Abstract
Context
Fibrous dysplasia/McCune-Albright syndrome (FD/MAS) is a rare genetic disorder. Incidence and prevalence are not well-studied. Epidemiological research is complicated by the rarity of FD/MAS, absence of registries, heterogeneous presentation, and possibly asymptomatic phenotype. FD/MAS may present with FGF23-mediated hypophosphatemia, of which the epidemiology is also unclear.
Objective
Evaluate incidence and prevalence of FD/MAS and FD/MAS-related hypophosphatemia.
Methods
This cohort study based on the nationwide Danish National Patient Registry from 1995-2018, included patients identified by ICD-10 codes M85.0 (monostotic FD [MFD]) and Q78.1 (polyostotic FD [PFD]/MAS). Incidence rates and prevalence were calculated and stratified by sex, age, calendar period, and diagnosis code. Cases were screened for FD-associated hypophosphatemia by diagnosis code E.83 (disorder of mineral metabolism) and dispatched vitamin D analogues.
Results
A total of 408 patients were identified, 269 with MFD (66%), 139 with PFD/MAS (34%), comparable between sexes. Incidence of FD/MAS demonstrated increasing secular trend with a rate of 3.6 per 1 000 000 person-years (95% CI: 2.9, 4.5) in 2015-2018. Incidence peaked between age 11 and 20. Prevalence of FD/MAS increased over time to 61.0 (95% CI: 54.6, 67.4) per 1 000 000 persons in 2018. The incidence rate of MFD was 1.5-fold that of PFD/MAS in the first decade, rising to 2.5-fold in the last decade. No FD/MAS cases were registered with diagnosis code or treatment for hypophosphatemia.
Conclusion
FD/MAS is rare, diagnosis peaks during adolescence without sex predominance, and MFD is most prevalent. Hypophosphatemia may be underdiagnosed and undertreated, or it may be underregistered, comparing this study to literature.
Keywords: incidence, prevalence, epidemiology, fibrous dysplasia/McCune-Albright syndrome, rare bone disorder, registry
Fibrous dysplasia/McCune-Albright syndrome (FD/MAS) is a rare disorder characterized by local replacement of bone by dysplastic tissue in a single skeletal site (monostotic FD [MFD]) or in several bones (polyostotic FD [PFD]). These fibrous bone lesions may lead to significant skeletal morbidity (1). The disorder is caused by postzygotic activating mutations of the GNAS gene, leading to increased cAMP and abnormal cellular responses (2). Due to the genetic mosaicism of the GNAS mutation, FD presents along a heterogenous clinical spectrum and bone lesions can coincide with hyperfunctioning endocrinopathies, most commonly precocious puberty or hyperthyroidism, or hyperpigmented skin macules in the McCune-Albright syndrome. Therefore, the disease is referred to as FD/MAS (3). In skeletal tissue, the maturation of osteoprogenitor cells into osteoblasts is hampered but proliferation is stimulated, causing the continuous formation of immature, woven bone (2). In addition, the phosphaturic hormone fibroblast growth factor 23 (FGF23) is produced and high serum levels of FGF23 may be present in patients with FD/MAS, leading to renal phosphate wasting and hypophosphatemia (4). The hypophosphatemia may cause osteomalacia, which is associated with pain, deformities, and fractures (5-7). Previously the incidence of hypophosphatemia in a cohort of severely affected FD patients was found to be 48% (20 of 42 cases) (4). However, the exact incidence of hypophosphatemia in all FD/MAS patients is lacking. The same holds for the incidence of FD/MAS itself, since the monostotic subtype is often diagnosed as an incidental finding without symptoms (3) and no epidemiological studies on FD/MAS have been published. Only estimations based on regional observations are available, but these numbers are not confirmed by research studies nor replicated in different cohorts (8). Incidence measures may provide useful information on risks by age or sex and may aid in screening and diagnostic decision making (9). In addition, epidemiological measures and the distribution of subtypes within FD/MAS are important for research purposes, to estimate the degree of bias and external validity (generalizability) of studies: if a study conducted in a tertiary referral center includes mainly patients with severe subtypes (PFD or MAS), the conclusions might not be applied to cohorts of less severely affected patients in smaller hospitals, but knowledge on the proportion of subtypes in FD/MAS is essential for this consideration. For these reasons, the present study was conducted to assess the incidence and prevalence of FD/MAS in Denmark with an additional interest in FD-associated hypophosphatemia, over a 25-year period, specified in calendar intervals, age intervals, by sex, and by subtype, and to describe the demographics of the FD/MAS cohort based on the national registry data of Denmark.
Materials and Methods
Data Source
This nationwide, observational cohort study used registry data of Denmark, currently having 5.9 million citizens (10). The Danish National Health Service provides tax-supported health care, ensuring unfettered access to general practitioners and hospitals for all Danish inhabitants. Accurate linkage of all administrative and medical registries is possible at the individual level. Every citizen of Denmark is automatically enrolled in the registries without requirement of informed consent. The Danish National Patient Registry was established in 1977 for inpatients and outpatients were included from 1995 (11). A disease is coded as primary diagnosis when comprising the main reason for the hospital contact (although financial considerations may not be ruled out) and as secondary when supplemental to the primary diagnosis or relevant for the hospital contact, for instance, underlying chronic diseases. The Danish National Patient Registry was used to identify patients with FD/MAS. In 1994 the tenth revision of the International Classification of Disease (ICD-10) was implemented and for these reasons the timeframe for data collection for this study was set at January 1, 1995, to December 31, 2018. Migration, demographic, and mortality data were available through individual-level linkage with the Danish Civil Registration System and allowed life-long follow-up and accurate determination of person-years at risk (11). The observation period ended when the subject was no longer resident in Denmark, at death, or on the 31st of December 2018, whichever occurred first. Data were also linked with the Danish National Prescriptions Registry, containing data regarding all prescription drugs dispensed at Danish community pharmacies (12). Important to note is that for registry analyses yielding 5 or fewer cases per category, the exact number of patients may not be disclosed and patient demographics may not be explored for privacy reasons. The project has been approved by Statistics Denmark, the Danish Health Data Board, the Danish Medicines Agency, and Danish Data Protection Agency, with record number 2016-051-000001/1880 assigned by Arhus University.
