiring, Neurospora, Synthetic Biology, Photoresponses
Subject terms: Biotechnology & Synthetic Biology; Chromatin, Transcription & Genomics
Synopsis

Eukaryotic circadian oscillators share a one-step transcription-translation negative-feedback loop (TTFL) circuit design, where a main transcriptional complex drives expression of a negative element that can inhibit its own expression. Here, a transcriptional rewiring strategy in the fungus N. crassa reveals that this basic circuit topology can be dramatically changed, while still yielding a functional semi-synthetic clock.
Placing the transcription of the negative component frq under the control of the output promoter con-10 alters its regulation, while still yielding rhythmic expression.
In the resulting functional semi-synthetic circuit, frq expression is now under the control of circadian clock downstream components.
FRQ phosphorylation has a more pronounced effect on circadian period length and temperature compensation than its transcriptional regulation.
In the semi-synthetic oscillator, the circadian rhythm phase is now determined by the moment the light is turned on.
Co-option of downstream transcription factors into the main transcriptional circuit of the circadian clock in the fungus Neurospora crassa leads to altered clock phase determination.

Introduction
Circadian rhythms are present in diverse organisms, from bacteria to mammals. These ~24-h rhythms, generated by circadian oscillators, share common features: they are maintained under constant conditions, are temperature compensated (period is stable within a physiological range of temperatures) and are entrained by environmental cues such as light and temperature (Dunlap, 1999; Rosbash and Hall, 1989). Circadian clocks allow organisms to anticipate daily changes, temporarily compartmentalizing diverse, and sometimes antagonizing, cellular processes (Hurley et al, 2014; Sancar et al, 2015b; Asher and Schibler, 2011; Hurley et al, 2018; Baek et al, 2019), while the absence of a functional clock or the genetic or environmental perturbations of its function, can compromise organismal fitness and physiology (Roenneberg and Merrow, 2016).
Circadian clocks have appeared independently at least three different times throughout evolution and, consequently, all core-clock components do not share sequence conservation throughout the tree of life (Loudon, 2012; Rosbash, 2009; Dunlap and Loros, 2018). Yet, how these molecular gears interact in terms of circuitry is highly preserved across taxa (Rosbash, 2009; Dunlap, 1999). Indeed, circadian oscillators in fungi and animals are based on a transcription-translation negative feedback loop (TTFL) where Positive Elements (WC-1/WC-2 in Neurospora; CYC/CLK in Drosophila; BMAL1/CLOCK in mammals) directly drive the transcription of Negative Elements (FRQ in Neurospora; TIM/PER in Drosophila; CRY/PER in mammals) that nucleate multiprotein complexes—always including casein kinase 1 (CK1)—which feedback to inhibit the Positive Elements, thereby shutting down their own expression (Dunlap, 1999).
In Neurospora, White Collar-1 (WC-1) and White Collar-2 (WC-2) form the White Collar Complex (WCC) (Crosthwaite et al, 1997; Dunlap, 2006; Ballario and Macino, 1997), which in constant darkness binds the clock box (c-box) in the frq promoter (Froehlich et al, 2003), activating its expression. FRQ is produced, dimerizes, and interacts with the RNA helicase FRH (Cheng et al, 2005) and CK1, promoting the inactivation of WCC through its phosphorylation (Cheng et al, 2005; Schafmeier et al, 2005; He et al, 2006; Wang et al, 2019). FRQ is the substrate of several kinases, until it reaches a hyperphosphorylated state where it can no longer inhibit the WCC, allowing a new cycle of transcription to start; subsequently, hyperphosphorylated FRQ is degraded via the proteasome (He et al, 2003; He and Liu, 2005). Indeed, progressive phosphorylation of FRQ leading to its hyperphosphorylation and inactivation, and not degradation per se, appears to be critical for circadian cycling (Larrondo et al, 2015; Liu et al, 2019). In addition, WC-1 is a photoreceptor that in response to light induces the expression of many genes, including frq (Chen et al, 2009). In the presence of light, WCC recognizes a distinct cis-element within the frq promoter (proximal light response element or pLRE), boosting frq transcription, and allowing the synchronization of the clock with the environment, in a process that is crucial for phase determination (Froehlich et al, 2002). Thus, the direct binding of the WCC to defined cis-elements within the promoter of the negative element frq, and the tight control over its regulation, is essential for proper circadian and light-induced expression of this core-clock component (Sancar et al, 2012; Belden et al, 2011, 2007b; Oehler et al, 2023).
In Neurospora, the central oscillator confers rhythmicity to diverse biological processes such as metabolism and conidiation (Dunlap, 1999). The information passes from the central oscillator to the output pathways, in part by a hierarchical arrangement of transcription factors that allows the rhythmic expression of an abundant cohort of clock-controlled genes (ccgs) (Hurley et al, 2016). con-10 is one of those ccgs, with an undefined function in development and conidiation (Berlin and Yanofsky, 1985; Roberts et al, 1988; Ebbole et al, 1991), which also exhibits a strong and acute transcriptional response to light (Lauter and Yanofsky, 1993). Nevertheless, despite its strong photo-response, neither its induction by light nor its rhythmic expression have been reported to be directly controlled by the WCC (Hurley et al, 2014; Smith et al, 2010), and instead it has been described to depend on a complex regulation involving other transcription factors (Sancar et al, 2015a, 2015b, 2011).
One of the fascinating aspects about fungal and animal circadian clocks is how, despite their divergence from a phylogenetic origin a billion years ago, they display an evolutionarily conserved design: a one-step TTFL where Positive Elements directly control the expression of Negative ones. Moreover, as circadian oscillators started to be described across phyla, it was suggested that such one-step TTFL circuitry might be “the only way you can make a clock” (Barinaga, 1998). Provoked by such concept, we sought to challenge the genetic topological plasticity of a circadian TTFL and attest if an alternative circuit design could actually yield a functional oscillator. Thus, through transcriptional rewiring, we extended the original TTFL topology by subjecting frq expression to the control of a ccg promoter. We called this semi-synthetic design a hybrid oscillator (HO), as it combines core-clock components with cogs and gears that are normally part of the output (ccg) pathways. Due to the abundance of molecular tools, straightforward genetics, and the absence of gene families and paralogues, Neurospora is a great platform in which to adopt synthetic biology strategies, such as the implementation of new circadian clock topologies or synthetic circuits (Tabilo-Agurto et al, 2023; Matsu-Ura et al, 2018). Herein we characterize the HO generated by rewiring the con-10 promoter to control frq expression, denominating it HO-10. HO-10 has a period close to 24 h in constant conditions, which can be dramatically shortened or lengthened by altering FRQ phosphorylation dynamics and, most remarkably, it is temperature compensated. Interestingly, other aspects such as responses to light and phase definition display novel and unexpected properties, revealing a “lights-on timer” behavior. Thus, this work uncovers that the evolutionarily conserved simple one-step TTFL is not the only possible functional circadian core-clock topology that could arise based on already existing cellular components. Analysis of this semi-synthetic oscillator also helps to underscore a key role for posttranslational regulation in properties such as temperature compensation and period determination, whereas transcriptional mechanisms appears as critical for clock phase determination and entrainment to light, revealing also an unanticipated property such as the emergence of a lights-on timer logic.
Results
Generation of a functional hybrid oscillator
To change the architecture of the central oscillator, we eliminated the native connection between the Positive (WCC) and the Negative Element (frq) by a transcriptional rewiring strategy. Thus, using homologous recombination we replaced the endogenous frq promoter, including its c-box, pLRE, and 1.5 kbp 5’ UTR, with different ccg promoters (including their respective 5’ UTRs). As indicated above, we denominated these semi-synthetic circuits as hybrid oscillators (HOs), as they conserve parts of the wild-type oscillator and incorporate new components that innately only serve a role in output pathways (Fig. 1A). As proof of concept, we first chose three different ccg promoters already known to drive rhythmic transcription under constant conditions (constant darkness, DD), as confirmed by luciferase transcriptional reporters (Appendix Fig S1). Importantly, most ccgs are not only subjected to clock-regulation but also to a myriad of additional transcriptional inputs related to their specific role in the biology of the organism.
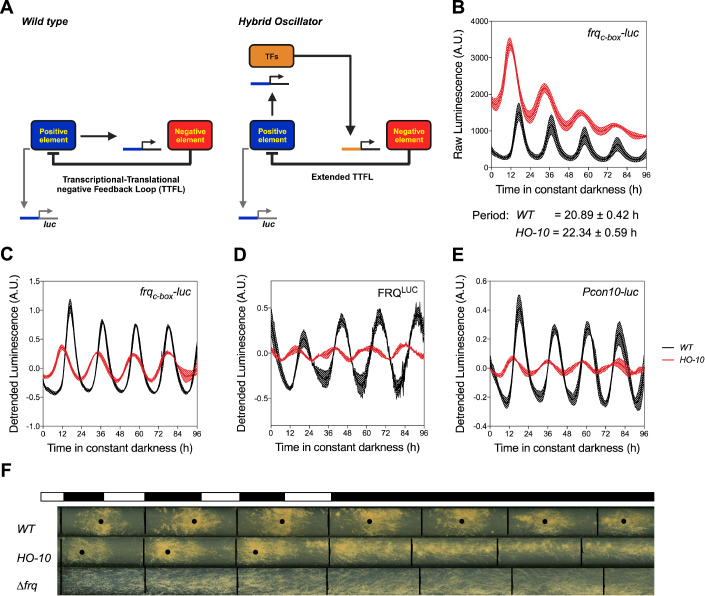
Figure 1. A semi-synthetic oscillator sustains molecular oscillations, but not rhythmic overt conidiation under constant darkness.
