Abstract
Background
Metabolic syndrome (MetS) is a cluster of interconnected risk factors that significantly increase the likelihood of cardiovascular disease and type 2 diabetes. Taurine has emerged as a potential therapeutic agent for MetS. This meta-analysis of randomized controlled trials (RCTs) aimed to evaluate the effects of taurine supplementation on MetS-related parameters.
Methods
We conducted electronic searches through databases like Embase, PubMed, Web of Science, Cochrane CENTRAL, and ClinicalTrials.gov, encompassing publications up to December 1, 2023. Our analysis focused on established MetS diagnostic criteria, including systolic blood pressure (SBP), diastolic blood pressure (DBP), fasting blood glucose (FBG), triglyceride (TG), and high-density lipoprotein cholesterol (HDL-C). Meta-regression explored potential dose-dependent relationships based on the total taurine dose administered during the treatment period. We also assessed secondary outcomes like body composition, lipid profile, and glycemic control.
Results
Our analysis included 1024 participants from 25 RCTs. The daily dosage of taurine in the studies ranged from 0.5 g/day to 6 g/day, with follow-up periods varying between 5 and 365 days. Compared to control groups, taurine supplementation demonstrated statistically significant reductions in SBP (weighted mean difference [WMD] = −3.999 mmHg, 95% confidence interval [CI] = −7.293 to −0.706, p = 0.017), DBP (WMD = −1.509 mmHg, 95% CI = −2.479 to −0.539, p = 0.002), FBG (WMD: −5.882 mg/dL, 95% CI: −10.747 to −1.018, p = 0.018), TG (WMD: −18.315 mg/dL, 95% CI: −25.628 to −11.002, p < 0.001), but not in HDL-C (WMD: 0.644 mg/dl, 95% CI: −0.244 to 1.532, p = 0.155). Meta-regression analysis revealed a dose-dependent reduction in DBP (coefficient = −0.0108 mmHg per g, p = 0.0297) and FBG (coefficient = −0.0445 mg/dL per g, p = 0.0273). No significant adverse effects were observed compared to the control group.
Conclusion
Taurine supplementation exhibits positive effects on multiple MetS-related factors, making it a potential dietary addition for individuals at risk of or already experiencing MetS. Future research may explore dose-optimization strategies and potential long-term benefits of taurine for MetS management.
Subject terms: Nutrition, Metabolic syndrome
Introduction
Metabolic syndrome (MetS) poses a significant global health challenge, affecting over one billion people [1]. This cluster of interconnected risk factors is diagnosed by the presence of: (1) abdominal obesity with increased waist circumference, (2) high blood pressure, (3) high fasting blood glucose (FBG) levels, (4) high triglyceride (TG) levels, and (5) a low level of high-density lipoprotein cholesterol (HDL-C) [2]. MetS significantly increases the risk of various health problems, including cardiovascular disease, stroke, and type 2 diabetes [3]. Its development is associated with factors like insulin resistance, chronic inflammation, and neurohormonal activation [3].
Taurine (2-aminoethanesulfonic acid), a sulfur-containing amino acid, has gained scientific interest due to its potential to modulate various physiological processes. It is primarily obtained through diet, particularly from foods like shellfish, dark meat, and some energy drinks [4]. Abundant in tissues like the heart, retina, liver, muscle, and platelets, taurine plays crucial roles in osmoregulation, mitochondrial function, maintenance of cell membrane stability, antioxidative defense mechanisms, and regulating cation balance [4].
In addition to its fundamental functions, taurine shows promise for regulating key metabolic parameters associated with MetS. It plays a significant role in controlling lipid metabolism by conjugating with bile salts [4]. Studies have also suggested its potential to improve glycemic markers, including FBG, serum insulin levels, and glycated hemoglobin (HbA1c) [5]. Moreover, taurine may exert anti-inflammatory effects by inhibiting the renin-angiotensin system, a key factor in the development of cardiovascular diseases and obesity [6]. Therefore, taurine has the potential to positively affect MetS.
Despite numerous clinical studies demonstrating the diverse health benefits of taurine, inconsistencies in clinical outcomes make it challenging to definitely determine whether taurine reduces the risk of MetS [7, 8]. To address this knowledge gap, we conducted a meta-analysis of randomized controlled trials (RCTs) to systematically analyze the effects of taurine on modifying the parameters associated with MetS.
Materials and methods
General guidelines
This meta-analysis adhered to the most recent revision of Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 guidelines (Table S1) [9]. The review was registered on Inplasy.com under number (INPLASY2023120081). Two authors (T.-C.C. and C.-L.Y.) conducted independent electronic searches in Embase, PubMed, Web of Science, Cochrane CENTRAL, and ClinicalTrials.gov databases using the following keywords (“taurine” OR “taufon”) AND (“metabolic syndrome” OR “diabetes mellitus” OR “obesity” OR “hypertension” OR “dyslipidemia” OR ‘hyperglycemia’). The search covered the inception of each database until December 1, 2023. The detailed search methodology is provided in the Supplementary Material (Table S2). The identified titles and abstracts were initially screened by the two authors to determine their eligibility, followed by a full-text review where necessary. We also manually searched additional databases and checked the reference lists of relevant meta-analyses. This study included publications in all languages.
Inclusion and exclusion criteria
This meta-analysis followed the PICO (population, intervention, comparison, and outcome) settings design: P, human participants; I, taurine supplementation; C, supplementation (including placebo) other than taurine; and O, parameters associated with the diagnosis of MetS.
We included (1) RCTs employing pure taurine and its compounds as the treatment arm, (2) trials with a comparative arm using interventions other than taurine, and (3) studies providing data for pre- and post-intervention assessments of at least one outcome related to MetS.
We excluded (1) non-RCTs, including quasi-experimental studies such as real world observations, trials without a comparing placebo, retrospective studies, cohort studies and case reports; (2) studies with short follow-up periods unlikely to capture effects on MetS (e.g., less than 24 h); (3) trials using herbal treatments with unclear active ingredients; (4) studies lacking data for pre- and post-intervention endpoints; and (5) studies not investigating outcomes of interest.
Trials with follow-up durations less than 24 h and trials with unclear active ingredients were excluded as they did not align with the purpose of the study. Studies lasting less than 24 h primarily focus on the immediate effects of energy drinks, such as changes in heart rate, so we have opted to exclude them. Furthermore, studies that lacked precise quantification of active ingredients or failed to provide a comprehensive list of effective ingredients were excluded since we could accurately attribute observed effects solely to taurine.
Methodological quality appraisal
The methodological quality of the included studies was assessed using the Cochrane risk of bias tool for RCTs (RoB 2, London, United Kingdom), which evaluates six main domains: randomization process, intervention adherence, missing outcome data, outcome measurement, selective reporting, and overall risk of bias [10]. Within the RoB 2 framework, intervention adherence can be assessed using two approaches: intention-to-treat and per-protocol. We opted for the per-protocol approach because most RCTs only report data from participants who completed the entire trial course [10].
Primary and secondary outcome
The primary outcomes of this investigation were changes in (1) systolic blood pressure (SBP), (2) diastolic blood pressure (DBP), (3) FBG, (4) TG, and (5) HDL-C. The secondary outcomes included: (1) body composition measures like body weight (BW) and body mass index (BMI), (2) lipid profiles including total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C), (3) glycemic profiles including HbA1c, homeostatic model assessment (HOMA), and fasting insulin, and (4) adverse effects. To accommodate studies with no reported adverse events, the number of cells with zero events was adjusted to 0.5 [11].
