Abstract
Context
In type 2 diabetes mellitus (T2DM), orthostatic hypotension (OH) is associated with cognition, but the mechanisms governing the link between OH and cognition are still unclear.
Objective
We sought to analyze Alzheimer’s disease (AD) biomarkers and the part of complement proteins in modulating the association of OH with cognitive impairment and examine whether OH could accelerate the clinical progression of mild cognitive impairment (MCI) to dementia in T2DM.
Methods
We recruited patients with T2DM with MCI and collected general healthy information and blood samples. Complement proteins of astrocyte-derived exosomes were isolated and AD biomarkers of neuronal cell-derived exosomes isolated were quantified by enzyme-linked immunosorbent assay. Cognitive assessments were performed at patient enrollment and follow-up.
Results
Mediation analysis showed that the influence of OH on cognition in T2DM was partly mediated by baseline AD biomarkers and complement proteins. Cox proportional-hazards regression proved the OH group had a higher risk of developing dementia compared to the T2DM without OH group.
Conclusion
In T2DM with MCI patients, AD biomarkers and complement proteins mediate the effects of OH on cognitive impairment and OH may be a risk factor of progression from MCI to dementia in T2DM.
Keywords: orthostatic hypotension, cognitive impairment, type 2 diabetes mellitus, complement protein, AD biomarkers
Orthostatic hypotension (OH) occurs when blood pressure has a significant drop due to posture changes from supine to the upright position, often with symptoms of cerebral hypoperfusion, such as lightheadedness, blurred vision, and somnolence, and has a highly prevalence of nearly 20% in older adults and 5% in middle-aged adults in the community setting (1). Studies have shown OH is associated with a long-term increased risk of dementia in the general population and delayed orthostatic hypotension (DOH) can increase the risk of clinical progression to mild cognitive impairment (MCI) or dementia during follow-up (2, 3). Previous studies showed that OH leads to cerebral blood flow hypoperfusion, which may lead to the deposition of amyloid-β (Aβ) and tau hyperphosphorylation in the brain if frequently occurring and further result in cognitive decline (4, 5).
Diabetes mellitus (DM) has become a major public health problem, and one study has predicted that the number of DM patients is projected to double from 2000 to 2030 (6). One of the common and serious complications of diabetes is autonomic dysfunction, and OH is a hallmark of diabetic autonomic neuropathy (7), with an incidence of 28% to 30.5% (8, 9). Massive epidemiological studies have shown that T2DM is associated with cognitive deficits, and may eventually lead to vascular dementia (VD) and Alzheimer disease (AD) (10-12), and inflammation may play a role in these relationships (13). Hyperglycemia is closely related to complement activation, and the complement has an important role in the progression of MCI to AD (14, 15). And we have previously found that T2DM with OH patients had transient, posture-mediated cognitive deficits and OH was independently related to the increase of amyloid beta42 (Aβ42), total tau (T-tau), and tau phosphorylated at threonine 181 (P-T181-tau) levels in neural-derived plasma exosomes in diabetic patients (16, 17). The research on the relationship between OH and the progression of cognitive impairment in type 2 DM (T2DM) is still lacking. Therefore, in this study, we analyzed the mediating effect of AD pathology and complement proteins on the relationship between OH and cognitive impairment and investigated the influence of OH on the progression of MCI to dementia in T2DM patients.
Materials and Methods
Study Participants
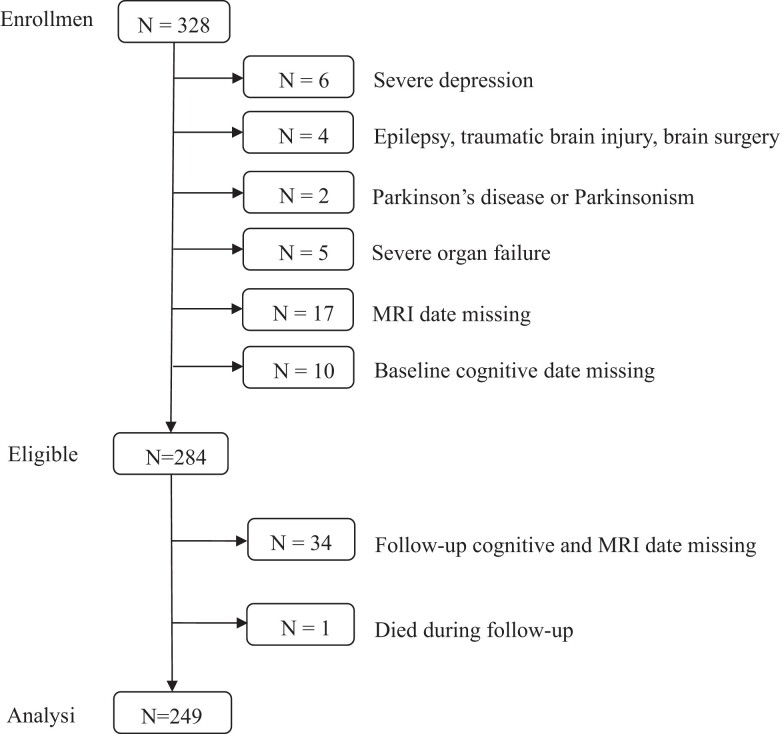
We enrolled T2DM participants with MCI at the Department of Endocrinology of Weihai Municipal Hospital between April 2017 and January 2018. The study followed the Declaration of Helsinki and was approved by the ethics committee of Weihai Municipal Hospital. In addition, written informed consent was obtained from every participant. The diagnosis of T2DM was based on the 1999 World Health Organization criteria. MCI was defined as a Montreal Cognitive Assessment (MoCA) score of less than 26 and met the following criteria: (1) a subjective cognitive impairment; (2) an objective impairment in one or more cognitive domains; (3) complex instrumental daily abilities can be slightly impaired but independent daily living abilities are maintained; and (4) no dementia (18, 19). We included the participants in the baseline based on the following criteria: (1) more than 3 years of T2DM duration; (2) age 55 years or older; and (3) long-term residents of Weihai City. The following are exclusion criteria: level 2 hypoglycemia (<3.0 mmol/L), stroke, peripheral arterial disease, epilepsy, traumatic brain injury, brain surgery, malignant tumor, neurological disorder potentially affecting cognition (eg, Parkinson disease), clinically severe heart, lung, liver, or kidney diseases affecting quality of life, and unable to finish the study. Patients with depression were excluded too (20). According to the aforementioned inclusion criteria, 328 T2DM with MCI patients were enrolled in our study. After excluding the ineligible and individuals with lost information, a total of 249 participants completed the study (see Fig. 1).
Figure 1.
Description of the study population. MRI, magnetic resonance imaging.
