Abstract
Background
Sphingolipids are implicated in neurodegeneration and neuroinflammation. We assessed the potential role of circulating ceramides and sphingomyelins in subclinical brain pathology by investigating their association with brain magnetic resonance imaging (MRI) measures and circulating biomarkers of brain injury, neurofilament light chain (NfL) and glial fibrillary acidic protein (GFAP) in the Cardiovascular Health Study (CHS), a large and intensively phenotyped cohort of older adults.
Methods
Brain MRI was offered twice to CHS participants with a mean of 5 years between scans, and results were available from both time points in 2,116 participants (mean age 76 years; 40% male; and 25% APOE ε4 allele carriers). We measured 8 ceramide and sphingomyelin species in plasma samples and examined the associations with several MRI, including worsening grades of white matter hyperintensities and ventricular size, number of brain infarcts, and measures of brain atrophy in a subset with quantitative measures. We also investigated the sphingolipid associations with serum NfL and GFAP.
Results
In the fully adjusted model, higher plasma levels of ceramides and sphingomyelins with a long (16-carbon) saturated fatty acid were associated with higher blood levels of NfL [β = 0.05, false-discovery rate corrected P (PFDR) = 0.004 and β = 0.06, PFDR = < 0.001, respectively]. In contrast, sphingomyelins with very long (20- and 22-carbon) saturated fatty acids tended to have an inverse association with levels of circulating NfL. In secondary analyses, we found an interaction between ceramide d18:1/20:0 and sex (P for interaction = <0.001), such that ceramide d18:1/20:0 associated with higher odds for infarcts in women [OR = 1.26 (95%CI: 1.07, 1.49), PFDR = 0.03]. We did not observe any associations with GFAP blood levels, white matter grade, ventricular grade, mean bilateral hippocampal volume, or total brain volume.
Conclusion
Overall, our comprehensive investigation supports the evidence that ceramides and sphingomyelins are associated with increased aging brain pathology and that the direction of association depends on the fatty acid attached to the sphingosine backbone.
Keywords: white matter hyperintensity, ventricular size, hippocampal atrophy, brain infarcts, neurofilament light chain, glial fibrillary acidic protein, ceramide, sphingomyelin
Introduction
Dementia is a major global public health emergency, and identifying its pathophysiological risk factors remains a key scientific priority (1–3). Subclinical vascular brain injury such as covert infarction and white matter changes contribute to cognitive decline and dementia (4–7). Additionally, cerebral small vessel disease (CSVD) has emerged as a major cause of stroke and the leading contributor to vascular dementia (8).
Sphingolipids are a group of lipids characterized by a sphingosine backbone to which a fatty acid is acylated. They are especially concentrated in myelin sheath in the brain and relay important signals for intra- and intercellular events (9–11). Circulating levels of sphingolipids have been associated with both cerebrovascular and neurodegenerative diseases such as stroke and dementia (12–14). One family of sphingolipids, the ceramides (Cer) has been shown to accumulate in tissues such as the liver and brain during aging (15–17), and emerging evidence indicates that Cer are the most dysregulated lipids in cerebrovascular diseases, including stroke and CSVD (18–21). Blood levels of Cer and sphingomyelins (SM), another class of sphingolipids that can be metabolized to Cer, have both been associated with hippocampal atrophy, white matter hyperintensities, and white matter microstructural changes (22–24).
Cer and SM with different fatty acid chain lengths may exhibit distinct biological activities (25, 26). Indeed, we have shown that higher levels of circulating Cer and SM with a saturated fatty acid chain length of 16-carbon (Cer-16 and SM-16) are directly associated with cardiovascular disease and mortality, while circulating Cer and SM with 22- and 24-carbon saturated fatty acid chain length (Cer-22, Cer-24, SM-22, SM-24) are inversely associated (27–29).
Here, we address the role of Cer and SM in subclinical brain pathology by investigating their association with a broad range of measures in the Cardiovascular Health Study (CHS), a large and extensively phenotyped cohort of older adults. Based on previous observations, we hypothesize that Cer and SM with different saturated fatty acids will differ in associations, thus this study focus on 4 Cer species with the fatty acids palmitic acid, arachidic acid, behenic acid and lignoceric acid (Cer-16, Cer-20, Cer-22, Cer-24, respectively) and 4 analogous SM species (SM-16, SM-20, SM-22, SM-24). We examined several magnetic resonance imaging (MRI) measurements such as white matter hyperintensities, ventricular size, and brain atrophy, and two circulating biomarkers of brain injury, neurofilament light chain (NfL) and glial fibrillary acidic protein (GFAP), to gain a comprehensive view of the associations of 8 sphingolipid species with these measures.
Methods
Study population
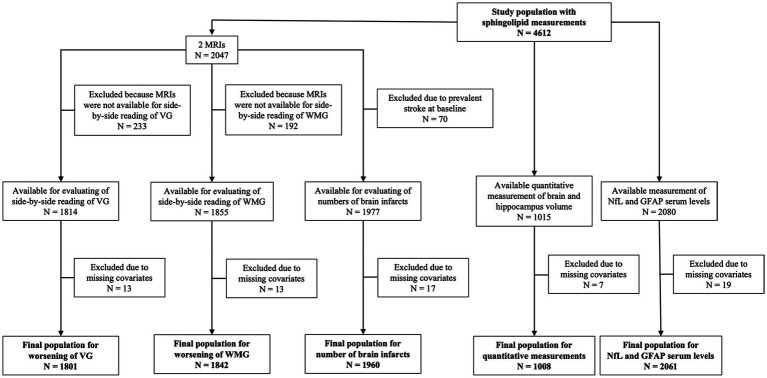
The CHS began in 1989–1990 and recruited 5,201 women and men aged 65 years in 4 different US communities: Forsyth County, NC; Sacramento County, CA; Washington County, MD; Allegheny CA. In 1992–1993, an additional 687 predominantly African American participants were recruited in 3 of the 4 original communities. Potential participants were randomly identified from Medicare eligibility lists. The study design and sampling methods have been previously described in detail (30). During the first decade of the study, participants underwent annual clinic visits, followed by semiannual phone contacts thereafter. Each clinic visit involved comprehensive physical examinations and the collection of demographics, anthropometry, blood pressure, psychosocial interviews, depression assessments, medical history, health behaviors, physical function evaluations, hematology, laboratory examinations, and medication records. For the present analyses, sphingolipids were measured in plasma samples from 586 participants from 1992 to 1993 visit and 4,026 participants from the 1994–1995 visit (total N = 4,612). The clinic visit from which sphingolipids were measured (1992–1993 or 1994–1995) formed the study baseline in the analyses. Figure 1 shows the flow and number of participants included in each of our analyses.
