Abstract
BACKGROUND
Synaptotagmins (SYTs) are a family of 17 membrane transporters that function as calcium ion sensors during the release of Ca2+-dependent neurotransmitters and hormones. However, few studies have reported whether members of the SYT family play a role in glucose uptake in diabetic retinopathy (DR) through Ca2+/glucose transporter-1 (GLUT1) and the possible regulatory mechanism of SYTs.
AIM
To elucidate the role of the SYT family in the regulation of glucose transport in retinal pigment epithelial cells and explore its potential as a therapeutic target for the clinical management of DR.
METHODS
DR was induced by streptozotocin in C57BL/6J mice and by high glucose medium in human retinal pigment epithelial cells (ARPE-19). Bioinformatics analysis, reverse transcriptase-polymerase chain reaction, Western blot, flow cytometry, ELISA, HE staining, and TUNEL staining were used for analysis.
RESULTS
Six differentially expressed proteins (SYT2, SYT3, SYT4, SYT7, SYT11, and SYT13) were found between the DR and control groups, and SYT4 was highly expressed. Hyperglycemia induces SYT4 overexpression, manipulates Ca2+ influx to induce GLUT1 fusion with the plasma membrane, promotes abnormal expression of the glucose transporter GLUT1 and excessive glucose uptake, induces ARPE-19 cell apoptosis, and promotes DR progression. Parkin deficiency inhibits the proteasomal degradation of SYT4 in DR, resulting in SYT4 accumulation and enhanced GLUT1 fusion with the plasma membrane, and these effects were blocked by oe-Parkin treatment. Moreover, dysregulation of the myelin transcription factor 1 (Myt1)-induced transcription of SYT4 in DR further activated the SYT4-mediated stimulus-secretion coupling process, and this process was inhibited in the oe-MYT1-treated group.
CONCLUSION
Our study reveals the key role of SYT4 in regulating glucose transport in retinal pigment epithelial cells during the pathogenesis of DR and the underlying mechanism and suggests potential therapeutic targets for clinical DR.
Keywords: Diabetic retinopathy, Glucose transporter-1, Synaptotagmin 4, Parkin, Myelin transcription factor 1
Core Tip: This study highlights the important role of synaptotagmin 4 (SYT4), which is a member of the SYTs family, in the regulation of glucose transport in retinal pigment epithelial cells during the development of diabetic retinopathy (DR). Hyperglycemia-induced overexpression of SYT4 perturbed calcium influx, resulting in the fusion of glucose transporter-1 (GLUT1) with the plasma membrane, abnormal expression of the glucose transporter GLUT1, and increased glucose uptake. Additionally, SYT4 contributed to apoptosis and inflammation, further exacerbating DR progression. This study also elucidated the molecular mechanisms by which Parkin and myelin transcription factor 1 modulate SYT4, providing new potential therapeutic targets for the clinical management of DR.
INTRODUCTION
Diabetic retinopathy (DR) is a common microvascular complication of diabetes mellitus and the leading cause of poor vision and blindness[1]. More than 400 million people worldwide have been diagnosed with diabetes, and approximately one-third of DR patients develop a vision-threatening form of the disease[2]. The development of DR is closely related to uncontrolled glucose-driven biochemical reactions[3]. Long-term hyperglycemia can lead to inflammation, retinal pigment epithelial cell damage, and a series of fundus lesions, such as microangioma, neovascularization, vitreous hyperplasia and even retinal detachment[4]. Studies have reported that the progression of retinopathy can be reversed or delayed by strict glycemic control[5,6]. Therefore, a better understanding of the mechanisms governing glucose transport is essential for inhibiting the uptake of glucose by retinal cells under hyperglycemic conditions, thereby alleviating the progression of DR.
Glucose is a major substrate for energy metabolism in retinal neurons. Like in most tissues, retinal cells produce ATP through the uptake of glucose and then through glycolysis in the cytoplasm or oxidative phosphorylation in mi-tochondria[7,8]. Glucose transporter (GLUT) proteins are monosaccharide transporters that belong to the major facilitator superfamily and mediate the transport of sugars across the cell membrane in mammals[9]. GLUT1 plays a key role in glucose uptake. The glucose transporter GLUT1 is responsible for the facilitated diffusion of glucose into red blood cells and supplies glucose to the brain and other organs[10]. Several studies have reported that GLUT1 is involved in DR progression. GLUT1 in the neural retina induces polyol accumulation and promotes early pathological changes in the diabetic retina[11]. GLUT1 promotes retinal inflammation triggered by excessive glucose uptake in microglia, thereby exacerbating DR progression[12]. In addition, recent studies have shown that GLUT1 expression and glucose uptake are Ca2+-dependent and that an increase in cellular Ca2+ after cell injury initiates information flow, which ultimately leads to the upregulation of the stress response gene GLUT1[13]. Perrini et al[14] reported that increased cytoplasmic Ca2+ concentrations stimulates GLUT1 translocation to the plasma membrane and increases glucose uptake in adipocytes, con-tributing to the progression of diabetes. Based on these findings, we hypothesize that the Ca2+ pathway may affect the uptake of glucose by retinal cells in DR by upregulating the expression of the glucose transporter GLUT1.
Synaptic receptors (SYTs) are abundant, evolutionarily conserved integral membrane proteins constituting a family of 17 isoforms (SYT1-SYT17); these proteins share a transmembrane domain near the N-terminus and a double C2 domain at the C-terminus, which binds Ca2+ to regulate Ca2+-dependent membrane docking and fusion[15,16].
SYTs act as calcium sensors during Ca2+-dependent neurotransmitter and hormone release[17,18]. These factors trigger and regulate the fusion of vesicles with the target membrane, participate in the strict regulation of neurotransmitter and hormone release, and regulate the transport of cellular proteins and membranes[15,19]. Dean et al[20] performed crystal structure analysis and showed that the C2B domains of Syt IV and Syt XI were confirmed to contain all five acidic residues involved in Ca2+ binding via lentivirus-mediated gene delivery. Huynh et al[21] identified Syt XI as a substrate of Parkin that plays a role in mediating neurotransmitter release by binding to its C2A and C2B domains, and Parkin-mediated polyubiquitination accelerates its turnover. In addition, several studies have reported that SYT family members play important roles in the pathogenesis and progression of human diseases[22,23]. However, few studies have shown that members of the SYT family further influence glucose uptake by DR retinal cells by regulating the fusion of GSVs with target membranes via Ca2+ and promoting GLUT1 membrane translocation.
Myelin transcription factor 1 (Myt1) is a founding member of the zinc finger protein family, which also includes MyT1 L and MyT3. Myt1 is highly expressed in developing neural tissue and was first shown to regulate neurogenesis in Xenopus gastrula[24]. Myt1 has since been shown to be involved in the migration of neuronal precursors to the sub-ventricular zone and the cortical plate in mammals[25]. In addition, Myt1 is closely related to endocrine function, and members of the Myt1 family promote neuroendocrine differentiation by antagonizing Notch activity[26]. Interestingly, recent studies have shown that the transcription factor Myt1 affects glucose-induced insulin secretion by regulating the transcription of the SYT4[27]. However, whether Myt1 affects the progression of DR by regulating the transcription of SYTs to mediate glucose uptake is unclear.
In this study, we investigated the expression of SYT family members in DR and their effects on glucose uptake in human retinal pigment epithelial cells (ARPE-19), and the results showed that SYT4 regulated the Ca2+ signaling pathway through GLUT1 to affect glucose uptake by ARPE-19 cells under high glucose conditions. In addition, we provide evidence that Parkin overexpression selectively induces the degradation of SYT4 and that Myt1 overexpression inhibits the transcription of SYT4, which plays a key role in the effects of GLUT1 on high glucose-induced cellular glucose uptake.
MATERIALS AND METHODS
Animals
A total of 60 male mice (C57BL/6J) weighing 18-20 g were purchased from the Animal Experimental Center of Kunming Medical University. A total of 15 sham-operated mice were fed a normal diet (control group), and 45 mice were fed a high-fat diet.
Model group: After fasting for 12 h, the mice were given intraperitoneal injections of 60 mg/kg streptozotocin (STZ) solubilized in 0.1 mol/L citrate buffer (pH = 4.5). After 72 h, blood samples were collected from the tail vein, and blood glucose levels were measured. Control mice were injected with an equal dose of citrate buffer, and blood glucose was measured using a blood glucose monitoring system (Glucotrend-2; Roche Diagnostics GmbH, Mannheim, Germany). Blood glucose levels greater than 16.7 mmol/L for one week indicated successful establishment of the DR model[28].
Model + si-SYT4 group: Model group mice were injected with 5 μL of si-SYT4 through the vitreous cavity.
Model + si-SYT4 + Bay K8644 group: Model group mice were injected with 5 μL of si-SYT4 through the vitreous cavity and were injected intraperitoneally with Bay K8644 (Sigma-Aldrich Corp., St. Louis, MO, United States) at a dose of 8 mg/kg.
The animal experiments were approved by the Animal Care and Use Committee of The People’s Hospital of ChuXiong Yi Autonomous Prefecture & The Fourth Affiliated Hospital of Dali University (approval No. 2022-08) and were conducted in accordance with the National Institutes of Health guidelines.
Cell culture and transfection
Thirty-two pairs of DR samples and adjacent normal retinal tissue were collected from the ophthalmology department of the First Affiliated Hospital of Kunming Medical University. ARPE-19 were purchased from Otwo Biotech Co., Ltd. (Shenzhen, China) and cultured in medium containing 10% fetal bovine serum, 100 U/mL penicillin and 100 μg/mL streptomycin; the medium was changed every 2-3 d. In addition, ARPE-19 cells were cultured in medium with 25 mmol/L glucose to establish a DR cell model (HG group)[29]. si-SYT4, oe-Parkin, or oe-MYT1 (Sangon Biotech, China) were transfected into ARPE-19 cells according to the instructions of the Lipofectamine 2000 transfection kit (Invitrogen, United States).
Cell viability assay
We used Cell Counting Kit-8 (CCK-8) assays (MCE, United States) to measure cell viability. First, the cells were added to a 96-well plate, after which 10 μL of CCK-8 solution was added, and the plate was incubated for 1 h. The absorbance at 450 nm was subsequently measured with a microplate reader (BioTek, United States).
