Abstract
Background:
Sublingual atropine is an effective treatment of clozapine-induced hypersalivation. This study aims to investigate the pharmacokinetics of atropine after sublingual and oral administration and study the dose effect of atropine on saliva secretion.
Methods:
An interventional cross-over clinical trial where participants received 0.6 mg and 1.2 mg atropine sulfate sublingual solution and 0.6 mg oral tablet. Atropine plasma concentration was measured over 9 hours with validated LC-MS/MS method. Atropine effects on saliva secretion rate, visual acuity and accommodation, and vital signs were assessed.
Results:
Four clozapine-treated and three healthy participants were enrolled in the study. The area under the atropine plasma concentration-time curve (AUC0-∞) was highest after the 1.2 mg sublingual solution administration in comparison with 0.6 mg tablet or sublingual solution (8.58±1.66 µg.L-1.h vs. 4.65±1.29 vs. 2.98±0.73 µg.L-1.h, respectively). The Cmax for the 0.6 mg and 1.2 mg sublingual solutions was 1.11±0.99 and 1.76±0.62 µg.L-1, and tmax was 2.18±0.59 and 1.9±0.71 h, respectively. In comparison with the 0.6 mg sublingual solution dose, the saliva secretion reduction was larger after the oral tablet administration (-40% (-59, -22%) vs. -69% (-80, -57)) and largest after the 1.2 mg sublingual solution administration (-79% (-93,-64)).
Conclusion:
Both the sublingual and oral atropine are effective in reducing the saliva secretion however at a lower plasma concentration after sublingual administration, with a dose-dependent effect. Both have significantly reduced the blood pressure and pulse rate over 3 hours without significant changes in vision. No major safety concerns were reported.
Keywords: Atropine, clozapine, hyoscyamine, hypersalivation, sialorrhea
Main Points
A highly sensitive and specific atropine assay was developed and applied for the measurement of atropine plasma concentration after a small sublingual and oral dose administration (limit of quantification is 0.025 µg.L-1)
Both sublingual and oral atropine, at the 0.6 mg and 1.2 mg doses tested, significantly reduced the saliva secretion with no significant effect on blood pressure or vision, or an increase in pulse rate.
Sublingual atropine significantly reduced the daytime saliva secretion in clozapine-treated and healthy adults in a dose dependent manner and at approximately 60% systemic exposure of that of the oral tablet.
Introduction
Clozapine Induced Hypersalivation (CIH) is a commonly reported adverse effect in patients with well-described mental and physical adverse consequences.1 A number of management strategies have been proposed to mitigate this adverse effect, including the administration of the anticholinergic drug atropine.
In a recent study, we found sublingual atropine sulfate solution to significantly reduce the saliva secretion in clozapine-treated patients when used for night-time hypersalivation.2 The successful use of atropine in the treatment of CIH was also reported in the systematic review of Van der Poorten et al.3
Atropine is an anticholinergic medication demonstrating nonselective blockade of muscarinic receptors. Nevertheless, in small oral and injectable doses, atropine has been found to have cholinomimetic effects.4 The pharmacokinetic profile of atropine after parenteral administration is described in multiple reports.5 Only one study by Kanto et al6 has investigated the plasma concentration of atropine after sublingual administration6 where three doses of sublingual atropine sulfate were administered with the largest dose of 0.07 mg/kg compared to a 0.02 mg/kg dose after subcutaneous and intramuscular administration. The participants were 12 women undergoing caesarean section under general anaesthesia. The short duration of blood sampling (3 h) is one major limitation of the study and the high dose tested is unlikely to be used for the treatment of CIH.
As for orally administered atropine, the pharmacokinetics in adults was investigated by Beermann et al7 using radiolabelled atropine. Two studies have investigated the oral absorption of atropine in children.8,9
There is a lack of information about the pharmacokinetics of sublingual atropine that can guide the dose selection of an effective yet safe doses of sublingual atropine, and the dose-response relationship on saliva secretion. Another investigated indication of sublingual atropine is in the treatment of organophosphate poisoning as an alternative to IM administration.10 Pharmacokinetic studies utilizing precise analytical methods to investigate atropine disposition in adults are warranted.
This study aimed to investigate the atropine pharmacokinetics, effects on daytime saliva flow, and the dose-effect relationship in clozapine-treated adults and compare that to healthy adults. The atropine effect on vision (acuity, accommodation and convergence), blood pressure and pulse rate were also investigated between the different doses.
Methods
Analytical Methods
The analytical methods were developed and validated at the Chemistry Laboratory at Royal Prince Alfred Hospital, Sydney, Australia.
Materials
Atropine was purchased from Sigma-Aldrich (Atropine A0132, purity ≥ 99%, Castle Hill, Australia). A second source of atropine (A794630, Purity=98%) as well as the internal standard, Atropine-d5 (A794627) were purchased from Toronto Research Chemicals (TRC, Supplied by PM Separations, Brisbane, Australia). Drug-free human plasma was sourced from the Blood Bank laboratory at Royal Prince Alfred Hospital.
Equipments
The chromatographic instrumentation used is described in Table 1.
Table 1.
LC-MS/MS Instrument Parameters
| Instrument | Shimadzu Nexera UHPLC system (Shimadzu Scientific, Rydalmere, Australia) |
| Column | ACE Excel C18-AR (50 mm x 2.1 mm x 1.7 µm particle size, Advanced Chromatography Technologies LTD, UK) with a Rheodyne in-line particulate filter |
| Column Temperature | 30 °C |
| Mobile Phase A | 0.01M ammonium formate buffer in water (pH=3.5) |
| Mobile Phase B | 100% methanol |
| Mobile Phase Profile | Linear gradient from 30% B at 0 min to 50% B at 4 min. These conditions were maintained for 2 min and then the mobile phase settings were returned to initial conditions (30% B) for a 2 min equilibration. The mobile phase flow rate was 0.35 mL/min |
| Run Time | 8 min |
| Autosampler Temperature | 4 °C |
The samples were analysed using multiple reaction monitoring (MRM) on a LCMS-8050 Triple Quadrupole Tandem Mass Spectrometer with data analysis performed via LabSolutions version 5.97 (Shimadzu Scientific, Rydalmere, Australia). One quantifying and one qualifying ion transitions were monitored for atropine and its internal standard, atropine-d5. The MRM (quantifying and qualifying transitions respectively, collision energies and quadrupole voltages) for atropine and atropine-d5 are 290.0 -> 124.1 (-20, -23, -24), 290.0 -> 92.9 (-20, -32, -34) for atropine, and 295.2 -> 124.1 (-20, -23, -24) and 295.2 -> 92.9 ((-20, -32, -34) for atropine-d5. The injection volume was 10µL.