Figure 1.
Flowchart of study populations. GFAP, glial fibrillary acidic protein; MRI, magnetic resonance imaging; NfL, neurofilament light chain; VG, ventricular grade; WMG, white matter grade.
Measurements of sphingolipids
Measurement of sphingolipid species were performed using EDTA-plasma samples that had been stored at −70°C. Plasma lipids were extracted, and sphingolipids quantified by liquid chromatography–tandem mass spectrometry. Previous work suggests that sphingolipid species are robust to storage at −70°C (31). A comprehensive description of the methods and quality control for sphingolipid measurement, including details on lipid extraction, sample plating, instrumentation, internal standards, and normalization, can be found in previous publications (27, 32). In the current study, we restricted analyses to 8 primary sphingolipids. This included 4 Cer species with the fatty acids palmitic acid, arachidic acid, behenic acid and lignoceric acid (Cer-16, Cer-20, Cer-22, Cer-24, respectively) and 4 analogous SM species (SM-16, SM-20, SM-22, SM-24).
Brain imaging
Brain MRI was offered twice to CHS participants in 1992–1994 and 1997–1999, as described earlier (33, 34). Results were available from both time points in 2,116 participants with a mean of 5 years between scans. Non-contrast MRI imaging included sagittal T1-weighted, spin-density, and T2-weighted images with 5-mm thickness and no interslice gaps (35). At a single reading center, neuroradiologists rated the MRI scans blinded to study information (33).
White matter hyperintensities
White matter hyperintensities (WMH) were identified on either axial T2-weighted or spin-density images scans and graded on a similar 10-point semiquantitative white matter grade (WMG), ranging from 0 (least) to 9 (most abnormal). WMG had an inter-reader interclass kappa correlation coefficient of 0.76 and intra-reader kappa coefficient of 0.89 (36, 37). To assess white matter grades over time, we used MRIs eligible for side-by-side re-reading. Because of technical problems, 197 of the original 2,116 pairs of scans could not be re-read, leaving 1,919 (91%) pairs (38). To document worsening WMG of 1 or more grades (yes vs. no), the neuroradiologists read all scans from the same participant side-by-side without knowledge of which scan was performed first (38).
Ventricular grade
Ventricular sizes were estimated from the T1-weighted axial images. The size of the lateral ventricles was estimated by the radiologist based on a referenced standard to create a ventricular grade (VG) ranging from 0 (least) to 9 (most abnormal) (39). Intra-reader agreement within 1 grade was 94% with a kappa of 0.89 for the VG (39). Worsening of VG by one or more grades (yes vs. no) was determined by neuroradiologists in side-by-side reading without knowledge of which scan was performed first.
Total brain volume and mean bilateral hippocampi volume
A subgroup of the follow-up MRIs was performed using a 1.5-tesla scanner and included 3D T1-weighted spoiled gradient-recall sequences, as described previously (40). These scans were processed with FreeSurfer software, which converted high-resolution T1-weighted images into 1x1x1 mm3 voxels and generated a surface-based reconstruction of the brain to estimate the quantitative volume of the hippocampus and total intracranial space, which were then converted to normalized brain volume (1-NBV) units. Volumetric measurements from the right and left hippocampus were averaged to compute the mean bilateral hippocampal volume.
Brain infarcts
Brain infarcts were defined as abnormal signal intensities 3 mm in size or larger, identified by neuroradiologists on MRI scans. Number of brain infarcts was defined as one or more new infarcts on the follow-up MRI.
Quantification of blood NfL and GFAP
Frozen fasting serum samples collected from participants at the 1996–1997 clinic visit were used to measure NfL and GFAP. The CHS Central Laboratory at the University of Vermont measured NfL and GFAP using the 4th generation single-molecule array Simoa™ Human Neurology 4-Plex A assay (N4PA, Quanterix™). As the measurements were funded as part of a metabolic ancillary study to CHS, they were limited to participants who underwent an oral glucose tolerance test that excluded participants with treated diabetes. The inter-assay coefficients of variation were 9.3% for NfL and 8.2% for GFAP.
Covariates
Information on age (years), race/ethnicity (Caucasian, African American), education (years), physical activity (kcal/week), alcohol intake (drinks/week), smoking status (never, former, current), medication-use, and CESD Depression score was based on self-report. Weight, height, and blood pressure were measured using standardized protocols. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared (kg/m2). Hypertension was defined by systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg or use of antihypertensive medication. Plasma lipids and glucose were measured on fasting blood samples using standard protocols (30). Prevalent diabetes mellitus was defined by a fasting glucose concentration of ≥7 mmol/L (126 mg/dL) or use of insulin or oral hypoglycemic agents. History of stroke and coronary heart disease was obtained from medical records, participant and/or proxy reports and adjudicated by committee (41, 42).
Statistical analysis
Baseline characteristics were presented for continuous variables as mean (SD) and for categorical variables as numbers (percentages). Sphingolipid species concentrations were log-transformed to reduce skewness. We used non-parametric testing to estimate correlations between sphingolipid species.
Logistic regression was used to assess associations with the dichotomous outcomes of VG and WMG worsening on follow-up MRI, using each sphingolipid specie concentration as a continuous variable [per one standard deviation (SD) in log sphingolipid species concentration (μM)]. We used ordinal logistic regression to model the associations with number of infarcts, where the number of infarcts was categorized as 0, 1 to 2, and ≥3 based on evaluation of the proportional odds assumption using a score test. We excluded all participants with prevalent stroke (N = 70) at baseline for this analysis. These analyses were initially adjusted for age (years), sex, race/ethnicity (Caucasian, African American), education (years), field center (4 sites), and baseline year (1992–1993 or 1994–1995) (Model 1). Next, we additionally included BMI (kg/m2), physical activity (kcal/week), alcohol intake (drinks/week), smoking status (never, former, current), depression score (continuous), diabetes (yes/no), coronary heart disease (yes/no), hypertension (yes/no), lipid-lowering medication use (yes/no), HDL-cholesterol (mg/dL), and LDL-cholesterol (mg/dL) (Model 2). All covariates were assessed at the respective baseline visit. Due to mutual correlation and contrasting biological properties, a third model was included in which Cer/SM with an acylated 16-carbon fatty acid were adjusted for Cer/SM with 22-carbon fatty acid, and Cer/SM with 20-, 22-, 24-carbon fatty acids were each adjusted for Cer/SM with a 16-carbon fatty acid (Model 3). This approach has been reported elsewhere (27–29, 43).
