Abstract
The intention-to-treat (ITT) analysis of the Applying Wolbachia to Eliminate Dengue (AWED) trial estimated a protective efficacy of 77.1% for participants resident in areas randomised to receive releases of wMel-infected Aedes aegypti mosquitoes, an emerging dengue preventive intervention. The limiting assumptions of ITT analyses in cluster randomised trials and the mobility of mosquitoes and humans across cluster boundaries indicate the primary analysis is likely to underestimate the full public health benefit. Using spatiotemporally-resolved data on the distribution of Wolbachia mosquitoes and on the mobility of AWED participants (n = 6306), we perform complier-restricted and per-protocol re-examinations of the efficacy of the Wolbachia intervention. Increased intervention efficacy was estimated in all analyses by the refined exposure measures. The complier-restricted analysis returned an estimated efficacy of 80.7% (95% CI 65.9, 89.0) and the per-protocol analysis estimated 82.7% (71.7, 88.4) efficacy when comparing participants with an estimated wMel exposure of 80% compared to those with <20%. These reanalyses demonstrate how human and mosquito movement can lead to underestimation of intervention effects in trials of vector interventions and indicate that the protective efficacy of Wolbachia is even higher than reported in the primary trial results.
Subject terms: Infectious diseases, Viral infection, Epidemiology, Randomized controlled trials
Introduction
A breakthrough in efforts to curtail the global spread of dengue has recently come in the form of the intracellular bacterium Wolbachia (wMel strain), which increases the resistance of Aedes aegypti mosquitoes—the primary vector of the dengue virus—to the replication and onward transmission of a number of arboviral diseases including dengue, Zika, chikungunya, and yellow fever1–4. Encouraging results from quasi-experimental field trials indicated that introgression of wMel into local Ae. aedes populations was associated with reduced incidence of notified dengue cases5–7. The efficacy of Wolbachia for dengue control was demonstrated experimentally in a gold standard parallel-arm cluster randomised trial (CRT) in Indonesia (the AWED trial, ‘Applying Wolbachia to Eliminate Dengue’), which reported a protective efficacy of 77.1% (95% CI 65.3%, 84.9%) against virologically-confirmed dengue in the primary intention-to-treat (ITT) analysis8.
While highly promising, the ITT estimate is unlikely to capture the full intervention effect due to aspects of the study design that are not well handled by an ITT analysis, resulting in an underestimated intervention efficacy9. In cluster randomised trials of community-delivered interventions, such as the wMel deployments in the AWED trial, individual participants’ true exposure status may be different to the cluster-allocated intervention status due to mobility of humans and spillover of the intervention across cluster boundaries. wMel coverage is heterogeneous across time and space, within “intervention” clusters themselves and with observed spillover into “untreated” clusters during the later portion of the study period. Further, humans move outside of their cluster of residence in their daily routines. When such mosquito and human mobility extends the protective effect of the intervention to individuals in the control clusters, and dilutes the exposure of ostensibly ‘treated’ individuals who spend time in control clusters, it can result in traditional ITT analyses that underestimate the full public health benefit.
A first effort at accounting for this variability in individuals’ Wolbachia exposure status within intervention and untreated arms, in order to better estimate the full protective effect of the Wolbachia intervention, was a pre-specified secondary ’per-protocol’ analysis of the AWED trial data in which participants’ exposure status was reclassified from a cluster-level binary to an individual-level weighted ‘Wolbachia Exposure Index’ (WEI) to account for self-reported mobility and measured differences in wMel establishment across the study area. In this initial ‘per-protocol’ analysis, reported together with the ITT analysis in the publication of trial results8, the WEI was estimated in two distinct ways: (1) based solely on the measured cluster-level wMel prevalence in Ae. aegypti mosquitoes in the participant’s cluster of residence during the calendar month of participant enrolment, and (2) based on the cluster-level wMel prevalence in each cluster the participant reported visiting in the week prior to illness onset, weighted by the proportion of total observed time the participant spent in each cluster. The methods are further described in the AWED study protocol10. The WEI based on cluster of residence alone resulted in very little exposure variability (i.e. most participants had WEI values near 1 or 0, see Figure S6C), making it difficult to perform reliable risk comparisons at intermediate levels of exposure. Dengue risk was significantly reduced in only the highest WEI stratum (WEI 80%) compared to the lowest (WEI < 20%), with efficacy estimated at 77.4% (95% CI 62.1%, 85.6%), very similar to the ITT efficacy estimate. The WEI based on wMel and travel history had a bit more variation in exposure for participants in each arm (Figure S6A), and estimated a dose-response effect, suggesting statistically significant differences in protective efficacy for individuals with at least a WEI of 40% as compared to those with WEI less than 20%8.
