Abstract
The naphthalene dioxygenase (NDO) system catalyzes the first step in the degradation of naphthalene by Pseudomonas sp. strain NCIB 9816-4. The enzyme has a broad substrate range and catalyzes several types of reactions including cis-dihydroxylation, monooxygenation, and desaturation. Substitution of valine or leucine at Phe-352 near the active site iron in the α subunit of NDO altered the stereochemistry of naphthalene cis-dihydrodiol formed from naphthalene and also changed the region of oxidation of biphenyl and phenanthrene. In this study, we replaced Phe-352 with glycine, alanine, isoleucine, threonine, tryptophan, and tyrosine and determined the activity with naphthalene, biphenyl, and phenanthrene as substrates. NDO variants F352W and F352Y were marginally active with all substrates tested. F352G and F352A had reduced but significant activity, and F352I, F352T, F352V, and F352L had nearly wild-type activities with respect to naphthalene oxidation. All active enzymes had altered regioselectivity with biphenyl and phenanthrene. In addition, the F352V and F352T variants formed the opposite enantiomer of biphenyl cis-3,4-dihydrodiol [77 and 60% (−)-(3S,4R), respectively] to that formed by wild-type NDO [>98% (+)-(3R,4S)]. The F352V mutant enzyme also formed the opposite enantiomer of phenanthrene cis-1,2-dihydrodiol from phenanthrene to that formed by biphenyl dioxygenase from Sphingomonas yanoikuyae B8/36. A recombinant Escherichia coli strain expressing the F352V variant of NDO and the enantioselective toluene cis-dihydrodiol dehydrogenase from Pseudomonas putida F1 was used to produce enantiomerically pure (−)-biphenyl cis-(3S,4R)-dihydrodiol and (−)-phenanthrene cis-(1S,2R)-dihydrodiol from biphenyl and phenanthrene, respectively.
The naphthalene dioxygenase (NDO) system (EC 1.14.12.12) catalyzes the first step in the degradation of naphthalene in Pseudomonas sp. NCIB 9816-4. In this reaction, both atoms of O2 are added to the aromatic ring to form (+)-cis-(1R,2S)-dihydroxy-1,2-dihydronaphthalene (naphthalene cis-dihydrodiol) (28, 29). NDO consists of three components. An iron-sulfur flavoprotein reductase and a Rieske iron-sulfur ferredoxin transfer electrons from NAD(P)H to the catalytic oxygenase component (15, 16, 21, 22). The oxygenase consists of large (α) and small (β) subunits that form an α3β3 native structure (31). Each α subunit contains a Rieske [2Fe-2S] center and mononuclear nonheme iron (15, 31). Electrons are transferred from the Rieske center in one α subunit to the mononuclear iron in an adjacent α subunit (31, 39), and this is the site of oxygen activation and catalysis.
NDO catalyzes the oxidation of a wide variety of aromatic compounds, and many of the products are enantiomerically pure chiral compounds (9, 24, 45). The use of dioxygenases to initiate biocatalytic routes for the production of pharmaceuticals and natural products has received significant attention of late (9, 12, 25, 42), and the possibility of generating new synthons with opposite stereochemistry is an attractive alternative to asymmetric chemical synthesis (7).
From the crystal structure of NDO, several amino acids were identified near the active site (31). Site-directed mutagenesis at nine positions near the mononuclear iron identified Phe-352 as an amino acid that plays an important role in determining the regioselectivity of biphenyl and phenanthrene oxidation and the stereochemistry of naphthalene cis-dihydrodiol formed from naphthalene (38). Valine and leucine substitutions at Phe-352 resulted in the largest specificity changes with several substrates. In this study, we generated and characterized six new enzymes with amino acid substitutions at Phe-352. Results show that Phe-352 controls enantioselectivity with naphthalene, biphenyl, phenanthrene, and anthracene as substrates. A previously undescribed compound, the (−)-enantiomer of biphenyl cis-3,4-dihydrodiol was identified and characterized.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Bacterial strains and plasmids used in this study are listed in Table 1. Escherichia coli strains DH5α and JM109(DE3) were used for subcloning and gene expression studies, respectively. Competent E. coli strains ES1301 and JM109 were purchased from Promega Corp., Madison, Wis., for use in generating site-directed mutants as described below.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Source or reference(s) |
|---|---|---|
| Bacterial strains | ||
| E. coli | ||
| DH5α | Δ(lacZYA-argF)U169 hsdR17 relA1 supE44 endA1 recA1 thi gyrA96 φ80dlacZΔM15 | Life Technologies, Gaithersburg, Md. |
| JM109 | endA1 recA1 gyrA96 thi hsdR17 relA1 supE44 Δ(lac-proAB) mcrA (F′ traD36 proAB+ lacIqZΔM15) | 55 |
| JM109(DE3) | endA1 recA1 gyrA96 thi hsdR17 relA1 supE44 Δ(lac-proAB) mcrA (F′ traD36 proAB+ lacIqZΔM15) λ(DE3) | Promega Corp., Madison, Wis. |
| ES1301 mutS | KmrlacZ53 mutS201::Tn5 thyA36 rha-5 metB1 deoC IN(rrnD-rrnE) | Promega Corp. |
| Pseudomonas putida | ||
| UV4 | Toluene-degrading strain | 6 |
| F1 | Toluene-degrading strain | 17, 18 |
| Sphingomonas yanoikuyae B8/36 | cis-Biphenyl dihydrodiol dehydrogenase mutant of strain B1 | 19, 32 |
| Plasmids | ||
| pDTG141 | Apr, nahAaAbAcAd (encoding the naphthalene dioxygenase components reductaseNAP, ferredoxinNAP, and large and small subunits of the oxygenase, respectively) under the control of the T7 promoter of pT7-5 | 52 |
| pT7-5 | Apr, T7 expression vector | 54 |
| pMASTER-1 | Tcr Aps, pALTER-1 carrying the KpnI-XbaI fragment of pDTG141 (nahAc′Ad) | 39 |
| pDTG511 | Kmr, pKT230 containing todD gene encoding cis-toluene dihydrodiol dehydrogenase from P. putida F1 | 56 |
| pDTG512 | Kmr, pKT230 containing truncated todD gene from P. putida F1 | 56 |
| pKT230 | Kmr Smr, broad-host-range cloning vector | 4 |
Kmr, kanamycin resistance; Apr, ampicillin resistance; Tcr, tetracycline resistance, Smr, streptomycin resistance.
Media and growth conditions.
Sphingomonas yanoikuyae (previously Beijerinckia sp.) B8/36 was grown at 30°C in minimal salts medium (MSB) (51) containing 40 mM pyruvate and 0.1% yeast extract. Cultures were induced for 8 h with m-xylene provided in the vapor phase. Pseudomonas putida F1 was grown in MSB with 40 mM pyruvate and toluene provided in the vapor phase. Except where indicated below, E. coli strains were grown at 37°C in Luria-Bertani medium (14) or Terrific Broth medium (34). For growth of E. coli strains harboring plasmids, antibiotics were added to the following final concentrations as appropriate: ampicillin, 150 μg/ml; tetracycline, 12.5 μg/ml; or kanamycin, 50 μg/ml. For biotransformation studies, E. coli JM109(DE3) strains carrying plasmids of interest were grown at 30°C in MSB containing 10 mM glucose, 0.1 mM thiamine, and the appropriate antibiotics. Isopropyl-β-d-thiogalactopyranoside (IPTG) was added to a final concentration of 100 μM when culture turbidity reached 0.6 to 0.8 at 660 nm. After a 2-h induction, biotransformations were initiated as described below. To produce solid media, MSB was solidified with 1.8% Noble agar (Difco Laboratories), and LB was solidified with 1.5% Bactoagar (Difco Laboratories).
For large-scale biotransformations, JM109(DE3) carrying the appropriate plasmid was grown at 27°C in MSB in a 10-liter Biostat B fermentor (B. Braun Biotech International, Melsungen, Germany). The pH was maintained at 7.3 by automated addition of NH4OH, and a slow glucose feed was used to maintain the dissolved O2 concentration at approximately 25% saturation. When the turbidity (660 nm) reached approximately 0.7, cultures were induced for 3 h with 150 μM IPTG.
Whole-cell biotransformations.
After induction, E. coli cultures were supplemented with 20 mM glucose and 80 mM phosphate buffer (pH 7.2). Solid substrates (naphthalene, biphenyl, or phenanthrene) were added to a final concentration of 0.025% (wt/vol). Tween 20 (0.005% [vol/vol]; Aldrich Chemical Co., Milwaukee, Wis.) was added to biotransformations with anthracene (0.0125% [wt/vol]) to increase substrate availability. Cultures were incubated at 30°C with shaking (250 rpm) for 15 to 18 h. Control biotransformations carried out under identical conditions with JM109(DE3)(pT7-5) did not result in product formation with any of the substrates tested. Large-scale (5.5-liter) biotransformations with biphenyl or phenanthrene were carried out in a 10-liter Biostat B fermentor. Induced cultures were incubated at 27°C for 14 to 17 h with 0.025% (wt/vol) substrate, high agitation (700 rpm), automated pH control (pH 7.3), and a slow glucose feed. S. yanoikuyae B8/36 biotransformations were carried out at 30°C with induced cultures (800 ml) supplemented with 40 mM pyruvate–0.025% (wt/vol) substrate.
Rates of product formation.
