Key Points
Question
What is the validity of the molecular targets and clinical benefits of US Food and Drug Administration–approved genome-targeted cancer drugs based on the results of pivotal clinical trials?
Findings
In this cohort study, 50 molecular-targeted drugs covering 84 indications were identified. Using an international grading system to evaluate molecular targetability strength (European Society for Medical Oncology Scale for Clinical Actionability of Molecular Targets) and a scale to assess clinical benefit in genome-targeted cancer therapies (European Society for Medical Oncology Magnitude of Clinical Benefit Scale), 24 indications (29%) supported high-benefit genomic-based cancer treatments.
Meaning
The therapeutic benefit grading frameworks used in this study can help stakeholders identify therapies with the greatest clinical potential.
Abstract
Importance
The number of new genome-targeted cancer drugs has increased, offering the possibility of personalized therapy, often at a very high cost.
Objective
To assess the validity of molecular targets and therapeutic benefits of US Food and Drug Administration–approved genome-targeted cancer drugs based on the outcomes of their corresponding pivotal clinical trials.
Design and Settings
In this cohort study, all genome-targeted cancer drugs that were FDA-approved between January 1, 2015, and December 31, 2022, were analyzed. From FDA drug labels and trial reports, key characteristics of pivotal trials were extracted, including the outcomes assessed.
Main Outcomes and Measures
The strength of evidence supporting molecular targetability was assessed using the European Society for Medical Oncology (ESMO) Scale for Clinical Actionability of Molecular Targets (ESCAT). Clinical benefit for their approved indications was evaluated using the ESMO–Magnitude of Clinical Benefit Scale (ESMO-MCBS). Substantial clinical benefit was defined as a grade of A or B for curative intent and 4 or 5 for noncurative intent. Molecular targets qualifying for ESCAT category level I-A and I-B associated with substantial clinical benefit by ESMO-MCBS were rated as high-benefit genomic-based cancer treatments.
Results
A total of 50 molecular-targeted drugs covering 84 indications were analyzed. Forty-five indications (54%) were approved based on phase 1 or phase 2 pivotal trials, 45 (54%) were supported by single-arm pivotal trials, and 48 (57%) were approved on the basis of subgroup analyses. By each indication, 46 of 84 primary end points (55%) were overall response rate (median [IQR] overall response rate, 57% [40%-69%]; median [IQR] duration of response, 11.1 [9.2-19.8] months). Among the 84 pivotal trials supporting these 84 indications, 38 trials (45%) had I-A ESCAT targetability, and 32 (38%) had I-B targetability. Overall, 24 of 84 trials (29%) demonstrated substantial clinical benefit via ESMO-MCBS. Combining these ratings, 24 of 84 indications (29%) were associated with high-benefit genomic-based cancer treatments.
Conclusions and Relevance
The results of this cohort study demonstrate that among recently approved molecular-targeted cancer therapies, fewer than one-third demonstrated substantial patient benefits at approval. Benefit frameworks such as ESMO-MCBS and ESCAT can help physicians, patients, and payers identify therapies with the greatest clinical potential.
This cohort study assesses the validity of molecular targets and therapeutic benefits of US Food and Drug Administration (FDA)–approved genome-targeted cancer drugs based on the outcomes of their corresponding pivotal clinical trials.
Introduction
Advances in tumor sequencing technologies and understanding of cancer biology have accelerated precision oncology as a cancer treatment strategy. Treatment selection based on biomarkers has the potential for substantial patient benefit by tailoring treatments for individual patients and avoiding unnecessary toxic effects without compromising efficacy. Next-generation sequencing technology defining the sequence variation status of hundreds of genes in a single test has rapidly expanded as an important component of cancer care. However, the safety and effectiveness of several US Food and Drug Administration (FDA)–approved molecular-targeted drugs have not necessarily matched their biochemical target profiles.1 To address these challenges, the European Society for Medical Oncology (ESMO) Translational Research and Precision Medicine Working Group recently developed the ESMO Scale for Clinical Actionability of Molecular Targets (ESCAT),2 a framework that classifies genomic alterations according to their actionability for available precision cancer medicines. This classification system is aimed at supporting clinicians in prioritizing cancer genomic alterations based on available evidence supporting their targetability and value as clinical targets.
