Abstract
True aneurysms of the pancreaticoduodenal artery (PDA) arcade are rare but require intervention due to the high risk of rupture. Historically, these aneurysms have been managed with open surgical methods. In this study, we describe a contemporary series of aneurysms treated using a modern approach that includes endovascular and hybrid techniques. All the patients with aneurysms of the PDA arcade in an institutional database were identified between 2008 and 2022. Patients with history of pancreatic resection were excluded. Data on demographics, presenting symptoms, imaging findings, operative approach, and outcomes were collected and reviewed. There were nine patients diagnosed with a PDA aneurysm, and all nine underwent endovascular intervention. Most were men (n = 5; 55.6%) and White (n = 7; 77.8%) and had American Society of Anesthesiologists class II or III. The median aneurysm size was 21 mm (range, 6-42 mm), and five (55.5%) were symptomatic. Of the five symptomatic cases, two presented with rupture and were treated urgently. The median time to intervention for the nonurgent cases was 30 days. All but one patient had concomitant celiac artery stenosis and two of the eight cases (25%) were due to extrinsic compression from median arcuate ligament syndrome. Both patients underwent median arcuate ligament syndrome release before endovascular intervention. Another patient required open surgical bypass before endovascular repair from the supraceliac aorta to hepatic artery using a Dacron graft to maintain hepatic perfusion. Among the eight patients with celiac axis stenosis, five (62.5%) required celiac stent placement within the same operation. Coil embolization of the aneurysm was used for all except for two patients (n = 7 of 9; 77.8%), with one patient receiving embolic plugs and another receiving an 8 × 38-mm balloon-expandable covered stent for aneurysm exclusion. The median operating room time was 134 minutes. All repairs were technically successful without any intraoperative or postoperative complications. The mean follow-up was 30 months. There was no morbidity, mortality, or unplanned secondary reinterventions within 6 months after aneurysm repair. Stent patency and aneurysm size remained stable at 2 years of follow-up. True pancreaticoduodenal artery arcade aneurysms can be safely and effectively treated using endovascular and hybrid techniques. Because many of these aneurysms have concomitant celiac artery stenosis, the use of endovascular technology allows for simultaneous treatment of both the aneurysm and the stenosis with exceptional results.
Keywords: Concomitant celiac artery stenosis, Endovascular repair of visceral aneurysms, True pancreaticoduodenal artery arcade aneurysms, Visceral aneurysms
Pancreaticoduodenal artery arcade (PDAA) aneurysms, including those aneurysms within the gastroduodenal artery (GDA) and pancreaticoduodenal artery (PDA), are very rare. PDAA aneurysms are exceptionally rare, accounting for 2% to 3% of all splanchnic artery aneurysms.1 Some aneurysms will present symptomatically with a clinical presentation of chronic mesenteric ischemia; however, these aneurysms are typically discovered incidentally when computed tomography (CT) or magnetic resonance imaging (MRI) has been performed for unrelated abdominal symptoms.2,3
Most aneurysms of the PDAA are pseudoaneurysms secondary to abdominal trauma, portal hypertension, or inflammation from pancreatitis.4,5 True aneurysms of the PDAA are a more infrequent occurrence and are usually seen in the setting of concomitant celiac artery stenosis, implicated in more than one half of identified PDAA aneurysms.6,7 Celiac axis stenosis is most commonly due to atherosclerosis or extrinsic compression from median arcuate ligament syndrome (MALS), causing intrinsic changes within the celiac artery.3,6,8, 9, 10, 11 High-grade stenosis at the celiac artery causes a reversal of blood flow, which, in turn, causes the development of collateral pathways that influence the formation of PDAA aneurysms from the altered hemodynamics of increased flow velocities.6,12, 13, 14 Some simulation studies have demonstrated that when the degree of celiac artery stenosis is >50%, flow reversal and increased flow velocity occurs within the PDAA of up to three times the normal rate.15
These lesions are unique in that the risk of rupture does not correlate with the aneurysm size.16,17 Previous case series have demonstrated that approximately 20% of ruptured PDAA aneurysms have occurred in PDAA aneurysms <1 cm in size, with an overall risk of rupture of between 7% and 15% reported.16,18, 19, 20 Moreover, rupture is associated with significant mortality, with a large case series of visceral artery aneurysms demonstrating a 1-month mortality rate of 10% and overall mortality approaching 20% for ruptured aneurysms of the PDAA. Due to the significant risk of hemorrhage and mortality, it is, therefore, recommended that all PDAA aneurysms are treated regardless of size at the time of recognition.6,16,21,22
Traditionally, PDAA aneurysms were repaired using open surgical techniques of aneurysm ligation. However, endovascular approaches, such as aneurysm coil embolization, are now more routinely used.6,23 Depending on the location and etiology of PDAA aneurysms, some might require a stepwise hybrid approach due to the need for MALS release or the maintenance of hepatic perfusion due to collateral flow from the aneurysm directly feeding into the hepatic vessels.23
Large retrospective reviews are limited due to the low incidence of PDAA aneurysms, with <10% of all reported cases of visceral artery aneurysms including PDAA aneurysms and even fewer including case series specifically of true PDAA aneurysms.24 In addition, the management of true aneurysms of the GDA and PDA has evolved from historically requiring open surgical intervention, with most of these aneurysms currently treated through endovascular and hybrid stepwise approaches.6,23 A recent European retrospective study by Illuminati et al23 was one of the first to compare the outcomes of elective open surgical and endovascular repair of PDAA aneurysms with celiac artery stenosis. However, no clear consensus has been reached on whether simultaneous celiac artery stenosis repair should be performed at the time of endovascular repair of the aneurysm, including after MALS release.3,6,23 Identifying the durability of modern endovascular and hybrid management strategies to treat PDAA aneurysms is critical for future management decisions and prognostication. We sought to evaluate the management and outcomes of patients presenting at our center with true GDA and PDA aneurysms, including those with concomitant celiac artery stenosis.