We collected the general health information of all the participants, including sex, age, educational attainment, body mass index (BMI), ischemic cardiomyopathy, hypertension, diabetes duration, hyperlipidemia, tobacco use (within the previous 3 months), alcohol consumption (≥300 g per week), and hypoglycemic drugs that could reduce the risk of developing dementia. We also measured the serum total cholesterol, low-density lipoprotein cholesterol (LDL-C), triglycerides, glucose levels, and glycated hemoglobin A1c (HbA1c). Hypertension was defined as systolic blood pressure greater than or equal to 140 mm Hg, or/and diastolic blood pressure greater than or equal to 90 mm Hg or/and current use of antihypertensive drugs in past 2 weeks. Hyperlipidemia was defined as total cholesterol levels greater than or equal to 200 mg/dL, triglyceride levels greater than or equal to 150 mg/dL, LDL-C levels greater than or equal to 130 mg/dL, or the use of lipid-lowering drugs. We scored them on the Mini-Mental State Examination (MMSE) and MoCA scale with the modifications of MoCA Beijing version at 6 time points: baseline, 12 months, 24 months, 36 months, 48 months, and 60 months.
Diagnosis of Dementia
The MMSE was used to exclude individuals with dementia: for illiterate, a MMSE score of less than 17; for participants with primary education, MMSE less than 20; for participants with junior secondary education and above, MMSE less than 24 (21). At follow-up, participants received cognitive assessment and the participants who converted to dementia underwent brain magnetic resonance imaging. The diagnosis of dementia was made independently by 2 physicians according to the revised Diagnostic and Statistical Manual of Mental Disorders-IV criteria. The diagnosis of AD was based on the diagnostic criteria for probable AD published by the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association Work Group (NINCDS-ADRDA) (22). The diagnosis of VD was made according to the diagnostic criteria for possible vascular dementia published by the National Institute of Neurological Disorders and Stroke–Association International pour la Recherche etl’Enseignementen Neurosciences (NINDS-AIREN) (23).
Measurement of Blood Pressure
To ensure the accuracy and consistency of the OH assessment, the day of the evaluation, participants continued to take their previously prescribed medication, had a light breakfast, and measured blood pressure at 8 Am. Prior to blood pressure measurement, the patients rested in the supine position for 20 minutes to establish psychophysiological balance. The same tester measured blood pressure and heart rate in a quiet, bright room with a completely automatic electronic sphygmomanometer (Omron HBP-1300; Omron Healthcare Inc) in a flat position and repeated at 1 and 3 minutes after standing. OH was defined as a fall in systolic blood pressure of 20 mm Hg or more and/or diastolic blood pressure of 10 mm Hg or more within 1 minute and/or 3 minutes after standing, and was classified as early OH (EOH) occurring only at 1 minute after standing and delayed and/or prolonged OH (DOH) occurring at 1 and 3 minutes after standing (24).
Extraction and Quantification of Alzheimer Disease Biomarkers and Complement Proteins
After a 12-hour fast, peripheral blood samples were taken from patients’ forearm vein between 6 and 7 Am. We centrifuged (3000g for 10 minutes) the blood samples to separate the upper serum within 30 minutes and stored them in a refrigerator at −80°C until assayed.
Specific astrocyte-derived exosomes (ADEs) were extracted according to our previously published method. We used the Exo Quick exosome precipitation solution (System Biosciences, EXOQ20A-1) to collect the total exosomes from serum. ADEs were obtained using glutamine aspartate transporter (GLAST) (ACSA-1) biotinylated antibody (Miltenyi Biotec; catalog No. 130-118-984, RRID:AB_2733473) and labeled with biotin by the EZ-Link sulfo-NHS-biotin system (Thermo Fisher Scientific; catalog No. 53117).
According to our published methods, specific neuronal-derived exosomes (NDEs) were separated. We collected the total exosome using the ExoQuick exosome precipitation solution (EXOQ, EXOQ20A-1, System Biosciences) and isolated the NDEs by coimmunoprecipitation using a rabbit anti-L1 cell adhesion molecule (L1CAM) antibody (eBiosciences; catalog No. 13-1719-82, RRID:AB_2043813), then labeled it with biotin by the EZ-Link sulfo-NHS-biotin system (Thermo Fisher Scientific; catalog No. 53117).We used Western blotting, transmission electron microscopy, and nanoparticle tracking analysis to confirm the success of exosomal collection according to our previous protocols (17, 25).
The levels of Aβ42, T-tau, and P-T181-tau in plasma NDEs and levels of C3b, C5b-9, and CD55 in serum ADEs were measured by enzyme-linked immunosorbent assay kits (Aβ42, Abexxa; catalog No. abx574166, RRID:AB_3076178; T-tau, Thermo Fisher Scientific; catalog No. KHB0041, RRID:AB_3076179; P-T181-tau, Thermo Fisher Scientific; catalog No. KHO0631, RRID:AB_3076180; C3b, Abexxa; catalog No. ABX252114, RRID:AB_3076175; C5b-9, Abexxa; catalog No. abx054346, RRID:AB_3076176; CD55, RayBiotech; catalog No. ELH-CD55, RRID:AB_3076177) according to our published methods. The mean value of CD81 measured in each assay group was set at 1.00 and the relative CD81 values for each sample were used to standardize their recovery rates. The intrabatch coefficient of variation was less than 10% and the interbatch coefficient of variation was less than 10% to 12%. The enzyme-linked immunosorbent assay microplates were read using a Varioskan LUX 3020 instrument (Thermo Fisher Scientific Oy, Ratastie 2). The same technician who was blinded to the clinical data analyzed the participants’ serum samples.
Apolipoprotein E Genotyping
Apolipoprotein E (APOE) was genotyped using the standard Sanger sequencing method as described previously (26). APOE genotypes were segregated into 2 groups, those carrying any ε4 allele and those not carrying the ε4 allele.
Statistical Analyses
IBM SPSS Statistics 25.0 (IBM) and R software version 4.2.1 were used for statistical analysis, and GraphPad Prism version 9.00 was used for figure preparation. Continuous data were tested by t test and categorical variables were analyzed using the χ2 test. All tests were 2-tailed, and the statistically significant difference was set at P less than .05. We developed a mediation model with the total MoCA scores as the dependent variable, with OH as the independent variable, and the ADE levels of C3b, C5b-9, and CD55, and the NDE levels of Aβ42, T-tau, and P-T181-tau, as mediator variables. All analyses used 10 000 bootstrapped iterations, and the models were corrected for age, sex, education, and APOEε4 genotyping. Mediation analyses were performed using the “Mediation” package in R version 4.2.1 software.
We estimated the associations of OH with the progression from MCI to dementia in T2DM using the hazard ratios (HRs) with 95% CIs calculated by Cox proportional-hazards models with adjustment for age, sex, education, and APOEε4 genotyping. The follow-up time was calculated from the start of the study to the time of dementia diagnosis, death, or last examination. Schoenfeld residual was used to test the proportional risk hypothesis. No breaches of the proportional risk assumption were observed.
