INTRODUCTION
The Randomized Comparison of the Clinical Outcomes of Single versus Multiple Arterial Grafts (ROMA) trial (NCT03217006) [1] is the largest randomized trial testing the hypothesis that multiple arterial grafting (MAG) provides superior clinical outcomes compared to single arterial grafting (SAG) in patients undergoing coronary artery bypass surgery (CABG).
Trial activities started in January 2017, the first patient was enrolled in January 2018, and the last one (# 4375) was enrolled on 14 April 2023.
Here, we summarize the first 7 years of ROMA activities, with particular focus on those aspects that may potentially be relevant for trialists interested in designing or implementing other large cardiac surgery trials. As the trial is ongoing and the primary analysis has not been performed yet, no outcomes or individual patient data are provided.
BACKGROUND
The idea of a multiple arterial grafting trial
The stimulus to design a second large MAG trial came after evaluating the results of the 5-year analysis of the Arterial Revascularization Trial (ART) in 2016 [2]. Following almost 50 years of observational MAG versus SAG comparisons that in general favoured MAG [3] and in the presence of convincing evidence of better mid- and long-term patency rates of arterial versus venous grafts [4] ART was generally expected to confirm the superiority of MAG and to move the field towards its larger adoption [5]. The 5-year analysis of ART was surprising due to the almost perfect overlap of the survival and event-free survival curves of the 2 treatment arms [2]; in addition, the frequent utilization of the radial artery as an additional arterial graft in the SAG group, and the high crossover rate (that likely reflected the lack of confidence of the ART surgeons with the use of the bilateral internal thoracic artery), raised key concerns over whether ART could meaningfully test the MAG hypothesis when the final data were available. This was the key motivation to design a new MAG trial, building on the lessons learned from ART—the collaboration with the ART investigators at the early stage was very important to inform the design of ROMA (Table 1).
Table 1:
Key differences in trial design between ROMA and ART
| ROMA | ART | |
|---|---|---|
| Primary outcome | Major adverse cardiovascular events (all-cause mortality, stroke, non-procedural myocardial infarction, repeat revascularization) | All-cause mortality |
| Experimental intervention | Multiple arterial grafting | Bilateral internal thoracic artery grafting |
| Population | 70 years or younger |
|
| Power | Event-driven | Underpowered |
| Deliverability of intervention |
|
|
ART: Arterial Revascularization Trial; ROMA: Randomized Comparison of the Clinical Outcomes of Single versus Multiple Arterial Grafts.
Protocol development
In early January 2017, after consultation with several leaders in the CABG field who were generally supportive of another MAG trial, protocol development started [1]. The first draft was written by the 2 trial Principal Investigators over 2 months and then circulated to a larger group of experts (many of whom became part of the trial Steering Committee). The protocol was finalized by June 2017 and subsequently submitted for publication in the European Journal of Cardio-Thoracic Surgery [1]. In October 2017, the trial was officially presented at the 31st annual meeting of the European Association of Cardio-thoracic Surgery, whose support over the course of the following years was critical in assuring continuous exposure of ROMA and maximizing the interest in the trial (a very important reason for its overall success).
Trial logistics considerations
At the beginning of trial activities, the key decision to start enrollment before securing external funding was made. This decision was motivated by (i) the long turnaround time for traditional grant applications, (ii) the consideration that any ROMA grant application could be seen with skepticism by funding agencies due to feasibility concerns and (iii) the lack of interest by industry in funding a trial testing surgical strategies rather than a device or drug.
Trial activities started using internal funding from the Department of Cardiothoracic Surgery of Weill Cornell Medicine to support the coordinating centre and all start-up procedures. Twenty-five international sites agreed to start enrollment without external support to generate preliminary data for subsequent grant applications (Vanguard centres).
