Abstract
Prolactin-producing pituitary tumor (PRLoma) is the most prevalent functional pituitary tumor. If the tumor becomes large, vision can be impaired. In contrast to other pituitary tumors, cabergoline (CAB) is extremely effective for PRLoma and has become the first-line treatment. In this study, we examined our experience with the pharmacological and surgical management of PRLomas with visual impairment (VI) to determine whether VI could be a surgical indication. Further, we discussed the function of surgery in situations where the gold standard of PRLoma treatment was CAB administration. Of the 159 patients with PRLomas (age, 13-77 [mean = 36.3] years; men, 29; women, 130) at Tokyo Women's Medical University Hospital from 2009 to 2021, 18 (age, 15-67 [mean = 35.8] years; men, 12; woman, 6) had VI (subjectively, 12; objectively, 6). They started CAB treatment immediately (maximum dose: 0.5 to 6 mg/week; average: 2.17 mg/week). VI improved in 16 patients (88.9%) but did not improve in 2 (11.1%) requiring surgeries. One of the two patients had a parenchymal tumor resistant to CAB, and the other had a cystic tumor due to intratumoral bleeding. Consequently, CAB is the first-line treatment for PRLomas with VI because of its significantly high rate of improvement. However, close and rigorous surveillance is necessary for cases resistant to CAB, and the correct decision is required regarding surgical interventions at proper timing and appropriate surgical approaches considering the purpose of surgery.
Keywords: prolactin-producing pituitary tumor, lactotroph PitNET, surgical indication, visual impairment, cabergoline
Introduction
Prolactin-producing pituitary tumor (PRLoma) or lactotroph pituitary neuroendocrine tumor (PitNET) is the most common functional pituitary tumor, accounting for nearly 40% of all PitNET. It can cause irregular menstruation and amenorrhea in women of childbearing age, and if the tumor grows big enough, vision can be impaired. The goals of PRLoma treatment are to normalize the endocrine function and cure neurological morbidity. Dopamine agonists (DAs) are extremely effective for PRLoma, and the new DA, cabergoline (CAB), is associated with a higher normalization rate of hyperprolactinemia and mild and temporary adverse effects compared to traditional DAs, such as bromocriptine and terguride. Consequently, in contrast to other functioning and nonfunctioning pituitary tumors, PRLomas are the only pituitary tumors for which medical treatment is the first line. CAB can significantly decrease serum prolactin (PRL) levels and reduce tumor mass. Generally, surgical treatment is considered only in cases of resistance and/or intolerance to DAs and cerebrospinal fluid (CSF) leakage.1-4) Conversely, the sole surgical indication for nonfunctioning pituitary tumor is visual impairment (VI). This is because the only way to improve the symptoms is surgical removal. However, the situation is different in PRLomas, where tumor shrinkage can be expected with CAB therapy. In this study, we examined our experience with the pharmacological and surgical management of PRLomas with VI to determine whether VI could be an indication for surgery and discuss the role of surgery in cases where the gold standard of PRLoma treatment was CAB administration.
Materials and Methods
We treated 159 patients with PRLoma (age 13-77 [mean = 36.3] years; men, 29; women, 130) at Tokyo Women's Medical University Hospital between 2009 and 2021. Overall, 132 (83%) patients underwent CAB treatment and 27 (17%) underwent surgery. Of the 159 patients, 18 (11.3%; age 15-67 [mean = 35.8] years; men, 12; woman, 6) had VI (Tables 1 and 2). The VI included subjective and objective symptoms that were identified based on examinations such as the Goldmann, Humphrey, or Octopus visual field tests. One patient (Case No. 1) declined a visual field test. All patients with VI started CAB treatment immediately after PRLoma diagnosis. We evaluated the condition of VI and the pharmacological and surgical outcomes (impact of CAB administration and VI enhancement) and developed improved surgical indications for PRLoma with VI.
Table 1.
Characteristic of 159 Prolactinomas without and with visual impairment (VI)
Table 2.
