Abstract
Phototherapy is the most commonly used treatment for neonatal hyperbilirubinemia (NH). Gut microbiota is involved in bilirubin metabolism; however, it is uncertain whether this is affected by phototherapy. The present study included 43 newborns with hyperbilirubinemia and collected fecal samples for high-throughput sequencing before and after phototherapy. Selection α diversity analysis was used to determine the differences in diversity and abundance between the two groups, whereas similarity was determined using β diversity analysis. Linear discriminant analysis effect size analysis was used to screen for markedly different bacteria. The structure of the gut microbiota in newborns with hyperbilirubinemia changed after phototherapy, with a significant decrease in abundance and diversity. The changes in the key bacterial species were characterized by an increase in the abundance of Streptococcus salivarius and a decrease in the abundance of Escherichia, Klebsiella pneumoniae, Rothia mucilaginosa and Streptococcus oralis. These changes mainly manifested as an increase in beneficial bacteria and a decrease in opportunistic bacteria, which may not be related to the side effects of phototherapy. These results can provide theoretical assistance for microbiological research on the later stages of NH.
Keywords: neonatal hyperbilirubinemia, phototherapy, gut microbiota, high-throughput sequencing
Introduction
Neonatal hyperbilirubinemia (NH) is one of the most common neonatal diseases and may lead to permanent neurological sequelae in severe cases (1). Phototherapy has become a necessary treatment option to quickly and effectively reduce unconjugated bilirubin and prevent bilirubin encephalopathy (2). Phototherapy causes photoisomerization of unconjugated bilirubin in superficial skin tissues to form configurational and structural isomers, both of which are water-soluble and can be directly excreted through bile and urine without liver metabolism, thereby reducing the concentration of bilirubin in the body (3). However, with the increasing popularity of phototherapy in clinical practice, there have been an increasing number of reports on its related side effects such as fever, diarrhea, rash and bronzed skin, which have gradually attracted the attention of doctors (4). Although it is well known that gut microbiota plays an important role in hyperbilirubin metabolism (5), its correlation with phototherapy has rarely been reported.
The present study used 16S rDNA amplicon sequencing technology to detect and analyze the gut microbiota of newborns with NH before and after phototherapy and screened for markedly altered bacterial species, which may provide some theoretical assistance for further microbiological research on the later stages of NH.
Materials and methods
Participants
Clinical data and fresh feces of newborns diagnosed with NH requiring phototherapy were collected from Suqian Hospital Affiliated to Xuzhou Medical University (Jiangsu, China) between December 2022 and June 2023. The present study included a total of 43 newborns (28 males and 15 females) diagnosed with NH who received phototherapy, with an age range of 1-13 days and a male to female ratio of 1.86:1. Their gestational age, age and birth weight were 270.53±7.34 d, 3.93±2.32 d and 3367.93±413.92 g, respectively. The inclusion criteria were as follows: i) The diagnostic and phototherapy indications for NH complied with the guidelines established by the American Academy of Pediatrics (2); ii) pregnancy was >35 weeks and age was <14 days; and iii) natural birth of the participants in the Suqian Hospital Affiliated to Xuzhou Medical University and breastfeeding after birth. Exclusion criteria were as follows: i) Previous use of antibiotics, probiotics, prebiotics, or synbiotics; ii) concomitant infectious, gastrointestinal, or congenital diseases; and iii) birth weight <2,500 g.
Phototherapy was performed in the neonatal jaundice treatment box (Ningbo David Medical Equipment Co., Ltd.), with a wavelength of 450-480 nm, temperature of 30-34˚C and humidity of 55-65%. The light source in the box was a double-sided LED and the distance between the lamp and skin was 33-50 cm. The duration of phototherapy was set to 24 h, during which the newborn's skin was exposed, except for their eyes and perineum. These newborns were assigned to the control group (before phototherapy) and observation group (after phototherapy).
Ethical approval
The parents or guardians of the children provided informed consent and the study design was approved by the Ethics Committee of Suqian Hospital Affiliated to Xuzhou Medical University (approval no. 2021022). All experiments were performed in accordance with guidelines of the declaration of Helsinki 2013.
Fecal DNA extraction
Fecal samples were collected by medical staff in the hospital within 1 h before and after phototherapy (using glycerin, if necessary). Prior to sampling, both hands were cleansed and no soap or hand sanitizer containing antibacterial ingredients was used. A stool sample (~3-5 g) was scooped with a sampling spoon and placed in a sterile freezer tube for storage at -80˚C. After all the samples were collected, DNA was extracted using the QIAamp PowerFecal Pro DNA Kit (Qiagen GmbH) according to the manufacturer's instructions. The extracted DNA was tested using a Qubit 3 (Thermo Fisher Scientific, Inc.) for concentration, Thermo NanoDrop 2000 (Thermo Fisher Scientific, Inc.) for purity and 1% agarose gel electrophoresis for integrity. Only DNA samples with c ≥100 ng/µl, 1.8<A260/280<2.0 and obvious master bands were considered qualified.
