Abstract
The treatment of musculoskeletal infections (MSIs), including periprosthetic joint infection (PJI) and fracture-related infection (FRI), is often complicated by biofilm-related challenges necessitating multiple revision surgeries and incurring substantial costs.
The emergence of antimicrobial resistance (AMR) adds to the complexity of the problem, leading to increased morbidity and healthcare expenses.
There is an urgent need for novel antibacterial strategies, with the World Health Organization endorsing non-traditional approaches like bacteriophage (phage) therapy.
Phage therapy, involving the targeted application of lytic potent phages, shows promise in the treatment of MSIs.
Although historical clinical trials and recent case studies present significant milestones in the evolution of phage therapy over the past century, challenges persist, including variability in study designs, administration protocols and phage selection. Efforts to enhance treatment efficacy consist of personalized phage therapy and combination with antibiotics.
Future perspectives entail addressing regulatory barriers, standardizing treatment protocols, and conducting high-quality clinical trials to establish phage therapy’s efficacy for the treatment of MSIs.
Initiatives like the PHAGEFORCE study and the PHAGEinLYON Clinic programme aim to streamline phage therapy, facilitating personalized treatment approaches and systematic data collection to advance its clinical utility in these challenging infections.
Keywords: fracture-related infection, periprosthetic joint infection, antimicrobial resistance, bacteriophage therapy
Introduction
Musculoskeletal infections (MSIs), including periprosthetic joint infection (PJI) and fracture-related infection (FRI), remain a devastating complication in modern trauma and orthopaedic surgery, with significant financial and psychosocial costs and increased morbidity (1, 2). PJI encompasses a range of infections, occurring in different locations (e.g. hip, knee and shoulder) and on different types of prosthesis (first-line prosthesis, or revision prosthesis that includes hinged prosthesis, resection prosthesis, prosthesis–arthrodesis and total femur) (3). For patients with PJI, it is necessary to avoid dead-end clinical situations, as transfemoral amputation or hip disarticulation are associated with a catastrophic functional outcome. FRI, on the other hand, can include both operatively and non-operatively treated bone fractures. For approximately 3–17% of the FRI patients with severe comorbidities or associated bone defects, amputation is the only option (4). While the incidence of PJI in most centres ranges between 0.5% and 2% (3, 5), the incidence for FRI varies widely, from 1% to 30%, depending to a large degree on injury severity (6). Given the ageing population, with a growing number of fragility fractures, and an increase in joint replacement surgery, it is expected that the incidence of MSIs will only increase in the upcoming years (3). These infections pose a serious burden on patients and healthcare systems as they often result in functional impairment, limited mobility, and a higher mortality rate compared to patients who do not develop this complication (3, 6). Management concepts consist of a combined surgical and antibiotic treatment approach. Because these infections are biofilm-related, treatment often necessitates multiple revision surgeries. This is not only costly but also demands considerable time and resources, with healthcare expenses for FRI patients being up to seven times higher compared to those who do not develop an FRI (7).
Although the biofilm is a well-known problem in MSIs, the increasing incidence of antimicrobial resistance (AMR) is potentially even more worrisome. The most frequently isolated pathogen in MSI is Staphylococcus aureus (30–42%) (8). Despite a decrease in the prevalence of methicillin-resistant S. aureus (MRSA) in Europe over the past decade, considerably higher rates persist in the USA, China, and the UK, indicating that it remains a substantial concern in these regions (6). Overall, the proportion of other resistant pathogens such as methicillin-resistant Staphylococcus epidermidis (MRSE) and multi-drug-resistant Pseudomonasaeruginosa is also increasing. Treatment becomes even more challenging in the case of these antibiotic-resistant pathogens (9, 10, 11), leading to increased morbidity, prolonged hospitalisation, and increased healthcare costs (3, 6). Patient-reported outcomes are worse for patients with resistant infections compared to infections with susceptible strains (11). It is clear that there is an urgent need for new or alternative antibacterial strategies. In their report, the World Health Organization (WHO) underlines the potential of non-traditional antibacterials, including bacteriophages (phages) and phage-derived enzymes (12, 13). Phage therapy involves the direct application of carefully selected lytic potent phages to patients, aiming to eradicate bacterial pathogens causing clinically significant infections (14). Numerous case reports and series have been published over the past decades, showing a low rate of adverse events and high efficacy rates (12, 13). However, there is substantial heterogeneity between studies regarding study design, adverse event reporting, administration protocols, and the selected phages (15). The aim of this review is to summarise the current evidence for phage therapy as a treatment modality for MSI.
Basic science
Phages are abundantly present in our natural environment. Furthermore, phages that target common MSI pathogens are often cultivable from the human microbiota. Despite the wide variety of potential phages, a relatively small number have been applied in preclinical or clinical trials. Regardless of the phage phylogeny, the life cycle of phage–host interaction is similar, with viral attachment, penetration, replication, and release leading to bacterial death and multiplication of phage particles (Fig. 1A and B) (16, 17).
Figure 1.
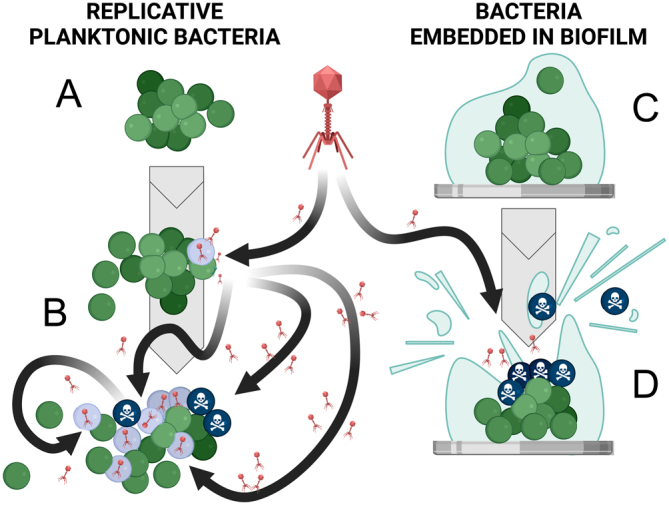
Bacteriophage activities against planktonic bacteria and against bacteria embedded in biofilm. A. Replicative bacteria in a planktonic state. B. Adhesion of an active phage at the bacterial surface followed by intrabacterial viral multiplication leading to bacterial lysis and propagation of the infection in the bacterial community. C. Bacteria embedded in biofilm at the surface of an implant. D. Destructuration of the biofilm thanks to the activity of phages and thanks to the activity of phage lysins.