TUNEL staining
Mouse retinal tissue was fixed in 1% formaldehyde and mixed with 0.2% Triton X-100. Then, the samples were treated with fluorescein 2’-deoxyuridine 5’-triphosphate (dUTP)-end labeling (ApoAlert DNA Fragment Analysis Kit; Clontech, Mountain View, CA, United States) and DAPI. Finally, the samples were observed and photographed by a fluorescence microscope (Nikon TE200-U, Tokyo, Japan).
Cell apoptosis was determined by flow cytometry
Apoptosis was assessed using the Annexin V-FITC/PI Apoptosis Detection Kit (American, Sigma-Aldrich). The cells were harvested and washed twice with cold phosphate-buffered saline. Then, 500 µL of binding buffer, 5 µL of Annexin V-FITC and 5 µL of PI were added according to the kit instructions. Subsequently, the cells were incubated for 10 min, after which the percentage of apoptotic cells was determined via flow cytometry.
Enzyme-linked immunosorbent assay
A multifactor ELISA kit (ExCell Biology, Shanghai, China) was used for analysis. Cell culture supernatants were collected and frozen at -80 °C. The levels of the inflammatory factor tumour necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), and IL-6 in each experimental group were quantified according to the manufacturer’s instructions.
Reverse transcriptase-polymerase chain reaction
Total RNA was extracted from tissues and cells by a one-step method with TRIzol reagent (Invitrogen, Carlsbad, CA, United States) and then reverse transcribed into cDNA using a PrimeScript™ RT kit (TaKaRa, Japan). The cDNA was subjected to reverse transcriptase-polymerase chain reaction (RT-qPCR) using SYBR Premix Ex Taq™ II (TaKaRa, Japan). The primer sequences are shown in Table 1.
Table 1.
Primer sequences
|
Gene
|
Forward (5′-3′)
|
Reverse (5′-3′) |
| Parkin | GGCTGACCAGTTGCGTGTGAT | GTGGTGAGTCCTTCCTGCTGTC |
| MYT1 | ACGAAGAGGAGGACGAGGAGGA | GGCAGAGGTGTGAGAGGTGTCT |
| SYT2 | TGCTTCTCACCTGCTGCTTCTG | CTGTCTCTGCGTCGTCGTCATC |
| SYT3 | CCAGTCACATCAGCAGGTCACA | GGTCTGAGAAGCCGTTGGAGTC |
| SYT4 | TCTCTGCTATCAGTCCACCACA | TTCCAGTGCTCTCCACCAGTTC |
| SYT7 | CCTCTGCCAACTCCATCATCGT | CCGCTCTTCCAGGACAGGTAGA |
| SYT11 | CACCTGCCGAAGATGGACATC | AGGTCGGTGGGGATGTCGTAG |
| SYT13 | CGCCTCCTGGTGGTGCTGATTA | CTTCCGAGCCTGGTGCTTCAAG |
| GAPDH | TGAGGACCAGGTTGTCTCCTGCG | CACCACCCTGTTGCTGTAGCCA |
SYT: Synaptotagmins; MYT1: Myelin transcription factor 1.
Western blot analysis
Total protein was extracted from tissues and cells in each experimental group using RIPA buffer (Sigma Aldrich, Cambridge, MA, United States), and the protein concentration was detected using a BCA kit (Sigma Aldrich, Cambridge, MA, United States). The proteins were next separated on a 10% SDS-Page gel and transferred to a PVDF membrane, which was then incubated with SYT2 (1/1000, EPR23920-2, ab259977), SYT3 (1/1000, ASV30, ab13259), SYT4 (1/100, ASV30, ab13259), SYT7 (1/1000, N275/14, ab174633), SYT11 (Anti-SYT11, 1/250, ab204589), SYT13 (1/500, Anti-SYT13, ab110520), GLUT4 (1/1000, EPR930(2), ab188317), GLUT1 (1/20000, EPR3915, ab115730), GLUT2 (1/10000, EPR16550, ab192599), GLUT3 (1/20000, EPR10508(N), ab191071), CAMK4 (1/1000000, EP2565AY, ab68218), p-CREB (1/500, E113, ab32096), Parkin (1/20000, PRK8, ab77924), MYT1 (1/50, anti-MyT1L, ab139732), and GAPDH (1/10000, EPR16891, ab181602) primary antibodies overnight at 4 °C. The membrane was then incubated with the appropriate secondary antibody for 1 h at room temperature, after which the optical density of the protein band was measured using an enhanced chemiluminescence Western blot Detection Kit (Bio-Rad, United States).
Glut1 localization assay
The cells were fixed in methanol for 10 min, washed twice with PBS, and then blocked in 1% goat serum/PBS for 1 h. Subsequently, the cells were incubated with GlUT1 antibody (1:250) for 1 h at room temperature, followed by washing steps and incubation with the secondary antibody (Alexa Fluor 594; 1:500; Life Technologies, Waltham, MA, United States) for 1 h at room temperature. The cells were then treated with DAPI. Images were obtained using a microscope (Nikon TE200-U, Tokyo, Japan).
SYT4 ubiquitination assay
The cells were cotransfected with SYT4 and Parkin-ubiquitin. Forty-eight hours after transfection, the cells were collected and lysed in RIPA buffer (Beyotime), and IP was performed on the lysate using anti-SYT4, followed by Western blot using anti-SYT4 antibodies.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 8 software (San Diego, CA, United States). The data were from at least three independent experiments and are shown as the mean ± SD. Differences between two groups were analyzed using Student’s t test, while differences between multiple groups were analyzed by one-way analysis of variance. P < 0.05 indicated a significant difference.
RESULTS
Expression of SYT family members in DR
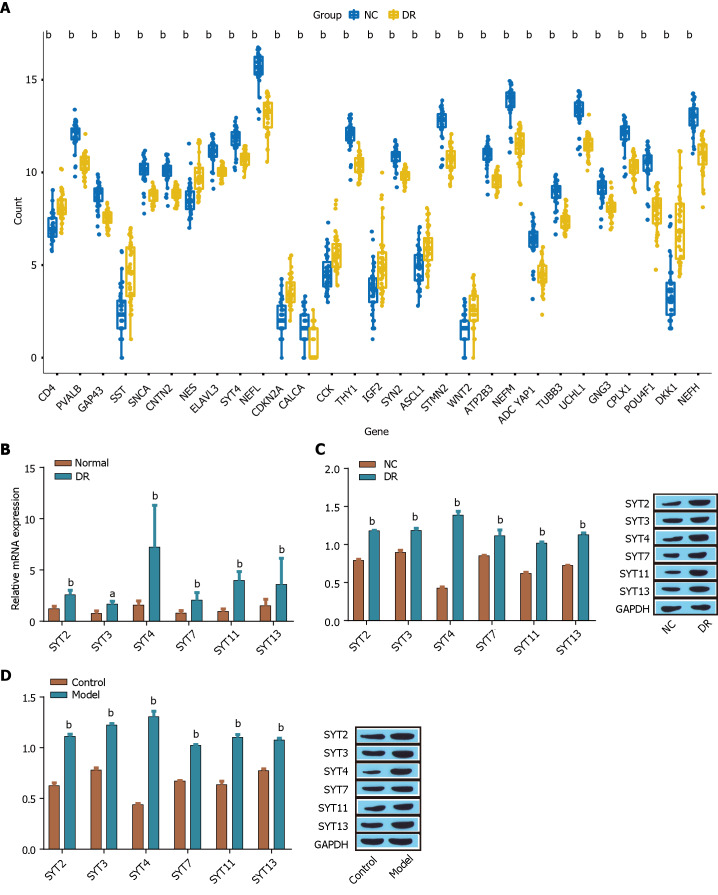
Six genes (SYT2, SYT3, SYT4, SYT7, SYT11, and SYT13) were differentially expressed in DR, as determined by a bioinformatics analysis of SYT family member (SYT1 to SYT17) expression in 32 pairs of human normal retinal tissues and diabetic retinal tissues (Figure 1A). Subsequently, the expression of SYT2, SYT3, SYT4, SYT7, SYT11, and SYT13 in 32 pairs of human normal retinal tissues and diabetic retinal tissues was analyzed by RT-qPCR, and the results showed that, compared with the other five differentially expressed genes, SYT4 was significantly upregulated in human diabetic retinal tissue (Figure 1B). In addition, we analyzed the expression of SYT2, SYT3, SYT4, SYT7, SYT11, and SYT13 in a DR ARPE-19 cell model induced by high glucose medium and a STZ-induced DR animal model by Western blot and found that the six differentially expressed proteins were highly expressed in the DR model groups compared with the control groups both in vitro and in vivo. SYT4 was significantly upregulated (Figure 1C and D). These results demonstrated that six members of the SYT family (SYT2, SYT3, SYT4, SYT7, SYT11, and SYT13) were differentially expressed in DR and that SYT4 was highly expressed in DR samples and in both in vitro and in vivo models of DR. Therefore, we next focused on the impact of SYT4 on the progression of DR.
Figure 1.
Expression of synaptotagmins family members in diabetic retinopathy. A: The expression of synaptotagmins 1 (SYT1)-SYT17 was analyzed to identify differentially expressed proteins; B: The expression of SYT2, SYT3, SYT4, SYT7, SYT11, and SYT13 was measured via reverse transcriptase-polymerase chain reaction; C and D: The expression of SYT2, SYT3, SYT4, SYT7, SYT11, and SYT13 in ARPE-19 cells (C) induced by high glucose was detected by Western blot, and the expression in the retinas of diabetic retinopathy model mice (D) induced by streptozotocin was detected by Western blot. aP < 0.01, bP < 0.001, vs the normal group, control group, NC group. SYT: Synaptotagmins; DR: Diabetic retinopathy.