Case presentation: details of PRLomas with visual impairment
| Case No. | Age/Sex | Visual impairment | Maximum vertical diameter of tumor (mm) | Pre- treatment PRL level (ng/mL) | Maximum dose of CAB (mg/week) | Normalization of serum PRL level | Visual inprovement after CAB | Outcome | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Symptoms(opportunity for detection) | VFD | Failing vision | ||||||||||
| left | right | left | right | |||||||||
| 1 | 59/man | subjective | / | hemi | blind | CVA↓ | 30 | 3905 | 2 | No (→400 →1450) | △ | TSS→Lt.△,Rt.○ |
| 2 | 17/man | subjective | hemi | hemi | 49 | 3393 | 4 | yes | ○ | |||
| 3 | 67/man | objective (brain infarction) | hemi | hemi | 29 | 371 | 0.5 | yes | ◎ | |||
| 4 | 29/man | subjective | quadrant | hemi | CVA↓ | 31 | 2314 | 1 | yes | ◎ | ||
| 5 | 20/woman | subjective | hemi | hemi | 26 | 2577 | 3 | yes | ○ | |||
| 6 | 47/man | subjective | hemi | quadrant | 30 | 2911 | 2 | yes | ◎ | |||
| 7 | 27/woman | objective (amenorrhea) | quadrant | quadrant | 28 | 2168 | 6 | yes | ◎ | |||
| 8 | 27/woman | subjective | hemi | hemi | 22 | 1744 | 6 | yes | ○ | |||
| 9 | 50/man | objective (brain check) | quadrant | 23 | 527 | 4 | yes | ◎ | ||||
| 10 | 35/man | objective (headache) | quadrant | 28 | 615 | 1 | yes | ◎ | ||||
| 11 | 15/woman | subjective | hemi | hemi | 31 | 1117 | 1 | yes | ○ | |||
| 12 | 41/man | subjective | hemi | hemi | 33 | 2519 | 1.5 | yes | ○ | |||
| 13 | 28/man | objective (infertility treatment) | quadrant | 25 | 5001 | 1.5 | yes | ◎ | ||||
| 14 | 23/woman | subjective | quadrant | hemi | 29 | 2320 | 0.5 | yes | ◎ | |||
| 15 | 70/man | objective (metastasis check) | quadrant | 25 | 767 | 0.5 | yes | ◎ | ||||
| 16 | 35/man | subjective | quadrant | hemi | 36 | 690 | 1.5 | No (→185) | × (finger perception) | TSS→○ | ||
| 17 | 44/man | subjective | hemi | hemi | CVA↓ | 32 | 2308 | 2 | yes | ○ | ||
| 18 | 20/woman | subjective | quadrant | quadrant | 24 | 780 | 1 | yes | ◎ | |||
| average: 29.5 | average: 2001.5 | average: 2.17 | ◎: 10, ○: 6, △: 1, ×: 1 | |||||||||
VFD: visual field deficit, CVA: corrected visual acuity, ◎: complete recovery, ○: subjectively improved with slight residual VFD, △: no change, ×: deteriorated, TSS: Trans-sphenoidal surgery
Serum PRL level was measured using an ELISA kit (Tosoh, Tokyo, Japan) for those treated until 2012. Since April 2012, ECLusis Prolactin III (Roche Diagnostics, Tokyo, Japan) has been used, with the Third International Reference Preparation World Health Organization Reference Standard-PRL (84/500) as the reference preparation. The normal ranges of serum PRL were as follows: premenopausal women, 4.91-29.32; postmenopausal women, 3.12-15.39; and men, 4.29-13.69 ng/mL.
Informed consent was obtained from all the patients or their families. This study was approved by the Human Investigation Committee of Tokyo Women's Medical University (2021-0063) and was performed in accordance with the Declaration of Helsinki.
Statistical analyses were conducted utilizing SPSS Statistics (version 25.0; IBM Corp., Armonk, NY, USA). Categorical variables were compared using the chi-square test, and the Mann-Whitney U test was used for continuous nonparametric variables. The level of statistical significance was set at p < 0.05.
Results
Table 1 shows the characteristics of the 159 patients with PRLoma treated between 2009 and 2021, including 141 (88.7%) without VI and 18 (11.3%) with VI. The male occupancy rates were 18.2% overall and were higher in PRLomas with VI than in those without VI (66.7% vs. 12.1%, p < 0.001). The pretreatment serum PRL levels were 50-7,534 ng/mL overall, 50-7,534 ng/mL without VI, and 371-5,001 ng/mL with VI. Average PRL levels were 562.9 ng/mL overall and were higher in PRLomas with VI than in those without VI (2001.5 vs. 379.3 ng/mL, p < 0.001).