(A) Scheme of the native transcriptional translational feedback loop (TTFL) where the Positive Element (WCC, blue) directly regulates the transcription of the Negative Element (frq, red). To generate a semi-synthetic oscillator the promoter of the Negative Element was replaced by the promoter of a clock-controlled gene (ccg, orange), giving rise to a hybrid oscillator (HO) that combines core (blue and red) and output (orange) components in an extended TTFL topology. In both cases, a minimal frq promoter controlling luc expression serves as a readout of the system. (B–E) Evaluation of HO-10, constructed with the promoter of the ccg known as con-10, under constant darkness (DD) by analyzing LUC activity. Prior to luciferase monitoring in DD the strains were grown for 3 days under cycles of 12 h of light and 12 h of darkness (LD 12:12). The analyzed luciferase reporters were frqc-box-luc (B) and (C), FRQLUC (D) and Pcon10-luc (E). While in (B) raw data are shown, (C), (D), and (E) correspond to normalized detrended data. Each luciferase trace corresponds to the average of three different wells ± SD. All experiments were run three independent times, and a representative set is shown. (F) Race tube analysis of WT, HO-10, and a frq-less strain. Strains were analyzed for three days under LD 12:12 cycles and then transferred, on day four, to constant darkness. All experiments were run three independent times, and a representative set is shown. Source data are available online for this figure.
The capacity of the different HOs to generate and sustain rhythms in DD was evaluated using frqc-box-luc which serves as a reporter for the activity of the TTFL Positive Element (WCC). Strains were grown in constant light (LL) for 24 h before monitoring them for luciferase activity in continuous darkness (DD). While WT strains showed strong and robust rhythms, the different rewired strains failed to exhibit a rhythmic behavior (Appendix Fig. S2). We then applied a LD12:12 entrainment protocol, for 3 days prior to their analyses in DD, observing that this allowed—particularly—one of the HOs to generate and sustain strong and stable oscillations under DD (Figs. 1B,C and EV1). We continued to characterize this HO, henceforth referred to as HO-10, as it was generated utilizing the promoter from the ccg known as con-10.
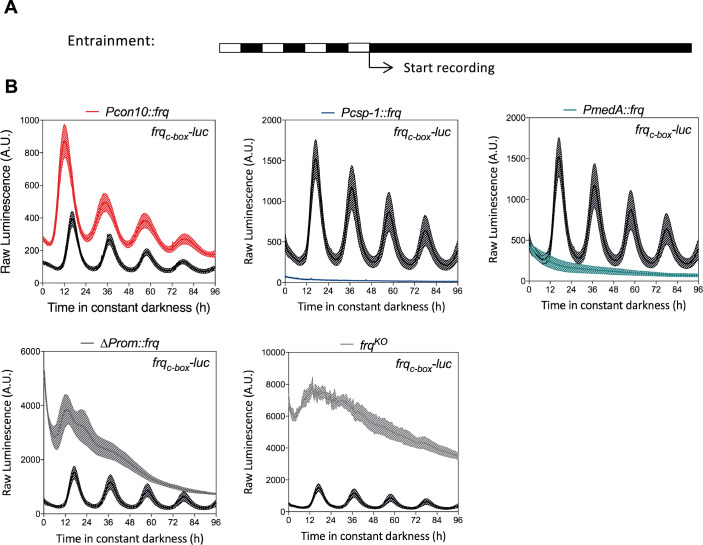
Figure EV1. Only one the tested Hybrid Oscillators sustains robust rhythms after a three days LD 12:12 entrainment.
(A) Entrainment protocol used to evaluate the different HOs. Prior to recording in DD, the strains were entrained for three days under 12:12 LD cycles. (B) Evaluation of HOs under DD conditions, by analyzing LUC activity coming from a frqc-box-luc reporter. The black traces represent a wild type strain, whereas the different HOs are depicted in color. A negative control without a promoter (∆Prom::frq only containing the resistance cassette, bar), as well as a frqKO were examined. In all cases, experiments were run three independent times, and a representative set is shown. Each luciferase trace corresponds to the average of three different wells ± SD.
The expression of FRQ was confirmed in the different HOs through Western blot assays in LL and DD conditions (Appendix Fig. S3), observing that in the latter FRQ levels are three times higher in HO-10 than in the WT control, whereas in two of the arrhythmic HOs (generated with the promoters of csp-1 and medA) FRQ levels were 5.2 and 6.5 times higher compared to WT, respectively (Appendix Fig S3). These results are compatible with the idea that in DD, high levels of FRQ such as the ones observed in the HOs with the csp-1 or medA promoters, exert a constant inhibition of WCC as evidenced by low and arrhythmic levels of frqc-box-luc expression (Appendix Fig. S2; Fig. EV1). In addition, as a negative control we also evaluated the effect of eliminating the native frq promoter, replacing it only by the selection marker cassette, confirming that when frq is not transcribed the system is arrhythmic, as seen in a Δfrq, which lacks its ORF (Appendix Figs. S2 and S3; Fig. EV1). We also examined FRQ levels in two additional HOs that exhibit oscillations, HO-tub and HO-vvd, generated with the tubulin and vivid promoter, confirming that FRQ levels in DD were similar (~1.1X) or lower (~0.5X) than the ones in HO-10 (Fig. EV2). HO-tub and HO-vvd were developed based on the promoters of a weakly and strongly rhythmic gene, respectively (Fig. EV2A,B). After LD entrainment HO-vvd exhibits only two peaks and then loses rhythmicity, whereas HO-tub shows low amplitude oscillations with an unstable period of 24.72 ± 3.06 h (Fig. EV2C,D).
Figure EV2. Hybrid oscillators with tubulin and vivid promoters exhibit weak rhythms after three days in LD 12:12 entrainment.
(A,B) Evaluation of tubulin (green (A)) and vivid (orange (B)) promoters under DD conditions. The strains were grown for three days under 12:12 LD cycles prior start recording in darkness. The black traces represent a frqc-box+pLRE-luc_PEST reporter. In all cases, experiments were run three independent times, and a representative set is shown. Each luciferase trace corresponds to the average of three different wells ± SD. (C,D) Evaluation of HOs under DD conditions, by analyzing LUC activity coming from a frqc-box-luc reporter. The black traces represent a wild type strain, whereas the different HOs are depicted in color. In green the tubulin promoter (C) and in orange the vivid promoter controlling frq transcription (D). In all cases, experiments were run three independent times, and a representative set is shown. Each luciferase trace corresponds to the average of three different wells ± SD. (E,F) Western blots showing the levels of FRQ in the different HOs, after growth in LL for 48 h (A) and after 24 h in DD (coming from 24 h in LL) (B). The name of each sample indicates the promoter that controls frq transcription.
In contrast, HO-10 exhibits robust oscillations with a period of 22.34 ± 0.59 h, which are slightly longer compared to the WT clock under these conditions (20.89 ± 0.42 h). In addition, we were able to confirm oscillations in FRQ protein levels utilizing a frqLUC reporter (Fig. 1D), providing additional evidence that the con-10 promoter can yield frq/FRQ rhythmic expression. When utilizing a Pcon10-luc reporter (Fig. 1E) we also observed that in HO-10 rhythms are being effectively passed to the output pathways, although overt rhythms in conidiation (a hallmark of Neurospora clock output) are not seen in DD when examined in race tube assays. The latter further confirms that—as inferred from different lines of evidence (Larrondo et al, 2015; Shi et al, 2007)—rhythms in frq/FRQ expression may not always be visualized by overt conidiation rhythms. In addition, the lack of rhythmic conidiation in DD may be attributed to the decreased amplitude of the HO-10 oscillations. Nevertheless, HO-10 exhibits a cyclic conidiation pattern under a LD12:12 entrainment, similar to WT, contrasting the absence of periodic bands in a ∆frq strain (Fig. 1F).
As a proof of concept that HO-10 differs in its molecular circuitry from a WT clock, we assessed the consequences of eliminating an output regulator. We chose CSP-1, a TF known to be under clock control and that is part of the output pathways, but that it is not essential for clock function. csp-1 encodes for a regulator that holds similarities to the yeast transcription repressors NRG1 and NRG2, and it has been described to have a large role in regulating metabolic genes in Neurospora (Sancar et al, 2011). Relevantly, it represents one of the best studied TF involved in circadian output, modulating a large number of ccgs, including con-10. In a WT clock devoid of csp-1, rhythms remain robust (Fig. EV3A) and period is unaltered under low sugar conditions (Sancar et al, 2012). In contrast, deletion of csp-1 in HO-10 causes arrhythmicity (Fig. EV3B) confirming that—by definition—this output transcription factor is now a bona fide core-clock component and, therefore, part of the extended TTFL topology of HO-10.