SYT4 promotes cellular glucose uptake by ARPE-19 cells under high glucose conditions
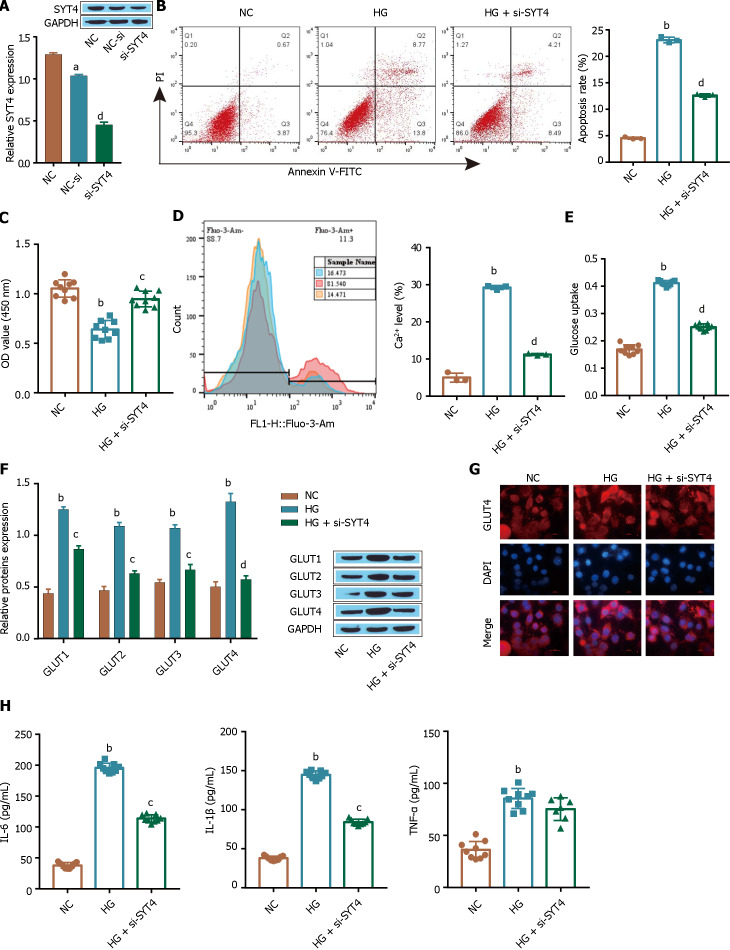
The transfection efficiency of SYT4 was examined by Western blot, and as shown in Figure 2A, knockdown of SYT4 in ARPE-19 cells significantly downregulated the expression of SYT4 compared with that in the control group (Figure 2A). Flow cytometry and CCK-8 assays showed that, compared with the control treatment, HG treatment significantly promoted ARPE-19 cell apoptosis (Figure 2B) and inhibited cell proliferation (Figure 2C), and these effects were reversed in the si-SYT4-treated group. In addition, the level of Ca2+ was significantly increased in HG-treated cells compared with control cells (Figure 2D), and glucose uptake by ARPE-19 cells was significantly increased under HG conditions (Figure 2E). Furthermore, Western blot showed that, compared with those in the control group, the expression of the transporter GLUT1 in ARPE-19 cells was significantly higher, and the protein expression level of GLUT1 was higher than that of the other three transporters (Figure 2F). Consistent with these findings, HG induced GLUT1 membrane translocation in ARPE-19 cells, as shown by immunofluorescence analysis (Figure 2G). Notably, these changes were also reversed in the si-SYT4-treated group. In addition, compared with the control treatment, HG significantly promoted the inflammatory response and increased the levels of the inflammatory factors TNF-α, IL-1β, and IL-6, as detected by ELISA (Figure 2H), and these changes were consistently blocked in the si-SYT4-treated group. These results suggest that under high glucose conditions, ARPE-19 cell proliferation is inhibited and ARPE-19 apoptosis is increased, which may be due to the SYT4-induced influx of intracellular Ca2+ triggered by high glucose concentrations, thus inducing excessive cellular glucose uptake and inflammation.
Figure 2.
Synaptotagmins 4 promotes high glucose-induced cellular glucose uptake by ARPE-19 cells. A: The expression of synaptotagmins 4 (SYT4) was detected via Western blot; B: Apoptosis was observed by flow cytometry; C: A Cell Counting Kit-8 assay was used to determine cell viability; D: Flow cytometry was used to determine intracellular Ca2+ levels; E: Glucose uptake levels were determined; F: Western blot was used to detect the glucose transporter; G: Immunofluorescence analysis was used to examine the extent of GLUT1 membrane translocation; H: The levels of inflammatory cytokines [tumour necrosis factor alpha, interleukin (IL)-1β, and IL-6] were measured via ELISA. aP < 0.05, bP < 0.001, vs the NC group; cP < 0.01, dP < 0.01 vs the NC group, HG group. SYT: Synaptotagmins; GLUT1: Glucose transporter-1; TNF-α: Tumour necrosis factor alpha; IL: Interleukin.
Ca2+ signaling is required for the increased glucose uptake by ARPE-19 cells in high glucose conditions
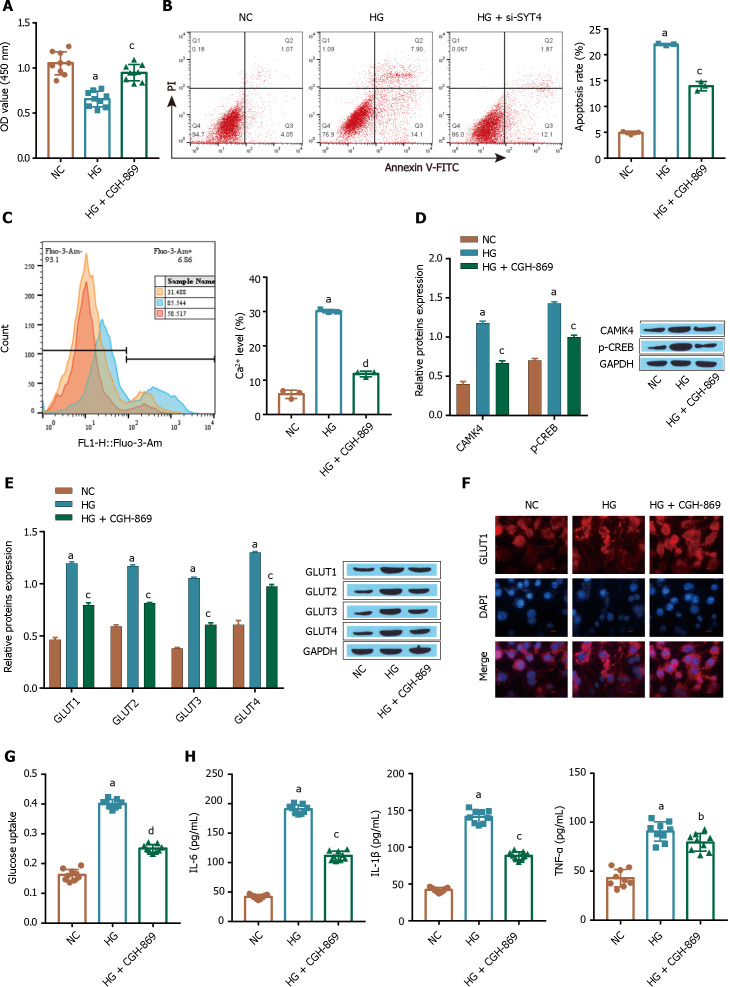
An increase in the intracellular Ca2+ concentration can upregulate the stress response gene GLUT1[13]. Next, we investigated whether the Ca2+ signaling pathway affects HG-induced cellular glucose uptake in ARPE-19 cells through GLUT1. The flow cytometry and CCK-8 results showed that compared to the control treatment, treatment with the Ca2+ signaling pathway inhibitor CGH-869 significantly promoted the proliferation of ARPE-19 cells (Figure 3A) and inhibited apoptosis (Figure 3B). The flow cytometry, Western blot and glucose assay results showed that treatment with the Ca2+ signaling pathway inhibitor CGH-869 reduced the intracellular Ca2+ levels induced by HG (Figure 3C) and inhibited the protein expression of CAMK4 and p-CREB, which are related to the Ca2+ signaling pathway (Figure 3D). Furthermore, treatment with a Ca2+ signaling pathway inhibitor (CGH-869) reduced the expression level of the transporter GLUT1 in HG-treated ARPE-19 cells (Figure 3E) and inhibited the GLUT1 membrane transfer induced by HG (Figure 3F), significantly alleviating the excessive uptake of glucose by ARPE-19 cells (Figure 3G). In addition, we found that CGH-869 significantly inhibited the HG-induced inflammatory response and reduced the levels of the inflammatory cytokines TNF-α, IL-1β, and IL-6 in the treatment group (Figure 3H). These results suggest that high glucose induces apoptosis and inhibits the proliferation of ARPE-19 cells, which may be achieved by GLUT1 translocation via the Ca2+ signaling pathway, excessive glucose uptake by ARPE-19 cells, and increased inflammation.
Figure 3.
Ca2+ signaling is required for the increased glucose uptake by ARPE-19 cells in high glucose conditions. A: A Cell Counting Kit-8 assay was used to determine cell viability; B: Flow cytometry was used to determine apoptosis; C: Intracellular Ca2+ levels were determined by flow cytometry; D: The Ca2+ signaling pathway-related proteins CAMK4 and p-CREB were detected via Western blot; E: The glucose transporter was detected via Western blot; F: The membrane translocation of GlUT1 was examined by immunofluorescence analysis; G: ELISA was used to measure the levels of inflammatory factors [tumour necrosis factor alpha, interleukin (IL)-1β, and IL-6]; H: ELISA was used to measure the level of glucose uptake. aP < 0.001, vs the NC group; bP < 0.01, cP < 0.01, dP < 0.001 vs the HG group. GLUT1: Glucose transporter-1; TNF-α: Tumour necrosis factor alpha; IL: Interleukin.