Results
Baseline Demographic and Biomarker Characteristics of Type 2 Diabetes Mellitus With Orthostatic Hypotension Group and Without Orthostatic Hypotension Group
The demographic features of the participants are shown in Table 1. We enrolled a total of 249 patients with T2DM and MCI, and the mean age of participants at baseline was 67.040 ± 7.057 years. There were 84 participants with OH in the study: 18 patients with EOH, and 66 patients with DOH. Among the participants, the T2DM with OH group had a longer period of education and diabetes (P < .05). The T2DM with OH group had lower MMSE and MoCA scores than the T2DM without OH group (P < .05). Between the groups, there were no statistically significant differences in age, sex, BMI, rate of hypertension, hyperlipidemia, ischemic cardiomyopathy, current drinking and smoking, fasting blood glucose, HbA1c, or hypoglycemic drugs used by patients, including sodium-dependent glucose transporters 2 (SGLT2) inhibitors, glucagon-like peptide-1 (GLP-1) receptor drugs, and thiazolidinediones (P > .05).
Table 1.
Baseline characteristics of demographics and clinical parameters between the type 2 diabetes mellitus with orthostatic hypotension group and without orthostatic hypotension group
| Total (n = 249) | With OH (n = 84) | Without OH (n = 165) | P | |
|---|---|---|---|---|
| Male/female | 124/125 | 35/49 | 89/76 | .068 |
| Age, y | 67.040 ± 7.057 | 66.320 ± 7.164 | 67.400 ± 6.995 | .255 |
| Education, y | 8.570 ± 3.406 | 9.262 ± 3.159 | 8.218 ± 3.482 | .022 |
| BMI | 25.790 ± 3.353 | 26.110 ± 3.602 | 25.626 ± 3.218 | .282 |
| Ischemic cardiomyopathy, % | 22.424 | 27.381 | 22.424 | .388 |
| Hypertension, % | 53.414 | 60.714 | 49.697 | .110 |
| Smoking, % | 12.048 | 10.714 | 12.723 | .645 |
| Drinking, % | 14.859 | 9.524 | 17.576 | .092 |
| Duration of T2DM, y | 10.890 ± 5.428 | 12.770 ± 5.803 | 9.930 ± 4.977 | < .001 |
| APOE ε4 positive, % | 23.695 | 22.619 | 24.242 | .776 |
| Triglycerides, mg/dL | 173.39 ± 143.944 | 179.260 ± 132.695 | 170.620 ± 149.387 | .700 |
| Cholesterol, mg/dL | 171.160 ± 62.741 | 179.920 ± 73.238 | 166.990 ± 56.910 | .186 |
| LDL-C, mg/dL | 106.350 ± 37.216 | 115.780 ± 44.533 | 101.780 ± 44.533 | .016 |
| Hyperlipidemia, % | 53.414 | 51.190 | 54.545 | .617 |
| Fasting blood glucose, mmol/L | 9.315 ± 2.907 | 9.584 ± 3.129 | 9.179 ± 2.787 | .300 |
| HbA1c, % | 8.591 ± 2.283 | 8.489 ± 1.758 | 8.642 ± 2.512 | .616 |
| Diabetic medication | ||||
| SGLT2 inhibitors, % | 26.104 | 22.619 | 27.879 | .492 |
| GLP-1 receptor drugs, % | 10.040 | 9.524 | 10.303 | .847 |
| Thiazolidinediones, % | 11.647 | 9.524 | 12.727 | .457 |
| MoCA | 20.740 ± 3.140 | 20.170 ± 3.146 | 21.040 ± 3.106 | .039 |
| MMSE | 25.900 ± 2.204 | 25.420 ± 2.001 | 26.150 ± 2.267 | .013 |
Data are expressed as mean ± SD or n (%) unless otherwise specified. Between-group comparisons were performed using t tests for parametric data and χ2 tests for proportions.
Abbreviations: APOE, apolipoprotein E; BMI, body mass index; GLP-1, glucagon-like peptide-1; HbA1c, glycated hemoglobin A1c; LDL-C, low-density lipoprotein cholesterol; MoCA, Montreal Cognitive Assessment; MMSE, Mini-Mental State Examination; OH, orthostatic hypotension; SGLT2, sodium-dependent glucose transporters 2; T2DM, type 2 diabetes mellitus.
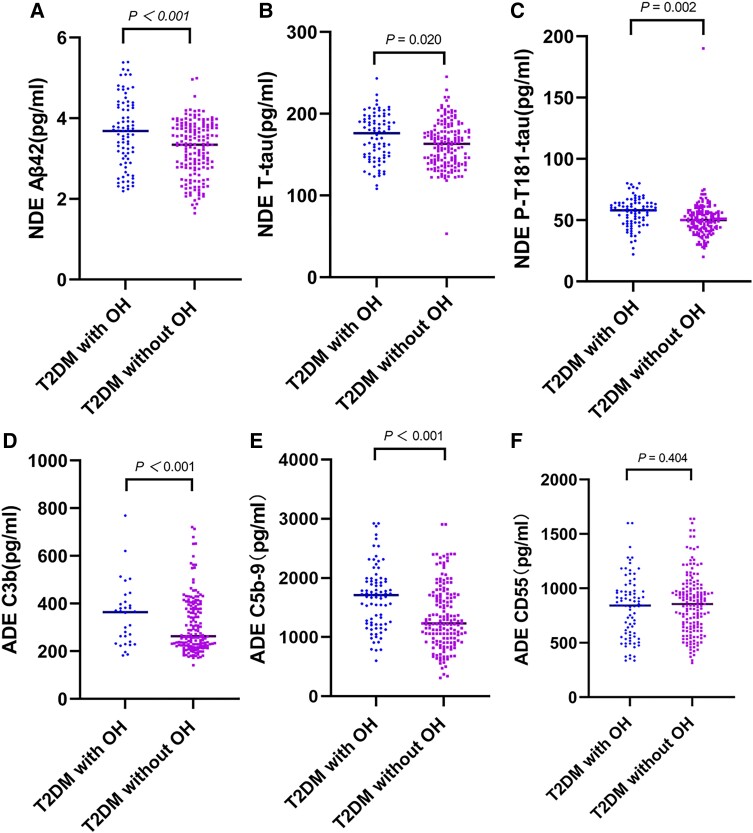
We compared the baseline plasma NDE levels of Aβ42, T-tau, and P-T181-tau and ADE levels of C3b, C5b-9, and CD55 between the 2 groups. Plasma baseline NDE levels of Aβ42, T-tau, and P-T181-tau in the T2DM with OH group were higher than the T2DM without OH group (3.644 ± 0.863 pg/mL vs 3.231 ± 0.679 pg/mL; P < .001; 170.890 ± 28.880 pg/mL vs 162.130 ± 27.400 pg/mL; P = .020; 56.250 ± 11.889 pg/mL vs 50.350 ± 15.042 pg/mL; P = .002). Higher baseline ADE levels of C3b and C5b-9 were found in the T2DM with OH patients than in the T2DM without OH patients (373.599 ± 135.735 pg/mL vs 310.792 ± 118.490 pg/mL; P < .001; 1670.379 ± 526.119 pg/mL vs 1340.059 ± 529.713 pg/mL; P < .001). There was no statistically significant difference in the levels of CD55 between the 2 groups (P = .404) (see Fig. 2).
Figure 2.