Figure EV3. Effect of the absence of csp-1 in the WT and HO-10 oscillators.

(A,B) Evaluation of the WT (A) and HO-10 oscillator (B) in the absence of csp-1. Strains were entrained for 3 days under 12:12 LD cycles prior to monitoring in DD. Strains contain a c-box-luc reporter. In (A), the black traces represent the WT strain and purple traces the ∆csp-1 in a WT clock background. In (B), the red traces represent the HO-10 and blue traces the ∆csp-1 in a HO-10 background. In all cases, experiments were run three independent times, and a representative set is shown. Each luciferase trace corresponds to the average of three different wells ± SD.
Period mainly depends on FRQ determinants
In order to further evaluate the contributions of different processes to period determination, we tested whether mutations known to affect FRQ phosphorylation-dynamics, and therefore period, would still do so in a context where frq transcriptional control had been dramatically changed. Thus, we reconstructed the HO-10 utilizing different frq alleles, some known to exhibit shorter (frq∆C-term and frqS900A) or longer (frq7 and frqS538A, S540A) periods. As expected, such alleles behaved as reported in a WT oscillator background (Fig. 2A–G). Brilliantly, the same behavior was also evident when those alleles were tested in HO-10 (Fig. 2A–G). Unexpectedly, frq5S→D, which has been associated with the rapid degradation of this FRQ allele, fails to yield rhythms in a WT background but, nevertheless, behaved rhythmically in the context of the HO-10 (Fig. 2A–G; Appendix Fig S4). A similar behavior had been previously observed for frq5S→D in a ∆fwd-1 background (which exhibits impaired FRQ degradation) leading to overall high levels of this Negative Element (Larrondo et al, 2015). The latter observation is consistent with the fact that in HO-10 FRQ levels are higher, allowing the maintenance of rhythms of a highly unstable FRQ mutant such as frq5S→D.
Figure 2. The period of the semi-synthetic Oscillator HO-10 is dependent on FRQ determinants.
(A–F) Different frq alleles, as indicated in the insets, were analyzed in the context of a WT (black) or the HO-10 semi-synthetic circuitry (red), under constant darkness (DD), utilizing frqc-box-luc as a reporter. (A–F) Samples were entrained for three days under 12:12 LD cycles, prior to monitoring. In all cases, experiments were run three independent times, and a representative set is shown. Each luciferase trace corresponds to the average of three different wells ± SD. (G) Period lengths of the different frq alleles were calculated for WT and HO-10 strains. Source data are available online for this figure.
Interestingly, independent of the frq allele that was analyzed, the first peak of the HO-10 always showed a phase advance and slightly longer period compared to its counterpart (Fig. 2) (see below). Thus, in the aggregate, the results indicate that while tampering with frq’s transcriptional control only appears to have a marginal effect on period, FRQ sequence changes known to modulate its phosphorylation dynamics and properties are, in return, a major variable impacting period length.
Temperature compensation is unlikely a network-wide process
As temperature compensation is a defining property of clocks, we tested whether this was the case in HO-10, determining period at 22 °C, 25 °C, and 28 °C and comparing the Q10 values for WT and HO-10. In our experiments we obtained a Q10 = 0.96 for the WT and a Q10 = 0.94 for the HO-10 (Fig. 3A), concluding that the rewired oscillator is also temperature compensated.
Figure 3. The semi-synthetic oscillator HO-10 is temperature compensated.
(A) Strains were grown for three days under LD 12:12 cycles at 25 °C, and then monitored in DD at three different temperatures 22 °C, 25 °C, and 28 °C, utilizing frqc-box-luc as a reporter, and period and Q values were calculated. Data leading to Q10 calculation were plotted as the average ± SD of three different measurements. (B) To evaluate the presence of s-FRQ and l-FRQ, strains were grown for 48 h in LL at 25 °C and proteins were extracted and treated with phosphatase, prior to SDS PAGE in the WT and HO-10 (HO), observing that the latter only expresses l-FRQ. * Unspecific band. Source data are available online for this figure.
These results reinforce the hypothesis that temperature compensation is molecularly encoded in FRQ posttranslational events, such as phosphorylation (Mehra et al, 2009; Hu et al, 2021), and does not depend on particular aspects of frq transcription, or even on the characteristics of the frq 5’UTR. Indeed, it has been described that the latter undergoes temperature-dependent splicing that regulates the amounts of short (s-FRQ) and long FRQ (l-FRQ) isoforms, where the former leads to a longer period and l-FRQ to a shorter one (Diernfellner et al, 2007). In HO-10, frq 5’UTR is absent, so its splicing is unaffected by temperature and only l-FRQ is produced, as confirmed by western blot (Fig. 3B). Thus, HO-10 is temperature compensated even though it has an imbalance of short/long FRQ ratio, while also exhibiting an overall longer period. Interestingly, expression of only l-FRQ should render a shorter period, therefore the rather longer period in HO-10 is likely due to the introduction of additional steps/delays in the system. Besides its role in temperature finetuning of FRQ isoforms, the frq 5’UTR has several micro-ORFs that have been postulated to regulate mRNA levels and FRQ translation efficiency (Liu et al, 1997; Diernfellner et al, 2005; Colot et al, 2005). While, the absence of the entire frq 5’UTR in this rewired semi-synthetic oscillator argues against the assigned importance of this element in the clockworks, higher levels of frq transcription in HO-10 could partially compensate for such effects. In toto, and considering that the HO-10 possesses a very different (and intricate) circuitry topology, the results strongly indicate that transcriptional-wide processes are not playing a key role, as predicted by a network model of temperature compensation (Kurosawa and Iwasa, 2005), and that instead such clock property is expected to depend mainly on translational/posttranslational mechanisms.
Unexpected properties of the semi-synthetic oscillator
Light is a main circadian input and, in Neurospora, it is signaled via the photoactivation of the WCC and its binding to the pLRE in the frq promoter, which leads to defined transcriptional dynamics (Froehlich et al, 2002; Oehler et al, 2023). Importantly, this region is no longer controlling frq expression in HO-10 and, moreover, a classic activation via WCC (at least with strong promoter binding) is not occurring in the con-10 promoter (Smith et al, 2010; Hurley et al, 2014; Sancar et al, 2015a); therefore, in HO-10 light information is conveyed to frq by additional steps, involving other regulators downstream from WCC activity. Importantly, it had not escaped our attention that HO-10 reporter activity in DD, or race tubes under 12:12 LD cycles, revealed phase advances compared to WT. To further dig into this, we grew Neurospora on race tubes under different photoperiods (LD16:8, LD12:12, LD8:16, LD4:20, and LD0.5:23.5). Using the moment when lights are turned off as a reference mark, the phase of conidiation in WT is at the center of the photoperiod independent of the entrainment (Fig. 4A), whereas in HO-10 the conidiation band occurs at different times, depending on the photoperiod being tested (Fig. 4B). To analyze the effect of these different entrainment photoperiods in the phase of LUC levels in DD, we entrained strains for three days in such distinct photoperiods, and then we released them into DD. Monitoring LUC activity, we observed that while WT exhibits the same phase after all entrainments (Fig. 4C), in HO-10 the phase varies according to the preceding photoperiod (Fig. 4D). These results demonstrate that like WT, the HO-10 is (i) capable of perceiving environmental information and (ii) retaining it after transfer to constant conditions, both being defining critical properties of circadian clocks. But remarkably, HO-10 shows a distinct difference compared to the WT oscillator. The latter fixes its phase at the moment lights are turned off, which can be clearly seen as a slope close to 0 when the phase of conidiation or LUC activity is plotted relative to photoperiod, indicating dusk dominance (Edwards et al, 2010). In contrast, HO-10 displays a slope close to −1 denoting a dawn dominance, which implies that it fixes its phase when the lights are turned on (Fig. 4E,F). To further confirm the concept that phase in HO-10 is determined at the moment lights are turned on, new experiments were conducted such that luciferase tracking was synchronized to the moment when lights would be turned on after the different entrainment regimes. When doing that and, as expected, LUC expression in HO-10 shows the same phase, whereas the phase of the WT clock is instead broadly distributed (Appendix Fig S5). Finally, we also performed the same experiments but now using race tubes, and providing reference marks at the lights-on transitions, observing that relative to these marks the conidial bands exhibit the same phase in HO-10, but not in WT (Appendix Fig. S5). In summary, these results indicate that the semi-synthetic HO-10 clock works with the logic of a lights-on timer.
Figure 4. The semi-synthetic HO-10 clock presents unexpected responses to light stimuli.
(A,B) The phase of HO-10 dramatically changes depending on the LD regime, as observed by analyzing race tubes grown under the indicated LD conditions, where daily marks were placed at the light to dark transitions. Experiments were run three independent times, and a representative set is shown. (C,D) Luciferase activity was monitored as cultures were transferred to DD after 3 days of the indicated entrainments. Experiments were run three independent times, and a representative set is shown. Each luciferase trace corresponds to the average of three different wells. (E,F) Plots depicting the phase (with symbols denoting average, and error bars the SD) of conidiation (race tubes) under LD conditions (A) and (B), and of luciferase activity under DD conditions after the different entrainments (C) and (D). (G,H) WT and HO-10 strains were entrained for three days under LD 12:12 cycles in four different incubators to set four different phases prior transferring to DD, when monitoring of luciferase started. After 48 h in DD a 30 min LP was applied, such that strains were at different circadian times (CTs) when the pulse was given. frqc-box+pLRE-luc-PEST was used as reporter. Experiments were run three independent times, and a representative set is shown. Each luciferase trace corresponds to the average of three different wells. Source data are available online for this figure.