Due to the size of the trial and budget constraints at most funding agencies, ROMA was designed as a pragmatic trial, minimizing deviation from standard of care and burden on the local study team. Quality of life, neurocognitive function and imaging outcomes, although admittedly important, were not included in the protocol and left to eventual ancillary applications to be submitted once the trial was successfully launched.
Funding
In September 2017, an application was submitted to the Canadian Institutes of Health Research (CIHR) to fund the ROMA pilot phase and it was awarded funding. The excellent results of the pilot phase were critical in subsequently obtaining funding from the same agency for the full-scale trial. Once funding for the main trial was secured and trial activity was ongoing, separate applications were successfully submitted to the National Institutes of Health to fund a quality of life, a neurocognitive and an echocardiographic study.
Trial activity
Regulatory and contract activities started in the summer of 2017 and the first patient was enrolled on 8 January 2018 in Innsbruck.
The pilot phase was aimed at enrolling 10% of the trial sample size in 1 year to prove feasibility and provide important data on recruitment rate and protocol adherence (the latter was considered particularly important due to the high crossover rate seen in ART). The planned 430 patients were enrolled in <9 months (from January to early September 2018) and the enrollment rate, protocol adherence, and completion of follow-up were all consistent with protocol estimates or even more favourable than anticipated. At the end of the pilot phase, the trial transitioned to the full-scale phase without interruption and the patients enrolled in the pilot phase were included in the main trial.
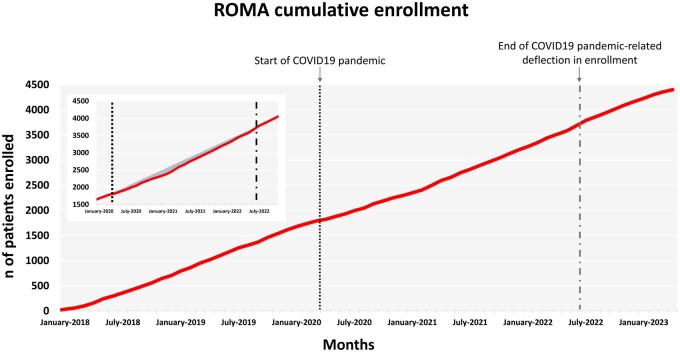
Over the course of 5 years, 81 centres in 21 countries were open and the enrollment of the planned 4375 patients was completed on 14 April 2023 (Fig. 1).
Figure 1:
ROMA cumulative enrollment and the effect of the COVID-19 pandemic (panel). COVID-19: coronavirus disease 2019; ROMA: Randomized Comparison of the Clinical Outcomes of Single versus Multiple Arterial Grafts.
The primary analysis of ROMA is event-driven and at the moment it is difficult to predict with good precision when it will be performed. Based on current event accrual, the needed number of events will occur in 2027, but those estimates are constantly revised and may change substantially with the accumulation of more data.
Crossover rate
The high crossover rate observed in ART was a critical concern when designing ROMA. In cardiac surgery trials, crossover, in particular from experimental to control, is often a sign of lack of confidence of participating surgeons with the tested interventions, and we have shown how a crossover rate from experimental to intervention ≥7% is generally associated with a neutral outcome [6].
In ROMA, the intraoperative electronic case report form captures crossover events in great detail (including timing, reason, and technical description); every episode of crossover generates an automatic alert to the research team and the clinical coordinating centre. The crossover rate for each participating site was monitored continuously over the course of enrollment and sites received detailed reminders of the importance of minimizing crossover at each event; sites with excessive crossover rate (over 2 standard deviations above the mean of the trial or 2 consecutive events) were temporarily closed until a remediation plan was agreed upon with the local team. Most of the crossover episodes were due to logistics (ie, miscommunication between the research team and the operating surgeon, randomization before assessment of the availability of both arterial and venous conduits) and could be easily avoided with a remediation plan that generally included only small corrections to the local study procedures. The final protocol non-adherence in the ROMA trial was well below the pre-specified 5% rate.