Baseline NDE levels of Aβ42 and tau proteins and ADE levels of complement proteins in T2DM with OH group and without OH group. The exosomal concentration of A, Aβ42; B, T-tau; and C, P-T181-tau; D, C3b; and E, C5b-9 in OH group was higher than the without OH group. There was no significant difference in the levels of F, CD55 between the two groups. Aβ42, amyloid beta42; ADE, astrocyte-derived exosomes; NDE, neuronal-derived exosomes; OH, orthostatic hypotension; P-T181-tau, tau phosphorylated at threonine 181; T-tau, total tau; T2DM, type 2 diabetes mellitus.
Mediation Effect of Orthostatic Hypotension on Cognitive Impairment via Alzheimer Disease Biomarkers and Complement Proteins
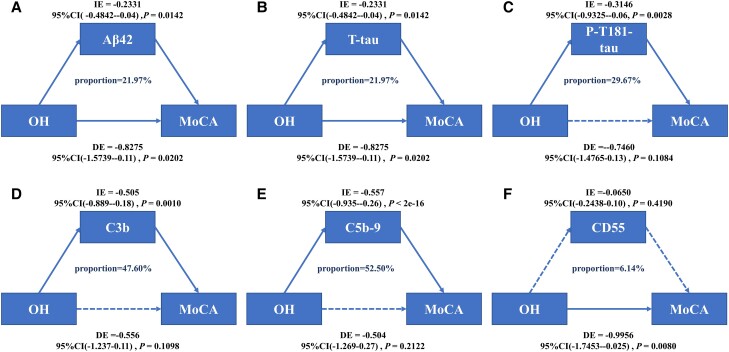
We next investigated whether OH affects cognition through baseline NDE levels of Aβ42, T-tau, and P-T181-tau and ADE levels of C3b, C5b-9, and CD55. After adjusting for age, sex, and education, and APOE ε4–positive status, we found the relationship between OH and cognitive impairment was partially mediated by baseline Aβ42 (21.97% of total effect), T-tau (21.97% of total effect), P-T181-tau (29.67% of total effect), C3b (47.60% of total effect), and C5b-9 (52.50% of total effect). But CD55 could not modulate the relationship between OH and cognition (see Fig. 3A-3F).
Figure 3.
Mediation analyses with MoCA as cognitive outcome. In this study, the relationship between OH and cognitive impairment was mediated by A, Aβ42; B, T-tau; C, P-T181-tau; D, C3b; and E, C5b-9; but F, CD55 cannot mediate the association between OH and cognition. Aβ42, amyloid beta42; DE, direct effect; IE, indirect effect; MoCA, Montreal Cognitive Assessment; OH, orthostatic hypotension; P-T181-tau, tau phosphorylated at threonine 181; T-tau, total tau.
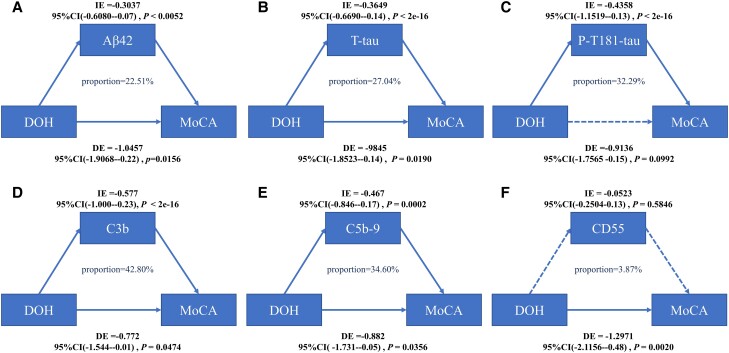
We then examined the effect of Aβ42, T-tau, and P-T181-tau of NDEs and C3b, C5b-9, and CD55 of ADEs on the relationship between EOH and DOH with cognitive impairment. The mediation analysis showed that the association between DOH and cognitive impairment was partially modulated by the baseline NDE levels of Aβ42 (22.51% of total effect), T-tau (27.04% of total effect), P-T181-tau (32.29% of total effect), the ADE levels of C3b (42.80% of total effect) and C5b-9 (34.60% of total effect) after adjusting for age, sex, education, and APOE ε4–positive status. We did not find a statistical interaction of CD55 in the association of DOH with cognitive impairment (see Fig. 4A-4F). Our results showed that there was no statistically significant correlation between EOH and cognitive impairment.
Figure 4.
Mediation analyses with MoCA as cognitive outcome. In this study, the relationship between DOH and cognitive impairment was mediated by A, Aβ42; B, T-tau; C, P-T181-tau; D, C3b; and E, C5b-9. F, CD55 cannot mediate the association between DOH and cognition. Aβ42, amyloid beta42; DE, direct effect; DOH, delayed orthostatic hypotension; IE, indirect effect; MoCA, Montreal Cognitive Assessment; P-T181-tau, tau phosphorylated at threonine 181; T-tau, total tau.
Relationship Between Orthostatic Hypotension and Progression From Mild Cognitive Impairment to Dementia in Type 2 Diabetes Mellitus
During the follow-up (mean 35.840 ± 12.161 months), 74 participants had progressed from MCI to dementia, including 39 patients with VD, 32 patients with AD, and 3 patients with mixed dementia. We divided the patients into a progression to dementia group and a nonprogression group. Table 2 shows the clinical and demographic characteristics of the 2 groups. Compared with the nonprogression group, the progression to dementia group was older, with a higher proportion of women and a lower number of years of education (P < .05), and had a higher rate of ischemic cardiomyopathy (P < .05). The incidence of OH was higher in the progression to dementia group than in the nonprogression group (P < .001), especially the rate of DOH (P < .001). There were no significant differences in current drinking and smoking, the rate of hypertension, hyperlipoidemia, incidence of EOH, APOE ε4 genotyping, fasting blood glucose, HbA1c, and hypoglycemic drugs used by patients, including SGLT2 inhibitors, GLP-1 receptor drugs, and thiazolidinediones (P > .05).
Table 2.