Sample Preparation
Plasma samples were prepared for analysis through mixing aliquots (200 µL) of the plasma with 800 µL of acetonitrile containing the internal standard. The nominal concentration of Atropine-d5 in acetonitrile was 0.5 µg.L-1. The mixture was vortex-mixed to precipitate plasma proteins, then centrifuged for 5 min at 4000 rpm. Extracts (900 µL) were evaporated to dryness then reconstituted with 200 µL of 10% methanol in water.
Method Validation
The assay was validated according to US FDA guidelines for bioanalytical studies.11
The calibrator solutions were prepared from pure atropine powder supplied by PM Separations where the spiking solutions (prepared in in 10% methanol) were diluted 1 in 10 with plasma to yield 7 Plasma calibrators ranges from 0.025 to 10.0 µg.L-1 of atropine. Quality control (QC) solutions were prepared in a similar fashion to the calibrator solutions but using the atropine powder purchased from Sigma-Aldrich.
The method was highly selective with no overlapping peaks or signal abnormalities that co-eluted at the atropine retention time. All runs returned a least-squares regression correlation coefficient (R2) higher than 0.998. Matrix effects were evaluated through post-column infusion of atropine.
Accuracy and precision were deemed acceptable if deviations at a nominal concentration were ≤ 15%, except at the Limit of Quantification (LOQ) which could deviate by up to 20%. Recovery and matrix effects were also investigated. The RE% for the inter-run study and within-run study ranged between -1.5 and +4.4 (CV% was 16.21 for the 0.025 µg.L-1 concentration and ranged between 2.7 and 10.93 for the other concentrations) and 4.6 to 11.7 (CV% between 2.7 and 8.9) respectively.
The recovery was calculated by comparing the calibration curve slope of the calibrators with that of the atropine concentration in 10% methanol in water. The mean recovery was found to be 96.1% (SD=5.91, CV%=6.16).
The LOQ was found to be the 0.025 µg.L-1 and displayed a maximum RE% of mean calculated concentration of 12.3% and maximum CV% of 13.7.
All calibrators and QCs were freshly prepared at the time of each analysis. In regards to the atropine stability in human plasma, atropine was reported to be stable for 6 months after storage at –20 °C.12 Also, haemolysis was found not have an effect on atropine bioanalysis.13 The stability of atropine after reconstitution with 10% methanol while in the autosampler was assessed by comparing the calculated atropine plasma concentration of the 0.1 µg.L-1 QC sample at the start of the run and 9 hours later in each of the participants’ samples runs. Nine hours was the longest duration of the participants’ samples run on any day. Stability of atropine in prepared samples in the autosampler over 24 h was reported by Siluk et al.14
Clinical Study
Participants:
The clinical study was carried at the Charles Perkins Centre at the University of Sydney, New South Wales, Australia. Written informed consent was obtained from each participant. A study poster was displayed at the University of Sydney, Sydney, Australia and Royal Prince Alfred Hospital, Sydney, Australia to invite healthy adults to participate in the study. Clozapine-treated adults attending the clozapine clinic at the Camperdown Community Mental Health Centre, Sydney, Australia, were invited to participate in the study.
The clinical trial was approved by the Sydney Local Health District Ethics Review Committee (RPAH Zone) (Ethics approval number: HREC/18/RPAH/687) and registered on the Australian New Zealand Clinical Trials Registry ANZCTR (Trial ID: ACTRN12618001817235).
Inclusion and Exclusion Criteria
All participants were at least 18 years old, if a female non-pregnant and non-breastfeeding, and normotensive with no signs of heart disease based on recent blood pressure, blood pathology, and ECG tests. Participants were excluded if they had known allergy to atropine, known kidney or liver impairment, were anemic, treated with an anticholinergic medication other than clozapine, treated with an anticoagulant, or had a contraindication to the use of the study medication.
Sample Size
The sample size calculation was based on the atropine effect on saliva secretion after the sublingual administration of 0.6 mg atropine sulfate. Assuming a saliva secretion reduction of 52% (SD±26),2 five participants are needed to be treated with sublingual 0.6 mg atropine sulfate to be able to reject the null hypothesis that the population saliva flow rate mean of the atropine sulfate and the baseline were equal (power 0.90). The type 1 error probability associated with this test of this null hypothesis is 0.05 (G*Power 3.1.9.7 software, difference between two dependent means).
Cotton rolls and saliva pads were used in measuring the saliva secretion in subjects (Cotton rolls: Henry Schein Dental Cotton Rolls®, size 2 (3.8 mm), Henry Schein Australia. Saliva pads: NeoDrys®, small size, Henry Schein Australia). The dry and wet cotton rolls and saliva pads were weighed using a METTLER TOLEDO, PL83-S scale.
The wall LogMAR chart (Proportionally spaced LogMAR Sloan Letters Chart, 3 metres, GOOD-LITE, USA), was used to assess the distance visual acuity at baseline and after the administration of atropine. The RAF Rule (Haag-Streit UK, Edinburgh Way, Harlow, Essex, UK) binocular gauge was used to measure visual accommodation, convergence, and near vision.
The vital signs (body temperature, blood pressure and pulse rate) were measured using a Welch Allyn Connex Spot Monitor.
Study Procedure
Study participants were instructed not to eat on the morning of the study after an overnight fast. Participants arrived at the study site at 7:30 am and immediately received a standardised breakfast and was provided with 500 mL of water. One hour after breakfast, a 5 min baseline saliva secretion rate was measured as described in a previously published study.2 Vision, body weight, height, and waist circumference were assessed. The saliva secretion was measured again at 2 h and 5 h after the administration of atropine.
The distance visual acuity test was carried out according to chart use instructions by the GOOD-LITE company. The vision tests were carried out by the use of the RAF Rule.15 They were carried out before and 2 h after the administration of atropine.