The associations with total brain volume and mean bilateral hippocampi volume were assessed using linear regression, adjusted for the same covariates as above and estimated total intracranial volume. The associations with circulating NfL and GFAP were also analyzed using linear regression models. Due to skewness, we winsorized 10 GFAP outliers to the value of 1,000 pg./mL and 9 NfL outliers to the value of 250 pg./mL and transformed their values to the natural logarithm (lognNfl or lognGFAP).
In secondary analyses, we tested for interactions with sex and race/ethnicity of the associations of sphingolipids with brain pathology by inclusion of multiplicative interaction terms in the respective models. Lastly, we performed stratified analyses based on APOE genotype, as APOEε4 carriers have increased risk of development of neurodegenerative diseases such as dementia. Analyses were limited to those with available DNA who consented to genetic studies.
To correct the analyses for multiple comparisons, we applied a false discovery rate (FDR) p-value correction in which p = 0.05 was considered significant. All statistical analyses were conducted using R version 4.1.3 (44).
Results
Figure 1 shows the number of CHS participants in each analysis, based upon outcome and exclusions ranging from N = 1,008 for quantitative analyses of brain volumes to N = 2,061 for circulating NfL and GFAP. No major differences in population characteristics between the five groups included in the different analyses were observed. Mean age was 76 years, and 57.9% to 60.4% of participants were female and 12.6% to 15.7% were African American, depending on the analyses (Supplementary Table S1). The measured sphingolipid species showed moderate inter-correlations, with stronger correlations observed between sphingolipids with very long chain fatty acid (20-, 22-, and 24-carbon) and coefficients of variation estimated over the whole study period ranged from 5.9 to 18.6% (Supplementary Table S2).
No associations with volumetric measurements, WMG, or VG worsening over 5 years
We observed no clear associations for any of the sphingolipid species with worsening in WMG, decrease in total brain volume, or mean bilateral hippocampal volume. In the fully adjusted model, elevated plasma levels of Cer-16 and SM-16 were associated with higher odds of worsening in VG, but this finding was not statistically significant upon p-value correction. In contrast, Cer-22 was associated with lower odds of worsening in VG, which also became non-significant upon p-value correction (data not shown).
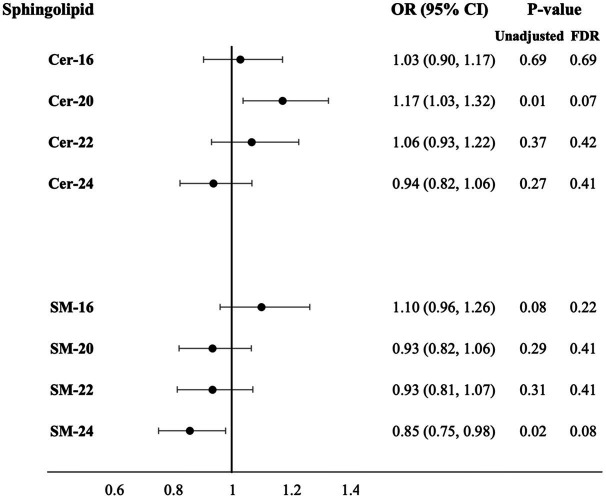
Cer-20 associates with higher number of brain infarcts in women
Higher plasma levels of Cer-20 associated with increased odds for more brain infarcts in Model 1 [OR = 1.18 (1.07, 1.31), PFDR = 0.008] and 2 [OR = 1.16 (1.04, 1.29), PFDR = 0.03], which attenuated upon p-value correction in Model 3 (OR = 1.17 (1.03, 1.32), PFDR = 0.07; Figure 2; Supplementary Table S3 for estimates of Model 1 and Model 2). In sex-stratified analysis, Cer-20 was associated with 26% higher odds [OR = 1.26 (1.07, 1.49), PFDR = 0.03, P for interaction = <0.001] in women and not in men (Table 1).
Figure 2.
Adjusted odds ratios for increase in number of brain infarcts per SD higher plasma log-sphingolipid concentrations, N = 1,960. Cer, ceramide; CI, confidence interval; FDR, false discovery rate; OR, odds ratio; SD, standard deviation; SM, sphingomyelins; 16, 20, 22, and 24 stand for the number of carbons of the saturated fatty acid acylated to the sphingolipid backbone. Odds ratios adjusted for age, sex, race/ethnicity, education, field center, year of blood sample measurement, BMI, physical activity, alcohol intake, smoking status, depression score, prevalent diabetes, prevalent coronary heart disease, prevalent hypertension, lipid-lowering medication use, HDL-cholesterol, LDL-cholesterol and mutual adjustment of sphingolipids with 16-carbon fatty acid chains with corresponding sphingolipids with 22-carbon fatty acid chains and sphingolipid with 20-, 22-, 24-carbon fatty acid chains with sphingolipids with 16-carbon fatty acid chains (Model 3).
Table 1.
Adjusted odds ratios for increase in number of brain infarcts per SD higher plasma log-sphingolipid concentrations in sex-stratified analysis.