Demographic and clinical characteristics of participants at baseline of progression to dementia and nonprogression group
| Progression to dementia (n = 74) | Nonprogression (n = 175) | P | |
|---|---|---|---|
| Male/female | 23/51 | 101/74 | < .001 |
| Age, y | 68.990 ± 6.820 | 66.210 ± 7.012 | .006 |
| Education, y | 7.568 ± 3.562 | 8.994 ± 3.256 | .02 |
| BMI | 26.518 ± 3.634 | 25.481 ± 3.188 | .025 |
| Ischemic cardiomyopathy, % | 33.784 | 20.000 | .020 |
| Hypertension, % | 58.108 | 51.429 | .283 |
| Smoking, % | 13.514 | 11.429 | .645 |
| Drinking, % | 10.811 | 16.571 | .244 |
| Duration of T2DM, y | 12.880 ± 6.204 | 10.050 ± 4.843 | < .001 |
| APOE ε4 positive, % | 31.081 | 20.571 | .075 |
| OH, % | 50.000 | 26.857 | < .001 |
| EOH, % | 8.824 | 6.857 | .822 |
| DOH, % | 41.892 | 20.000 | < .001 |
| Follow-up time, mo | 35.840 ± 12.161 | 41.350 ± 12.179 | .001 |
| Triglycerides, mg/dL | 162.900 ± 112.483 | 178.120 ± 156.225 | .502 |
| Cholesterol, mg/dL | 190.050 ± 62.675 | 162.590 ± 61.103 | .005 |
| LDL-C, mg/dL | 113.550 ± 42.202 | 103.110 ± 34.430 | .076 |
| Hyperlipidemia, % | 58.108 | 51.429 | .335 |
| Fasting blood glucose, mmol/L | 9.475 ± 2.963 | 9.248 ± 2.888 | .575 |
| HbA1c, % | 8.315 ± 1.396 | 8.707 ± 2.562 | .216 |
| Diabetic medication | |||
| SGLT2 inhibitors, % | 31.081 | 24.000 | .169 |
| GLP-1 receptor drugs, % | 12.162 | 9.143 | .470 |
| Thiazolidinediones, % | 13.514 | 10.857 | .551 |
| MoCA | 17.720 ± 2.816 | 22.020 ± 2.277 | < .001 |
| MMSE | 24.860 ± 2.343 | 26.340 ± 1.993 | < .001 |
| NDE Aβ42, pg/mL | 3.583 ± 0.838 | 3.281 ± 0.723 | .004 |
| NDE T-tau, pg/mL | 172.090 ± 28.652 | 162.130 ± 27.494 | .010 |
| NDE P-T181-tau, pg/mL | 56.000 ± 12.489 | 50.790 ± 14.774 | .008 |
| ADE C3b, pg/mL | 399.901 ± 150.244 | 303.259 ± 104.983 | < .001 |
| ADE C5b-9, pg/mL | 1743.400 ± 06.342 | 1328.057 ± 474.979 | < .001 |
| ADE CD55, pg/mL | 797.734 ± 313.128 | 865.853 ± 264.553 | .080 |
Data are expressed as mean ± SD or n (%) unless otherwise specified. Between-group comparisons were performed using t tests for parametric data and χ2 tests for proportions.
Abbreviations: Aβ42, amyloid beta42; ADE, astrocyte-derived exosome; APOE, apolipoprotein E; BMI, body mass index; DOH, delayed orthostatic hypotension; EOH, early orthostatic hypotension; GLP-1, glucagon-like peptide-1; HbA1c, glycated hemoglobin A1c; LDL-C, low-density lipoprotein cholesterol; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; NDE, neuronal cell-derived exosome; OH, orthostatic hypotension; P-T181-tau, tau phosphorylated at threonine 181; SGLT2, sodium-dependent glucose transporters 2; T-tau, total tau; T2DM, type 2 diabetes mellitus.
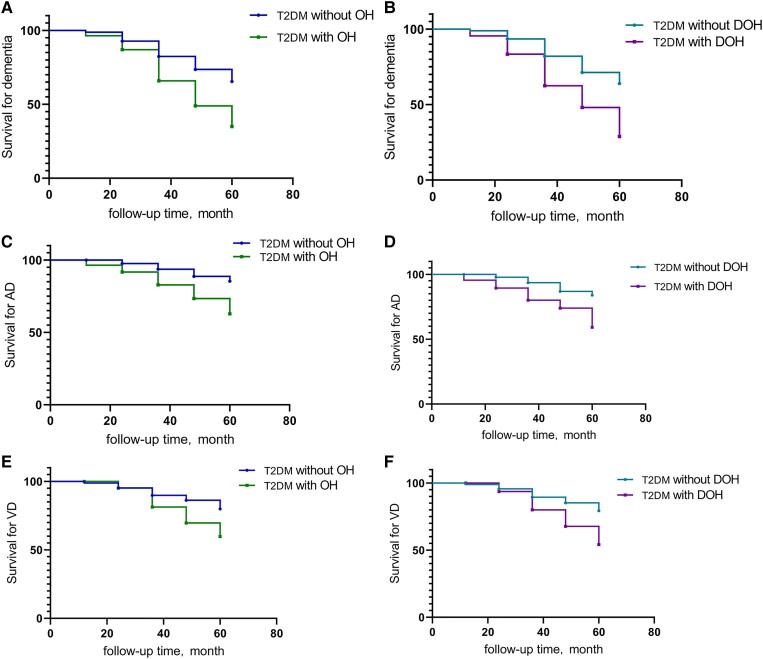
We analyzed whether OH predicted progression from MCI to dementia in T2DM with Cox proportional-hazard regression analysis. After adjusting for age, sex, education, and APOE ε4–positive status, OH patients were at a higher risk for progression from MCI to dementia than the T2DM without OH patients (HR = 2.409; 95% CI, 1.485-3.910; P < .001). We respectively analyzed the effect of EOH and DOH on progression of MCI to dementia in T2DM. Participants with DOH were associated with a higher risk of developing dementia than the without DOH group (HR = 2.474; 95% CI, 1.516-4.036; P < .001). We did not find that EOH can promote the development of dementia in T2DM patients with MCI (Table 3, Fig. 5A and 5B).
Table 3.
Associations of orthostatic hypotension, early orthostatic hypotension, and delayed orthostatic hypotension with progression from mild cognitive impairment to dementia, Alzheimer disease, and vascular dementia in type 2 diabetes mellitus as assessed by Cox proportional hazard regression analysis
| Predictor | Dementia | AD | VD | |||
|---|---|---|---|---|---|---|
| HRs (95%CI) | P | HRs (95%CI) | P | HRs (95%CI) | P | |
| Non-OH | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| OH | 2.409 (1.485-3.910) | < .001 | 3.324 (1.552-7.116) | .002 | 2.257 (1.166-4.370) | .016 |
| EOH | 1.165 (.498-2.725) | .725 | 1.123 (.326-3.867) | .854 | 1.173 (.357-3.856) | .793 |
| DOH | 2.474 (1.516-4.036) | < .001 | 3.456 (1.638-7.295) | .001 | 2.309 (1.180-4.517) | .015 |
Adjusted for age, sex, education, and APOE ε4 positive.
Abbreviations: AD, Alzheimer disease; APOE, apolipoprotein E; DOH, delayed orthostatic hypotension; EOH, early orthostatic hypotension; HRs, hazard ratios; OH, orthostatic hypotension; T2DM, type 2 diabetes mellitus; VD, vascular dementia.
Figure 5.
A, OH, and B, DOH predicted the progression of MCI to dementia. Survival from AD as a function of C, OH, and D, DOH are shown. E, OH, and F, DOH, as a risk factor of progression from MCI to VD. Analyses were adjusted for age, sex, education, and APOE ε4 positive. AD, Alzheimer disease; APOE, apolipoprotein E; MCI, mild cognitive impairment; OH, orthostatic hypotension; DOH, delayed orthostatic hypotension; T2DM, type 2 diabetes mellitus; VD, vascular dementia.