16S rDNA amplicon sequencing
The highly variable V4 region was selected as the segment for 16S rDNA amplification. A total of 30 ng of qualified genomic DNA samples and the corresponding primers were configured using the PCR system and amplified. The following primers were used: 515F (5'-GTGCCAGCMGCCGCGGTAA-3') and 806R (5'-GGACTACHVGGGTWTCTAAT-3'). Reactions were performed in a volume of 50 µl using Dream Taq Green PCR Master Mix (Thermo Fisher Scientific, Inc.). The PCR program included denaturation at 95˚C for 2 min, followed by 27 cycles at 98˚C for 10 sec, annealing at 62˚C for 30 sec, and extension at 68˚C for 30 sec, and a final extension for 10 min at 68˚C. The amplification products were electrophoresed on 2% agarose gels with ethidium bromide staining and purified using Agencourt AMPure XP Beads (A&D Technology), dissolved in Elution Buffer (Thermo Fisher Scientific, Inc.) and labeled to complete library construction. Fragment length and concentration of the library were measured using an Agilent 2100 Bioanalyzer (Agilent Technologies, Inc.). Qualified libraries were sequenced using the HiSeq X Ten (Illumina, Inc.) according to the size of the inserted fragment. All raw reads were deposited in the NCBI Sequence Read Archive database (accession no. PRJNA1096251; https://www.ncbi.nlm.nih.gov/sra/?term=PRJNA1096251).
Bioinformatic data collection
The raw data obtained after sequencing was processed to obtain clean data as follows: i) A window of 25 bp was set and if the average quality value of the window was <20, the backend base was intercepted from the window; if the read length after truncation was <75% of the raw read length, the entire sequence was removed; ii) contaminated reads were removed from the connector (default adapter sequence had a 15 bp overlap with the read sequence, was set to 15 bp and three mismatches were allowed); iii) reads containing N were removed; and iv) low complexity reads were removed (default reads with continuous base length ≥10 were considered low complexity reads). Duplicate reads were removed with Picard Tools 3.1.1 (http://picard.sourceforge.net). Trimmomatic (version 0.32; http://www.usadellab.org/cms/index.php?page=trimmomatic) was used to remove adaptor sequences and trim bases with quality <20 (Phred 33 quality scores). The allowed number of mismatches between barcode sequences and sequencing reads was 0 bp.
Sequence stitching was performed using FLASH software (version 1.2.11; www.cbcb.umd.edu/software/flash). Based on the overlapping relationship, pairs of reads obtained by double-terminal sequencing were assembled into a sequence to obtain tags in the highly variable region. The stitching requirements were as follows: i) the minimum matching length was 15 bp and ii) the allowable mismatch rate of the overlapping area was 0.1.
USEARCH software (version 7.0.1090; http://www.drive5.com/usearch/) was used to cluster the assembled tags into operational taxonomic unit (OTUs). The main process was as follows: i) the representative sequence of OTUs was obtained by clustering with 97% similarity using UPARSE (version 7.0.1001; http://drive5.com/uparse/) and ii) the chimera generated by PCR amplification was removed from the OTU representative sequence using UCHIME (version 4.2.40; https://www.drive5.com/uchime/uchime_download.html). The usearch_global method was used to compare all tags back to the OTU representative sequence to obtain the OTU abundance statistics table for each sample.
OTU abundance was normalized to the lowest number of sequences obtained from a single sample and assessed for α diversity (ACE and Shannon indices) and β diversity (unifrac distances weighted by principal component analysis) in quantitative insights into microbial ecology (QIIME) software (version 1.9.1; http://www.wernerlab.org/software/macqiime) (6-8). Linear discriminant analysis (LDA), followed by LDA effect size (LEfSe) analysis, was also conducted.
Statistical analysis
Data analysis was conducted using the SPSS software (version 26.0; IBM Corp.). Measurement data are reported as mean ± standard deviation unless otherwise stated. The normality of the data was tested using the Kolmogorov-Smirnova method. A paired t-test was used if the data conformed to a normal distribution; otherwise, a rank-sum test was used. P<0.05 was considered to indicate a statistically significant difference.
Results
α and β diversity analysis
The total number of clustered OTUs in the observation and control groups was 354, with 139 in the observation group and 215 in the control group, of which 81 were shared (Fig. 1). α diversity analysis showed that the diversity (Shannon index, P<0.001; Fig. 2A) and abundance (ACE index, P=0.016; Fig. 2B) of the observation group were significantly lower compared to the control group. β diversity analysis showed a statistically significant difference in the similarity between the two groups based on unweighted and weighted unifrac distance (both P<0.001; Fig. 3A and B).
Figure 1.
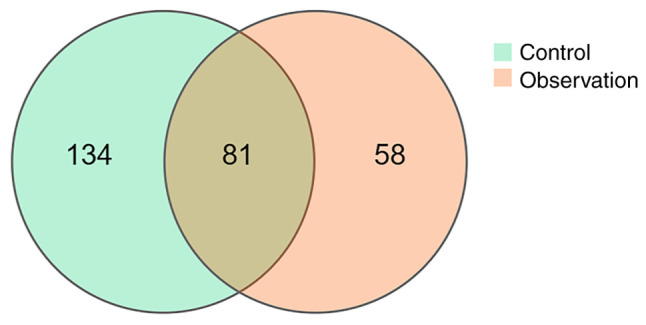
Comparison of OTUs between control and observation groups. The number in the overlapping part is the number of OTUs shared between the two groups. OTUs, operational taxonomic units.