The strength and quality of the available evidence supporting genome-targeted cancer drug approvals varies.3,4,5,6 An increasing number of cancer drugs have received regulatory approval based on improvements in surrogate measures, including disease-free survival (DFS) and progression-free survival (PFS) or, more recently, durable response rates, rather than clinical end points such as overall survival (OS) or quality of life (QoL).7 In addition, as a result of an enhanced understanding of actionable molecular targets, the FDA has granted several molecular therapy drug approvals based on expansion cohorts in phase 1 clinical trials, or, more recently, with basket trial designs supporting tissue agnostic approvals. This new paradigm for agents that have shown promising efficacy in early clinical development, raises questions about the clinical benefits of the drugs.
To help physicians and patients differentiate highly effective therapies from less effective ones, oncology professional societies have developed frameworks to distinguish different levels of benefit. The ESMO–Magnitude of Clinical Benefit Scale (ESMO-MCBS)8,9 is a validated framework that has been used to guide physicians, patients, and health technology assessments in selecting high-value treatments.10,11 The most updated version of the scale (version 1.1)9 covered randomized clinical trials (RCTs) as well as single-arm trials and disseminated composite measures of clinical benefit based on drug efficacy, safety, and QoL outcomes. Originally, the ESCAT framework was a clinical benefit–centered system that ranked genomic alterations ready for routine use (ESCAT evidence class I-A and I-B) as targets for cancer precision medicine associated with a meaningful improvement as defined by the ESMO-MCBS, version 1.1.9 More recently, with widespread use of the scale, the magnitude of benefit is not specifically considered to reach a level of evidence I-A or I-B but only a demonstration of an outcome improvement in a survival end point in prospective RCTs for evidence class I-A and from prospective non-RCT data for evidence class I-B.12,13 With the increasing implementation of next-generation sequencing assays to guide treatment decisions, several prospective trials have explored the association of multigene sequencing in daily practice without showing a significant association with patient outcomes.14,15,16,17,18
Because the validity and value of the targets and surrogate measures underlying FDA genome-targeted cancer drug approvals are uncertain, we assessed the actionability of molecular targets related to genome-targeted cancer therapies approved by the FDA between January 1, 2015, and December 31, 2022. Then, we evaluated the evidence supporting genome-targeted cancer drug approvals and their association with clinical benefit.
Methods
In this cohort study, we searched Drugs@FDA19 to identify all new and supplemental indications for genome-targeted drugs approved for the treatment of solid tumors from January 1, 2015, to December 31, 2022.20 Genome-targeted drugs were defined as those approved based on a genomic test in which the drug targeted a given genomic alteration. We excluded chemotherapy regimens and nongenome-targeted drugs.20,21,22 This study was exempt from ethical approval, and informed consent was waived because it exclusively used publicly available data. It followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.23
Data Extraction
We collected the name of the drug, the date of FDA approval, treatment indication, and data on genomic alterations used to define trial enrollment and the treatment regimens. We then extracted the following regulatory information from the FDA website19: product regulatory category (new drug application or biologic licensing application), initial vs supplemental approval, and key special regulatory pathways (priority or standard review,24 breakthrough therapy designation,25 Orphan Drug Act designation,26 and accelerated approval.27 For accelerated approvals, if conversion to regular approval was later granted, we used the confirmatory trial as the pivotal trial.28
Pivotal trial characteristics and outcomes were collected from FDA drug approval package/product labeling and other reports of pivotal trials in the literature.29,30 These included number of trials supporting the approval, sample size, study design (randomized vs single-arm), blinding (blinded vs open-label), phase of clinical trial, treatment intent (curative vs noncurative), type of sponsorship (industry/for-profit vs nonprofit), primary efficacy end points supporting the approval (OS vs intermediate end points [eg, PFS, DFS, overall response rate [ORR], and duration of response [DOR]), and whether approval was based on subgroup analysis of the pivotal trial.