Since phage therapy has been shown to support improved outcomes in MSI, the preclinical and basic science fields have continued to pursue improvements in treatment efficacy (16, 17, 18, 19, 20, 21). In addition, efforts to commercialise and translate phage therapy to a wider patient population have also been a driving factor for innovation in phage therapy.
With respect to improvements in antibacterial efficacy, preclinical studies have successfully shown that phages can be ‘trained’ to increase activity against biofilm-growing bacteria (22, 23, 24), which is, as previously mentioned, a critical aspect of MSI (Fig. 1C and D). By taking advantage of the high mutation frequency and tendency for recombination when multiple different phages are infecting the same pathogen, mutant or recombinant phages that display greater biofilm-killing activity may then be isolated in laboratory settings (22). Although this approach has not yet been applied to MSI in human cases, preclinical studies have shown some potential. Improvements in efficacy have also been seen by applying phage cocktails, combining them with antibiotics, or delivery within biomaterials such as hydrogels or within coatings. The preclinical data exceed the clinical data with regard to these approaches, with clinical benefit yet to be firmly established (25).
Another important limitation of phage therapy, particularly when considering industrial scale-up and clinical implementation, is the host range of any given phage or phage cocktail. Currently, selectivity of phages against bacterial hosts is determined by the phage receptor-binding proteins and their specificity for receptors on the bacterial surface (16, 17). Classically, this is considered a positive attribute, as it limits off-target effects of phage application on other species within the microbiome. This contrasts with antibiotic therapy, which can often have cross-species effects. However, this specificity makes it challenging to manage a biobank of suitable phages to target the range of pathogens encountered in clinical routine (16, 26). Any given phage may maximally target a proportion of clinical isolates of one species. This, therefore, requires curation and routine testing of panels of phages to cover medically important bacteria. One approach to overcome this is by engineering the phage tail fibres and the receptor-binding proteins to broaden the host range (26, 27). This approach may make production and clinical implementation more achievable since fewer phages may be required; however, there may potentially be additional complications due to the use of these genetically modified organisms (26).
Although relatively poorly described for human patients, amongst the numerous challenges faced by clinical implementation of phage therapy is the issue of phage neutralisation (18, 28). As the phage particle is proteinaceous, there are numerous antigens on the surface of the phage that may elicit an immune response, ultimately leading to antigen-specific phage neutralisation and more rapid elimination after administration than the baseline elimination that occurs in a non-specific manner. This may limit the ability to provide repeated doses of the same phage over a few weeks (29, 30). Although clinical data are limited, and no clinical data confirm any effect in MSIs to date, efforts are underway in the basic science field to also address this. For example, serum inactivation may be minimised by encapsulation of phage, and there are also early indications that incorporating phages in biomaterials masks or delays the induction of a secondary immune response (18). Cross-reactivity of immune memory from one phage to others remains poorly understood at the present time.
Finally, although phages were discovered more than a century ago, many basic science questions remain. With phage therapy being an active research topic in the field of MSI today, basic scientists are hopeful that they can assist in improving phage therapy in daily clinical practice in the near future.
General indications
Historical applications and indications
As mentioned above, the application of phage therapy has a long history, stretching back over 100 years. Initial records, dating as far back as the 1920s, largely focussed on wound infections, with Staphylococci as the predominant pathogens (31, 32). At this time, phages were directly applied to the infected site. Over time, indications broadened to include prophylaxis and treatment of other infectious diseases using different routes of administration. A significant milestone emerged from the Tbilisi Institute of Vaccine and Sera (now the Eliava Institute) in Georgia, where a method for preparing Staphylococcal phages for intravenous use was pioneered in the late 1970s (33). In one of their early trials, over 650 patients were treated for various infectious diseases including sepsis, peritonitis, osteomyelitis, purulent arthritis, lung infections and urogenital infections (34). Although overall outcomes for all medical indications favoured the combination of phages and antibiotic therapy, the interpretation of the trial data is challenging due to the lack of (English) data on population characteristics and an imbalance in group sizes (16). Similarly, in Poland, large case series from the 1980s reported a 90% success rate in patients with ‘pyogenic arthritis and myositis’, ‘osteomyelitis of long bones’ and ‘osteitis of long bones after fracture’ (35, 36). Application protocols included topical and oral applications, but no further details were reported concerning treatment duration, dosage and formulation, for example (16, 25).
While these historical studies present significant milestones in the evolution of phage therapy over the past century, a critical concern arises from the heterogeneous nature of the presented patient populations, characterised by varying causative pathogens, types and severity of infection. Moreover, these studies lack comprehensive details regarding critical aspects such as production, formulation, administration route, dosing and follow-up time.