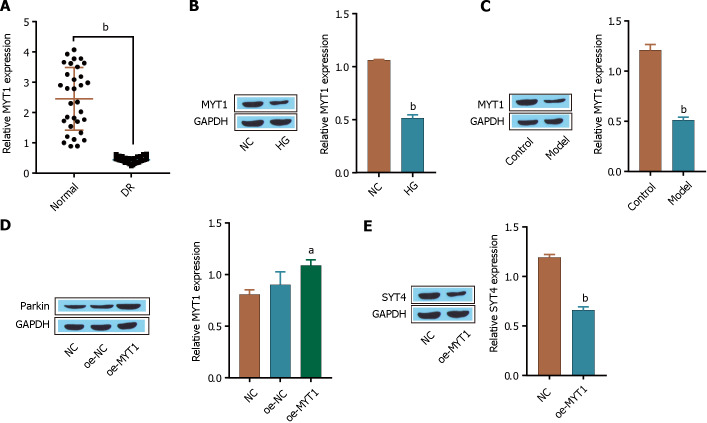
Parkin promotes the ubiquitination and degradation of SYT4
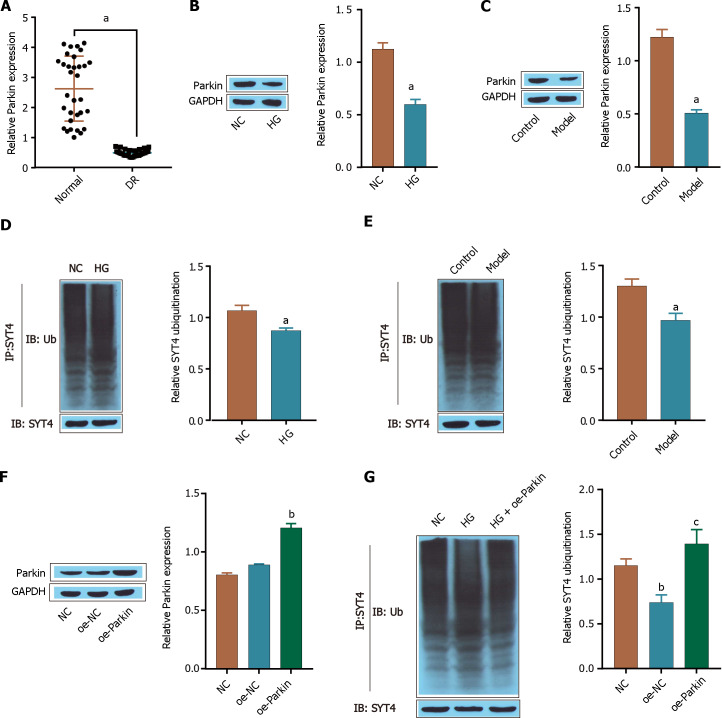
Parkin is an E3 ubiquitin ligase. In Parkinson’s disease (PD), parkin interacts with and polyubiquitinates SYT4, thereby accelerating its proteoglycan degradation[30]. In this study, we investigated whether Parkin plays a role in the ubiquitination-mediated degradation of SYT4 in the context of DR. A total of 32 pairs of human normal and diabetic retinal tissues were analyzed via RT-qPCR, which revealed that Parkin was downregulated in human diabetic retinal tissue clinical samples (Figure 4A). Furthermore, Western blot revealed that Parkin was expressed at low levels in high glucose-treated ARPE-19 cells and in STZ-induced DR model mice (Figure 4B and C). We also found that the ubiquitination-mediated degradation of SYT4 was significantly inhibited in high glucose-treated ARPE-19 cells compared with that in the NC group (Figure 4D). Consistently, the ubiquitination-mediated degradation of SYT4 was significantly inhibited in STZ-induced DR mouse retinal tissue compared to that in control tissue (Figure 4E). In addition, oe-Parkin significantly upregulated the expression of Parkin compared with that in the oe-NC group (Figure 4F). Western blot further revealed that oe-Parkin significantly promoted the ubiquitination-mediated degradation of SYT4 compared with that in the HG group (Figure 4G). These results indicate that Parkin is downregulated in DR and that oe-Parkin promotes the ubiquitination of SYT4.
Figure 4.
Parkin promotes the ubiquitination and degradation of synaptotagmins 4. A: reverse transcriptase-polymerase chain reaction was used to measure the expression of Parkin; B: Western blot was used to detect the expression of Parkin in cells; C: Western blot was used to determine the expression of Parkin in the tissues of the mice; D: Western blot was used to determine the level of synaptotagmins 4 (SYT4) ubiquitination in cells; E: Western blot was used to determine the level of SYT4 ubiquitination in the tissues of mice; F: The transfection efficiency of oe-Parkin was detected via Western blot; G: The level of SYT4 ubiquitination in cells was detected via Western blot. aP < 0.05, bP < 0.01, cP < 0.001, vs normal group, control group, NC group; cP < 0.001, vs HG group. SYT: Synaptotagmins; DR: Diabetic retinopathy; TNF-α: Tumour necrosis factor alpha; IL: Interleukin.
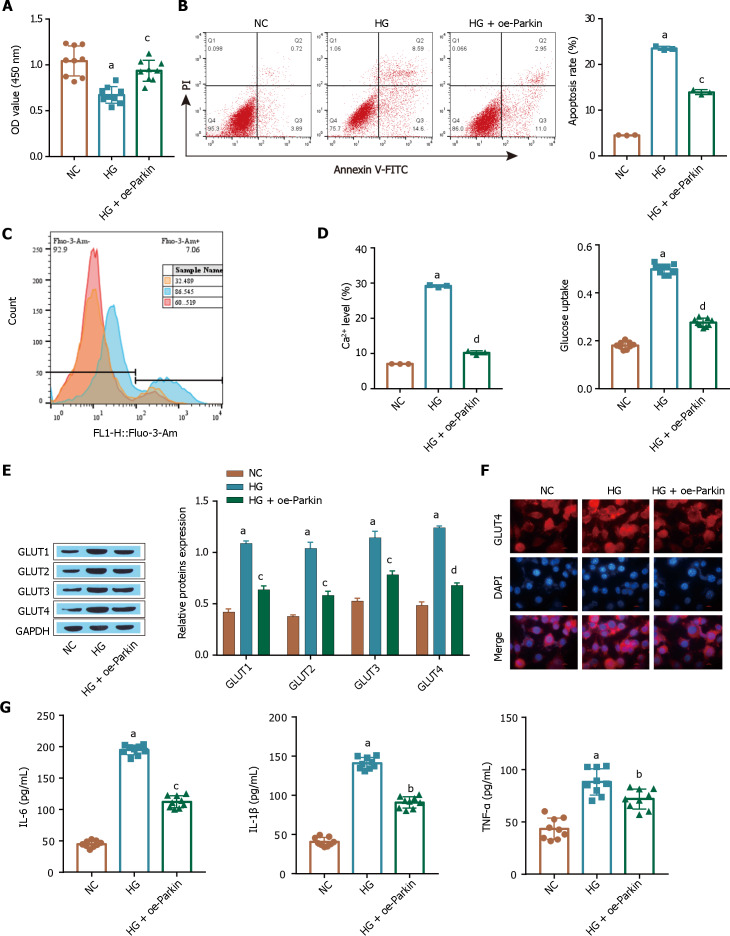
Parkin overexpression inhibits the increased glucose uptake by ARPE-19 cells induced by high glucose
The flow cytometry and CCK-8 results showed that HG treatment inhibited ARPE-19 cell proliferation (Figure 5A) and promoted apoptosis (Figure 5B), and these changes were reversed in the oe-Parkin treatment group. Compared with those in the HG group, the HG-induced intracellular Ca2+ levels in the oe-Parkin treatment group were reduced (Figure 5C), and oe-Parkin alleviated the excessive glucose uptake by ARPE-19 cells (Figure 5D). Oe-Parkin treatment downregulated the expression of the transporter GLUT1 in ARPE-19 cells, as shown by the Western blot and immunofluorescence analyses, compared to that in the control cells under HG conditions (Figure 5E), and inhibited HG-induced GLUT1 translocation in ARPE-19 cells (Figure 5F). In addition, the HG-induced inflammatory response was inhibited in the oe-Parkin treatment group, and the levels of the inflammatory factors TNF-α, IL-1β, and IL-6 were significantly inhibited (Figure 5G). These results suggest that high glucose induces apoptosis and inhibits the proliferation of ARPE-19 cells, possibly through the inhibition of Parkin expression, which further increases intracellular Ca2+ influx and induces excessive cellular glucose uptake and an inflammatory response.
Figure 5.
Parkin overexpression inhibits the excessive glucose uptake by ARPE-19 cells induced by high glucose. A: A Cell Counting Kit-8 assay was used to determine cell viability; B: Flow cytometry was used to determine apoptosis; C: Intracellular Ca2+ levels were determined by flow cytometry; D: Glucose uptake was detected by a kit; E: GLUT1 expression was detected via Western blot; F: The extent of glucose transporter-1 membrane transfer was examined by immunofluorescence analysis; G: The levels of inflammatory factors [tumour necrosis factor alpha, interleukin (IL)-1β, and IL-6] were measured via ELISA. aP < 0.001, vs the NC group; bP < 0.01, cP < 0.01, dP < 0.001 vs the HG group. GLUT1: Glucose transporter-1; TNF-α: Tumour necrosis factor alpha; IL: Interleukin.
The transcription factor MYT1 regulates SYT4 expression
The SYT and transcription factor SYT4 has been reported to be regulated by Myt1 in neonatal mammalian islet beta cells[27]. In this study, we aimed to investigate whether the expression of SYT4 in DR is regulated by the transcription factor MYT1. A total of 32 pairs of human normal and diabetic retinal tissues were analyzed by RT-qPCR, which revealed that MYT1 was downregulated in clinical DR samples (Figure 6A). Furthermore, Western blot showed that MYT1 expression was low in ARPE-19 cell treated with high glucose medium and in the DR model mice (Figure 6B and C). In addition, oe-MYT1 significantly upregulated the expression of MYT1 compared with that in the oe-NC group (Figure 6D). Furthermore, Western blot showed that SYT4 was significantly low expressed in the oe-MYT1 group compared to the HG group (Figure 6E). These results indicate that MYT1 is downregulated in DR and that oe-MYT1 inhibits SYT4 transcription.
Figure 6.