Methods
All the patients from a single institution between June 2008 and July 2022 with a radiographic diagnosis of a PDAA aneurysm, including aneurysms from the GDA and PDA, were identified. The patients were identified using the International Classification of Diseases, 9th revision, diagnosis code 442.84 and International Classification of Diseases, 10th revision, diagnosis code I72.8 for an aneurysm of other specified arteries to include all patients with visceral artery aneurysms. The patients were further reviewed to distinguish only those with PDAA aneurysms, including PDA and GDA aneurysms. The institutional review board approved this study protocol and waived the requirement for patient consent because this was a retrospective medical record review.
The inclusion criteria were patient age >18 years and a confirmed true PDAA aneurysm identified through various imaging modalities, including, but not limited to, ultrasound, CT, MRI, and/or conventional angiography. Patients with a visceral aneurysm other than within the PDAA, patients with thrombosed PDA or GDA aneurysms on axial imaging before intervention, and patients with pseudoaneurysms secondary to a procedural complication from pancreatic resection or prior abdominal trauma were excluded to ensure the homogeneity of true PDAA aneurysms and minimize potential confounding factors related to prior surgical interventions.
Data collection
Data were collected retrospectively using the institutional database, which was queried with the established inclusion and exclusion criteria to include only those patients with true PDAA aneurysms. Pertinent patient data, including demographics, clinical presentation, radiographic characteristics, operative approach, and postprocedural follow-up outcomes, were collected. Each patient record was independently reviewed by two designated research fellows (N.S. and M.B.) and a vascular surgery attending (P.C.) to ensure accuracy and completeness of the dataset.
Clinical and imaging parameters
The clinical presentation of the patients included symptoms at presentation, the presence of rupture, and case urgency. Patients who were categorized as symptomatic had the presence of either nonspecific abdominal pain, chronic mesenteric ischemia, or gastrointestinal bleeding such as hemorrhage from aneurysm rupture. Patients who were categorized as asymptomatic had aneurysms that were incidentally found through imaging without any associative abdominal symptoms. Rupture was identified through preoperative imaging or from operative findings. Case urgency was categorized as emergent or nonemergent.
The imaging findings and associated radiology reports were obtained from the preoperative CT and MRI scans and were systematically reviewed to assess the aneurysm size, location, and morphology and associated celiac axis stenosis. The aneurysm size was measured in millimeters and represented the maximum diameter of the lesion, capturing its full extent. Concomitant celiac artery stenosis was defined as imaging-proven stenosis >50% of the celiac artery either from atherosclerosis or MALS. The criteria for either celiac artery atherosclerosis or MALS are as follows: celiac artery atherosclerosis was designated if CT imaging demonstrated calcification or plaque at the origin of the celiac artery, and MALS was designated if CT imaging confirmed the classic radiographic signs of celiac compression with evidence of diaphragmatic crura surrounding the celiac axis, post-stenotic celiac artery dilation, superior notching, and proximal celiac artery acute downward angulation.16
Treatment approach and outcomes
The operative approach and outcomes were reviewed for each patient. The operative approach was defined as the primary planned treatment modality at PDAA recognition, which included a total endovascular approach, total surgical approach, or hybrid endovascular approach. A total endovascular approach included transcatheter arterial access of the mesenteric vessels and performing either aneurysm embolization or aneurysm exclusion via stenting. A total surgical approach included open surgical ligation of the aneurysm, open mesenteric bypass, and/or MALS release without any endovascular treatment. A hybrid endovascular approach included stepwise open surgical repair—this included first performing open MALS release and/or maintenance of collateral flow with mesenteric bypass and subsequent endovascular repair of the PDAA aneurysm and celiac artery stenosis when present. MALS release at our institution was typically performed robotically, without the possibility for simultaneous ligation of the PDA aneurysm. As such, we elected to perform a staged repair, with subsequent endovascular intervention for treatment of the aneurysm. The short-term outcomes included technical success, postoperative complications, and 30-day readmission from the time of endovascular repair. Technical success was defined as nonvisualization of the PDA aneurysm and stent patency on completion angiography without intraoperative complications. The long-term outcomes included unplanned 6-month secondary reintervention, 2-year status of aneurysm sac exclusion/thrombosis with stent patency, and overall mortality.
Statistical analysis
The primary outcomes of this study are to evaluate and characterize the clinical presentation, operative strategies, and clinical outcomes such as technical success, reintervention rates, and mortality of those patients presenting with PDAA aneurysms. Descriptive statistics were used to summarize patient demographics, clinical characteristics, and treatment outcomes. Categorical variables are presented as frequencies and percentages and continuous variables as the mean ± standard deviation or median with the total or interquartile range, depending on the data distribution.
Ethical considerations
This study adhered to the ethical guidelines and regulations outlined by our institutional review board. All patient data were de-identified and securely stored to protect patient privacy and confidentiality.
Results
There were nine total patients identified with a true PDAA aneurysm during the study period, with seven (77.8%) presenting with an intact aneurysm and two (22.2%) with a ruptured aneurysm. The median age was 65 years (range, 50-85 years), with a normal median body mass index (22.6 kg/m2; range, 18.4-34.3 kg/m2). Most patients were men (55.6%) and White (77.8%) and had American Society of Anesthesiologists class II or III (Table I). Comorbidities within the cohort included hypertension, coronary artery disease, atrial fibrillation, congestive heart disease, and hepatitis (Table I).