Relationship Between Orthostatic Hypotension and Progression From Mild Cognitive Impairment to Alzheimer Disease in Type 2 Diabetes Mellitus
In the OH cohort, a total of 18 (21.429%) people converted to AD from MCI during the follow-up, a significantly higher proportion than the T2DM without OH patients. In multiadjusted Cox regression models (adjusted for age, sex, education, and APOE ε4–positive status), OH predicted the progression from MCI to AD (HR = 3.324; 95% CI, 1.552-7.116; P = .002). We found that DOH was also associated with a significantly increased risk of AD incident (HR = 3.456; 95% CI, 1.638-7.295; P = .001). But we did not find that EOH has a similar effect (P = .854) (see Table 3 and Fig. 5C and 5D).
Relationship Between Orthostatic Hypotension and Progression From Mild Cognitive Impairment to Vascular Dementia in Type 2 Diabetes Mellitus
The type of dementia that occurred most frequently in our cohort was VD, and 19 of the patients had OH. After adjusting for age, sex, education, and APOE ε40150–positive status, OH could be a predictor of progression from MCI to VD in T2DM (HR = 2.257; 95% CI, 1.166-4.370; P = .016). Only DOH significantly predicted progression from MCI to VD (HR = 2.309; 95% CI, 1.180-4.517; P = .015) (see Table 3 and Fig. 5E and 5F).
Discussion
As far as we know, we are the first study to examine the relationship between OH and progression from MCI to dementia in T2DM. In this study, the results showed that (1) the baseline NDEs AD pathology biomarkers and ADEs complement proteins of the OH group were higher than in the without OH group; (2) the influence of OH on cognitive impairment was partially mediated by NDEs Aβ42, T-tau, P-T181-tau, and ADEs C3b and C5b-9; (3) the NDE levels of AD biomarkers and ADE levels of complement proteins of patients who are progressive to dementia were higher than those with nonprogression at baseline; and (4) in patients with T2DM, OH could accelerate the progression from MCI to dementia.
A previous study has demonstrated that OH was associated with cognitive decline longitudinally (27), but the mechanisms governing the link between OH and cognition are still unclear. One possibility is that OH leads to cerebral hypoperfusion, with subsequent consequences on Aβ and tau protein levels. Many studies have shown that cerebral hypoperfusion could induce HIF-1 expression, which then binds to the promoter of β-secretase and consequently increases its expression (28), and could also significantly increase β-/γ-secretase activity, consequently increasing Aβ production in the brain (4, 5, 29). In addition to increased Aβ generation, hypoperfusion affects peptidases that degrade Aβ peptides, thus reducing Aβ clearance (30, 31). Another study demonstrated that lower cerebral blood flow was associated with higher Aβ load, and cerebral blood flow was reduced in dementia patients in most brain regions as shown by florbetapir positron emission tomography (32). In addition, cerebral hypoperfusion may inhibit the activity of protein phosphatase 2A or induce the decrease of brain glucose metabolism to trigger the downregulation of tau O-GlcNAcylation, finally leading to hyperphosphorylation of tau protein (33-36). Studies have shown that both Aβ accumulation and hyperphosphorylation of tau are related to AD and VD (37-39). Our previous study indicated that OH was independently associated with increased levels of Aβ42, T-tau, and P-T181-tau from neuronal cell-derived exosome in T2DM patients (17). In the present study, we proved that the NDEs Aβ42, T-tau, and P-T181-tau modulate the relationship of OH with cognition via mediation effects in patients with T2DM.
Additionally, the mediation analysis we established also indicated that OH might affect cognition via ADE complement proteins. OH can cause cerebral hypoperfusion, which, if frequently occurring, may result in destruction of the blood-brain barrier (BBB), triggering excitotoxicity, oxidative stress, and inflammation, subsequently leading to brain injury and cognitive decline (40-42). And chronic cerebral hypoperfusion (CCH) enhanced C3 expression in astroglias of neurovascular units and promotes inflammation in the AD brain (43). Studies have also reported that, in chronic cerebral ischemia, complement component C5 and the C3-C3aR pathway are involved in white matter injury and neuroinflammation (44, 45). As we know, astrocytes have been identified as an important mediator of BBB component and function, and can secrete complement proteins involved in neuroinflammation (46, 47). Exosomes can carry complement proteins secreted by astrocytes across the BBB into the blood, and astrocyte-derived exosome levels of C3b and C5b-9 are significantly higher for AD patients (48, 49). In our study, we found the ADE complement proteins C3b and C5b-9 in the OH group were higher than in those who without OH among participants, then we established a mediation analysis to explore whether ADE complement proteins could mediate the relationship between OH and cognitive impairment of participants at enrollment. According to our data, we could infer that ADE C3b and C5b-9 modulate the relationship of OH with cognition via mediation effects in T2DM patients, which is consistent with previous research that diabetes may synergistically promote cognitive impairment induced by CCH through neuroinflammation (50).
Several population-based studies of middle-aged and older people have found that OH is associated with an increased risk for dementia and cognitive decline longitudinally (27, 51, 52). Our previous study demonstrated that the T2DM patients with OH had transient, posture-mediated cognitive deficits compared with individuals with diabetes without OH (16). Zhang et al (50) found that CCH combined with DM exacerbates neuroinflammation and cognitive deficits. The present study results are consistent with the aforementioned research, showing that in T2DM patients OH was associated with cognitive impairment, and DOH could be a risk factor of progression from MCI to dementia, but we did not find that EOH has the same effect. This is probably because patients with DOH are more likely to suffer from longer periods of cerebral hypoperfusion compared to patients with EOH. Our study also proved that OH promotes progression from MCI to AD and VD in T2DM patients. In the future, more research is needed to explore the mechanisms of OH promoting the progression from MCI to dementia.
Study limitations should be addressed. First, our sample was single and small, and more studies are needed in the future to replicate and validate our findings in large numbers of patients. Second, we lacked some information, such as blood samples at follow-up and cerebrospinal fluid data to assess the dynamics of exosomal proteins and confirm our results. Third, our study was limited to patients with T2DM over 55 years, and it is not applicable to the general population. Fourth, as there are various forms of L1CAM, both membrane-bound and soluble, and L1CAM is expressed outside the brain as well, it is controversial for LICAM to be used as a specific marker to isolate NDEs. We will actively look for other markers to isolate NDEs.
In conclusion, we demonstrate that in patients with T2DM and MCI, OH is associated with cognitive impairment, and mediated by baseline Aβ42, T-tau, P-T181-tau, C3b, and C5b-9. OH can be used as a risk factor of dementia in T2DM with MCI. As the prevalence of OH is high in patients with T2DM, early identification and treatment of OH may greatly reduce the risk of dementia in T2DM patients with MCI.