| Sphingolipid | OR for increase in number of brain infarcts (95% CI) | |||||
|---|---|---|---|---|---|---|
| Women (N = 1,175) | Men (N = 785) | |||||
| Model 3 | P-value | Model 3 | P-value | |||
| Unadjusted | FDR | Unadjusted | FDR | |||
| Cer-16 | 0.97 (0.82, 1.15) | 0.74 | 0.82 | 1.11 (0.90, 1.36) | 0.34 | 0.75 |
| Cer-20 | 1.26 (1.07, 1.49) | 0.004 | 0.03 | 1.06 (0.88, 1.28) | 0.50 | 0.75 |
| Cer-22 | 1.14 (0.96, 1.37) | 0.14 | 0.22 | 0.96 (0.77, 1.20) | 0.75 | 0.75 |
| Cer-24 | 0.98 (0.83, 1.16) | 0.82 | 0.82 | 0.87 (0.71, 1.07) | 0.14 | 0.75 |
| SM-16 | 1.15 (0.96, 1.37) | 0.05 | 0.10 | 1.03 (0.82, 1.29) | 0.71 | 0.75 |
| SM-20 | 0.83 (0.69, 0.99) | 0.03 | 0.09 | 1.09 (0.89, 1.34) | 0.40 | 0.75 |
| SM-22 | 0.92 (0.77, 1.10) | 0.38 | 0.50 | 0.94 (0.75, 1.17) | 0.57 | 0.75 |
| SM-24 | 0.82 (0.70, 0.98) | 0.03 | 0.09 | 0.90 (0.73, 1.12) | 0.34 | 0.75 |
Cer, ceramide; CI, confidence interval; FDR, false discovery rate; OR, odds ratio; SM, sphingomyelins; 16, 20, 22, and 24 stand for the number of carbons of the saturated fatty acid acylated to the sphingolipid backbone. Model 3 adjusted for age, sex, race/ethnicity, education, field center, year of blood sample measurement, BMI, physical activity, alcohol intake, smoking status, depression score, prevalent diabetes, prevalent coronary heart disease, prevalent hypertension, lipid-lowering medication use, HDL-cholesterol, and LDL-cholesterol, mutual adjustment of sphingolipids with 16-carbon fatty acid chains with sphingolipids with 22-carbon fatty acid chains and sphingolipids with 20-, 22-, 24-carbon fatty acid chains with sphingolipids with 16-carbon fatty acid chains. Cer-20, P for sex interaction = <0.001.
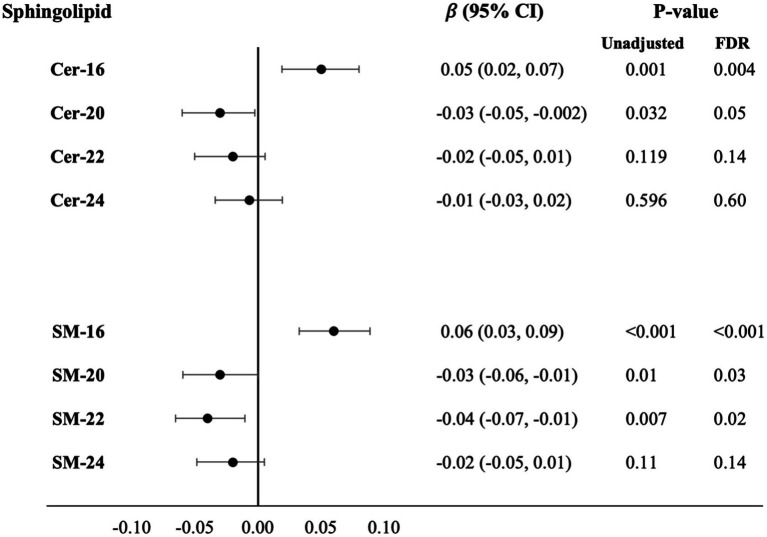
Cer-16 and SM-16 associates with higher circulating NfL levels
Higher plasma levels of Cer and SM with long fatty acids were associated with higher levels of circulating NfL: Cer-16 (β = 0.05, PFDR = 0.004) and SM-16 (β = 0.06, PFDR = <0.001). In contrast, higher levels of several Cer and SM with very long chain fatty acids were associated with lower levels of circulating NfL; Cer-20 (β = −0.03, PFDR = 0.05), SM-20 (β = −0.03, PFDR = 0.03), and SM-22 (β = −0.04, PFDR = 0.02; Figure 3; Supplementary Table S4 for estimates of Model 1 and Model 2). In APOE genotype stratified analysis, higher levels of SM-16 in APOE ε4 carriers associated with higher levels of circulating NfL (β = 0.07, PFDR = 0.03), whereas the association was not statistically significant in APOE ε4 non-carriers (β = 0.03, PFDR = 0.22; Supplementary Table S5). For associations with levels of circulation GFAP, no associations were observed after covariate adjustments and p-value correction.
Figure 3.
Cross-sectional associations with difference in serum NfL levels per SD higher plasma log-sphingolipid concentrations, N = 2,061. Cer, ceramide; CI, confidence interval; FDR, false discovery rate; NfL, neurofilament light chain; SD, standard deviation; SM, sphingomyelins; 16, 20, 22, and 24 stand for the number of carbons of the saturated fatty acid acylated to the sphingolipid backbone. Linear regression beta coefficients adjusted for age, sex, race/ethnicity, education, field center, year of blood sample measurement, BMI, physical activity, alcohol intake, smoking status, depression score, prevalent diabetes, prevalent coronary heart disease, prevalent hypertension, lipid-lowering medication use, HDL-cholesterol, LDL-cholesterol and mutual adjustment of sphingolipids with 16-carbon fatty acid chains with corresponding sphingolipids with 22-carbon fatty acid chains and sphingolipid with 20-, 22-, 24-carbon fatty acid chains with sphingolipids with 16-carbon fatty acid chains (Model 3).
Discussion
Overall, our comprehensive investigation supports the evidence that Cer and SM species with a bound (acylated) 16-carbon long fatty acid are associated with increased signs of degenerative changes on MRI. In this prospective study in older adults, higher plasma levels of Cer-16 and SM-16 were associated with higher blood levels of NfL, a biomarker of brain injury, and tended to associate with worsening of VG over 5 years, however, non-significant upon adjustment for multiple comparisons. In APOE ε4 carriers, higher levels of SM-16 associated with higher levels of circulating NfL, and no associations were observed in APOE ε4 non-carriers. Additionally, Cer and SM with a very long (20- and 22-carbon) chain fatty acids show an inverse association with levels of circulating NfL. However, Cer-20 also associated with higher odds for infarcts in women in secondary analyses. We did not observe any associations with levels of GFAP, WMG or mean bilateral hippocampal volume.
Previous epidemiological studies have found that higher plasma levels of Cer-16 associate with greater WMH volumes (23) and higher plasma levels of Cer-22 and Cer-24 associate with greater right hippocampal volume loss 1 year later in 17 patients with amnesiac mild cognitive impairment (22). We did not confirm either of these associations, although we did find that Cer-16 appears to be associated with ongoing brain injury and global atrophy. Part of the difference in these results may relate to the prospective nature of our white matter findings, which specifically examined progression late in life. Another possibility might be that Cer-16 confounded the associations of Cer-22 and Cer-24.