Cultures (50 ml in 500-ml flasks) were grown and induced, and biotransformations with naphthalene or biphenyl were initiated as described above. Samples (1 ml each) were taken at 30-min intervals over a period of 5 h. Cells were removed by centrifugation, and pellets were stored at −20°C for protein determinations. Naphthalene cis-dihydrodiol formation was monitored at 262 nm (ɛ = 8114 M−1 cm−1 [28]). Biphenyl cis-2,3-dihydrodiol formation was monitored at 303 nm (ɛ = 13,600 M−1 cm−1 [19]). Biphenyl cis-3,4-dihydrodiol formation was monitored at 276 nm (ɛ = 4340 M−1 cm−1 [38]) using a correction for the absorbance of biphenyl cis-2,3-dihydrodiol at this wavelength. The extinction coefficient of biphenyl cis-2,3-dihydrodiol at 276 nm (the λmax of biphenyl cis-3,4-dihydrodiol) was determined to be 7950 M−1 cm−1 using purified biphenyl cis-2,3-dihydrodiol produced by S. yanoikuyae B8/36 (19). The concentration of biphenyl cis-3,4-dihydrodiol was calculated using the ratios of products formed by each mutant enzyme (see results) and subtracting the contribution of biphenyl cis-2,3-dihydrodiol. Absorbance readings obtained from control naphthalene or biphenyl biotransformations with JM109(DE3)(pT7-5) were subtracted at each time point to eliminate the contributions of the substrates as they dissolved. Protein concentrations were determined by the method of Bradford (10) after boiling cell pellets for 1 h in 0.1 N NaOH. Bovine serum albumin was used as the standard. Reported rates are the averages of three independent experiments.
Indigo formation.
E. coli JM109(DE3) strains carrying plasmids of interest were grown overnight at 37°C on nitrocellulose filters placed on MSB agar plates containing glucose, thiamine, and ampicillin. Whatman no. 1 filter papers were soaked in a 10% solution of indole dissolved in acetone, dried, and placed in the covers of inverted petri dishes after colony formation. Production of indigo from indole vapor was observed as colonies turned blue. Cultures were not induced.
Molecular techniques.
Plasmid DNA was purified as previously described (34) or with the Qiagen Midi Kit (Qiagen, Inc., Santa Clarita, Calif.). DNA to be used for nucleotide sequencing was further purified using a Centricon-100 filter unit (Amicon, Inc., Beverly, Mass.). Restriction digests were performed as suggested by the enzyme suppliers (New England Biolabs, Inc., Beverly, Mass.; Promega Corp.). DNA fragments were purified from gel slices using the GeneClean Spin Kit according to the manufacturer's instructions (BIO101, Vista, Calif.). Ligation reactions, E. coli transformations, and agarose gel electrophoresis were performed by standard methods (49).
Site-directed mutagenesis.
Mutagenesis of nahAc was carried out with the Altered Sites II in vitro mutagenesis system according to the manufacturer's instructions (Promega Corp.). Plasmid pMASTER-1 (39) carries the 3′ end of the nahAc gene and the complete nahAd gene (encoding the α and β subunits of NDO, respectively) and was used as the template for mutagenesis. Each mutagenic oligonucleotide (Table 2) was designed with a silent mutation that altered the restriction pattern of the plasmid (eliminating an AclI site) to facilitate mutant screening. Phosphorylated oligonucleotides used for mutagenesis were synthesized by Genosys Biotechnologies Inc., Midland, Tex. The nucleotide sequences of both strands of each insertion in pMASTER-1 were determined for each mutant. Fluorescent, automated DNA sequencing was carried out at the University of Iowa DNA Facility using an Applied Biosystems 373A automated DNA sequencer. The 1.5-kb KpnI-XbaI fragments carrying each mutation were individually cloned into KpnI-XbaI-digested pDTG141, and the resulting plasmids were introduced into E. coli strain JM109(DE3) for expression studies. After this subcloning step, the presence of each mutation was verified by restriction and sequence analyses.
TABLE 2.
Amino acid substitutions in the α subunit of NDO generated by site-directed mutagenesis
| Mutation | Mutagenic oligonucleotidea | Indigo formationb |
|---|---|---|
| F352G | 5′-GTTCAGCGAACGGGCGGGCCTGCTGG-3′ | + |
| F352A | 5′-GTTCAGCGAACGGCCGGGCCTGCTGG-3′ | + |
| F352T | 5′-GTTCAGCGAACGACCGGGCCTGCTGG-3′ | ++ |
| F352I | 5′-GTTCAGCGAACGATCGGGCCTGCTG-3′ | ++ |
| F352Lc | 5′-TTCAGCGAACGCTCGGGCCTGC-3′ | ++ |
| F352Vc | 5′-TTCAGCGAACGGTCGGGCCTGC-3′ | + |
| F352W | 5′-GTTCAGCGAACGTGGGGGCCTGCTGG-3′ | − |
| F352Y | 5′-TTCAGCGAACGTACGGGCCTGCTGG-3′ | − |
Underlined bases indicate the position of the eliminated restriction site, AclI. Base changes are in bold.
Indigo formation was monitored after 8 h as described in Materials and Methods. ++, colonies dark blue; +, colonies light blue; −, no blue color [corresponds to negative control, JM109(DE3)(pT7-5)].
The construction of the F352L and F352V mutations was previously reported (38).
Separation and identification of products.
Culture supernatants from whole-cell biotransformation experiments were extracted with sodium hydroxide-washed ethyl acetate and analyzed by thin-layer chromatography (TLC) (47). Phenyl boronic acid derivatives (23) were prepared and analyzed by gas chromatography-mass spectrometry (GC-MS) as previously described (44). Naphthalene cis-dihydrodiol and anthracene cis-dihydrodiol were purified by preparative-layer chromatography (PLC) with chloroform-acetone (8:2) (44). Regioisomers of biphenyl cis-dihydrodiol were separated by PLC or radial-dispersion chromatography using a Chromatotron (Harrison Research, Palo Alto, Calif.) as previously described (38).
Chiral stationary-phase liquid chromatography was used to resolve the enantiomers of naphthalene cis-dihydrodiol with a Chiralcel OJ column (Chiral Technologies, Exton, Pa.) as described previously (47). Under the conditions used, the (+)-(1R,2S)- and (−)-(1S,2R)-enantiomers of naphthalene cis-dihydrodiol eluted with retention times of 30 and 33 min, respectively. Using the same column and elution conditions, the (+)- and (−)-enantiomers of biphenyl cis-2,3-dihydrodiol eluted at approximately 35 and 40 min, respectively, and the (+)- and (−)-enantiomers of biphenyl cis-3,4-dihydrodiol eluted at approximately 34 and 30 min, respectively. Product identifications were based on comparisons to standards with the exception of biphenyl cis-3,4-dihydrodiol (see below). Enantiomeric purity (or enantiomeric composition) is defined herein as the mole percent of the major enantiomer.
Proton (1H) nuclear magnetic resonance (NMR) spectra were acquired on the Bruker AMX 600-MHz NMR spectrometer at 600.14 MHz in the University of Iowa High-Field NMR Facility and on a Bruker Avance DPX 500-MHz spectrometer at the Queen's University of Belfast. All spectra were obtained using a 14-s recovery delay, a 4.06-s acquisition time, a spectral width of 13.4 ppm and a 90° pulse width of 7.5 μs. Chiral methoxyethylphenyl boronic acid (MPBA) derivatives were prepared as previously described (46, 47). Optical rotations were determined at 25°C using a Jasco P1020 polarimeter with a 589-nm-wavelength Na lamp. Circular dichroism (CD) spectra were obtained with a Jasco J-720 instrument using spectroscopic-grade methanol. Concentrations used were ca. 0.0005 g/ml, and Δɛ values were given per mole per cubic decimeter per centimeter.
Chemicals.
Naphthalene was obtained from Fisher Scientific Co., Pittsburgh, Pa. Indole, biphenyl, phenanthrene, and anthracene were purchased from Aldrich Chemical Co. 3,4-Dihydroxybiphenyl was obtained from Ultra Scientific, North Kingstown, R.I. Synthetic (±)-naphthalene cis-dihydrodiol and homochiral (+)-naphthalene cis-dihydrodiol were prepared as previously described (27–29). The (+)-enantiomer of biphenyl cis-2,3-dihydrodiol was produced by S. yanoikuyae B8/36 (19) and the (−)-enantiomer by Pseudomonas stutzeri C250 (43). Synthetic phenanthrene cis-9,10-dihydrodiol having the reported (50) characteristics (mp 170 to 171°C) was prepared using the method previously described for K-region cis-dihydrodiols of chrysene, benzo[c]phenanthrene and 7,12-dimethylbenz[a]anthracene (5). (+)-Anthracene cis-1,2-dihydrodiol was prepared as previously described using S. yanoikuyae B8/36 (30).
Synthesis of (+)-biphenyl cis-3,4-dihydrodiol.
(+)-Biphenyl cis-3,4-dihydrodiol [(+)-cis(1S,2R)-1,2-dihydroxy-4-phenylcyclohexa-3,5-diene] was synthesized in a five-step procedure as described below and as outlined in Fig. 1. 1-Bromo-2-iodobenzene (2.0 g, 7.07 mmol [compound 1]) was oxidized by P. putida UV4 using the standard procedure (7, 8), to yield the major cis-diol, (+)-cis-(1S,2R)-1,2-dihydroxy-4-bromo-3-iodocyclohexa-3,5-diene (compound 2) (0.8 g, 36%), mp, 101 to 103°C (EtOAc-hexane), [α]D + 75 (c 2.96, MeOH), δH (500 MHz, CDCl3), 4.38 (d, 1H, J2,1 5.9, H-2), 4.48 to 4.50 (m, 1H, H-1), 5.98 (dd, 1H, J5,6 9.7, J5,1 3.5, H-5), 6.07 (dd, 1H, J6,1 1.4, J6,5 9.7, H-6); found C, 22.6; H, 1.7; C6H6IBrO2 requires C, 22.7; H, 1.9.