In ROMA, the choice between the second internal thoracic artery and the radial artery in the MAG arm as well as all the key technical details of the CABG operation were left to the operating surgeon’s discretion; the fact that the operating surgeons had the flexibility to choose the technique that they considered the most appropriate and the close supervision of protocol adherence were key reasons for the low crossover rate. In addition, this design will allow important comparisons between the 2 complementary arterial conduits used in the most appropriate scenario based on the operating surgeon’s judgement.
Surgeon experience
Because of the described concerns regarding the deliverability of the MAG operation and the high crossover rate seen in ART, ROMA surgeons were selected either based on their level of experience with MAG (at least 250 cases as primary operator—this threshold is 5 times higher than the one used in ART) or personal vetting by the trial Principal Investigators. While this may affect the external validity and the generalizability of ROMA results, it does minimize the risk that technical factors and suboptimal delivery, rather than biological reasons, may determine the trial outcome.
Coronavirus disease 2019 pandemic
The coronavirus disease 2019 pandemic occurred when ROMA was at approximately half of enrollment (see Fig. 1). The reaction of the ROMA coordinating centre to the widespread reduction of clinical research activity around the world was to increase the number of participating sites from the planned 50 to over 80. This was done to increase geographic diversity, as different world regions were impacted differently and at different times by the pandemic, but also with the aim of enrolling at a faster pace once the pandemic was resolved. The pragmatic design of the trial and the minimal deviation from local standards of care allowed most sites to remain active even during 2020 and 2021 so that, while many cardiovascular trials were prematurely stopped or severely delayed [7, 8], ROMA enrollment experienced only a modest reduction (see Fig. 1). In the final analysis plan, strategies to assess a potential coronavirus disease 2019 effect of ROMA outcomes will be included.
Current relevance of the multiple arterial grafting question
Since 2017, when the ROMA protocol was designed [1], only few MAG trials and meta-analyses have published their results. The Radial Artery Database International Alliance Investigators (RADIAL) have published the extension 10-year follow-up that confirmed a statistically significant reduction in the risk of cardiac events with the use of the radial artery compared with the saphenous vein [hazard ratio (HR) 0.73, 95% confidence interval (CI) 0.61–0.88] [9]. The Radial Artery Patency and Clinical Outcomes (RAPCO) trial investigators have published the 10-year (primary analysis) and 15-year (extension follow-up) results, showing a better patency rate at 10 years for the radial artery compared with the saphenous vein (HR for graft failure 0.40, 95% CI 0.15–1.00) and the right internal thoracic artery (HR for graft failure 0.45, 95% CI 0.23–0.88) and a statistically significant reduction of the risk of cardiac events at 15 years using the radial artery (HR 0.71, 95% CI 0.52–0.98 versus the saphenous vein and 0.74, 95% CI 0.55–0.97 versus the right internal thoracic artery) [10, 11]. The 15-year extension follow-up of the ART trial is currently ongoing. Current myocardial revascularization guidelines assign a Class I recommendation to the use of the radial artery and a Class IIA recommendation to the use of the right internal thoracic artery, both with low level of evidence (Level of Evidence B) [12, 13].
Overall, the MAG question is still highly relevant, and no definitive evidence has been published since the start of ROMA, so that the trial results remain very important for patients and providers.
ROMA: Women and other trials: establishment of the ROMA network
The ROMA trial provided a unique opportunity to leverage the existing trial infrastructure including clinical trial unit, database, case report forms, randomization system, site training resources, informed consent forms, regulatory approvals, contractual agreements, Clinical Events Committee processes/personnel, network of participating sites and study coordinators to realize ROMA: Women, the first cardiac surgery trial dedicated to women, that is currently ongoing and actively recruiting [14, 15]. This is particularly relevant as there are known sex differences in CABG outcomes and in the MAG effect that have only been marginally investigated [16].