Both definitions of WEI used a somewhat crude estimation of time spent under protective wMel cover, relying on cluster-level wMel proportions in the month of enrolment; a more geographically and temporally localised wMel proportion may more accurately capture an individual’s protective exposure. Simulation work by others demonstrates that a greater protective efficacy is expected than observed in the initial ITT and per-protocol analyses9. Human and mosquito mobility occur on much finer scales than the 1 km2 geographic regions (“clusters”) randomised to treatment allocation. Ignoring the contamination effect of this movement has been shown to bias the efficacy estimate towards the null9.
The present study re-estimates AWED trial participants’ individual-level wMel exposure using spatially and temporally resolved data on the distribution of Wolbachia mosquitoes and on trial participants’ mobility collected during the AWED trial, in order to perform an improved per-protocol and a complier-restricted re-examination of Wolbachia efficacy in the AWED trial. This granular exposure data accounts explicitly for human mobility, as well as the spatial heterogeneity in wMel coverage particularly arising from spillover of wMel mosquitoes across the boundaries between treated and untreated clusters. By reducing misclassification in individuals’ Wolbachia exposure status, these reanalyses of data from the AWED cluster randomised trial provide the most unbiased experimental estimate to date of the true efficacy of Wolbachia for dengue control.
Results
Human mobility
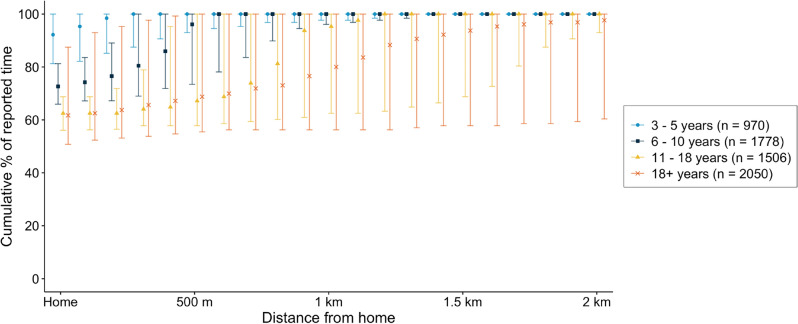
wMel-infected Ae. aegypti mosquitoes were released into 12 intervention clusters (of 24 total clusters) in Yogyakarta between March and December 2017. Patients presenting to local health clinics (puskesmas) between January 2018 and March 2020 with undifferentiated acute febrile illness of 1 to 4 days duration and aged between 3 and 45 years were invited to enrol in the AWED trial10. Those who consented to enrol had a blood sample collected for dengue diagnostic testing and were asked about their movements during daytime hours (5 am–9 pm) in each of the 3 to 10 days prior to their illness onset, corresponding to the incubation period of a dengue virus infection. The 6306 participants included in the AWED analysis dataset reported spending the majority of their time at home (Fig. 1, median 68.8%, interquartile range 57.8–85.2%), consistent with the results of a baseline mobility survey in Yogyakarta11.
Figure 1.
Mobility of AWED trial participants in Yogyakarta City during the 3–10 days prior to illness onset, based on self-reported travel (5 AM–9 PM). The graph shows the median and interquartile range of the time participants within each age group spent at home and cumulatively at increasing distances from home (children aged 3–5 years, 6–10 years, 11-18 years, and greater than 18 years).
Though 87.2% of participants left their residence at least once during the 8-day period (Figure S1A), typically to attend school (56.8%) or work (19.2%) (Figure S2), 86% of the reported locations visited were within 1km of a participant’s residence (Figure S3). The number of unique locations visited did not differ between adults and children, between study arm of residence, or between dengue cases and test-negative controls (Figure S1B–D). However, the aggregate time that participants spent at increasing distances from home increased with age, with only 28.5% (276/970) of young children 3–5 years and 32.1% (571/1778) children 6-10 years reporting any time spent further than 1km from home, compared to 54.5% (821/1506) adolescents 11–18 years and 64.9% (1330/2050) adults. More than a third of participants (39%) stayed within their cluster of residence throughout all 8 days. A median of 98.4% (IQR 68.0–100.0%) of participants’ time was spent under the same intervention arm as their cluster of residence. The proportion of time under the intervention assignment showed no apparent differences for individuals in the intervention versus untreated arm.
wMel heterogeneity
Adult mosquitoes were collected from a fixed network of 455 traps approximately monthly throughout the 27-month trial period in order to monitor wMel prevalence in Ae. aegypti over time in the intervention clusters and contamination in untreated clusters. Between January 2018 and March 2020, there were a total of 10,432 mosquito trapping events, only 10 of which had no Ae. aegypti present. A total of 87,679 Ae. aegypti mosquitoes were screened; 49,266 (56.19%) of which were detected to have wMel.