To the best of our knowledge, our results are novel, as no previous study has reported on the associations of sphingolipids and VG or blood levels of NfL. Neuronal cell death is a hallmark of brain atrophy, including ventricular enlargement. Ceramides have been implicated in promoting apoptosis and neuronal loss through various mechanisms, including mitochondrial dysfunction, activation of pro-apoptotic pathways, and disruption of neuronal survival signaling (45, 46). Both Cer-16 and SM-16 have been associated with neurodegenerative diseases such as dementia including Alzheimer’s disease (22, 47–55), Huntington’s and Parkinson’s disease (56–59) as well as neuroinflammation (60, 61). Our study also showed that Cer-16 and SM-16, but not other species of Cer and SM, associated with higher levels of NfL in blood, which is a nonspecific biomarker for neuronal damage. Recent studies in CHS have shown that serum NfL and GFAP were associated with substantially higher risk of incident dementia and dementia mortality (62) and WMG worsening over 5 years (37). Altered sphingolipid metabolism may similarly reflects increased neurodegeneration, neuroinflammation or both and might be valuable as a biomarker in conjunction with NfL and GFAP. The reason for the differences observed in our findings regarding NfL and GFAP is unclear. However, our previous research has shown a tendency for NfL to exhibit a higher correlation with vascular brain injuries compared to GFAP in CHS (37).
Our findings provide crucial evidence that the direction of association of Cer and SM with several brain pathology outcome depends on the fatty acid attached to the sphingosine backbone. Our research demonstrates that elevated baseline plasma levels of Cer-16 and SM-16 are associated with a higher degree of brain pathology, while higher baseline plasma levels of Cer and SM with very-long chain fatty acids are independently inversely associated. Several cell and animal studies suggest that Cer have different biological properties dependent on the length of the fatty acid chain (25, 26). Diverse Cer can alter cell membrane properties, where fluidity increases with higher levels of Cer-16 and lower amounts of Cer-22 and Cer-24 (25, 63). The enzyme ceramide synthases 2 (CerS2) produces very-long chain ceramides and a study in CerS2 null mice showed that these mice had defective myelin sheath with lower levels of Cer-24 and higher levels Cer-16 (64). Another study of downregulation of CerS2 observed induced cell autophagy with a reduction in the levels of Cer-24 and increase in Cer-16 (65). Overexpression of ceramide synthases 6 (CerS6), which produces long-chain ceramides (C14-C18), increased the secretion of the inflammatory cytokine TNF-α in the liver (66). In addition, several studies report that Cer-16 promotes apoptosis, while Cer-22 and Cer-24 appear to protect from apoptosis (14, 25, 26, 67, 68). Most studies have investigated ceramides; however, ceramides and sphingomyelins are highly interrelated and can be generated from each other.
This study has several strengths, which involve the large sample size, and the comprehensive assessment of multiple complementary subclinical brain pathology measurements including two MRIs performed 5 years apart, enabling longitudinal analysis. We also included circulating neuronal biomarkers to complement the radiological findings. Several limitations include the study participants being older and primarily Caucasian, thus our findings may not generalize to other ethnicities or younger populations. Additionally, the MRI measurements in CHS were performed in the 1990s and did not include imaging of fractional anisotropy. Also, additional brain imaging studies, such as PET scanning for proteinopathies, were not performed.
Lastly, further studies are warranted to determine whether the timing of the measurements (midlife vs. late life) is important. The levels of sphingolipids change with age (69–71), thus multiple measurements over a long time will be needed to fully understand the dynamics of sphingolipid metabolism with relation to disease.
Conclusion
In this study of older adults, Cer and SM differed in association with several measurements of subclinical brain pathology including number of brain infarcts, and NfL blood levels, which were dependent on the length of the fatty acid chain bound. Confirmation of these results in other studies with repetitive measurements of sphingolipids, MRI, levels of circulating NfL and GFAP is necessary to establish their role biomarkers of degenerative and vascular brain injury.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: access to data can be done through CHS. Requests to access these datasets should be directed to CHS-NHLBI.org.
Ethics statement
The studies involving humans were approved by the Institutional Review Board at University of Washington. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
KFM: Conceptualization, Formal analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing. JH: Writing – original draft, Writing – review & editing. AF: Writing – original draft, Writing – review & editing. LD: Writing – original draft, Writing – review & editing. WL: Writing – original draft, Writing – review & editing. OL: Writing – original draft, Writing – review & editing. AH: Writing – original draft, Writing – review & editing. MJ: Supervision, Writing – original draft, Writing – review & editing. RL: Writing – original draft, Writing – review & editing. KJM: Supervision, Writing – original draft, Writing – review & editing.
Acknowledgments
A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and 75N92021D00006 and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). Support for individual investigators include K24AG065525 from the NIA to KJM and the Novo Nordic Foundation Challenge Programme: Harnessing the Power of Big Data to Address the Societal Challenge of Aging (NNF17OC0027812) to KFM and MJ.