Table I.
Patient demographics (n = 9)
| Variable | Median (range) or No. (%) |
|---|---|
| Age, years | 65 (50-85) |
| Male gender | 5 (55.6) |
| BMI, kg/m2 | 22.6 (18.4-34.3) |
| White race | 7 (77.8) |
| Comorbidity | |
| Hypertension | 3 (33.3) |
| Coronary artery disease | 2 (22.2) |
| Atrial fibrillation | 2 (22.2) |
| Congestive heart failure | 1 (11.1) |
| Hepatitis | 1 (11.1) |
| Gastroesophageal reflux disease | 1 (11.1) |
BMI, Body mass index.
All aneurysms were within the PDAA, with a similar distribution for the aneurysm location between the PDA and GDA (n = 5 [55.6%] vs n = 4 [44.4%], respectively). The median aneurysm size at presentation was 21 mm (range, 6-42 mm; Table II). There was also a similar distribution in the clinical presentation between symptomatic cases (n = 5; 55.6%) and incidental cases (n = 4; 44.4%). Symptomatic patients presented with abdominal pain, back pain, or chronic mesenteric ischemia characterized as postprandial abdominal pain. Among the five symptomatic patients in our study, two (40%) presented with associated ruptured aneurysms and were treated urgently (Table II). The median time to primary intervention of nonurgent cases was 29 days (range, 8-150 days; Table II). All except for one patient (n = 8; 88.9%) were found to have concomitant celiac artery stenosis. Of the cases of concomitant celiac artery stenosis, most were due to intrinsic atherosclerosis (n = 6 of 8; 75%), with two (25%) caused by extrinsic compression of the celiac artery due to median arcuate ligament syndrome (Table II).
Table II.
Summary of patient data
| Pt. No. | Age, years; gender | Aneurysm location | Size, mm | Presentation | Symptoms | Concomitant celiac axis stenosis | Cause of stenosis | Intervention approach | Emergent case | Time to primary intervention, days |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 65; M | GDA | 21 | Incidental | – | Yes | Atherosclerosis | Endovascular | No | 28 |
| 2 | 56; M | GDA | 25 | Incidental | – | Yes | Atherosclerosis | Hybrid | No | 70 |
| 3 | 82; F | PDA | 6 | Symptomatic, ruptured | Abdominal pain | Yes | Atherosclerosis | Endovascular | Yes | 1 |
| 4 | 78; M | GDA | 42 | Incidental | – | No | – | Endovascular | No | 8 |
| 5 | 85; F | PDA | 15 | Symptomatic, Ruptured | Abdominal pain; back pain | Yes | Atherosclerosis | Endovascular | Yes | 1 |
| 6 | 55; F | PDA | 17 | Symptomatic | Back pain; postprandial abdominal pain | Yes | MALS | Hybrid | No | 22 |
| 7 | 58; M | GDA | 20 | Symptomatic | Postprandial abdominal pain | Yes | MALS | Hybrid | No | 358a |
| 8 | 50; F | PDA | 35 | Symptomatic | Abdominal pain | Yes | Atherosclerosis | Endovascular | No | 30 |
| 9 | 72; M | PDA | 25 | Incidental | – | Yes | Atherosclerosis | Endovascular | No | 150 |
F, Female; GDA, gastroduodenal artery; M, male; MALS, median arcuate ligament syndrome; PDA, pancreaticoduodenal artery; Pt. No., patient number.
Outlier.
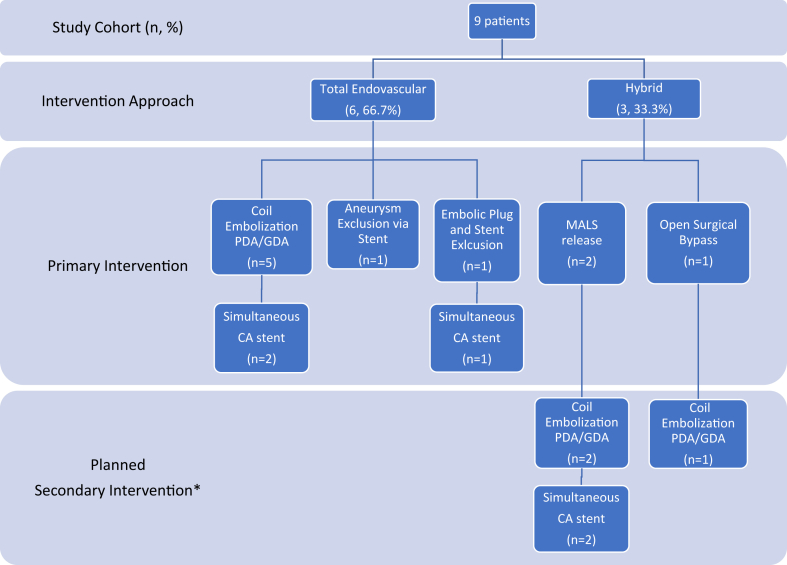
In terms of the operative approach, the patients either underwent a total endovascular approach (n = 6; 66.7%) or hybrid endovascular approach (n = 3; 33.3%; Table II; Fig 1). Of the three patients who required a hybrid endovascular approach (33.3%), two underwent planned robotic MALS release (patients 6 and 7) and one underwent open surgical mesenteric bypass from the supraceliac aorta to the hepatic artery using a Dacron graft to maintain adequate hepatic perfusion (patient 2; Table III; Fig 1). All surgical procedures were performed <3 months before the endovascular intervention. No patient underwent a total open surgical approach.
Fig 1.