With over 100 sites currently participating and an efficient infrastructure in place for regulatory and contract activities as well as database development, the ROMA network offers an ideal platform to rapidly launch new cardiac surgery trials. The network is currently being leveraged for several large international randomized studies on different topics, such as The Canadian CABG or PCI in Patients with Ischaemic Cardiomyopathy (STICH3C) trial (NCT05427370), the One-Month DAPT (Dual Antiplatelet Therapy) in CABG Patients (ODIN) trial (NCT05997693) and the upcoming Effect of left posterior Pericardiotomy In Cardiac surgery (EPIC) trial.
CONCLUSIONS
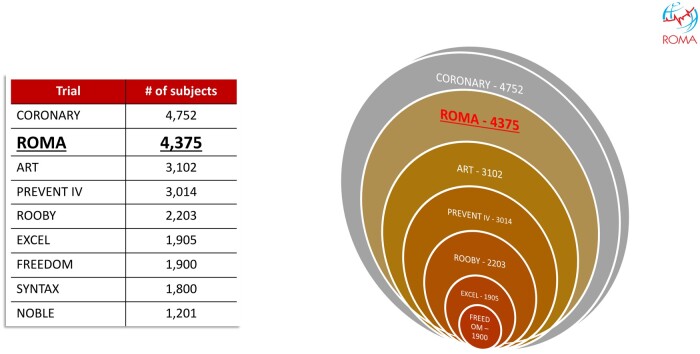
In 7 years, ROMA completed enrollment of over 4300 patients and became the second largest CABG trial (Fig. 2) and a global network for large cardiac surgery clinical trials (Table 2). The results of ROMA will provide a definitive answer to the MAG question and the ROMA network is being now leveraged for other large cardiac surgery trials; we hope that the development of the ROMA network may be used as a model by investigators interested in clinical trials even in other fields.
Figure 2:
Large contemporary CABG trials. ART: Arterial Revascularization Trial; CORONARY: Coronary Artery Bypass Surgery Off or On Pump Revascularization Study; EXCEL: Evaluation of XIENCE versus Coronary Artery Bypass Graft Surgery for Effectiveness of Left Main Revascularizaton trial; FREEDOM: Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease trial; NOBLE: Nordic Baltic British Left Main Revascularization trial; PREVENT IV: The PRoject of Ex vivo Vein graft ENgineering via Transfection IV trial; ROOBY: Randomized On/Off Bypass trial; ROMA: Randomized Comparison of the Clinical Outcomes of Single versus Multiple Arterial Grafts trial; SYNTAX: Synergy Between PCI with Taxus and Cardiac Surgery trial.
Table 2:
The ROMA trial participating centres by continent
| Hospital | City | Country | Local principal investigator |
|---|---|---|---|
| Asia | |||
| Jilin Heart Hospital | Changchun | China | Massimo Lemma |
| Fuwai Hospital | Beijing | China | Zhe Zheng |
| Teda Hospital | Tianjin | China | Guo-Wei He |
| National Taiwan University Hospital | Taipei City | Taiwan | Yi-Sharng Chen |
| Ruijin Hospital Shanghai Jiao Tong USM | Shanghai | China | Qiang Zhao |
| Star Hospital | Hyderabad | India | Gopi Chand Mannam, Lokeswara Rao Sajja |
| G Kuppuswamy Naidu Memorial Hospital | Coimbatore | India | Chandra Padmanabhan |
| Saitama Medical University | Saitama | Japan | Hiroyuki Nakajima |
| Tokyo Medical and Dental University | Tokyo | Japan | Hirokuni Arai |
| National University Hospital | Singapore | Singapore | Theodoros Kofidis |
| Severance Cardiovascular Hospital | Seoul | South Korea | Kyung-Jong Yoo |
| Seoul National University Hospital | Seoul | South Korea | Ho Young Hwang |
| Europe | |||
| MU Innsbruck | Innsbruck | Austria | Elfriede Ruttman-Ulmer |
| MU Vienna | Vienna | Austria | Sigrid Sandner |
| Krankenhaus Nord Vienna | Vienna | Austria | Berhard Winkler |
| MU Graz | Graz | Austria | Daniel Zimpfer |