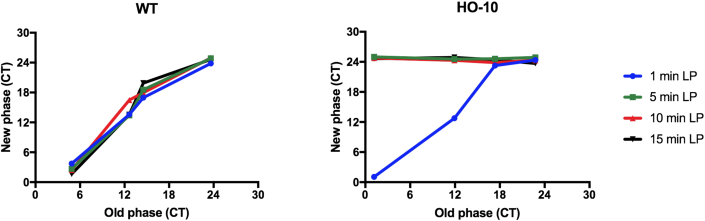
Since HO-10 revealed particular ways of processing light information, we also evaluated the effect of a discrete saturating 30 min light pulse (LP) (Crosthwaite et al, 1995). Importantly, the LP was applied on strains that have been in DD for 48 h, but which displayed different circadian times (CT). The LP produces clear phase shifts in the WT oscillator (Fig. 4G), whereas the clock in HO-10 is completely reset to early subjective day independent of the CT at which the pulse was given (Fig. 4H). Thus, while a WT clock exhibits a type 1 Phase Transition Curve (PTC), the hybrid HO-10 displays a type 0 PTC (Winfree, 1970). This was true for short LP of even 5 min under our tested conditions, yet when a 1 min LP was administrated the HO-10 exhibited a type 1 PTC (Fig. EV4). This may be explained by the fact that in response to light the con-10 promoter has a stronger induction pattern than frq’s native one (Tan et al, 2004; Wu et al, 2014). Notably, the effect of a LP was also evaluated when HO-10 was in an arrhythmic state, such as when transferred to DD after only 24 h in LL (Appendix Fig. S2) evidencing that a single 30 min LP is capable of taking this semi-synthetic oscillator from an arrhythmic to a rhythmic orbit (Fig. EV5A). Likewise, LPs of 5 min or longer are capable of generating strong oscillations, when the system is in an arrhythmic state (Fig. EV5). All this indicates that the HO-10 clock has a hypersensitivity to light stimuli.
Figure EV4. HO-10 mainly exhibits type 0 PTCs.
The phase shift produced by discrete light pulses (LPs) of different duration was evaluated in a WT (left panel) and HO-10 (right panel) clock. Strains were entrained as in Fig. 4G,H and after 48 h in DD and LP of 1, 5 10, and 15 min were administrated. While for all different tested LPs the WT clock exhibited a Type 1 PTC, the HO-10 depicted a type 0 PTC for LPs of 5, 10, and 15 min. Only for the shorter LP (of 1 min) a Type 1 PTC was obtained. The reported phase shifts were calculated by comparing the new phase after LP to the phase of the same strain without LP. frqc-box+pLRE-luc-PEST was used as reporter. In all cases, experiments were run three independent times, and a representative set is shown. Each luciferase trace corresponds to the average of three different wells. Standard deviation is not plotted to help visualization of each independent PTC.
Figure EV5. A 5-min LP is sufficient to trigger oscillations in HO-10.
(A–E) After identifying that a 30 min LP could “jump start” the HO-10 (A), we evaluated the effect of different duration LPs in WT (black) and HO-10 (red) strains. Strains were transfer from 24 h LL and start monitoring LUC activity in DD, after 48 h a LP of 1 (B), 5 (C), 10 (D), 15 (E) min was administrated. frqc-box+pLRE-luc-PEST was used as a reporter. In all cases, experiments were run three independent times, and a representative set is shown. Each luciferase trace corresponds to the average of three different wells ± SD.
Mathematical modeling of the semi-synthetic HO-10
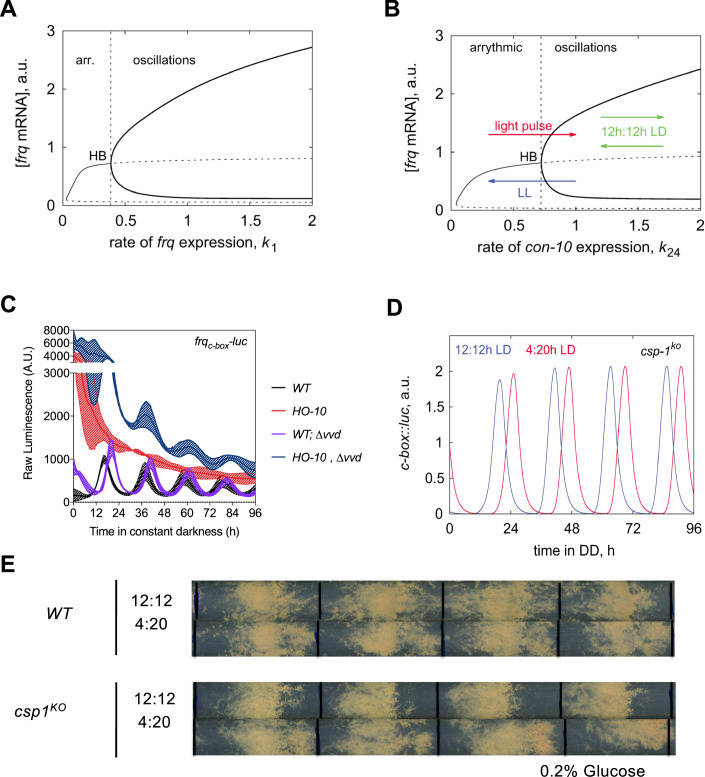
Finally, we modified the mathematical model implemented by Dovzhenok and cols (Dovzhenok et al, 2015), to better understand the behavior of this semi-synthetic oscillator under different entrainment conditions. Briefly, we abstracted the wiring of HO-10 as described in Fig. 1A, and considered different light-dependent kinetics of con-10 vs frq promoters based on reported time-series data (Tan et al, 2004). Specifically, con-10 mRNA shows rapid induction followed by photoadaptation when Neurospora crassa is transferred from dark to light. Hence, we assumed that the rate of con-10 expression (k24) undergoes transient light-dependent increase. Importantly, such abstraction allowed us also to prescind of the exact topology of the HO-10 circuitry. Our analysis indicates that HO-10 resides in a non-oscillatory domain when it’s transferred from LL to DD, since the rate of frq transcription is significantly reduced in LL due to the lower activity of con-10 promoter, which is caused by its rapid photoadaptation (Lauter and Yanofsky, 1993; Tan et al, 2004). However, a 30 min LP in DD boosts the activity of this promoter increasing the rate of frq expression (Fig. EV5), which can push the system into an oscillatory domain (Fig. 5A,B). Specifically, a light pulse increases the rate of con-10 expression (k24) pushing the system from a stable steady state to an unstable steady state with a stable periodic limit cycle domain enabling autonomous oscillations (red arrow, in Fig. 5B). On the other hand, if HO-10 is grown in LL, then the rate of con-10 expression (k24) is decreased due to the lower activity of con-10 promoter moving the system to a region of stable steady states (blue arrow, Fig. 5B). Based on this model, we predicted that a HO-10 strain deficient in photoadaptation, such as ∆vvd, would be rhythmic after a direct LL to DD transfer, as frq levels would be higher in LL, allowing efficient inhibition of WCC once in DD, a prediction that was successfully confirmed (Fig. 5C).
Figure 5. A mathematical model helps explaining the properties of the HO-10 and to predict new properties of the WT oscillator.
(A,B) Bifurcation diagrams for expression rates, where vertical dashed lines represent the critical expression rates that separate arrhythmic from oscillatory regions (HB = Hopf bifurcation). Thin solid (dashed) curves represent stable (unstable) steady states of the model. Thick black curves show the envelope (max and min) of the oscillatory solution. Diagrams for frq expression rate in the WT clock (A) and HO-10 (B). The blue arrow represents the reduction of con-10 expression below the critical value when HO-10 is transferred from LL to DD. The red arrow represents the strong activation of con-10 expression when HO-10 is subjected to a LP, pushing HO-10 into the oscillatory region. The green arrow represents HO-10 in the oscillatory region when is entrained using 12:12 LD. (C) The strains were grown for 24 h in LL and transferred to DD where LUC activity coming from a c-box-luc reporter was evaluated. The black traces represent a WT strain, in purple ∆vvd, in red the HO-10 and in blue the HO-10, ∆vvd. Each luciferase trace corresponds to the average of three different wells ± SD. (D) Model simulations predict that the phase-correcting mechanism in the WT clock is CSP-1-dependent. (E) Experimental data (based on three independent experiments, of which a representative set is presented) confirms the model prediction (D). The strains were grown in race tube with 0.2% glucose medium under the indicated light regime LD 12:12 or LD 4:20. Every 24 h the growth front was marked. Source data are available online for this figure.