Data extraction and management
Two independent authors (T.-C.C. and C.-L.Y.) extracted data from the reviewed studies, including demographics, research design, details of taurine and control regimens, and outcome values. To avoid misinterpretation of effects, they carefully considered the direction of the scales used in each trial. In cases where data was missing from published studies, attempts were made to contact the corresponding authors to obtain the original data. Data extraction, conversion, and merging of outcomes from different study arms with varying taurine dosages were performed in accordance with the Cochrane Handbook for Systematic Review of Interventions and relevant medical literature [12–14]. For statistical analysis, the outcomes reported after the intervention were extracted, assuming data were available for multiple time points post-treatment.
Statistical analyses
This meta-analysis was conducted using Comprehensive Meta-Analysis software (version 3; Biostat, Englewood, NJ, United States) due to the heterogeneous nature of the study populations [15]. For all continuous outcomes, the weighted mean difference (WMD) and its 95% confidence interval (CI) were calculated. Odds ratios and their corresponding 95% CIs were used to analyze categorical outcomes (i.e., the rates of treatment-related adverse events), such as the rates of treatment-related adverse events. The effects of outcomes were assessed using WMD, with respective units dependent on the variable. This metric illustrates the magnitude of change observed across the entire taurine intervention, regardless of dose and duration.
Heterogeneity between studies was assessed using I2 and Cochran’s Q statistics. I2 values of 25%, 50%, and 75% were considered indicative of low, moderate, and high heterogeneity, respectively [16]. To investigate potential dose-dependent relationships between taurine and primary outcomes, meta-regression analyses were conducted using the total taurine dose administered throughout the treatment period and the daily dosage. The total dose was calculated as the product of the duration of intake multiplied by the dosage per day. Thus, the coefficient represents the average effect per gram of administered taurine.
A one-study removal sensitivity analysis was conducted to assess whether excluding a specific trial significantly altered the overall effect size [11]. To investigate potential publication bias, we visually examined the distribution of effect sizes in a funnel plot and assessed the statistical significance of Egger’s regression test [9].
Results
Study selection
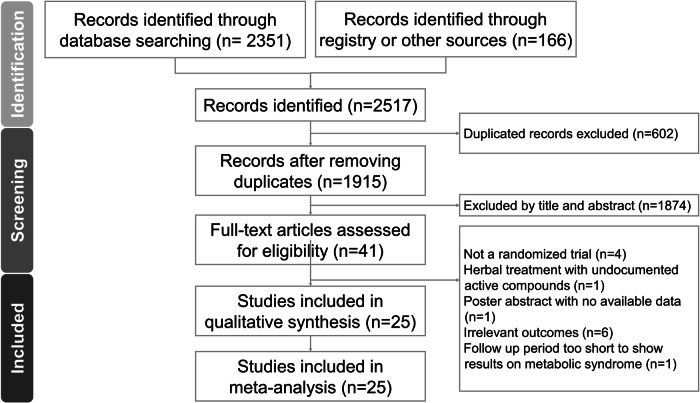
Our initial search yielded 2517 publications. After removing duplicates and screening titles and abstracts, we deemed 2476 articles irrelevant and discarded them. We then conducted a full-text review of the remaining 41 studies.
Thirteen articles were excluded for various reasons (Table S3): four weren’t RCTs, one used an herbal treatment with unverified active compounds, one was a poster abstract lacking data, six did not report outcomes aligned with our research focus, and one only administered a single dose of the intervention. This resulted in the inclusion of 25 studies [7, 8, 17–39] in our final quantitative analysis (Fig. 1). Data extraction details for these RCTs are presented in Tables 1 and 2.
Fig. 1.
The PRISMA flow diagram of the screening and review process.
Table 1.
Summary of trials retrieved to investigate the impact of taurine on metabolic syndrome.
| First author & year | Country | Population | Participants (F/M) | Age | Funding/grants/support |
|---|---|---|---|---|---|
| Azuma [22] | Japan | Congestive heart failure | 58 (30/28) | 38 - 89 | N/A |
| Azuma [21] | Japan | Congestive heart failure | 14 (5/9) | 68.71 ± 9.10 | Osaka University Medical School |
| Fujita [27] | Japan | Borderline hypertension | 19 (N/A) | 20 - 25 | N/A |
| Azuma [23] | Japan | Idiopathic dilated cardiomyopathy | 17 (6/11) | N/A | N/A |
| Mizushima [30] | Japan | Healthy male volunteers | 20 (0/20) | 18 - 29 | N/A |
| Zhang [8] | Japan | Overweight or obese non-diabetic subjects | 30 (16/14) |
Taurine group 20.1 ± 2.0 Placebo group 20.4 ± 1.1 |
Taisho Pharmaceutical Co., Ltd., Japan |
| Spohr [7] | Denmark | Type 2 diabetes mellitus | 18 (0/18) | 40 ± 8 | Steno Diabetes Center, Gentofte, Denmark, Aase and Ejnar Danielsens Foundation, Lyngby, Denmark |
| Moloney [31] | Ireland | Type 1 diabetes mellitus | 19 (0/19) | 28.0 ± 2.0 | N/A |
| Beyranvand [25] | Iran | Heart failure with left ventricular ejection fraction less than 50% | 29 (3/26) | 60.57 ± 6.54 |
Shahid Beheshti Medical University |
| Arrieta [19] | Spain | Patients require amino-acid supplement after major surgical procedure | 74 (21/53) |
Taurine group: 48 – 90 Placebo group: 50 - 113 |
N/A |
| Hsieh [28] | China | Chronic alcoholic patients | 30 (17/13) |
Taurine group 59 ± 13 Placebo group 60 ± 12 |
Asia University (100-A-06), Chung Shan MedicalUniversity (CSMU-INT-102-12). |
| Rosa [34] | Brazil | Obese women | 16 (16/0) | 32 ± 2 | National Council of Scientific and Technological Development (CNPq), State of Sao Paulo Research Foundation (FAPESP). |
| Averin [20] | Russia | Coronary heart disease/Heart valve defects | 48 (12/36) |
Taurine group: 49.79 ± 1.4 Placebo group 48.65 ± 1.5 |
N/A |
| Ra [33] | Japan | Healthy men | 29 (0/29) | Taurine group: 25. 4 ± 1.0Placebo group: 25.2 ± 1.0 | Japan Society for the Promotion of Science (JSPS) |
| Sun [37] | China | Prehypertensive individuals | 86 (44/42) | 56.75 ± 8.26 | National Basic Research Program of China, National Natural Science Foundation of China |
| Ahmadian [17] a a (Journal of Dietary Supplements) | Iran | Heart failure | 16 (N/A) |
Taurine group: 60.12 ± 5.4 Placebo group: 60.13 ± 8.3 |
N/A |
| Ahmadian [18] b a (Ther Adv Cardiovasc Dis) | Iran | Heart failure | 16 (N/A) |
Taurine group: 60.12 ± 5.4 Placebo group: 60.13 ± 8.3 |
N/A |
| Schwarzer [35] | Austria | Patients with hepatic venous pressure gradient ≥12 mmHg | 22 (8/14) | 52 ± 11 | N/A |
| Batitucci [24] | Brazil | Obese women | 22 (22/0) | 36.6 ± 6.4 | Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES); The Sao Paulo Research Foundation (FAPESP) |
| Shari [36] | Iraq | Type 2 diabetes mellitus | 80 (34/46) |
Taurine group: 48.8 + 3.1 Placebo group: 50.2 ± 3.7 |
N/A |
| Van Hove [38] | USA | Inherited cystathionine -β-synthase deficient homocystinuria | 36 (19/17) | 8 - 50 | Food and Drug Administration, Walter S. and Lucienne Driskill Foundation |
| Maleki [29] | Iran | Type 2 diabetes mellitus | 45 (30/15) |
Taurine group: 41.61 ± 6.63 Placebo group: 43.86 ± 7.06 |
Tabriz University of Medical Sciences, Tabriz, Iran |
| Esmaeili [26] | Canada | Type 2 diabetes mellitus | 46 (32/14)b |
Taurine group: 42.74 ± 7.21 Placebo group: 43.52 ± 6.94 |
N/A |
| Zaki [39] | Egypt | Peripartum cardiomyopathy | 40 (40/0) |
Taurine group: 31.1 ± 2.64 Placebo group: 30.85 ± 3.07 |
N/A |
| Moludi [32] | Iran | Type 2 diabetes mellitus | 120 (97/23) |
Taurine group: 52.13 ± 8.1 Placebo group: 53.08 ± 8.8 |
N/A |
AA amino acid, RCT randomized controlled trial, N/A not applicable.