The transcription factor myelin transcription factor 1 regulates synaptotagmins 4 expression. A: reverse transcriptase-polymerase chain reaction was used to measure the expression of myelin transcription factor 1 (MYT1); B: Western blot was used to detect the expression of MYT1 in cells; C: Western blot was used to determine the expression of MYT1 in mouse tissues; D: Western blot was used to determine the transfection efficiency of MYT1 in cells; E: The expression of synaptotagmins 4 was detected by Western blot. aP < 0.05, bP < 0.001, vs normal group, control group, NC group. DR: Diabetic retinopathy; MYT1: Myelin transcription factor 1.
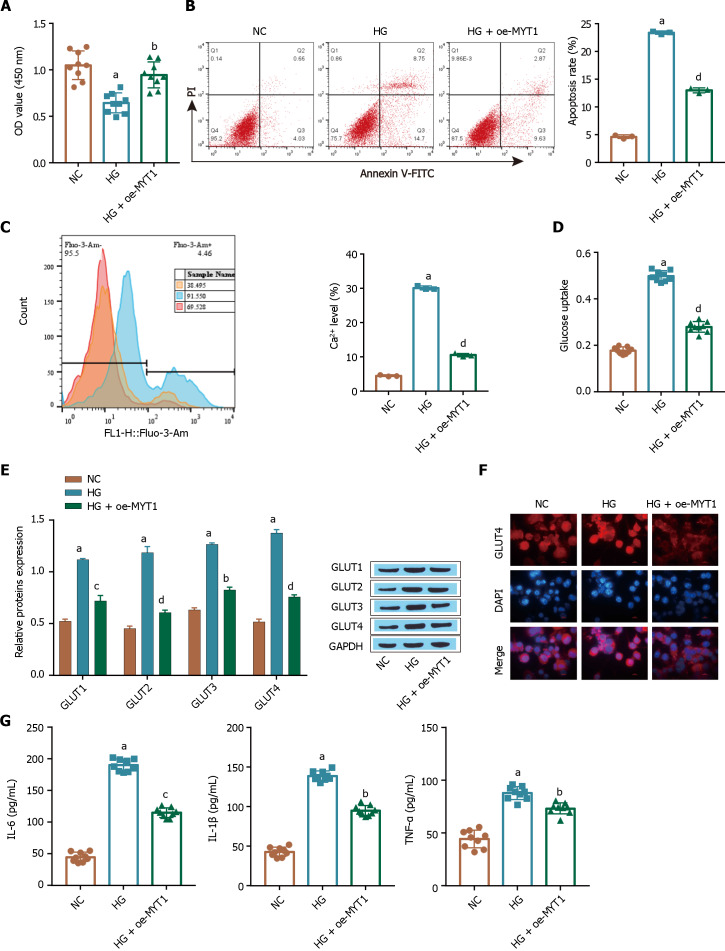
Overexpression of MYT1 inhibits excessive glucose uptake by ARPE-19 cells under high glucose conditions
The flow cytometry and CCK-8 results showed that compared with the HG treatment, the oe-MYT1 treatment significantly promoted the proliferation of ARPE-19 cells (Figure 7A) and reduced the apoptosis of ARPE-19 cells (Figure 7B). In addition, compared with those in the HG-treated cells, the HG-induced intracellular Ca2+ levels in the oe-MYT1-treated cells were decreased (Figure 7C), and oe-MYT1 treatment alleviated the excessive glucose uptake by ARPE-19 cells (Figure 7D), as shown by flow cytometry, Western blot analysis and glucose assays. Compared with the HG treatment, the oe-MYT1 treatment downregulated the expression of the transporter GLUT1 in ARPE-19 cells (Figure 7E) and inhibited HG-induced GLUT1 translocation in ARPE-19 cells (Figure 7F), as shown by Western blot and immunofluorescence analysis. In addition, the HG-induced inflammatory response was inhibited in the oe-MYT1 treatment group, and the levels of the inflammatory factors TNF-α, IL-1β, and IL-6 were significantly reduced (Figure 7G). These results suggest that high glucose induces apoptosis and inhibits the proliferation of ARPE-19 cells, which may be caused by the inhibition of MYT1 expression, which further increases intracellular Ca2+ influx, induces excessive cellular glucose uptake, and increases inflammation.
Figure 7.
Myelin transcription factor 1 overexpression inhibits high glucose-induced cellular glucose uptake by ARPE-19 cells. A: A Cell Counting Kit-8 assay was used to determine cell viability; B: Flow cytometry was used to determine apoptosis; C: Intracellular Ca2+ levels were determined by flow cytometry; D: Glucose uptake was detected by a kit; E: Glucose transporter-1 (GLUT1) expression was detected via Western blot; F: The extent of GlUT1 membrane transfer was examined by immunofluorescence analysis; G: The levels of inflammatory factors [tumour necrosis factor alpha, interleukin (IL)-1β, and IL-6] were measured via ELISA. aP < 0.001, vs the NC group; bP < 0.01, cP < 0.01, dP < 0.001 vs the HG group. MYT1: Myelin transcription factor 1; GLUT1: Glucose transporter-1; TNF-α: Tumour necrosis factor alpha; IL: Interleukin.
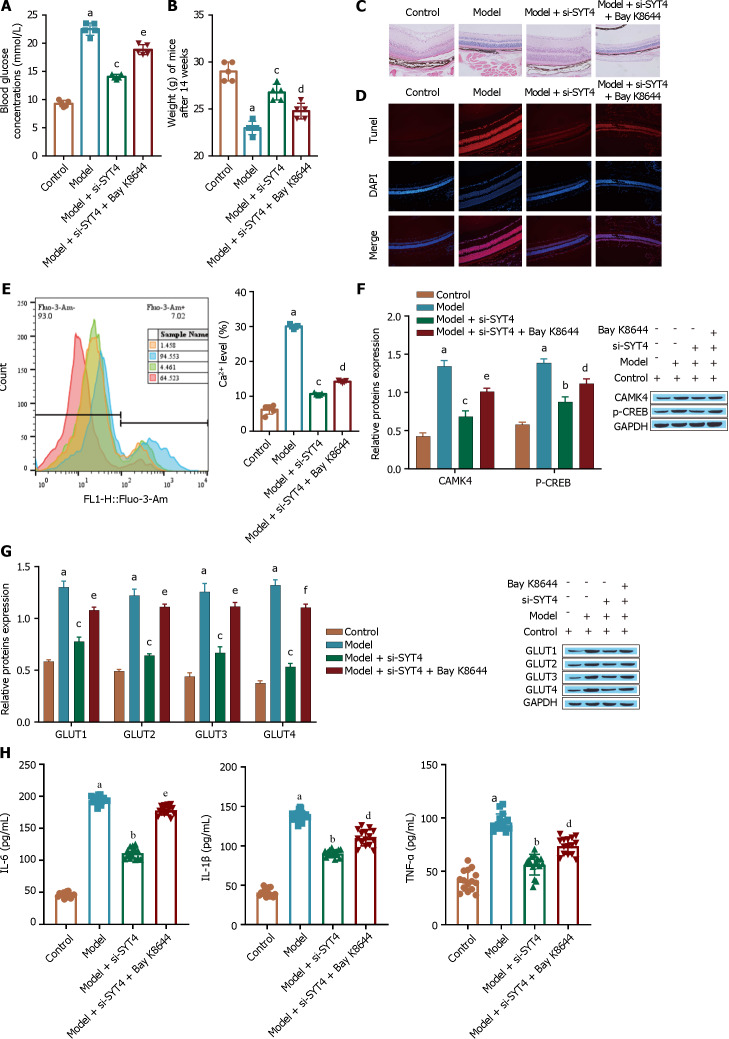
Protective effect of SYT4 knockdown against DR
We verified the effect of SYT4 on DR in animal experiments. Compared to mice in the model group, mice in the si-SYT4-treated group had significantly lower blood glucose levels (Figure 8A) and significantly higher body weights (Figure 8B). HE staining of mouse retinopathy tissue showed that, compared with those in the model group, the retinas in the si-SYT4 treatment group were significantly thicker, the cells in the entire retinal layer were neatly arranged, and the tissue morphology was significantly restored (Figure 8C). Moreover, si-SYT4 treatment significantly inhibited STZ-induced apoptosis (Figure 8D). However, these changes were abrogated by a calcium channel agonist (Bay K8644). In addition, compared with the model group, the si-SYT4 treatment group exhibited reduced levels of Ca2+ (Figure 8E), downregulated expression of the Ca2+ signaling pathway-related proteins CAMK4 and p-CREB (Figure 8F), and significantly inhibited expression of the transporter GLUT1 (Figure 8G). These changes were abrogated in the Bay K8644 treatment group. In addition, si-SYT4 treatment significantly inhibited the inflammatory response and reduced the levels of the inflammatory cytokines TNF-α, IL-1β, and IL-6 compared to those in the model group (Figure 8H), as measured by ELISA. Consistent with these findings, these changes were blocked by Bay K8644 treatment. These experimental results indicate that SYT4 knockdown protects against DR.
Figure 8.
Animal experiment to verify the inhibition of diabetic retinopathy by synaptotagmins 4 knockdown. A: A kit was used to measure the blood glucose levels of the mice; B: Electronic weighing was used to evaluate the mice; C: HE staining was used to observe retinal structure; D: TUNEL staining was performed to evaluate cell apoptosis; E: Flow cytometry was used to determine Ca2+ levels; F: Western blot was used to detect the Ca2+ signaling pathway-related proteins CAMK4 and p-CREB; G: The glucose transporter was detected via Western blot; H: The levels of inflammatory cytokines [tumour necrosis factor alpha, interleukin (IL)-1β, and IL-6] were measured via ELISA. aP < 0.001, vs the control group; bP < 0.01, cP < 0.001 vs the model group; dP < 0.05, eP < 0.01, fP < 0.001 vs the model + si- synaptotagmins 4 group. SYT: Synaptotagmins; TNF-α: Tumour necrosis factor alpha; IL: Interleukin.