Figure 2.
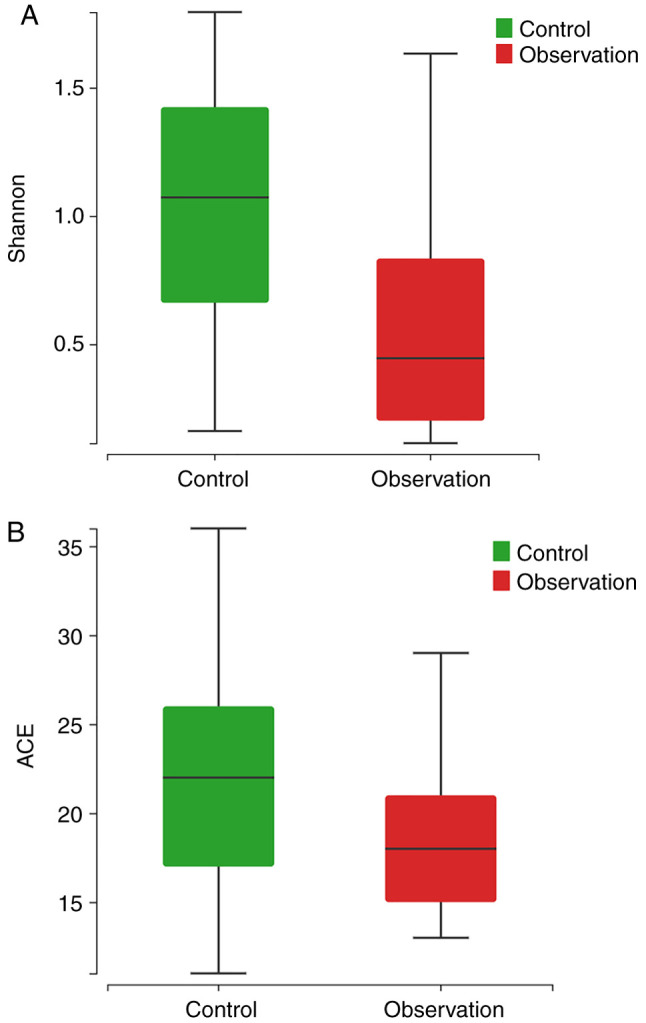
α diversity of gut microbiota within newborns with neonatal hyperbilirubinemia before (control group) and after phototherapy (observation group): (A) Shannon index and (B) ACE index.
Figure 3.
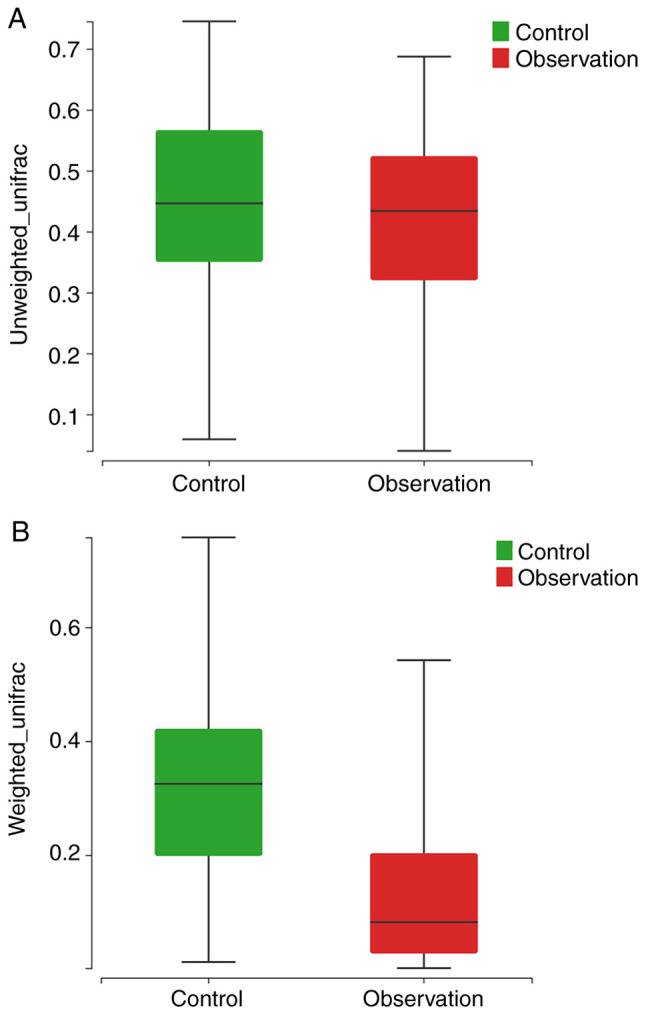
β diversity test between two groups based on unweighted (A) and weighted (B) unifrac distance.