Vital signs were recorded at baseline then, at least, at the time of each blood sample collection between 9 am and 1 pm. All participants had the same standardised meal for lunch (at 1 pm, after the 3 h blood sample collection) with a cup of coffee immediately after lunch. Vital signs after 180 min were recorded but not compared due to the variation in the time participants finished their lunch and the possible effect of coffee on the recorded vital signs.
All participants had a 12-lead ECG at the end of all blood samples collection.
All study procedures were carried out at the Charles Perkins Centre.
Atropine Dosing
Three atropine doses were administered; a single dose of 60 µL of atropine sulfate 1% drops solution (equivalent to 0.6 mg of atropine sulfate, Bausch & Lomb Australia PTY LTD) administered sublingually on first visit, a single dose of 0.6 mg atropine sulfate tablet (Wockhardt UK Ltd, United Kingdom) administered orally on the second visit, and a single dose of 120 µL of atropine sulfate 1% drops solution administered sublingually on the third visit. One participant only was tested on any study day. There was a washout period of at least 3 days between visits. The participant was instructed to keep the atropine solution in the mouth as long as possible before swallowing. The atropine tablet was placed at the back of the tongue then immediately swallowed with 200 mL of plain water. No food was allowed between breakfast and lunch time. Only water was allowed after the collection of the 60 min blood sample.
Blood Sampling
A baseline blood sample (2 mL, Vacutainer® EDTA tube) was collected via indwelling cannula into the antecubital fossa prior to the administration of the study medication. Subsequent blood samples were collected at 5, 10, 20, 30, 40, 60, and 90 min, then 2, 2.5, 3, 4, 5, 5.5, 6, 6.5, 7, 7.5, 8, 8.5, 9 h after the administration of atropine. The tube was inverted at least 8 times, immediately refrigerated then centrifuged within 3 hours from collection for 10 min at 2000 g at 4°C (Heraeus Megafuge 16R, ThermoFisher SCIENTIFIC). Harvested plasma was transferred to a 2 mL Eppendorf tube (POCD, Artarmon, NSW, Australia) and frozen at –20°C until analysis. The participants’ samples were analysed once so were not exposed to repeated freeze/thaw cycles. The maximum storage time for the participants’ plasma samples was 4 months which is less than the 6-month stability period reported for atropine in plasma when stored at –20 °C.12
Data Analysis
Non-compartmental pharmacokinetic analysis were performed using PKanalix (version 2019R1, Lixoft SAS, Abtony, France). The area under the atropine plasma concentration-time curve (AUC) was calculated using the linear log trapezoidal method. The maximum atropine plasma concentration (Cmax) and the time to Cmax (tmax) was determined by observation. The terminal elimination rate constant (kel) was estimated as the slope of the terminal portion of the log concentration-time plot using at least three points. The time needed for the atropine concentration to decrease by half during the elimination phase (half-life, t1/2) was calculated by dividing 0.693 by the elimination rate constant (kel) for each participant. Relative bioavailability was calculated by comparison of dose-corrected AUC. All parameters were summarized using geometric means±SD.
Statistical analysis was carried out using the SPSS statistical package (IBM SPSS Statistics for Windows, version 26 (IBM Corp., Armonk, N.Y., USA)). A Generalised Estimating Equation (GEE) procedure was used to compare the means of pharmacokinetic parameters, saliva secretion at 2 and 5 h, vision at 2 h, and the vital signs over 3 h between the three atropine doses. The GEE comparison tables were checked for the time at which the medication has made a significant change then the difference in the mean change was compared to that produced by the 0.6 mg SL solution at the particular time.
A Spearman Ranking Test was used to measure the correlation between the AUC with the saliva secretion change and between each of the pharmacokinetic parameters and vital signs change over 3 hours.
All analyses were performed using SPSS software version 26.0 (IBM Corp., Armonk, NY, USA).
Results
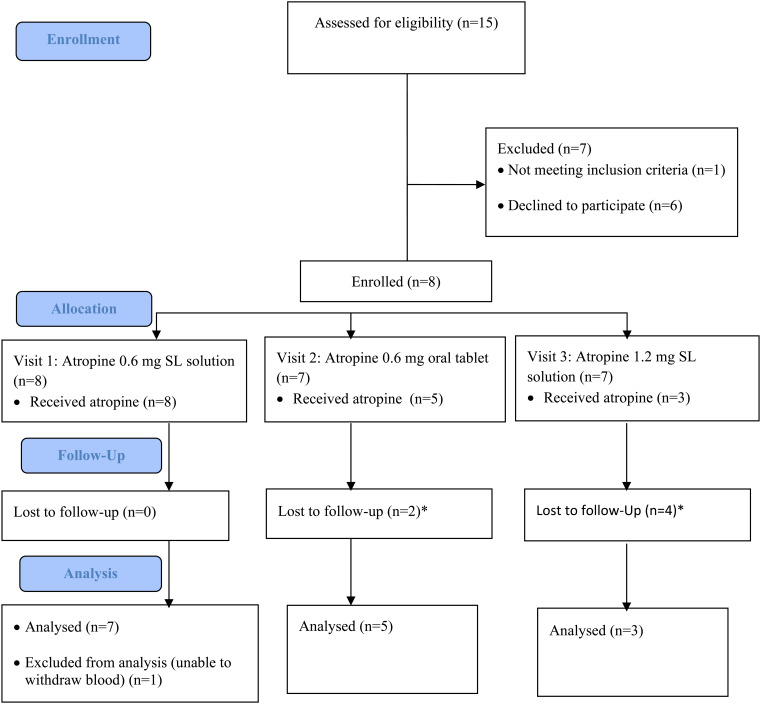
Four clozapine-treated patients and four healthy adults were enrolled in the study. One healthy participant did not comply with the study procedure and was excluded from analysis. The remaining 7 participants (3 females) had a mean age (± SD) of 36 ± 9 years, mean body weight 86 ± 18 kg, and mean Body Mass Index (BMI) 26±6 kg/m2 (Figure 1). All clozapine-treated participants were not currently employed. The three healthy participants were healthcare professionals.
Figure 1.
Study flow diagram (CONSORT flow chart). *Participants refused to return for later study visits due to the COVID pandemic. SL, sublingual.