| University Hospital Dubrava | Zagreb | Croatia | Igor Rudez |
| University Hospital Hradec Kralove | Hradec Kralove | Czech Republic | Jan Vojacek |
| General University Hospital Prague | Prague | Czech Republic | Tomas Prskavec |
| University Hospital of Giessen and Marburg | Giessen | Germany | Andreas Boening |
| HDZ NRW Bad Oeynhausen | Bad Oeynhausen | Germany | Marcus-Andre Deutsch |
| Robert-Bosch-Hospital Stuttgart | Stuttgart | Germany | Marc Albert |
| University Hospital Erlangen | Erlangen | Germany | Ehab Nooh |
| Heart Center Leipzig | Leipzig | Germany | Michael Borger |
| Duisburg Heart Center | Duisburg | Germany | Jochen Borgermann |
| Essen University | Essen | Germany | Matthias Klaus Thielmann |
| Frankfurt-Goethe University | Frankfurt | Germany | Tomas Holubec |
| Krankenhaus der Barmherzigen Bruder Trier | Trier | Germany | Terrence John Donovan |
| Duesseldorf University Hospital | Dusseldorf | Germany | Alexander Assman |
| Jena University Hospital | Jena | Germany | Torsten Doenst |
| University Hospital of Goettingen | Goettingen | Germany | Bernhard Danner |
| University Herzzentrum Freiburg | Freiburg im Breisgau | Germany | Martin Czerny |
| Kerckhoff-Klinik Bad Nauheim | Bad Nauheim | Germany | Yeong-Hoon Choi |
| European Hospital | Roma | Italy | Ruggero De Paulis |
| Fondazione Poliambulanza | Brescia | Italy | Gianni Troise |
| Anthea Hospital | Bari | Italy | Giuseppe Nasso |
| Ospedale Le Molinette | Turin | Italy | Mauro Rinaldi |
| Universita Cattolica del Sacro Cuore | Rome | Italy | Massimo Massetti |
| Maria Cecilia Hospital | Cotignola | Italy | Alberto Tripodi |
| Humanitas Gavazzeni | Bergamo | Italy | Alfonso Agnino |
| Maastricht University Medical Center | Maastricht | Netherlands | Roberto Lorusso |
| Medical University of Silesia—Katowice | Katowice | Poland | Marek Deja |
| Zbigniew Religa Lower Silesian Heart Disease Centre—Medinet | Zabrze | Poland |
|
| University Hospital Coimbra | Coimbra | Portugal |
|
| Centro Hospitalar e Universitario Sao Joao | Porto | Portugal | Adelino Leite-Moreira |
| Centro Hospitalar Lisboa Ocidental | Lisbon | Portugal | Miguel Sousa Uva |
| Dedinje Cardiovascular Institute | Belgrade | Serbia | Milan Milojevic |
| Hospital Universitario del Vinalopo | Alicante | Spain | Jose Albors Martin |
| Hospital Clinic de Barcelona | Barcelona | Spain | Jorge Alcocer |
| Hospital Clinico de San Carlos | Madrid | Spain | Paula Campelos |
| NavarraBiomed | Pamplona | Spain | Javier De Diego Candela |
| North America | |||
| Universite Laval Quebec (CRIUCPQ) | Quebec | Canada | Mohamed Marzouk |
| Hamilton General Hospital | Hamilton | Canada | Andre Lamy |
| Sunnybrook Health Science | Toronto | Canada | Stephen Fremes |
| London Health Sciences Ontario | London | Canada | David Nagpal |
| University of Ottawa Heart Institute | Ottawa | Canada | Marc Ruel |
| Toronto General Hospital | Toronto | Canada | Vivek Rao |
| McGill University Health | Montreal | Canada | Kevin Lachapelle |
| University Hospital of Montreal (CHUM) | Montreal | Canada | Nicolas Noiseux |
| St. Boniface General Hospital | Winnipeg | Canada | Nitin Ghorpade |