aDescribes the same set of subjects, but provides different outcomes.
bBefore loss of follow up.
Table 2.
Summary of taurine interventions administered in the treatment arms of the trials.
| First author & year | Daily Taurine dose (N) | Control (N) | Population | Duration | Taurine product/manufacturer |
| Azuma [22] | 6 g/day (58) | Matching placebo (58) | Congestive heart failure | 4 weeksc | Not mentioned |
| Azuma [21] | 6 g/day (14) | Matching placebo (14) | Congestive heart failure | 4 weeksc | Not mentioned |
| Fujita [27] | 6 g/day (10) | Matching placebo (9) | Borderline hypertension | 7 days | Not mentioned |
| Azuma [23] | 3 g/day (7) | Active placebo (10) | Idiopathic dilated cardiomyopathy | 6 weeks | Taurine sachet/Not mentioned |
| Mizushima [30] | 6 g/day (11) | Matching placebo (9) | Healthy male volunteers | 3 weeks | Taurine capsules/Taisho Pharmaceutical Co. Ltd. |
| Zhang [8] | 3 g/day (15) | Comparable placebo (15) | Overweight or obese non-diabetic subjects | 7 weeks | Taurine capsules/Jianli Pham Co., Beijing, China |
| Spohr [7] | 1.5 g/day (18) | Matching placebo (44) | Type 2 diabetes mellitus | 8 weeksc | Taurine capsules/Not mentioned |
| Moloney [31] | 1.5 g/day (9) | Matching placebo (10) | Type 1 diabetes mellitus | 14 daysc | Taurine tablet/Twinlab |
| Beyranvand [25] | 1.5 g/day (15) | Matching placebo (14) | Heart failure with left ventricular ejection fraction less than 50% | 2 weeks | Taurine capsules/Solgar, Leonia, NJ, USA |
| Arrieta [19] | 1.6 g/dayb (35) | Comparable placebo (39) | Patients require amino-acid supplement after major surgical procedure | 24 days | Tauramin 10%,/Laboratorios Grifols SA, Barcelona, Spain |
| Hsieh [28] | 6 g/day (15) | Matching placebo (15) | Chronic alcoholic patients | 3 months | Taurine capsules/Dokui Chemical Company (Taiwan) |
| Rosa [34] | 3 g/day (8) | Matching placebo (8) | Obese women | 8 weeks | Taurine capsules/Ajinomoto CO., INC., Limeira, SP,Brazil |
| Averin [20] | 0.5 g/day (24) | Matching placebo (24) | Coronary heart disease/Heart valve defects | 3 months | Taurine capsules/Pik-Pharma, Russian Federation |
| Ra [33] | 6 g/day (15) | Matching placebo (14) | Healthy men | 15 days | Taurine capsules/Taisho Pharmaceutical Co., Ltd., Japan |
| Sun [37] | 1.6 g/day (42) | Matching placebo (20) | Prehypertensive individuals | 12 weeks | Taurine capsules/Not mentioned |
| Ahmadian [17]a (Journal of Dietary Supplements) | 1.5 g/day (8) | Matching placebo (8) | Heart failure | 2 weeks | Taurine capsules/Solgar, Leonia, NJ, USA |
| Ahmadian [18]a (Ther Adv Cardiovasc Dis) | 1.5 g/day (8) | Matching placebo (8) | Heart failure | 2 weeks | Taurine capsules/Solgar, Leonia, NJ, USA |
| Schwarzer [35] | 6 g/day (12) | Matching placebo (10) | Patients with hepatic venous pressure gradient ≥12 mm Hg | 4 weeks | Taurine capsules/Not mentioned |
| Batitucci [24] | 3 g/day (14) | Matching placebo (8) | Obese women | 8 weeks | Taurine powder/Aminoethylsulfonic Acid, Ajinomoto®, São Paulo, SP |
| Shari [36] | 1 g/day (40) | Matching placebo (40) | Type 2 diabetes mellitus | 3 months | Taurine capsules/Jarrow’s formulas |
| Van Hove [38] | 5 g/day (14) | Matching placebo (22) | Inherited cystathionine -β-synthase deficient homocystinuria | 5 days | Not mentioned |
| Maleki [29] | 3 g/day (23) | Matching placebo (22) | Type 2 diabetes mellitus | 8 weeks | Taurine capsules/Karen Pharma and Food Supplement Co., Iran, |
| Esmaeili [26] | 3 g/day (23) | Matching placebo (23) | Type 2 diabetes mellitus | 8 weeks | Taurine capsules/Karen Pharmaceutical Co |
| Zaki [39] | 0.6 g/day (20) | Comparable placebo (18) | Peripartum cardiomyopathy | 5 days | 10 ml/kg taurine solution/10% (Aminoven®, Fresenius‑Kabi, Egypt) |
| Moludi [32] | 3 g/day (60) | Matching placebo (60) | Type 2 diabetes mellitus | 8 weeks | Taurine capsules/Karen Food Supplement Co, Iran |
aDescribes the same set of subjects, but provides different outcomes.
b1.5 g amino acid/kg bw/d, subjects are 69 kg in average.
cTreatment period of placebo or taurine in a crossover study.
Study characteristic
Key features of the 25 RCTs, involving 1,024 participants, are summarized in Table 1. Conducted between 1983 and 2021 in diverse locations (Russia, Iran, Japan, Spain, Brazil, Canada, Ireland, China, Austria, Iraq, Denmark, the USA, and Egypt), the studies enrolled participants aged 8–113 years with a wide range of conditions. These included healthy individuals, post-surgical patients, and individuals with conditions such as heart failure, hypertension, coronary heart disease, heart valve defects, cardiomyopathy, type 1 diabetes mellitus, type 2 diabetes mellitus, obesity, alcoholism, and homocystinuria.
Quality assessment
Eighteen studies [8, 17–25, 27, 28, 30, 33–38] lacked information on allocation concealment, putting them at risk of bias. The remaining seven studies [7, 26, 29, 31, 32, 35, 39] had a low risk of bias, and none had a high risk of bias (Fig. S1, Table 3).