FIG. 1.
Strategy for the synthesis of (+)-biphenyl cis-3,4-dihydrodiol. Compound 1, 1-bromo-2-iodobenzene; compound 2, (+)-cis-(1S,2R)-1,2-dihydroxy-4-bromo-3-iodocyclohexa-3,5-diene; compound 3, (+)-cis-(1S,2R)-1,2-dihydroxy-4-bromocyclohexa-3,5-diene; compound 4, (−)-cis-(1S,2R)-1,2-di-tert-butyldimethylsilyloxy-4-bromocyclohexa-3,5-diene; compound 5, (+)-cis-(1S,2R)-1,2-di-tert-butyldimethylsilyloxy-4-phenylcyclohexa-3,5-diene; compound 6, (+)-cis-(1S,2R)-1,2-dihydroxy-4-phenylcyclohexa-3,5-diene [(+)-biphenyl cis-(3R,4S)-dihydrodiol].
To a solution of the (+)-cis-diol (compound 2; 0.12 g, 0.38 mmol) in methanol (8 ml) was added sodium acetate trihydrate (0.105 g), quinoline (75 μl), and 3% Pd/C (0.016 g). The reaction mixture was stirred in an atmosphere of hydrogen at ambient temperature. On completion of the hydrogenolysis reaction (4 h), monitored by TLC (EtOAc-hexane, 1:2), the catalyst was removed by filtration, and the filtrate was concentrated under reduced pressure. Purification of the concentrated reaction mixture by passing its solution (6% MeOH in CHCl3) through a pad of silica gel followed by PLC (Si gel; EtOAc-hexane, 1:2) gave (+)-cis-(1S,2R)-1,2-dihydroxy-4-bromocyclohexa-3,5-diene (compound 3) as colorless plates (0.043 g, 60%), mp, 69 to 71°C (ethanol), [α]D + 11 (c 1.3, MeOH); δH (500 MHz, CDCl3), 4.13 (dd, 1H, J2,3 4.6, J2,1 6.3, H-2), 4.22 (ddd, 1H, J1,2 6.3, J1,6 3.6, J1,5 1.7, H-1), 5.84 (dd, 1H, J6,5 9.9, J6,1 3.6, H-6), 5.95 (ddd, 1H, J5,6 9.9, J5,1 = J5,3 1.7, H-5), 6.18 (dd, 1H, J3,2 4.6, J3,5 1.7, H-3); found M+, 189.96209; C6H779BrO2 requires 189.96294.
tert-Butyldimethylsilyltrifluoromethanesulfonate (TBDMS-triflate) (0.4 ml) was added dropwise, at 0°C under nitrogen, to a solution of the (+)-cis-diol (compound 3; 0.150 g, 0.79 mmol) in dichloromethane (5 ml) containing triethylamine (0.3 ml). The reaction mixture was stirred for 0.5 h before quenching with 5% aqueous sodium bicarbonate (10 ml). The organic layer was diluted with dichloromethane (10 ml), separated, washed with water, and dried over anhydrous Na2SO4. Removal of the solvent gave a crude product that was purified by PLC (Si gel; ether-hexane, 1:19) to yield the di-TBDMS derivative (−)-cis-(1S,2R)-1,2-di-tert-butyldimethylsilyloxy-4-bromocyclohexa-3,5-diene (compound 4) as a colorless viscous oil (0.280 g, 85%), [α]D − 5 (c 1.54, CHCl3), δH (500 MHz, CDCl3), 0.06 (s, 6H, 2× Si-CH3), 0.08 (s, 6H, 2× Si-CH3), 0.88 [s, 9H Si-C(CH3)3], 0.90 [s, 9H Si-C(CH3)3], 4.16 (dd, 1H, J2,1 5.2, J2,3 4.8, H-2), 4.24 (ddd, 1H, J1,2 5.2, J1,6 3.2, J1,5 1.8, H-1), 5.84 (dd, 1H, J6,5 9.9, J6,1 3.2, H-6), 5.93 (dd, 1H, J5,6 9.9, J5,3 1.8, H-5), 6.17 (dd, 1H, J3,2 4.8, J3,5 1.8, H-3); found M+, 418.13555; C18H3579BrO2Si2 requires 418.13589.
A stirred solution of the (−)-di-TBDMS derivative (compound 4; 0.150 g, 0.36 mmol) in dry ether (10 ml) under nitrogen at 0°C, containing nickel (II) acetylacetonate (0.005 g), was treated dropwise with a solution of phenylmagnesium bromide (1 M in diethylether, 0.5 ml). The reaction mixture was stirred for 3 h at 0°C and then at ambient temperature for a further 3 h. A saturated solution of ammonium chloride (5 ml) was added to terminate the reaction; the ethereal layer was separated, and the remaining aqueous layer was extracted with ether (15 ml). The combined ether extract was dried over Na2SO4 and concentrated, and the residue obtained was purified by PLC (Si gel, hexane) to yield the phenyl cis-diol derivative (+)-cis-(1S,2R)-1,2-di-tert-butyldimethylsilyloxy-4-phenylcyclohexa-3,5-diene (compound 5), as a colorless viscous oil (0.065 g, 44%), [α]D + 13 (c 2.0, CHCl3), δH (500 MHz, CDCl3), 0.10 (s, 6H, 2× Si-CH3), 0.11 (s, 3H, Si-CH3), 0.12 (s, 3H, Si-CH3), 0.91 [s, 9H Si-C(CH3)3], 0.92 [s, 9H Si-C(CH3)3], 4.25 (ddd, 1H, J1,2 5.4, J1,6 3.6, J1,5 1.6, H-1), 4.28 (dd, 1H, J2,1 5.4, J2,3 4.2, H-2), 6.05 (dd, 1H, J6,5 9.8, J6,1 3.6, H-6), 6.10 (dd, 1H, J3,2 4.2, J3,5 1.6, H-3), 6.30 (ddd, 1H, J5,6 9.8, J5,3 = J5,1 1.6, H-5), 7.41 to 7.52 (m, 5H, Ar); found M+, 416.25624; C24H40Si2O2 requires 416.25646.
Tetrabutylammonium fluoride solution (1.0 M in tetrahydrofuran [THF], 0.4 ml) was added to a stirred solution of the (+)-di-TBDMS derivative (compound 5) (0.050 g, 0.12 mmol) in THF (4 ml) at 0°C. The reaction mixture was maintained at 0°C for 3 h. The crude product, obtained after removal of most of the THF solvent, was purified by PLC (Si gel; EtOAc-hexane, 1:1) to yield (+)-cis-(1S,2R)-1,2-dihydroxy-4-phenylcyclohexa-3,5-diene (compound 6) as tiny colorless plates (0.02 g, 88%), mp, 80 to 82°C, (CHCl3-hexane), [α]D + 84 (c 0.4, CHCl3), δH (500 MHz, CDCl3), 2.17 (m, 1H, OH), 2.22 (m, 1H, OH), 4.32 (m, 1H, J1,2 6.3, J1,6 3.8, J1,3 = J1,5 1.6, H-1), 4.39 (ddd, 1H, J2,1 6.3, J2,3 4.3, J2,6 0.7, H-2), 6.13 (ddd, 1H, J6,5 9.8, J6,1 3.8, J6,2 0.7, H-6), 6.17 (ddd, 1H, J3,2 4.3, J3,1 = J3,5 1.6, H-3), 6.40 (ddd, 1H, J5,6 9.8, J5,3 = J5,1 1.6, H-5); found M+, 188.08338; C12H12O2 requires 188.08373.
Gel electrophoresis and Western blot analyses.
Cell pellets (from 1-ml suspensions) were resuspended in 200 μl of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer (2) and boiled for 10 min, and proteins (approximately 20 μg of total protein per lane) were separated by SDS-12% PAGE (2). The gel was subjected to Western blotting as described previously using a monoclonal antibody specific for the α subunit of NDO (22, 36). Antigens were visualized using alkaline phosphatase-conjugated goat anti-mouse immunoglobulin G (Pierce, Rockford, Ill.).
Partial purification of toluene cis-dihydrodiol dehydrogenase.
Toluene-grown cells of P. putida F1 were harvested by centrifugation (10 min, 8,000 × g, 4°C) and resuspended in Tris buffer (50 mM Tris-HCl [pH 7.2]–20 μg of DNase I per ml–1.0 mM phenylmethylsulfonyl fluoride). Cell extracts were prepared by passage of cell suspensions through a chilled French pressure cell (20,000 lb/in2), followed by ultracentrifugation (40,000 × g, 60 min, 4°C). Enzyme purification was carried out at 4°C with a Bio-Rad BioLogic chromatography system (Bio-Rad, Hercules, Calif.). Cell extracts were applied to a DE-52 anion exchange column equilibrated with 25 mM Tris-HCl buffer (pH 7.2). The column was washed with the same buffer, and protein was eluted with a linear gradient (0 to 0.8 M KCl) in 25 mM Tris-HCl (pH 7.2). Fractions containing toluene cis-dihydrodiol dehydrogenase activity eluted at approximately 0.4 M KCl. These were pooled and concentrated by ultrafiltration with a 30-kDa cut-off membrane filter (Amicon, Danvers, Mass.). The partially purified dehydrogenase preparation, which did not oxidize 3-methylcatechol, was stored at −70°C. The toluene cis-dihydrodiol dehydrogenase (TodD) expressed by IPTG-induced JM109(DE3)(pDTG141-F352V)(pDTG511) was partially purified by an analogous procedure. Protein concentrations were determined by the method of Bradford with bovine serum albumin as standard (10).
cis-Dihydrodiol dehydrogenase assays.