| Montreal Heart Institute | Montreal | Canada | Philippe Demers |
| Health Sciences North | Sudbury | Canada | Bindu Bittira |
| Horizon Health Network—Saint John Regional Hospital | Saint John | Canada | Craig Brown |
| St. Michael's Hospital | Toronto | Canada | Gianluigi Bisleri |
| Weill Cornell Medicine—New York Presbyterian | New York | United States | Mario Gaudino |
| New York Presbyterian—Queens | New York | United States | Charles Mack |
| Northwell Health | New York | United States | Nirav Patel |
| Cleveland Clinic Foundation | Cleveland | United States | Faisal Bakaeen |
| Allegheny General Hospital | Pittsburgh | United States | Scott Halbreiner |
| Baystate Health | Springfield | United States | Daniel Engelman |
| Mount Sinai Hospital—St. Luke's | New York City | United States | John Puskas |
| Nebraska Heart Hospital | Lincoln | United States | Omar Nass |
| University of Nebraska Medical Center | Omaha | United States | Aleem Siddique |
| University of Colorado Health-Anschutz Medical Center | Denver | United States | Jessica Rove |
| Ohio State University Wexner Medical Center | Columbus | United States | John Bozinovski |
| South America | |||
| Heart Institute Medical School of the University of Sao Paulo | Sao Paulo | Brazil | Fabio Biscegli |
| Porto Alegre RS | Porto Alegre | Brazil | Renato Kalil |
| Instituto Dante Pazzanese | Sao Paulo | Brazil | Renato Tabellini |
ROMA: Randomized Comparison of the Clinical Outcomes of Single versus Multiple Arterial Grafts.
Contributor Information
Mario Gaudino, Department of Cardiothoracic Surgery, Weill Cornell Medicine, New York, NY, USA.
Massimo Lemma, Department of Cardiac Surgery, Jilin Heart Hospital, Changchun, China.
Sigrid Sandner, Department of Cardiac Surgery, Medical University of Vienna, Vienna, Austria.
Andreas Boening, Department of Cardiovascular Surgery, University Hospital Giessen, Giessen, Germany.
Lamia Harik, Department of Cardiothoracic Surgery, Weill Cornell Medicine, New York, NY, USA.
Marc Albert, Department of Cardiovascular Surgery, Robert-Bosch Hospital, Stuttgart, Germany.
Jose Albors Martin, Department of Cardiac Surgery, Hospital Universitario del Vinalopo, Alicante, Spain.
Jorge Alcocer, Department of Cardiac Surgery, Hospital Clinic de Barcelona, Barcelona, Spain.
John H Alexander, Duke Clinical Research Institute, Division of Cardiology, Duke University, Durham, NC, USA.
Deepak L Bhatt, Department of Medicine, Mount Sinai Fuster Heart Hospital, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Nikolaos Bonaros, Department of Cardiac Surgery, Medical University of Innsbruck, Innsbruck, Austria.
Michael Borger, Department of Cardiac Surgery, Leipzig Heart Center, Leipzig, Germany.
Bernhard C Danner, Department of Thoracic and Cardiovascular Surgery, University Hospital of Goettingen, Goettingen, Germany.
Piroze Davierwala, Department of Surgery, Division of Cardiac Surgery, Toronto General Hospital, Toronto, ON, Canada.
Marek A Deja, Department of Cardiac Surgery, Medical University of Silesia-Katowice, Katowice, Poland.
Ruggero De Paulis, Department of Cardiac Surgery, European Hospital, Unicamillus University, Rome, Italy.