Table 3.
Detailed quality assessment of the included studies using Cochrane risk of bias 2 tool.
| First Author | Year | Randomization process | Intervention adherence | Missing outcome data | Outcome measurement | Selective reporting | Overall RoB |
|---|---|---|---|---|---|---|---|
| Azuma | 1983 | Sa | L | L | L | L | S |
| Azuma | 1985 | Sa | L | L | L | L | S |
| Fujita | 1987 | Sa | L | L | L | L | S |
| Azuma | 1992 | Sa | L | L | L | L | S |
| Mizushima | 1996 | Sa | L | L | L | L | S |
| Zhang | 2004 | Sa | L | L | L | L | S |
| Spohr | 2005 | L | L | L | L | L | L |
| Moloney | 2010 | L | L | L | L | L | L |
| Beyranva-nd | 2011 | Sa | L | L | L | L | S |
| Arrieta | 2014 | Sa | L | L | L | L | S |
| Hsieh | 2014 | Sa | L | L | L | L | S |
| Rosa | 2014 | Sa | L | L | L | L | S |
| Averin | 2015 | Sa | L | L | L | L | S |
| Ra | 2016 | Sa | L | L | L | L | S |
| Sun | 2016 | Sa | L | L | L | L | S |
| Ahmadian | 2017 | Sa | L | L | L | L | S |
| Ahmadian | 2017 | Sa | L | L | L | L | S |
| Schwarzer | 2018 | L | L | L | L | L | L |
| Batitucci | 2019 | Sa | L | L | L | L | S |
| Shari | 2019 | Sa | L | L | L | L | S |
| Van Hove | 2019 | Sa | L | L | L | L | S |
| Maleki | 2020 | L | L | L | L | L | L |
| Esmaeili | 2021 | L | L | L | L | L | L |
| Zaki | 2021 | L | L | L | L | L | L |
| Moludi | 2022 | L | L | L | L | L | L |
H high risk of bias, L low risk of bias, RoB risk of bias, S some risk of bias.
aThe studies did not provide allocation concealment details.
Primary outcomes
Effects of taurine on SBP/DBP
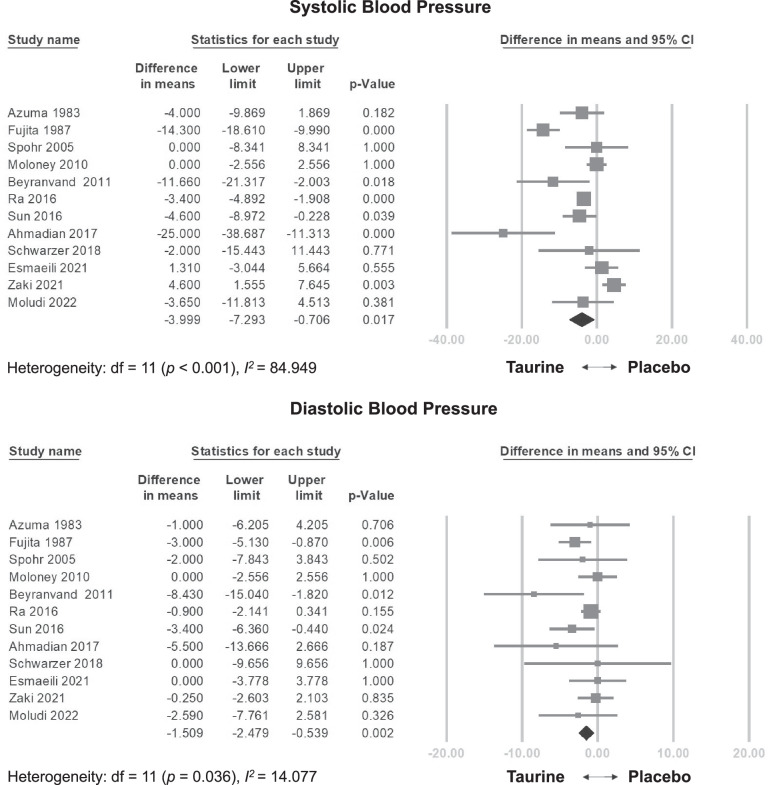
Taurine supplementation significantly reduced SBP compared to the control group (WMD = −3.999 mmHg, 95% CI = −7.293 to −0.706, p = 0.017, I2 = 84.949) (Fig. 2a). This effect remained consistent even after excluding individual studies on the sensitivity analysis (Fig. S2a). Meta-analysis regression did not reveal a statistically significant linear relationship between total dose and SBP (coefficient = −0.024 mmHg per g, p = 0.113) (Fig. S3a), and a significant relationship between daily dose and SBP (coefficient = −1.1258 mmHg per g/day, p = 0.0055) (Fig. S4a).
Fig. 2.
Forest plot of overall effects of taurine on systolic blood pressure (SBP) and diastolic blood pressure (DBP).
Taurine significantly reduced DBP levels (WMD = −1.509 mmHg, 95% CI = −2.479 to −0.539, p = 0.002, I2 = 14.077) (Fig. 2b). Similar to SBP, this DBP reduction persisted in the sensitivity analysis (Fig. S2b). Moreover, meta-regression analysis showed a significant correlation between total dose and decreased DBP (coefficient = −0.014 mmHg per g, p = 0.026) (Fig. S3b), and a significant relationship between daily dose and DBP (coefficient = −0.3247 mmHg per g/day, p = 0.0182) (Fig. S4b).
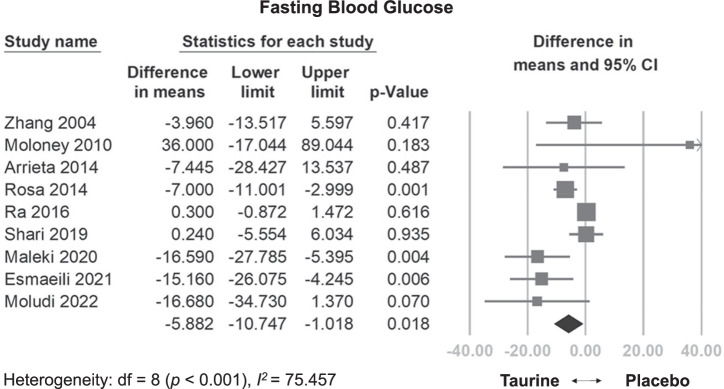
Effects of taurine on FBG
Overall, taurine supplementation significantly reduced FBG levels compared to the control group (WMD: −5.882 mg/dL, 95% CI: −10.747 to −1.018, p = 0.018, I2 = 75.457) (Fig. 3). This effect remained consistent even after excluding individual studies in the sensitivity analysis (Fig. S5). Interestingly, meta-regression revealed a significant correlation between total dose and decreased FBG levels (coefficient = −0.495 mg/dL per g, p = 0.0011) (Fig. S6), but no significant relationship between daily dose and FBG (coefficient = −1.5146 mg/dL per g/day, p = 0.0703) (Fig. S7).
Fig. 3.
Forest plot of overall effects of taurine on fasting blood glucose (FBG).