Clozapine-treated participants reported untreated night-time but not daytime drooling. All were prescribed clozapine for the treatment of treatment resistant schizophrenia. The median daily clozapine dose was 375 mg. The number of blood samples collected ranged between ten and nineteen samples per participant for each study visit.
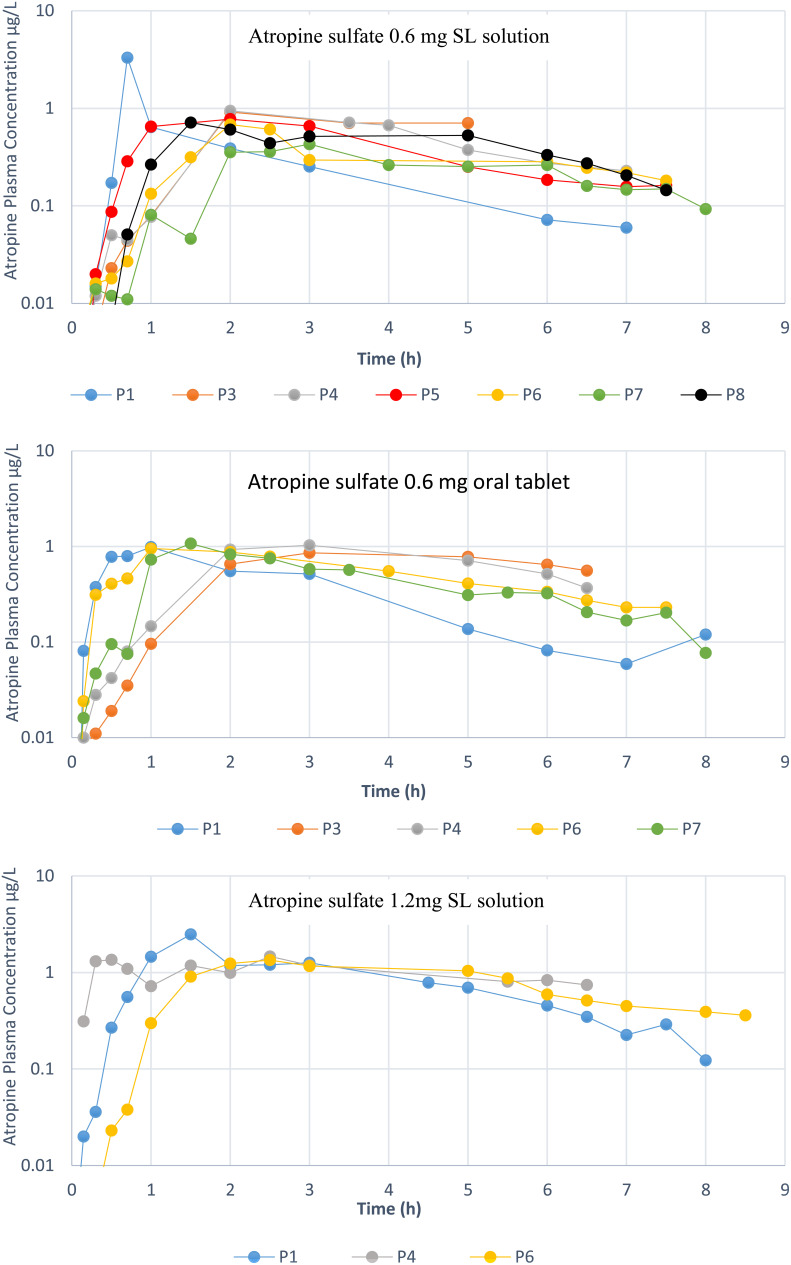
The plasma concentration versus time of the atropine after sublingual solution and oral tablet administration is shown in Figure 2.
Figure 2.
Atropine plasma concentration-time profiles after the administration of (a) 0.6 mg atropine sulfate sublingual solution, (b) 0.6 mg atropine sulfate tablet and (c) 1.2 mg atropine sulfate sublingual solution in patients receiving clozapine (P1,P4,P6,P8) and healthy participants (P3,P5,P7). Note: Participant 2 was excluded.
The mean (±SD) atropine Cmax for the 0.6 mg (n=7) and 1.2 mg (n=3) sublingual solutions was 1.11±0.99 and 1.76±0.62 µg.L-1 respectively, and tmax was 2.18±0.59 and 1.9±0.71 h, respectively. For the atropine 0.6 mg tablet (n=5), the Cmax and tmax were 0.98±0.08 µg.L-1 and 1.9±1.03 h, respectively.
The mean values of the main pharmacokinetic parameters are summarized in Table 2.
Table 2.
Pharmacokinetic Parameters of atropine After Sublingual (SL) and Oral Tablet Administration (n=number of participants)
| Parameter | 0.6 mg SL Solution. | 1.2 mg SL Solution | 0.6 mg Oral Tablet | ||||
|---|---|---|---|---|---|---|---|
| Clozapine- treated (n=4) | Healthy (n=3) | All (n=7) | Clozapine-treateda (n=3) | Clozapine-treated (n=3) | Healthy (n=2) | All (n=5) | |
| AUC0-∞ (µg.L-1.h) | 3.16 (0.63) | 2.63 (1.06) | 2.98 (0.73) | 8.58 (1.66) | 4.45 (1.25) | 4.95 (1.80) | 4.65 (1.29) |
| Cmax (µg.L-1) | 1.41 (1.27) | 0.71 (0.25) | 1.11 (0.99) | 1.76 (0.62) | 0.99 (0.039) | 0.96 (0.15) | 0.98 (0.08) |
| tmax (h) | 1.61 (0.70) | 2.28 (0.63) | 1.9 (0.71) | 2.18 (0.59) | 1.67 (1.15) | 2.26 (1.07) | 1.9 (1.03) |
| t1/2 (h) | 1.93 (0.64) | 2.90 (1.83) | 2.26 (1.08) | 3.05 (1.54) | 2.24 (0.34) | 2.57 (0.73) | 2.37 (0.47) |
Mean (SD) data presented.
aOnly clozapine-treated participants participated in the 1.2 mg SL solution study.
AUC0-∞: Area under the atropine plasma concentration versus time curve over time zero extrapolated to infinite time, Cmax: Maximum plasma concentration, tmax: Time to maximum plasma concentration. Elimination half-life (t1/2) was calculated by dividing 0.693 by the elimination rate constant (kel) for each participant.