Furthermore, mathematical modeling of this rewired oscillator, also provided unexpected insights into the understanding of the WT system, particularly regarding how transcriptional regulation of the core oscillator modulates light-dependent responses. CSP-1 is a known light-inducible transcriptional repressor, and our computer simulations revealed that light-induced CSP-1 exerts stronger repression on WC-1 in LD12:12 compared to LD4:20 (Appendix Fig. S6A,B). This results in reduced expression of frq mRNA and nuclear FRQ (FRQn) in LD12:12 compared to LD4:20, and a phase shift when comparing these two entrainment conditions (Appendix Fig. S6A,B). In contrast, HO-10 shows no phase difference under the two entrainment regimens due to the strong light induction of the con-10 promoter driving the expression of frq in the hybrid oscillator (Appendix Fig. S6C,D). The above data suggest a potential role of CSP-1 regulating the phase of circadian rhythms. Hence, we tested in silico the consequences of not having csp-1 with respect to phase information. Our simulations indicate that removal of csp-1 in the model results in a phase shift that resembles the behavior of HO-10 in DD conditions. Specifically, in ∆csp-1 we observe a phase difference of ~8-h under free-running DD conditions after LD12:12 and LD4:20 entrainment regimens (Fig. 5D; Appendix Fig. S7). To validate the above model prediction, we tested conidiation rhythms using race tubes of ∆csp-1 under free-running conditions after LD12:12 and LD4:20 entrainment regimens. Our experimental data confirmed that, as predicted in our model, a LD12:12 regime causes a significant phase advance compared to LD4:20 (~3 h), albeit of a smaller magnitude than expected (Fig. 5E; Appendix Fig. S7). These data suggest that the underlying transcriptional regulation of the WT system includes intricate aspects controlling light responses via the frq promoter, integrating signals from WCC and CSP-1. While CSP-1 had already been implicated in metabolic compensation, these new results uncover its unexpected role in modulating frq expression and subsequent phase changes of circadian rhythms in different photoperiods. Such role could be the sum of CSP-1 effects on the wc-1 and frq promoters, where the absence of csp-1 is evidenced by an altered phase in DD following distinct entrainment conditions. Notably, the behavior of a WT clock devoid of csp-1 resembles HO-10, which due to its rewired transcriptional circuitry processes light information in a different fashion having, as a consequence, altered phase responses. Importantly, this ancillary role of CSP-1 in the WT core-clock contrasts the essential role that CSP-1 plays in the HO-10 oscillator.
Discussion
While several efforts have sought to establish fully functional synthetic circadian oscillators, this has proven a daunting task in part due to the underlying complexity of the clockworks (Elowitz and Leibier, 2000; Purcell et al, 2010). Instead of building a fully synthetic clock, our transcriptional rewiring approach allowed implementing a semi-synthetic one, which we denominated hybrid as it combines, as core-components, parts from the native oscillator and elements from the output pathways. Thus, the results show that we can significantly modify the circuit topology of an evolutionarily conserved circadian TTFL and still have a functional clock. In this new architecture the canonical c-box and pLRE sequences (key in regulating native frq) are no longer controlling its expression and importantly, frq transcription (commanded now by the con-10 promoter) ceases to be simply and directly regulated by the WCC. Although multiple ChIP-Seq datasets, including our own unpublished ones, have failed to detect WCC occupancy at the con-10 promoter either under DD conditions or after a light-pulse (Smith et al, 2010; Hurley et al, 2014; Sancar et al, 2015a), we cannot fully discard a transient action of WCC on the con-10 promoter (i.e., WCC acting as a pioneer TF facilitating the recruitment of additional components). Thus, a limitation of the current study is that part of the resulting circuitry may still relay on a direct effect of WCC, which would mean that the core-clock topology would be an admixture of a classic TTFL circuit, entangled with multiple output nodes. Nevertheless, it is clear that the rewired TTFL presents dynamics that are quite different from the canonical frq promoter (given by its native elements), which exhibits distinct refractoriness or complex transcriptional and chromatin remodeling dynamics (Oehler et al, 2023; Cesbron et al, 2015). Future efforts will be focused on providing a detailed transcriptional landscape of this rewired oscillator, including the main TFs acting as bona fide positive elements, particularly unravelling the exact direct contribution of WCC. As shown in Fig. EV3, deletion of a TF known to be part of the output pathways, such as CSP-1, confirms that although this TF is not essential for a WT clock, its absence causes arrhythmicity in the context of HO-10: in other words, CSP-1 is now, by definition, a genuine core-clock component. Although FRQ can still directly inhibit WCC activity, the latter now also controls transcription of frq via output components that act upon the con-10 promoter, such as CSP-1, and likely many clock-controlled TFs such as SUB-1 (Sancar et al, 2015a). Notably, despite this altered topology, circadian behavior is still achieved. Importantly, as hinted earlier, this rewired architecture scrambles several essential parameters described as key to achieving proper clock function such as the transcriptional rates of the Negative Element (Froehlich et al, 2002), post-transcriptional regulation mediated by frq 5’UTR (Liu et al, 1997; Garceau et al, 1997), chromatin remodeling processes associated with the c-box (Wang et al, 2014; Gai et al, 2017; Belden et al, 2011) and, most remarkable, the basic and broadly conserved topology of eukaryotic circadian TTFLs (Ode and Ueda, 2018). It is notable that systematic deletion of different regulators in the WT oscillator, have failed to reveal any other TF (besides WC-1 and WC-2) essential for the clockworks (Muñoz-Guzmán et al, 2021), which contrasts with the acquired crucial role of CSP-1 in HO-10 rhythmicity. It is also noteworthy that one of the other semi-synthetic oscillators tested in this work (HO-tub) exhibited an oscillatory behavior (Fig EV2), despite bearing a promoter that is loosely connected to the output pathways, reinforcing the idea of the genetic plasticity of a functional circadian oscillator. In contrast HO-vvd, which is based on the vvd promoter (a direct target of WCC that displays rhythmic transcription (Cesbron et al, 2013)), exhibits limited oscillations, reflecting that it is not all just about the wiring of the circuit, but also dependent on the transcriptional landscape and kinetics governing expression of the Negative Element (Oehler et al, 2023). In our current limited experience, period of the HOs appears to be longer than WT, and although the two peaks seen in HO-vvd may be suggestive of a shorter period, we cannot discard the effect of transients that may obscure proper period determination. We foresee that detailed study of these and other semi-synthetic circuits will continue to yield important circadian insights.
Indeed, the characterization of a functional HO, such as HO-10, allows separation or decoupling of clock properties that are determined by transcriptional- versus posttranslational- mechanisms. In this work we have shown that phase determination and sensitivity to light responses are highly dependent on transcriptional mechanisms, whereas period determination and temperature compensation are mainly dictated by posttranslational mechanisms. Indeed, it is extremely informative to confirm that a fascinating property such as temperature compensation remains intact in HO-10. These results further indicate that this emergent property is unlikely to depend on a transcriptional network property, as expected from a network model of temperature compensation (Kidd et al, 2015; Kurosawa and Iwasa, 2005) and that, instead, it strictly relies on posttranslational mechanisms (Hu et al, 2021; Mehra et al, 2009; Wang et al, 2023).
Another interesting observation is that HO-10 exhibits robust molecular oscillations, whereas overt conidiation rhythms are impaired, as seen also in some other mutants (Larrondo et al, 2015; Shi et al, 2007). Thus, the core-oscillator appears more resilient to changes in state variables compared to the clock output, which could be interpreted as that the oscillator cogwheels need to be of a minimal size (i.e., amplitude) to properly engage with the cogs controlling overt output. While we have interpreted our results based on relative FRQ levels, we are not oblivious to the fact that quality of FRQ (i.e., timing on phospho-forms) may be, potentially, playing even stronger effects.
Importantly, although the semi-synthetic oscillator is still able to perceive and entrain to light cues, it processes this information with different dynamics, behaving as a lights-on instead of a lights-off timer. Indeed, the HO demonstrates that to switch the same basic oscillator from dusk to dawn-synchronization it is necessary only to strongly repress or modify the direct action of light on the Negative Element (i.e., frq pLRE), and to replace this with a new controller that depicts very distinct light-dynamics (con-10 promoter). The co-existence of dawn and dusk-synchronized clocks in different cells of the same organism has been noted both in plants (e.g., Edwards et al, 2010) and in the SCN in mammals (e.g., Inagaki et al, 2007), and it may be that the HO provides insight into how this could be achieved. The initial action of light to reset the mammalian clock is the rapid light-induction of Per1 (Shigeyoshi et al, 1997). If this transduction pathway was repressed in a cell-type-specific manner and replaced by rapid light-induction of a transcription factor that secondarily activates Per1 (analogous to the replacement of the simple direct action of WCC on frq seen in WT Neurospora with a more complex action of light on con-10 which also secondarily acts on frq in the HO), an oppositely-synchronized clock might emerge. With no doubt, the molecular dissection of semi- or/and fully-synthetic oscillators will help further uncovering the clockworks of timing-circuits. Such an approach should also provide a unique opportunity to examine the physiological impact of changing clock systemic properties, such as phase determination, eventually leading to the proximate and ultimate causes underlying phase selection in a given species.