Case Definition
A subject was classified as having FD/MAS in case of at least one outpatient hospital visit encounter or inpatient hospital stay coded as monostotic fibrous dysplasia (ICD-10: M85.0), polyostotic fibrous dysplasia/McCune-Albright syndrome (ICD-10: Q78.1), or craniofacial fibrous dysplasia (ICD-10: K10.8), either as primary or secondary diagnosis. Although the latter diagnosis code also includes other fibrous lesions of the jaw, it was planned to include the diagnosis code initially and decide post hoc whether to include or exclude the registered cases. No data exist on validity of these codes specifically, but in general the positive predictive value of three-digit ICD-10 codes in the Danish National Patient Registry is 88% (13). The index date was defined as the date of the first hospital contact that yielded the FD/MAS diagnosis. FD/MAS is a chronic and incurable disease, and the duration of disease was considered as lifetime.
Statistical Analysis
Demographic data and epidemiological measures were calculated using R 4.2.2 and SAS software, version 9.4. Tables and graphs were designed in R 4.2.2.
Incidence
We calculated incidence rates per 1 000 000 person-years with 95% CIs using the exact Poisson distribution. Incidence data were stratified by sex, 10-year age intervals, age and calendar period, age and sex, FD subtypes (code for MFD vs PFD/MAS) and calendar period, and FD subtype and age. To analyze temporal trends, incidence rates were calculated for intervals of 5 calendar years.
Prevalence
Prevalence was calculated as the ratio of persons having the disease in the calendar year. This cumulative measure included patients being incident at or before the calendar year and who remained alive. The prevalence was referenced to 1 000 000 persons alive at mid-year and the confidence interval was estimated using the normal approximation of the binomial distribution. Prevalences were stratified by sex, 10-year age intervals, age, and 5-year calendar period with prevalence for every fifth year, age, and sex, FD subtypes (code for MFD vs PFD/McCune-Albright syndrome) and age.
Hypophosphatemia
After identifying cases with FD/MAS, all cases were screened for the presence of an encounter coded as “Hypophosphatemia” (ICD-10 code: E83.3), and more generally coded as “Disorders of mineral metabolism” (ICD-10: E.83). In addition, all prescriptions of alphacalcidol (ATC-code A11CC03), other active vitamin D analogues (ATC: AC11CC), and phosphate supplementation (ATC: A12CD02) to cases were identified in the Danish National Prescriptions Registry. Statistical analyses to calculate epidemiological measures of hypophosphatemia in FD/MAS were similar as stated above.
Differentiation Between PFD and MAS
For patients coded with ICD-10 code Q78.1, further differentiation between disease subtypes PFD and MAS was attempted by identifying all encounters coded for MAS-related extraskeletal manifestations (endocrinopathies and café au lait spots) and all prescribed drugs used in the treatment for endocrinopathies. All ICD-10 codes and ATC codes are provided in the supplemental file, Supplementary Table SA (14).
Results
The total number of uniquely identified patients between 1995 and 2018 was 408. Thirty-one patients received codes for both MFD and PFD/MAS. They were regarded as having PFD/MAS and were excluded from the monostotic cohort, which eventually comprised 269 cases (65.9%). The PFD/MAS cohort included 139 cases (34.1%). In total 188 male individuals (46%) and 220 female individuals (54%) were included.
The cohort of patients with craniofacial disease consisted of 2098 cases. A high number of non-FD fibrous jaw lesions was suspected and patients with this code were therefore excluded from further analyses.
Incidence
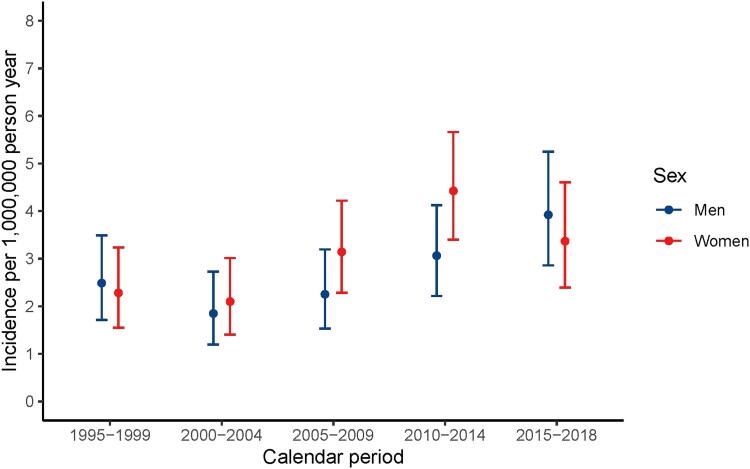
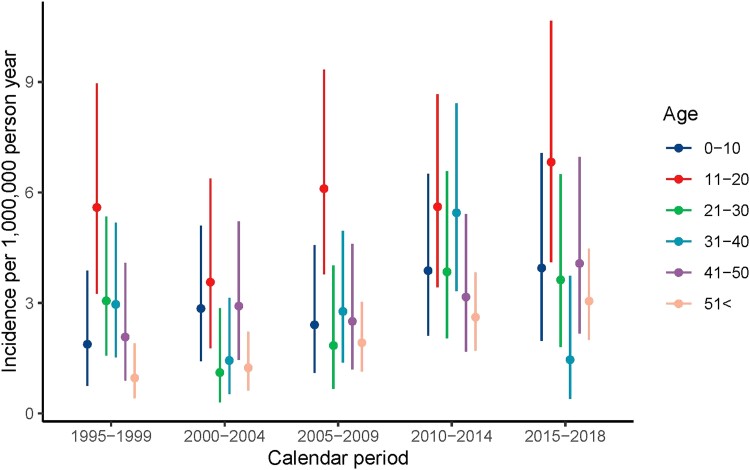
A slight upward trend in incidence rate was observed over time: the incidence rate of 2.4 (95% CI: 1.8, 3.0) per 1 000 000 person-years in 1995-1999 increased over time to reach a plateau after 2010, with a rate of 3.6 (95% CI: 2.9, 4.5) in 2015-2018, comparable for men and women (Table 1, Fig. 1). In general, incidence rates rose over time for all age categories, and in all calendar periods the highest incidence rates were observed in age category 11 to 20 years, the lowest rates in age older than 51 years (Fig. 2). No shift toward earlier diagnosis in later calendar years was observed.