Dehydrogenase activities with biphenyl cis-3,4-dihydrodiol were measured spectrophotometrically by following the reduction of NAD+ at 340 nm. Since biphenyl cis-2,3-dihydrodiol shows a slight absorbance at 340 nm, activities with this substrate were measured at 350 nm using an extinction coefficient of 5170 M−1 cm−1 for NADH. Reaction mixtures were contained in a final volume of 1.0 ml of 25 mM Tris-HCl (pH 7.2), NAD+ (2.6 μmol), and enzyme (0.5 to 1.0 mg of protein). Reactions were started by adding biphenyl cis-dihydrodiol (0.1 to 0.3 μmol).
RESULTS
Activity of modified NDO proteins.
The formation of indigo from indole was used to screen for NDO activity. Freshly grown cells of JM109(DE3) carrying modified pDTG141 plasmids were incubated in the presence of indole. Strains producing NDO enzymes with the mutations F352W and F352Y formed white colonies, suggesting that these enzymes were inactive or that indole was no longer a substrate for the modified enzymes. All other NDO variants constructed in this study were active (Table 2).
Production of mutant NDO α subunits.
Formation of mutant α subunits was demonstrated in Western blots using whole-cell protein samples from induced JM109(DE3) strains expressing the mutant NDO genes. Use of the monoclonal antibody specific for the α subunit of NDO (37) demonstrated that all mutant constructs resulted in the formation of full-length α subunits (Fig. 2). The results show that the inability of NDO variants F352Y and F352W to transform the various substrates (see below) was not due to the absence of protein.
FIG. 2.
Western blot showing α subunits formed by JM109(DE3) carrying pDTG141 derivatives with the amino acid substitutions indicated below. A monoclonal antibody specific for the α subunit of NDO was used as described in Materials and Methods. Lanes: M, prestained molecular mass markers (Bio-Rad Laboratories); 1, purified wild-type NDO (2 μg); 2, wild-type NDO (pDTG141); 3, vector control (pT7-5); 4, F352G; 5, F352A; 6, F352I; 7, F352T; 8, F352W; 9, F352Y.
Regioselectivity of modified NDO proteins.
Biotransformation products were identified by comparison to standards in GC-MS analyses. Biotransformations with naphthalene result in the formation of naphthalene cis-dihydrodiol by wild-type NDO from Pseudomonas sp. NCIB 9816-4 (28) and by JM109(DE3)(pDTG141), which carries the cloned naphthalene dioxygenase genes from NCIB 9816-4 (38). All NDO variants with substitutions at position 352 also formed naphthalene cis-dihydrodiol except the F352Y mutant, which formed no product, and F352W, which formed only a trace amount of naphthalene cis-dihydrodiol.
Wild-type NDO oxidizes biphenyl to a 87:13 mixture of biphenyl cis-2,3-dihydrodiol and biphenyl cis-3,4-dihydrodiol (38). However, a major change in regioselectivity with biphenyl was seen when amino acid substitutions were introduced at position 352. Most of the mutant NDO enzymes with changes at this position formed biphenyl cis-3,4-dihydrodiol as the major product (Fig. 3A). The F352Y enzyme formed no detectable product from biphenyl, and the F352W variant formed only a trace amount of biphenyl cis-2,3-dihydrodiol.
FIG. 3.
Regiochemistry and relative product distributions (%) of compounds formed by wild-type and mutant NDO enzymes (A) with biphenyl as substrate and (B) with phenanthrene as substrate. Data for F352V and F352L mutants are from reference 38. Absolute stereochemistry of the biphenyl and phenanthrene dihydrodiols is not intended.
Wild-type NDO and variants F352G, F352A, F352T, F352I, and F352L formed phenanthrene cis-3,4-dihydrodiol as the major product from phenanthrene, although product ratios varied significantly depending on the enzyme (Fig. 3B). As noted previously (38), the F352V mutant had the opposite regioselectivity, forming primarily (83%) phenanthrene cis-1,2-dihydrodiol. In addition, the F352L mutant formed a new product, phenanthrene cis-9,10-dihydrodiol. NDO mutants F352W and F352Y did not form detectable amounts of product from phenanthrene.
Anthracene was converted to anthracene cis-1,2-dihydrodiol by both the wild type and the F352V variant of NDO. The same result was obtained with the NDO from P. putida strain 119 (30). Product formation with anthracene was not tested with the other NDO mutant enzymes.
Relative activities of the mutant NDO enzymes.
The in vivo rates of formation of naphthalene cis-dihydrodiol by wild-type and mutant NDO enzymes are shown in Table 3. The F352L enzyme produced naphthalene cis-dihydrodiol at wild-type rates, while the F352T, F352V, and F352I mutant enzymes were slightly less efficient, with rates 77 to 83% of wild-type NDO. The F352G and F352A mutant enzymes were the least efficient in catalyzing this reaction. A similar trend is seen in the rates of formation of biphenyl cis-3,4-dihydrodiol from biphenyl by the enzymes with substitutions at position 352 (Table 3). The F352T, V, I, and L mutant enzymes formed biphenyl cis-3,4-dihydrodiol at slightly reduced rates compared to wild-type NDO, while the F352A variant was significantly slower, and rates with the F352G mutant were not measurable. In contrast, all enzymes with substitutions at position 352 were severely defective in forming biphenyl cis-2,3-dihydrodiol from biphenyl (Table 3). These studies demonstrate that the amino acid substitutions at position 352 result in enzymes with a decreased tendency to oxidize at the 2,3 position of biphenyl. Although the regioselectivity was changed in the mutant enzymes, the rates of oxidation at the 3,4 position of biphenyl by the F352T, V, I, and L mutants were similar to that observed with the wild-type enzyme.
TABLE 3.
Rates of product formation by wild-type and mutant NDO enzymes
| NDO enzyme | Naphthalene cis-dihydrodiol formationa
|
Biphenyl cis-2,3-dihydrodiol formationa
|
Biphenyl cis-3,4-dihydrodiol formationa
|
|||
|---|---|---|---|---|---|---|
| sp act (nmol/min/mg) | Relative activity (%) | sp act (nmol/min/mg) | Relative activity (%) | sp act (nmol/min/mg) | Relative activity (%) | |
| Wild type | 20.4 | 100 | 4.81 | 100 | 0.95 | 100 |
| F352G | 7.4 | 37 | <0.05 | <1 | <0.25 | <26 |
| F352A | 9.9 | 49 | 0.07 | 1 | 0.25 | 26 |
| F352T | 15.6 | 77 | 0.14 | 3 | 0.73 | 77 |
| F352V | 16.9 | 83 | 0.15 | 3 | 0.94 | 99 |
| F352I | 16.5 | 81 | 0.16 | 3 | 0.74 | 78 |
| F352L | 19.5 | 96 | 0.21 | 4 | 0.82 | 86 |
The formation of naphthalene and biphenyl cis-dihydrodiols was measured spectrophotometrically as described in Materials and Methods.
Enantioselectivity of modified NDO proteins.
The enantiomeric purities of naphthalene cis-dihydrodiol and biphenyl cis-2,3- and cis-3,4-dihydrodiols were determined by chiral stationary-phase high-performance liquid chromatography (HPLC) analysis as described in Materials and Methods. In contrast to wild-type NDO, all enzymes with amino acid substitutions at position 352 formed small amounts of the (−)-enantiomer of naphthalene cis-dihydrodiol from naphthalene (Table 4). The enantiomeric composition of biphenyl cis-2,3-dihydrodiol was unaffected by amino acid substitutions at this position, but that of the biphenyl cis-3,4-dihydrodiol was significantly different in all cases from that formed by the wild-type enzyme (Table 4). It is of interest that the NDO variants F352V and F352T formed the opposite enantiomer of biphenyl cis-3,4-dihydrodiol to that formed by wild-type NDO (Table 4).
TABLE 4.
Enantiomeric composition of products formed by wild-type and mutant NDO enzymes
| NDO enzyme | Enantiomeric compositiona of:
|
||
|---|---|---|---|
| Naphthalene cis-1,2-dihydrodiol | Biphenyl cis-2,3-dihydrodiol | Biphenyl cis-3,4-dihydrodiol | |
| Wild type | >99% (+)-(1R,2S)- | >95% (+)-(2R,3S)- | >98% (+)-(3R,4S) |
| F352G | 98% (+)-(1R,2S)- | >95% (+)-(2R,3S)- | 60% (+)-(3R,4S) |
| F352A | 96% (+)-(1R,2S)- | >95% (+)-(2R,3S)- | 65% (+)-(3R,4S) |
| F352T | 93% (+)-(1R,2S)- | >95% (+)-(2R,3S)- | 60% (−)-(3S,4R) |
| F352V | 92% (+)-(1R,2S)- | NDb | 77% (−)-(3S,4R) |
| F352I | 94% (+)-(1R,2S)- | >95% (+)-(2R,3S)- | 53% (+)-(3R,4S) |
| F352L | 96% (+)-(1R,2S)- | >95% (+)-(2R,3S)- | 70% (+)-(3R,4S) |
Enantiomeric composition was determined by chiral stationary-phase HPLC as described in Materials and Methods.
ND, not determined.