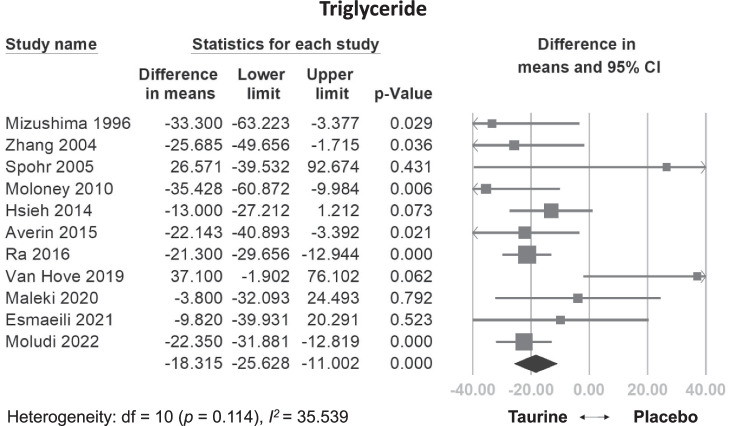
Effects of taurine on TG
Taurine supplementation significantly reduced TG levels compared to the control group (WMD: −18.315 mg/dL, 95% CI: −25.628 to −11.002, p < 0.001, I2 = 35.539) (Fig. 4). This effect remained consistent even after excluding individual studies in a sensitivity analysis (Fig. S8). While meta-regression did not reveal a statistically significant dose-dependent relationship between total dose and TG reduction (coefficient = −0.0522 mg/dL per g, p = 0.0730) (Fig. S9), it revealed a significant relationship between daily dose and TG (coefficient = −3.3600 mg/dL per g/day, p = 0.0062) (Fig. S10).
Fig. 4.
Forest plot of overall effects of taurine on triglyceride (TG).
Effects of taurine on HDL-C
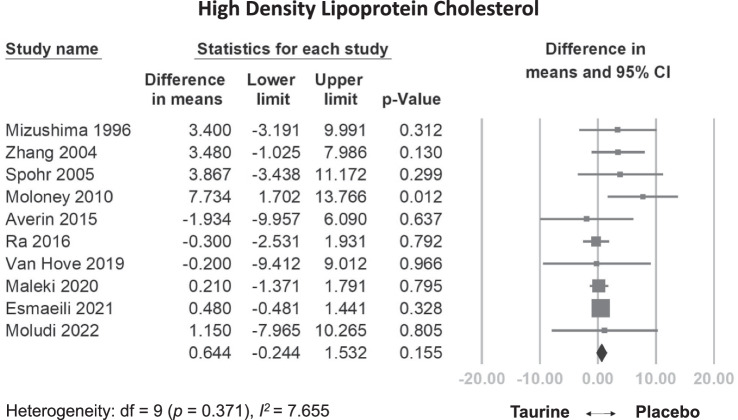
Overall, taurine supplementation did not significantly increase HDL-C levels compared to the control group (WMD: 0.644 mg/dL, 95% CI: −0.244 to 1.532, p = 0.155, I2 = 7.655) (Fig. 5). This observation remained consistent in the sensitivity analysis (Fig. S11). Similarly, meta-regression did not show a statistically significant dose-dependent relationship between total dose and HDL-C levels (coefficient = 0.0037 mg/dL per g, p = 0.2729) (Fig. S12). Moreover, it didn’t reveal a significant relationship between daily dose and HDL-C (coefficient = 0.1370 mg/dL per g/day, p = 0.3200) (Fig. S13).
Fig. 5.
Forest plot of overall effects of taurine on high density lipoprotein-cholesterol (HDL-C).
Publication bias
Funnel plot analysis for all investigated outcomes (SBP, DBP, FBG, TG, and HDL-C) indicated no evidence of publication bias. The distribution effect sizes were symmetric, as confirmed by Egger’s regression test, with p values exceeding 0.5 for all outcomes (p = 0.439, p = 0.213, p = 0.083, p = 0.166, and p = 0.158, respectively) (Figs. S14–S17).
Secondary outcomes
Effects of taurine on body composition
Taurine supplementation did not significantly impact BW or BMI compared to the control group. The pooled effect size for BW change was minimal and non-significant (WMD: −0.642 kg, 95% CI: −1.494 to 0.209, p = 0.139) (Fig. S18a). Similarly, the effect size for BMI change was not statistically significant (WMD: −0.296 kg, 95% CI: −0.889 to 0.296, p = 0.327) (Fig. S18b). These findings were further supported by a sensitivity analysis with consistent non-significant effects of taurine on both BW and BMI (Fig. S18c, d).
Effects of taurine on lipid profiles
Taurine demonstrated a significant beneficial effect on lipid profiles. Compared to the control group, taurine supplementation significantly reduced both TC and LDL-C levels. The pooled effect size showed a notable decrease in TC (WMD: −8.305 mg/dL, 95% CI: −13.771 to −2.929, p = 0.003) (Fig. S19a), and a similar statistically significant effect was observed for LDL-C levels (WMD: −6.495 mg/dL, 95% CI: −10.912 to −2.079, p = 0.004) (Fig. S19b). These findings were further validated by a sensitivity analysis showing consistent significant effects of taurine on both TC and LDL-C reduction (Fig. S19c and Fig. S19d).
Effects of taurine on glycemic status
Taurine supplementation positively impacted several glycemic markers. Pooled effect sizes revealed significant reductions in HbA1c (WMD: −0.341%, 95% CI: −0.709 to −0.028, p = 0.070) (Fig. S20a), HOMA index (WMD: −0.693, 95% CI: −1.133 to −0.252, p = 0.002) (Fig. S20b), and fasting insulin levels (WMD: −1.521 mU/L, 95% CI: −2.591 to −0.451, p = 0.005) (Fig. S20c) compared to the control group. A sensitivity analysis showed a consistently non-significant effect on HbA1c reduction (Fig. S20d) but maintained consistent significant effects on both HOMA and fasting insulin (Fig. S20e, f).
Adverse effects
Meta-analysis of the treatment-associated adverse effect rates showed no significant differences between the taurine and control groups (odds ratio = 1.481, 95% CI = 0.843–2.604, p = 0.172) (Fig. S17).
Discussion
Our meta-analysis found that taurine supplementation significantly reduced SBP, DBP, FBG, and TG levels, suggesting an improvement in risk factors associated with MetS. A dose-dependent effect is evident on SBP, DBP, FBG, and TG levels, as influenced by both dose per day and total dose. Even though not all parameters reached statistical significance, the consistent trends point to a higher physiological response to administered substance doses. However, our analysis revealed no discernible dose-dependent effect on HDL-C levels, irrespective of dose per day or total dose. Notably, taurine did not exhibit any apparent adverse effects.
Compared to the control group, taurine demonstrated a significant lowering of both SBP and DBP. The hypotensive effect may be attributed to several mechanisms, primarily through increased nitric oxide availability [15] and enhanced hydrogen sulfide production [14], ultimately leading to improved blood flow dilation [13]. Our findings also revealed a remarkable ability of taurine to effectively reduce FBG levels. This suggests a positive impact on glycemic control, potentially via various mechanisms. These include: reduced hepatic glucose production, inhibition of glucagon activity, elevated uncoupling protein 1 levels [40], enhanced insulin clearance by insulin-degrading enzymes [41], and support for the health of beta-pancreatic cells [42]. Furthermore, taurine may upregulate adiponectin mRNA expression and increase blood adiponectin levels, thereby improving insulin sensitivity [43] and contributing to overall metabolic health.