Table 1.
Incidence per calendar period for men and women
| Sex | Calendar period | Incidence rate per 1 000 000 person-years | Lower CI (95%) | Upper CI (95%) |
|---|---|---|---|---|
| Men | 1995-1999 | 2.486 | 1.711 | 3.492 |
| Men | 2000-2004 | 1.848 | 1.196 | 2.728 |
| Men | 2005-2009 | 2.252 | 1.530 | 3.197 |
| Men | 2010-2014 | 3.063 | 2.217 | 4.126 |
| Men | 2015-2018 | 3.923 | 2.861 | 5.249 |
| Women | 1995-1999 | 2.281 | 1.550 | 3.238 |
| Women | 2000-2004 | 2.100 | 1.406 | 3.016 |
| Women | 2005-2009 | 3.143 | 2.284 | 4.219 |
| Women | 2010-2014 | 4.425 | 3.400 | 5.661 |
| Women | 2015-2018 | 3.367 | 2.395 | 4.603 |
Figure 1.
Incidence by calendar period and sex.
Figure 2.
Incidence by age and calendar period.
Incidence rates were in all age categories comparable for male and female patients, with a peak incidence in the age interval of 11 to 20 years, 5.4 (95% CI: 3.9, 7.3) in male and 5.7 (95% CI: 4.1, 7.6] in female patients (Supplementary Figure SA, Supplementary Table SB (14)).
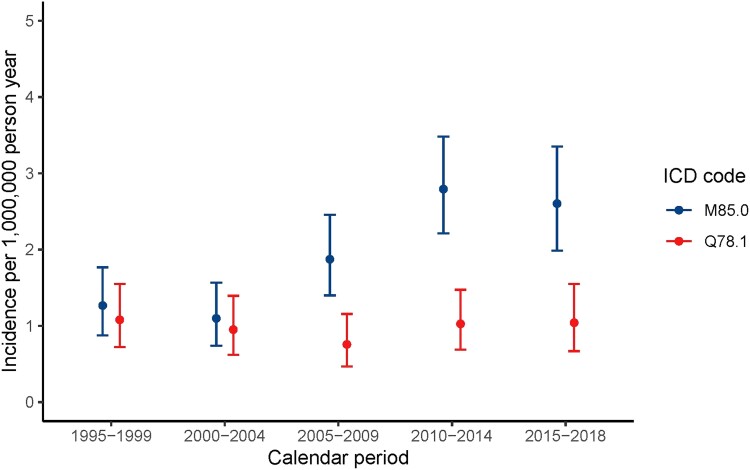
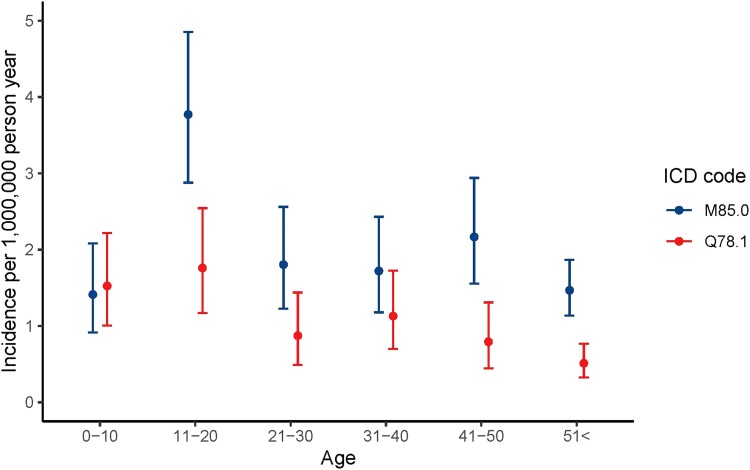
In 2015-2018, MFD was more frequently diagnosed compared with PFD/MAS, with an incidence rate of 2.6 (95% CI: 2.0, 3.4) for MFD and 1.0 (95% CI: 0.7, 1.6] for PFD/MAS (Table 2, Fig. 3). For MFD, this most recent incidence rate is considerably higher compared to the first calendar interval of 1995 to 1999 (1.3 [95% CI: 0.9-1.8]), whereas incidence rates were rather constant over time for PFD/MAS. In 1995-1999, the incidence rate of MFD was 1.5 times the rate of PFD/MAS; in the last decade this has risen to 2.5 times, with 71.4% of diagnosis being MFD and 28.6% PFD/MAS. The peak incidence rate for MFD was between ages 11 and 20 (3.8 [95% CI: 2.9-4.9]). In all other age intervals, rates were lower and comparable (Table 3, Fig. 4). Polyostotic FD/MAS was most frequently diagnosed in the first 2 decades, with a rate of 1.5 (95% CI: 1.0-2.2) between age 0 and 10 and 1.8 (95% CI: 1.2-2.5) between age 11 and 20, and rates decreased thereafter to 0.5 (95% CI: 0.3-0.8) above age 51.