Abbreviations
- Aβ42
amyloid beta42
- AD
Alzheimer disease
- ADE
astrocyte-derived exosome
- APOE
apolipoprotein E
- BBB
blood-brain barrier
- BMI
body mass index
- CCH
chronic cerebral hypoperfusion
- DM
diabetes mellitus
- DOH
delayed orthostatic hypotension
- EOH
early orthostatic hypotension
- GLP-1
glucagon-like peptide-1
- HbA1c
glycated hemoglobin A1c
- HR
hazard ratio
- L1CAM
anti-L1 cell adhesion molecule
- LDL-C
low-density lipoprotein cholesterol
- MCI
mild cognitive impairment
- MMSE
Mini-Mental State Examination
- MoCA
Montreal Cognitive Assessment
- NDE
neuronal-derived exosome
- OH
orthostatic hypotension
- P-T181-tau
tau phosphorylated at threonine 181
- SGLT2
sodium-dependent glucose transporters 2
- T2DM
type 2 diabetes mellitus
- T-tau
total tau
- VD
vascular dementia
Contributor Information
Qiao Xiong, Department of Clinical Medicine, Weifang Medical University, Weifang, Shandong 261053, China; Department of Neurology, Weihai Municipal Hospital, Cheeloo College of Medicine, Shandong University, Weihai, Shandong 264200, China.
Fang Li, Department of Neurology, The First Affiliated Hospital of Jinzhou Medical University, Jinzhou, Liaoning 121017, China.
Haiyan Chi, Department of Endocrinology, Weihai Municipal Hospital, Cheeloo College of Medicine, Shandong University, Weihai, Shandong 264200, China.
Yachao Yang, Department of Endocrinology, Weihai Municipal Hospital, Cheeloo College of Medicine, Shandong University, Weihai, Shandong 264200, China.
Mengfan Li, Department of Neurology, Weihai Municipal Hospital, Cheeloo College of Medicine, Shandong University, Weihai, Shandong 264200, China.
Yingxiao Liu, Department of Endocrinology, Weihai Municipal Hospital, Cheeloo College of Medicine, Shandong University, Weihai, Shandong 264200, China.
Yupan Zhang, Department of Endocrinology, Weihai Municipal Hospital, Cheeloo College of Medicine, Shandong University, Weihai, Shandong 264200, China.
Bing Leng, Department of Neurology, Weihai Municipal Hospital, Cheeloo College of Medicine, Shandong University, Weihai, Shandong 264200, China.
Xiaoxiao Qi, Department of Clinical Medicine, Weifang Medical University, Weifang, Shandong 261053, China; Department of Neurology, Weihai Municipal Hospital, Cheeloo College of Medicine, Shandong University, Weihai, Shandong 264200, China.
Hairong Sun, Department of Neurology, Weihai Municipal Hospital, Cheeloo College of Medicine, Shandong University, Weihai, Shandong 264200, China.
Zhenguang Li, Department of Neurology, Weihai Municipal Hospital, Cheeloo College of Medicine, Shandong University, Weihai, Shandong 264200, China.
Jinbiao Zhang, Department of Neurology, Weihai Municipal Hospital, Cheeloo College of Medicine, Shandong University, Weihai, Shandong 264200, China.
Funding
This work was supported by the Key Projects of Discipline Climbing Project of Weihai Municipal Hospital (FH-2021-XC034) and the Traditional Chinese Medicine Science and Technology Project of Weihai (2022N-16).
Author Contributions
Q.X.: data curation and writing–original draft preparation; F.L.: conceptualization, methodology, and software; H.C., Y.Y., and M.L.: data curation and formal analysis; Y.L. and Y.Z.: investigation and validation; B.L.: formal analysis and resources; X.Q. and R.Y.: methodology and resources; H.S.: supervision and validation; Z.L.: funding acquisition and project administration; and J.Z.: writing–reviewing and editing. The corresponding author attests that all listed authors meet the authorship criteria. All authors read and approved the final manuscript.
Disclosures
The authors have no relevant financial or nonfinancial interests to disclose.
Data Availability
Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Kim MJ, Farrell J. Orthostatic hypotension: a practical approach. Am Fam Physician. 2022;105(1):39‐49. [PubMed] [Google Scholar]
- 2. Wolters FJ, Mattace-Raso FU, Koudstaal PJ, et al. Orthostatic hypotension and the long-term risk of dementia: a population-based study. PLoS Med. 2016;13(10):e1002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kleipool EEF, Trappenburg MC, Rhodius-Meester HFM, et al. Orthostatic hypotension: an important risk factor for clinical progression to mild cognitive impairment or dementia. The Amsterdam dementia cohort. J Alzheimers Dis. 2019;71(1):317‐325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Koike MA, Green KN, Blurton-Jones M, et al. Oligemic hypoperfusion differentially affects tau and amyloid-beta. Am J Pathol. 2010;177(1):300‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhiyou C, Yong Y, Shanquan S, et al. Upregulation of BACE1 and beta-amyloid protein mediated by chronic cerebral hypoperfusion contributes to cognitive impairment and pathogenesis of Alzheimer’s disease. Neurochem Res. 2009;34(7):1226‐1235. [DOI] [PubMed] [Google Scholar]
- 6. Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047‐1053. [DOI] [PubMed] [Google Scholar]
- 7. Azmi S, Ferdousi M, Kalteniece A, et al. Diagnosing and managing diabetic somatic and autonomic neuropathy. Ther Adv Endocrinol Metab. 2019;10:2042018819826890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Van Hateren KJJ, Kleefstra N, Blanker MH, et al. Orthostatic hypotension, diabetes, and falling in older patients: a cross-sectional study. Br J General Pract. 2012;62(603):e696‐e702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bouhanick B, Meliani S, Doucet J, et al. Orthostatic hypotension is associated with more severe hypertension in elderly autonomous diabetic patients from the French Gerodiab study at inclusion. Ann Cardiol Angeiol (Paris). 2014;63(3):176‐182. [DOI] [PubMed] [Google Scholar]
- 10. Zilliox LA, Chadrasekaran K, Kwan JY, et al. Diabetes and cognitive impairment. Curr Diab Rep. 2016;16(9):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jayaraj RL, Azimullah S, Beiram R. Diabetes as a risk factor for Alzheimer’s disease in the Middle East and its shared pathological mediators. Saudi J Biol Sci. 2020;27(2):736‐750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lyu F, Wu D, Wei C, et al. Vascular cognitive impairment and dementia in type 2 diabetes mellitus: an overview. Life Sci. 2020;254:117771. [DOI] [PubMed] [Google Scholar]
- 13. Dove A, Shang Y, Xu W, et al. The impact of diabetes on cognitive impairment and its progression to dementia. Alzheimer’s & Dementia. 2021;17(11):1769‐1778. [DOI] [PubMed] [Google Scholar]
- 14. Li Z, Zhang W, Gao F, et al. Different complement activation pathways underly cognitive impairment and type 2 diabetes Mellitus combined with cognitive impairment. Front Aging Neurosci. 2022;14:810335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Winston CN, Goetzl EJ, Schwartz JB, et al. Complement protein levels in plasma astrocyte-derived exosomes are abnormal in conversion from mild cognitive impairment to Alzheimer’s disease dementia. Alzheimers Dement (Amst). 2019;11:61‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang J, Chi H, Wang T, et al. Effects of orthostatic hypotension on cognition in type 2 diabetes mellitus. Ann Neurol. 2019;86(5):754‐761. [DOI] [PubMed] [Google Scholar]