When comparing the total amount of atropine absorbed after the administration of the three doses, the mean (± SD) dose adjusted relative bioavailability of the 1.2 mg SL solution and 0.6 mg tablet relative to the 0.6 mg SL solution was 0.98±0.01 and 1.54±0.30, respectively.
The mean AUC0-∞ after the administration of the 1.2 mg SL solution was statistically significantly larger than that after the administration of the 0.6 mg SL solution and 0.6 mg oral tablet (mean difference in AUC0-∞ = 5.49 µg.L-1.h (CI:4.69, 6.29) and 4.07 µg.L-1.h (CI:3.61, 4.52) respectively).
Also, more atropine was absorbed after the administration of 0.6 mg tablet in comparison with the same dose after sublingual solution administration (relative bioavailability of sublingual solution, 64%).
There was no significant difference in the mean tmax observed between the clozapine-treated participants and healthy participants after oral atropine administration (p=0.69) or for all study days (p=0.28). The mean estimated atropine t1/2 for all doses was 2.47 h±0.99.
Effect on Saliva Secretion
The majority of participants (6 out of 7) had a significant decrease (mean percentage change -40.5%, CI: -59.3, -21.7%) in saliva secretion (Table 3) after the 0.6 mg SL atropine solution administration.
Table 3.
Change in Total Saliva Secretion in All Participants
| Atropine Dose | Change in total saliva secretion (%), mean (CI) |
|---|---|
| 0.6 mg SL solution, n=7 | -22.85 (-57.77, 12.08)* |
| 0.6 mg oral tablet, n=5 | -68.60 (-80.40, -56.79) |
| 1.2 mg SL solution n=3 | -78.93 (-93.28, -64.57) |
SL soln.: sublingually administered solution, n=number of participants.
*Statistically non-significant change.
CI: confidence interval.
One participant receiving clozapine (participant 1 in Figure 2) had an increase in saliva flow and also had shortest tmax and largest Cmax of atropine. The participant also demonstrated a higher atropine Cmax after the administration of 1.2 mg sublingual atropine sulfate. To eliminate a larger atropine elimination rate in this participant, the atropine apparent clearance (CL/F) was found to be within the range observed for other participants (250 L.h-1 vs. 152-319 L.h-1 in other participants).
There was a statistically significant larger decrease in total saliva secretion in all participants after the administration of the 0.6 mg atropine sulfate tablet and 1.2 mg SL atropine sulfate solution in comparison with the 0.6 mg SL solution (Table 4).
Table 4.
Difference in the Saliva Secretion Change Between the Atropine Sulfate Tablet and 1.2 mg SL Solution to the Atropine Sulfate 0.6 mg SL Solution (n=7)
| Atropine Dose | Mean difference in saliva flow ratea (%) (CI) | P (α=0.05) |
|---|---|---|
| 0.6 mg oral tablet (n=5) | -46 (-76, -15) | 0.003 |
| 1.2 mg SL solution (n=3) | -56 (-98,-14) | 0.008 |
SL soln.: sublingually administered solution, Tab.: tablet, n=number of participants.
aMean difference is calculated using Generalised Estimating Equation.
CI: confidence interval.
However, after the exclusion of the participant who experienced an increase in saliva secretion, the change was statistically significant with only the 1.2 mg SL solution relative to the 0.6 mg SL solution (mean estimated difference: -23.00, CI: -44.71, -1.30). As for the dose-effect relationship, no significant correlation was found between the change in saliva secretion and AUC0-2h or AUC0-2h/dose.
No statistically significant change in saliva secretion was found at 5 h after the atropine sulfate administration. However, the saliva secretion was only measured in 9 out of the 15 atropine administration occasions.
Atropine Safety
Atropine Effect on Vital Signs:
All three atropine doses caused a statistically significant reduction in standing and sitting pulse rate from baseline. However, none of the changes recorded with any one dose were statistically significantly different from those recorded with the other doses. The maximum change in standing diastolic BP was -6.27 (-10.60,-1.94) mmHg, standing pulse was -14.12 (-21.91, -6.34) beats per min (bpm), sitting systolic BP was -9.33 (-17.91, -0.76) mmHg, sitting diastolic BP was -5.73 (-9.28, -2.25) mmHg, and sitting pulse was -21.75 (-28.12, -15.38) bpm. A statistically non-significant change in standing systolic BP was recorded.
Atropine Effect on Vision:
The study of the effect of atropine on visual acuity found no changes in near vision test in any of the participants. No statistically significant change in distance vision was found after the administration of any of the atropine doses or forms in any group of participants.
For the visual accommodation and convergence testing, a statistically significant change (increase) in Near Point Accommodation (NPA) was found in the healthy participants (mean: 1.00 cm, CI: 0.17, 1.83) and in the clozapine-treated participants after the administration of the 1.2 mg SL atropine only. The change in the Near Point of Convergence (NPC) was not statistically significant in any of the study groups.
Cardiac Conduction Effects:
No significant changes in the 12-lead ECG were recorded between the end of blood collection time and baseline.
Discussion
This study is the first to investigate plasma concentration of atropine following sublingual administration in people treated with clozapine. This study found that systemic exposure after sublingual administration of an atropine solution was lower when compared to the oral tablet of the drug. No major safety concerns were found.
The sublingual absorption of atropine in adults was previously described by Kanto et al6 However, in the Kanto et al6 study, the participants were anaesthetised over the first one hour after the administration of the atropine sulfate dose which may have prolonged the retention time of atropine in the mouth and therefore the absorption phase. The short blood collection time makes the reported AUC of atropine over the three hours incomparable with that in our study.
The 36% higher bioavailability after the oral tablet administration relative to the SL route may be due to a reduced absorption of the sublingual atropine due to incomplete swallowing of the solution or a decrease in the permeation of atropine across buccal cavity membranes. The interaction of saliva components such as peptides and proteins with sublingually administered atropine will need to be investigated.16
The comparable atropine plasma concentration after oral and sublingual administration indicates that the same maximum daily oral atropine tablet dose of 2 mg may also apply to sublingual atropine solution when used in the treatment of CIH. It is noteworthy that the pharmacokinetics of atropine after IV administration is reported to be nonlinear after IV administration of 0.5 to 4 mg of atropine.17 This was also found in this study between the 0.6 mg and 1.2 mg sublingual atropine.