Current modes of application and indications
Although phage therapy is still widely used in countries like Georgia, Russia and Poland (37), the lack of a rigorous evidence basis has led regulatory bodies in other regions to still consider phage therapy as experimental. This has restricted more recent applications of phage therapy primarily to ‘last resort’ scenarios or patients with difficult-to-treat infections that may have experienced recurrent infection despite prolonged therapy. A recent systematic review evaluated the literature to discern evidence on the safety and efficacy of phage therapy over the course of the past two decades (15). In total, 59 studies were included, covering nine medical disciplines: pneumology, urology, dermatology, otorhinolaryngology, ophthalmology, gastroenterology, cardiology, intensive care medicine and orthopaedics. Most of these studies were case studies of patients for whom all standard treatments had proven ineffective and for whom phage therapy was a final option. The authors concluded that phage therapy is generally considered safe, with a low incidence of adverse events. However, there was an important heterogeneity between studies, by which the evidence quality of studies could only be scored as low to moderate (15). Notably, the majority of these studies used a personalised approach, whereby a phagogram was obtained prior to the start of treatment to establish the sensitivity of patient samples to available phages. If, according to the phagogram, none of the available phages are active, another way of personalisation is to tailor phage therapy based on the causative strains. New phages can be searched specifically for the patient’s isolate from other sources or phages can be ‘trained’ by undergoing co-evolution with the pathogenic strain (38, 39). Indeed, phages can either be applied as a targeted dose based on phage susceptibility testing, or alternatively as a fixed phage cocktail designed to cover as broad a range of strains as possible (37, 40). In general, it is recommended to use targeted or tailored phage products, possibly in combination with antibiotics, to fully leverage and enhance the properties that phages offer compared to conventional antimicrobials. However, such an approach induces significant variability, thus complicating the conduct of clinical trials (15). Furthermore, in some (acute) cases, it may be challenging to isolate the causative pathogens and await phage susceptibility testing. In those cases, the empirical administration of a fixed phage cocktail covering a wide range of bacterial strains could be an option. To optimise treatment, this approach can later be complemented with personalised phage preparations once phage susceptibility data become available. Georgia and Russia have decades of empirical experience with the constitution of commercial phage cocktails (12). Several phage cocktails against a broad spectrum of pathogens are currently available over the counter at specialised pharmacies. Examples are the IntestiPhage and PyoPhage cocktails produced by the Eliava Institute. As the activity spectrum of these cocktails is known to decline over time, the constituent phages are regularly revised to cover emerging virulent bacteria (40). From a regulatory point of view, the issue with this method lies in the lack of precise knowledge regarding the composition of these cocktails and the fact that the individual phages have not undergone individual investigations before their inclusion in the product (41). Furthermore, most regulatory bodies would require that these phage cocktails are subjected to costly phase I–IV clinical trials and that the process is repeated after each modification, which significantly constrains the practicality of these cocktails (25, 41).
Fracture-related infection
Multiple case reports demonstrate the potential for phage therapy in the treatment of difficult-to-treat FRI (e.g. antibiotic-resistant pathogens, recurrent infections despite adequate surgical and antibiotic treatment, or patients with severe comorbidities). Eskenazi et al. describe the treatment of a patient with an FRI of the femur caused by pandrug-resistant Klebsiella pneumoniae (42). The phage (vB_Kp M1) was applied locally through a draining system in the form of 20 mL 108 PFU/mL three times per day for 5 days (20 mL), while antibiotic therapy was continued for another 3 months. After the cessation of antibiotic therapy, the patient recovered, and there was no recurrence of the infection (42).
Nir-Paz et al. describe the treatment of a patient who developed an FRI after suffering a Grade IIIA open fracture of the tibia (43). The infection was polymicrobial, caused by an extensively drug-resistant (XDR) Acinetobacter baumannii and multidrug-resistant (MDR) K. pneumoniae. Two phages were selected, targeting both causative pathogens. Phage therapy was administered intravenously, three times per day over 35 min, in the form of 1 mL 5 × 107 PFU/mL for a total duration of 5 days, after which A. baumannii was isolated again. One week later, a second treatment course was started for 6 days. The patient received concomitant intravenous antibiotics. After completion of the combined treatment, the wounds remained closed, and no signs of infection occurred. The patient was followed up for 8 months.
Onsea et al. described the treatment of four cases with difficult-to-treat FRI (44). The infection was polymicrobial in three out of four cases and caused by a combination of P. aeruginosa and S. epidermidis or Streptococcus agalactiae and S. aureus. One patient had a monomicrobial infection with Enterococcus faecalis. The treatment plan was set up by a ‘multidisciplinary phage task force’, a team consisting of infectious disease specialists, pharmacists, microbiologists, surgeons and phage scientists. Phages were selected based on the isolated strains, while one patient received commercial phages (Pyo bacteriophage) from the Eliava Institute. Phages were applied intraoperatively and three times daily through a draining system in the form of 20–40 mL 107 PFU/mL for up to 10 days postoperatively. All patients received concomitant antibiotics for a total duration of 3 months. No severe adverse effects were found, and all patients recovered and remain infection-free. The same authors recently published a study protocol (PHAGEFORCE) to standardise phage therapy and prospectively collect data on patients (44). In this study, patient eligibility for phage therapy is determined by a multidisciplinary team referred to as the Coordination group for Bacteriophage therapy Leuven (CBL). Patients are eligible when there are no standard curative antibiotic and/or surgical treatment options available and phages are available against the isolated pathogens (44). The treatment course for MSI is standardised and involves the intraoperative placement of a drain through which phages are administered three times per day for up to 10 days (Figs 2 and 3). The use of the draining system allows treatment optimisation as it facilitates sampling during treatment to monitor phage titers and isolate bacteria. In this way, resistance development can be detected in an early stage. No adverse events have been associated with the use of an external drain, which is removed after the final phage administration. Patients are followed up for a minimum of 1 year postoperatively.
Figure 2.
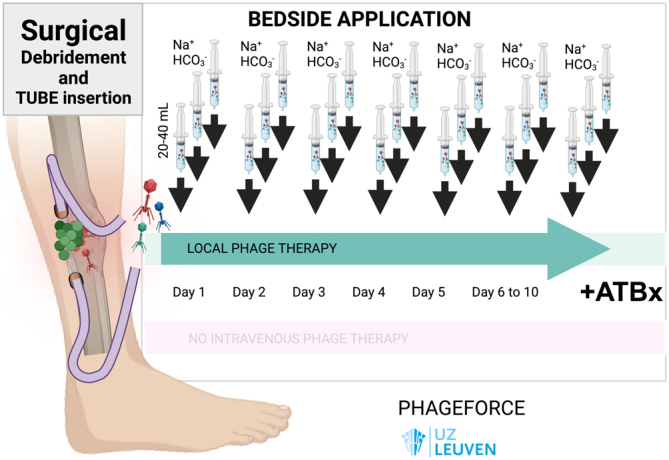
Typical phage treatment course in patients with FRI included in the PHAGEFORCE study, with surgical debridement, surgical tube (drain) insertion, and then administration of 20–40 mL of active phages three times per day for 7–10 days. Prior to each phage application, the drain is rinsed with sodium bicarbonate (NaHCO3) 1.4%. All patients receive concomitant antibiotics.