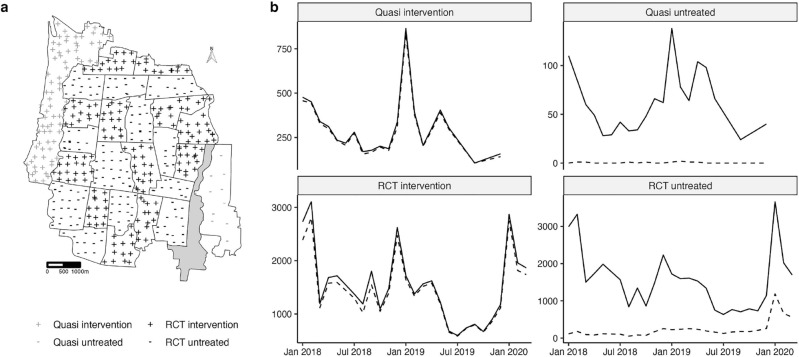
The majority of BG traps were located within the AWED RCT study boundaries (Fig. 2A), with 181 traps in the intervention region and 186 traps in the untreated region. An additional 11 traps were located in an untreated area on the southeast boundary of the RCT study site and 77 traps in a Wolbachia-treated area on the northwest boundary of the RCT study site; these areas had served as the untreated control and intervention area, respectively, in a quasi-experimental Wolbachia release study prior to the AWED randomised trial5. Overall, 96.1% and 93.6% of mosquitoes screened in the quasi-intervention and RCT intervention areas contained wMel, respectively. Only 9% of the mosquitoes captured in the quasi-untreated and 15% of mosquitoes from the RCT untreated areas had wMel present. In the RCT intervention and quasi-intervention areas, the marginal monthly capture and detection of wMel in mosquitoes was fairly consistent across time. In the RCT untreated area, there was a marked increase in the proportion and absolute number of wMel-infected mosquitoes detected in the final year of the study (Fig. 2B).
Figure 2.
(a) Map of Yogyakarta City with geolocated trap locations. Shape is used to distinguish intervention areas where wMel releases occurred (‘+’) and untreated areas where wMel releases did not occur (‘−’). Traps within the AWED RCT study boundaries are denoted in black, while those in the quasi-experimental areas are marked in gray. (b) The total number of mosquitoes screened (solid line) and the number of mosquitoes with wMel detected (dashed line) per month by study area.
Complier-restricted reanalysis of Wolbachia efficacy
To examine the efficacy of Wolbachia among those who received the intervention as randomly assigned, we restricted the analysis to those participants who reported spending all of the potential DENV exposure period under the intervention assignment concordant with their cluster of residence (“compliers”). Among the 6306 participants in the AWED primary analysis data set, 3114 participants (49%) stayed strictly within the RCT study area and within clusters in the same study arm as their cluster of residence, during the 3 to 10 days prior to illness onset: 1359 test-negatives and 33 dengue cases in the intervention arm and 1530 test-negatives and 192 dengue cases in the untreated arm. Applying the same modified odds ratio approach used in the primary analysis of the AWED trial8 and described in an earlier methods paper12, intervention efficacy in the complier-restricted subgroup was estimated at 80.6% (95% confidence interval 65.9%, 89.0%), about 3 percentage points higher than that estimated in the primary analysis.
If we expand the definition of compliers to include participants who left the RCT study area but remained in areas with concordant treatment assignment to their cluster of residence, an additional 772 participants are included: 64 additional test-negatives in the intervention arm and 651 test-negatives and 57 dengue cases in the untreated arm. Intervention efficacy in this subgroup was similarly estimated at 79.7% (95% confidence interval 67.2%, 87.4%).
Per-protocol reanalysis of Wolbachia efficacy
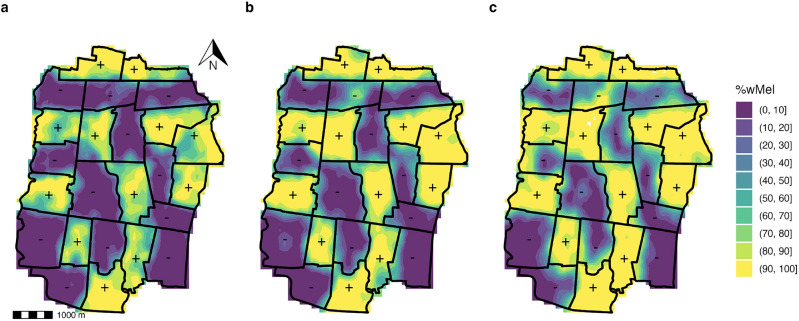
To examine the protective effect of Wolbachia exposure on dengue risk, regardless of compliance with intervention assignment, we constructed an interpolated wMel surface to estimate each individual participant’s Wolbachia exposure level. Figure 3 shows the inverse density weighted maps of wMel coverage aggregated monthly across a 100100 m grid overlaid on the AWED study site. Across each panel, it is evident that wMel is established at a high level (light regions) in intervention areas and, as suggested by Figure 2, begins to creep across the boundaries between the intervention and untreated regions by years 2 and 3 of the trial, leaving the centers of the untreated clusters uncontaminated (dark regions). The results from the leave-one-out cross-validation method used to determine the optimal hyperparameters for interpolation are provided in the Supplemental Material.
Figure 3.