The Cmax recorded in this study is very similar to that reported by Kozelj et al13 after the ingestion of meals with different amounts of atropine (Cmax = 0.55 and 1.6 µg.L-1 after the intake of 3.58 µg.kg-1 body weight and 12.1 µg.kg-1 body weight of atropine respectively).13 It is worth noting that all 5 participants had a reasonably close Cmax which was smaller than that for the sixth participant.
Some participants in the sublingual atropine study have shown double absorption peaks consistent with two-parallel absorption phases. The lack of such peaks after oral tablet administration suggests a partial absorption from the buccal cavity after the SL solution administration and not an enterohepatic recirculation. A second concentration peak may lead to prolonged antimuscarinic action. The variable absorption among the participants may be due to a variable surface area and permeability of the mucosal membrane in the buccal cavity, saliva volume, and the saliva and mucous membrane pH.18
A decrease in the pH at the absorption site in the mouth or an increase in the amount of saliva initially secreted to wash out the bitter tasting atropine solution would reduce the absorption rate based on the buccal medications absorption models proposed in the literature.19,20 The acidic pH of the atropine eye drops solution (pH=3.0-4.2; personal communication, Atropine Minims, Bausch and Lomb) and short retention time may reduce, or delay, the absorption of atropine from the buccal cavity. Atropine is metabolised in the liver through glucuronidation with around 60% of the administered dose excreted unchanged in the urine.21 It is unknown if any of the esterase enzymes secreted into the saliva in humans has any hydrolysing effect on the atropine molecule.22 A lower buccal bioavailability was reported with other drugs such as nicotine.23 The large Cmax in participant 1 after sublingual atropine administration may be due to a possibly reduced integrity of the buccal mucosal membrane due to injury, for example, or a higher saliva pH in comparison with the other participants. The use of a tasteless buccal or sublingual oral dispersible tablet is suggested for future studies testing the atropine buccal absorption. Such tablets would increase the uniformity of the absorption process between participants. The elimination of the momentary increase in saliva secretion due to atropine’s bitter taste may reduce the chances of an interaction between atropine and the saliva components. The incorporation of permeation enhancers and sodium bicarbonate (increasing the medium pH) or mucoadhesive into atropine sublingual preparations may increase the atropine buccal absorption.24
In this study, the tmax is found to be longer and the t1/2 range is wider than that reported by Beermann et al7 after oral atropine administration in adults (tmax: 1.96 vs. 1 h, t1/2: 0.53-4.41 vs. 2.5-3 h).7 Yet, the atropine half-life in the present study (2.47±0.99 h) is in close agreement with the half-life reported by other researchers after the parenteral administration of atropine; 2.56±0.46 h and 4.3±1.7 h after IV administration25 and 2.4±0.6 h and 3.1±0.6 h after IM administration.26 In children, the oral atropine tmax reported has ranged between 1.5 and 2 hours.8,9
In terms of the effect of atropine on gastrointestinal motility, a single 0.25 mg dose of sublingual atropine was previously reported to significantly reduce the colonic motility in participants undergoing elective colonoscopy.27 Reduced gastric motility was similarly reported after IV atropine administration.28 Despite the well-known gastrointestinal hypomotility in clozapine-treated patients, no statistically significant difference in the tmax was found between the clozapine-treated and healthy participants in the present study. This implies a rapid anticholinergic effect of atropine on gastric emptying after a single dose administration, or failure to show a difference due to the multiple atropine peak concentrations found after the atropine solution administration.
As for the effect on saliva secretion, both the atropine sublingual solution and oral tablet significantly reduced the saliva secretion. The percentage change in the 0.6 mg SL solution group became statistically significant after the exclusion of the one participant who experienced the increase in saliva secretion (mean= -40.48, CI: -59.28, -21.67). This confirms the findings in our previous study including a possible paradoxical increase in saliva secretion in a small percentage of clozapine-treated patients.2 In comparison with our previous study, the reduction in this study is slightly less (-40.5% vs. -52.4%) which may be referred to the different testing time (daytime vs. nigh-time) and that all participants had hypersalivation at the time of testing in the previous study but none of the participants in this study. These findings suggest that sublingual atropine solution may be effective for the treatment of daytime hypersalivation as was the case for nigh-time hypersalivation.
A dose dependent decrease in saliva secretion after sublingual administration was observed in this study similar to that reported by other researchers over the atropine oral dose range of 0.25 mg to 1 mg.29 The atropine tablet is a more convenient option than the sublingual solution and the 1.2 mg dose may be used in patients who fail to respond to the smaller 0.6mg dose. In comparison with SL solution, the larger amount of atropine that was absorbed after oral tablet administration without a significantly larger decrease in saliva secretion warrants close monitoring of systemic anticholinergic adverse effects after the administration of oral atropine tablets especially when given in the presence of clozapine.
Atropine treatment decreased pulse rate after all atropine doses in this study opposite to that reported after the administration of high doses of parenteral or oral atropine reported.30 The finding in this study is of clinical significance in clozapine-treated patients who commonly experience tachycardia.
In regards to the visual acuity after atropine administration, no significant changes were recorded. This is in keeping with the findings in other studies after the administration of 1mg oral or 1 and 2 mg IM atropine.31 Despite a reported significant pupillary dilatation after the administration of 2 mg of atropine but not with smaller doses,29 no change in near vision was recorded.
A strength of this study is the novel insights into atropine systemic exposure in both healthy participants and people taking clozapine after sublingual administration.
An important feature of this research is the development and application of a highly sensitive and specific atropine assay which allowed the quantitation of atropine concentrations after very low doses administration. Among the different analytical methods for atropine, liquid chromatography-tandem mass spectrometry methods are found to have highest analyte selectivity and sensitivity.13 The lowest reported LOQ of atropine was 0.05 µg.L-1 using a liquid chromatography electrospray ionization tandem mass spectrometry (LC-ESI MS/MS).32 The LOQ reported in the present study is 0.025 µg.L-1.