Figure 3.
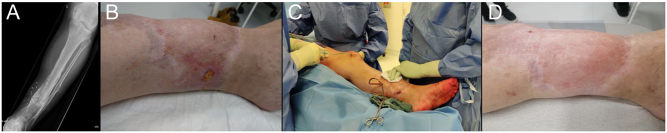
Phage treatment course for a patient with FRI of the tibia caused by MRSA, included in the PHAGEFORCE study. Based on susceptibility testing, the patient received 20 mL of phage ISP (107 PFU/mL) through each drain, three times per day for 10 days postoperatively. Concomitant antibiotics were administered for a total duration of 3 months. A. Preoperative radiograph. B. Preoperative clinical image showing the presence of a draining fistula. C. Intraoperative application of phage therapy through a draining system. D. Clinical status assessed at 1-year follow-up.
Periprosthetic joint infection
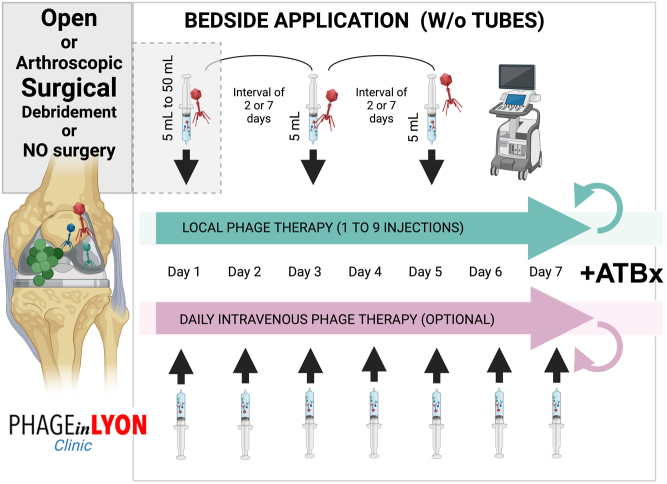
Multiple case reports demonstrate the potential for phage therapy in patients with complex and relapsing PJI (45, 46, 47, 48, 49, 50). From one-shot administration of phage therapy during conservative surgery to exclusive intravenous injections, several modes of administration have been described. Ferry et al. described the PhagoDAIR procedure in 2020, and since then, different treatment modalities for the treatment of complex PJI have been standardised through the PHAGEinLYON Clinic programme (supported by the Hospices Civils de Lyon Foundation) and based on the patient’s medical history and clinical presentation (17, 48). A national multidisciplinary team meeting, funded by the French health care ministry, has been set up at CRIOAc Lyon (https://www.crioac-lyon.fr) to determine if there is an indication for phage therapy in patients with complex MSI, and if yes, another dedicated multidisciplinary team meeting determines which phages would be the most appropriate and thereafter propose a personalised way of administration, depending on the clinical presentation. Patients are eligible when prosthesis exchange is not feasible, or when this is associated with life-threatening complications, and for whom a conservative approach seems to be mandatory. Of note, it was decided not to use drains for administration due to the potential risk of superinfection of the prosthesis. The treatment course for PJI is standardised based on three local injections with a 1-week interval, the first one can be done during an open or arthroscopic DAIR (with a maximum PFU diluted in 30–50 mL), and the subsequent ones can be done under sonography (dilution 5 mL) (Figs. 4 and 5). For non-surgical patients, exclusive injections under sonography are proposed, and an intensive regimen has been set up with injections each Monday–Wednesday–Friday under sonography for 3 weeks (totalling 9 injections). Intravenous injections of phages for 1–3 weeks can be applied in nonsurgical patients with abscesses (17), also in combination with antibiotics.
Figure 4.
Typical phage treatment course in patients with PJI included in the PHAGEinLYON Clinic programme. As adjuvant treatment to antibiotics, based on susceptibility testing, the patient received a first local injection of phages in the joint cavity during surgery (volume 30–50 mL) or after the surgery under sonography (5 mL), and then subsequent injections under sonography exclusively (5 mL), without using tubes. The number of injections and the time interval between each injection are depending on the clinical presentation. Daily intravenous injections of phages could be prescribed in non-surgical patients.
Figure 5.
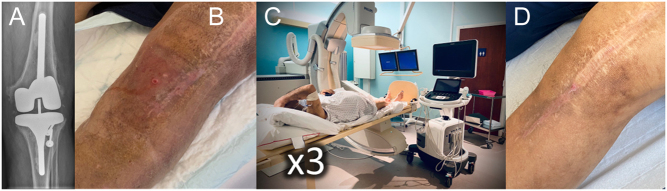
Phage treatment course for a patient with knee PJI caused by MSSA, included in the PHAGEinLYON Clinic programme. No surgery was performed, and Based on susceptibility testing, the patient received 5 mL of phage PP1815 (109 PFU/mL) under sonography, with a total of three injections with an interval of 1 week, in combination with antibiotics. A. Preoperative radiograph. B. Clinical image showing the presence of a draining fistula. C. Phage therapy with injection under sonography. D. Clinical status assessed at 18-month follow-up.
Future perspectives
While phage therapy is still widely used for various infectious diseases in Georgia, Russia and Poland, in Western Europe, phage therapy appears to be more restricted to certain indications.
Although recent case reports offer promising results, the variability in patients, administration routes (IV vs local instillation), dosages and treatment duration, coupled with the lack of details on phage production, formulation and stability, make it difficult to draw conclusions on the optimal phage therapy protocol for MSI. Standardised data on these topics are therefore crucial for the implementation of phage therapy in our daily clinical practice. Therefore, future high-quality clinical trials are critical (15).