Proportion of gut microbiota abundance
At the phylum level (Fig. 4A), the control group was composed mainly of Firmicutes (53.58%), Proteobacteria (35.33%), Actinobacteria (7.33%) and Bacteroidetes (3.73%), whereas the observation group was composed of Firmicutes (87.44%), Proteobacteria (10.40%) and Actinobacteria (2.07%). At the genus level (Fig. 4B), the key bacterial genera and their abundance ratios in the control group were Streptococcus (30.89%), Escherichia (19.58%), Klebsiella (13.56%), Enterococcus (13.30%), Veillonella (4.22%), Bacteroides (3.12%), Rothia (3.03%), Clostridium (1.77%), Salmonella (1.51%) and Bifidobacterium (4.24%). Whereas, in the observation group, the key bacterial genera and their abundance ratios were Streptococcus (74.81%) Enterococcus (9.89%), Escherichia (6.21%), Klebsiella (4.08%), Veillonella (2.05%), Bifidobacterium (1.31%), Rothia (0.76%), Clostridium (0.47%), Lactobacillus (0.10%) and Bacteroides (0.05%).
Figure 4.
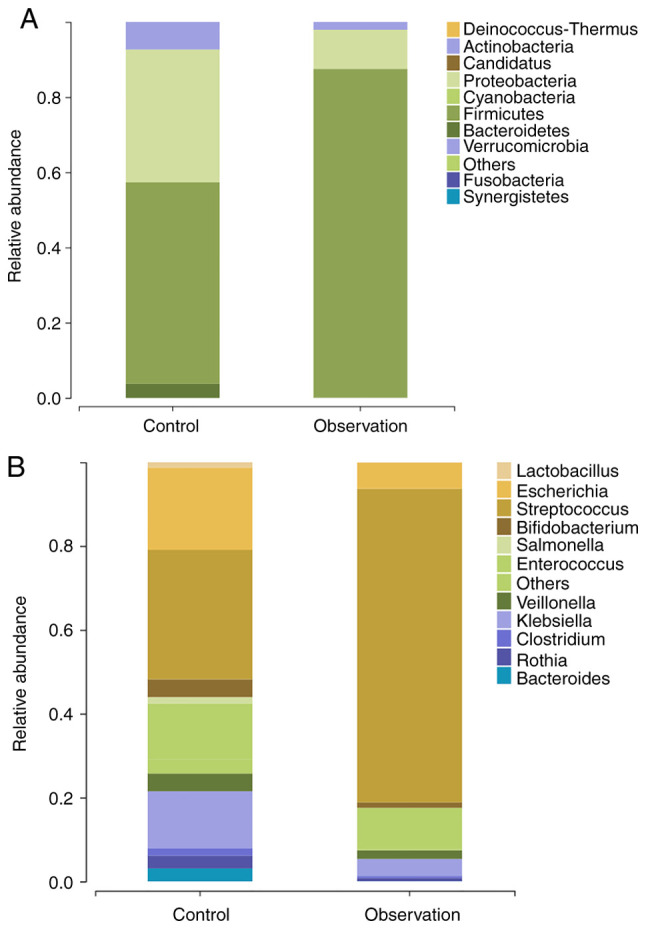
Bacterial composition of gut microbiota in patients with neonatal hyperbilirubinemia before (control group) and after phototherapy (observation group). Distribution of the predominant bacteria at the (A) phylum and (B) levels are shown.
Key differential species selection (biomarkers)
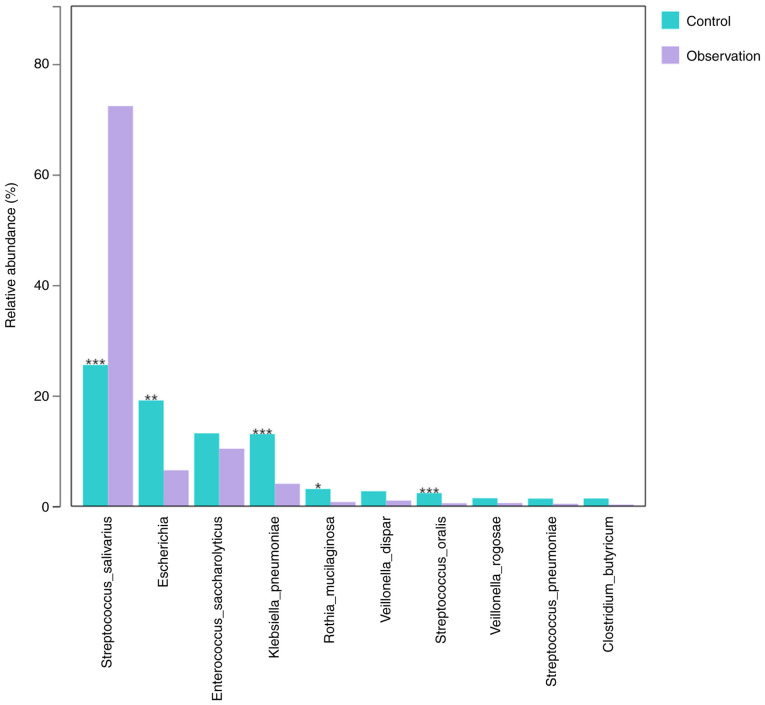
The default (LDA) value was 2.0 and those with scores greater than this value were considered microbial communities with statistical differences. The results revealed significant differences in the microbial communities between the two groups at the phylum, class, order, family and genus levels (Fig. 5). In addition, the key species between the two groups were compared. The results showed that at the species level, the abundance of Streptococcus salivarius (P<0.001) significantly increased, whereas the abundance of Escherichia (P=0.002), Klebsiella pneumoniae (P<0.001), Rothia mucilaginosa (P=0.012) and Streptococcus oralis (P<0.001) significantly decreased (Fig. 6) in the observation group compared to the control group.