The trial or cohort reported in section 14 of the labeling was chosen as the pivotal study supporting the indication.31 When multiple studies supported a single approval, each was reviewed separately. When approval was based on subgroup analyses defined by the FDA as clinically relevant subgroups of the patient population of the pivotal trial,32 each subgroup was also considered separately; subgroups included expansion cohorts in phase 1/2 clinical trials or exploratory or preplanned subgroup analysis in RCTs. Pivotal trials, supplementary trials, and subgroup analyses were globally considered as data points supporting approval (eTable 1 in Supplement 1).
Data Synthesis and Scoring
For each pivotal trial or data point supporting the approval of genome-targeted drug indications, we used the ESCAT framework to evaluate the strength of clinical evidence supporting molecular targetability.2 ESCAT provides a class I-A designation based on improved survival (PFS, DFS, or OS) in a prospective randomized trial. Class I-B indicates an outcome benefit (ORR, DOR, or PFS) derived from prospective nonrandomized data. Class I-C evidence was connected to basket trials showing similar clinical benefits across tumor types. Drugs approved based on retrospective studies alone were classified as class II-A (eTable 2 in Supplement 1).
For each pivotal trial or subgroup supporting approval of genome-targeted drug indications, ESMO-MCBS grades were applied and extracted from the ESMO website33 or using the publicly available forms if scorecards were not available.34 This framework is designed to apply to both RCTs and single-arm trials and uses a different scale for curative and noncurative settings. First, a preliminary score quantifies gains in the outcome under evaluation. For RCTs, hazard ratios with associated outcome gains are linked to a particular grade in a prespecified manner; for single-arm trials, when the primary outcome was PFS or ORR, gains on these outcomes were also associated with a particular grade. Second, positive and negative adjustments of the preliminary score were completed based on toxic effect and QoL data, as well as any other relevant information (eTable 3 in Supplement 1). ESMO-MCBS grades are ranked from A (high) to C (low) for the curative setting and 1 (low) to 5 (high) for the noncurative setting.9 Substantial clinical benefit was defined as grade A/B for curative trials and grade 4/5 for noncurative trials. Molecular targets associated with substantial clinical benefit by ESMO-MCBS and qualifying for ESCAT category level I-A and I-B were rated as high-benefit genomic-based cancer treatments.2
Analysis
Using descriptive analysis, data were reported as proportions, medians, and IQRs. The main analysis was focused on pivotal trials supporting approval. A secondary analysis considered separately each data point: pivotal trials, supplemental trials, and subgroup analyses.
Results
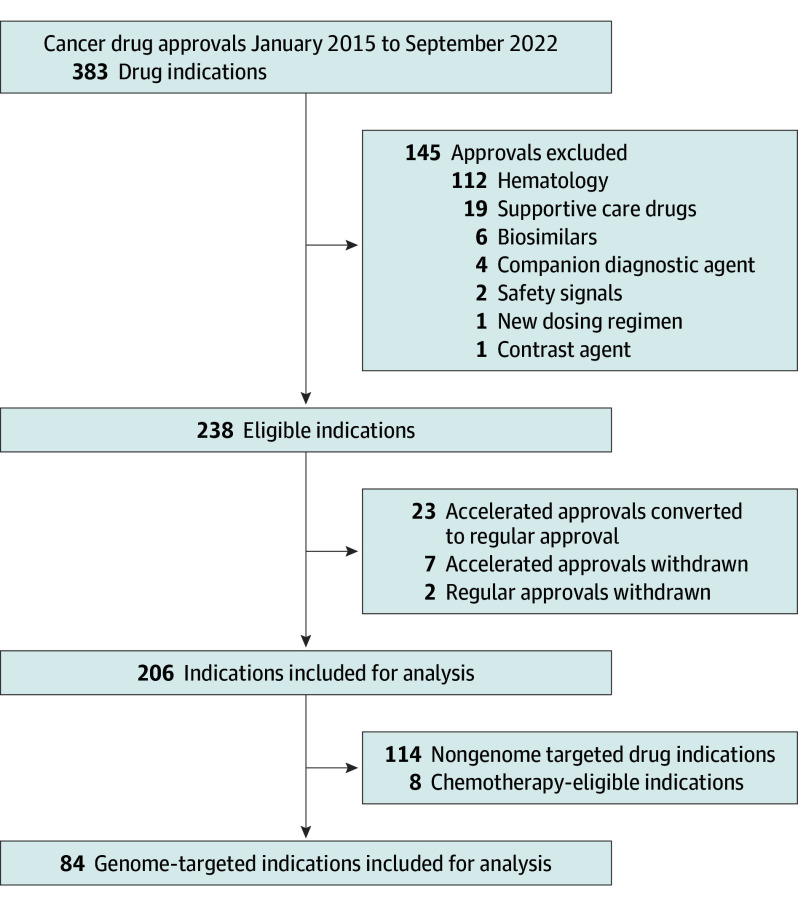