The details of 18 PRLomas with VI are summarized in Table 2. Their VI symptoms were either subjective (12 patients) or objective (6 patients). Twelve patients had hemianopsia, six had quadrantanopia, and three had failing vision based on corrected visual acuity (CVA). The maximum vertical diameter of tumors with VI ranged from 24 to 49 (average, 29.5) mm. The maximum dose of CAB ranged from 0.5 to 6 (average, 2.17) mg/week. VI improved in 16 patients (88.9%) following CAB administration, including full recovery in 10 patients and subjective improvement with minor residual visual field deficit (VFD) in 6 patients. However, VI did not improve in two patients (11.1%; Case No. 1, no change; Case No. 16, deteriorated to finger perception) whose tumors were resistant to CAB and needed surgery. Case No. 1 showed improvement regarding the right VFD and CVA following transsphenoidal surgery (TSS), and Case No. 16 demonstrated total improvement in the visual acuity and nearly complete improvement in the VFD after TSS.
Case presentation of PRLoma with VI showing significant response to CAB treatment (Case No. 4)
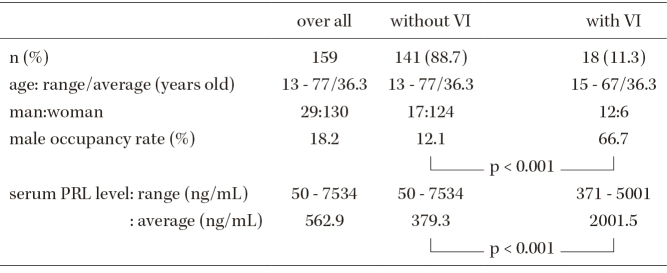
A 29-year-old man presented with left quadrantanopia, right temporal hemianopsia, and reduced right CVA (0.2). Magnetic resonance imaging (MRI) demonstrated a large pituitary tumor compressing the optic nerves (left < right), and the serum PRL level was abnormally high (2,314 ng/mL). He started treatment with CAB (0.25 mg/week) immediately, and the VI subjectively improved 2 weeks after treatment began. The dose of CAB was increased to 1 mg/week, the serum PRL level dropped to 5.6 ng/mL, the tumor shrunk, and the VI improved fully (Fig. 1).
Fig. 1.
Case No. 4.
(a) Coronal T1-weighted magnetic resonance imaging (MRI) with gadolinium enhancement (Gd) revealed a pituitary tumor compressing the optic nerves. (b) Sagittal T1-weighted MRI with Gd, (c) sagittal T1-weighted MRI, (d) sagittal T2-weighted MRI, and (e) visual field deficit in the Goldmann perimetry and bilateral visions before cabergoline (CAB) administration. (f) Coronal T1-weighted MRI with Gd, (g) sagittal T1-weighted MRI with Gd, (h) sagittal T1-weighted MRI, and (i) sagittal T2-weighted MRI 3 months after CAB administration. (j) Visual field deficit in the Goldmann perimetry and bilateral visions were improved.
Case presentation of PRLoma with VI treated using CAB and required surgery (Case No. 1)
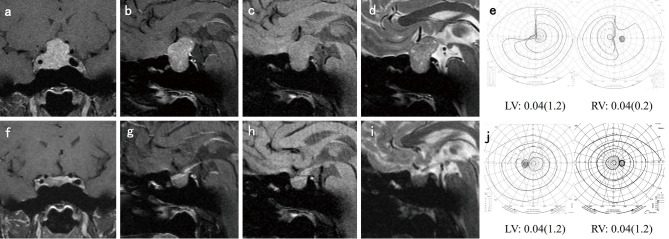
A 54-year-old man demonstrated left blindness and right hemianopsia and failing vision. He had received CAB therapy at a prior facility for 5 years, but his compliance was poor. He was referred to the endocrinologist at our hospital for further CAB treatment. The PRL level initially decreased from 3,905 to 400 ng/mL after taking a dose of 2 mg/week. However, VI did not improve and it subsequently increased to 1,450 ng/mL. Finally, at the age of 59 years, he was referred for neurosurgery. The tumor and fibrous tissue were subtotally resected by TSS (Fig. 2). PRL reduced to 456 ng/mL, and CVA in the right eye improved from 0.1 to 0.3. CAB administration (1 mg/week) following surgery ensured that a normal range of PRL level was achieved.