Figure 5.
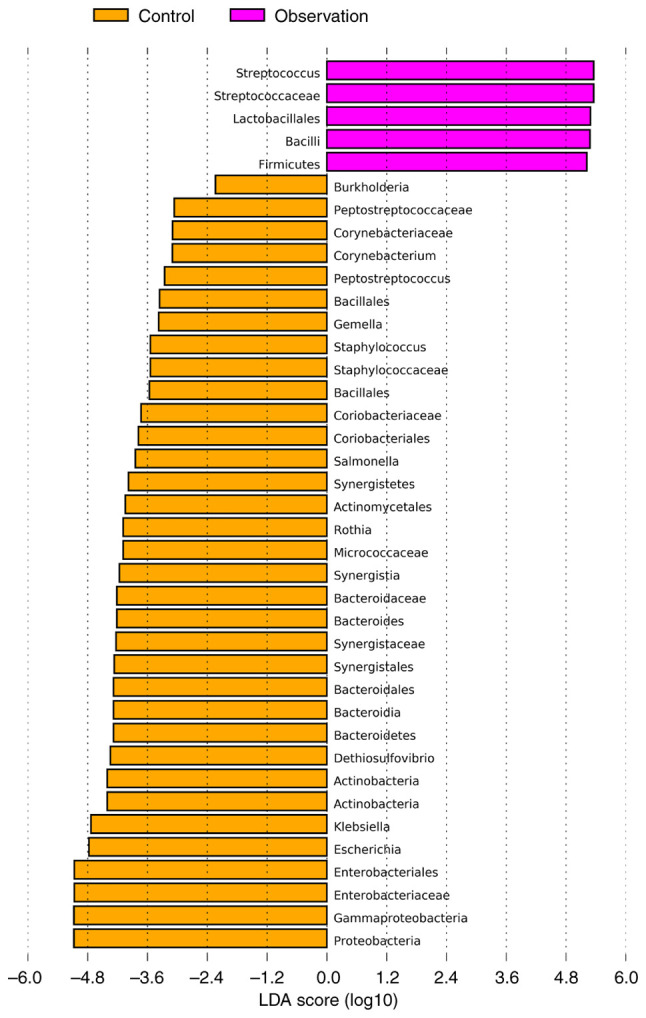
The most differentially abundant taxa between control and observation groups (LDA score >2) which was generated from LEfSe analysis. LDA, linear discriminant analysis; LEfSe, LDA effect size.
Figure 6.
The relative abundance of the top 10 species was compared between the control and observation groups. *P<0.05, **P<0.01 and ***P<0.001 vs. control group. No mark of significance indicates P>0.05.
Discussion
The present study used 16S rDNA amplicon sequencing technology to detect the gut microbiota in newborns with hyperbilirubinemia who received phototherapy. It was observed that phototherapy markedly altered the structure of the gut microbiota, leading to a significant decrease in both diversity and abundance. The changes in the key bacterial species were characterized by an increase in the abundance of S. salivarius and a decrease in the abundance of Escherichia, K. pneumoniae, R. mucilaginosa and S. oralis.
Homeostasis of the gut microbiota is the foundation of a number of physiological activities in the human body, such as food digestion, nutritional metabolism, body defense and development and maturation of the immune system (9-11). Therefore, several diseases are associated with gut microbiota disorders, including NH (5). An increasing number of studies have indicated that the gut microbiota regulates bilirubin metabolism through enterohepatic circulation (12-14). Early evidence from sterile animals suggests that the gut microbiota is necessary for bilirubin metabolism in the intestine (15). The results showed that average fecal urobilin production in sterile rodents was 0 mmol, whereas that in wild rats was 3.4 mmol (15). In one study, when rats were fed clindamycin or neomycin to combat anaerobic bacteria, urobilin disappeared, whereas serum bilirubin continued to rise (16). These data indicate that the gut microbiota are important participants in the bilirubin metabolism. An increase in diversity and abundance is a common manifestation of gut microbiota disorders in NH (17,18). The present study found that 24 h of phototherapy markedly reduced the diversity and abundance of intestinal flora, suggesting that phototherapy may also partially reverse the microflora changes caused by NH.
The increase in the abundance of S. salivarius after phototherapy was unexpected. S. salivarius is a well-known oral probiotic and its formulations have been widely used in clinical practice (19-22). Studies have shown that this bacterium exerts beneficial effects on the gastrointestinal tract. S. salivarius can release bacteriocins (salivary A5 and B) (23-25), thereby antagonizing opportunistic or pathogenic bacteria. As aforementioned, the diversity and abundance of the gut microbiota in newborns with hyperbilirubinemia are increased and contain a number of opportunistic pathogens (17,18). Therefore, the increase in the abundance of S. salivarius after phototherapy may partially improve intestinal defense function. In addition, S. salivarius can strengthen the tight junctions of intestinal epithelial surface cells (26). NH often has high levels of unbound bilirubin, which can not only increase the permeability of the intestinal epithelium, but also reduce the expression of epithelial tight junction proteins, which leads to more bilirubin secretion into the enterohepatic circulation (5,27). Therefore, an increase in the abundance of S. salivarius after phototherapy is beneficial for decreasing the bilirubin levels.