Of 206 initial and supplemental solid tumor drug indications approved by the FDA between January 1, 2015, and December 31, 2022, 84 (40%) targeted the genome (Figure 1). For the 84 genome-targeted cancer drug approvals, 50 molecular-targeted drugs were identified and matched to genomic alterations (eFigure in Supplement 1). As of December 2022, 22 genes comprising at least 48 genomic alterations guided therapy selection for the 84 indications. Included applications were supported by 84 qualifying pivotal trials.
Figure 1. Flow Chart Showing Search Results.
Characteristics of Indications
As shown in Table 1, of the 84 genome-targeted approved indications for treating patients with cancer, 73 (87%) were granted priority review, 50 (60%) received breakthrough therapy designation, and 58 (69%) received orphan drug designation. In addition, 32 (38%) were granted accelerated approval, with 52 (62%) receiving regular approval. Among them, 10 indications were first designated as accelerated approval and then converted to regular approval by the end of 2022. Among the 52 regular approvals, 13 (25%) were based on single-arm data.
Table 1. Characteristics of Included Indications Supporting Genome-Targeted Drug Approvals.
| Characteristic | No. (%) |
|---|---|
| Application | 84 (100) |
| Application type | |
| New drug | 67 (80) |
| Biologic licensing | 17 (20) |
| Indication type | |
| Initial | 37 (44) |
| Supplemental | 47 (56) |
| Approval type | |
| Regulara | 52 (62) |
| Accelerated | 32 (38) |
| Orphan drug–designated approval | |
| Yes | 58 (69) |
| No | 26 (31) |
| Priority review | |
| Yes | 73 (87) |
| No | 11 (13) |
| Breakthrough therapy designation | |
| Yes | 50 (60) |
| No | 34 (40) |
| No. of trials supporting approval | |
| 1 | 72 (86) |
| >1 | 12 (14) |
Ten indications initially approved through the accelerated approval pathway were further converted to regular approval. Only the confirmatory trial was retained and reviewed.
Characteristics of Pivotal Trials
The characteristics of 84 pivotal trials supporting genome-targeted drug approvals are shown in Table 2. Among them, 45 of 84 approvals (54%) were based on phase 1 or phase 2 data, 45 of 84 (54%) were supported by single-arm trials, 67 of 84 (80%) stemmed from an open-label trial, and 48 (57%) were approved on the basis of subgroup analyses of the pivotal trials, including 35 cohorts in phase 1/2 clinical trials and 13 exploratory or preplanned subgroup analyses of RCTs. The median (IQR) sample size was 130 (61-378). Approvals were based on ORR in 46 trials (55%), with a median (IQR) DOR of 11.1 (9.2-19.8) months. In 25 of the 46 trials (54%), the median DOR was not reached at approval. In 19 of 46 trials (41%), the end point was having an ORR superior to 60%, with a median (IQR) ORR of 57% (40%-69%). Seven indications (8%) were supported by trials demonstrating an improvement in OS. All 84 of the included studies reported sponsorship/external funding by the for-profit industry, and of these, 13 studies (15%) also reported partial sponsorship/funding by nonprofit organizations.