Absolute stereochemistry of biphenyl cis-3,4-dihydrodiol.
The optical rotation of the biphenyl cis-3,4-dihydrodiol produced by the F352V mutant was previously reported ([α]D −37.5) (38). The absolute stereochemistry of the (−)-biphenyl cis-3,4-dihydrodiol was unequivocally identified as the (3S,4R) enantiomer by preparing the compound through chemoenzymatic synthesis as described in Materials and Methods and outlined in Fig. 1. The synthesis led to the preparation of (+)-biphenyl cis-(3R,4S)-dihydrodiol which was identical in structure but had chiroptical properties opposite to those of the bacterial metabolite (Fig. 4).
FIG. 4.
CD spectra of chemoenzymatically derived (+)-biphenyl-cis-(3R,4S)-dihydrodiol (99% enantiomeric purity, bold line), and enzymatically derived (−)-biphenyl-cis-(3S,4R)-dihydrodiol (75% enantiomeric purity, dashed line).
The formation of diastereomeric MPBA derivatives of the biphenyl cis-3,4-dihydrodiol produced by the F352V enzyme provided another method for determining the enantiomeric purity of the compound. The results also allowed an empirical prediction of absolute configuration based on trends for vicinal cis-diols with a benzylic hydroxymethine (46). These trends were employed in the absence of MPBA directional-shift data for a series of cis-3,4-dihydrodiols of known absolute configuration, which are presently unavailable. 1H-NMR analysis (d6-benzene) of the derivative formed with the (−)-biphenyl cis-3,4-dihydrodiol and (S)-MPBA showed that the methoxy signal was shifted downfield (Δδ + 21 ppb; 3.1987 ppm) relative to the corresponding signal of the (R)-MPBA derivative (Table 5). The enantiomeric purity of the major diol was approximately 75%, based on integration of the methoxy groups of the major and minor MPBA diastereomers. This result confirms the data obtained by chiral HPLC analysis (Table 4) and CD spectroscopy (Fig. 4). The downfield shifted methoxy signal for the (S)-MPBA derivative would indicate an S-configuration at the benzylic carbon for a 2,3-dihydrodiol. Application of this trend to the hydroxymethine nearest to the benzylic position allows the prediction of S-stereochemistry at C-3 and an absolute configuration of (−)-biphenyl cis-(3S,4R)-dihydrodiol. This result is consistent with the correlation to the synthetic compound of known stereochemistry.
TABLE 5.
Determination of the absolute configuration and enantiomeric purity of biphenyl-, phenanthrene-, and anthracene-cis-dihydrodiols by 1H-NMR analysis of diastereomeric MPBA estersa
| Compound | Enzyme | Compound relative yield (%) | −OCH3
|
−CH3
|
||||
|---|---|---|---|---|---|---|---|---|
| Derivative type | δ (ppm) CDCl3 [C6D6] | Δδ (ppb) CDCl3 [C6D6] | δ (ppm) CDCl3 [C6D6] | δ (ppb) CDCl3 [C6D6] | cis-Dihydrodiol absolute configurationb (enantiomeric purity [%]) | |||
| (−)-cis-3,4-Dihydroxy-3,4- dihydrobiphenyl | NDO-F352V | 96% | (S)-MPBA | 3.238 [3.187] | +6 [+21] | 1.4195 [1.584] | −5 [−20] | 3S,4R (75) |
| (R)-MPBA | 3.232 [3.165] | —c | 1.4245 [1.601] | — | ||||
| (−)-cis-1,2-Dihydroxy-1,2- dihydrophenanthrene | NDO-F352V | 83% | (S)-MPBA | 3.220 | +72 | 1.368 | −74 | 1S,2R (91) |
| (R)-MPBA | 3.148 | — | 1.442 | — | ||||
| (+)-cis-3,4-Dihydroxy-3,4- dihydrophenanthrene | NDO-F352V | 17% | (S)-MPBA | 3.115 | −126 | NDc | — | 3S,4R (>95) |
| (R)-MPBA | 3.241 | — | ND | — | ||||
| (+)-cis-3,4-Dihydroxy-3,4- dihydrophenanthrene | BPDO (B8/36) | 90% | (S)-MPBA | 3.115 | −126 | 1.452 | +159 | 3S,4R (>98) |
| (R)-MPBA | 3.241 | — | 1.293 | — | ||||
| (+)-cis-1,2-Dihydroxy-1,2- dihydrophenanthrened | BPDO (B8/36) | 10% | (S)-MPBA | 3.148 | −72 | ND | — | 1R,2S (>98) |
| (R)-MPBA | 3.220 | — | ND | — | ||||
| (+)-cis-1,2-Dihydroxy-1,2- dihydroanthracene | NDO-F352V | 100% | (S)-MPBA | 3.166 | −66 | 1.442 | +64 | 1R,2S (96.5) |
| (R)-MPBA | 3.232 | — | 1.378 | — | ||||
Chemical shifts (δ) are reported for the (−)-(S)- and (+)-(R)-MPBA esters of the cis-dihydrodiols formed from biphenyl (recorded at 360 MHz) and from phenanthrene (recorded at 600 MHz). The Δδ represents the distance and direction of the (−)-(S)-MPBA ester chemical shift from the corresponding signal of the boronate ester formed with (+)-(R)-MPBA. A negative Δδ value indicates that the signal of interest is upfield from the corresponding signal of the diastereomer formed with the opposite boronic acid; a positive Δδ means that the signal of interest is downfield from the corresponding signal of the ester formed with the opposite boronic acid.
The absolute configuration predicted for the cis-3,4-dihydroxy-3,4-dihydrobiphenyl was based on the trends in directional shifts observed for a series of 2,3-dihydrodiols. The absolute configuration of the phenanthrene cis-3,4- and cis-1,2-dihydrodiols formed by NDO-F352V was determined by stereochemical correlation with standards of known absolute stereochemistry prepared by strain B8/36 biphenyl dioxygenase (BPDO).
—, not applicable; ND, not determined.
The (+) rotation was inferred from the [α]D of the (−)-phenanthrene cis-1,2-dihydrodiol produced by JM109(DE3)(pDTG141-F352V)(pDTG511).
Absolute stereochemistry of phenanthrene cis-3,4-dihydrodiol, phenanthrene cis-1,2-dihydrodiol, and anthracene cis-1,2-dihydrodiol.
For the phenanthrene cis-1,2-dihydrodiol formed by the F352V mutant of NDO, the methoxy signal of the (S)-MPBA derivative was observed downfield (+72 ppb) from the corresponding signal of the opposite diastereomer formed with (R)-MPBA and predicted an S-configuration at the benzylic center (Table 5). Based on previously documented trends (46), the absolute configuration of the major dihydrodiol formed by the F352V mutant from phenanthrene is phenanthrene cis-(1S,2R)-dihydrodiol (91% enantiomeric purity; 83% relative yield). The facial selectivity in this case was opposite to that shown for wild-type biphenyl dioxygenase from S. yanoikuyae B8/36. Analysis of the (±)-MPBA derivative of the isolated phenanthrene cis-dihydrodiol fraction formed by strain B8/36 showed resolution of the mixed racemates (of 3,4- and 1,2-diols) with minor methoxy signals of the 1,2-diol at 3.148 and 3.220 ppm. The same sample, when derivatized with (S)-MPBA, showed the upfield shifted methoxy signal at 3.148 ppm which corresponds to an R-configuration at the benzylic center, a result consistent with the (1R,2S) configuration that was previously determined (33). The results of the above stereochemical correlation also suggest that the empirical application of the trends in the directional shifts of polycyclic aromatic diols are valid for both the “bay-region” cis-3,4- and “non-bay region” cis-1,2-dihydrodiols of phenanthrene.
The minor diol formed from phenanthrene by the NDO F352V mutant was identified as phenanthrene cis-(3S,4R)- dihydrodiol (>95% enantiomeric purity, 17% relative yield). This assignment is based on the correlation of the methoxy signal at 3.115 ppm (but not 3.241 ppm) in the (S)-MPBA derivative of the F352V minor phenanthrene cis-3,4-dihydrodiol with that of the identical directional shifts of the known B8/36 phenanthrene cis-3,4-dihydrodiol derivatives (Table 5).
Biotransformation reactions with the F352V mutant of NDO using anthracene (200 mg) as substrate yielded 22 mg of cis-1,2-dihydroxy-1,2-dihydroanthracene (anthracene cis-1,2-dihydrodiol) as the sole product following extraction and PLC purification. The diol was identified by GC-MS and 1H-NMR analysis and comparison with authentic compound formed by wild-type NDO and by S. yanoikuyae B8/36 (30). The diol had a specific rotation [α]D of +134 (c 0.5, methanol), and 1H-NMR analysis of diastereomeric MPBA-esters (46) provided independent confirmation of its (1R,2S) absolute configuration and indicated the enantiopurity to be >96% (Table 5). A minor amount of the opposite enantiomer was observed. In contrast, the products formed by wild-type NDO from P. putida strain 119 and B8/36 were enantiomerically pure (1, 30).
Preparation of enantiopure (−)-biphenyl cis-(3S,4R)-dihydrodiol and (−)-phenanthrene cis-(1S,2R)-dihydrodiol.