A previous meta-analysis by Guan et al. [44] found no significant difference in FBG between taurine and control groups, our results revealed a notable reduction. This discrepancy may be due to our inclusion of more studies involving individuals with diabetes, where baseline glucose levels were already elevated [19, 26, 29, 32]. This suggests that taurine’s impact might be less pronounced in those with normal blood sugar regulation.
Taurine significantly reduced TG levels. This is likely due to its ability to enhance TG removal into the bile by stimulating the production of bile acid through heightened hepatic cholesterol 7α-hydroxylase activity. Additionally, taurine increases LDL-C receptor activity, aiding in LDL-C clearance from the blood [45].
While our meta-analysis did not show a statistically significant increase in HDL-C levels, the observed trends suggest potential benefits. HDL serves as a crucial form of endogenous lipid storage by removing excess cholesterol from peripheral tissues. Hepatocytes and intestinal cells are the main internal biosynthesisers that control its levels [46]. Taurine has known effects on promoting cholesterol catabolism, particularly by increasing CYP7A1 activity and bile acid synthesis, which enhances cholesterol removal through feces. Additionally, taurine decreases the expression of ApoB-100 and ApoE, which are major receptors for LDL and VLDL, further aiding in cholesterol clearance [47]. It’s likely that taurine efficiently removes cholesterol, displacing the need for extra HDL clearance. Furthermore, although taurine has been shown to raise serum HDL levels in rats raised on a high-cholesterol diet [47], human trials usually do not include purposefully high-fat diets, and the majority of participants are not obese. Therefore, in contrast to animal research, the effects of taurine on HDL levels in human trials may be less apparent. It is noteworthy that these trends towards elevated HDL-C may have a noteworthy effect on lowering the atherosclerosis index, even in the absence of statistically significant results [48].
Beyond FBG, taurine demonstrates effectiveness in both glycemic control and lipid profiles, with significant reductions observed in TC, LDL-C, fasting insulin, and HOMA levels. Although our analysis revealed only marginal benefits on HbA1c (WMD -0.341 [95% CI: −0.709, −0.28], p = 0.07), this may be attributed to the limited duration and intensity of protocols in the included studies (dosages ranging from 3 to 168 g with tracking periods of eight weeks to three months). Conversely, a meta-analysis conducted by Tao et al. exclusively on patients with diabetes reported significant reductions in HbA1c levels (WMD −0.41 [95% CI: −0.74, −0.09], p = 0.01) [5]. All four of the included trials [26, 29, 32, 36] assessing HbA1c as an endpoint involved individuals with type 2 diabetes, indicating a lack of evidence regarding HbA1c level changes in participants with diabetes. The lack of studies conducted on populations without diabetes could be because these changes are unlikely in this population. Future research may delve deeper into this topic. Since HbA1c not only offers a trustworthy indicator of chronic hyperglycemia but also strongly correlates with the risk of long-term diabetes complications [49], more research is required to fully understand its impact on long-term glycemic control in larger populations.
Taurine did not appear to significantly affect BW or BMI. In the only trial demonstrating a decrease in body mass, Shari et al. [36] administered 1 g taurine daily for 3 months to participants with type 2 diabetes. However, findings from other trials consistently showed negligible effects on body mass within their respective protocols. This suggests that factors like hypocaloric diet and exercise likely have a greater impact on body weight in this context [8].
Taurine is classified as “generally considered as safe” by the United States Food and Drug Administration [50]. In line with this, our study did not reveal any significant differences in treatment-related adverse effects between the taurine and control groups. All reported events were mild and transient, involving primarily gastrointestinal issues, headaches, and fatigue. Notably, no instances of moderate or severe events were associated with taurine supplementation [19, 35, 38].
While previous studies have examined similar endpoints [5, 44], ours is the first to conduct a meta-regression analysis on dose-response relationships, highlighting the effectiveness of taurine in mitigating MetS risk factors in the general population. However, some limitations warrant further consideration.
The observed heterogeneity has the potential to attenuate the true effect of taurine, introducing variability across different populations that may result in diminished statistical significance for parameters such as TG and HDL-C. Several factors contribute to this heightened heterogeneity. Firstly, there is variation in the selection of participants across studies, where individuals with diabetes and obesity, although at high risk for metabolic diseases, may not necessarily be diagnosed with specific disorders. Secondly, inconsistencies in study protocols play a role, with some participants adhering to their regular diet and activity levels [8], while others follow calorie-modified plans [32], potentially influencing results and increasing heterogeneity. Thirdly, our included trials suggest that taurine is more effective in moderating metabolism among studies that exclusively recruit patients with obesity or diabetes, as their TG levels are inherently higher and more prone to decrease compared to those with cardiovascular disease or no definite metabolic disorder.
When examining the impact of taurine on lipid profiles in patients with cardiovascular disease, confounding factors such as concurrent medication use must be considered. Given that a large number of the included studies focus on patients with heart failure, it is important to remember that medications like diuretics, angiotensin-converting enzyme (ACE), and vasodilators, which are commonly prescribed for cardiovascular conditions, can affect lipid levels. Long-term use of ACE inhibitors like enalapril has shown to significantly reduce TC, TG, and Very Low-density lipoprotein (VLDL) in patients [51]. Thiazide-type diuretics, often used for hypertension, may increase TC and VLDL levels in the short term, while HDL-C levels remain unchanged [52].
The authors of some included trials [26, 29, 32] were more aware of the impact of lipid level resulting from concurrent medication use, and specifically instructed participants to maintain their standard therapeutic regimen, diet, and lifestyle, with careful monitoring and controlled for. To better understand taurine’s effects on lipid profiles, future clinical trials should carefully consider and possibly exclude patients using medications known to significantly impact lipid levels.
Direct data on waist circumference, another key MetS criterion, were unavailable for any of the included trials. Despite this, we incorporated BMI as a secondary outcome, which could indirectly reflect the potential impact of taurine on waist circumference due to their strong correlation [53].
Another critical issue is that the majority of included studies on taurine’s effects typically have short durations, lasting no more than two months, with only a few extending up to a year at most. This limited timeframe emphasizes the necessity for longer-term studies to validate taurine’s efficacy thoroughly. Future studies should conduct more future studies to ascertain the duration of taurine’s effects after its cessation. Such extended investigations are crucial to explore its potential incorporation into novel clinical guidelines for the management of MetS and related conditions.
Conclusion
In conclusion, our meta-analysis of RCTs highlights taurine supplementation’s significant potential in mitigating key MetS risk factors, including reductions in SBP, DBP, FBG, and TG levels. This underscores its potential as a complementary therapeutic agent for MetS management, offering a multifaceted approach to glycemic control and cardiovascular health. Future clinical trials should focus on determining optimal taurine dosage and treatment duration, especially in MetS-susceptible populations. Addressing limitations in existing trials, such as variations in dosage, trial duration, sample size, disease severity, and participant characteristics, is crucial for generating robust evidence. As taurine is an inexpensive and easily obtainable supplement, further research can fill knowledge gaps, supporting clinical guidelines on its use as a nutraceutical for MetS prevention and management. Integrating taurine supplementation with established pharmacological interventions may enhance treatment outcomes and overall cardiovascular health in MetS patients.