Table 2. Characteristics of Included Pivotal Trials and Data Points Supporting Genome-Targeted Drug Indications.
| Characteristic | No. (%) | |
|---|---|---|
| Trials | Data pointsa | |
| Trials | 84 (100) | 179 (100) |
| Decision basis | ||
| Subgroup analysis | 48 (57) | 141 (79) |
| Entire study population | 36 (43) | 37 (21) |
| Sample size, median (IQR) | 130 (61-378) | 29 (4-177) |
| Tumor type | ||
| Lung cancer | 27 (32) | 33 (18) |
| Breast cancer | 12 (14) | 17 (9) |
| Colon cancer | 5 (6) | 9 (5) |
| Prostate cancer | 2 (2) | 5 (3) |
| Other | 38 (45) | 115 (64) |
| Study design | ||
| RCT | 39 (46) | 43 (24) |
| Single-arm | 45 (54) | 136 (76) |
| Study phase | ||
| 1 | 8 (9) | 24 (13) |
| 1/2 | 16 (19) | 51 (28) |
| 2 | 21 (25) | 61 (34) |
| 3 | 38 (45) | 42 (23) |
| Real-world | 1 (1) | 1 (1) |
| Blinding | ||
| Open-label | 67 (80) | 160 (89) |
| Double-blind | 17 (20) | 19 (11) |
| Primary end point | ||
| Overall survival | 7 (8) | 8 (4) |
| Intermediate end point | 77 (92) | 171 (96) |
| ORR | 46 (55) | 137 (77) |
| PFS | 26 (31) | 29 (16) |
| DFS | 5 (6) | 5 (3) |
| Funding | ||
| For-profit industry (full) | 71(85) | NA |
| For-profit industry (partial) | 13 (15) | NA |
| Nonprofit organization | 0 | NA |
Abbreviations: DFS, disease-free survival; NA, not applicable; ORR, overall response rate; PFS, progression-free survival; RCT, randomized clinical trial.
Data points included pivotal trials, supplemental trials, and subgroup analyses.
Level of Evidence Attributed to Molecular Targets and Magnitude of Clinical Benefit
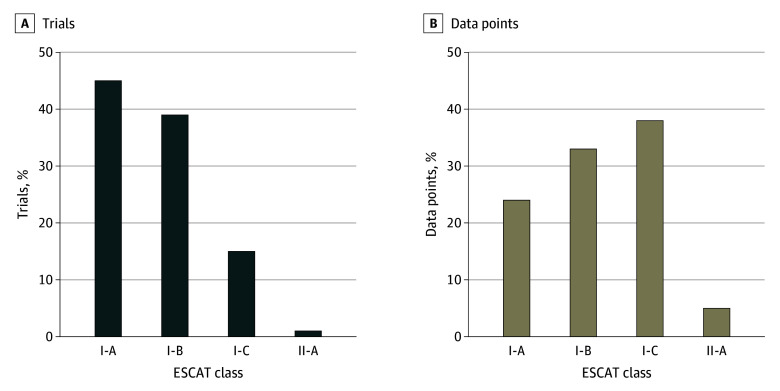
When ESCAT was applied to the 84 trials supporting genome-targeted indication approvals, 38 pivotal trials (45%) were graded as class I-A (prospective randomized); 32 (38%) as class I-B (prospective nonrandomized); 13 (16%) as class I-C (basket); and 1 (1%) as class II-A (retrospective) (Figure 2A).
Figure 2. ESCAT Class Distribution of Molecular Targets.
This figure shows the European Society for Medical Oncology Scale for Clinical Actionability of Molecular Targets (ESCAT) class distribution of the molecular targets of genome-targeted drugs approved by the US Food and Drug Administration from 2015 to 2022. Class I-C evidence was connected to basket trials showing similar clinical benefits across tumor types, and class II-A represents drugs approved based on retrospective studies alone. The pivotal trial supporting an indication is the trial or cohort reported in section 14 of the labeling (A). Data points included pivotal trials, supplemental trials, and subgroup analyses (B).