Spatiotemporal inverse density weighted maps of wMel% interpolated across a meter grid overlaid on the AWED trial surface on January 1 each year of (a) 2018, (b) 2019, and (c) 2020, with RCT intervention areas (“+”) and RCT untreated areas (“−”) labeled.
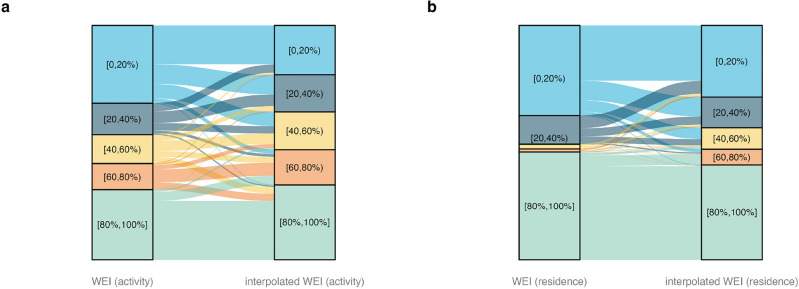
We approach per-protocol re-estimation of efficacy in two ways, mirroring the previous efforts towards obtaining individual-level WEI8,10 (Figures S5, S6). For all participants, in both intervention and untreated arms, we first estimate an individual’s Wolbachia-exposure index (WEI) based on the spatiotemporally interpolated proportion of wMel mosquitoes at the individual’s residence on the reported day of illness onset, referred to as ‘interpolated WEI (residence)’. Second, we use a weighted sum of the individual’s interpolated WEI over their travel history and residence in the 3 to 10 days prior to illness onset, referred to as ‘interpolated WEI (activity)’. A moving sum of the ultimate and penultimate trap collections (relative to a participant’s illness onset) was used in both interpolation procedures in order to stabilise the highly variable individual trap collections. A comparison of the original individual-level per-protocol WEI values and the new individual-level WEI values based on the interpolated surface (Fig. 4) demonstrates the reclassification of participants’ WEI, primarily away from the limits and towards more moderate values.
Figure 4.
Changes in participant-level WEI values based on the method of estimation. (a) The original per-protocol individual-level WEI (activity) values (left) are compared to the individual-level WEI (activity) values from the interpolated surface (right). (b) The original per-protocol individual-level WEI (residence) values (left) are compared to the individual-level WEI (residence) values from the interpolated surface (right).
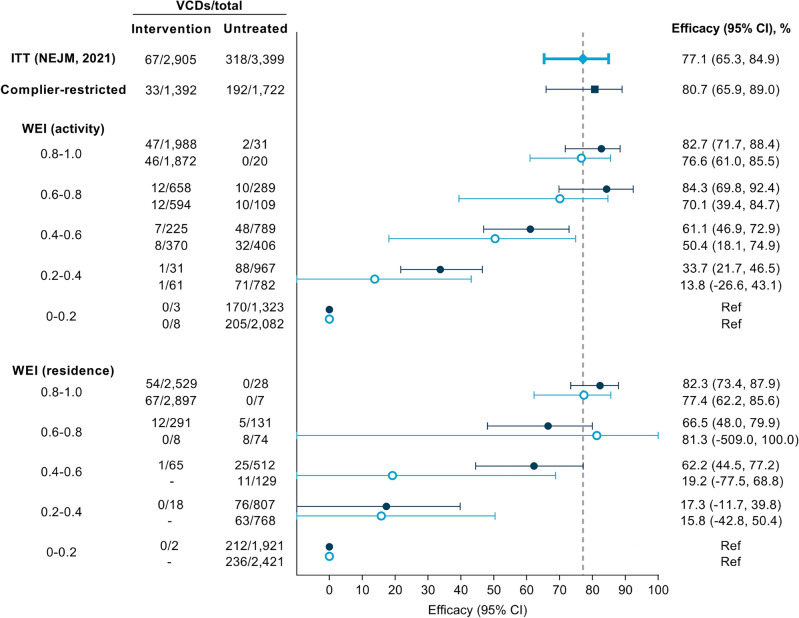
The per-protocol reanalysis based on the interpolated WEI (residence) estimated the Wolbachia efficacy to reach 82.3% (95% CI 73.4%, 87.9%) when comparing those whose residential WEI was above 0.8 to those whose residential WEI was below 0.2 (Fig. 5). Figure 5 further shows that a monotonic dose-response relationship with efficacy was observed as the interpolated WEI (residence) increased. Statistical significance was found at every contrasting WEI (residence) strata when WEI was at or above 0.4. A similar trend was observed with interpolated WEI (activity), where statistically significant effects were observed in every WEI stratum, with a maximum efficacy estimate of 82.7% (95% CI 71.7%, 88.4%) for WEI greater than 0.8 and minimum efficacy estimate of 33.7% (95% CI 21.7%, 46.5%) observed at WEI within [0.2, 0.4), compared to WEI less than 0.2 (Fig. 5). Compared to the original per-protocol results based on cluster-aggregate WEI, the reanalysis using interpolated values of individual WEI resulted in an increase in the estimated intervention efficacy and (for the WEI based on residence only) a more pronounced dose-response relationship. Further, the precision around the stratum-specific estimates was increased reflecting the larger number of participants in the intermediate categories.