Fig. 2.
Case No. 1.
(a) Coronal T1-weighted magnetic resonance imaging (MRI) with gadolinium enhancement (Gd) revealed a pituitary tumor compressing the optic nerves. (b) Sagittal T1-weighted MRI with Gd. (c) Coronal T1-weighted MRI with Gd. (d) Sagittal T1-weighted MRI with Gd after cabergoline administration. (e) *: tumor. (f) ×: fibrous tissue after tumor resection under endoscopic view. (g) Coronal T1-weighted MRI. (h) Sagittal T1-weighted MRI after surgery.
Case presentation of PRLoma with VI treated using CAB and required surgery (Case No. 16)
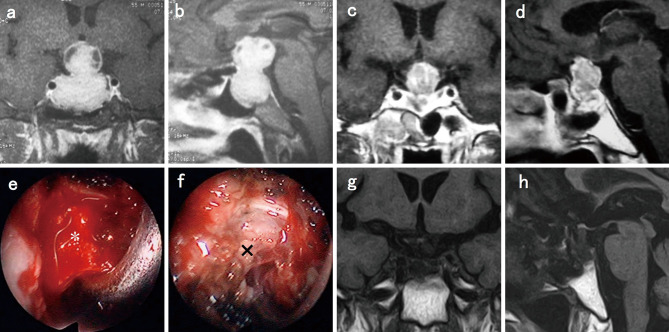
A 35-year-old man presented with right hemianopsia, left quadrantanopia, and hyperprolactinemia (690 ng/mL). CAB administration was started immediately and was titrated rapidly to 1.5 mg/week. The serum PRL level decreased to 185 ng/mL, but the tumor did not shrink. He complained of a headache behind his right eye, and the VI worsened to right finger perception 3 months after the start of CAB therapy. The patient underwent urgent TSS. The surgical findings revealed that the tumor comprised an old hematoma (liquid) of the cystic lesion and a fresh hematoma (clot) caudal to the right optic nerve. The serum PRL level was reduced to 8.7 ng/mL, and the VI improved drastically after gross total resection of the tumor (Fig. 3). The PRL level rose to 47 ng/mL 3 months following the surgery, but a minimum dose of CAB (0.25 mg/week) restored the PRL levels to the normal range.
Fig. 3.
Case No. 16.
(a) Coronal T1-weighted magnetic resonance imaging (MRI) with gadolinium enhancement (Gd) revealed a pituitary tumor compressing the optic nerves. (b) Sagittal T1-weighted MRI with Gd, (c) sagittal T1-weighted MRI, (d) sagittal T2-weighted MRI, and (e) visual field deficit in the Goldmann perimetry and bilateral visions (CPA) before cabergoline (CAB) administration. (f) Coronal T1-weighted MRI with Gd, (g) sagittal T1-weighted MRI with Gd, (h) sagittal T1-weighted MRI, (i) sagittal T2-weighted MRI, and (j) deteriorated visual function status 3 months after CAB administration. (k) *: tumor and △: old hematoma (liquid) of the cystic lesions and (l) *: tumor and ☆: fresh hematoma (clot) caudal to the right optic nerve under endoscopic view. (m) Coronal T1-weighted MRI with Gd, (n) sagittal T1-weighted MRI with Gd, and (o) visual field deficit in the Goldmann perimetry and bilateral visions (CPA) after surgery.