Among the 84 trials supporting genome-targeted indication approvals, 24 (29%) met the ESMO-MCBS threshold for substantial clinical benefit. Specifically, of the 45 single-arm trials assessable with the ESMO-MCBS, 27 (60%) received a grade 3, 12 (27%) received a grade 2, 2 (4%) a grade 1, and 3 (6%) a grade 0. Only 1 trial achieved a grade of 4.
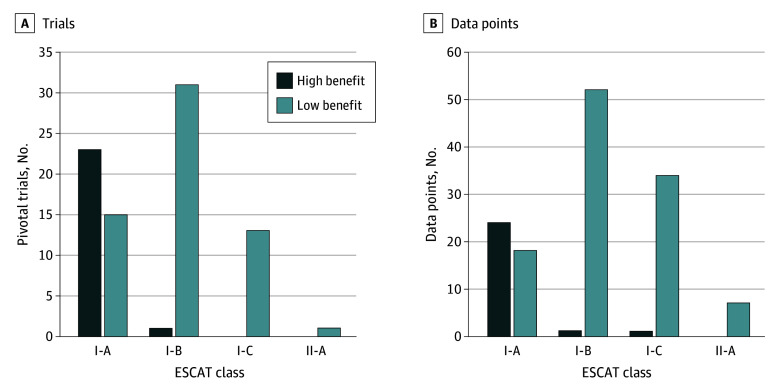
Of the 38 class I-A genome-targeted drugs, 23 (60%) demonstrated substantial clinical benefit. Of the 32 class I-B genome-targeted drugs, only 1 drug was based on a trial meeting the ESMO-MCBS thresholds for substantial clinical benefit. Among class I-C or class II-A genome-targeted drugs, none were supported by trials meeting the ESMO-MCBS thresholds for substantial clinical benefit. Overall, molecular targets from ESCAT category levels I-A and I-B associated with substantial clinical benefit by ESMO-MCBS covered 24 trials (29%) (Figure 3A).
Figure 3. ESCAT Class Distribution of Molecular Targets by High Benefit vs Low Benefit.
This figure shows the European Society for Medical Oncology Scale for Clinical Actionability of Molecular Targets (ESCAT) class distribution of the molecular targets of genome-targeted drugs approved by the US Food and Drug Administration from 2015 to 2022 stratified by high-benefit vs low-benefit classifications. The pivotal trial supporting an indication is the trial or cohort reported in section 14 of the labeling (A). Data points included pivotal trials, supplemental trials, and subgroup analyses (B).
Secondary Analysis
Among the 84 pivotal trials, 12 included multiple subgroups, and 2 were also supported by efficacy supplemental trials suitable for analysis. Consequently, considering separately each data point (pivotal trials, supplemental trials, and subgroup analyses), a total of 179 sets of results were available for analysis.
Characteristics of all analyzable results supporting genome-targeted drug approvals are shown in Table 2. A total of 136 approvals (76%) were based on phase 1 or phase 2 data, 136 (76%) were supported by single-arm trials, 160 (89%) were open-label trials, and 141 (79%) were approved based on subgroup analyses of the pivotal trials. The median (IQR) sample size was 29 (4-177). Approvals were based on ORR in 137 cases (77%). Among the 94 with an evaluable ORR (69%), the median (IQR) ORR was 46% (36%-69%), with 33 of 94 indications (35%) with an ORR superior to 60%. Eight (4%) of the expanded cohort of trial results showed significant improvement in OS.
The level of evidence attributed to molecular targets among this larger cohort of trial data was weaker than the primary cohort (Figure 2B). In this analysis, 42 molecular targets (23%) achieved a 1-A ESCAT level of evidence; 59 (33%) were rated I-B; 68 (38%) were rated I-C; and 10 (6%) were rated II-A.
ESMO-MCBS was applied to 135 sets of results (75%). It was not applied to 44 subgroup data points: 43 subgroups from single-arm trials in which the number of patients was too small to report an ORR benefit and for 1 preplanned cohort from an RCT with no differences between arms. Twenty-six (19%) met the threshold for substantial clinical benefit, of which one was class I-C (Figure3B). Thus, high-value molecular targets that have earned an ESCAT category level I-A or I-B and are associated with substantial clinical benefit by ESMO-MCBS were limited to 25 of 135 (19%) in the expanded cohort of clinical trials.