We advise clinicians to alert the patient to the bitter taste of the atropine drops. A sublingual 0.6 mg of atropine sulfate at bed-time for night-time hypersalivation or drooling is proposed as a treatment option in the management of clozapine-induced hypersalivation or drooling. The same dose may be used during the daytime if other non-pharmacologic options fail. The atropine dose may be increased to 1.2 mg in patients experiencing a paradoxical increase in saliva secretion or unsatisfactory response to the smaller dose. A metered sublingual spray is recommended for the administration of sublingual atropine solution. Dental cotton rolls and saliva pads used in this study may be used in measuring the atropine effect on saliva secretion. Given the dose-dependent effect type and extent of atropine on heart rate, we recommend measuring the pulse rate before starting the atropine therapy and 2 hours after the first dose. Patients should be counselled on the potential adverse effects of atropine if administered at an amount larger than the prescribed dose.
This study has a number of limitations. A small number of participants were investigated at each atropine dose. A larger number of participants (24 participants for an effect size of 0.6, G*Power 3.1.9.7 software) would have allowed for a better description of the inter-individual differences of the atropine pharmacokinetics with all doses. The use of tasteless atropine dispersible tablets that allows for maximum retention of atropine in the buccal cavity could have allowed for a better study of any atropine absorption through the membranes in the buccal cavity. This study was carried out in the fed state due to the long blood collection time. A study conducted under fasting condition would have potentially reduced the intra- and inter-individual variation in atropine pharmacokinetics.
In conclusion, significantly less atropine is absorbed into the systemic circulation after sublingual administration as a liquid in comparison with administration as an oral tablet. In this study sublingually and orally administered atropine significantly reduced saliva secretion in clozapine-treated patients and healthy participants. A decrease in pulse rate without a significant effect on vision was recorded. In general, atropine was well tolerated which supports the safe and appropriate use in reducing the burden of hypersalivation in people treated with clozapine at daytime in addition to the night-time found in a previous study. A larger number of participants is suggested in future studies to better describe the absorption phase of sublingual atropine. The findings of this study supports the use of sublingual atropine as a safe option in the treatment of hypersalivation.
Data Availability
The data that support the findings of this study are presented within this publication. Further data may be requested from the corresponding author.
Funding Statement
The study was partially funded by the Sydney Local Health District. Omar Mubaslat received a Sydney Local Health District Allied Health Small Grant.
Footnotes
Ethics Committee Approval: Ethical committee approval was received from the Sydney Local Health District Ethics Review Committee (RPAH Zone) (HREC/18/RPAH/687).
Informed Consent: Written informed consent was obtained from all participant who participated in this study.
Peer-review: Externally peer-reviewed.
Acknowledgements: Mario D’Souza is acknowledged for advice on statistical analysis. We thank all study participants, Dr Carolyn Stoney and other psychiatrists for recruiting participants, Charles Perkins Centre-RPA clinic staff, A/Prof David Sullivan (RPA Chemistry Lab) and Dr Vincent Chow (Cardiologist) for their support.
Author Contributions: Concept - O.M., A.M., T.L.; Design - O.M., A.M., T.L.; Supervision - M.F., A.M., T.L.; Resource - O.M., M.F.; Materials - O.M., M.F.; Data Collection - O.M., M.F., T.L.; Data Analysis - O.M., M.F., A.M., T.L.; Literature Search - O.M.; Writing - O.M., M.F.; Critical Review - A.M., T.L.
Declaration of Interests: The authors have no conflicts of interest to declare.
Clinical Trial Registration: Australian New Zealand Clinical Trials Registry ANZCTR (Trial ID: ACTRN12618001817235).
References
- 1. Rogers DP, Shramko JK. Therapeutic options in the treatment of clozapine induced sialorrhea. Pharmacotherapy. 2000;20(9):1092 1095. 10.1592/phco.20.13.1092.35036 [DOI] [PubMed] [Google Scholar]
- 2. Mubaslat O, Lambert T. The effect of sublingual atropine sulfate on clozapine-induced hypersalivation: a multicentre, randomised placebo-controlled trial. Psychopharmacology. 2020;237(10):2905 2915. 10.1007/s00213-020-05627-4 [DOI] [PubMed] [Google Scholar]
- 3. Van der Poorten T, De Hert M. The sublingual use of atropine in the treatment of clozapine induced sialorrhea: a systematic review. Clin Case Rep. 2019;7(11):2108 2113. 10.1002/ccr3.2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scheinin H, Helminen A, Huhtala S.et al. Spectral analysis of heart rate variability as a quantitative measure of parasympatholytic effect-integrated pharmacokinetics and pharmacodynamics of three anticholinergic drugs. Ther Drug Monit. 1999;21(2):141 151. 10.1097/00007691-199904000-00001 [DOI] [PubMed] [Google Scholar]
- 5. Kanto J, Klotz U. Pharmacokinetic implications for the clinical use of atropine, scopolamine and glycopyrrolate. Acta Anaesthesiol Scand. 1988;32(2):69 78. 10.1111/j.1399-6576.1988.tb02691.x [DOI] [PubMed] [Google Scholar]
- 6. Kanto J, Pihlajamäki K. Oropharyngeal absorption of atropine. Int J Clin Pharmacol Ther Toxicol. 1986;24(11):627 629. [PubMed] [Google Scholar]
- 7. Beermann B, Hellström K, Rosén A. The gastrointestinal absorption of atropine in man. Clin Sci. 1971;40(1):95 106. 10.1042/cs0400095 [DOI] [PubMed] [Google Scholar]
- 8. Saarnivaara L, Kautto UM, Iisalo E, Pihlajamäki K. Comparison of pharmacokinetic and pharmacodynamic parameters following oral or intramuscular atropine in children: atropine overdose in two small children. Acta Anaesthesiol Scand. 1985;29(5):529 536. 10.1111/j.1399-6576.1985.tb02248.x [DOI] [PubMed] [Google Scholar]