Discussion
This study aimed to examine cases of PRLoma with VI that truly required surgery in order to avoid meaningless or unnecessary surgery. We reviewed our experience with the pharmacological and surgical care of 18 PRLomas with VI and 141 PRLomas without VI to establish whether VI could be a sign for surgery and to understand the function of surgery in the treatment of PRLoma. The gold standard of recent PRLoma treatment is the administration of CAB, with the exception of pituitary carcinoma.5) This is because CAB is effective in normalizing serum PRL levels and reducing tumor mass and has less adverse effects compared to traditional DAs.1-4) Therefore, the surgical indications for PRLomas are usually restricted. Klibanski A determined the indications for neurosurgery in patients with prolactinomas as follows2): growing tumor size despite optimal medical treatment, pituitary apoplexy, incapacity to tolerate DA therapy, DA-resistant macroadenoma, DA-resistant microadenoma in women seeking fertility when ovulation induction is inappropriate, persistent chiasmal compression despite optimal medical therapy, medically unresponsive cystic prolactinoma, macroadenomas near the optic chiasm despite optimal medical therapy in women seeking fertility (where prepregnancy debulking is advised), CSF leakage during DA administration, and macroadenomas in patients with mental disorders in which DAs are contraindicated. Of these indications, the clear and absolute surgical indications for PRLoma were extremely restricted: the resistance and/or intolerance to CAB and CSF leakage due to tumor invasion. Surgery can only be chosen if it has advantages superior to medical treatment. Furthermore, as a prerequisite for performing surgery, a high-level surgical technique that can minimize complications is required.2) Gamma Knife robotic microradiosurgery for PRLomas is considered only in situations where medical care and surgery are ineffective.6,7)
It is evident that the longer the tumor compresses the optic nerve, the more difficult it is for the optic function to improve and recover. There would be an irreversible point beyond which the visual function would fail to recover to its initial state, but this is challenging to determine.8,9) Therefore, it is better to release the compression on the optic nerve as soon as possible if the VI advances aggressively and rapidly. The advantages of surgery are that symptoms improve immediately after surgery; however, the disadvantages are that surgery requires hospitalization, it is often associated with complications, and the surgical procedure may damage the optic nerve or superior hypophyseal artery (SHA), which may worsen visual function. This is more likely to happen when the optic nerve is severely compressed by the tumor and weakens. First of all, the purpose of surgery for PRLoma with VI is not total removal of the tumor and normalization of serum PRL levels but to relieve tumor compression of the optic nerve and rescue visual function. The goal should be to remove the tumor or hematoma surrounding the optic nerve, which causes deterioration of visual function. In Case No. 1, the tumor and fibrous tissue were resected subtotally after 5 years of CAB treatment, but there was little improvement in VI because of long term severe compression of the optic nerve by the tumor (Fig. 2). In Case No. 16, the clot and tumor on the caudal side of the right optic nerve caused severe visual impairment of finger perception, and we promptly excised the lesion. Immediately after surgery, the VI improved almost completely, and the PRL level reduced to the normal range (Fig. 3). The tumor did not need to be entirely removed. Of note, we must prevent complications of surgery,10-12) skill up the endoscopic procedure, develop instruments for TSS,13-16) and utilize high-definition endoscopes,17) which can help preserve the optic nerves, SHA, and normal pituitary gland. Visual and pituitary functions must not be compromised, and excessive removal must be strongly prevented.
Conversely, the advantage of CAB treatment is that it is not invasive and does not potentially damage the optic nerve or SHA. The drawbacks are that mild and transient side effects occur and visual functions improve gradually (1-3 months). However, all cases that showed improvement in our series began to improve perceptibly within 1 month after treatment initiation. A limitation of this study was that the ultimate level of improvement could not be compared between surgery and CAB. However, it can be expected that there would be almost no difference if CAB was effective, given that even in cases of severe VI (Case No. 4), where the CVA was reduced, visual acuity was almost fully restored, even to the extent that the patient presented with subjective improvement with a slight residual VFD (Fig. 1).