DISCUSSION
The retinal pigment epithelium (RPE) plays a crucial role in retinal homeostasis by influencing the function and maintenance of photoreceptors and the capillary endothelium[31]. Dysfunction of the RPE barrier caused by excessive cellular uptake of glucose through the insulin-independent GLUT is the leading cause of DR[32]. Previous studies have shown that insulin is secreted in response to glucose stimulation and is accompanied by increased intracellular ATP levels during cellular glucose uptake[33]. An increase in ATP levels leads to membrane depolarization and the opening of Ca2+ channels, resulting in the influx of extracellular Ca2+. An increase in intracellular Ca2+ promotes the fusion of GLUT1 with the plasma membrane, mediating excessive glucose uptake[34-36]. In this study, in vivo and in vitro experiments revealed that high expression of the Ca2+ signaling pathway-related proteins CAMK4 and p-CREB and high Ca2+ influx induced the rapid fusion of GLUT1 vesicles with the plasma membrane, resulting in high expression of the glucose transporter GLUT1 and promoting the excessive uptake of glucose by ARPE-19 cells, further impairing ARPE-19 cells, and promoting inflammation. Our results indicate that the Ca2+ signaling pathway promotes high glucose uptake by ARPE-19 cells through GLUT1, resulting in ARPE-19 injury and inflammation and accelerating DR progression.
The SYT protein family plays a key role in regulating membrane trafficking at neuronal synapses. In recent years, SYT family members have been shown to be involved in the pathogenesis and progression of human diseases. Specifically, Sung et al[37] demonstrated that SYT2 was upregulated in ovarian cancer, promoted the migration and invasion of ovarian cancer cells and was associated with poor survival in ovarian cancer patients. SYT7 is overexpressed in lung cancer, colorectal cancer and glioma and promotes cell proliferation and inhibits apoptosis, resulting in poor prognosis[38,39]. SYT4 is upregulated in triple-negative breast cancer, confers resistance to paclitaxel, and contributes to a poor prognosis[40]. However, few studies have assessed the impact of SYT family members on DR progression. In this study, we investigated the effect of SYT family members on DR. Bioinformatics analysis (SYT1-SYT17) revealed the differential expression of 6 members of the SYT family: SYT2, SYT3, SYT4, SYT7, SYT11, and SYT13. In particular, SYT4 was highly expressed in the tissues and cells of DR patients. Therefore, we focused on the effect of SYT4 on glucose transport in RPE cells during DR.
Accumulating evidence suggests that a transient increase in intracellular Ca2+ triggers and accelerates clathrin-dependent and clathrin-independent endocytosis in neurons and neuroendocrine cells[41,42]. Several endocytic Ca2+ sensors and effectors can initiate and mediate Ca2+-dependent endocytosis. Among them, SYTs are a family of type I membrane proteins with an evolutionarily conserved cytoplasmic tandem C2 domain (C2A and C2B) and are well-characterized Ca2+ sensors[17]. SYT4, which is a member of the SYT family, has been reported to be present in vesicles that contain synaptic plasticity and growth regulators, mediating the delivery of Ca2+-dependent factors to postsynaptic cells via exosomal anterograde delivery of SYT4 and regulating the fusion pores and fusion patterns of endocrine cells and neurons[43,44]. In Drosophila, the Ca2+-dependent retrograde signaling pathway is dependent on the postsynaptic Ca2+ sensor SYT4, and the loss of SYT4 results in abnormal development and function of the NMJ[45]. In addition, SYT4 is involved in the regulation of secretory events in several other mammalian cell types, including insulin-stimulated glucose transporter delivery to the plasma membrane in adipocytes and glucose-stimulated insulin secretion by pancreatic beta cells[27]. Consistently, in the present study, we found that SYT4 induced an increase in Ca2+ levels in RPE cells under high glucose conditions, promoting GLUT1 vesicle fusion with the plasma membrane and cellular glucose uptake.
Ubiquitination is a posttranslational modification that mediates the degradation of proteins, typically through the covalent attachment of ubiquitin and ubiquitin chains to lysine residues or to the N-terminal amino group of the substrate protein[46]. In the ubiquitination pathway, E3 ubiquitin ligases typically provide most of the specificity and regulation by recognizing substrates and controlling activity[47]. Parkin is an E3 ubiquitin ligase that induces proteasomal degradation of its substrates by polyubiquitination[48] and mitochondrial autophagy[49]. It has been suggested that abnormal accumulation of Parkin substrates can contribute to DA-related neuronal degeneration in PD patients and is associated with Parkin mutations[50]. In addition, SYTs have been extensively studied as Parkin substrates in PD. Wang et al[51] showed that parkin dysfunction leads to the accumulation of Syt11, which inhibits endocytosis, and the release of DA by DA neurons, ultimately initiating the pathogenesis of PD. Huynh et al[21] identified Syt XI as a substrate of Parkin whose turnover is accelerated by Parkin-mediated polyubiquitination. Interestingly, recent studies have also reported that Syt IV, which is an isoform that is highly homologous to Syt XI, can also bind to the C2A and C2B domains of Syt IV and promote its proteasomal degradation through parkin polyubiquitination[30]. Therefore, we examined whether the excessive accumulation of SYT4 in DR was related to Parkin. As expected, we found that Parkin expression was significantly downregulated in DR. Furthermore, in cells transfected with the Parkin overexpression plasmid, we found that Parkin overexpression promoted the ubiquitination and degradation of SYT4 and significantly reduced SYT4-induced Ca2+ levels, GLUT1 membrane translocation, glucose uptake, apoptosis, and inflammation. Our data suggest that Parkin deficiency is an important factor in the excessive accumulation of SYT4 in DR and that Parkin deficiency inhibits SYT4 proteasomal degradation and promotes GLUT1 membrane translocation, which in turn promotes cellular glucose uptake.
In recent years, SYT4 has attracted much attention as a new intervention target in diabetes-related diseases[52,53]. However, the mechanism regulating SYT4 expression has not yet been determined. A previous study showed that SYT4 expression affects pancreatic beta cell insulin secretion and that the expression of SYT4 may be regulated by the transcription factor Myt1[27]. Therefore, in this study, we focused on whether the high expression of SYT4 in DR was related to the transcription factor MYT1. Overexpression of MYT1 inhibited SYT4 transcription in ARPE-19 cells, which in turn inhibited Ca2+-induced GLUT1 fusion with the plasma membrane, attenuated GLUT1 translocation, and downregulated the protein expression of the glucose transporter GLUT1. High glucose-induced glucose uptake by ARPE-19 cells was inhibited. Notably, SYTs often act synergistically by binding to multiple family members, so it is not clear whether MYT1 directly inhibits SYT4 transcription in ARPE-19 cells. Mall et al[25] showed that MYT1L binds to the promoters of several SYT genes (SYT1, 2, 3, 7, and 12) and downregulates their expression in fibroblasts. We hypothesized that MYT1 inhibits SYT4 expression via a similar mechanism in ARPE-19 cells. Furthermore, different cell types use different signals to activate SYT4 expression[52]. Therefore, further identification of the unknown factors and mechanisms controlling SYT4 expression is needed to fully understand SYT4-mediated stimulus-secretion coupling in DR.
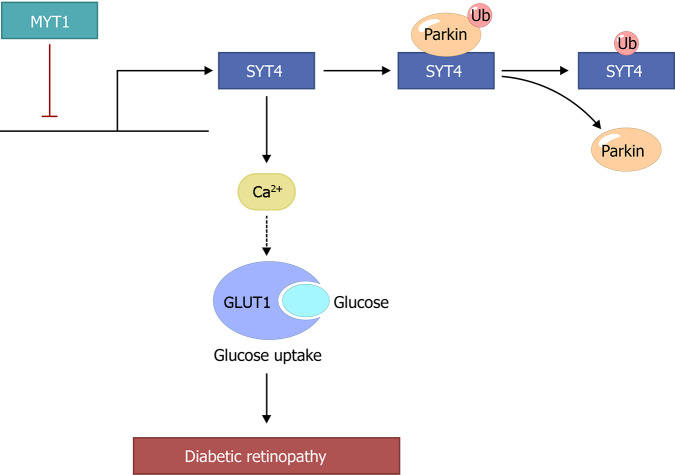
In this study, we provide evidence that hyperglycemia-mediated overexpression of SYT4 manipulates Ca2+ influx to induce the fusion of GLUT1 to the plasma membrane, promote abnormal glucose transporter expression and cellular glucose uptake, and enhance the inflammatory response in cells, which in turn promotes DR progression (Figure 9). In addition, our results showed that in our DR cell model, the lack of Parkin inhibited the proteasome-mediated degradation of SYT4 and promoted the fusion of GLUT1 with the plasma membrane, which upregulated the expression of GLUT1. Furthermore, we also found that the regulatory mechanism of the abnormally high SYT4 expression was related to the dysregulation of the transcription factor MYT1 in DR and that the transcription of SYT4 was repressed by MYT1, playing a key role in inhibiting SYT4-mediated stimulus-secretion coupling.
Figure 9.
The mechanism of action of synaptotagmins 4 in diabetic retinopathy. SYT: Synaptotagmins; MYT1: Myelin transcription factor 1; GLUT1: Glucose transporter-1.
CONCLUSION
In conclusion, our study reveals the key role of SYT4 in regulating glucose transport in retinal pigment epithelial cells during the pathogenesis of DR and provides insights into potential therapeutic targets for clinical DR.
ARTICLE HIGHLIGHTS
Research background
Synaptotagmins (SYTs) are a family of 17 membrane transporters that function as calcium ion sensors during the release of Ca2+-dependent neurotransmitters and hormones. However, few studies have investigated whether members of the SYT family play a role in glucose uptake in diabetic retinopathy (DR) through Ca2+/glucose transporter-1 (GLUT1) and the possible related mechanisms.
Research motivation
To elucidate the role of the SYT family in the regulation of glucose transport in retinal pigment epithelial cells and explore its potential as a therapeutic target for the clinical management of DR.
Research objectives
To elucidate the role of SYT4 in the regulation of glucose transport in retinal pigment epithelial cells.
Research methods
DR was induced by streptozotocin in C57BL/6J mice in vivo and by high glucose medium in human retinal pigment epithelial cells (ARPE-19) in vitro. Bioinformatics analysis, reverse transcriptase-polymerase chain reaction, Western blot, flow cytometry, ELISA, HE staining and TUNEL staining were used for analysis.