Figure 5.
Forest plot comparing Wolbachia efficacy estimates from the primary analysis and reanalysis of the AWED trial in Yogyakarta, Indonesia. Included are the original ITT estimates8 (blue rhombus), the complier-restricted analysis results performed here (navy blue square), the new per-protocol estimates based on individuals’ spatio-temporally interpolated WEI (navy blue round), and the original per-protocol estimates based on individuals’ cluster-aggregate WEI (blue circle).
Discussion
Our reanalysis of data from a cluster randomised trial of Wolbachia-infected mosquito releases for the control of dengue showed that improving the classification of participants’ individual-level exposure, by accounting for human movement and intervention contamination across cluster boundaries, resulted in an increased estimate of intervention efficacy compared to the primary analyses published previously. Participants’ observed movement patterns were strongly age-dependent, highlighting the need for consideration of human mobility in both the design and—where possible—the analysis of trials for interventions delivered at a community level. Substantial spillover of Wolbachia mosquitoes into several untreated clusters during the 27-month period of clinical enrolment complicated the measurement of the intervention effect, but also suggests that small initial gaps in Wolbachia coverage under programmatic deployment could fill themselves in with time without further intervention.
In randomised controlled trials, misclassification of participants’ exposure can occur if they do not receive—or are non-compliant with—the intervention they were randomly allocated to receive. This exposure misclassification produces an estimate of intervention efficacy that is biased towards the null when the comparison groups are more similar in their exposure status than dictated by the randomised treatment allocation. In the specific case of cluster-randomised trials of area-level interventions without buffer areas between clusters, contamination by human movement and/or spillover of the intervention across cluster boundaries can lead to an underestimate of efficacy in the ITT analysis. In the AWED trial, most participants reported spending the majority of their time prior to illness onset under their intervention assignment, however half of participants spent at least 15% of their time either outside the trial area or in a location with a discordant intervention assignment. In addition, a marked increase in wMel-infected mosquito spillover into untreated clusters was observed in the final year of the trial. These contamination risks were recognised at the design stage in the AWED trial, in which 12 of 24 contiguous clusters were randomised to receive releases of Wolbachia-infected mosquito releases for control of dengue10, but could not be accounted for fully in the initial intention-to-treat or per-protocol analyses, which relied on cluster-level summary values of Wolbachia prevalence in Ae. aegypti mosquitoes. By deriving spatiotemporally interpolated values of Wolbachia prevalence at participants’ homes and other visited locations, and combining these with detailed travel history data to calculate individual-level WEI in order to reduce biases in exposure measurement, we have estimated the intervention efficacy to be more than five percentage points higher than in the ITT analysis (82.7% [95% CI 71.7, 88.4] vs 77.1% [65.3, 84.9]), among participants with a Wolbachia exposure index ≥ 80%.
The results of our reanalysis suggest that exposure misclassification from both human mobility and spillover of wMel mosquitoes contributed to the underestimate of Wolbachia intervention effect in the ITT analysis. In the complier-restricted analysis, the point estimate of intervention effect increased by three percentage points compared to the ITT analysis after removing only the biasing effect of human mobility by restricting the analysis to those participants who spent all of their time within the assigned intervention arm, while still considering cluster-level Wolbachia status as a binary (treated vs untreated). The further increase in efficacy measured in the per-protocol reanalysis—both relative to the ITT analysis and to the original per-protocol analysis based on cluster-level wMel prevalence - indicates that within-cluster variability in wMel prevalence was also contributing to the misclassification of individual participants’ wMel exposure status. The estimated intervention effect for participants in the highest stratum of wMel exposure (80–100%), relative to the lowest stratum (0–20%) was very similar regardless of whether or not an individual’s exposure was based on interpolated wMel prevalence at their primary residence only, or weighted by time spent at other visited locations. This observation further supports the home as the primary location of dengue transmission risk, at least in Yogyakarta city.
Recent work13 used geolocated residence, date of illness onset, and DENV serotype information to estimate small-scale spatiotemporal dependence among the dengue cases detected in the AWED trial and showed, for the first time, that the focal clustering of dengue cases is interrupted by the presence of wMel-infected mosquitoes. Those results support the proposition that the true wMel intervention effect was larger than measured in the primary analysis of the AWED trial. Area-wide deployment of Wolbachia across whole cities diminishes the issue of human mobility that arose in the AWED trial, because the vast majority of individuals’ routine movements will occur within the Wolbachia-treated area. The results of area-wide Wolbachia deployment across a continuous urban population of 3.3 million people in Colombia showed that notified dengue incidence was reduced by >95% after Wolbachia was established at a prevalence of 60% or greater in the local mosquito population14, further supporting i) a greater wMel intervention effect under real-world conditions than was measurable in the AWED trial, and ii) a significant reduction in dengue incidence even at intermediate Wolbachia exposure levels, as was observed in the current study.