Finally, HO-10 constitutes in itself evidence that other circuitries (different from a one-step closed TTFL, and based fully on rewiring endogenous components), can indeed act as functional circadian oscillators. Therefore, from a topological perspective, there are different ways of making a clock yet, by parsimony, evolution appears to have always chosen the simplest design.
Methods
Plasmids and strains
All the plasmids were constructed using yeast recombination cloning using PCR amplification products, and the Neurospora strains were transformed by electroporation with the dialyzed PCR products obtained with Phusion Flash, following a similar protocol as already described (Colot et al, 2006). The primers used to generate the transcriptional reporters are detailed in Table EV1. To generate the different rewired strains to be tested as HOs, we eliminated the control of frq by its native promoter, and replaced it by selected ccgs promoters. The primers used to create the constructs and plasmids are detailed in Table EV2. The allelic replacement of the frq promoter was conducted as described (Larrondo et al, 2009), and correct integration of the genetic constructs were confirmed by PCR.
Neurospora was grown at 25 °C in constant light (LL) on slants with minimal Vogel’s 1X media supplemented with 2% sucrose w/v and 1.5% agar w/v (Vogel, 1956). The strains x654-14a (ras-1bd; mus-51rip; his-3::frqc-box-luc, a), x658-8a (ras-1bd; mus-51rip; frqLUC; a), xc1783-4a (ras-1bd; mus-51rip; csr-1::frqc-box+pLRE-luc_PEST, a) and x383-4A (ras-1bd; mus-51rip; his-3::Pcon10-luc; A) were utilized as the recipient strains for the rewiring of frq expression, whereas x654-1a (ras-1bd; mus-51rip; a) was used to generate the promoter-luc reporters.
The different strains containing frqc-box-luc and different frq alleles (frqV5; frqΔC-term; frqS900A; frqS538A,S540A and frq5S->D) were previously described (Larrondo et al, 2015), and herein were crossed to x654-16A (ras-1bd; mus-51rip; his-3::frqc-box-luc, a), in order to obtain xc2116-10 (frqV5, ras-1bd; mus-51rip; his-3::frqc-box-luc, a), xc2117-12 (frqS900A, ras-1bd; mus-51rip; his-3::frqc-box-luc, A), xc2118-6 (frqS538A,S540A, ras-1bd; mus-51rip; his-3::frqc-box-luc, a), xc2119-1(frq∆C-term, ras-1bd; mus-51rip; his-3::frqc-box-luc, a), xc2121-7(frq5S->D, ras-1bd; mus-51rip; his-3::frqc-box-luc, a). The frq7 strain was obtained by a sexual cross between x578-9 (his-3::frqc-box-luc, ras-1bd, frq7, a) and x654-7A (ras-1bd; mus-51rip; A) to obtain strain xc1755-3a (ras-1bd, mus-51rip, his-3::frqc-box-luc, frq7, a). These strains were utilized to build the HO-10 (replacing the frq promoter by the one of con-10), in genetic backgrounds bearing different frq alleles.
Homokarionization by microconidiation
To avoid ripping (Selker and Garrett, 1988; Watters et al, 1999), the HOs were homokarionized by microconidiation. Neurospora was inoculated on a slant with 6 mL of microconidiation media (0.5% sucrose w/v, 0.1X Westergaard w/v, 2% agar w/v) supplemented with fresh sterile 60 μl of iodoacetate 0.1 M. Strains grew for 12 days in 12:12 LD cycle previous harvest using 2 mL of sterile water and filter with a 5-μm pore size syringe filter (EMD Millipore™ SLSV025LS). 150 μl were plated with the corresponding selective media, grew overnight at 30 °C and colonies were picked and transferred to slant with the selective media. Homokarionization was confirmed by PCR with the primers detailed in Table EV3.
Culture conditions
In vivo bioluminescence was conducted, as already reported (Larrondo et al, 2015; Muñoz-Guzmán et al, 2021) in 96-wells plates with LNN-CCD media (0.03% glucose, 0.05% arginine, 50 ng/ml biotin, 1.5% agar, and 25 μM luciferin) supplemented with quinic acid (QA 0.01 M) at 25 °C, unless otherwise specified. The different LD entrainments are indicated in each figure. Strains for Western blot were grown in petri dishes with LNN-CCD + QA as in (Larrondo et al, 2015). Race tube analyses were conducted as reported (Belden et al, 2007a) and for ∆csp-1 experiments media included glucose 0.2%. All experiments were performed in Percival incubators equipped with white cool light fluorescent tubes (light intensity up to 100 μM/m2/s; wavelength 400–720 nm).
Luciferase-based analysis
Strains were incubated according to the entrainments specified in each experiment. Data acquisition was conducted as described (Larrondo et al, 2015). Period and phase analyses were performed in BioDare2 (Zielinski et al, 2014) as previously indicated (Muñoz-Guzmán et al, 2021). For strains of different genotypes, several clones (in general 3) were selected, after which a representative one was utilized for the different analyses. Experiments were performed at least three independent times and in each one samples were inoculated in triplicate. When plotted each line corresponds to the average of three different wells ± SD.
DNA analysis
DNA extraction was performed as previously described (Cenis, 1992), but using conidia as starting material. All strains were confirmed by PCR.
Protein extraction and western blotting
Proteins were extracted by TCA method and Western blot was performed loading 40 μg of total protein in 4–20% Mini-PROTEAN® TGX™ precast protein gels and FRQ antisera was used. Phosphatase treatment was performed as manufacture instructions (New England Biolabs p0753s). All experiments were performed three times.
Mathematical modeling
The Neurospora circadian clock model (Dovzhenok et al, 2015) was adapted to include genes downstream of the core clock. An unknown transcription factor X (tfx) mRNA expression was included with the rate constant k20 (k20 = 1.5 h−1), TFX protein synthesis with the rate constant k22 (k22 = 5 h−1), and con-10 mRNA expression with rate constant k24 (k24 = 1 h−1). tfx mRNA, TFX, and con-10 mRNA degrade with the rate constants k21, k23, and k25, respectively (k21 = 2.8 h−1, k23 = 2.8 h−1, k25 = 2.8 h−1).
To model the hybrid oscillator, the promoter of frq was substituted with the promoter of con-10. This modification rewires and extends the core negative feedback loop that drives circadian oscillations to include genes downstream of the core clock (such as tfx). All the mathematical equations and model parameters are detailed in Extended methods.
Mathematical modeling was carried out using XPP-AUT computer program (Ermentrout, 2002).
Code is available upon request.
Supplementary information
Acknowledgements
This work was founded by ANID-Millennium Science Initiative Program-Millennium Institute for Integrative Biology (iBio ICN17_022), grant number ANID/FONDECYT 1211715, the International Research Scholar Program of the Howard Hughes Medical Institute, and The Richard Lounsbery Foundation. Additional funding was provided by grant number ANID/FONDECYT Postdoctorado 3220747 to AG. JCD and JJL were supported by NIH grants R35GM118021 and R35GM118022, respectively.
Expanded view
Author contributions
Alejandra Goity: Conceptualization; Funding acquisition; Investigation; Visualization; Methodology; Writing—original draft; Writing—review and editing. Andrey Dovzhenok: Resources; Investigation; Visualization; Methodology; Writing—review and editing. Sookkyung Lim: Investigation; Visualization; Methodology; Writing—review and editing. Christian Hong: Supervision; Funding acquisition; Investigation; Visualization; Methodology; Writing—review and editing. Jennifer Loros: Supervision; Funding acquisition; Writing—review and editing. Jay C Dunlap: Supervision; Funding acquisition; Writing—review and editing. Luis F Larrondo: Conceptualization; Supervision; Funding acquisition; Methodology; Writing—original draft; Project administration; Writing—review and editing.
Data availability
All data are available in the main text and the expanded view materials. Raw data files are available upon request.
Disclosure and competing interests statement
The authors declare no competing interests.