Table 2.
Incidence per calendar period and ICD
| Calendar period | M85.0 | Q78.1 | ||||
|---|---|---|---|---|---|---|
| Incidence rate per 1 000 000 person-years | Lower CI (95%) | Upper CI (95%) | Incidence rate per 1 000 000 person-years | Lower CI (95%) | Upper CI (95%) | |
| 1995-1999 | 1.27 | .88 | 1.77 | 1.08 | .72 | 1.55 |
| 2000-2004 | 1.10 | .74 | 1.57 | 0.95 | .62 | 1.39 |
| 2005-2009 | 1.87 | 1.40 | 2.46 | 0.76 | .47 | 1.16 |
| 2010-2014 | 2.79 | 2.21 | 3.48 | 1.03 | .69 | 1.47 |
| 2015-2018 | 2.60 | 1.99 | 3.35 | 1.04 | .67 | 1.55 |
Figure 3.
Incidence by calendar period and ICD.
Table 3.
Incidence by age and ICD
| Age at incidence | M85.0 | Q78.1 | ||||
|---|---|---|---|---|---|---|
| Incidence rate per 1 000 000 person-years | Lower CI (95%) | Upper CI (95%) | Incidence rate per 100 000 person-years | Lower CI (95%) | Upper CI (95%) | |
| 0-10 | 1.41 | 0.91 | 2.08 | 1.52 | 1.00 | 2.22 |
| 11-20 | 3.77 | 2.88 | 4.85 | 1.76 | 1.17 | 2.54 |
| 21-30 | 1.80 | 1.23 | 2.56 | 0.87 | 0.49 | 1.44 |
| 31-40 | 1.72 | 1.18 | 2.43 | 1.13 | 0.70 | 1.73 |
| 41-50 | 2.17 | 1.56 | 2.94 | 0.79 | 0.44 | 1.31 |
| ≥51 | 1.47 | 1.13 | 1.87 | 0.51 | 0.32 | 0.77 |
Figure 4.
Incidence by age and ICD.
Prevalence
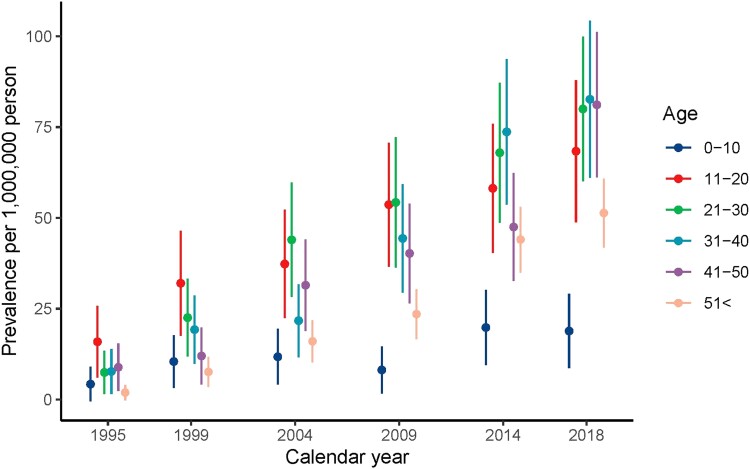
Prevalence demonstrated an increasing trend, from below 0.1 per 1 000 000 persons at the start of the study period, gradually inclining to 61.0 (95% CI: 54.6, 67.4) in 2018 (Supplementary Figure SB (14)). In all age-stratified analyses, prevalence was lowest in the age category of 0 to 10 years, slightly higher in category >51 years, and highest in the second to fifth decade. In 2018, prevalence was 18.9 (95% CI: 8.6-29.1) per 1 000 000 persons for age category 0 to 10 years and highest between age of 31 and 40 years (82.6 [95% CI: 61.0-104.3]) (Table 4). In all age groups, prevalence increased during the study period, except for age 0 to 10 years (Fig. 5).
Table 4.
Prevalence by age in 2018
| Age | Prevalence per 1 000 000 person-years | Lower CI (95%) | Upper CI (95%) |
|---|---|---|---|
| 0-10 | 18.9 | 8.6 | 29.1 |
| 11-20 | 68.4 | 48.8 | 87.9 |
| 21-30 | 80.0 | 60.1 | 99.9 |
| 31-40 | 82.6 | 61.0 | 104.3 |
| 41-50 | 81.1 | 61.1 | 101.2 |
| ≥51 | 51.3 | 41.8 | 60.8 |
Figure 5.
Prevalence by age and calendar year.
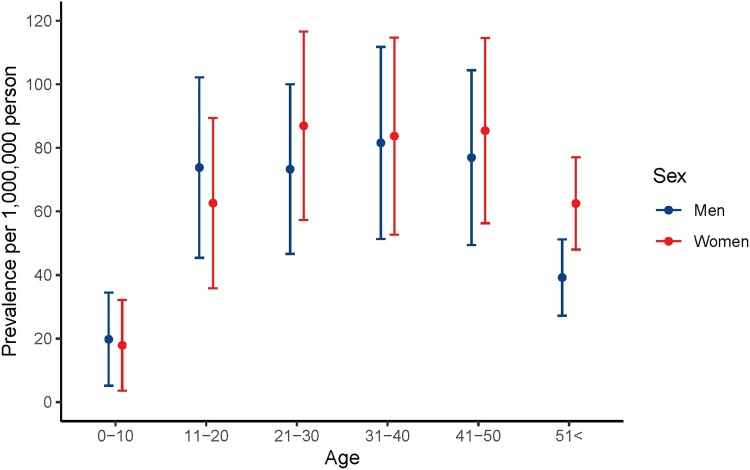
In 2018, prevalence was comparable between men and women in all age intervals (Table 5, Fig. 6). The prevalence of MFD was 1.4- to 1.7-fold higher compared to PFD/MAS in the first 4 decades, and 2.4- to 2.9-fold higher in the 2 oldest age groups (Supplementary Table SC (14)).