Supplementary information
Author contributions
C.-C.T. and C.-K.V. were responsible for designing the review protocol. C.-C.T. and L.-Y.C. were responsible for screening potentially eligible studies and extracting data. C.-C.T., L.-L.H., and W.-W.T. contributed to conducting the analysis and interpreting the results. C.-C.T. and L.-Y.C. wrote the manuscript. C.-K.V. and L.Ö. provided feedback on the report. L.-T.Y. and W.-W.T. helped created the tables. All authors contributed to the manuscript editing.
Funding
This study was funded by the National Taiwan University Hospital, Bei-Hu Branch (11302, 113-BH002, 11304 and 113-BH004), Ministry of Science and Technology, Taiwan (MOST 106-2314-B-002-180-MY3 and MOST 109-2314-B-002-114-MY3) and National Science and Technology, Taiwan (NSTC 112-2314-B-002-134).
Data availability
All data included in this study are shown in this article or supplementary information, any further request is available by contacting the corresponding authors.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41387-024-00289-z.
References
- 1.Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20:12. doi: 10.1007/s11906-018-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 3.Wilson PW, D’Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–72. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 4.Wright CE, Tallan HH, Lin YY, Gaull GE. Taurine: biological update. Annu Rev Biochem. 1986;55:427–53. doi: 10.1146/annurev.bi.55.070186.002235. [DOI] [PubMed] [Google Scholar]
- 5.Tao X, Zhang Z, Yang Z, Rao B. The effects of taurine supplementation on diabetes mellitus in humans: a systematic review and meta-analysis. Food Chem. 2022;4:100–106. doi: 10.1016/j.fochms.2022.100106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qaradakhi T, Gadanec LK, McSweeney KR, Abraham JR, Apostolopoulos V, Zulli A. The anti-inflammatory effect of taurine on cardiovascular disease. Nutrients. 2020;12:2847.. doi: 10.3390/nu12092847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spohr C, Brøns C, Winther K, Dyerberg J, Vaag A. No effect of taurine on platelet aggregation in men with a predisposition to type 2 diabetes mellitus. Platelets. 2005;16:301–5. doi: 10.1080/09537100400020575. [DOI] [PubMed] [Google Scholar]
- 8.Zhang M, Bi LF, Fang JH, Su XL, Da GL, Kuwamori T, et al. Beneficial effects of taurine on serum lipids in overweight or obese non-diabetic subjects. Amino Acids. 2004;26:267–71. doi: 10.1007/s00726-003-0059-z. [DOI] [PubMed] [Google Scholar]
- 9.Page MJ, Higgins JPT, Sterne JAC. Assessing risk of bias due to missing results in a synthesis. In Cochrane handbook for systematic re views of interventions. 2021;2023:Version 6.2 edn UK: Cochrane Training.
- 10.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 11.Deeks JJ, Higgins JPT, Altman DG. Analysing data and undertaking meta-analyses. In Cochrane handbook forsystematic reviews of interventions. Version 6.2 vol. 2023, 2021 UK: Cochrane Training.
- 12.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JPT, Li T, Deeks JJ. Choosing effect measures and computing estimates of effect. Cochrane handbook for systematic reviews of interventions. 2021;2023: Version 6.2. UK: Cochrane Training.
- 14.Higgins JPT, Li T, Deeks, AJ. Choosing effect measures and computing estimates of effect. Cochrane handbook for systematic reviews of interventions, vol. 2023, 2021.
- 15.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1:97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmadian M, Roshan VD, Aslani E, Stannard SR. Taurine supplementation has anti-atherogenic and anti-inflammatory effects before and after incremental exercise in heart failure. Ther Adv Cardiovasc Dis. 2017;11:185–94. doi: 10.1177/1753944717711138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmadian M, Dabidi Roshan V, Ashourpore E. Taurine supplementation improves functional capacity, myocardial oxygen consumption, and electrical activity in heart failure. Journal of dietary supplements. 2017;14:422–32. doi: 10.1080/19390211.2016.1267059. [DOI] [PubMed] [Google Scholar]
- 19.Arrieta F, Balsa JA, de la Puerta C, Botella JI, Zamarron I, Elias E, et al. Phase IV prospective clinical study to evaluate the effect of taurine on liver function in postsurgical adult patients requiring parenteral nutrition. Nutr Clin Pract. 2014;29:672–80. doi: 10.1177/0884533614533610. [DOI] [PubMed] [Google Scholar]
- 20.Averin E. Use of taurine during rehabilitation after cardiac surgery. Adv Exp Med Biol. 2015;803:637–49. doi: 10.1007/978-3-319-15126-7_51. [DOI] [PubMed] [Google Scholar]
- 21.Azuma J, Sawamura A, Awata N, Ohta H, Hamaguchi T, Harada H, et al. Therapeutic effect of taurine in congestive heart failure: a double-blind crossover trial. Clin Cardiol. 1985;8:276–82. doi: 10.1002/clc.4960080507. [DOI] [PubMed] [Google Scholar]
- 22.Azuma J, Sawamura A, Awata N. Double-blind randomized crossover trial of taurine in congestive heart failure. Curr Ther Res Clin Exp. 1983;34:543–57. [Google Scholar]
- 23.Azuma J, Sawamura A, Awata N. Usefulness of taurine in chronic congestive heart failure and its prospective application. Jpn Circ J. 1992;56:95–99. doi: 10.1253/jcj.56.95. [DOI] [PubMed] [Google Scholar]
- 24.Batitucci G, Brandao CFC, De Carvalho FG, Marchini JS, Pfrimer K, Ferrioli E, et al. Taurine supplementation increases irisin levels after high intensity physical training in obese women. Cytokine. 2019;123:154741. doi: 10.1016/j.cyto.2019.154741. [DOI] [PubMed] [Google Scholar]
- 25.Beyranvand MR, Khalafi MK, Roshan VD, Choobineh S, Parsa SA, Piranfar MA. Effect of taurine supplementation on exercise capacity of patients with heart failure. J Cardiol. 2011;57:333–7. doi: 10.1016/j.jjcc.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Esmaeili F, Maleki V, Kheirouri S, Alizadeh M. The effects of taurine supplementation on metabolic profiles, pentosidine, soluble receptor of advanced glycation end products and methylglyoxal in adults with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Can J Diabetes. 2021;45:39–46. doi: 10.1016/j.jcjd.2020.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Fujita T, Ando K, Noda H, Ito Y, Sato Y. Effects of increased adrenomedullary activity and taurine in young-patients with borderline hypertension. Circulation. 1987;75:525–32. doi: 10.1161/01.CIR.75.3.525. [DOI] [PubMed] [Google Scholar]
- 28.Hsieh Y-L, Yeh Y-H, Lee Y-T, Huang C-Y. Effect of taurine in chronic alcoholic patients. Food Funct. 2014;5:1529–35. doi: 10.1039/C3FO60597C. [DOI] [PubMed] [Google Scholar]