Overview of pancreaticoduodenal artery arcade (PDAA) aneurysm treatment approach. CA, Celiac artery; GDA, gastroduodenal artery; MALS, median arcuate ligament syndrome; PDA, pancreaticoduodenal artery. ∗There were no unplanned secondary or unplanned reinterventions within 6 months after the initial intervention approach.
Table III.
Summary of patient operative data
| Pt. no. | Primary intervention | Secondary intervention | Aneurysm location | Access | Maximum sheath size, F | Cannulation (in order) | Aneurysm embolization or exclusion technique | Embolic material | Celiac artery stent placed | Stents placed; location | Stent size; type |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Coil embolization | – | GDA | Right femoral artery | 8 | SMA; celiac artery | IE > OE packing; SMA stent | Microcoils and packing coils | Yes | Celiac artery; SMA | 8 × 20 mm BE; 8 × 29 mm BE |
| 2 | Celiac artery bypass | Coil embolization | GDA | Right femoral artery | 5 | Celiac artery bypass; SMA | OE packing | Microcoils and packing coils | Noa | None | – |
| 3 | Coil embolization | – | PDA | Right femoral artery | 6.5 | Celiac artery; SMA | IE > OE packing | Microcoils and packing coils | Yes | Celiac artery | 7 × 15 mm BE |
| 4 | Coil embolization | – | GDA | Right femoral artery | 5 | Celiac artery | IE | Thrombogenic microcoils | Nob | – | – |
| 5 | Coil embolization | – | PDA | Right femoral artery | 5 | SMA | OE > IE packing | Microcoils and packing coils | Noc | None | – |
| 6 | MALS release | Coil embolization | PDA | Right femoral artery | 8.5 | Celiac artery; SMA | OE > IE packing; SMA stent | Microcoils and packing coils | Yes | Celiac artery; SMA | 7 × 29 mm BE; 8 × 29 mm BE |
| 7 | MALS release | Coil embolization | GDA | Right femoral artery | 6.5 | Celiac artery; SMA | IE > OE packing; celiac artery stent | Microcoils and packing coils | Yes | Celiac artery | 5 × 22 mm BE |
| 8 | Embolic plug; stent exclusion | – | PDA | Left axillary artery | 6 | SMA; celiac artery | OE > IE plug; PDA stent | Vascular plug | Yes | Celiac artery; PDA | 8 × 20 mm BE; 7 × 25 mm BE |
| 9 | Stent exclusion | – | PDA | Right femoral artery | 7 | SMA | PDA stent | None | Noc | PDA | 8 × 38 mm BE |
BE, Balloon expandable; GDA, gastroduodenal artery; IE, inflow embolization; MALS, median arcuate ligament syndrome; PDA, pancreaticoduodenal artery; Pt. No., patient number; SMA, superior mesenteric artery.
No celiac stent was placed because the celiac artery was bypassed with a prior supraceliac aorta to hepatic artery extra-anatomical bypass.
No stenosis of the celiac artery.
Unable to cannulate the stenosis at the celiac artery.
In the review of the procedural details for endovascular intervention, the median operating room (OR) time for endovascular repair was 134 minutes (range, 115-211 minutes; Table III). All but one of the nine patients underwent their endovascular procedure under local anesthesia with monitored anesthesia care (89%). The median blood loss was minimal, with no patient requiring any transfusion products in the OR. The median OR volume resuscitation was 1000 mL (range, 500-2500 mL; Table III). Access for the endovascular procedures was via the femoral artery for a large majority (n = 8; 88.9%), and one had access via the left axillary artery (patient 8). The superior mesenteric artery (SMA) and celiac artery were both cannulated to achieve successful embolization or stent exclusion (Table III). An endovascular technique of coil embolization of PDA and GDA aneurysms was used seven of the nine patients (77.8%). Of the two patients, one (patient 8) received embolic plugs for PDA aneurysm exclusion using a 7 × 25-mm balloon-expandable covered stent, and one (patient 9) received PDA aneurysm exclusion via an 8 × 38-mm balloon-expandable covered stent (Table III). Balloon-expanded covered stents were chosen because the aneurysm was more pedunculated in anatomical feature. The balloon-expandable stent was used to allow for greater juxtaposition of the stent with the target vessel. Of the eight patients with celiac axis stenosis, five (65%) had simultaneous celiac artery angioplasty and balloon-expandable stent placement within the same endovascular intervention (Table III). For those patients without celiac artery stent placement, either the patient had a prior celiac artery bypass to maintain hepatic circulation (patient 2) or cannulation of the celiac artery could not be performed due to complete celiac artery occlusion (patients 5 and 9), and bypass was determined to be unnecessary because they had sufficient collateral flow.
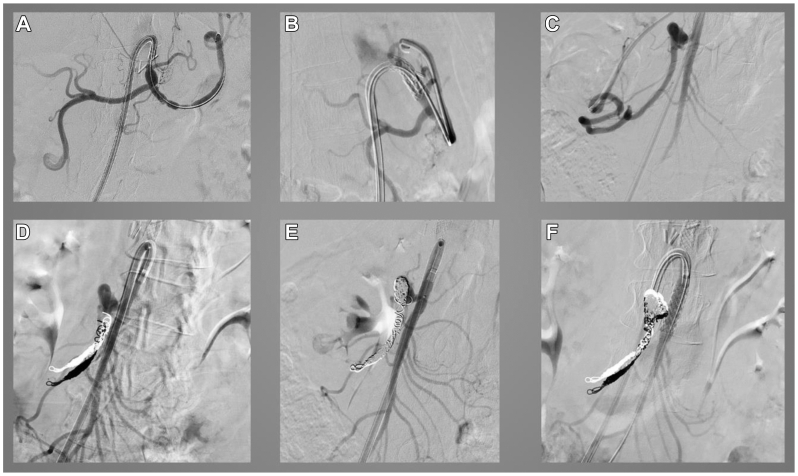
Fig 2 demonstrates a sample angiographic series from patient 6 with a PDA aneurysm and celiac artery stenosis secondary to MALS. Angiograms were taken after the patient had undergone robotic MALS release surgery 1 month prior. In this case, transcatheter arterial access via the right femoral artery was performed to first cannulate the celiac axis to perform balloon angioplasty and stenting (Fig 2, A and B). The PDA aneurysm was then identified (Fig 2, C) and coiled with successful embolization using multiple coils (Fig 2, D and E). Finally, the SMA was cannulated and stented because the aneurysm had a broad base (Fig 2, F).