- 9. Gervais HW, el Gindi M, Radermacher PR.et al. Plasma concentration following oral and intramuscular atropine in children and their clinical effects. Paediatr Anaesth. 1997;7(1):13 18. 10.1046/j.1460-9592.1997.d01-40.x [DOI] [PubMed] [Google Scholar]
- 10. Aodah A, Rawas-Qalaji M, Bafail R, Rawas-Qalaji M. Effect of fast-disintegrating tablets’ characteristics on the sublingual permeability of atropine sulfate for the potential treatment of organophosphates toxicity. AAPS PharmSciTech. 2019;20(6):229. 10.1208/s12249-019-1420-1 [DOI] [PubMed] [Google Scholar]
- 11. United States Food and Drug Administration. Bioanalytical method validation guidance for industry May 2018. Available at: https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf. Accessed on January 2021. [Google Scholar]
- 12. Xu A, Havel J, Linderholm K, Hulse J. Development and validation of an LC/MS/MS method for the determination of L-hyoscyamine in human plasma. J Pharm Biomed Anal. 1995;14(1-2):33 42. 10.1016/0731-7085(95)01630-9 [DOI] [PubMed] [Google Scholar]
- 13. Koželj G, Perharič L, Stanovnik L, Prosen H. Simple validated LC–MS/MS method for the determination of atropine and scopolamine in plasma for clinical and forensic toxicological purposes. J Pharm Biomed Anal. 2014;96:197 206. 10.1016/j.jpba.2014.03.037 [DOI] [PubMed] [Google Scholar]
- 14. Siluk D, Mager DE, Gronich N, Abernethy D, Wainer IW. HPLC–atmospheric pressure chemical ionization mass spectrometric method for enantioselective determination of R, S-propranolol and R, S-hyoscyamine in human plasma. J Chromatogr B. 2007;859(2):213 221. 10.1016/j.jchromb.2007.09.035 [DOI] [PubMed] [Google Scholar]
- 15. Sharma IP. RAF near point rule for near point of convergence--a short review. Ann Eye Sci. 2017;2(16):16 16. 10.21037/aes.2017.02.05 [DOI] [Google Scholar]
- 16. Pedersen AML, Sørensen CE, Proctor GB, Carpenter GH, Ekström J. Salivary secretion in health and disease. J Oral Rehabil. 2018;45(9):730 746. 10.1111/joor.12664 [DOI] [PubMed] [Google Scholar]
- 17. Hospira USA. Ansyr™ plastic syringes (atropine sulfate injection) prescribing information. FDA; D; rugs; 2015. Accesed February 2021. [Google Scholar]
- 18. Patel VF, Liu F, Brown MB. Advances in oral transmucosal drug delivery. J Control Release. 2011;153(2):106 116. 10.1016/j.jconrel.2011.01.027 [DOI] [PubMed] [Google Scholar]
- 19. Goswami T, Kokate A, Jasti BR, Li X. In silico model of drug permeability across sublingual mucosa. Arch Oral Biol. 2013;58(5):545 551. 10.1016/j.archoralbio.2012.09.020 [DOI] [PubMed] [Google Scholar]
- 20. Amores S, Lauroba J, Calpena A, Colom H, Gimeno A, Domenech J. A comparative ex vivo drug permeation study of beta-blockers through porcine buccal mucosa. Int J Pharm. 2014;468(1-2):50 54. 10.1016/j.ijpharm.2014.03.050 [DOI] [PubMed] [Google Scholar]
- 21. Hinderling PH, Gundert-Remy U, Schmidlin O. Integrated pharmacokinetics and pharmacodynamics of atropine in healthy humans I: pharmacokinetics. J Pharm Sci. 1985;74(7):703 710. 10.1002/jps.2600740702 [DOI] [PubMed] [Google Scholar]
- 22. Yamahara H, Lee VHL. Drug metabolism in the oral cavity. Adv Drug Deliv Rev. 1993;12(1-2):25 39. 10.1016/0169-409X(93)90039-7 [DOI] [Google Scholar]
- 23. Olsson Gisleskog PO, Perez Ruixo JJ, Westin Å, Hansson AC, Soons PA. Nicotine population pharmacokinetics in healthy smokers after intravenous, oral, buccal and transdermal administration. Clin Pharmacokinet. 2021;60(4):541 561. 10.1007/s40262-020-00960-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bafail RS. Enhancing the Sublingual Permeability of Atropine Sulfate: Effect of pH and Penetration Enhancers of pH and Penetration Enhancers. USA: Nova Southeastern University; 2019. [Google Scholar]
- 25. Aaltonen L, Kanto J, Iisalo E, Pihlajamäki K. Comparison of radioreceptor assay and radioimmunoassay for atropine: pharmacokinetic application. Eur J Clin Pharmacol. 1984;26(5):613 617. 10.1007/BF00543495 [DOI] [PubMed] [Google Scholar]
- 26. Corcoran TE, Venkataramanan R, Hoffman RM.et al. Systemic delivery of atropine sulfate by the microdose dry-powder inhaler. J Aerosol Med Pulm Drug Deliv. 2013;26(1):46 55. 10.1089/jamp.2011.0948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chaptini LA, Janec EM, Seltzer G, Peikin S, Elfant AB. Sublingual hyoscyamine spray as premedication for colonoscopy: a randomized double-blinded placebo-controlled trial. Am J Surg. 2008;196(1):51 55. 10.1016/j.amjsurg.2007.06.030 [DOI] [PubMed] [Google Scholar]
- 28. Rashid MU, Bateman DN. Effect of intravenous atropine on gastric emptying, paracetamol absorption, salivary flow and heart rate in young and fit elderly volunteers. Br J Clin Pharmacol. 1990;30(1):25 34. 10.1111/j.1365-2125.1990.tb03739.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mirakhur RK. Comparative study of the effects of oral and im atropine and hyoscine in volunteers. Br J Anaesth. 1978;50(6):591 598. 10.1093/bja/50.6.591 [DOI] [PubMed] [Google Scholar]
- 30. Wellstein A, Pitschner HF. Complex dose-response curves of atropine in man explained by different functions of M1-and M2-cholinoceptors. Naunyn-Schmiedebergs Arch Pharmacol. 1988;338(1):19 27. 10.1007/BF00168807 [DOI] [PubMed] [Google Scholar]
- 31. Kay CD, Morrison JD. The effects of a single intramuscular injection of atropine sulphate on visual performance in man. Hum Toxicol. 1987;6(2):165 172. 10.1177/096032718700600210 [DOI] [PubMed] [Google Scholar]
- 32. John H, Binder T, Höchstetter H, Thiermann H. LC-ESI MS/MS quantification of atropine and six other antimuscarinic tropane alkaloids in plasma. Anal Bioanal Chem. 2010;396(2):751 763. 10.1007/s00216-009-3209-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are presented within this publication. Further data may be requested from the corresponding author.



 Content of this journal is licensed under a
Content of this journal is licensed under a