Escherichia, K. pneumoniae, R. mucilaginosa and S. oralis are common opportunistic pathogens that can cause infectious diseases, such as pneumonia, enteritis, peritonitis, arthritis, encephalitis and sepsis (28-33). The abundance of these species was markedly higher in the guts of neonates with hyperbilirubinemia before phototherapy compared with after phototherapy. The incidence of infection is relatively high in newborns owing to their immature immune function. Therefore, reducing the abundance of opportunistic pathogenic bacteria through phototherapy may be beneficial for preventing enterogenous infections in newborns with hyperbilirubinemia. In addition to the risk of infection, opportunistic pathogenic bacteria participate in the intestinal bilirubin metabolism. In a prospective cohort study, Tang et al (34) found a significant decrease in Escherichia abundance in NH after treatment, accompanied by a decrease in fecal β-glucuronidase activity and the two were positively correlated. β-glucosidase can promote the conversion of conjugated bilirubin to unbound bilirubin, which is then absorbed by the intestinal mucosa and returned to the liver, delaying bilirubin excretion (35,36). Therefore, a decrease in the abundance of Escherichia in the intestine after phototherapy is beneficial for the excretion of bilirubin.
The mechanism by which beneficial changes in gut microbiota structure occur in newborns with NH after phototherapy is still unclear. Previous studies have shown that phototherapy can not only directly stimulate the proliferation of a variety of Gram-positive and Gram-negative bacteria, but also indirectly affect the intestinal microbiota by regulating cytokines, transcription factors and metabolites (37-39). However, the light source used in these studies is laser or red light, which is completely different from blue light used for NH. Therefore, further research is needed to clarify whether blue light directly affects the structure of gut microbiota or indirectly by reducing bilirubin concentration.
In summary, the present study demonstrated that phototherapy can alter the structure, diversity and abundance of intestinal flora using 16S rDNA amplicon sequencing. This change mainly manifested as an increase in beneficial bacteria and a decrease in opportunistic bacteria, which may not be related to the side effects of phototherapy. However, the limitation of the present study was that the duration of phototherapy is 24 h, so the results cannot represent the effects of other periods of phototherapy on gut microbiota. In addition, the present study used the latest guidelines for NH developed by the American Academy of Pediatrics as the inclusion criteria and screened for biomarkers before and after phototherapy at the species level (2). These results may provide theoretical assistance for microbiological research on later stages of NH.
Acknowledgements
The authors thank Dr Jing Zhang and Dr Youqing Shen (Department of Pediatrics, Suqian Hospital Affiliated to Xuzhou Medical University, Suqian, China) for their helpful guidance during the writing of the manuscript.
Funding Statement
Funding: The present study was supported by Jiangsu Province Maternal and Child Health Research Project (grant nos. F201941 and F202153) and the Suqian Science and Technology Plan Project (grant no. K202118).
Availability of data and materials
The data generated in the present study may be requested from the corresponding author. All raw reads were deposited in the NCBI Sequence Read Archive database (accession no. PRJNA1096251; https://www.ncbi.nlm.nih.gov/sra/?term=PRJNA1096251).
Authors' contributions
RW, YJ and JY wrote the manuscript and analyzed the data. JQ and SZ designed the study. SL, HY and NS performed the experiments and collected the data. SZ and JQ confirmed the authenticity of the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The parents or guardians of the children provided informed consent and the study design was approved by the Ethics Committee of Suqian Hospital Affiliated to Xuzhou Medical University (approval no. 2021022). All experiments were performed in accordance with guidelines of the declaration of Helsinki 2013.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Par EJ, Hughes CA, DeRico P. Neonatal hyperbilirubinemia: Evaluation and treatment. Am Fam Physician. 2023;107:525–534. [PubMed] [Google Scholar]
- 2.Kemper AR, Newman TB, Slaughter JL, Maisels MJ, Watchko JF, Downs SM, Grout RW, Bundy DG, Stark AR, Bogen DL, et al. Clinical practice guideline revision: Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2022;150(e2022058859) doi: 10.1542/peds.2022-058859. [DOI] [PubMed] [Google Scholar]
- 3.Pu R, Wang Z, Zhu R, Jiang J, Weng TC, Huang Y, Liu W. Investigation of ultrafast configurational photoisomerization of bilirubin using femtosecond stimulated raman spectroscopy. J Phys Chem Lett. 2023;14:809–816. doi: 10.1021/acs.jpclett.2c03535. [DOI] [PubMed] [Google Scholar]