Supplementary information
Expanded view data, supplementary information, appendices are available for this paper at 10.1038/s44318-024-00088-3
References
- Asher G, Schibler U. Review crosstalk between components of circadian and metabolic cycles in mammals. Cell Metab. 2011;13:125–137. doi: 10.1016/j.cmet.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Baek M, Virgilio S, Lamb TM, Ibarra O, Moreira J. Circadian clock regulation of the glycogen synthase (gsn) gene by WCC is critical for rhythmic glycogen metabolism in Neurospora crassa. Proc Natl Acad Sci USA. 2019;116:10435–10440. doi: 10.1073/pnas.1815360116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballario P, Macino G (1997) White collar proteins: PASsing the light signal in Neurospora crassa. Trends Microbiol 5(11):458–62 [DOI] [PubMed]
- Barinaga M. BIOLOGICAL CLOCKS: new timepiece has a familiar ring. Science. 1998;281:1429–1430. doi: 10.1126/science.281.5382.1429. [DOI] [PubMed] [Google Scholar]
- Belden WJ, Larrondo LF, Froehlich AC, Shi M, Chen C-HH, Loros JJ, Dunlap JC. The band mutation in Neurospora crassa is a dominant allele of ras-1 implicating RAS signaling in circadian output. Genes Dev. 2007;21:1494–1505. doi: 10.1101/gad.1551707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belden WJ, Lewis ZA, Selker EU, Loros JJ, Dunlap JC. CHD1 remodels chromatin and influences transient DNA methylation at the clock gene frequency. PLoS Genet. 2011;7:e1002166. doi: 10.1371/journal.pgen.1002166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belden WJ, Loros JJ, Dunlap JC. Execution of the circadian negative feedback loop in Neurospora requires the ATP-dependent chromatin-remodeling enzyme CLOCKSWITCH. Mol Cell. 2007;25:587–600. doi: 10.1016/j.molcel.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Berlin V, Yanofsky C. Isolation and characterization of genes differentially expressed during conidiation of Neurospora crassa. Mol Cell Biol. 1985;5:849–855. doi: 10.1128/mcb.5.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenis JL. Rapid extraction of fungal DNA for PCR amplification. Nucleic Acids Res. 1992;20:2380. doi: 10.1093/nar/20.9.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesbron F, Brunner M, Diernfellner ACR. Light-dependent and circadian transcription dynamics in vivo recorded with a destabilized luciferase reporter in Neurospora. PLoS ONE. 2013;8:1–6. doi: 10.1371/journal.pone.0083660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesbron F, Oehler M, Ha N, Sancar G, Brunner M. Transcriptional refractoriness is dependent on core promoter architecture. Nat Commun. 2015;6:6753. doi: 10.1038/ncomms7753. [DOI] [PubMed] [Google Scholar]
- Chen C-H, Ringelberg CS, Gross RH, Dunlap JC, Loros JJ. Genome-wide analysis of light-inducible responses reveals hierarchical light signalling in Neurospora. EMBO J. 2009;28:1029–1042. doi: 10.1038/emboj.2009.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P, He Q, He Q, Wang L, Liu Y. Regulation of the Neurospora circadian clock by an RNA helicase. Genes Dev. 2005;19:234–241. doi: 10.1101/gad.1266805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colot HV, Loros JJ, Dunlap JC. Temperature-modulated alternative splicing and promoter use in the circadian clock gene frequency. Mol Biol Cell. 2005;16:5563–5571. doi: 10.1091/mbc.e05-08-0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colot HV, Park G, Turner GE, Ringelberg C, Crew CM, Litvinkova L, Weiss RL, Borkovich KA, Dunlap JC. A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc Natl Acad Sci USA. 2006;103:10352–10357. doi: 10.1073/pnas.0601456103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosthwaite SK, Dunlap JC, Loros JJ. Neurospora wc-1 and wc-2: transcription, photoresponses, and the origins of circadian rhythmicity. Science. 1997;276:763–769. doi: 10.1126/science.276.5313.763. [DOI] [PubMed] [Google Scholar]
- Crosthwaite SK, Loros JJ, Dunlap JC. Light-induced resetting of a circadian clock is mediated by a rapid increase in frequency transcript. Cell. 1995;81:1003–1012. doi: 10.1016/S0092-8674(05)80005-4. [DOI] [PubMed] [Google Scholar]
- Diernfellner A, Colot HV, Dintsis O, Loros JJ, Dunlap JC, Brunner M. Long and short isoforms of Neurospora clock protein FRQ support temperature-compensated circadian rhythms. FEBS Lett. 2007;581:5759–5764. doi: 10.1016/j.febslet.2007.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diernfellner ACR, Schafmeier T, Merrow MW, Brunner M. Molecular mechanism of temperature sensing by the circadian clock of Neurospora crassa. Genes Dev. 2005;19:1968–1973. doi: 10.1101/gad.345905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovzhenok AA, Baek M, Lim S, Hong CI. Mathematical modeling and validation of glucose compensation of the Neurospora circadian clock. Biophys J. 2015;108:1830–1839. doi: 10.1016/j.bpj.2015.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap JC. Molecular bases for circadian clocks. Cell. 1999;96:271–290. doi: 10.1016/S0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- Dunlap JC. Proteins in the Neurospora circadian clockworks. J Biol Chem. 2006;281:28489–28493. doi: 10.1074/jbc.R600018200. [DOI] [PubMed] [Google Scholar]
- Dunlap JC, Loros JJ. Essay just-so stories and origin myths: phosphorylation and structural disorder in circadian clock proteins. Mol Cell. 2018;69:165–168. doi: 10.1016/j.molcel.2017.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbole DJ, Paluh JL, Plamann M, Sachs MS, Yanofsky C. CPC-1, the general regulatory gene for genes of amino acid biosynthesis in Neurospora crassa, is differentially expressed during the asexual life cycle. Mol Cell Biol. 1991;11:928–934. doi: 10.1128/mcb.11.2.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards KD, Akman OE, Knox K, Lumsden PJ, Thomson AW, Brown PE, Pokhilko A, Kozma-Bognar L, Nagy F, Rand DA, et al. Quantitative analysis of regulatory flexibility under changing environmental conditions. Mol Syst Biol. 2010;6:1–11. doi: 10.1038/msb.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elowitz MB, Leibier S. A synthetic oscillatory network of transcriptional regulators. Nature. 2000;403:335–338. doi: 10.1038/35002125. [DOI] [PubMed] [Google Scholar]
- Ermentrout B (2002) Simulating, analyzing, and animating dynamical systems - a guide to XPPAUT for researchers and students. Software, environments, tools
- Froehlich AC, Liu Y, Loros JJ, Dunlap JC. White Collar-1, a circadian blue light photoreceptor, binding to the frequency promoter. Science. 2002;297:815–819. doi: 10.1126/science.1073681. [DOI] [PubMed] [Google Scholar]
- Froehlich AC, Loros JJ, Dunlap JC. Rhythmic binding of a WHITE COLLAR-containing complex to the frequency promoter is inhibited by FREQUENCY. Proc Natl Acad Sci USA. 2003;100:5914–5919. doi: 10.1073/pnas.1030057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gai K, Cao X, Dong Q, Ding Z, Wei Y, Liu Y, Liu X, He Q. Transcriptional repression of frequency by the IEC-1-INO80 complex is required for normal Neurospora circadian clock function. PLoS Genet. 2017;13:1–18. doi: 10.1371/journal.pgen.1006732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garceau NY, Liu Y, Loros JJ, Dunlap JC. Alternative initiation of translation and time-specific phosphorylation yield multiple forms of the essential clock protein FREQUENCY. Cell. 1997;89:469–476. doi: 10.1016/S0092-8674(00)80227-5. [DOI] [PubMed] [Google Scholar]
- He Q, Cha J, He Q, Lee HC, Yang Y, Liu Y. CKI and CKII mediate the FREQUENCY-dependent phosphorylation of the WHITE COLLAR complex to close the Neurospora circadian negative feedback loop. Genes Dev. 2006;20:2552–2565. doi: 10.1101/gad.1463506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Cheng P, Yang Y, He Q, Yu H, Liu Y. FWD1-mediated degradation of FREQUENCY in Neurospora establishes a conserved mechanism for circadian clock regulation. EMBO J. 2003;22:4421–4430. doi: 10.1093/emboj/cdg425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Liu Y. Degradation of the Neurospora circadian clock protein FREQUENCY through the ubiquitin–proteasome pathway. Biochem Soc Trans. 2005;33:953–956. doi: 10.1042/BST0330953. [DOI] [PubMed] [Google Scholar]