Abbreviations
Cer, Ceramide; GFAP, Glial fibrillary acidic protein; MRI, Magnetic resonance imaging; NfL, Neurofilament light chain; SM, Sphingomyelin; VG, Ventricular grade; WMH, White matter hyperintensities
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1385623/full#supplementary-material
References
- 1.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of Disease study 2017. Lancet. (2018) 392:1789–858. doi: 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.2021 Alzheimer's disease facts and figures. Alzheimers Dement. (2021) 17:327–406. doi: 10.1002/alz.12328 [DOI] [PubMed] [Google Scholar]
- 3.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet. (2020) 396:413–46. doi: 10.1016/S0140-6736(20)30367-6, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emery VO, Gillie EX, Smith JA. Noninfarct vascular dementia and Alzheimer dementia spectrum. J Neurol Sci. (2005) 229-230:27–36. doi: 10.1016/j.jns.2004.11.016, PMID: [DOI] [PubMed] [Google Scholar]
- 5.Ott A, Breteler MMB, van Harskamp F, Claus JJ, van der Cammen TJM, Grobbee DE, et al. Prevalence of Alzheimer's disease and vascular dementia: association with education. The Rotterdam study. BMJ. (1995) 310:970–3. doi: 10.1136/bmj.310.6985.970, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Román GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN international workshop. Neurology. (1993) 43:250–60. doi: 10.1212/WNL.43.2.250 [DOI] [PubMed] [Google Scholar]
- 7.Corriveau RA, Bosetti F, Emr M, Gladman JT, Koenig JI, Moy CS, et al. The science of vascular contributions to cognitive impairment and dementia (VCID): a framework for advancing research priorities in the cerebrovascular biology of cognitive decline. Cell Mol Neurobiol. (2016) 36:281–8. doi: 10.1007/s10571-016-0334-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lam BYK, Cai Y, Akinyemi R, Biessels GJ, van den Brink H, Chen C, et al. The global burden of cerebral small vessel disease in low- and middle-income countries: a systematic review and meta-analysis. Int J Stroke. (2023) 18:15–27. doi: 10.1177/17474930221137019, PMID: [DOI] [PubMed] [Google Scholar]
- 9.Gault CR, Obeid LM, Hannun YA. Sphingolipids as Signaling and regulatory molecules. New York: Springer; (2010). [Google Scholar]
- 10.Zheng W, Kollmeyer J, Symolon H, Momin A, Munter E, Wang E, et al. Ceramides and other bioactive sphingolipid backbones in health and disease: lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochim Biophys Acta. (2006) 1758:1864–84. doi: 10.1016/j.bbamem.2006.08.009, PMID: [DOI] [PubMed] [Google Scholar]
- 11.Gault CR, Obeid LM, Hannun YA. An overview of sphingolipid metabolism: from synthesis to breakdown. Adv Exp Med Biol. (2010) 688:1–23. doi: 10.1007/978-1-4419-6741-1_1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan H, Zhu B, Li C, Zhao Z. Ceramide in cerebrovascular diseases. Front Cell Neurosci. (2023) 17:1191609. doi: 10.3389/fncel.2023.1191609, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baloni P, Arnold M, Buitrago L, Nho K, Moreno H, Huynh K, et al. Multi-Omic analyses characterize the ceramide/sphingomyelin pathway as a therapeutic target in Alzheimer's disease. Commun Biol. (2022) 5:1074. doi: 10.1038/s42003-022-04011-6, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crivelli SM, Giovagnoni C, Visseren L, Scheithauer AL, de Wit N, den Hoedt S, et al. Sphingolipids in Alzheimer's disease, how can we target them? Adv Drug Deliv Rev. (2020) 159:214–31. doi: 10.1016/j.addr.2019.12.003 [DOI] [PubMed] [Google Scholar]
- 15.Cutler RG, Mattson MP. Sphingomyelin and ceramide as regulators of development and lifespan. Mech Ageing Dev. (2001) 122:895–908. doi: 10.1016/S0047-6374(01)00246-9, PMID: [DOI] [PubMed] [Google Scholar]
- 16.Lightle SA, Oakley JI, Nikolova-Karakashian MN. Activation of sphingolipid turnover and chronic generation of ceramide and sphingosine in liver during aging. Mech Ageing Dev. (2000) 120:111–25. doi: 10.1016/S0047-6374(00)00191-3, PMID: [DOI] [PubMed] [Google Scholar]
- 17.Trayssac M, Hannun YA, Obeid LM. Role of sphingolipids in senescence: implication in aging and age-related diseases. J Clin Invest. (2018) 128:2702–12. doi: 10.1172/JCI97949, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gui YK, Li Q, Liu L, Zeng P, Ren RF, Guo ZF, et al. Plasma levels of ceramides relate to ischemic stroke risk and clinical severity. Brain Res Bull. (2020) 158:122–7. doi: 10.1016/j.brainresbull.2020.03.009, PMID: [DOI] [PubMed] [Google Scholar]
- 19.Ighodaro ET, Graff-Radford J, Syrjanen JA, Bui HH, Petersen RC, Knopman DS, et al. Associations between plasma ceramides and cerebral microbleeds or lacunes. Arterioscler Thromb Vasc Biol. (2020) 40:2785–93. doi: 10.1161/ATVBAHA.120.314796, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hannawi Y, Yanek LR, Kral BG, Becker LC, Vaidya D, Haughey NJ, et al. White matter Injury is associated with reduced manual dexterity and elevated serum ceramides in subjects with cerebral small vessel Disease. Cerebrovasc Dis. (2021) 50:100–7. doi: 10.1159/000511937, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee TH, Cheng CN, Chao HC, Lee CH, Kuo CH, Tang SC, et al. Plasma ceramides are associated with outcomes in acute ischemic stroke patients. J Formos Med Assoc. (2022) 121:43–50. doi: 10.1016/j.jfma.2021.01.006, PMID: [DOI] [PubMed] [Google Scholar]
- 22.Mielke MM, Haughey NJ, Bandaru VVR, Schech S, Carrick R, Carlson MC, et al. Plasma ceramides are altered in mild cognitive impairment and predict cognitive decline and hippocampal volume loss. Alzheimers Dement. (2010) 6:378–85. doi: 10.1016/j.jalz.2010.03.014, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mielke MM, Syrjanen JA, Bui HH, Petersen RC, Knopman DS, Jack CR, et al. Elevated plasma ceramides are associated with higher white matter hyperintensity volume-brief report. Arterioscler Thromb Vasc Biol. (2019) 39:2431–6. doi: 10.1161/ATVBAHA.119.313099, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez CE, Venkatraman VK, An Y, Landman BA, Davatzikos C, Ratnam Bandaru VV, et al. Peripheral sphingolipids are associated with variation in white matter microstructure in older adults. Neurobiol Aging. (2016) 43:156–63. doi: 10.1016/j.neurobiolaging.2016.04.008, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mu J, Lam SM, Shui G. Emerging roles and therapeutic potentials of sphingolipids in pathophysiology --emphasis on fatty acyl heterogeneity. J Genet Genomics. (2023) 51:268–78. doi: 10.1016/j.jgg.2023.06.006 [DOI] [PubMed] [Google Scholar]