Marcus-Andre Deutsch, Department of Thoracic and Cardiovascular Surgery, HDZ NRW, University Hospital Ruhr-University Bochum, Bad Oeynhausen, Germany.
Marcus Flather, Department of Medicine, Norwich Medical School, University of East Anglia, Norwich, UK.
Pieter Kappetein, Medtronic Bakken Research Center, Maastricht, Netherlands.
Paul Kurlansky, Department of Surgery, Columbia University College of Physicians and Surgeons, New York, NY, USA.
Andre Lamy, Department of Surgery, McMaster University, Hamilton, ON, Canada.
Roberto Lorusso, Department of Cardio-Thoracic Surgery, Maastricht University Medical Center and Cardiovascular Research Maastricht, Maastricht, Netherlands.
Gopi Chand Mannam, Department of Cardiothoracic Surgery, Star Hospitals, Hyderabad, India.
Mohamed Marzouk, Department of Cardiovascular Surgery, IUCPQ, Universite Laval, Quebec City, QC, Canada.
Ruth Masterson Creber, Columbia University School of Nursing, New York, NY, USA.
Milan Milojevic, Department of Cardiac Surgery and Cardiovascular Research, Dedinje Cardiovascular Institute, Belgrade, Serbia; Department of Cardiothoracic Surgery, Erasmus MC, Rotterdam, Netherlands.
Giuseppe Nasso, Department of Cardiac Surgery, Anthea Hospital, Bari, Italy.
Nirav Patel, Department of Cardiovascular and Thoracic Surgery, Northwell Health, New Hyde Park, NY, USA.
Ivana Petrovic, Department of Cardiosurgery, Center of Excellence, Dedinje Cardiovascular Institute, Belgrade, Serbia.
Eduard Quintana, Department of Cardiac Surgery, Hospital Clinic de Barcelona, Barcelona, Spain.
Lokeswara Rao Sajja, Department of Cardiothoracic Surgery, Star Hospitals, Hyderabad, India.
Mauro Rinaldi, Department of Cardiac Surgery, University of Turin, Turin, Italy.
Lisa Rong, Department of Anesthesia, Weill Cornell Medicine, New York, NY, USA.
Igor Rudez, Department of Cardiac and Transplant Surgery, Dubrava University Hospital, Zagreb, Croatia.
Marc Ruel, Division of Cardiac Surgery, University of Ottawa Heart Institute, Ottawa, ON, Canada.
Elfriede Ruttmann-Ulmer, Department of Cardiac Surgery, Medical University of Innsbruck, Innsbruck, Austria.
Pierre Voisine, Division of Cardiac Surgery, University of Ottawa Heart Institute, Ottawa, ON, Canada.
Qiang Zhao, Department of Cardiac Surgery, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China.
Zhe Zheng, Department of Cardiac Surgery, Fuwai Hospital, Beijing, China.
Stephen E Fremes, Division of Cardiac Surgery, Schulich Heart Centre, Department of Surgery, Sunnybrook Health Sciences Centre, University of Toronto, Toronto, ON, Canada.
FUNDING
The ROMA trial receives grant support from the National Institutes of Health (ROMA Quality of Life: 1 R01HL152021-01 and ROMA Cognition: 1R01NS123639-01) and the Canadian Institutes of Health and Research (ROMA main trial: PJT-162085).
Conflict of interest: none declared.
DATA AVAILABILITY
All relevant data are within the manuscript and its Supporting Information files.