Fig 2.
Angiographic series from a patient with a pancreaticoduodenal artery (PDA) aneurysm and celiac artery (CA) stenosis due to median arcuate ligament syndrome (MALS). A, Angiography of celiac axis via selective catheterization of CA demonstrating prior clips from median arcuate ligament release and CA stenosis. B, Placement of CA stent using a 7 × 29-mm balloon-expandable covered stent. C, Visualization of inferior PDA aneurysm via selective cannulation of CA to hepatic artery (HA) to gastroduodenal artery (GDA) using an angled Glidewire (Terumo Interventional Systems). D, Outflow coil embolization of PDA aneurysm deployed via a lantern microcatheter. E, Inflow coil embolization and packing of remainder of PDA aneurysm. F, Selective cannulation of superior mesenteric artery (SMA) via lantern microcatheter and transcend wire, with deployment of an 8 × 28-mm balloon-expandable covered stent over the SMA due to the broad base of the PDA aneurysm.
All endovascular repairs were technically successful with no postoperative complications (Table IV). The median length of stay after endovascular intervention for those patients whose aneurysms were not ruptured was 2 days (range, 1-5 days; n = 7; 78%). The median length of stay for those patients with ruptured aneurysms was 4 days (range, 3-5 days; n = 2; 22%; Table IV). No patients were readmitted within 30 days after the endovascular intervention (Table IV). The mean duration of follow-up was 30 months. Eight of the nine patients (88.9%) received follow-up imaging within 6 months after intervention. The remaining patient was lost to follow-up. None of the patients required reintervention for treatment of their aneurysm within 6 months after treatment (Table IV). The patients were prescribed an antiplatelet regimen if not already taking an antiplatelet agent preoperatively, with 67% receiving aspirin-only therapy and 22% receiving dual antiplatelet with aspirin and clopidogrel. All the patients with follow-up imaging available were found to have no aneurysm enlargement and the stents placed remained patent. No major adverse cardiac events occurred within 30 days and no mortality had occurred at 30 days, 6 months, or 2 years (Table IV). Two patients had undergone their procedures much earlier and had significantly longer follow-up (8 and 10 years) than the remainder of the cohort. Stent patency was 100% at the last follow-up with no increase in aneurysm size. On long-term follow-up, two patients required an additional unplanned intervention for the development of SMA stenosis, which was diagnosed 4 years after aneurysm repair (Table IV). Of these two patients, one had endovascular repair with SMA angioplasty and stenting (patient 4), and the other refused subsequent intervention (patient 9).
Table IV.
Summary of clinical outcomes after endovascular intervention
| Variable | Median (range) or No. (%) |
|---|---|
| Short-term outcomes (n = 9) | |
| Length of stay, days | |
| Nonruptured patients | 2 (1-5) |
| Ruptured patients | 4 (3-5) |
| Technically successful | 9 (100) |
| Postoperative complication | 0 (0) |
| Readmission at 30 days | 0 (0) |
| MACE at 30 days | 0 (0) |
| Mortality at 30 days | 0 (0) |
| Mortality at 6 months | 0 (0) |
| Reintervention for aneurysm (≤6 months) | 0 (0) |
| 6-Month follow-up | 8 (88.9) |
| Long-term outcomes (n = 8) | |
| Aneurysm enlargement (2 years) | 0 (0) |
| Stent patency at 2 years | 8 (100) |
| Any additional interventiona (>2 years) | 2 (25) |
| Mortality (2 years) | 0 (0) |
MACE, Major adverse cardiac events.
Two patients required additional intervention during long-term follow-up for superior mesenteric artery stenosis, which was diagnosed >4 years after aneurysm repair. Patient 4 underwent successful superior mesenteric artery angioplasty plus stent placement; patient 9 refused additional intervention.
Discussion
In this study, we investigated the management and outcomes of patients with aneurysms of the GDA and PDA, with a particular focus on those with concomitant celiac artery stenosis. Our small sample size of only nine cases within a 15-year window at a tertiary referral center is consistent with prior reports that these aneurysms are exceptionally rare, accounting for only 2% to 3% of all visceral artery aneurysms.1
A review of the patient demographics revealed that our study did not have some of the typical comorbidities that have been noted in prior literature in patients with PDAA, including a history of portal hypertension, pancreatitis, or fibromuscular dysplasia.6 This might suggest that our study cohort developed PDAA aneurysms due to the direct pathophysiology of a change in flow hemodynamics and not from inflammatory causes. Overall, patients with PDAA tend to be healthy individuals with few comorbidities, and our cohort was consistent with that trend.