- 29.Maleki V, Alizadeh M, Esmaeili F, Mahdavi R. The effects of taurine supplementation on glycemic control and serum lipid profile in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Amino Acids. 2020;52:905–14. doi: 10.1007/s00726-020-02859-8. [DOI] [PubMed] [Google Scholar]
- 30.Mizushima S, Nara Y, Sawamura M, Yamori Y. Effects of oral taurine supplementation on lipids and sympathetic nerve tone. Adv Exp Med Biol. 1996;403:615–22. doi: 10.1007/978-1-4899-0182-8_68. [DOI] [PubMed] [Google Scholar]
- 31.Moloney MA, Casey RG, O’Donnell DH, Fitzgerald P, Thompson C, Bouchier-Hayes DJ. Two weeks taurine supplementation reverses endothelial dysfunction in young male type 1 diabetics. Diabetes Vasc Dis Res. 2010;7:300–10. doi: 10.1177/1479164110375971. [DOI] [PubMed] [Google Scholar]
- 32.Moludi J, Qaisar SA, Kadhim MM, Ahmadi Y, Davari M. Protective and therapeutic effectiveness of taurine supplementation plus low calorie diet on metabolic parameters and endothelial markers in patients with diabetes mellitus: a randomized, clinical trial. Nutr Metab. 2022;19:49. doi: 10.1186/s12986-022-00684-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ra SG, Choi Y, Akazawa N, Ohmori H, Maeda S. Taurine supplementation attenuates delayed increase in exercise-induced arterial stiffness. Appl Physiol Nutr Metab. 2016;41:618–23. doi: 10.1139/apnm-2015-0560. [DOI] [PubMed] [Google Scholar]
- 34.Rosa FT, Freitas EC, Deminice R, Jordão AA, Marchini JS. Oxidative stress and inflammation in obesity after taurine supplementation: a double-blind, placebo-controlled study. Eur J Nutr. 2014;53:823–30. doi: 10.1007/s00394-013-0586-7. [DOI] [PubMed] [Google Scholar]
- 35.Schwarzer R, Kivaranovic D, Mandorfer M, Paternostro R, Wolrab D, Heinisch B, et al. Randomised clinical study: the effects of oral taurine 6g/day vs placebo on portal hypertension. Aliment Pharmacol Ther. 2018;47:86–94. doi: 10.1111/apt.14377. [DOI] [PubMed] [Google Scholar]
- 36.Shari FH, Dawood H, Hassan JK, Aljazeari QA, Najm MAA, Salahuddin A, et al. To study the effect of taurine on the effects of vital bones and regulate the level of glucose in type II diabetes. Int J Res Pharm Sci. 2019;10:2545–2551. doi: 10.26452/ijrps.v10i3.1508. [DOI] [Google Scholar]
- 37.Sun Q, Wang B, Li Y, Sun F, Li P, Xia W, et al. Taurine supplementation lowers blood pressure and improves vascular function in prehypertension: randomized, double-blind, placebo-controlled study. Hypertension. 2016;67:541–9. doi: 10.1161/HYPERTENSIONAHA.115.06624. [DOI] [PubMed] [Google Scholar]
- 38.Van Hove JLK, Freehauf CL, Ficicioglu C, Pena LDM, Moreau KL, Henthorn TK, et al. Biomarkers of oxidative stress, inflammation, and vascular dysfunction in inherited cystathionine -synthase deficient homocystinuria and the impact of taurine treatment in a phase 1/2 human clinical trial. J Inherit Metab Dis. 2019;42:424–37. doi: 10.1002/jimd.12085. [DOI] [PubMed] [Google Scholar]
- 39.Zaki HV, Sweed MS, Ali RM, Abdelhafeez MA. Taurine as an adjunct therapy for early left ventricular recovery in peripartum cardiomyopathy. J Obstetr Anaesth Crit Care. 2021;11:9–14. doi: 10.4103/joacc.JOACC_36_20. [DOI] [Google Scholar]
- 40.Ribeiro RA, Bonfleur ML, Batista TM, Borck PC, Carneiro EM. Regulation of glucose and lipid metabolism by the pancreatic and extra-pancreatic actions of taurine. Amino Acids. 2018;50:1511–24. doi: 10.1007/s00726-018-2650-3. [DOI] [PubMed] [Google Scholar]
- 41.Camargo RL, Branco RC, de Rezende LF, Vettorazzi JF, Borck PC, Boschero AC, et al. The effect of taurine supplementation on glucose homeostasis: the role of insulin-degrading enzyme. Adv Exp Med Biol. 2015;803:715–24. doi: 10.1007/978-3-319-15126-7_57. [DOI] [PubMed] [Google Scholar]
- 42.Vettorazzi JF, Ribeiro RA, Santos-Silva JC, Borck PC, Batista TM, Nardelli TR, et al. Taurine supplementation increases K(ATP) channel protein content, improving Ca2+ handling and insulin secretion in islets from malnourished mice fed on a high-fat diet. Amino Acids. 2014;46:2123–36. doi: 10.1007/s00726-014-1763-6. [DOI] [PubMed] [Google Scholar]
- 43.Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, et al. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004;279:12152–62. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- 44.Guan L, Miao P. The effects of taurine supplementation on obesity, blood pressure and lipid profile: a meta-analysis of randomized controlled trials. Eur J Pharmacol. 2020;885:173533. doi: 10.1016/j.ejphar.2020.173533. [DOI] [PubMed] [Google Scholar]
- 45.Stephan ZF, Lindsey S, Hayes KC. Taurine enhances low density lipoprotein binding. Internalization and degradation by cultured Hep G2 cells. J Biol Chem. 1987;262:6069–73. doi: 10.1016/S0021-9258(18)45538-X. [DOI] [PubMed] [Google Scholar]
- 46.Dong Y, Li X, Liu Y, Gao J, Tao J. The molecular targets of taurine confer anti-hyperlipidemic effects. Life Sciences. 2021;278:119579. doi: 10.1016/j.lfs.2021.119579. [DOI] [PubMed] [Google Scholar]
- 47.Chen W, Guo J, Zhang Y, Zhang J. The beneficial effects of taurine in preventing metabolic syndrome. Food Funct. 2016;7:1849–63. doi: 10.1039/C5FO01295C. [DOI] [PubMed] [Google Scholar]
- 48.Schonfeld G. Lipoproteins in atherogenesis. Artery. 1979;5:305–29. [PubMed] [Google Scholar]
- 49.Sherwani SI, Khan HA, Ekhzaimy A, Masood A, Sakharkar MK. Significance of HbA1c test in diagnosis and prognosis of diabetic patients. Biomark Insights. 2016;11:95–104. doi: 10.4137/BMI.S38440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.GRAS Exemption Claim for Taurine for Use in Enhanced Water Beverages GRAS Notice (GRN), vol. 2023, No. 822. edn. U.S. Food & Drug Administration. GRAS Notice Inventory.
- 51.Williams LL, Lopez LM, Thorman AD, Quay GP, Stein GH, Mehta JL. Plasma lipid profiles and antihypertensive agents: effects of lisinopril, enalapril, nitrendipine, hydralazine, and hydrochlorothiazide. Drug Intell Clin Pharm. 1988;22:546–50. doi: 10.1177/106002808802200704. [DOI] [PubMed] [Google Scholar]
- 52.Ames R. Effects of diuretic drugs on the lipid profile. Drugs. 1988;36:33–40. doi: 10.2165/00003495-198800362-00007. [DOI] [PubMed] [Google Scholar]
- 53.Andreacchi AT, Griffith LE, Guindon GE, Mayhew A, Bassim C, Pigeyre M, et al. Body mass index, waist circumference, waist-to-hip ratio, and body fat in relation to health care use in the Canadian Longitudinal Study on Aging. Int J Obes. 2021;45:666–76. doi: 10.1038/s41366-020-00731-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data included in this study are shown in this article or supplementary information, any further request is available by contacting the corresponding authors.