Research results
Six proteins (SYT2, SYT3, SYT4, SYT7, SYT11, and SYT13) were found to be differentially expressed in DR, and SYT4 was highly expressed. Hyperglycemia induces the overexpression of SYT4, manipulates Ca2+ influx to induce the fusion of GLUT1 with the plasma membrane, promotes the abnormal expression of the glucose transporter GLUT1 and excessive cellular glucose uptake, induces ARPE-19 cell apoptosis, and promotes the progression of DR. Parkin deficiency inhibits the proteasomal degradation of SYT4 in DR, resulting in SYT4 accumulation and promoting GLUT1 fusion to the plasma membrane, and this process is blocked by oe-Parkin treatment. Moreover, dysregulation of Myelin transcription factor 1 (Myt1)-induced transcription of SYT4 in DR further activated the SYT4-mediated stimulus-secretion coupling process, and this process was inhibited in the oe-MYT1-treated group.
Research conclusions
The hyperglycemia-mediated overexpression of SYT4 manipulates Ca2+ influx to induce the fusion of GLUT1 to the plasma membrane, promote abnormal glucose transporter expression and cellular glucose uptake, and enhance the inflammatory response in cells, which in turn promotes DR progression. A lack of Parkin inhibited the proteasome-mediated degradation of SYT4 and promoted the fusion of GLUT1 with the plasma membrane, which upregulated the expression of GLUT1. Furthermore, the regulatory mechanism of the abnormally high expression of SYT4 was related to the dysregulation of the transcription factor Myt1.
Research perspectives
Our study reveals the key role of SYT4 in regulating glucose transport in retinal pigment epithelial cells during the pathogenesis of DR and the underlying mechanism and suggests potential therapeutic targets for clinical DR.
Footnotes
Institutional review board statement: This study was reviewed and approved by the Institutional Review Board of The People’s Hospital of Chuxiong Yi Autonomous Prefecture (Approval No. 2022-08).
Institutional animal care and use committee statement: All procedures involving animals were approved by the Animal Care and Use Committee of the People’s Hospital of Chuxiong Yi Autonomous Prefecture (Approval No. 2022-08).
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
ARRIVE guidelines statement: The authors have read the ARRIVE guidelines, and the manuscript was prepared and revised according to the ARRIVE guidelines.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: October 19, 2023
First decision: January 12, 2024
Article in press: March 11, 2024
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sun XD, China; Ye Y, China S-Editor: Chen YL L-Editor: A P-Editor: Chen YX
Contributor Information
Hong Xu, Department of Ophthalmology, The People’s Hospital of Chuxiong Yi Autonomous Prefecture & The Fourth Affiliated Hospital of Dali University, Chuxiong Yi Autonomous Prefecture 675000, Yunnan Province, China.
Li-Bo Zhang, Department of Ophthalmology, The People’s Hospital of Chuxiong Yi Autonomous Prefecture & The Fourth Affiliated Hospital of Dali University, Chuxiong Yi Autonomous Prefecture 675000, Yunnan Province, China.
Yi-Yi Luo, Precision Medicine Center of Chuxiong Yi Autonomous Prefecture, The People’s Hospital of Chuxiong Yi Autonomous Prefecture & The Fourth Affiliated Hospital of Dali University, Chuxiong Yi Autonomous Prefecture 675000, Yunnan Province, China.
Ling Wang, Department of Endocrinology, The People’s Hospital of Chuxiong Yi Autonomous Prefecture & The Fourth Affiliated Hospital of Dali University, Chuxiong Yi Autonomous Prefecture 675000, Yunnan Province, China.
Ye-Pin Zhang, Department of Pathology, The People’s Hospital of Chuxiong Yi Autonomous Prefecture & The Fourth Affiliated Hospital of Dali University, Chuxiong Yi Autonomous Prefecture 675000, Yunnan Province, China.
Pei-Qi Chen, Department of Endocrinology, The People’s Hospital of Chuxiong Yi Autonomous Prefecture & The Fourth Affiliated Hospital of Dali University, Chuxiong Yi Autonomous Prefecture 675000, Yunnan Province, China.
Xue-Ying Ba, Precision Medicine Center of Chuxiong Yi Autonomous Prefecture, The People’s Hospital of Chuxiong Yi Autonomous Prefecture & The Fourth Affiliated Hospital of Dali University, Chuxiong Yi Autonomous Prefecture 675000, Yunnan Province, China.
Jian Han, Precision Medicine Center of Chuxiong Yi Autonomous Prefecture, The People’s Hospital of Chuxiong Yi Autonomous Prefecture & The Fourth Affiliated Hospital of Dali University, Chuxiong Yi Autonomous Prefecture 675000, Yunnan Province, China.
Heng Luo, Department of Ophthalmology, The People’s Hospital of Chuxiong Yi Autonomous Prefecture & The Fourth Affiliated Hospital of Dali University, Chuxiong Yi Autonomous Prefecture 675000, Yunnan Province, China; Precision Medicine Center of Chuxiong Yi Autonomous Prefecture, The People’s Hospital of Chuxiong Yi Autonomous Prefecture & The Fourth Affiliated Hospital of Dali University, Chuxiong Yi Autonomous Prefecture 675000, Yunnan Province, China. lh18987837533@163.com.
Data sharing statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
- 1.Tseng ST, Chou ST, Low BH, Su FL. Risk factors associated with diabetic retinopathy onset and progression in diabetes patients: a Taiwanese cohort study. Int J Clin Exp Med. 2015;8:21507–21515. [PMC free article] [PubMed] [Google Scholar]
- 2.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 3.Sharma Y, Saxena S, Mishra A, Saxena A, Natu SM. Apolipoprotein A-I and B and Subjective Global Assessment relationship can reflect lipid defects in diabetic retinopathy. Nutrition. 2017;33:70–75. doi: 10.1016/j.nut.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Chen S, Feng B, Thomas AA, Chakrabarti S. miR-146a regulates glucose induced upregulation of inflammatory cytokines extracellular matrix proteins in the retina and kidney in diabetes. PLoS One. 2017;12:e0173918. doi: 10.1371/journal.pone.0173918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White NH, Waltman SR, Krupin T, Santiago JV. Reversal of abnormalities in ocular fluorophotometry in insulin-dependent diabetes after five to nine months of improved metabolic control. Diabetes. 1982;31:80–85. doi: 10.2337/diab.31.1.80. [DOI] [PubMed] [Google Scholar]
- 6.Lingam G, Wong TY. Systemic medical management of diabetic retinopathy. Middle East Afr J Ophthalmol. 2013;20:301–308. doi: 10.4103/0974-9233.120010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong-Riley MT. Energy metabolism of the visual system. Eye Brain. 2010;2:99–116. doi: 10.2147/EB.S9078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen LH, NOELL WK. Glucose catabolism of rabbit retina before and after development of visual function. J Neurochem. 1960;5:253–276. doi: 10.1111/j.1471-4159.1960.tb13363.x. [DOI] [PubMed] [Google Scholar]
- 9.Chen LQ, Cheung LS, Feng L, Tanner W, Frommer WB. Transport of sugars. Annu Rev Biochem. 2015;84:865–894. doi: 10.1146/annurev-biochem-060614-033904. [DOI] [PubMed] [Google Scholar]
- 10.Deng D, Xu C, Sun P, Wu J, Yan C, Hu M, Yan N. Crystal structure of the human glucose transporter GLUT1. Nature. 2014;510:121–125. doi: 10.1038/nature13306. [DOI] [PubMed] [Google Scholar]
- 11.Holoman NC, Aiello JJ, Trobenter TD, Tarchick MJ, Kozlowski MR, Makowski ER, De Vivo DC, Singh C, Sears JE, Samuels IS. Reduction of Glut1 in the Neural Retina But Not the RPE Alleviates Polyol Accumulation and Normalizes Early Characteristics of Diabetic Retinopathy. J Neurosci. 2021;41:3275–3299. doi: 10.1523/JNEUROSCI.2010-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang T, Ouyang H, Mei X, Lu B, Yu Z, Chen K, Wang Z, Ji L. Erianin alleviates diabetic retinopathy by reducing retinal inflammation initiated by microglial cells via inhibiting hyperglycemia-mediated ERK1/2-NF-κB signaling pathway. FASEB J. 2019;33:11776–11790. doi: 10.1096/fj.201802614RRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dominguez JH, Song B, Liu-Chen S, Qulali M, Howard R, Lee CH, McAteer J. Studies of renal injury. II. Activation of the glucose transporter 1 (GLUT1) gene and glycolysis in LLC-PK1 cells under Ca2+ stress. J Clin Invest. 1996;98:395–404. doi: 10.1172/JCI118805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perrini S, Natalicchio A, Laviola L, Belsanti G, Montrone C, Cignarelli A, Minielli V, Grano M, De Pergola G, Giorgino R, Giorgino F. Dehydroepiandrosterone stimulates glucose uptake in human and murine adipocytes by inducing GLUT1 and GLUT4 translocation to the plasma membrane. Diabetes. 2004;53:41–52. doi: 10.2337/diabetes.53.1.41. [DOI] [PubMed] [Google Scholar]