- 17. Zhang J, Chi H, Wang T, et al. Altered amyloid-beta and tau proteins in neural-derived plasma exosomes of type 2 diabetes patients with orthostatic hypotension. J Alzheimers Dis. 2021;82(1):261‐272. [DOI] [PubMed] [Google Scholar]
- 18. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695‐699. [DOI] [PubMed] [Google Scholar]
- 19. Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256(3):240‐246. [DOI] [PubMed] [Google Scholar]
- 20. Palmer K, Di Iulio F, Varsi A E, et al. Neuropsychiatric predictors of progression from amnestic-mild cognitive impairment to Alzheimer’s disease: the role of depression and apathy. J Alzheimers Dis. 2010;20(1):175‐183. [DOI] [PubMed] [Google Scholar]
- 21. Li H, Jia J, Yang Z. Mini-Mental state examination in elderly Chinese: a population-based normative study. J Alzheimers Dis. 2016;53(2):487‐496. [DOI] [PubMed] [Google Scholar]
- 22. Mckhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34(7):939‐944. [DOI] [PubMed] [Google Scholar]
- 23. Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN international workshop. Neurology. 1993;43(2):250‐260. [DOI] [PubMed] [Google Scholar]
- 24. Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21(2):69‐72. [DOI] [PubMed] [Google Scholar]
- 25. Chi H, Yao R, Sun C, et al. Blood neuroexosomal mitochondrial proteins predict Alzheimer disease in diabetes. Diabetes. 2022;71(6):1313‐1323. [DOI] [PubMed] [Google Scholar]
- 26. Sun Y, Wang X, Wang Y, et al. Anxiety correlates with cortical surface area in subjective cognitive decline: aPOE epsilon4 carriers versus APOE epsilon4 non-carriers. Alzheimers Res Ther. 2019;11(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zimmermann M, Wurster I, Lerche S, et al. Orthostatic hypotension as a risk factor for longitudinal deterioration of cognitive function in the elderly. Eur J Neurol. 2020;27(1):160‐167. [DOI] [PubMed] [Google Scholar]
- 28. Zhang X, Zhou K, Wang R, et al. Hypoxia-inducible factor 1α (HIF-1α)-mediated hypoxia increases BACE1 expression and β-amyloid generation. J Biol Chem. 2007;282(15):10873‐10880. [DOI] [PubMed] [Google Scholar]
- 29. Pluta R, Furmaga-Jabłońska W, Maciejewski R, et al. Brain ischemia activates β- and γ-secretase cleavage of amyloid precursor protein: significance in sporadic Alzheimer’s disease. Mol Neurobiol. 2012;47(1):425‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miners JS, Palmer JC, Tayler H, et al. Abeta degradation or cerebral perfusion? Divergent effects of multifunctional enzymes. Front Aging Neurosci. 2014;6:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shang J, Yamashita T, Tian F, et al. Chronic cerebral hypoperfusion alters amyloid-beta transport related proteins in the cortical blood vessels of Alzheimer’s disease model mouse. Brain Res. 2019;1723:146379. [DOI] [PubMed] [Google Scholar]
- 32. Mattsson N, Tosun D, Insel P S, et al. Association of brain amyloid-beta with cerebral perfusion and structure in Alzheimer’s disease and mild cognitive impairment. Brain. 2014;137(Pt 5):1550‐1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Song B, Ao Q, Wang Z, et al. Phosphorylation of tau protein over time in rats subjected to transient brain ischemia. Neural Regen Res. 2013;8(34):3173‐3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu F, Iqbal K, Grundke-Iqbal I, et al. O-GlcNAcylation regulates phosphorylation of tau: a mechanism involved in Alzheimer’s disease. Proc Natl Acad Sci U S A. 2004;101(29):10804‐10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu F, Shi J, Tanimukai H, et al. Reduced O-GlcNAcylation links lower brain glucose metabolism and tau pathology in Alzheimer’s disease. Brain. 2009;132(Pt 7):1820‐1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhao Y, Gu JH, Dai CL, et al. Chronic cerebral hypoperfusion causes decrease of O-GlcNAcylation, hyperphosphorylation of tau and behavioral deficits in mice. Front Aging Neurosci. 2014;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yu W, Li Y, Hu J, et al. A study on the pathogenesis of vascular cognitive impairment and dementia: the chronic cerebral hypoperfusion hypothesis. J Clin Med. 2022;11(16):4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang H, Lu F, Liu P, et al. A direct interaction between RhoGDIalpha/Tau alleviates hyperphosphorylation of Tau in Alzheimer’s disease and vascular dementia. J Neuroimmune Pharmacol. 2022;18(1-2):58‐71. [DOI] [PubMed] [Google Scholar]
- 39. Raz L, Knoefel J, Bhaskar K. The neuropathology and cerebrovascular mechanisms of dementia. J Cereb Blood Flow Metab. 2016;36(1):172‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Veerhuis R, Nielsen HM, Tenner AJ. Complement in the brain. Mol Immunol. 2011;48(14):1592‐1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang L, Du Y, Wang K, et al. Chronic cerebral hypoperfusion induces memory deficits and facilitates aβ generation in C57BL/6J mice. Exp Neurol. 2016;283:353‐364. [DOI] [PubMed] [Google Scholar]
- 42. Morgan BP. Complement in the pathogenesis of Alzheimer’s disease. Semin Immunopathol. 2018;40(1):113‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shi X, Ohta Y, Liu X, et al. Chronic cerebral hypoperfusion activates the coagulation and complement cascades in Alzheimer’s disease mice. Neuroscience. 2019;416:126‐136. [DOI] [PubMed] [Google Scholar]
- 44. Cordula M S, Liu Q, He S, et al. White matter injury due to experimental chronic cerebral hypoperfusion is associated with C5 deposition. PLoS One. 2013;8(12):e84802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang L-Y, Pan J, Mamtilahun M, et al. Microglia exacerbate white matter injury via complement C3/C3aR pathway after hypoperfusion. Theranostics. 2020;10(1):74‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015;7(1):a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Verkhratsky A, Nedergaard M. Physiology of astroglia. Physiol Rev. 2018;98(1):239‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Saint-Pol J, Gosselet F, Duban-Deweer S, et al. Targeting and crossing the blood-brain barrier with extracellular vesicles. Cells. 2020;9(4):851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Goetzl E J, Schwartz J B, Abner EL, et al. High complement levels in astrocyte-derived exosomes of Alzheimer disease. Ann Neurol. 2018;83(3):544‐552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang J, Liu Y, Zheng Y, et al. TREM-2-p38 MAPK signaling regulates neuroinflammation during chronic cerebral hypoperfusion combined with diabetes mellitus. J Neuroinflammation. 2020;17(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rawlings A M, Juraschek S P, Heiss G, et al. Association of orthostatic hypotension with incident dementia, stroke, and cognitive decline. Neurology. 2018;91(8):e759‐ee68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Peters R, Anstey K J, Booth A, et al. Orthostatic hypotension and symptomatic subclinical orthostatic hypotension increase risk of cognitive impairment: an integrated evidence review and analysis of a large older adult hypertensive cohort. Eur Heart J. 2018;39(33):3135‐3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.