Table 5.
Prevalence by age and sex in 2018
| Age | Men | Women | ||||
|---|---|---|---|---|---|---|
| Prevalence per 1 000 000 person-years | Lower CI (95%) | Upper CI (95%) | Prevalence per 1 000 000 person-years | Lower CI (95%) | Upper CI (95%) | |
| 0-10 | 19.8 | 5.1 | 34.4 | 17.9 | 3.6 | 32.2 |
| 11-20 | 73.8 | 45.4 | 102.2 | 62.6 | 35.8 | 89.4 |
| 21-30 | 73.3 | 46.6 | 100.0 | 87.0 | 57.3 | 116.6 |
| 31-40 | 81.6 | 51.4 | 111.8 | 83.7 | 52.7 | 114.7 |
| 41-50 | 76.9 | 49.4 | 104.5 | 85.4 | 56.3 | 114.5 |
| ≥51 | 39.2 | 27.2 | 51.2 | 62.5 | 48.0 | 77.1 |
Figure 6.
Prevalence by age and sex in 2018.
Hypophosphatemia
No FD/MAS patients were coded with an ICD-10 code for hypophosphatemia or other disorders of mineral metabolism in the Danish National Patient Registry. No prescriptions were identified in the registers for vitamin D analogues or phosphate supplementation in FD/MAS. Therefore, the incidence and prevalence of hypophosphatemia in patients with FD/MAS could not be calculated.
Differentiation Between PFD and MAS
Of the total of 408 patients, 29 (7.1%) were diagnosed with at least one of the ICD-10 codes of Table A, 9 (2.2%) were prescribed at least one of the drugs, and 35 (8.6%) had at least one encounter of either an ICD-10 code or one of the drugs. Several extraskeletal manifestations were diagnosed in 5 or fewer patients. The number of patients with a diagnosis for precocious puberty or with puberty-delaying therapy was 11 (2.6%). For pituitary hyperfunctioning, the number of diagnosed patients was 9 (2.2%), with no records of treatment. Hyperthyroidism was coded in 15 patients (3.6%) without records of treatment, hypercortisolism in ≤5 patients with merely ketoconazole recorded in ≤5 patients, hyperparathyroidism was diagnosed in ≤5 patients, without records of treatment, and café au lait spots were coded in ≤5 patients.
Discussion
This study provides insight in the incidence and prevalence of FD/MAS in Denmark. In recent years, the overall incidence of FD/MAS was 3.6 (95% CI: 2.9, 4.5) per 1 000 000 person-years, meeting the definition of the EU for a rare disease (15). The observed prevalence of FD/MAS was 61.0 (95% CI: 54.6, 67.4) per 1 000 000 persons, equivalent to 1 case per 16 500 (95% CI: 14.837, 18.315) persons. Previous papers estimated FD to account for 5-7% of all benign bone tumors (16, 17), although these numbers were not based on scientific data. Research on FD/MAS in general is mostly conducted in specialized academic hospitals, where bias is imminent due to inclusion of mainly severely affected cases, which prevents accurate epidemiological research. Registry-based studies are less affected by referral or selection bias, and our cohort study is the first to investigate incidence and prevalence of FD/MAS in a nationwide setting. In the Danish health care system, hospital care is free and universal, limiting selection bias regarding income, health insurance systems, age, or hospital-specific cohorts (13). The Danish National Patient Registry has a nationwide coverage and data are prospectively collected. In general, the validity of ICD-10 coding in this registry is high, demonstrated by high completeness and high positive predictive value (11), although no studies have investigated these measures for FD/MAS specifically. However, a limitation of our study is that the prevalence calculations were affected by the revision of the ICD-8 to ICD-10 version in 1994, prior to the start of this study in 1995. The ICD revision caused left-truncation and brought a decreased prevalence of FD/MAS in older age groups: patients diagnosed with FD/MAS in the ICD-8 coding system, not repeatedly coded in ICD-10, were missed in our study. Specifically, older patients with FD/MAS have lived more life years before 1994 than younger patients, leading to a higher chance to be diagnosed with FD/MAS before 1994 in the ICD-8 coding system and not (again) after 1994 in the ICD-10 coding system. Thus, the older patients are, the higher the chance on missing an ICD-10 diagnosis, and the larger the underestimation (Fig. 7). This phenomenon was merely absent in the age category 0 to 10 after 2004, as these patients were born after the revision, which therefore reflects the true prevalence (Fig. 5). Additionally, patients with merely craniofacial FD are missed. Since a very high number of cases were diagnosed with this code, we suspected a considerable dilution of this subgroup with patients with other fibrous jaw lesions and therefore a low positive predictive value of this code. Weighing the consequences of over- and underdiagnosis, we excluded patients with this code. This problem in registry-based studies can only be fixed by creating a diagnosis code specifically for craniofacial fibrous dysplasia. Further analyses would also benefit from assigning separate codes to polyostotic disease as well as to McCune-Albright syndrome. In a similar manner, asymptomatic nondiagnosed cases are likely to be missed, a limitation hardly possible to address in any kind of research regarding FD/MAS. Despite these limitations, the epidemiology measures resulting from this study do provide understanding on the distribution of clinically apparent FD/MAS and are useful for various purposes. This may aid in screening and counseling of patients and may improve planning and budgeting of health care. Notably, consequences for planning and budgeting health care are not affected by the missingness of asymptomatic cases, as these patients do not require health care. Our results also allow comparison with other rare bone diseases/skeletal dysplasias. The incidence and prevalence of FD/MAS are low compared to osteogenesis imperfecta (OI) in Denmark (incidence 150 per 1 000 000 births, with birth and population prevalence 218 and 106, respectively, per 1 000 000 persons (18, 19). Incidence is vastly lower than in X-linked hypophosphatemic rickets (XLH) (39 per 1 000 000 children of age 0-15 years) although the prevalence is similar (48 per 1 000 000 children) (20). Prevalence of FD/MAS is similar to birth prevalence of achondroplasia (46 per 1 000 000) (21) but lower than prevalence of hypophosphatasia (157 per 1 000 000) (22). Although most cases of OI, XLH, and achondroplasia are diagnosed at birth, prevalence in FD/MAS may not be directly comparable to birth or childhood prevalence due to methodological differences. The incidence of FD/MAS is a fraction compared to OI and XLH, but the prevalence is similar to XLH and achondroplasia, and approximately half of OI and hypophosphatasia. This is probably since FD/MAS is diagnosed in a broad age range, while other skeletal dysplasias are diagnosed in early childhood. Lastly, future studies may benefit from the results of this study: the impact of future therapeutic targets or other interventions may be estimated according to the prevalence of FD/MAS reported in this study. Prevalence monotonously increased from one calendar year to the next, as expected for a chronic disease that is not believed to materially affect life expectancy, with increasing incidence rates.
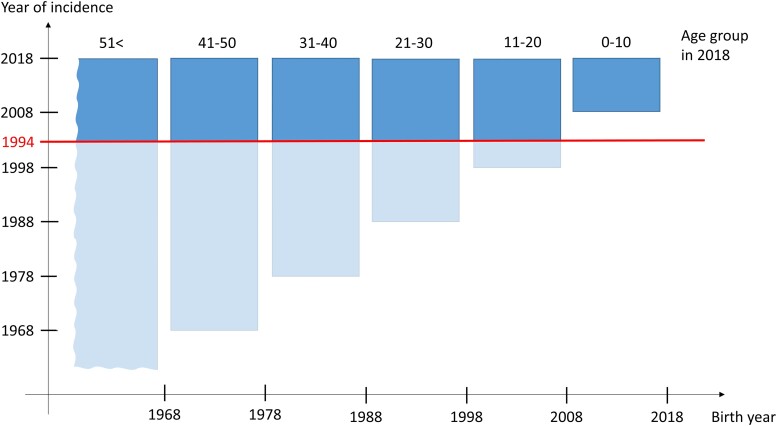
Figure 7.
Year of incidence by birth year for all age groups. The blue area indicates the birth year and year of incidence range for each age group separately. For example, FD/MAS cases belonging to age group 11 to 20 in 2018 were born between 1998 and 2008 and were incident between 1998 and 2018. The total blue bar represents the total of the patients with FD/MAS in the age group. The red line marks the year 1994, where the ICD coding changed from the ninth to the tenth revision. The dark blue component of the bar represents the patients actually diagnosed in the ICD-10 coding system and included in this study. The light blue component represents the patients with FD/MAS coded by ICD-9 before 1994. These patients are missed in the current study if they were not reportedly diagnosed in the ICD-10 system. The chart reflects that in older age groups, higher ratios of cases are missed in this study, that is, the underestimation is higher for older age groups.
Apart from incidence and prevalence in general, our study provided insight into temporal change. The incidence rate of FD/MAS was higher in the second half of the study compared to the first half, with a rise in incidence around 2007 and a stable rate in the last 10 years. This trend may be due to growing awareness, intensified screening protocols, improvements in technical abilities or detection methods, or increased usage of radiology for other conditions. We had expected these factors to diminish diagnostic delay and result in diagnosis at younger age in later calendar intervals, but surprisingly this is not supported by our data: in all calendar years, the highest incidence was observed in age group 11 to 20 years, followed by 0 to 10 years, with consistently lower rates in adulthood. Probably children and teenagers do not receive radiographic assessment in absence of (severe) complaints. Yet small decreases in diagnostic lag time could have been missed due to our 10-year age intervals. The age-specific pattern of diagnosis is consistent with previous studies, demonstrating most disease progression (21, 22) and fractures (23) in FD/MAS in the first 2 decades of life. The rise in incidence is mainly due to an increased rate of MFD diagnoses in last 10 years, as incidence of PFD/MAS was stable over time. Specifically, for MFD, innovations in imaging and histologic techniques, including GNAS mutation screening, may have augmented differentiation between a MFD lesion and other bone lesions, while the diagnosis of PFD/MAS is less dependent on radiology and pathology, due to the more severe phenotype of multiple lesions and shorter list of possible differential diagnoses. Additionally, incidental diagnosis of clinically silent MFD is presumably enhanced over time by the broader use and improved quality of radiographic imaging for musculoskeletal complaints in general, which does not apply for the mainly symptomatic subtypes PFD and MAS. In the final years of the study, 66% of patients were diagnosed with MFD compared to 34% with PFD/MAS. Previous studies have reported McCune-Albright syndrome to represent 7% to 80% of cases (23-25), heavily dependent on the type of hospital and degree of bias. In our study, the identification of patients with MAS was attempted by evaluation of diagnoses codes for extraskeletal manifestations of MAS and drug codes for their treatment. This analysis demonstrated that 8.6% of patients were diagnosed with or treated for extraskeletal disease, which is at the lower range of the proportion found in literature. Unfortunately, these analyses were of limited value due to many categories with fewer than 5 cases and due to several limitations. To capture as many cases as possible, a wide range of possible ICD-10 codes was selected, yet underdiagnosing and underreporting might be a confounding issue. Other limitations include absence of data on endocrine disease frequency in the general Danish population, preventing formal comparison, and the absence of clinical data to confirm these endocrinopathies to be MAS-related and confirm these drugs to be prescribed for MAS-related endocrinopathies. For these reasons, this study merely provides valuable data on the epidemiological differences between MFD and PFD/MAS, which may benefit assessment of bias in cohort studies. An important difference between MFD and PFD/MAS subgroups was age of diagnosis: cases diagnosed at older age were mostly patients with monostotic disease, with a proportion certainly comprising incidental findings in asymptomatic patients. Polyostotic FD/MAS was less common, but more frequently diagnosed in the first decade of life due to the more severe phenotype. Therefore, it should be considered that the majority of patients will not develop the severe consequences of FD/MAS reported in some studies conducted in a severely affected population of FD/MAS. Especially when patients are diagnosed at older age, the risk for a severe phenotype and associated osteomalacia, progressive deformity, nerve compression, or fractures diminishes (24, 26).