The gold standard for clinical studies is a randomised controlled trial (RCT). According to clinicaltrials.gov, there is currently one RCT planned focussing on the treatment of chronic PJI of the hip or knee after failed revision surgery. Patients receive DAIR and phage therapy or DAIR and placebo. Phages are applied intraoperatively (locally) and intravenously against one or two of following pathogens: S. aureus, S. epidermidis, Staphylococcus lugdunensis, Streptococcus spp., Enterococcus faecium, E. faecalis, Escherichia coli, P. aeruginosa and K. pneumoniae. However, conducting such trials can be expensive due to the requirement of producing phage preparations in accordance with good manufacturing practices, which precludes the feasibility of a personalised approach (37). To address this gap, initiatives such as the PHAGEFORCE study and PHAGEinLYON Clinic programme were set up in Europe (17, 51). In Belgium, phage therapy is implemented in the regulatory framework for magistral preparations. Within this framework, PHAGEFORCE builds on the multidisciplinary approach established by the ‘multidisciplinary phage task force’ and aims to further standardise phage therapy and prospectively collect data. Patients with musculoskeletal infections, chronic rhinosinusitis, skin infections, sepsis and pulmonary infections are treated according to a predefined protocol (52), and data regarding treatment and treatment outcomes are systematically collected. In France, a similar approach, the PHAGEinLYON Clinic programme, aims to develop the access to pharmaceutical-grade phages for French patients, who are primarily included in clinical trials or are treated with phages in a compassionate prescription framework or in an individual compassionate access authorisation, under the supervision of the French healthcare authority. Here, multidisciplinary team meetings facilitate and personalise phage therapy to treat patients with severe bacterial infections such as MSIs, complex lung infections and endocarditis.
Conclusion
The treatment of MSIs can present a serious challenge in modern trauma and orthopaedic surgery. The complexity of these infections, coupled with the emergence of AMR, underscores the need for innovative treatment strategies. Although the potential of phage therapy has been demonstrated in numerous case reports and case series over the past decades, knowledge gaps remain regarding the optimal treatment protocol. For FRI, phage therapy is typically applied locally through a draining system, although intravenous applications have been described. Such a draining system allows therapeutic phage monitoring and facilitates treatment optimisation. After the final phage administration, the drain is removed, thereby minimising the risk of superinfection. For PJI, phages are typically applied using local injections during an open or closed DAIR procedure. Given the complexity of these cases, it is recommended to have a dedicated multidisciplinary team to design and monitor the phage treatment plan. If this is not feasible, the treating physician should consider referral to an expert centre with a dedicated multidisciplinary team. For both disease entities, there is an absence of a universally accepted treatment protocol, and it is key that future studies collect treatment-related and outcome data in a standardised way.
In general, it is recommended to use targeted or tailored phage products, possibly in combination with antibiotics, to fully leverage and enhance the properties that phages offer compared to conventional antimicrobials. Such an approach depends on susceptibility testing of the bacterial isolates (i.e. the phagogram). However, there currently is no consensus or guideline regarding the clinical breakpoints to be used for this susceptibility test (cf. the standard antibiogram). Future studies should report the parameters (or cut-off values) that were used for the selection of phages according to the phagogram.
In some (acute) cases, it may be challenging to isolate the causative pathogens and await phage susceptibility testing. In those cases, the empirical administration of a fixed phage cocktail covering a wide range of bacterial strains could be an option. However, as phages are considered as a medicinal products (EU) or drugs (USA), direct requirements from the health agencies are that the products follow the same processes as general antibiotics to obtain marketing authorisation for human use (53). This implies that they should be subjected to costly phase I–IV clinical trials, which is especially problematic for phage cocktails, as the process should be repeated after each modification made to the original phage cocktail (54). It also implies that final phage products would need to be produced according to Good Manufacturing Practice rules and should fulfil quality controls of production batches before administration (53, 54).
As a result, different initiatives have been issued by the member states. For instance, in Belgium, phage therapy has been implemented in the regulatory framework for magistral preparations, where a monograph was established to define characteristics and quality standards of phage active substances for applications in humans. A similar approach is applied in France, where phages could be prescribed with a compassionate prescription framework or in an individual compassionate access authorisation under the supervision of the French healthcare authority, and they become magistral preparations before administration. Based on these developments, phage therapy regulations in other parts of the world are moving forward (31).
In conclusion, although the use of phage therapy in MSIs seems to be safe and clinical results are promising, challenges persist, including the variability in study designs, administration protocols and phage selection. Future perspectives entail addressing regulatory barriers, standardising treatment protocols and conducting high-quality clinical trials to establish phage therapy’s efficacy for the treatment of MSIs.
ICMJE Conflict of Interest Statement
Tristan Ferry is the principal investigator of the PhagoDAIR I clinical trial (Phaxiam Therapeutics, no direct funding). The remaining authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this article.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Acknowledgements
We acknowledge members of the PHAGEFORCE study group (formed of Willem-Jan Metsemakers, Jolien Onsea, Isabel Spriet, Yves Debaveye, Laura Van Gerven, Rob Lavigne, Vera Van Noort). This study is funded by KU Leuven (Interdisciplinary Networks, IDN/20/024). Furthermore, we acknowledge the members of the Coordination group for Bacteriophage therapy Leuven (CBL) including Willem-Jan Metsemakers, Jolien Onsea, Laura Bessems, Isabel Spriet, David Devolder, Melissa Depypere, Paul De Munter, Yves Debaveye, Laura Van Gerven, Saartje Uyttebroek, Lieven Dupont, Rob Lavigne, Jean-Paul Pirnay. We acknowledge members of the PHAGEinLYON Clinic Study Group (composed of Tristan Ferry, Myrtille Le Bouar, Thomas Briot, Tiphaine Roussel-Gaillard, Camille Kolenda and Karine Dallosto) and the Lyon Bone and Joint Infection Study Group (Coordinator: Tristan Ferry). The PHAGEinLYON Clinic programme is funded by the ‘Fondation Hospices Civils de Lyon’ and we particularly acknowledge Bruno Lacroix, Sophie Mérigot, Cécile Duthyer from the ‘Fondation Hospices Civils de Lyon’ and all founders and patrons who participated and made it possible. We also acknowledge the ‘Direction Général de l’Offre de Soin’ from the French health ministry, the ‘Agence Nationale de Sécurité du Médicament et des produits de santé’ team, and the ‘Haute Autorité de Santé’. Finally, we also greatly acknowledge public and private phage producers who are able to provide pharmaceutical-grade phages to treat our patients.