- 26.Grosch S, Schiffmann S, Geisslinger G. Chain length-specific properties of ceramides. Prog Lipid Res. (2012) 51:50–62. doi: 10.1016/j.plipres.2011.11.001, PMID: [DOI] [PubMed] [Google Scholar]
- 27.Lemaitre RN, Jensen PN, Hoofnagle A, McKnight B, Fretts AM, King IB, et al. Plasma ceramides and sphingomyelins in relation to heart failure risk. Circ Heart Fail. (2019) 12:e005708. doi: 10.1161/CIRCHEARTFAILURE.118.005708, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jensen PN, Fretts AM, Hoofnagle AN, Sitlani CM, McKnight B, King IB, et al. Plasma ceramides and sphingomyelins in relation to atrial fibrillation risk: the cardiovascular health study. J Am Heart Assoc. (2020) 9:e012853. doi: 10.1161/JAHA.119.012853, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fretts AM, Jensen PN, Hoofnagle AN, McKnight B, Sitlani CM, Siscovick DS, et al. Circulating ceramides and sphingomyelins and risk of mortality: the cardiovascular health study. Clin Chem. (2021) 67:1650–9. doi: 10.1093/clinchem/hvab182, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, et al. The cardiovascular health study: design and rationale. Ann Epidemiol. (1991) 1:263–76. doi: 10.1016/1047-2797(91)90005-W [DOI] [PubMed] [Google Scholar]
- 31.Brunkhorst R, Pfeilschifter W, Patyna S, Büttner S, Eckes T, Trautmann S, et al. Preanalytical biases in the measurement of human blood sphingolipids. Int J Mol Sci. (2018) 19:1390. doi: 10.3390/ijms19051390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lemaitre RN, Yu C, Hoofnagle A, Hari N, Jensen PN, Fretts AM, et al. Circulating sphingolipids, insulin, HOMA-IR and HOMA-B: the strong heart family study. Diabetes. (2018) 67:1663–72. doi: 10.2337/db17-1449, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Longstreth WT, Bernick C, Manolio TA, Bryan N, Jungreis CA, Price TR. Lacunar infarcts defined by magnetic resonance imaging of 3660 elderly people: the cardiovascular health study. Arch Neurol. (1998) 55:1217–25. doi: 10.1001/archneur.55.9.1217, PMID: [DOI] [PubMed] [Google Scholar]
- 34.Longstreth WT, Dulberg C, Manolio TA, Lewis MR, Beauchamp NJ, O’Leary D, et al. Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly: the cardiovascular health study. Stroke. (2002) 33:2376–82. doi: 10.1161/01.STR.0000032241.58727.49, PMID: [DOI] [PubMed] [Google Scholar]
- 35.Bryan RN, Manolio TA, Schertz LD, Jungreis C, Poirier VC, Elster AD, et al. A method for using MR to evaluate the effects of cardiovascular disease on the brain: the cardiovascular health study. AJNR Am J Neuroradiol. (1994) 15:1625–33. PMID: [PMC free article] [PubMed] [Google Scholar]
- 36.Longstreth WT, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. Stroke. (1996) 27:1274–82. doi: 10.1161/01.STR.27.8.1274, PMID: [DOI] [PubMed] [Google Scholar]
- 37.Fohner AE, Bartz TM, Tracy RP, Adams HHH, Bis JC, Djousse L, et al. Association of Serum Neurofilament Light Chain Concentration and MRI findings in older adults: the cardiovascular health study. Neurology. (2022) 98:e903–11. doi: 10.1212/WNL.0000000000013229, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Longstreth WT, Arnold AM, Beauchamp NJ, Manolio TA, Lefkowitz D, Jungreis C, et al. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: the cardiovascular health study. Stroke. (2005) 36:56–61. doi: 10.1161/01.STR.0000149625.99732.69, PMID: [DOI] [PubMed] [Google Scholar]
- 39.Kuller LH, Arnold AM, Longstreth WT, Manolio TA, O’Leary DH, Burke GL, et al. White matter grade and ventricular volume on brain MRI as markers of longevity in the cardiovascular health study. Neurobiol Aging. (2007) 28:1307–15. doi: 10.1016/j.neurobiolaging.2006.06.010, PMID: [DOI] [PubMed] [Google Scholar]
- 40.Raji CA, Lopez OL, Kuller LH, Carmichael OT, Longstreth WT, Gach HM, et al. White matter lesions and brain gray matter volume in cognitively normal elders. Neurobiol Aging. (2012) 33:834.e7–834.e16. doi: 10.1016/j.neurobiolaging.2011.08.010, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Longstreth WT, Bernick C, Fitzpatrick A, Cushman M, Knepper L, Lima J, et al. Frequency and predictors of stroke death in 5,888 participants in the cardiovascular health study. Neurology. (2001) 56:368–75. doi: 10.1212/WNL.56.3.368, PMID: [DOI] [PubMed] [Google Scholar]
- 42.Psaty BM, Delaney JA, Arnold AM, Curtis LH, Fitzpatrick AL, Heckbert SR, et al. Study of cardiovascular health outcomes in the era of claims data: the cardiovascular health study. Circulation. (2016) 133:156–64. doi: 10.1161/CIRCULATIONAHA.115.018610, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jensen PN, Fretts AM, Hoofnagle AN, McKnight B, Howard BV, Umans JG, et al. Circulating ceramides and sphingomyelins and the risk of incident cardiovascular disease among people with diabetes: the strong heart study. Cardiovasc Diabetol. (2022) 21:167. doi: 10.1186/s12933-022-01596-4, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.R Core Team . R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; (2021). [Google Scholar]
- 45.Gulbins E, Li PL. Physiological and pathophysiological aspects of ceramide. Am J Physiol Regul Integr Comp Physiol. (2006) 290:R11–26. doi: 10.1152/ajpregu.00416.2005, PMID: [DOI] [PubMed] [Google Scholar]
- 46.Pettus BJ, Chalfant CE, Hannun YA. Ceramide in apoptosis: an overview and current perspectives. Biochim Biophys Acta. (2002) 1585:114–25. doi: 10.1016/S1388-1981(02)00331-1, PMID: [DOI] [PubMed] [Google Scholar]