- 4.Shoris I, Gover A, Toropine A, Iofe A, Zoabi-Safadi R, Tsuprun S, Riskin A. ‘Light’ on phototherapy-complications and strategies for shortening its duration, a review of the literature. Children (Basel) 2023;10(1699) doi: 10.3390/children10101699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen K, Yuan T. The role of microbiota in neonatal hyperbilirubinemia. Am J Transl Res. 2020;12:7459–7474. [PMC free article] [PubMed] [Google Scholar]
- 6.Hackmann TJ. Accurate estimation of microbial sequence diversity with distanced. Bioinformatics. 2020;36:728–734. doi: 10.1093/bioinformatics/btz668. [DOI] [PubMed] [Google Scholar]
- 7.Finn DR. A metagenomic alpha-diversity index for microbial functional biodiversity. FEMS Microbiol Ecol. 2024;100(fiae019) doi: 10.1093/femsec/fiae019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall M, Beiko RG. 16S rRNA gene analysis with QIIME2. Methods Mol Biol. 2018;1849:113–129. doi: 10.1007/978-1-4939-8728-3_8. [DOI] [PubMed] [Google Scholar]
- 9.Winston JA, Theriot CM. Diversification of host bile acids by members of the gut microbiota. Gut Microbes. 2020;11:158–171. doi: 10.1080/19490976.2019.1674124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan L, Liu R . The role of diet and gut microbiota interactions in metabolic homeostasis. Adv Biol (Weinh) 2023;7(e2300100) doi: 10.1002/adbi.202300100. [DOI] [PubMed] [Google Scholar]
- 11.Shim JA, Ryu JH, Jo Y, Hong C. The role of gut microbiota in T cell immunity and immune mediated disorders. Int J Biol Sci. 2023;19:1178–1191. doi: 10.7150/ijbs.79430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tuzun F, Kumral A, Duman N, Ozkan H. Breast milk jaundice: Effect of bacteria present in breast milk and infant feces. J Pediatr Gastroenterol Nutr. 2013;56:328–332. doi: 10.1097/MPG.0b013e31827a964b. [DOI] [PubMed] [Google Scholar]
- 13.Samaddar A, van Nispen J, Armstrong A, Song E, Voigt M, Murali V, Krebs J, Manithody C, Denton C, Ericsson AC, Jain AK. Lower systemic inflammation is associated with gut firmicutes dominance and reduced liver injury in a novel ambulatory model of parenteral nutrition. Ann Med. 2022;54:1701–1713. doi: 10.1080/07853890.2022.2081871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou S, Wang Z, He F, Qiu H, Wang Y, Wang H, Zhou J, Zhou J, Cheng G, Zhou W, et al. Association of serum bilirubin in newborns affected by jaundice with gut microbiota dysbiosis. J Nutr Biochem. 2019;63:54–61. doi: 10.1016/j.jnutbio.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 15.Gustafsson BE, Lanke LS. Bilirubin and urobilins in germfree, ex-germfree and conventional rats. J Exp Med. 1960;112:975–981. doi: 10.1084/jem.112.6.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vítek L, Zelenka J, Zadinová M, Malina J. The impact of intestinal microflora on serum bilirubin levels. J Hepatol. 2005;42:238–243. doi: 10.1016/j.jhep.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Guo Q, Liu X, Cui M, Li X, Yang C, Zhao S, Pan L, Peng X, Wang L, Liu P. Characteristics of intestinal microbiota in infants with late-onset breast milk jaundice. Front Nutr. 2023;10(1119768) doi: 10.3389/fnut.2023.1119768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding J, Ma X, Han L, Zhao X, Li A, Xin Q, Lian W, Li Z, Ren H, Ren Z. Gut microbial alterations in neonatal jaundice pre- and post-treatment. Biosci Rep. 2021;41(BSR20210362) doi: 10.1042/BSR20210362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarlin S, Koskela U, Honkila M, Tähtinen PA, Pokka T, Renko M, Tapiainen T. Streptococcus salivarius probiotics to prevent acute otitis media in children: A randomized clinical trial. JAMA Netw Open. 2023;6(e2340608) doi: 10.1001/jamanetworkopen.2023.40608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choudhary P, Kraatz HB, Lévesque CM, Gong SG. Microencapsulation of probiotic Streptococcus salivarius LAB813. ACS Omega. 2023;8:12011–12018. doi: 10.1021/acsomega.2c07721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wescombe PA, Hale JD, Heng NC, Tagg JR. Developing oral probiotics from Streptococcus salivarius. Future Microbiol. 2012;7:1355–1371. doi: 10.2217/fmb.12.113. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Fields FR, Ho M, Marshall-Hudson A, Gross R, Casser ME, Naito M. Safety assessment of Streptococcus salivarius DB-B5 as a probiotic candidate for oral health. Food Chem Toxicol. 2021;153(112277) doi: 10.1016/j.fct.2021.112277. [DOI] [PubMed] [Google Scholar]