Although also set up to provide an answer on the incidence of hypophosphatemia, no patients were coded with an ICD-10 code for hypophosphatemia nor were any identified via prescriptions for vitamin D analogues or phosphate supplementation. This is contrary to literature cohorts (4, 7, 26, 27) and we would have expected a match in at least the PFD group. This can reasonably be explained by the manner of registration or prescription coding, as it is not mandatory to register secondary diagnoses in Denmark, but it might also be due to underdiagnosis and undertreatment of hypophosphatemia in persons with FD/MAS. Biochemistry data could be useful to identify cases of FD/MAS with hypophosphatemia and to determinate the underlying mechanism for the lack of registration, but these were not available for this study.
In conclusion, the incidence rate of FD/MAS in Denmark was 3.6 (95% CI: 2.9, 4.5) per 1 000 000 person-years and the prevalence was 61.0 (95% CI: 54.6, 67.4) per 1 000 000 persons. Most diagnoses were established between the age of 11 and 20 years, although MFD was more often diagnosed at older ages than PFD/MAS. Two-thirds of the FD/MAS population were diagnosed with MFD, and one-third with PFD/MAS. These epidemiology measures provide understanding on the distribution of disease, may aid in screening for FD/MAS, in planning health care, and in counseling patients, and may benefit future research.
Acknowledgments
We would like to thank prof. Henrik Toft Sørensen for his contribution to this paper.
Contributor Information
Maartje E Meier, Department of Orthopedic Surgery, Leiden University Medical Center, 2333 ZA Leiden, The Netherlands.
Emese Vágó, Department of Clinical Epidemiology, Aarhus University, 8200 Aarhus N, Denmark.
Bo Abrahamsen, OPEN Patient Data Explorative Network, University of Southern Denmark, 5000 Odense C, Denmark; Department of Medicine, Holbaek Hospital, 4300 Holbaek, Denmark.
Olaf M Dekkers, Department of Clinical Epidemiology, Aarhus University, 8200 Aarhus N, Denmark; Department of Clinical Epidemiology, Leiden University Medical Center, 2333 ZA Leiden, The Netherlands; Department of Internal Medicine, Division of Endocrinology, Leiden University Medical Center, 2333 ZA Leiden, The Netherlands.
Erzsébet Horváth-Puhó, Department of Clinical Epidemiology, Aarhus University, 8200 Aarhus N, Denmark.
Lars Rejnmark, Department of Endocrinology and Internal Medicine, Aarhus University Hospital, 8200 Aarhus N, Denmark.
Natasha M Appelman-Dijkstra, Department of Internal Medicine, Division of Endocrinology, Leiden University Medical Center, 2333 ZA Leiden, The Netherlands.
Disclosures
N.M.A.-D. received an unrestricted research grant from Kyowa Kirin Pharmaceutical Development for the present study. B.A. reports institutional research contracts with UCB, Pharmacosmos, and Kyowa Kirin Pharmaceutical Development outside the present study, and personal speaker’s fees or consulting fees from Amgen, UCB Pharma, Gedeon Richter, Kyowa Kirin Pharmaceutical Development. and Pharmacosmos. L.R. has, outside the present study, received institutional research grants and is research investigator and/or advisory board member of Takeda, Amolyt, Kyowa Kirin Pharmaceutical Development, Ascendis Pharma, and Calcilytix Therapeutics.
Data Availability
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
Grants
M.E.M. was supported by a grant from the Bontius Foundation, a nonprofit institution promoting research within the LUMC, and a grant of the European Joint Programme on Rare Diseases for a Research Mobility Fellowship.