- 47.Mielke MM, Bandaru VVR, Haughey NJ, Xia J, Fried LP, Yasar S, et al. Serum ceramides increase the risk of Alzheimer disease: the Women's health and aging study II. Neurology. (2012) 79:633–41. doi: 10.1212/WNL.0b013e318264e380, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mielke MM, Bandaru VVR, Haughey NJ, Rabins PV, Lyketsos CG, Carlson MC. Serum sphingomyelins and ceramides are early predictors of memory impairment. Neurobiol Aging. (2010) 31:17–24. doi: 10.1016/j.neurobiolaging.2008.03.011, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McGrath ER, Himali JJ, Xanthakis V, Duncan MS, Schaffer JE, Ory DS, et al. Circulating ceramide ratios and risk of vascular brain aging and dementia. Ann Clin Transl Neurol. (2020) 7:160–8. doi: 10.1002/acn3.50973, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Toledo JB, Arnold M, Kastenmüller G, Chang R, Baillie RA, Han X, et al. Metabolic network failures in Alzheimer's disease: a biochemical road map. Alzheimers Dement. (2017) 13:965–84. doi: 10.1016/j.jalz.2017.01.020, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huynh K, Lim WLF, Giles C, Jayawardana KS, Salim A, Mellett NA, et al. Concordant peripheral lipidome signatures in two large clinical studies of Alzheimer’s disease. Nat Commun. (2020) 11:5698. doi: 10.1038/s41467-020-19473-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mielke MM, Haughey NJ, Bandaru VVR, Weinberg DD, Darby E, Zaidi N, et al. Plasma sphingomyelins are associated with cognitive progression in Alzheimer's disease. J Alzheimers Dis. (2011) 27:259–69. doi: 10.3233/JAD-2011-110405, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mielke MM, Haughey NJ, Bandaru VVR, Zetterberg H, Blennow K, Andreasson U, et al. Cerebrospinal fluid sphingolipids, beta-amyloid, and tau in adults at risk for Alzheimer's disease. Neurobiol Aging. (2014) 35:2486–94. doi: 10.1016/j.neurobiolaging.2014.05.019, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mielke MM, Haughey NJ, Han D, An Y, Bandaru VVR, Lyketsos CG, et al. The association between plasma ceramides and sphingomyelins and risk of Alzheimer’s Disease differs by sex and APOE in the Baltimore longitudinal study of aging. J Alzheimers Dis. (2017) 60:819–28. doi: 10.3233/JAD-160925, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Varma VR, Oommen AM, Varma S, Casanova R, An Y, Andrews RM, et al. Brain and blood metabolite signatures of pathology and progression in Alzheimer disease: a targeted metabolomics study. PLoS Med. (2018) 15:e1002482. doi: 10.1371/journal.pmed.1002482, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin G, Wang L, Marcogliese PC, Bellen HJ. Sphingolipids in the pathogenesis of Parkinson's Disease and parkinsonism. Trends Endocrinol Metab. (2019) 30:106–17. doi: 10.1016/j.tem.2018.11.003 [DOI] [PubMed] [Google Scholar]
- 57.Esfandiary A, Finkelstein DI, Voelcker NH, Rudd D. Clinical sphingolipids pathway in Parkinson's Disease: from GCase to integrated-biomarker discovery. Cells. (2022) 11:1353. doi: 10.3390/cells11081353, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Phillips GR, Saville JT, Hancock SE, Brown SHJ, Jenner AM, McLean C, et al. The long and the short of Huntington's disease: how the sphingolipid profile is shifted in the caudate of advanced clinical cases. Brain Commun. (2022) 4:fcab303. doi: 10.1093/braincomms/fcab303, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.di Pardo A, Basit A, Armirotti A, Amico E, Castaldo S, Pepe G, et al. De novo synthesis of sphingolipids is defective in experimental models of Huntington's Disease. Front Neurosci. (2017) 11:698. doi: 10.3389/fnins.2017.00698, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morrow A, Panyard DJ, Deming YK, Jonaitis E, Dong R, Vasiljevic E, et al. Cerebrospinal fluid sphingomyelins in Alzheimer's Disease, neurodegeneration, and neuroinflammation. J Alzheimers Dis. (2022) 90:667–80. doi: 10.3233/JAD-220349, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee JY, Jin HK, Bae JS. Sphingolipids in neuroinflammation: a potential target for diagnosis and therapy. BMB Rep. (2020) 53:28–34. doi: 10.5483/BMBRep.2020.53.1.278, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cronjé HT, Liu X, Odden MC, Moseholm KF, Seshadri S, Satizabal CL, et al. Serum NfL and GFAP are associated with incident dementia and dementia mortality in older adults: the cardiovascular health study. Alzheimers Dement. (2023) 19:5672–80. doi: 10.1002/alz.13367, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pewzner-Jung Y, Park H, Laviad EL, Silva LC, Lahiri S, Stiban J, et al. A critical role for ceramide synthase 2 in liver homeostasis: I. Alterations in lipid metabolic pathways. J Biol Chem. (2010) 285:10902–10. doi: 10.1074/jbc.M109.077594, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Imgrund S, Hartmann D, Farwanah H, Eckhardt M, Sandhoff R, Degen J, et al. Adult ceramide synthase 2 (CERS2)-deficient mice exhibit myelin sheath defects, cerebellar degeneration, and hepatocarcinomas. J Biol Chem. (2009) 284:33549–60. doi: 10.1074/jbc.M109.031971, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spassieva SD, Mullen TD, Townsend DM, Obeid LM. Disruption of ceramide synthesis by CerS2 down-regulation leads to autophagy and the unfolded protein response. Biochem J. (2009) 424:273–83. doi: 10.1042/BJ20090699, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim MH, Ahn HK, Lee EJ, Kim SJ, Kim YR, Park JW, et al. Hepatic inflammatory cytokine production can be regulated by modulating sphingomyelinase and ceramide synthase 6. Int J Mol Med. (2017) 39:453–62. doi: 10.3892/ijmm.2016.2835, PMID: [DOI] [PubMed] [Google Scholar]
- 67.Deng X, Yin X, Allan R, Lu DD, Maurer CW, Haimovitz-Friedman A, et al. Ceramide biogenesis is required for radiation-induced apoptosis in the germ line of C. elegans. Science. (2008) 322:110–5. doi: 10.1126/science.1158111, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hartmann D, Lucks J, Fuchs S, Schiffmann S, Schreiber Y, Ferreirós N, et al. Long chain ceramides and very long chain ceramides have opposite effects on human breast and colon cancer cell growth. Int J Biochem Cell Biol. (2012) 44:620–8. doi: 10.1016/j.biocel.2011.12.019, PMID: [DOI] [PubMed] [Google Scholar]
- 69.Mielke MM, Bandaru VVR, Han D, An Y, Resnick SM, Ferrucci L, et al. Demographic and clinical variables affecting mid- to late-life trajectories of plasma ceramide and dihydroceramide species. Aging Cell. (2015) 14:1014–23. doi: 10.1111/acel.12369, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mielke MM, Bandaru VVR, Han D, An Y, Resnick SM, Ferrucci L, et al. Factors affecting longitudinal trajectories of plasma sphingomyelins: the Baltimore longitudinal study of aging. Aging Cell. (2015) 14:112–21. doi: 10.1111/acel.12275, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Couttas TA, Kain N, Tran C, Chatterton Z, Kwok JB, Don AS. Age-dependent changes to sphingolipid balance in the human hippocampus are gender-specific and may sensitize to neurodegeneration. J Alzheimers Dis. (2018) 63:503–14. doi: 10.3233/JAD-171054, PMID: [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data analyzed in this study is subject to the following licenses/restrictions: access to data can be done through CHS. Requests to access these datasets should be directed to CHS-NHLBI.org.