PRLoma with VI comprised a higher proportion of men and a higher average PRL level. This indicated that the symptoms of PRLoma remained undetected in men until they had VI, and the tumors impairing vision were larger and were associated with higher PRL levels (Tables 1 and 2). If the patients are not aware of VI in spite of objective VI, treatments need not to be hastened. However, VI can also be a subjective symptom, and if it becomes severe to the extent of deteriorating CVA, the decision regarding whether to administer CAB treatment, the rate of titrating the CAB dose, or the need for surgery becomes crucial. The CAB dose and therapy objectives differed from case to case, and we need individual considerations for each case. The maximum CAB doses for PRLomas with VI ranged from 0.5 to 6 mg/week in our series. Although the dose of CAB can be increased to 12 mg/week to be effective against the tumor,3) the degree to which it can be increased depends on the existence and severity of VI. If there is no VI, the dose of CAB can be increased gradually and slowly; however, in cases with VI, the dose must be up-titrated rapidly, considering the side effects.2) Moreover, a decline in serum PRL levels does not always correspond with a reduction in tumor size, as in Case Nos. 1 and 16. Case No.1 involved a solid parenchymal tumor that initially exhibited reduction in PRL levels with CAB treatment; however, later on the PRL levels rose and VI was not improved. Case No. 16 involved a partially solid, mainly cystic tumor. Cystic PRLoma is thought to be a result of chronic intratumoral bleeding and tends to be resistant to CAB.2,18,19) The reason the tumor did not shrink and the PRL level did not decrease enough was due to chronic intratumoral hemorrhage. Moreover, acute intratumoral hemorrhage is implicated in the process by which the size of the tumor increases and compresses the optic nerve, and intratumoral hemorrhage causes aggressive and severe VI.20) Consequently, it should be noted that cystic PRLomas may worsen VI. In this instance, the progressive, severe VI was fortunately improved through early surgical intervention based on close observation. Therefore, patients should be informed about the possibility of emergency surgery before CAB administration. To prevent losing the appropriate decision and timing to switch from CAB to surgery, the MRI and objective visual state must be evaluated frequently, and the patients should be informed that whenever they feel any subjective worsening of VI, they should not fail to notify their physicians. The indications and timing of surgery for PRLoma with VI should be assessed appropriately and comprehensively at a high-volume center, with the cooperation of experienced neurosurgeons, endocrinologists, and ophthalmologists.
In summary, in this study, the surgical criteria for PRLoma were reexamined focusing on VI. In contrast to other pituitary tumors, CAB is extremely effective for PRLoma. It helped in tumor shrinkage and markedly improved the VI (88.9%). Consequently, we suggest that CAB administration should be considered a first-line treatment for PRLoma even with VI. However, close and careful surveillance is needed for instances resistant to CAB, particularly cystic cases, which require correct decisions regarding surgical intervention at a moderate timing and appropriate surgical procedures considering the purpose of surgery.
Abbreviations
CAB, cabergoline
CSF, cerebrospinal fluid
CVA, corrected visual acuity
DA, dopamine agonist
MRI, magnetic resonance imaging
NFPA, nonfunctioning pituitary adenoma
PitNET, pituitary neuroendocrine tumor
PRL, prolactin
PRLoma, prolactin-producing pituitary adenoma
TSS, transsphenoidal surgery
VFD, visual field deficit
VI, visual impairment
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interest Disclosure
none
Acknowledgments
The authors would like to thank the endocrinologists at our hospital for providing extensive endocrine examinations and Dr. Atsushi Fukui for statistical analysis.