References
- 1.Cook GE Markel DC Ren W Webb LX McKee MD & Schemitsch EH. Infection in orthopaedics. Journal of Orthopaedic Trauma 201529(Supplement 12) S19–S23. ( 10.1097/BOT.0000000000000461) [DOI] [PubMed] [Google Scholar]
- 2.Lee C Mayer E Bernthal N Wenke J & O’Toole RV. Orthopaedic infections: what have we learned?. OTA International 20236(Supplement) e250. ( 10.1097/OI9.0000000000000250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel R. Periprosthetic joint infection. New England Journal of Medicine 2023388251–262. ( 10.1056/NEJMra2203477) [DOI] [PubMed] [Google Scholar]
- 4.Metsemakers WJ, Moriarty TF, Morgenstern M, Marais L, Onsea J, O’Toole RV, Depypere M, Obremskey WT, Verhofstad MHJ, McNally M, et al.The global burden of fracture-related infection: can we do better? Lancet. Infectious Diseases 2023. ( 10.1016/S1473-3099(2300503-0);(S1473-3099(23)00503-0) [DOI] [PubMed] [Google Scholar]
- 5.Tan TL Maltenfort MG Chen AF Shahi A Higuera CA Siqueira M & Parvizi J. Development and evaluation of a preoperative risk calculator for periprosthetic joint infection following total joint arthroplasty. Journal of Bone and Joint Surgery 2018100777–785. ( 10.2106/JBJS.16.01435) [DOI] [PubMed] [Google Scholar]
- 6.Moriarty TF Metsemakers WJ Morgenstern M Hofstee MI Vallejo Diaz A Cassat JE Wildemann B Depypere M Schwarz EM & Richards RG. Fracture-related infection. Nature Reviews. Disease Primers 2022867. ( 10.1038/s41572-022-00396-0) [DOI] [PubMed] [Google Scholar]
- 7.Iliaens J Onsea J Hoekstra H Nijs S Peetermans WE & Metsemakers WJ. Fracture-related infection in long bone fractures: a comprehensive analysis of the economic impact and influence on quality of life. Injury 2021523344–3349. ( 10.1016/j.injury.2021.08.023) [DOI] [PubMed] [Google Scholar]
- 8.Depypere M Sliepen J Onsea J Debaveye Y Govaert GAM IJpma FFA Zimmerli W & Metsemakers WJ. The microbiological etiology of fracture-related infection. Frontiers in Cellular and Infection Microbiology 202212934485. ( 10.3389/fcimb.2022.934485) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casenaz A Piroth L Labattut L Sixt T Magallon A Guilloteau A Neuwirth C & Amoureux L. Epidemiology and antibiotic resistance of prosthetic joint infections according to time of occurrence, a 10-year study. Journal of Infection 202285492–498. ( 10.1016/j.jinf.2022.07.009) [DOI] [PubMed] [Google Scholar]
- 10.Boucher HW & Corey GR. Epidemiology of methicillin-resistant Staphylococcus aureus. Clinical Infectious Diseases 200846(Supplement 5) S344–S349. ( 10.1086/533590) [DOI] [PubMed] [Google Scholar]
- 11.van Duin D & Paterson DL. Multidrug-resistant bacteria in the community: an update. Infectious Disease Clinics of North America 202034709–722. ( 10.1016/j.idc.2020.08.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCallin S, Drulis-Kawa Z, Ferry T, Pirnay JP, Nir-Paz R. & ESGNTA. ESCMID study group for non-traditional antibacterials . Phages and phage-borne enzymes as new antibacterial agents. Clinical Microbiology and Infection 2023. (S1198-743X(23)00528-1) [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. Geneva 2022. Antibacterial Agents in Clinical and Preclinical Development: an Overview and Analysis 2022. Available at https://iris.who.int/bitstream/handle/10665/354545/9789240047655-eng.pdf?sequence=1 [Google Scholar]
- 14.Ali Y Inusa I Sanghvi G Mandaliya VB & Bishoyi AK. The current status of phage therapy and its advancement towards establishing standard antimicrobials for combating multi drug-resistant bacterial pathogens. Microbial Pathogenesis 2023181106199. ( 10.1016/j.micpath.2023.106199) [DOI] [PubMed] [Google Scholar]
- 15.Uyttebroek S, Chen B, Onsea J, Ruythooren F, Debaveye Y, Devolder D, Spriet I, Depypere M, Wagemans J, Lavigne R, et al.Safety and efficacy of phage therapy in difficult-to-treat infections: a systematic review. Lancet. Infectious Diseases 202222e208–e220. ( 10.1016/S1473-3099(2100612-5) [DOI] [PubMed] [Google Scholar]
- 16.Onsea J Wagemans J Pirnay JP Di Luca M Gonzalez-Moreno M Lavigne R Trampuz A Moriarty TF & Metsemakers WJ. Bacteriophage therapy as a treatment strategy for orthopaedic-device-related infections: where do we stand? European Cells and Materials 202039193–210. ( 10.22203/eCM.v039a13) [DOI] [PubMed] [Google Scholar]
- 17.Ferry T. A review of phage therapy for bone and joint infections. Methods in Molecular Biology 20242734207–235. ( 10.1007/978-1-0716-3523-0_14) [DOI] [PubMed] [Google Scholar]
- 18.Rotman SG Sumrall E Ziadlou R Grijpma DW Richards RG Eglin D & Moriarty TF. Local bacteriophage delivery for treatment and prevention of bacterial infections. Frontiers in Microbiology 202011538060. ( 10.3389/fmicb.2020.538060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sumrall ET, Hofstee MI, Arens D, Röhrig C, Baertl S, Gehweiler D, Schmelcher M, Loessner MJ, Zeiter S, Richards RG, et al.An enzybiotic regimen for the treatment of methicillin-resistant Staphylococcus aureus orthopaedic device-related infection. Antibiotics (Basel) 2021101186. ( 10.3390/antibiotics10101186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tkhilaishvili T Lombardi L Klatt AB Trampuz A & Di Luca M. Bacteriophage Sb-1 enhances antibiotic activity against biofilm, degrades exopolysaccharide matrix and targets persisters of Staphylococcus aureus. International Journal of Antimicrobial Agents 201852842–853. ( 10.1016/j.ijantimicag.2018.09.006) [DOI] [PubMed] [Google Scholar]