Toluene cis-dihydrodiol dehydrogenase from P. putida F1 (48) was partially purified to remove 3-methylcatechol 2,3-dioxygenase. This was necessary to eliminate the absorbance at 340 nm by the 3-methylcatechol ring fission product. The partially purified dehydrogenase was examined for its ability to oxidize the enantiomers of biphenyl cis-2,3- and cis-3,4-dihydrodiols. As shown in Table 6, the enzyme specifically oxidized the (+)-enantiomers of both dihydrodiols. Extracts of an E. coli strain carrying pDTG511 (56), which expresses toluene cis-dihydrodiol dehydrogenase from P. putida F1, gave similar results (Table 6). After extraction and analysis by TLC, dihydroxybiphenyl products were detected in all reaction mixtures that contained (+)-biphenyl cis-dihydrodiols. Although a slight amount of NADH was formed in assays with (−)-biphenyl 2,3-dihydrodiol (Table 6), we did not detect the formation of any 2,3-dihydroxybiphenyl. On the basis of these findings, a recombinant E. coli strain was constructed which expresses both the P. putida todD gene encoding toluene cis-dihydrodiol dehydrogenase (56) and the NDO F352V mutant enzyme. This strain, JM109(DE3)(pDTG141-F352V)(pDTG511), was used in whole-cell biotransformation experiments. Incubation of this organism with biphenyl as described in Materials and Methods led to the isolation of 32 mg of homochiral (−)-biphenyl cis-(3S-4R)-dihydrodiol after purification by PLC. The (+)-enantiomers of biphenyl cis-2,3-dihydrodiol and biphenyl cis-3,4-dihydrodiol were completely converted to their respective catechols, compounds which were easily separated from the (−)-biphenyl cis-3,4-dihydrodiol by PLC. The specific rotation [α]D −84.4 (c 0.3, methanol), of the (−)-biphenyl cis-3,4-dihydrodiol was, with the exception of the sign of rotation, identical to that of the synthetic biphenyl cis-3,4-dihydrodiol. Control E. coli strain JM109(DE3)(pDTG141-F352V)(pDTG512), which contains a truncated todD gene, and strain JM109(DE3)(pDTG141-F352V)(pKT230), which does not contain the todD gene, both formed a 96:4 ratio of biphenyl cis-3,4-dihydrodiol and biphenyl cis-2,3-dihydrodiol.
TABLE 6.
Enantioselectivity of toluene cis-dihydrodiol dehydrogenasea
| Substrateb | μmol of NADH produced/μmol of substratec
|
|
|---|---|---|
| TDD (F1) | TDD (E. coli) | |
| (+)-Biphenyl cis-2,3-dihydrodiol | 1.09 ± 0.02 | 0.94 ± 0.01 |
| (−)-Biphenyl cis-2,3-dihydrodiol | 0.07 ± 0.01 | 0.08 ± 0.01 |
| 50:50 (±)-biphenyl cis-2,3-dihydrodiol | 0.51 ± 0.01 | 0.44 ± 0.01 |
| 70:30 (±)-biphenyl cis-3,4-dihydrodiol | 0.73 ± 0.06 | 0.65 ± 0.03 |
Reactions were initiated by the addition of 0.1 to 0.3 μmol of the substrates indicated. The amount of NADH was measured at 340 nm (biphenyl cis-3,4-dihydrodiol) or 350 nm (biphenyl cis-2,3-dihydrodiol) until no further increase in absorbance was observed.
(+)-Biphenyl cis-2,3-dihydrodiol (>99% enantiomeric purity) was generated by S. yanoikuyae B8/36; (−)-biphenyl cis-2,3-dihydrodiol (>95% enantiomeric purity) was generated by P. stutzeri C250; 50:50 (±)-biphenyl cis-2,3-dihydrodiol was a mixture of the above two substrates; 70:30 (±)-biphenyl cis-3,4-dihydrodiol was generated by the F352L mutant of NDO.
TDD (F1), toluene cis-dihydrodiol dehydrogenase partially purified from P. putida F1; TDD (E. coli), toluene cis-dihydrodiol dehydrogenase in extracts from a recombinant E. coli strain expressing the todD gene. Values are means and standard deviations for three separate experiments.
In a similar experiment to that described above, JM109(DE3)(pDTG141-F352V)(pDTG511) oxidized phenanthrene to homochiral (−)-phenanthrene cis-(1R,2S)-dihydrodiol [33 mg; [α]D −64.7 (c 0.23, methanol)].
In these experiments, no attempt was made to optimize the yields of (−)-biphenyl cis-3,4-dihydrodiol and (−)-phenanthrene cis-1,2-dihydrodiol. Nevertheless, it is clear that toluene cis-dihydrodiol dehydrogenase can be used to kinetically resolve enantiomeric mixtures of both dihydrodiols and thus provide novel routes to the pure (−)-biphenyl cis-3,4-dihydrodiol and (−)-phenanthrene cis-1,2-dihydrodiol enantiomers.
DISCUSSION
NDO has a relaxed substrate specificity that allows the oxidation of more than 70 substrates (45). Previous work has shown that NDO can accept amino acid substitutions at several positions near the active site without losing activity. Most of the resulting enzymes had, at most, minor changes in regioselectivity and no differences in enantioselectivity with the substrates tested (38). In this study, a series of amino acid substitutions at position 352 demonstrated that the amino acid at this position in the α subunit of NDO is critical in determining both the regio- and enantioselectivity of the enzyme with a variety of substrates. In addition, the surrounding amino acids also influence the substrate specificity of NDO. This can be seen by comparing specificities of the NDO mutants F352I and F352T with those of 2-nitrotoluene dioxygenase (2NTDO) from Pseudomonas sp. strain JS42 (3, 35, 36) and 2,4-dinitrotoluene dioxygenase (DNTDO) from Burkholderia sp. strain DNT (53). The deduced amino acid sequences of the α subunits of 2NTDO and DNTDO are 84 and 80% identical to the NDO α subunit, respectively (35, 53). At the position corresponding to Phe-352 in NDO, 2NTDO and DNTDO have an isoleucine and a threonine, respectively. 2NTDO catalyzes the oxidation of 2-nitrotoluene to nitrite and 3-methylcatechol. In a similar reaction, DNTDO converts 2,4-dinitrotoluene to 4-methyl-5-nitrocatechol and nitrite. However, the F352I and F352T mutants of NDO cannot catalyze either reaction (data not shown). In addition, whereas 2NTDO forms 70% (+)-naphthalene cis-(1R,2S)-dihydrodiol, the F352I mutant of NDO forms the cis-dihydrodiol in much higher enantiomeric purity [94% (+)-(1R,2S); Table 4]. The reactions catalyzed by DNTDO and the NDO F352T mutant are similar, producing 96 and 93% (+)-naphthalene cis-(1R,2S)-dihydrodiol, respectively. Biphenyl and phenanthrene are not substrates for 2NTDO and DNTDO (38) but are oxidized by the F352I and F352T mutants of NDO (Fig. 3; Table 3). These results suggest that while the amino acid at position 352 is critical, other amino acids must also play a role in determining substrate specificity in these enzymes.
Introduction of threonine, a smaller and more polar amino acid than phenylalanine, at position 352, resulted in an enzyme with slightly reduced activity (Table 3). However, replacement of Phe-352 with glycine resulted in a defective enzyme, particularly when biphenyl was provided as the substrate (Table 3). Introduction of this small amino acid may have destabilized the protein as suggested in other studies (11). Similarly, substitution with the next smallest amino acid, alanine, resulted in an enzyme with lowered activity. In contrast, hydrophobic amino acids of intermediate size were tolerated at position 352 and resulted in enzymes with good activity (Table 3). Substitution at position 352 by tyrosine or tryptophan, two amino acids that are larger than phenylalanine, resulted in enzymes with little or no measurable activity, suggesting that the active-site pocket is of limited size and cannot tolerate a larger residue at this position.
The asymmetric chemoenzymatic synthesis of (+)-biphenyl cis-(3R,4S)-dihydrodiol (Fig. 1) of known absolute stereochemistry allowed unequivocal assignment of the (−)-cis-(3S,4R) configuration to the regioisomer produced by the F352V mutant. The accurate prediction of cis-(3S,4R)-absolute stereochemistry for the same metabolite through analysis of MPBA-esters suggests that the trends observed for these derivatives with a series of polycyclic cis-2,3-dihydrodiols (46) may be applied to cis-3,4-dihydrodiols in the manner described here. Additional data for a series of cis-3,4-dihydrodiols are needed to establish whether this empirical correlation is generally valid. Assignments of absolute stereochemistry for the phenanthrene cis-1,2- and cis-3,4-dihydrodiols and anthracene cis-1,2-dihydrodiol were based on direct comparisons of 1H-NMR chemical shifts of their MPBA derivatives to those of known MPBA derivatives prepared using biphenyl dioxygenase (Table 5). Distinct chemical shifts allowed the analysis of a mixture of phenanthrene cis-3,4- and cis-1,2-dihydrodiols.
Of the eight amino acid replacements made at position 352 in NDO, the F352V mutant enzyme had an overall substrate specificity that was most different from wild-type NDO. A detailed characterization of the products formed by the F352V mutant demonstrated that the enzyme had the opposite regioselectivity with biphenyl (Fig. 3A) and phenanthrene (Fig. 3B; 38) and a slight change in enantioselectivity with naphthalene (Table 4) and anthracene as substrates (Table 5). In addition, the opposite enantiomers of biphenyl cis-3,4-dihydrodiol and phenanthrene cis-1,2-dihydrodiol were formed in contrast to the enantiomer formed by wild-type NDO (Tables 4 and 5).