While this work aimed to reduce exposure misclassification by taking a finer spatiotemporal snapshot of an individual’s WEI, lingering complications in estimation persist. Fogelson et al.15 find that estimates of intervention efficacy from mixed effects models, rather than the generalised linear models applied here, can be biased when within-cluster exposure levels are highly homogeneous. When this estimator bias is properly handled, their work estimates a greater than 90% intervention effect from the WEI activity and residence individual-level exposures. However, the Fogelson et al analysis modelled WEI as a continuous variable such that the efficacy estimate is based on a contrast of 100% versus 0% WEI, whereas our analysis contrasts the highest (80–100%) versus the lowest (<20%) WEI stratum.
Theoretical and empirical studies of wMel invasion into Ae. aegypti populations have predicted slow but steady spread at a rate of 100-200 metres per year in northern Australia16,17. wMel spread was predicted to be slower in tropical regions with higher Ae. aegypti densities, with variation in mosquito population density and geographical barriers contributing to variations in dispersal. This is consistent with the observed spread of wMel across the boundaries of several untreated clusters by 27 months after the end of releases.
Following the successful demonstration in the AWED trial of the efficacy of wMel-infected mosquito deployments in reducing dengue incidence, wMel deployments into the untreated areas of Yogyakarta city were completed between October 2020 and January 2021. Between August 2021 and November 2022, wMel deployments were expanded to the districts of Sleman and Bantul adjacent to Yogyakarta city, reaching a cumulative estimated population of 1.8 million people in a contiguous area of 540 km2. A recent secondary analysis of the AWED trial18 has shown that a Wolbachia intervention effect equivalent to the AWED result was measurable from interrupted time series analysis of routine dengue case notification data. The area-wide coverage of Wolbachia in and around Yogyakarta city now provides an opportunity to evaluate the feasibility for wMel to lead to sustained suppression, or even local elimination19, of dengue in Yogyakarta.
Methods
Data source
The Applying Wolbachia to Eliminate Dengue (AWED) trial8,10 was a cluster randomised, parallel arm study with test-negative sampling carried out in Yogyakarta, Indonesia for a period of 27 consecutive months, beginning in January 2018 and ending during the emergence of the global coronavirus pandemic in March 2020. The city of Yogyakarta was divided into 24 contiguous clusters each approximately 1km2, randomised 1:1 to receive releases of wMel-infected mosquitoes between March and December 2017 or no intervention. Routine vector control efforts continued throughout the study in both arms.
Participant data
Patients between the ages of 3 and 45 years old and resident in the study area who presented to a network of primary care clinics across the study area with undifferentiated febrile illness and a date of fever onset 1 to 4 days prior to the day of presentation were eligible for enrolment in the study. Participants enrolled in the AWED trial provided demographic information (age and sex), a geolocated residential address, and a detailed travel history for the hours between 5 AM and 9PM over the three to ten days before illness onset. Additionally, at enrolment a single blood sample was taken from all consenting participants and used to differentiate test-positives (virologically confirmed DENV cases) from test-negatives (controls). Further details can be found in the protocol10.
A total of 116,473 unique movement events were reported by the 6306 individuals in the analysis sample. The majority of reported locations fell within the RCT study area (92.9%). There were 8277 movement events outside of the RCT area: 1091 (<1%) and 533 (<1%) fell in the quasi-experimental intervention and untreated areas, respectively. The remaining 6653 (5.7%) events fell outside of any study area.
Entomological monitoring
A network of 367 BG Sentinel traps (Biogents, Germany) collected adult mosquitoes throughout the AWED trial area between January 2018 and March 2020, such that median trap density was 16.4 BG/km2 in the intervention clusters and 15.3 BG/km2 in the untreated clusters8. An additional 88 BG Sentinel traps monitored Wolbachia prevalence in two regions adjacent to the AWED trial site: one comprised of seven kelurahans (urban villages) on the northwestern perimeter of Yogyakarta City, where Wolbachia-infected mosquitoes had been released between August 2016 and March 2017 in a quasi-experimental field trial, and a second region comprised of three kelurahans on the southeastern perimeter, which had served as an untreated control area in that trial (Fig. 2A). The study and its results have been described elsewhere5. The median trap density was 15.7 BG/km2 in the quasi-experimental intervention area and 3.6 BG/km2 in the quasi-experimental non-release area (Fig. 2A)5. All traps were monitored on staggered weekly schedules. The number of mosquitoes caught in each BG trap was recorded by species, sex, and in total. Ae. aegypti were stored at − 20∘C in 80% ethanol until testing for wMel infection5,8.