- 15.Moghadam PK, Jackson MB. The functional significance of synaptotagmin diversity in neuroendocrine secretion. Front Endocrinol (Lausanne) 2013;4:124. doi: 10.3389/fendo.2013.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Südhof TC. Calcium control of neurotransmitter release. Cold Spring Harb Perspect Biol. 2012;4:a011353. doi: 10.1101/cshperspect.a011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pang ZP, Südhof TC. Cell biology of Ca2+-triggered exocytosis. Curr Opin Cell Biol. 2010;22:496–505. doi: 10.1016/j.ceb.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernández-Chacón R, Königstorfer A, Gerber SH, García J, Matos MF, Stevens CF, Brose N, Rizo J, Rosenmund C, Südhof TC. Synaptotagmin I functions as a calcium regulator of release probability. Nature. 2001;410:41–49. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- 19.Wolfes AC, Dean C. The diversity of synaptotagmin isoforms. Curr Opin Neurobiol. 2020;63:198–209. doi: 10.1016/j.conb.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Dean C, Liu H, Dunning FM, Chang PY, Jackson MB, Chapman ER. Synaptotagmin-IV modulates synaptic function and long-term potentiation by regulating BDNF release. Nat Neurosci. 2009;12:767–776. doi: 10.1038/nn.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huynh DP, Scoles DR, Nguyen D, Pulst SM. The autosomal recessive juvenile Parkinson disease gene product, parkin, interacts with and ubiquitinates synaptotagmin XI. Hum Mol Genet. 2003;12:2587–2597. doi: 10.1093/hmg/ddg269. [DOI] [PubMed] [Google Scholar]
- 22.Boo HJ, Min HY, Jang HJ, Yun HJ, Smith JK, Jin Q, Lee HJ, Liu D, Kweon HS, Behrens C, Lee JJ, Wistuba II, Lee E, Hong WK, Lee HY. The tobacco-specific carcinogen-operated calcium channel promotes lung tumorigenesis via IGF2 exocytosis in lung epithelial cells. Nat Commun. 2016;7:12961. doi: 10.1038/ncomms12961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L, Fan B, Zheng Y, Lou Y, Cui Y, Wang K, Zhang T, Tan X. Identification SYT13 as a novel biomarker in lung adenocarcinoma. J Cell Biochem. 2020;121:963–973. doi: 10.1002/jcb.29224. [DOI] [PubMed] [Google Scholar]
- 24.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Südhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mall M, Kareta MS, Chanda S, Ahlenius H, Perotti N, Zhou B, Grieder SD, Ge X, Drake S, Euong Ang C, Walker BM, Vierbuchen T, Fuentes DR, Brennecke P, Nitta KR, Jolma A, Steinmetz LM, Taipale J, Südhof TC, Wernig M. Myt1 L safeguards neuronal identity by actively repressing many non-neuronal fates. Nature. 2017;544:245–249. doi: 10.1038/nature21722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bellefroid EJ, Bourguignon C, Hollemann T, Ma Q, Anderson DJ, Kintner C, Pieler T. X-MyT1, a Xenopus C2HC-type zinc finger protein with a regulatory function in neuronal differentiation. Cell. 1996;87:1191–1202. doi: 10.1016/s0092-8674(00)81815-2. [DOI] [PubMed] [Google Scholar]
- 27.Huang C, Walker EM, Dadi PK, Hu R, Xu Y, Zhang W, Sanavia T, Mun J, Liu J, Nair GG, Tan HYA, Wang S, Magnuson MA, Stoeckert CJ Jr, Hebrok M, Gannon M, Han W, Stein R, Jacobson DA, Gu G. Synaptotagmin 4 Regulates Pancreatic β Cell Maturation by Modulating the Ca(2+) Sensitivity of Insulin Secretion Vesicles. Dev Cell. 2018;45:347–361.e5. doi: 10.1016/j.devcel.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kashihara N, Haruna Y, Kondeti VK, Kanwar YS. Oxidative stress in diabetic nephropathy. Curr Med Chem. 2010;17:4256–4269. doi: 10.2174/092986710793348581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J, Hou Y, Lin L, Yu N, Zhang Y. MicroRNA-5195-3p alleviates high glucoseinduced injury in human ARPE-19 cells by targeting GMFB. PLoS One. 2021;16:e0260071. doi: 10.1371/journal.pone.0260071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kabayama H, Tokushige N, Takeuchi M, Kabayama M, Fukuda M, Mikoshiba K. Parkin promotes proteasomal degradation of synaptotagmin IV by accelerating polyubiquitination. Mol Cell Neurosci. 2017;80:89–99. doi: 10.1016/j.mcn.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- 32.Gnana-Prakasam JP, Veeranan-Karmegam R, Coothankandaswamy V, Reddy SK, Martin PM, Thangaraju M, Smith SB, Ganapathy V. Loss of Hfe leads to progression of tumor phenotype in primary retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2013;54:63–71. doi: 10.1167/iovs.12-10312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou Q, Yang X, Xiong M, Xu X, Zhen L, Chen W, Wang Y, Shen J, Zhao P, Liu QH. Chloroquine Increases Glucose Uptake via Enhancing GLUT4 Translocation and Fusion with the Plasma Membrane in L6 Cells. Cell Physiol Biochem. 2016;38:2030–2040. doi: 10.1159/000445562. [DOI] [PubMed] [Google Scholar]
- 34.Bryan J, Crane A, Vila-Carriles WH, Babenko AP, Aguilar-Bryan L. Insulin secretagogues, sulfonylurea receptors and K(ATP) channels. Curr Pharm Des. 2005;11:2699–2716. doi: 10.2174/1381612054546879. [DOI] [PubMed] [Google Scholar]
- 35.Ashcroft FM, Harrison DE, Ashcroft SJ. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature. 1984;312:446–448. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- 36.Sharma B, Singh S, Kanwar SS. L-methionase: a therapeutic enzyme to treat malignancies. Biomed Res Int. 2014;2014:506287. doi: 10.1155/2014/506287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sung HY, Han J, Ju W, Ahn JH. Synaptotagmin-like protein 2 gene promotes the metastatic potential in ovarian cancer. Oncol Rep. 2016;36:535–541. doi: 10.3892/or.2016.4835. [DOI] [PubMed] [Google Scholar]
- 38.Wang K, Xiao H, Zhang J, Zhu D. Synaptotagmin7 Is Overexpressed In Colorectal Cancer And Regulates Colorectal Cancer Cell Proliferation. J Cancer. 2018;9:2349–2356. doi: 10.7150/jca.25098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao B, Li J, Fan Y, Ye M, Lv S, Xu B, Chai Y, Zhou Z, Wu M, Zhu X. Downregulation of SYT7 inhibits glioblastoma growth by promoting cellular apoptosis. Mol Med Rep. 2017;16:9017–9022. doi: 10.3892/mmr.2017.7723. [DOI] [PubMed] [Google Scholar]
- 40.Liu XY, Jiang W, Ma D, Ge LP, Yang YS, Gou ZC, Xu XE, Shao ZM, Jiang YZ. SYTL4 downregulates microtubule stability and confers paclitaxel resistance in triple-negative breast cancer. Theranostics. 2020;10:10940–10956. doi: 10.7150/thno.45207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun T, Wu XS, Xu J, McNeil BD, Pang ZP, Yang W, Bai L, Qadri S, Molkentin JD, Yue DT, Wu LG. The role of calcium/calmodulin-activated calcineurin in rapid and slow endocytosis at central synapses. J Neurosci. 2010;30:11838–11847. doi: 10.1523/JNEUROSCI.1481-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leitz J, Kavalali ET. Ca2+ Dependence of Synaptic Vesicle Endocytosis. Neuroscientist. 2016;22:464–476. doi: 10.1177/1073858415588265. [DOI] [PubMed] [Google Scholar]
- 43.Harris KP, Zhang YV, Piccioli ZD, Perrimon N, Littleton JT. The postsynaptic t-SNARE Syntaxin 4 controls traffic of Neuroligin 1 and Synaptotagmin 4 to regulate retrograde signaling. Elife. 2016;5 doi: 10.7554/eLife.13881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Korkut C, Li Y, Koles K, Brewer C, Ashley J, Yoshihara M, Budnik V. Regulation of postsynaptic retrograde signaling by presynaptic exosome release. Neuron. 2013;77:1039–1046. doi: 10.1016/j.neuron.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshihara M, Adolfsen B, Galle KT, Littleton JT. Retrograde signaling by Syt 4 induces presynaptic release and synapse-specific growth. Science. 2005;310:858–863. doi: 10.1126/science.1117541. [DOI] [PubMed] [Google Scholar]
- 46.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 47.Dye BT, Schulman BA. Structural mechanisms underlying posttranslational modification by ubiquitin-like proteins. Annu Rev Biophys Biomol Struct. 2007;36:131–150. doi: 10.1146/annurev.biophys.36.040306.132820. [DOI] [PubMed] [Google Scholar]
- 48.Heo JM, Ordureau A, Paulo JA, Rinehart J, Harper JW. The PINK1-PARKIN Mitochondrial Ubiquitylation Pathway Drives a Program of OPTN/NDP52 Recruitment and TBK1 Activation to Promote Mitophagy. Mol Cell. 2015;60:7–20. doi: 10.1016/j.molcel.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamamoto A, Yue Z. Autophagy and its normal and pathogenic states in the brain. Annu Rev Neurosci. 2014;37:55–78. doi: 10.1146/annurev-neuro-071013-014149. [DOI] [PubMed] [Google Scholar]
- 50.Shulman JM, De Jager PL, Feany MB. Parkinson’s disease: genetics and pathogenesis. Annu Rev Pathol. 2011;6:193–222. doi: 10.1146/annurev-pathol-011110-130242. [DOI] [PubMed] [Google Scholar]
- 51.Wang C, Kang X, Zhou L, Chai Z, Wu Q, Huang R, Xu H, Hu M, Sun X, Sun S, Li J, Jiao R, Zuo P, Zheng L, Yue Z, Zhou Z. Synaptotagmin-11 is a critical mediator of parkin-linked neurotoxicity and Parkinson’s disease-like pathology. Nat Commun. 2018;9:81. doi: 10.1038/s41467-017-02593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rahimi M, Vinciguerra M, Daghighi M, Özcan B, Akbarkhanzadeh V, Sheedfar F, Amini M, Mazza T, Pazienza V, Motazacker MM, Mahmoudi M, De Rooij FW, Sijbrands E, Peppelenbosch MP, Rezaee F. Age-related obesity and type 2 diabetes dysregulate neuronal associated genes and proteins in humans. Oncotarget. 2015;6:29818–29832. doi: 10.18632/oncotarget.4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakano K, Yanobu-Takanashi R, Shimizu Y, Takahashi Y, Hiura K, Watanabe M, Sasaki H, Okamura T, Sasaki N. Genetic locus responsible for diabetic phenotype in the insulin hyposecretion (ihs) mouse. PLoS One. 2020;15:e0234132. doi: 10.1371/journal.pone.0234132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.