Although most PDAA aneurysms are found incidentally, our case series had a more even distribution of symptomatic and asymptomatic cases. The symptoms also did not correlate with aneurysm size. The largest PDAA aneurysm in our case series was found incidentally and was a GDA aneurysm measuring 42 mm. Among the five symptomatic cases, two (40%) presented with rupture and gastrointestinal hemorrhage. Our incidence of rupture is in line with prior reviews demonstrating a range of ruptured presentation from approximately 20% to 46%.6,21,25 The literature has also demonstrated that the risk of rupture for PDAA aneurysms does not correlate with aneurysm size, and our study confirms the size discordant pathophysiology of this disease process.16,17,21 Those patients with the smallest diameter aneurysms of 6 mm (patient 3) and 15 mm (patient 5) had presented with ruptured aneurysms. This underlines the importance of identifying PDAA aneurysms at any size and treating them because the risk of rupture does not correlate with aneurysm size.
Regarding the time to intervention on recognition of a PDAA aneurysm, our study cohort had a median time of <1 month to the primary intervention and were appropriately treated on recognition. All patients who presented with PDAA aneurysms were treated, and none elected for observation. Moreover, both patients who presented with rupture were treated urgently at presentation. An interval of >30 days before the primary intervention was either due to the wait time for surgical planning for open surgical intervention with either mesenteric bypass or MALS release or patient preference to obtain a second opinion before aneurysm repair.
One of the most important aspects of this case series is the large majority of cases of concomitant celiac artery stenosis in our patient population (n = 8 of 9; 88.9%). Our incidence of concomitant celiac axis stenosis is consistent with prior reports implicating celiac artery narrowing in more than one half of all PDAA aneurysms.6,7,12,26 However, most of our celiac artery stenosis cases were due to intrinsic atherosclerosis and not extrinsic compression from MALS. Celiac artery compression attributed to MALS was observed in just 20% of our patients. However, published reports vary, with some reviews indicating MALS as the cause for 10% to 25% of cases of celiac axis stenosis, and others suggesting a range of 50% to 80%.1,12,16,23 Ligament release was performed in all patients with a history of MALS before addressing aneurysm repair.
The management of simultaneous celiac artery stenosis during true PDAA aneurysm repair remains in question. Although only 62.5% of our patients with celiac axis stenosis had celiac artery angioplasty and stent placement during the same endovascular intervention, it was attempted for all patients with celiac axis stenosis. Those patients who did not have celiac artery stenosis addressed either had a prior celiac artery bypass or we were unable to cannulate the celiac artery. Our patients did not have any associated morbidities or mortalities with simultaneous celiac axis intervention, and stent patency remained at 2 years of follow-up.
When comparing our findings with the available literature, diverse recommendations emerge regarding the approach to addressing celiac axis stenosis. Older reports have suggested that endovascular intervention for celiac trunk stenosis can increase the risk to patients.27 However, more contemporary series have shown that celiac trunk treatment can be safely performed.6,8,23 Corey et al6 indicated that 97% of their patient cohort presented with concomitant celiac stenosis, with 20% of their cohort having concomitant celiac axis stenting. Although this is a lower total percentage than that in our study, the patients who did not have celiac axis stenosis addressed had appropriate retrograde flow to the hepatic circulation and, thus, further intervention was considered unnecessary.6 Additionally, for those who underwent celiac axis stenting, there was good stent patency at their short-term follow-up.6 In contrast, a long-term follow-up study by Illuminati et al23 demonstrated a 50% failure rate for patency of the celiac axis after treatment in a cohort of 12 patients. However, the failed patency rate was of a small cohort and one third of the failed patency was observed immediately after endovascular intervention. Our study has demonstrated success with simultaneous celiac artery treatment; however, further long-term follow-up studies are warranted for more definitive recommendations.
Conflicting reports exist regarding the management of celiac artery stenosis after MALS release. Due to the intrinsic arterial changes from chronic extrinsic celiac artery compression from the median arcuate ligament, even after release, the celiac artery has persistent fibrotic changes with associated changes in hemodynamics; thus, celiac artery stenosis should be addressed after MALS release surgery.6,8,14,15,28 However, some literature suggests that after release of the median arcuate ligament, there is normal celiac artery regression, and celiac artery release is not necessary.23,29 A study by Boll et al29 demonstrated that patients who did not receive treatment of celiac artery stenosis did not develop any mesenteric morbidity during 6 months of follow-up. However, they did not consider those patients with MALS who had already developed aneurysmal changes in the PDAA.29 These alterations in hemodynamics would suggest that patients should have simultaneous intervention of the celiac artery to prevent aneurysm reformation—as was seen in the lack of aneurysm sac enlargement during follow-up of our patients treated for celiac axis stenosis. Overall, our study supports that concurrent celiac axis intervention can be performed safely and should be considered when addressing aneurysm repair.
Considering the perfusion effects is crucial in the event of aneurysm exclusion, because the PDAA could play a substantial role in hepatic perfusion. Hepatic malperfusion is more commonly observed in GDA aneurysms than in PDA aneurysms, primarily because the hepatic artery tends to receive preferential blood flow from the GDA.6,23 In our study, one patient had a GDA aneurysm affecting liver perfusion, necessitating completion of a supraceliac aorta to hepatic artery bypass before aneurysm repair. Our positive results can be attributed to having appropriate workup and understanding the etiology of the disease process before intervention.
Finally, endovascular coil embolization was the primary treatment modality used in our study to address aneurysm repair. All patients underwent endovascular intervention, and one third required a hybrid approach due to their etiology of MALS or anatomical considerations of collateral flow due to the location of their aneurysm. Overall, the short-term outcomes were excellent through the total endovascular and hybrid approach, with no treatment-related morbidity, mortality, or unplanned secondary reinterventions within 2 years after repair. Moreover, all patients with follow-up imaging showed patent stents and no aneurysm enlargement, indicating the durability of the endovascular interventions at short-term follow-up. Even with the opportunity to consider the long-term outcomes for some patients at 4 years after repair, aneurysm dissolution and stent patency remained. However, two of eight patients (25%) did present with stenosis of the SMA after 4 years of follow-up. Neither of these patients underwent SMA intervention or exhibited SMA stenosis during the index procedure. Celiac axis stenosis was only identified in one patient, attributed to atherosclerosis, and both patients received aspirin and statin therapy after the endovascular procedure. Persistent long-term imaging surveillance is crucial, given the potential emergence of other mesenteric arterial issues in this patient population.