Descriptive analyses
Human mobility
Descriptive analyses of participant mobility were performed examining (1) the proportion of cumulative observation time at varying distances from home by age group (Fig. 1), (2) number of unique locations visited (Figure S1), and (3) type of location (Figure S2).
wMel Spatiotemporal Heterogeneity
In the previously reported per-protocol analysis, wMel spatiotemporal heterogeneity was incorporated into the efficacy estimate by taking the proportion of trapped Ae. aegypti with wMel Wolbachia detected, in either the cluster of residence or as a time-weighted average across the clusters visited in the month of enrolment10. Here, we leverage the extensive and frequently monitored BG trap network to obtain estimated wMel proportions at much greater proximity to residential and visited locations. Specifically, based on an individuals’ reported residence and, in the case of WEI (activity), their travel history, we identify a network of the seven most proximate traps to each geolocated location and interpolate the individual-specific WEI using inverse-density weighting of the observed proportion of trapped Ae. aegypti with wMel Wolbachia detected within the two most recent trap events, where proximity is determined by both space and time. Further details can be found in the Supplemental Material and the code necessary to recreate the interpolated surface is available at the GitHub repository maintained by the first author (https://github.com/sdufault15/yogya-wei-spatial).
Complier-restricted analysis
The complier-restricted analysis restricted the analytic dataset to those who spent all reported time under the intervention assignment determined by their cluster of residence (n = 3114). Further detail on how this was determined can be found in the Supplemental Material.
Intervention efficacy was estimated with the modified odds ratio approach used in the primary analysis from the NEJM manuscript8, as described in the study protocol10 and earlier methods paper12.
Per-protocol reanalysis
We used the interpolated wMel surface to estimate individual levels of exposure. Just as in the initial secondary analysis of the AWED trial data8, we first estimated an individual’s Wolbachia-exposure index (WEI) based on the spatiotemporally interpolated proportion of wMel mosquitoes at the individual’s residence on the reported day of illness onset. Second, we used a weighted sum of the individual’s interpolated WEI over their travel history and residence in the 3 to 10 days prior to illness onset, corresponding to the incubation period of a dengue virus infection10,20.
Intervention efficacy was estimated with the same approach as in the initial report8, using a generalised linear model and balanced bootstrap resampling approach based on cluster residence. Acknowledging that WEI is not a highly precise measure, all per-protocol analyses are performed based on the strata of WEI rather than the individual-level estimates, as pre-specified in the AWED protocol10.
Ethical approval
The trial protocol for the Applying Wolbachia to Eliminate Dengue (AWED) trial study was approved by the Universitas Gadjah Mada (UGM) ethics committee (approval number KE/FK/105/EC/2016) and Monash University Human Research Ethics Committee (approval number 0960) and all research was performed in accordance with relevant guidelines/regulations. Written informed consent was obtained from participants (or their guardian where the participant is a minor). In addition, participants between 13 and 17 years of age were invited to sign a consent form indicating that they understood the research and agreed to participate.
Supplementary Information
Acknowledgements
We acknowledge the contribution of all investigators in the AWED Study Group to the implementation of the trial. We thank the leadership and residents of Yogyakarta for their support and participation in the trial. We gratefully acknowledge the Tahija Foundation as funders of the AWED trial, and the Wellcome Trust and the Bill and Melinda Gates Foundation, which provided financial support to the World Mosquito Program.
Author contributions
All authors were involved in reviewing the final manuscript. S.M.D contributed to the conceptualization, analysis, writing of the original draft, and revision of the final manuscript. S.K.T. contributed to the conceptualization, analysis, writing of the original draft, and revision of the final manuscript. C.I. contributed to the data curation, investigation, and provision of resources. A.U. contributed funding acquisition and project administration. R.A.A contributed resources and project administration. N.P.J. contributed to the conceptualization, analysis, and editing of the manuscript. C.P.S. contributed to the conceptualization, project administration, and editing of the manuscript. K.L.A. contributed to the conceptualization, project administration, writing of the original draft, and revision of the final manuscript.
Data availibility
Participants’ residential address was collected and stored under the ethical approval of the AWED trial protocol. Because the geolocated residence is considered personally identifiable information, individuals seeking access to this data should contact Katherine Anders (katie.anders@worldmosquito.org) to discuss obtaining ethical approval or accessing a deidentified version of the dataset. Trap-level entomological data is available for download at the GitHub repository maintained by the first author (https://github.com/sdufault15/yogya-wei-spatial). All analyses were performed with R and the analytical code can be found on GitHub (https://github.com/sdufault15/yogya-wei-spatial).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-60896-9.