- 23.Lawrence GW, McCarthy N, Walsh CJ, Kunyoshi TM, Lawton EM, O'Connor PM, Begley M, Cotter PD, Guinane CM. Effect of a bacteriocin-producing Streptococcus salivarius on the pathogen Fusobacterium nucleatum in a model of the human distal colon. Gut Microbes. 2022;14(2100203) doi: 10.1080/19490976.2022.2100203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hyink O, Wescombe PA, Upton M, Ragland N, Burton JP, Tagg JR. Salivaricin A2 and the novel lantibiotic salivaricin B are encoded at adjacent loci on a 190-kilobase transmissible megaplasmid in the oral probiotic strain Streptococcus salivarius K12. Appl Environ Microbiol. 2007;73:1107–1113. doi: 10.1128/AEM.02265-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wescombe PA, Upton M, Dierksen KP, Ragland NL, Sivabalan S, Wirawan RE, Inglis MA, Moore CJ, Walker GV, Chilcott CN, et al. Production of the lantibiotic salivaricin A and its variants by oral streptococci and use of a specific induction assay to detect their presence in human saliva. Appl Environ Microbiol. 2006;72:1459–1466. doi: 10.1128/AEM.72.2.1459-1466.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cosseau C, Devine DA, Dullaghan E, Gardy JL, Chikatamarla A, Gellatly S, Yu LL, Pistolic J, Falsafi R, Tagg J, Hancock REW. The commensal Streptococcus salivarius K12 downregulates the innate immune responses of human epithelial cells and promotes host-microbe homeostasis. Infect Immun. 2008;76:4163–4175. doi: 10.1128/IAI.00188-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansen TWR, Wong RJ, Stevenson DK. Molecular physiology and pathophysiology of bilirubin handling by the blood, liver, intestine, and brain in the newborn. Physiol Rev. 2020;100:1291–1346. doi: 10.1152/physrev.00004.2019. [DOI] [PubMed] [Google Scholar]
- 28.Komsani MR, Almaghlouth NK, Charla S, Li J, Mileno MD, Neill MA, Hong T, Lonks JR. Escherichia coli meningitis in a 72-year-old woman. R I Med J (2013) 2024;107:12–14. [PubMed] [Google Scholar]
- 29.Cui M, Sun W, Xue Y, Yang J, Xu T. Hepatitis E virus and Klebsiella pneumoniae co-infection detected by metagenomics next-generation sequencing in a patient with central nervous system and bloodstream Infection: A case report. BMC Infect Dis. 2024;24(33) doi: 10.1186/s12879-023-08850-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao C, Zheng Y, Hang Y, Chen Y, Liu Y, Zhu J, Fang Y, Xiong J, Hu L. Risk factors for 30-day mortality in patients with bacteremic pneumonia caused by Escherichia coli and Klebsiella pneumoniae: A retrospective study. Int J Gen Med. 2023;16:6163–6176. doi: 10.2147/IJGM.S447354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ling CW, Sud K, Lee VW, Peterson GM, Van C, Zaidi STR, Patel RP, Castelino RL. Treatment and outcomes of peritonitis due to Rothia species in patients on peritoneal dialysis: A systematic review and multicentre registry analysis. Perit Dial Int. 2023;43:220–230. doi: 10.1177/08968608221140227. [DOI] [PubMed] [Google Scholar]
- 32.Gomila-Sard B, Téllez-Castillo CJ, Sabater-Vidal S, Moreno-Muñoz R. Early prosthetic joint infection due to Streptococcus oralis. Enferm Infecc Microbiol Clin. 2009;27:547–548. doi: 10.1016/j.eimc.2008.12.006. (In Spanish) [DOI] [PubMed] [Google Scholar]
- 33.Cleary E, Boudou M, Garvey P, Aiseadha CO, McKeown P, O'Dwyer J, Hynds P. Spatiotemporal dynamics of sporadic shiga toxin-producing Escherichia coli enteritis, Ireland, 2013-2017. Emerg Infect Dis. 2021;27:2421–2433. doi: 10.3201/eid2709.204021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang W, Lu HY, Sun Q, Xu WM. Characteristics of gut microbiota and its association with the activity of β-glucuronidase in neonates with hyperbilirubinemia. Zhongguo Dang Dai Er Ke Za Zhi. 2021;23:677–683. doi: 10.7499/j.issn.1008-8830.2102039. (In Chinese) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ince Z, Coban A, Peker I, Can G. Breast milk beta-glucuronidase and prolonged jaundice in the neonate. Acta Paediatr. 1995;84:237–239. doi: 10.1111/j.1651-2227.1995.tb13621.x. [DOI] [PubMed] [Google Scholar]
- 36.Osnes T, Sandstad O, Skar V, Osnes M. beta-Glucuronidase in common duct bile, methodological aspects, variation of pH optima and relation to gallstones. Scand J Clin Lab Invest. 1997;57:307–315. doi: 10.3109/00365519709099404. [DOI] [PubMed] [Google Scholar]
- 37.Karu TJ, Tiphlova OA, Letokhov VS, Lobko VV. Stimulation of E. coli growth by laser and incoherent red light. Il Nuovo Cimento D. 1983;2:1138–1144. [Google Scholar]
- 38.Huang YY, Chen ACH, Carroll JD, Hamblin MR. Biphasic dose response in low level light therapy. Dose Response. 2009;7:358–383. doi: 10.2203/dose-response.09-027.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liebert A, Bicknell B, Johnstone DM, Gordon LC, Kiat H, Hamblin MR. ‘Photobiomics’: Can light, including photobiomodulation, alter the microbiome? Photobiomodul Photomed Laser Surg. 2019;37:681–693. doi: 10.1089/photob.2019.4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated in the present study may be requested from the corresponding author. All raw reads were deposited in the NCBI Sequence Read Archive database (accession no. PRJNA1096251; https://www.ncbi.nlm.nih.gov/sra/?term=PRJNA1096251).