Our short-term outcomes after endovascular repair were comparable to those reported in other case series, including one of the largest single-institution studies of 35 patients by Corey et al.6 The results showed >95% technical and clinical success.6 However, one recent European multicenter study of 57 patients included a comparison of endovascular and open surgical techniques for PDAA aneurysms with long-term follow-up of 6 years and demonstrated that coil embolization was associated with the need for recanalization and a reintervention rate of 11%.23 The same study also concluded that open surgical repair is favored for those aneurysms located on the GDA and anterior PDA, although endovascular embolization might be preferred for aneurysms located on the posterior PDA.23 In our study, we did not have the opportunity to compare our results with those from a total open surgical approach, and embolization was successfully performed for both GDA and PDA aneurysms. Furthermore, the endovascular approach has the advantage of concurrently addressing celiac axis stenosis if present through a less invasive method than if a total open approach were pursued. Again, a need exists for more longitudinal studies to appropriately evaluate the long-term success rates of endovascular intervention.
Study limitations and future directions
The study limitations include the retrospective single-institution design and lack of direct comparison with open surgical ligation. Further studies, especially prospective multi-institutional ones, are warranted to establish optimal management strategies and long-term durability of interventions for this rare patient population.
Conclusions
Our study provides valuable insights into the management and outcomes of patients with true aneurysms of the GDA and PDA, particularly of those with concomitant celiac artery stenosis. The appropriate use of a total or hybrid approach of endovascular technology appears to be a safe and effective treatment approach for these aneurysms, with excellent short-term outcomes. Simultaneous celiac artery repair was not associated with any additional risk of morbidity or mortality in our study, and the repairs of the celiac axis remained patent through the duration of follow-up. Continued long-term surveillance is necessary to detect and address other mesenteric arterial issues that could arise after aneurysm repair. Further research is needed to compare endovascular interventions with open surgical ligation to establish the optimal management strategy for this rare and challenging patient population.
Disclosures
S.E. is a consultant to Penumbra, Inc. B.D. is an advisor, a consultant, and a speaker to Abbott and Medtronic; and a consultant to Bard and Boston Scientific. The remaining authors report no conflicts.
Footnotes
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Tien Y.W., Kao H.L., Wang H.P. Celiac artery stenting: a new strategy for patients with pancreaticoduodenal artery aneurysm associated with stenosis of the celiac artery. J Gastroenterol. 2004;39:81–85. doi: 10.1007/s00535-003-1251-3. [DOI] [PubMed] [Google Scholar]
- 2.Pasha S.F., Gloviczki P., Stanson A.W., Kamath P.S. Splanchnic artery aneurysms. Mayo Clin Proc. 2007;82:472–479. doi: 10.4065/82.4.472. [DOI] [PubMed] [Google Scholar]
- 3.Katsura M., Gushimiyagi M., Takara H., Mototake H. True aneurysm of the pancreaticoduodenal arteries: a single institution experience. J Gastrointest Surg. 2010;14:1409–1413. doi: 10.1007/s11605-010-1257-0. [DOI] [PubMed] [Google Scholar]
- 4.Paty P.S.K., Cordero J.A., Clement Darling R., Chang B.B., Shah D.M., Leather R.P. Aneurysms of the pancreaticoduodenal artery. J Vasc Surg. 1996;23:710–713. doi: 10.1016/s0741-5214(96)80054-1. [DOI] [PubMed] [Google Scholar]
- 5.Granke K., Hollier L.H., Bowen J.C. Pancreaticoduodenal artery aneurysms: changing patterns. South Med J. 1990;83:918–921. doi: 10.1097/00007611-199008000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Corey M.R., Ergul E.A., Cambria R.P., et al. The presentation and management of aneurysms of the pancreaticoduodenal arcade. J Vasc Surg. 2016;64:1734–1740. doi: 10.1016/j.jvs.2016.05.067. [DOI] [PubMed] [Google Scholar]
- 7.Quandalle P., Chambon J.P., Marache P., Saudemont A., Maes B. Pancreaticoduodenal artery aneurysms associated with celiac axis stenosis: report of two cases and review of the literature. Ann Vasc Surg. 1990;4:540–545. doi: 10.1016/S0890-5096(06)60835-2. [DOI] [PubMed] [Google Scholar]
- 8.Duffy A.J., Panait L., Eisenberg D., Bell R.L., Roberts K.E., Sumpio B. Management of median arcuate ligament syndrome: a new paradigm. Ann Vasc Surg. 2009;23:778–784. doi: 10.1016/j.avsg.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Goodall R., Langridge B., Onida S., Ellis M., Lane T., Davies A.H. Median arcuate ligament syndrome. J Vasc Surg. 2020;71:2170–2176. doi: 10.1016/j.jvs.2019.11.012. [DOI] [PubMed] [Google Scholar]
- 10.Jimenez J.C., Harlander-Locke M., Dutson E.P. Open and laparoscopic treatment of median arcuate ligament syndrome. J Vasc Surg. 2012;56:869–873. doi: 10.1016/j.jvs.2012.04.057. [DOI] [PubMed] [Google Scholar]