References
- 1.Moreira LA, et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, chikungunya, and plasmodium. Cell. 2009;139:1268–1278. doi: 10.1016/j.cell.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 2.Walker T, et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature. 2011;476:450–453. doi: 10.1038/nature10355. [DOI] [PubMed] [Google Scholar]
- 3.Aliota MT, Peinado SA, Velez ID, Osorio JE. The wMel strain of Wolbachia reduces transmission of Zika virus by Aedes aegypti. Sci. Rep. 2016;6:1–7. doi: 10.1038/srep28792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carrington LB, et al. Field-and clinically derived estimates of Wolbachia-mediated blocking of dengue virus transmission potential in Aedes aegypti mosquitoes. Proc. Natl. Acad. Sci. 2018;115:361–366. doi: 10.1073/pnas.1715788115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Indriani C, et al. Reduced dengue incidence following deployments of Wolbachia-infected Aedes aegypti in Yogyakarta, Indonesia: A quasi-experimental trial using controlled interrupted time series analysis. Gates Open Res. 2020 doi: 10.1101/2020.03.15.20036566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinto SB, et al. Effectiveness of Wolbachia-infected mosquito deployments in reducing the incidence of dengue and other aedes-borne diseases in Niterói, Brazil: A quasi-experimental study. PLoS Negl. Trop. Dis. 2021;15:e0009556. doi: 10.1371/journal.pntd.0009556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dos Santos GR, et al. Estimating the effect of the wMel release programme on the incidence of dengue and chikungunya in Rio de Janeiro, Brazil: a spatiotemporal modelling study. Lancet. Infect. Dis. 2022;22:1587–1595. doi: 10.1016/S1473-3099(22)00436-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Utarini A, et al. Efficacy of Wolbachia-infected mosquito deployments for the control of dengue. N. Engl. J. Med. 2021;384:2177–2186. doi: 10.1056/NEJMoa2030243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavany S, et al. Does ignoring transmission dynamics lead to underestimation of the impact of interventions against mosquito-borne disease? BMJ Glob. Health. 2023;8:e012169. doi: 10.1136/bmjgh-2023-012169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anders KL, et al. Update to the AWED (Applying Wolbachia to Eliminate Dengue) trial study protocol: A cluster randomised controlled trial in Yogyakarta, Indonesia. Trials. 2020;21:1–5. doi: 10.1186/s13063-020-04367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Indriani C, et al. Baseline characterization of dengue epidemiology in Yogyakarta city, Indonesia, before a randomized controlled trial of Wolbachia for arboviral disease control. Am. J. Trop. Med. Hyg. 2018;99:1299. doi: 10.4269/ajtmh.18-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jewell NP, Dufault S, Cutcher Z, Simmons CP, Anders KL. Analysis of cluster-randomized test-negative designs: Cluster-level methods. Biostatistics. 2019;20:332–346. doi: 10.1093/biostatistics/kxy005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dufault SM, et al. Disruption of spatiotemporal clustering in dengue cases by wMel Wolbachia in Yogyakarta, Indonesia. Sci. Rep. 2022;12:1–11. doi: 10.1038/s41598-022-13749-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Velez ID, et al. Reduced dengue incidence following city-wide wMel Wolbachia mosquito releases throughout three Colombian cities: Interrupted time series analysis and a prospective case-control study. PLoS Negl. Trop. Dis. 2023;17:e0011713. doi: 10.1371/journal.pntd.0011713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fogelson A, Landsiedel K, Dufault SM, Jewell NP. As treated analysis of cluster-randomized trials. Ann. Appl. Stat. 2024;18:1506. doi: 10.1214/23-AOAS1846. [DOI] [Google Scholar]
- 16.Turelli M, Barton NH. Deploying dengue-suppressing Wolbachia: Robust models predict slow but effective spatial spread in Aedes aegypti. Theor. Popul. Biol. 2017;115:45–60. doi: 10.1016/j.tpb.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt TL, et al. Local introduction and heterogeneous spatial spread of dengue-suppressing Wolbachia through an urban population of Aedes aegypti. PLoS Biol. 2017;15:e2001894. doi: 10.1371/journal.pbio.2001894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Indriani C, et al. Impact of randomised wMel Wolbachia deployments on notified dengue cases and insecticide fogging for dengue control in Yogyakarta City. Glob. Health Action. 2023;16:2166650. doi: 10.1080/16549716.2023.2166650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bannister-Tyrrell M, et al. Utility of surveillance data for planning for dengue elimination in Yogyakarta, Indonesia: A scenario-tree modelling approach. BMJ Glob. Health. 2023;8:e013313. doi: 10.1136/bmjgh-2023-013313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan M, Johansson MA. The incubation periods of dengue viruses. PLoS ONE. 2012;7:e50972. doi: 10.1371/journal.pone.0050972. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Participants’ residential address was collected and stored under the ethical approval of the AWED trial protocol. Because the geolocated residence is considered personally identifiable information, individuals seeking access to this data should contact Katherine Anders (katie.anders@worldmosquito.org) to discuss obtaining ethical approval or accessing a deidentified version of the dataset. Trap-level entomological data is available for download at the GitHub repository maintained by the first author (https://github.com/sdufault15/yogya-wei-spatial). All analyses were performed with R and the analytical code can be found on GitHub (https://github.com/sdufault15/yogya-wei-spatial).