REFERENCES
- 1. Gaudino M, Alexander JH, Bakaeen FG, Ballman K, Barili F, Calafiore AM. et al. Randomized comparison of the clinical outcome of single versus multiple arterial grafts: the ROMA trial—rationale and study protocol. Eur J Cardiothorac Surg 2017;52:1031–40. [DOI] [PubMed] [Google Scholar]
- 2. Taggart DP, Altman DG, Gray AM, Lees B, Gerry S, Benedetto U. et al. ; ART Investigators. Randomized trial of bilateral versus single internal-thoracic-artery grafts. N Engl J Med 2016;375:2540–9. [DOI] [PubMed] [Google Scholar]
- 3. Gaudino M, Bakaeen F, Benedetto U, Rahouma M, Di Franco A, Tam DY. et al. Use rate and outcome in bilateral internal thoracic artery grafting: insights from a systematic review and meta‐analysis. JAHA 2018;7:e009361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Benedetto U, Raja SG, Albanese A, Amrani M, Biondi-Zoccai G, Frati G.. Searching for the second best graft for coronary artery bypass surgery: a network meta-analysis of randomized controlled trials. Eur J Cardiothorac Surg 2015;47:59–65; discussion 65. [DOI] [PubMed] [Google Scholar]
- 5. Persson M, Sartipy U.. Bilateral versus single internal thoracic artery grafts. Curr Cardiol Rep 2018;20:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gaudino M, Fremes SE, Ruel M, Di Franco A, Di Mauro M, Chikwe J et al Prevalence and impact of treatment crossover in cardiac surgery randomized trials: a meta-epidemiologic study. J Am Heart Assoc 2019;8:e013711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McNally EM, Elkind MSV, Benjamin IJ, Chung MK, Dillon GH, Hernandez AF. et al. Impact of the COVID-19 pandemic on cardiovascular science: anticipating problems and potential solutions: a presidential advisory from the American Heart Association. Circulation 2021;144:e461–e471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Audisio K, Lia H, Robinson N, Rahouma M, Soletti G, Cancelli G. et al. Impact of the COVID-19 pandemic on non-COVID-19 clinical trials. JCDD 2022;9:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gaudino M, Benedetto U, Fremes S, Ballman K, Biondi-Zoccai G, Sedrakyan A. et al. ; RADIAL Investigators. Association of radial artery graft vs saphenous vein graft with long-term cardiovascular outcomes among patients undergoing coronary artery bypass grafting: a systematic review and meta-analysis. JAMA 2020;324:179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buxton BF, Hayward PA, Raman J, Moten SC, Rosalion A, Gordon I. et al. ; RAPCO Investigators. Long-Term Results of the RAPCO Trials. Circulation 2020;142:1330–8. [DOI] [PubMed] [Google Scholar]
- 11. Hamilton GW, Raman J, Moten S, Matalanis G, Rosalion A, Dimagli A. et al. Radial artery vs. internal thoracic artery or saphenous vein grafts: 15-year results of the RAPCO trials. Eur Heart J 2023;44:ehad108–2408. [DOI] [PubMed] [Google Scholar]
- 12. Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM. et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022;145:e4–e17. [DOI] [PubMed] [Google Scholar]
- 13. Sousa-Uva M, Neumann F-J, Ahlsson A, Alfonso F, Banning AP, Benedetto U. et al. ; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur J Cardiothorac Surg 2019;55:4–90. [DOI] [PubMed] [Google Scholar]
- 14. Gaudino M, Fremes SE, Mehran R, Bairey Merz N; on behalf of the ROMA:Women Steering Committee and Investigators. ROMA-Women: innovative approaches for the first cardiac surgery trial in women. Circulation 2023;148:1289–91. [DOI] [PubMed] [Google Scholar]
- 15. Gaudino M, Bairey Merz CN, Sandner S, Creber RM, Ballman KV, O'Brien SM. et al. Randomized Comparison of the Outcome of Single Versus Multiple Arterial Grafts trial (ROMA): women—a trial dedicated to women to improve coronary bypass outcomes. J Thorac Cardiovasc Surg 2023;167:1316–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gaudino M, Di Franco A, Cao D, Giustino G, Bairey Merz CN, Fremes SE. et al. Sex-related outcomes of medical, percutaneous, and surgical interventions for coronary artery disease. J Am Coll Cardiol 2022;79:1407–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.