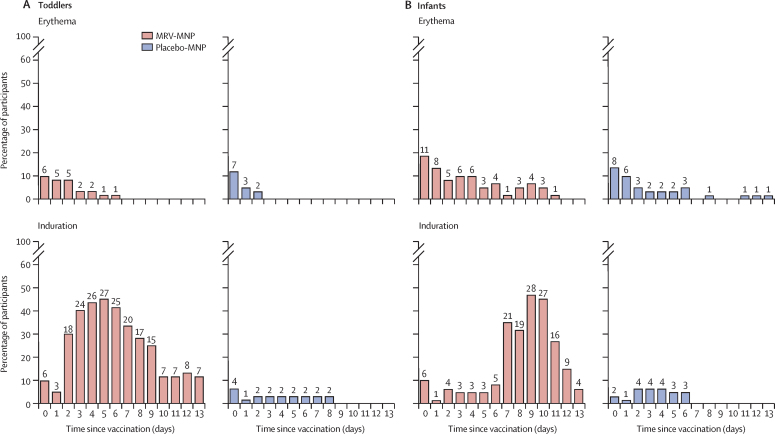
Figure 2.
Local solicited adverse events—toddler and infant cohorts
(A) Toddler cohort (B) Infant cohort. Numbers represent the absolute number of participants, from among the 60 in each randomisation group and cohort, affected on each day. All local reactions were mild in severity. In addition, one toddler had mild tenderness on day 8 following MRV-MNP and one toddler had mild tenderness on day 1 following placebo-MNP (data not shown graphically). MNP=microneedle patch. MRV=measles and rubella vaccine. SC=subcutaneous.