- Hu Y, Liu X, Lu Q, Yang Y, He Q, Liu Y, Liu X. Frq-ck1 interaction underlies temperature compensation of the Neurospora circadian clock. MBio. 2021;12:e0142521. doi: 10.1128/mBio.01425-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley JM, Dasgupta A, Emerson JM, Zhou X, Ringelberg CS, Knabe N, Lipzen AM, Lindquist EA, Daum CG, Barry KW, et al. Analysis of clock-regulated genes in Neurospora reveals widespread posttranscriptional control of metabolic potential. Proc Natl Acad Sci USA. 2014;111:16995–17002. doi: 10.1073/pnas.1418963111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley JM, Jankowski MS, Santos HDL, Baker SE, Loros JJ, Dunlap JC, Hurley JM, Jankowski MS, Santos HDL, Crowell AM, et al. Circadian proteomic analysis uncovers mechanisms of post-transcriptional regulation in metabolic article circadian proteomic analysis uncovers mechanisms of post-transcriptional regulation in metabolic pathways. Cell Syst. 2018;7:613–626.e5. doi: 10.1016/j.cels.2018.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley JM, Loros JJ, Dunlap JC. Circadian oscillators: around the transcription–translation feedback loop and on to output. Trends Biochem Sci. 2016;41:834–846. doi: 10.1016/j.tibs.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki N, Honma S, Ono D, Tanahashi Y, Honma KI. Separate oscillating cell groups in mouse suprachiasmatic nucleus couple photoperiodically to the onset and end of daily activity. Proc Natl Acad Sci USA. 2007;104:7664–7669. doi: 10.1073/pnas.0607713104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd PB, Young MW, Siggia ED. Temperature compensation and temperature sensation in the circadian clock. Proc Natl Acad Sci USA. 2015;112:201511215. doi: 10.1073/pnas.1511215112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosawa G, Iwasa Y. Temperature compensation in circadian clock models. J Theor Biol. 2005;233:453–468. doi: 10.1016/j.jtbi.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Larrondo LF, Colot HV, Baker CL, Loros JJ, Dunlap JC. Fungal functional genomics: tunable knockout-knock-in expression and tagging strategies. Eukaryot Cell. 2009;8:800–804. doi: 10.1128/EC.00072-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrondo LF, Olivares-Yañez C, Baker CL, Loros JJ, Dunlap JC. Decoupling circadian clock protein turnover from circadian period determination. Science. 2015;347:1257277. doi: 10.1126/science.1257277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauter FR, Yanofsky C. Day/night and circadian rhythm control of con gene expression in Neurospora. Proc Natl Acad Sci USA. 1993;90:8249–8253. doi: 10.1073/pnas.90.17.8249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Chen A, Caicedo-Casso A, Cui G, Du M, He Q, Lim S, Kim HJ, Hong CI, Liu Y. FRQ-CK1 interaction determines the period of circadian rhythms in Neurospora. Nat Commun. 2019;10:1–13. doi: 10.1038/s41467-019-12239-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Garceau NY, Loros JJ, Dunlap JC. Thermally regulated translational control of FRQ mediates aspects of temperature responses in the Neurospora circadian clock. Cell. 1997;89:477–486. doi: 10.1016/S0092-8674(00)80228-7. [DOI] [PubMed] [Google Scholar]
- Loudon ASI. Circadian biology: a 2.5 billion year old clock. Curr Biol. 2012;22:570–571. doi: 10.1016/j.cub.2012.06.023. [DOI] [PubMed] [Google Scholar]
- Matsu-Ura T, Dovzhenok AA, Coradetti ST, Subramanian KR, Meyer DR, Kwon JJ, Kim C, Salomonis N, Glass NL, Lim S, et al. Synthetic gene network with positive feedback loop amplifies cellulase gene expression in Neurospora crassa. ACS Synth Biol. 2018;7:1395–1405. doi: 10.1021/acssynbio.8b00011. [DOI] [PubMed] [Google Scholar]
- Mehra A, Shi M, Baker CL, Colot HV, Loros JJ, Dunlap JC. A role for casein kinase 2 in the mechanism underlying circadian temperature compensation. Cell. 2009;137:749–760. doi: 10.1016/j.cell.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Guzmán F, Caballero V, Larrondo LF. A global search for novel transcription factors impacting the Neurospora crassa circadian clock. G3: Genes Genomes Genet. 2021;11:jkab100. doi: 10.1093/g3journal/jkab100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ode KL, Ueda HR. Design principles of phosphorylation-dependent timekeeping in eukaryotic circadian clocks. Cold Spring Harb Perspect Biol. 2018;10:a028357. doi: 10.1101/cshperspect.a028357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehler M, Geisser L, Diernfellner ACR, Brunner M. Transcription activator WCC recruits deacetylase HDA3 to control transcription dynamics and bursting in Neurospora. Sci Adv. 2023;9:1–13. doi: 10.1126/sciadv.adh0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell O, Savery NJ, Grierson CS, Di Bernardo M. A comparative analysis of synthetic genetic oscillators. J R Soc Interface. 2010;7:1503–1524. doi: 10.1098/rsif.2010.0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AN, Berlin V, Hager KM, Yanofsky C. Molecular analysis of a Neurospora crassa gene expressed during conidiation. Mol Cell Biol. 1988;8:2411–2418. doi: 10.1128/mcb.8.6.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenneberg T, Merrow M. The circadian clock and human health. Curr Biol. 2016;26:R432–R443. doi: 10.1016/j.cub.2016.04.011. [DOI] [PubMed] [Google Scholar]
- Rosbash M. The implications of multiple circadian clock origins. PLoS Biol. 2009;7:e62. doi: 10.1371/journal.pbio.1000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosbash M, Hall JC. The molecular biology of circadian rhythms. Neuron. 1989;3:387–398. doi: 10.1016/0896-6273(89)90199-2. [DOI] [PubMed] [Google Scholar]
- Sancar C, Ha N, Yilmaz R, Tesorero R, Fisher T, Brunner M, Sancar G. Combinatorial control of light induced chromatin remodeling and gene activation in Neurospora. PLoS Genet. 2015;11:1–26. doi: 10.1371/journal.pgen.1005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar C, Sancar G, Ha N, Cesbron F, Brunner M. Dawn- and dusk-phased circadian transcription rhythms coordinate anabolic and catabolic functions in Neurospora. BMC Biol. 2015;13:17. doi: 10.1186/s12915-015-0126-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar G, Sancar C, Brügger B, Ha N, Sachsenheimer T, Gin E, Wdowik S, Lohmann I, Wieland F, Höfer T, et al. A global circadian repressor controls antiphasic expression of metabolic genes in Neurospora. Mol Cell. 2011;44:687–697. doi: 10.1016/j.molcel.2011.10.019. [DOI] [PubMed] [Google Scholar]
- Sancar G, Sancar C, Brunner M. Metabolic compensation of the Neurospora clock by a glucose-dependent feedback of the circadian repressor CSP1 on the core oscillator. Genes Dev. 2012;26:2435–2442. doi: 10.1101/gad.199547.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafmeier T, Haase A, Káldi K, Scholz J, Fuchs M, Brunner M. Transcriptional feedback of Neurospora circadian clock gene by phosphorylation-dependent inactivation of its transcription factor. Cell. 2005;122:235–246. doi: 10.1016/j.cell.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Selker EU, Garrett PW. DNA sequence duplications trigger gene inactivation in Neurospora crassa. Proc Natl Acad Sci USA. 1988;85:6870–6874. doi: 10.1073/pnas.85.18.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Larrondo LF, Loros JJ, Dunlap JC. A developmental cycle masks output from the circadian oscillator under conditions of choline deficiency in Neurospora. Proc Natl Acad Sci USA. 2007;104:20102–20107. doi: 10.1073/pnas.0706631104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeyoshi Y, Taguchi K, Yamamoto S, Takekida S, Yan L, Tei H, Moriya T, Shibata S, Loros JJ, Dunlap JC, et al. Light-induced resetting of a mammalian circadian clock is associated with rapid induction of the mPer1 transcript. Cell. 1997;91:1043–1053. doi: 10.1016/S0092-8674(00)80494-8. [DOI] [PubMed] [Google Scholar]
- Smith KM, Sancar G, Dekhang R, Sullivan CM, Li S, Tag AG, Sancar C, Bredeweg EL, Priest HD, McCormick RF, et al. Transcription factors in light and circadian clock signaling networks revealed by genomewide mapping of direct targets for Neurospora white collar complex. Eukaryot Cell. 2010;9:1549–1556. doi: 10.1128/EC.00154-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabilo-Agurto C, Del Rio-Pinilla V, Eltit-Villarroel V, Goity A, Muñoz-Guzmán F, Larrondo LF. Developing a temperature-inducible transcriptional rheostat in Neurospora crassa. MBio. 2023;14:e0329122. doi: 10.1128/mbio.03291-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y, Dragovic Z, Roenneberg T, Merrow M. Entrainment dissociates transcription and translation of a circadian clock gene in Neurospora. Curr Biol. 2004;14:433–438. doi: 10.1016/j.cub.2004.02.035. [DOI] [PubMed] [Google Scholar]
- Vogel HJ. A convenient growth medium for Neurospora crassa. Microb Genet Bull. 1956;13:42–47. [Google Scholar]
- Wang B, Kettenbach AN, Gerber SA, Loros JJ, Dunlap JC. Neurospora WC-1 recruits SWI/SNF to remodel frequency and initiate a circadian cycle. PLoS Genet. 2014;10:e1004599. doi: 10.1371/journal.pgen.1004599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Kettenbach AN, Zhou X, Loros JJ, Dunlap JC. The phospho-code determining circadian feedback loop closure and output in Neurospora. Mol Cell. 2019;74:771–784.e3. doi: 10.1016/j.molcel.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Stevenson EL, Dunlap JC. Functional analysis of 110 phosphorylation sites on the circadian clock protein FRQ identifies clusters determining period length and temperature compensation. G3: Genes Genomes Genet. 2023;13:1–16. doi: 10.1093/g3journal/jkac334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watters MK, Randall TA, Margolin BS, Selker EU, Stadler DR (1999) Action of repeat-induced point mutation on both strands of a duplex and on tandem duplications of various sizes in Neurospora. Genetics 153(2):705–14 [DOI] [PMC free article] [PubMed]
- Winfree AT. Integrated view of resetting a circadian clock? J Theor Biol. 1970;28:327–374. doi: 10.1016/0022-5193(70)90075-5. [DOI] [PubMed] [Google Scholar]
- Wu C, Yang F, Smith KM, Peterson M, Dekhang R, Zhang Y, Zucker J, Bredeweg EL, Mallappa C, Zhou X, et al. Genome-wide characterization of light-regulated genes in Neurospora crassa. G3: Genes Genomes Genet. 2014;4:1731–1745. doi: 10.1534/g3.114.012617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski T, Moore AM, Troup E, Halliday KJ, Millar AJ. Strengths and limitations of period estimation methods for circadian data. PLoS ONE. 2014;9:e96462. doi: 10.1371/journal.pone.0096462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the main text and the expanded view materials. Raw data files are available upon request.