- 11.Järvinen O., Laurikka J., Sisto T., Salenius J.P., Tarkka M.R. Atherosclerosis of the visceral arteries. Vasa. 1995;24:9–14. [PubMed] [Google Scholar]
- 12.Degheili J.A., El Chediak A., Dergham M.Y.R., Al-Kutoubi A., Hallal A.H. Pancreaticoduodenal artery aneurysm associated with celiac trunk stenosis: case illustration and literature review. Case Rep Radiol. 2017;2017 doi: 10.1155/2017/6989673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Endo Y., Sekino H., Ishii S., et al. Two cases of pancreaticoduodenal aneurysm with median arcuate ligament syndrome treated with coil embolization and median arcuate ligament incision. Radiol Case Rep. 2022;17:3663–3668. doi: 10.1016/j.radcr.2022.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mano Y., Takehara Y., Sakaguchi T., et al. Hemodynamic assessment of celiaco-mesenteric anastomosis in patients with pancreaticoduodenal artery aneurysm concomitant with celiac artery occlusion using flow-sensitive four-dimensional magnetic resonance imaging. Eur J Vasc Endovasc Surg. 2013;46:321–328. doi: 10.1016/j.ejvs.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 15.Miyahara K., Hoshina K., Nitta J., Kimura M., Yamamoto S., Ohshima M. Hemodynamic simulation of pancreaticoduodenal artery aneurysm formation using an electronic circuit model and a case series analysis. Ann Vasc Dis. 2019;12:176–181. doi: 10.3400/avd.oa.19-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki K., Tachi Y., Ito S., et al. Endovascular management of ruptured pancreaticoduodenal artery aneurysms associated with celiac axis stenosis. Cardiovasc Intervent Radiol. 2008;31:1082–1087. doi: 10.1007/s00270-008-9343-3. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X., Zhang W., Zhou W., Zhou W. Endovascular treatment of ruptured pancreaticoduodenal artery aneurysm with celiac Axis stenosis. Ann Vasc Surg. 2019;57:273.e1–273.e5. doi: 10.1016/j.avsg.2018.09.033. [DOI] [PubMed] [Google Scholar]
- 18.Chiang K.S., Johnson C.M., McKusick M.A., Maus T.P., Stanson A.W. Management of inferior pancreaticoduodenal artery aneurysms: a 4-year, single center experience. Cardiovasc Intervent Radiol. 1994;17:217–221. doi: 10.1007/BF00571539. [DOI] [PubMed] [Google Scholar]
- 19.De Perrot M., Berney T., Deléaval J., Bühler L., Mentha G., Morel P. Management of true aneurysms of the pancreaticoduodenal arteries. Ann Surg. 1999;229:416–420. doi: 10.1097/00000658-199903000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neschis D.G., Safford S.D., Golden M.A. Management of pancreaticoduodenal artery aneurysms presenting as catastrophic intraabdominal bleeding. Surgery. 1998;123:8–12. [PubMed] [Google Scholar]
- 21.Shukla A.J., Eid R., Fish L., et al. Contemporary outcomes of intact and ruptured visceral artery aneurysms. J Vasc Surg. 2015;61:1442–1448. doi: 10.1016/j.jvs.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Moore E., Matthews M.R., Minion D.J., et al. Surgical management of peripancreatic arterial aneurysms. J Vasc Surg. 2004;40:247–253. doi: 10.1016/j.jvs.2004.03.045. [DOI] [PubMed] [Google Scholar]
- 23.Illuminati G., Hostalrich A., Pasqua R., Nardi P., Chaufour X., Ricco J.B. Outcomes after open and endovascular repair of non-ruptured true pancreaticoduodenal and gastroduodenal artery aneurysms associated with coeliac artery compression: a multicentre retrospective study. Eur J Vasc Endovasc Surg. 2021;61:945–953. doi: 10.1016/j.ejvs.2021.02.023. [DOI] [PubMed] [Google Scholar]
- 24.Barrionuevo P., Malas M.B., Nejim B., et al. A systematic review and meta-analysis of the management of visceral artery aneurysms. J Vasc Surg. 2020;72:40S–45S. doi: 10.1016/j.jvs.2020.05.018. [DOI] [PubMed] [Google Scholar]
- 25.Shanley C.J., Shah N.L., Messina L.M. Uncommon splanchnic artery aneurysms: pancreaticoduodenal, gastroduodenal, superior mesenteric, inferior mesenteric, and colic. Ann Vasc Surg. 1996;10:506–515. doi: 10.1007/BF02000601. [DOI] [PubMed] [Google Scholar]
- 26.Vandy F.C., Sell K.A., Eliason J.L., Coleman D.M., Rectenwald J.E., Stanley J.C. Pancreaticoduodenal and gastroduodenal artery aneurysms associated with celiac artery occlusive disease. Ann Vasc Surg. 2017;41:32–40. doi: 10.1016/j.avsg.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 27.Ducasse E., Roy F., Chevalier J., et al. Aneurysm of the pancreaticoduodenal arteries with a celiac trunk lesion: current management. J Vasc Surg. 2004;39:906–911. doi: 10.1016/j.jvs.2003.09.049. [DOI] [PubMed] [Google Scholar]
- 28.Takach T.J., Livesay J.J., Reul G.J., Cooley D.A. Celiac compression syndrome: tailored therapy based on intraoperative findings. J Am Coll Surg. 1996;183:606–610. [PubMed] [Google Scholar]
- 29.Boll J.M., Sharp K.W., Garrard L.C., Naslund T.C., Curci J.A., Valentine J.R. Does management of true aneurysms of peripancreatic arteries require repair of associated celiac artery stenosis? J Am Coll Surg. 2017;224:199–203. doi: 10.1016/j.jamcollsurg.2016.10.030. [DOI] [PubMed] [Google Scholar]