Enantioselective diol dehydrogenases have been used to kinetically resolve mixtures of cis-dihydrodiols to form enantiomerically pure compounds (2, 13, 41). Naphthalene-grown whole cells of P. putida NCIMB 8859 selectively oxidized several cis-dihydrodiols, including (+)-naphthalene cis-dihydrodiol, and several substituted benzene dihydrodiols (2). Naphthalene cis-dihydrodiol dehydrogenase purified from P. putida NP exclusively oxidized the (+)-enantiomer of naphthalene cis-dihydrodiol (28). Results from this study indicate that toluene cis-dihydrodiol dehydrogenase from P. putida F1 catalyzes enantioselective reactions with biphenyl cis-2,3-dihydrodiol and biphenyl cis-3,4-dihydrodiol. Only the (+)-enantiomers of each dihydrodiol were oxidized by partially purified enzyme preparations from toluene-induced cells of P. putida F1 and IPTG-induced cells of a recombinant E. coli strain expressing the todD gene (Table 6). Similar enantioselective activities were obtained with partially purified biphenyl and naphthalene cis-dihydrodiol dehydrogenases from Pseudomonas sp. strain LB400 (26) and Pseudomonas sp. strain NCIB 9816-4 (40; data not shown).
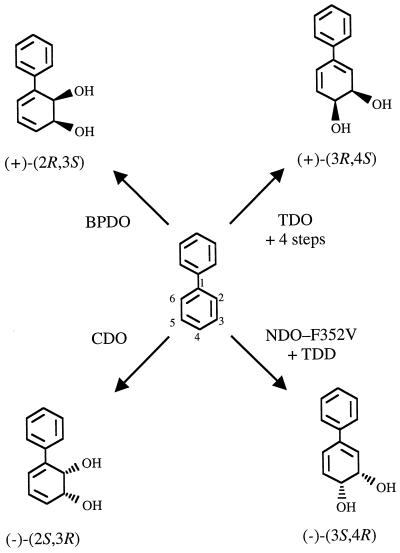
The results presented here establish procedures for the production of enantiomers of both biphenyl cis-2,3- and cis-3,4-dihydrodiol (Fig. 5). (+)-Biphenyl cis-(2R,3S)-dihydrodiol is the sole product formed from biphenyl by biphenyl dioxygenase (19, 20), and (−)-biphenyl cis-(2S,3R)-dihydrodiol is the major product formed by carbazole dioxygenase (43; Resnick and Gibson, Abstr. 96th Gen. Meet. Am. Soc. Microbiol., 1996, abstr. O-11, p. 355, 1996). The results presented provide methods for the enzymatic or chemoenzymatic synthesis of the (+)- and (−)-enantiomers of biphenyl cis-3,4-dihydrodiol. A five-step chemoenzymatic synthesis scheme (Fig. 1) for the formation of (+)-biphenyl cis-(3R,4S)-dihydrodiol utilizes toluene dioxygenase to catalyze the initial step. The combination of the NDO F352V mutant enzyme and toluene cis-dihydrodiol dehydrogenase allowed the formation of (−)-biphenyl cis-(3S,4R)-dihydrodiol from biphenyl in one step and required a single purification step. The same strain construction provided a simple method for the enzymatic production of (−)-phenanthrene cis-(1S,2R)-dihydrodiol. Formation of these two compounds as sole products in high enantiopurity demonstrates the utility of constructed strains expressing mutated NDO with altered substrate specificity in combination with the regio- and enantioselective resolution afforded by a cis-dihydrodiol dehydrogenase.
FIG. 5.
Biosynthetic routes to both enantiomers of biphenyl cis-2,3- and cis-3,4-dihydrodiols from biphenyl. BPDO, biphenyl dioxygenases from Pseudomonas sp. LB400 or S. yanoikuyae B8/36 form 100% (+)-biphenyl cis-(2R,3S)-dihydrodiol; CDO; carbazole dioxygenase from P. stutzeri C250 forms >95% (−)-biphenyl cis-(2S,3R)-dihydrodiol; TDO, toluene dioxygenase from P. putida UV4 and four synthetic steps (Fig. 1 and Materials and Methods) resulted in 100% (+)-biphenyl cis-(3R,4S)-dihydrodiol; the F352V mutant of NDO and toluene cis-dihydrodiol dehydrogenase (TDD) from P. putida F1, followed by one purification step, resulted in 100% (−)-biphenyl cis-(3S,4R)-dihydrodiol.
ACKNOWLEDGMENTS
This work was supported by U.S. Public Health Service grant GM29909 from the National Institute of General Medical Sciences.
We thank Tracy Walendy for assistance in constructing site-directed mutants and Juan Parales for assistance in the cis-dihydrodiol dehydrogenase purifications.
REFERENCES
- 1.Akhtar M N, Boyd D R, Thompson N J, Koreeda M, Gibson D T, Mahadevan V, Jerina D M. Absolute stereochemistry of the dihydroanthracene-cis- and -trans-1,2-diols produced from anthracene by mammals and bacteria. J. Chem. Soc. Perkin Trans. I. 1975. pp. 2506–2511. [PubMed] [Google Scholar]
- 2.Allen C C R, Boyd D R, Dalton H, Sharma N D, Brannigan I, Kerley N A, Sheldrake G N, Taylor S C. Enantioselective bacterial biotransformation routes to cis-diol metabolites of monosubstituted benzenes, naphthalene and benzocycloalkenes of either absolute configuration. J. Chem. Soc. Chem. Commun. 1995. pp. 117–118. [Google Scholar]
- 3.An D, Gibson D T, Spain J C. Oxidative release of nitrite from 2-nitrotoluene by a three-component enzyme system from Pseudomonas sp. strain JS42. J Bacteriol. 1994;176:7462–7467. doi: 10.1128/jb.176.24.7462-7467.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagdasarian M, Lurz R, Ruckert B, Franklin F C H, Bagdasarian M M, Frey J, Timmis K N. Specific-purpose cloning vectors. II. Broad-host-range high copy number, RSF1010 derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene. 1981;16:237–247. doi: 10.1016/0378-1119(81)90080-9. [DOI] [PubMed] [Google Scholar]
- 5.Balani S K, van Bladeren P J, Cassidy E S, Boyd D R, Jerina D M. Synthesis of the enantiomeric K-region arene 5,6-oxides derived from chrysene, 7,12-dimethylbenz[a]anthracene and benzo[c]phenanthrene. J Org Chem. 1987;52:137–144. [Google Scholar]
- 6.Ballard D G, Courtis A, Shirley J M, Taylor S C. A biotech route to polyphenylene. J. Chem. Soc. Chem. Commun. 1983. pp. 954–955. [Google Scholar]
- 7.Boyd D R, Sharma N D, Barr S A, Dalton H, Chima J, Whited G, Seemayer R. Chemoenzymatic synthesis of the 2,3- and 3,4-cis-dihydrodiol enantiomers of monosubstituted benzenes. J Am Chem Soc. 1994;116:1147–1148. [Google Scholar]
- 8.Boyd D R, Sharma N D, Dorrity M R J, Hand M V, McMordie R A S, Malone J F, Porter H P, Chima J, Dalton H, Sheldrake G N. Structure and stereochemistry of cis-dihydrodiol and phenol metabolites of bicyclic azaarenes from Pseudomonas putida UV4. J. Chem. Soc. Perkin Trans. I. 1993. pp. 1065–1071. [Google Scholar]
- 9.Boyd D R, Sheldrake G N. The dioxygenase-catalysed formation of vicinal cis-diols. Nat Prod Rep. 1998;15:309–324. [Google Scholar]
- 10.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 11.Caffrey M S. Strategies for the study of cytochrome c structure and function by site-directed mutagenesis. Biochimie. 1994;76:622–630. doi: 10.1016/0300-9084(94)90139-2. [DOI] [PubMed] [Google Scholar]
- 12.Carless H A J. The use of cyclohexa-3,5-diene-1,2-diols in enantiospecific synthesis. Tetrahedron: Asymmetry. 1992;3:795–826. [Google Scholar]
- 13.Connors N, Prevoznak R, Chartrain M, Reddy J, Singhvi R, Patel Z, Olewinski R, Salmon P, Wilson J, Greasham R. Conversion of indene to cis-(1S,2R)-indandiol by mutants of Pseudomonas putida F1. J Ind Microbiol Biotechnol. 1997;18:353–359. [Google Scholar]
- 14.Davis R W, Botstein D, Roth J R. Advanced bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1980. [Google Scholar]
- 15.Ensley B D, Gibson D T. Naphthalene dioxygenase: purification and properties of a terminal oxygenase component. J Bacteriol. 1983;155:505–511. doi: 10.1128/jb.155.2.505-511.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ensley B D, Gibson D T, Laborde A L. Oxidation of naphthalene by a multicomponent enzyme system from Pseudomonas sp. strain NCIB 9816. J Bacteriol. 1982;149:948–954. doi: 10.1128/jb.149.3.948-954.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finette B A, Subramanian V, Gibson D T. Isolation and characterization of Pseudomonas putida PpF1 mutants defective in the toluene dioxygenase enzyme system. J Bacteriol. 1984;160:1003–1009. doi: 10.1128/jb.160.3.1003-1009.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibson D T, Koch J R, Kallio R E. Oxidative degradation of aromatic hydrocarbons by microorganisms. I. Enzymatic formation of catechol from benzene. Biochemistry. 1968;7:2653–2661. doi: 10.1021/bi00847a031. [DOI] [PubMed] [Google Scholar]
- 19.Gibson D T, Roberts R L, Wells M C, Kobal V M. Oxidation of biphenyl by a Beijerinckia species. Biochem Biophys Res Commun. 1973;50:211–219. doi: 10.1016/0006-291x(73)90828-0. [DOI] [PubMed] [Google Scholar]
- 20.Haddock J D, Gibson D T. Purification and characterization of the oxygenase component of biphenyl 2,3-dioxygenase from Pseudomonas sp. strain LB400. J Bacteriol. 1995;177:5834–5839. doi: 10.1128/jb.177.20.5834-5839.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haigler B E, Gibson D T. Purification and properties of ferredoxinNAP, a component of naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816. J Bacteriol. 1990;172:465–468. doi: 10.1128/jb.172.1.465-468.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haigler B E, Gibson D T. Purification and properties of NADH-ferredoxinNAP reductase, a component of naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816. J Bacteriol. 1990;172:457–464. doi: 10.1128/jb.172.1.457-464.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herbert, A. B., G. N. Sheldrake, P. J. Somers, and J. A. Meredith. Jan. 1990. European Patent 0379300A2.