- 21.Fanaei Pirlar R Wagemans J Ponce Benavente L Lavigne R Trampuz A & Gonzalez Moreno M. Novel bacteriophage specific against Staphylococcus epidermidis and with antibiofilm activity. Viruses 2022141340. ( 10.3390/v14061340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rohde C, Resch G, Pirnay JP, Blasdel BG, Debarbieux L, Gelman D, Górski A, Hazan R, Huys I, Kakabadze E, et al.Expert opinion on three phage therapy related topics: bacterial phage resistance, phage training and prophages in bacterial production strains. Viruses 201810178. ( 10.3390/v10040178) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friman VP Soanes-Brown D Sierocinski P Molin S Johansen HK Merabishvili M Pirnay JP De Vos D & Buckling A. Pre-adapting parasitic phages to a pathogen leads to increased pathogen clearance and lowered resistance evolution with Pseudomonas aeruginosa cystic fibrosis bacterial isolates. Journal of Evolutionary Biology 201629188–198. ( 10.1111/jeb.12774) [DOI] [PubMed] [Google Scholar]
- 24.Betts A Vasse M Kaltz O & Hochberg ME. Back to the future: Evolving bacteriophages to increase their effectiveness against the pathogen Pseudomonas aeruginosa PAO1. Evolutionary Applications 201361054–1063. ( 10.1111/eva.12085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Briot T, Kolenda C, Ferry T, Medina M, Laurent F, Leboucher G, Pirot F. & PHAGEinLYON study group. Paving the way for phage therapy using novel drug delivery approaches. Journal of Controlled Release 2022347414–424. ( 10.1016/j.jconrel.2022.05.021) [DOI] [PubMed] [Google Scholar]
- 26.Hussain W Yang X Ullah M Wang H Aziz A Xu F Asif M Ullah MW & Wang S. Genetic engineering of bacteriophages: key concepts, strategies, and applications. Biotechnology Advances 202364108116. ( 10.1016/j.biotechadv.2023.108116) [DOI] [PubMed] [Google Scholar]
- 27.Payaslian F Gradaschi V & Piuri M. Genetic manipulation of phages for therapy using BRED. Current Opinion in Biotechnology 2021688–14. ( 10.1016/j.copbio.2020.09.005) [DOI] [PubMed] [Google Scholar]
- 28.Kulshrestha M Tiwari M & Tiwari V. Bacteriophage therapy against ESKAPE bacterial pathogens: current status, strategies, challenges, and future scope. Microbial Pathogenesis 2024186106467. ( 10.1016/j.micpath.2023.106467) [DOI] [PubMed] [Google Scholar]
- 29.Dąbrowska K. Phage therapy: what factors shape phage pharmacokinetics and bioavailability? Systematic and critical review. Medicinal Research Reviews 2019392000–2025. ( 10.1002/med.21572) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gembara K & Dąbrowska K. Phage-specific antibodies. Current Opinion in Biotechnology 202168186–192. ( 10.1016/j.copbio.2020.11.011) [DOI] [PubMed] [Google Scholar]
- 31.Metsemakers WJ Onsea J Moriarty TF Pruidze N Nadareishvili L Dadiani M & Kutateladze M. Bacteriophage therapy for human musculoskeletal and skin/soft tissue infections. Clinical Microbiology and Infection 202329695–701. ( 10.1016/j.cmi.2023.01.011) [DOI] [PubMed] [Google Scholar]
- 32.Ferry T Kolenda C Gustave CA Lustig S Josse J Batailler C Pirot F Leboucher G & Laurent F. Phage therapy in bone and joint infection: history, scientific basis, feasibility and perspectives in France. Virologie 2020244–11. ( 10.1684/vir.2020.0810) [DOI] [PubMed] [Google Scholar]
- 33.Kutateladze M & Adamia R. Bacteriophages as potential new therapeutics to replace or supplement antibiotics. Trends in Biotechnology 201028591–595. ( 10.1016/j.tibtech.2010.08.001) [DOI] [PubMed] [Google Scholar]
- 34.Kutateladze M. Experience of the Eliava Institute in bacteriophage therapy. Virologica Sinica 20153080–81. ( 10.1007/s12250-014-3557-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slopek S Weber-Dabrowska B Dabrowski M & Kucharewicz-Krukowska A. Results of bacteriophage treatment of suppurative bacterial infections in the years 1981–1986. Archivum Immunologiae et Therapiae Experimentalis 198735569–583. [PubMed] [Google Scholar]
- 36.Weber-Dabrowska B Mulczyk M & Górski A. Bacteriophage therapy of bacterial infections: an update of our institute’s experience. Archivum Immunologiae et Therapiae Experimentalis 200048547–551. [PubMed] [Google Scholar]
- 37.Pirnay JP, De Vos D, Verbeken G, Merabishvili M, Chanishvili N, Vaneechoutte M, Zizi M, Laire G, Lavigne R, Huys I, et al.The phage therapy paradigm: prêt-à-porter or sur-mesure? Pharmaceutical Research 201128934–937. ( 10.1007/s11095-010-0313-5) [DOI] [PubMed] [Google Scholar]
- 38.Borin JM Avrani S Barrick JE Petrie KL & Meyer JR. Coevolutionary phage training leads to greater bacterial suppression and delays the evolution of phage resistance. Proceedings of the National Academy of Sciences of the United States of America 2021118e2104592118. ( 10.1073/pnas.2104592118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Terwilliger AL Gu Liu C Green SI Clark JR Salazar KC Hernandez Santos H Heckmann ER Trautner BW Ramig RF & Maresso AW. Tailored antibacterials and innovative laboratories for phage (Φ) research: personalized infectious disease medicine for the most vulnerable at-risk patients. PHAGE 2020166–74. ( 10.1089/phage.2020.0007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villarroel J Larsen MV Kilstrup M & Nielsen M. Metagenomic analysis of therapeutic PYO phage cocktails from 1997 to 2014. Viruses 20179328. ( 10.3390/v9110328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pirnay JP Merabishvili M De Vos D & Verbeken G. Bacteriophage production in compliance with regulatory requirements. Methods in Molecular Biology 2024273489–115. ( 10.1007/978-1-0716-3523-0_6) [DOI] [PubMed] [Google Scholar]