References
- 1. Boyce AM, Collins MT. Fibrous dysplasia/McCune-albright syndrome: a rare, mosaic disease of gα s activation. Endocr Rev. 2019;41(2):345‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM. Activating mutations of the stimulatory G protein in the McCune–albright syndrome. N Engl J Med. 1991;325(24):1688‐1695. [DOI] [PubMed] [Google Scholar]
- 3. Javaid MK, Boyce A, Appelman-Dijkstra N, et al. Best practice management guidelines for fibrous dysplasia/McCune-Albright syndrome: a consensus statement from the FD/MAS international consortium. Orphanet J Rare Dis. 2019;14(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Collins MT, Chebli C, Jones J, et al. Renal phosphate wasting in fibrous dysplasia of bone is part of a generalized renal tubular dysfunction similar to that seen in tumor-induced osteomalacia. J Bone Miner Res. 2001;16(5):806‐813. [DOI] [PubMed] [Google Scholar]
- 5. Riminucci M, Collins MT, Fedarko NS, et al. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest. 2003;112(5):683‐692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benhamou J, Gensburger D, Messiaen C, Chapurlat R. Prognostic factors from an epidemiologic evaluation of fibrous dysplasia of bone in a modern cohort: the FRANCEDYS study. J Bone Miner Res. 2016;31(12):2167‐2172. [DOI] [PubMed] [Google Scholar]
- 7. Leet AI, Chebli C, Kushner H, et al. Fracture incidence in polyostotic fibrous dysplasia and the McCune-Albright syndrome. J Bone Miner Res. 2004;19(4):571‐577. [DOI] [PubMed] [Google Scholar]
- 8. Boyce AM, Florenzano P, de Castro LF, Collins MT. Fibrous dysplasia/McCune-Albright syndrome. In: Adam MP, Everman DB, Mirzaa GM, et al., eds. GeneReviews(®) [Internet]. University of Washington; 2015. https://www.ncbi.nlm.nih.gov/books/NBK274564/ [Google Scholar]
- 9. Noordzij M, Dekker FW, Zoccali C, Jager KJ. Measures of disease frequency: prevalence and incidence. Nephron Clin Pract. 2010;115(1):c17‐c20. [DOI] [PubMed] [Google Scholar]
- 10. Statistics Denmark . Population. Accessed September 12, 2022. https://www.dst.dk/en/Statistik/emner/priser-og-forbrug/forbrugerpriser
- 11. Schmidt M, Schmidt SAJ, Adelborg K, et al. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol. 2019;11:563‐591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wallach Kildemoes H, Toft Sørensen H, Hallas J. The Danish national prescription registry. Scand J Public Health. 2011;39(7_suppl):38‐41. [DOI] [PubMed] [Google Scholar]
- 13. Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The danish national patient registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meier ME, Vágó E, Abrahamsen B, et al. Data from: incidence and prevalence of fibrous dysplasia/McCune-Albright syndrome—a nationwide registry-based study in Denmark. Gen Digital Reposit. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.European Commision - Rare diseases. https://research-and-innovation.ec.europa.eu/research-area/health/rare-diseases_en
- 16. Florenzano P, Boyce A, De Castro L, Collins M. Fibrous dysplasia/McCune-albright Syndrome. In: Adam MP Ardinger HH Pagon RA Wallace SE Bean LJH Stephens K and Amemiya A, eds. GeneReviews® University of Washington; 2018:1993‐2018. [PubMed] [Google Scholar]
- 17. Chapurlat RD, Meunier PJ. Fibrous dysplasia of bone. Baillieres Best Pract Res Clin Rheumatol. 2000;14(2):385‐398. [DOI] [PubMed] [Google Scholar]
- 18. Folkestad L, Hald JD, Canudas-Romo V, et al. Mortality and causes of death in patients with osteogenesis Imperfecta: a register-based nationwide cohort study. J Bone Miner Res. 2016;31(12):2159‐2166. [DOI] [PubMed] [Google Scholar]
- 19. Andersen PE, Jr., Hauge M. Osteogenesis imperfecta: a genetic, radiological, and epidemiological study. Clin Genet. 1989; 36(4):250‐255. [DOI] [PubMed] [Google Scholar]
- 20. Beck-Nielsen SS, Brock-Jacobsen B, Gram J, Brixen K, Jensen TK. Incidence and prevalence of nutritional and hereditary rickets in southern Denmark. Eur J Endocrinol. 2009;160(3):491‐497. [DOI] [PubMed] [Google Scholar]
- 21. Foreman PK, van Kessel F, van Hoorn R, van den Bosch J, Shediac R, Landis S. Birth prevalence of achondroplasia: a systematic literature review and meta-analysis. American J Med Genet Part A. 2020;182(10):2297‐2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mornet E, Yvard A, Taillandier A, Fauvert D, Simon-Bouy B. A molecular-based estimation of the prevalence of hypophosphatasia in the European population. Ann Hum Genet. 2011;75(3):439‐445. [DOI] [PubMed] [Google Scholar]
- 23. Meier ME, Hagelstein-Rotman M, van de Ven AC, et al. A multidisciplinary care pathway improves quality of life and pain in patients with fibrous dysplasia/McCune-albright syndrome: a multicenter prospective observational study. Orphanet J Rare Dis. 2022;17(1):439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hart ES, Kelly MH, Brillante B, et al. Onset, progression, and plateau of skeletal lesions in fibrous dysplasia and the relationship to functional outcome. J Bone Miner Res. 2007;22(9):1468‐1474. [DOI] [PubMed] [Google Scholar]
- 25. Yang L, Wu H, Lu J, Teng L. Prevalence of different forms and involved bones of craniofacial fibrous dysplasia. J Craniofac Surg. 2017;28(1):21‐25. [DOI] [PubMed] [Google Scholar]
- 26. Meier ME, Appelman-Dijkstra NM, Collins MT, et al. Coxa vara deformity in fibrous dysplasia/McCune-Albright syndrome: prevalence, natural history and risk factors: a two-center study. J Bone Miner Res. 2023;38(7):968‐975. [DOI] [PubMed] [Google Scholar]
- 27. Geels RES, Meier ME, Saikali A, Tsonaka R, Appelman-Dijkstra NM, Boyce AM. Long bone fractures in fibrous dysplasia/McCune-albright syndrome: prevalence, natural history, and risk factors. J Bone Miner Res. 2022;37(2):236‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.