- 24.Hudlicky T, Gonzalez D, Gibson D T. Enzymatic dihydroxylation of aromatics in enantioselective synthesis: expanding asymmetric methodology. Aldrichimica Acta. 1999;32:35–62. [Google Scholar]
- 25.Hudlicky T, Reed J W. An evolutionary perspective of microbial oxidations of aromatic compounds in enantioselective synthesis: history, current status, and perspectives. In: Hassner A, editor. Advances in asymmetric synthesis. Vol. 1. Greenwich, Conn: JAI Press Inc.; 1995. pp. 271–312. [Google Scholar]
- 26.Hülsmeyer M, Hecht H-J, Niefend K, Hofer B, Eltis L D, Timmis K N, Schomburg D. Crystal structure of cis-biphenyl-2,3-dihydrodiol-2,3-dehydrogenase from a PCB degrader at 2.0 Å resolution. Protein Sci. 1998;7:1286–1293. doi: 10.1002/pro.5560070603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeffrey A M, Yeh H J C, Jerina D M. Synthesis of cis-1,2-dihydroxy-1,2-dihydronaphthalene and cis-1,4-dihydroxy-1,4-dihydronaphthalene. J Org Chem. 1974;39:1405–1407. [Google Scholar]
- 28.Jeffrey A M, Yeh H J C, Jerina D M, Patel T R, Davey J F, Gibson D T. Initial reactions in the oxidation of naphthalene by Pseudomonas putida. Biochemistry. 1975;14:575–583. doi: 10.1021/bi00674a018. [DOI] [PubMed] [Google Scholar]
- 29.Jerina D M, Daly J W, Jeffrey A M, Gibson D T. cis-1,2-Dihydroxy-1,2-dihydronaphthalene: a bacterial metabolite from naphthalene. Arch Biochem Biophys. 1971;142:394–396. doi: 10.1016/0003-9861(71)90298-0. [DOI] [PubMed] [Google Scholar]
- 30.Jerina D M, Selander H, Yagi H, Wells M C, Davey J F, Mahadevan V, Gibson D T. Dihydrodiols from anthracene and phenanthrene. J Am Chem Soc. 1976;98:5988–5996. doi: 10.1021/ja00435a035. [DOI] [PubMed] [Google Scholar]
- 31.Kauppi B, Lee K, Carredano E, Parales R E, Gibson D T, Eklund H, Ramaswamy S. Structure of an aromatic ring-hydroxylating dioxygenase-naphthalene 1,2-dioxygenase. Structure. 1998;6:571–586. doi: 10.1016/s0969-2126(98)00059-8. [DOI] [PubMed] [Google Scholar]
- 32.Khan A A, Wang R-F, Cao W-W, Franklin W, Cerniglia C E. Reclassification of a polycyclic aromatic hydrocarbon-metabolizing bacterium, Beijerinckia sp. strain B1, as Sphingomonas yanoikuyae by fatty-acid analysis, protein pattern-analysis, DNA-DNA hybridization, and 16S ribosomal DNA-sequencing. Int J Syst Bacteriol. 1996;46:466–469. doi: 10.1099/00207713-46-2-466. [DOI] [PubMed] [Google Scholar]
- 33.Koreeda M, Akhtar M N, Boyd D R, Neill J D, Gibson D T, Jerina D M. Absolute stereochemistry of cis-1,2-, trans-1,2-, and cis-3,4-dihydrodiol metabolites of phenanthrene. J Org Chem. 1978;43:1023–1027. [Google Scholar]
- 34.Lee S-Y, Rasheed S. A simple procedure for maximum yield of high-quality plasmid DNA. BioTechniques. 1990;9:676–679. [PubMed] [Google Scholar]
- 35.Parales J V, Kumar A, Parales R E, Gibson D T. Cloning and sequencing of the genes encoding 2-nitrotoluene dioxygenase from Pseudomonas sp. JS42. Gene. 1996;181:57–61. doi: 10.1016/s0378-1119(96)00462-3. [DOI] [PubMed] [Google Scholar]
- 36.Parales J V, Parales R E, Resnick S M, Gibson D T. Enzyme specificity of 2-nitrotoluene 2,3-dioxygenase from Pseudomonas sp. strain JS42 is determined by the C-terminal region of the α subunit of the oxygenase component. J Bacteriol. 1998;180:1194–1199. doi: 10.1128/jb.180.5.1194-1199.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parales R E, Emig M D, Lynch N A, Gibson D T. Substrate specificities of hybrid naphthalene and 2,4-dinitrotoluene dioxygenase enzyme systems. J Bacteriol. 1998;180:2337–2344. doi: 10.1128/jb.180.9.2337-2344.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parales R E, Lee K, Resnick S M, Jiang H, Lessner D J, Gibson D T. Substrate specificity of naphthalene dioxygenase: effect of specific amino acids at the active site of the enzyme. J Bacteriol. 2000;182:1641–1649. doi: 10.1128/jb.182.6.1641-1649.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parales R E, Parales J V, Gibson D T. Aspartate 205 in the catalytic domain of naphthalene dioxygenase is essential for activity. J Bacteriol. 1999;181:1831–1837. doi: 10.1128/jb.181.6.1831-1837.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel T R, Gibson D T. Purification and properties of (+)-cis-naphthalene dihydrodiol dehydrogenase of Pseudomonas putida. J Bacteriol. 1974;119:879–888. doi: 10.1128/jb.119.3.879-888.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raschke H, Fleischmann T, van der Meer J R, Kohler H-P. cis-Chlorobenzene dihydrodiol dehydrogenase (TcbB) from Pseudomonas sp. strain P51, expressed in Escherichia coli DH5α(pTCB149), catalyzes enantioselective dehydrogenase reactions. Appl Environ Microbiol. 1999;65:5242–5246. doi: 10.1128/aem.65.12.5242-5246.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reddy J, Lee C, Neeper M, Greasham R, Zhang J. Development of a bioconversion process for production of cis-1S,2R-indandiol from indene by recombinant Escherichia coli constructs. Appl Microbiol Biotechnol. 1999;51:614–620. doi: 10.1007/s002530051440. [DOI] [PubMed] [Google Scholar]
- 43.Resnick S. Ph.D. thesis. Iowa City: The University of Iowa; 1997. [Google Scholar]
- 44.Resnick S M, Gibson D T. Regio- and stereospecific oxidation of 9,10-dihydroanthracene and 9,10-dihydrophenanthrene by naphthalene dioxygenase: structure and absolute stereochemistry of metabolites. Appl Environ Microbiol. 1996;62:3355–3359. doi: 10.1128/aem.62.9.3355-3359.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Resnick S M, Lee K, Gibson D T. Diverse reactions catalyzed by naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816. J Ind Microbiol. 1996;17:438–457. [Google Scholar]
- 46.Resnick S M, Torok D S, Gibson D T. Chemoenzymatic synthesis of chiral boronates for the 1H NMR determination of the absolute configuration and enantiomeric excess of bacterial and synthetic cis-diols. J Org Chem. 1995;60:3546–3549. [Google Scholar]
- 47.Resnick S M, Torok D S, Lee K, Brand J M, Gibson D T. Regiospecific and stereoselective hydroxylation of 1-indanone and 2-indanone by naphthalene dioxygenase and toluene dioxygenase. Appl Environ Microbiol. 1994;60:3323–3328. doi: 10.1128/aem.60.9.3323-3328.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rogers J E, Gibson D T. Purification and properties of cis-toluene dihydrodiol dehydrogenase from Pseudomonas putida. J Bacteriol. 1977;130:1117–1124. doi: 10.1128/jb.130.3.1117-1124.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sambrook J, Fritch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 50.Shudo K, Okamoto T. Electronic effects of some epoxides and cyclopropanes. Hyperconjugative electron withdrawal. Tetrahedron. 1977;33:1717–1719. [Google Scholar]
- 51.Stanier R Y, Palleroni N J, Doudoroff M. The aerobic pseudomonads; a taxonomic study. J Gen Microbiol. 1966;43:159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- 52.Suen W-C. Ph.D. thesis. Iowa City: The University of Iowa; 1991. [Google Scholar]
- 53.Suen W-C, Haigler B E, Spain J C. 2,4-Dinitrotoluene dioxygenase from Burkholderia sp. strain DNT: similarity to naphthalene dioxygenase. J Bacteriol. 1996;178:4926–4934. doi: 10.1128/jb.178.16.4926-4934.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tabor S, Richardson C C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci USA. 1985;82:1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 56.Zylstra G J, Gibson D T. Aromatic hydrocarbon degradation: a molecular approach. Genet Eng. 1991;13:183–203. doi: 10.1007/978-1-4615-3760-1_8. [DOI] [PubMed] [Google Scholar]