- 42.Eskenazi A, Lood C, Wubbolts J, Hites M, Balarjishvili N, Leshkasheli L, Askilashvili L, Kvachadze L, van Noort V, Wagemans J, et al.Combination of pre-adapted bacteriophage therapy and antibiotics for treatment of fracture-related infection due to pandrug-resistant Klebsiella pneumoniae. Nature Communications 202213302. ( 10.1038/s41467-021-27656-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nir-Paz R, Gelman D, Khouri A, Sisson BM, Fackler J, Alkalay-Oren S, Khalifa L, Rimon A, Yerushalmy O, Bader R, et al.Successful treatment of antibiotic-resistant, poly-microbial bone infection with bacteriophages and antibiotics combination. Clinical Infectious Diseases 2019692015–2018. ( 10.1093/cid/ciz222) [DOI] [PubMed] [Google Scholar]
- 44.Onsea J, Soentjens P, Djebara S, Merabishvili M, Depypere M, Spriet I, De Munter P, Debaveye Y, Nijs S, Vanderschot P, et al.Bacteriophage application for difficult-to-treat musculoskeletal infections: development of a standardized multidisciplinary treatment protocol. Viruses 201911. ( 10.3390/v11100891) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferry T, Leboucher G, Fevre C, Herry Y, Conrad A, Josse J, Batailler C, Chidiac C, Medina M, Lustig S, et al.Salvage debridement, antibiotics and implant retention (“DAIR”) with local injection of a selected cocktail of bacteriophages: is it an option for an elderly patient with relapsing Staphylococcus aureus prosthetic-joint infection? Open Forum Infectious Diseases 20185ofy269. ( 10.1093/ofid/ofy269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferry T, Batailler C, Petitjean C, Chateau J, Fevre C, Forestier E, Brosset S, Leboucher G, Kolenda C, Laurent F, et al.The potential innovative use of bacteriophages within the DAC® hydrogel to treat patients with knee megaprosthesis infection requiring “debridement antibiotics and implant retention” and soft tissue coverage as salvage therapy. Frontiers in Medicine 20207342. ( 10.3389/fmed.2020.00342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferry T, Batailler C, Souche A, Cassino C, Chidiac C, Perpoint T, Corvaisier C, Josse J, Gaillard R, Roger J, et al.Arthroscopic “Debridement And Implant Retention” (DAIR) with local administration of Exebacase (Lysin CF-301) (LysinDAIR) followed by suppressive tedizolid as salvage therapy in elderly patients for relapsing multidrug-resistant (MDR) S. epidermidis prosthetic knee infection LysinDAIR for prosthetic knee infection. Frontiers of Medicine 2021. ( 10.3389/fmed.2021.550853) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferry T, Kolenda C, Batailler C, Gustave CA, Lustig S, Malatray M, Fevre C, Josse J, Petitjean C, Chidiac C, et al.Phage therapy as adjuvant to conservative surgery and antibiotics to salvage patients with relapsing S. aureus prosthetic knee infection. Frontiers in Medicine 20207570572. ( 10.3389/fmed.2020.570572) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Doub JB Johnson AJ Nandi S Ng V Manson T Lee M & Chan B. Experience using adjuvant bacteriophage therapy for the treatment of 10 recalcitrant periprosthetic joint infections: A case series. Clinical Infectious Diseases 202376e1463–e1466. ( 10.1093/cid/ciac694) [DOI] [PubMed] [Google Scholar]
- 50.Cano EJ, Caflisch KM, Bollyky PL, Van Belleghem JD, Patel R, Fackler J, Brownstein MJ, Horne B, Biswas B, Henry M, et al.Phage therapy for limb-threatening prosthetic knee Klebsiella pneumoniae infection: case report and in vitro characterization of anti-biofilm activity. Clinical Infectious Diseases 202173e144–e151. ( 10.1093/cid/ciaa705) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Onsea J, Uyttebroek S, Chen B, Wagemans J, Lood C, Van Gerven L, Spriet I, Devolder D, Debaveye Y, Depypere M, et al.Bacteriophage therapy for difficult-to-treat infections: the implementation of a multidisciplinary phage task force (the PHAGEFORCE study protocol). Viruses 2021131543. ( 10.3390/v13081543) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uyttebroek S, Bessems L, Metsemakers W-J, Debaveye Y, Van Gerven L, Dupont L, Depypere M, Wagemans J, Lavigne R, Merabishvili M, et al.Stability of magistral phage preparations before therapeutic application in patients with chronic rhinosinusitis, sepsis, pulmonary, and musculoskeletal infections. Microbiology Spectrum 202311e0290723. ( 10.1128/spectrum.02907-23) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bretaudeau L Tremblais K Aubrit F Meichenin M & Arnaud I. Good manufacturing practice (GMP) compliance for phage therapy medicinal products. Frontiers in Microbiology 2020111161. ( 10.3389/fmicb.2020.01161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pirnay JP Verbeken G Ceyssens PJ Huys I De Vos D Ameloot C & Fauconnier A. The magistral phage. Viruses 20181006. ( 10.3390/v10020064) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a