References
- 1). Casanueva FF, Molitch ME, Schlechte JA, et al. : Guidelines of the Pituitary Society for the diagnosis and management of prolactinomas. Clin Endocrinol (Oxf) 65: 265-273, 2006 [DOI] [PubMed] [Google Scholar]
- 2). Klibanski A: Clinical practice. Prolactinomas. N Engl J Med 362: 1219-1226, 2010 [DOI] [PubMed] [Google Scholar]
- 3). Ono M, Miki N, Kawamata T, et al. : Prospective study of high-dose cabergoline treatment of prolactinomas in 150 patients. J Clin Endocrinol Metab 93: 4721-4727, 2008 [DOI] [PubMed] [Google Scholar]
- 4). Ono M, Miki N, Amano K, et al. : Individualized high-dose cabergoline therapy for hyperprolactinemic infertility in women with micro- and macroprolactinomas. J Clin Endocrinol Metab 95: 2672-2679, 2010 [DOI] [PubMed] [Google Scholar]
- 5). Hirohata T, Asano K, Ogawa Y, et al. : DNA mismatch repair protein (MSH6) correlated with the responses of atypical pituitary adenomas and pituitary carcinomas to temozolomide: the national cooperative study by the Japan Society for Hypothalamic and Pituitary Tumors. J Clin Endocrinol Metab 98: 1130-1136, 2013 [DOI] [PubMed] [Google Scholar]
- 6). Hayashi M, Chernov M, Tamura N, et al. : Gamma knife robotic microradiosurgery of pituitary adenomas invading the cavernous sinus: treatment concept and results in 89 cases. J Neurooncol 98: 185-194, 2010 [DOI] [PubMed] [Google Scholar]
- 7). Hayashi M, Chernov M, Horiba A, Tamura N, Amano K, Kawamata T: Gamma knife radiosurgery or pituitary adenomas invading the cavernous sinus: Tokyo Women's Medical University experience. Acta Neurochir Suppl 128: 29-41, 2021 [DOI] [PubMed] [Google Scholar]
- 8). Suri A, Narang KS, Sharma BS, Mahapatra AK: Visual outcome after surgery in patients with suprasellar tumors and preoperative blindness. J Neurosurg 108: 19-25, 2008 [DOI] [PubMed] [Google Scholar]
- 9). Jahangiri A, Lamborn KR, Blevins L, Kunwar S, Aghi MK: Factors associated with delay to pituitary adenoma diagnosis in patients with visual loss. J Neurosurg 116: 283-289, 2012 [DOI] [PubMed] [Google Scholar]
- 10). Amano K, Kawamata T, Hori T, Okada Y: Repair of cerebrospinal fluid leakage in transsphenoidal surgery. No Shinkei Geka 38: 599-611, 2010. (Japanese) [PubMed] [Google Scholar]
- 11). Amano K, Kawamata T, Hori T, Okada Y: Complications of transsphenoidal surgery. No Shinkei Geka 40: 1119-1129, 2012. (Japanese) [PubMed] [Google Scholar]
- 12). Amano K, Hori T, Kawamata T, Okada Y: Repair and prevention of cerebrospinal fluid leakage in transsphenoidal surgery: a sphenoid sinus mucosa technique. Neurosurg Rev 39: 123-131, 2016 [DOI] [PubMed] [Google Scholar]
- 13). Kawamata T, Amano K, Hori T: Novel flexible forceps for endoscopic transsphenoidal resection of pituitary tumors: technical report. Neurosurg Rev 31: 65-68, 2008 [DOI] [PubMed] [Google Scholar]
- 14). Amano K, Okada Y, Kawamata T: Usefulness of the knot-tightener device following dural suturing in endonasal transsphenoidal surgery: technical report. Neurosurg Rev 42: 593-598, 2019 [DOI] [PubMed] [Google Scholar]
- 15). Suzuki S, Kobayashi E, Hododuka K, Amano K, Masamune K, Muragaki Y: Development of a semiautomatic dura mater suturing device for preventing cerebrospinal fluid leakage in transsphenoidal surgery. Surg Innov 28: 374-377, 2021 [DOI] [PubMed] [Google Scholar]
- 16). Kawamata T, Amano K: Novel bendable ring curette for endoscopic transsphenoidal surgery for pituitary tumors. technical note. World Neurosurg 151: 284-289, 2021 [DOI] [PubMed] [Google Scholar]
- 17). Amano K, Aihara Y, Tsuzuki S, Okada Y, Kawamata T: Application of indocyanine green fluorescence endoscopic system in transsphenoidal surgery for pituitary tumors. Acta Neurochir (Wien) 161: 695-706, 2019 [DOI] [PubMed] [Google Scholar]
- 18). Ogiwara T, Horiuchi T, Nagm A, Goto T, Hongo K: Significance of surgical management for cystic prolactinoma. Pituitary 20: 225-230, 2017 [DOI] [PubMed] [Google Scholar]
- 19). Nakhleh A, Shehadeh N, Hochberg I, et al. : Management of cystic prolactinomas: a review. Pituitary 21: 425-430, 2018 [DOI] [PubMed] [Google Scholar]
- 20). Oda Y, Amano K, Masui K, Kawamata T: Clinical features of pituitary or parasellar tumor onset with cranial nerve palsy: surgical intervention considerations. World Neurosurg 175: e832-e840, 2023 [DOI] [PubMed] [Google Scholar]