Discussion
In this cohort study involving 84 indications supporting genome-targeted cancer drug approvals between January 1, 2015, and December 31, 2022, and covering 50 molecular targets, approximately half were approved based on single-arm trials with response rate as a main end point. Despite high and durable ORRs, fewer than one-third of drugs met the threshold of substantial clinical benefit according to ESMO-MCBS criteria, and fewer than one-tenth demonstrated an OS benefit. When the evaluation of the molecular targets was added to the analysis, combining ESMO-MCBS and ESCAT scores, high-benefit molecular targets made up less than one-third of pivotal clinical trials supporting these drugs.
Genomic testing is increasingly used in cancer care. Since the first approval of a genomic-targeted drug in 1998 (trastuzumab), the number of new drug indications granted for genome-targeted drug approvals in the US has sharply increased,35 representing 40% of all solid cancer drug indications during the study period. To our knowledge, this study is the first to systematically evaluate the clinical benefit associated with FDA-approved indications for genome-targeted drugs and their corresponding targets. We applied the ESMO-MCBS and ESCAT value frameworks to identify therapies and molecular targets providing high clinical value that should be widely available to patients. This approach could enable payers, governments, and individual patients to prioritize the availability of high-value molecular-targeted therapies over other drugs. However, we found that drug indications supported by these characteristics represent a minority of cancer drug approvals in recent years. Our findings follow earlier29,36,37 and more recent30,38,39,40 research that assessed cancer treatments without taking the mechanism of action and targetability into account and evaluating clinical benefit using only the ESMO-MCBS framework. These studies demonstrated that approximately 30% of drugs achieved substantial clinical benefit according to the ESMO-MCBS, a percentage quite similar to our findings (eTable 4 in Supplement 1).
Previous data suggest that the FDA is more likely to approve cancer drugs with early but promising data if these therapies are associated with a genome-based companion diagnostic test.41 That study showed that, although the number of trials meeting substantial benefit thresholds using the ESMO-MCBS at the time of FDA cancer drug approval is low, drugs approved with a companion diagnostic test were associated with greater clinical benefit during postapproval use.41 Overall, this reflects a continued shift toward surrogate measures as end points in clinical trials and less reliance on an RCT at the time of approval, which is more pronounced for molecular-targeted agents. In this context, the ESMO-MCBS Working Group developed a clinical benefit scale for single-arm studies.9 To attain the highest efficacy score (grade 3), a single-arm trial must demonstrate either median PFS of more than 6 months, ORR of more than 60%, or ORR between 20% and 60% coupled with DOR of 9 months or longer. Preliminary scores are subject to downgrades due to safety concerns or upgrades predicated on QoL improvements or confirmatory phase 4 data availability. Although 60% of single-arm studies in the present cohort attained a grade 3, only 1 trial achieved a grade 4. Overall, these findings highlight that more than half of single-arm trials present moderate and promising yet unconfirmed clinical benefits before approval. Capturing substantial clinical benefit within the ESMO-MCBS scale remains challenging due to the limitations of single-arm trials in evaluating QoL data and the unavailability of confirmatory phase 4 data at the time of approval.
The use of surrogate measures for cancer drug approvals based on small numbers of study patients increases uncertainty around the actual benefit of those products.42 Such end points do not directly measure how patients feel or how long they survive and may be more sensitive to selection bias. In general, the greater uncertainty about the end points used to assess clinical benefit suggests that more data should be required to support approval, including larger effect size, internal consistency using secondary end points, randomized data, supportive clinical trials, and, for drugs approved under the accelerated-approval pathway, confirmatory randomized postmarketing trials. However, often this is not the case, as in the present study, which demonstrated that 16% of fully approved indications were given based on single-arm trials, many with small sample sizes or stemming from exploratory analyses. This observation also leads to a more limited understanding of their clinical benefit and considerable uncertainty for policymakers; furthermore, it raises several questions.43 For example, how do genome-targeted drugs affect health-related QoL or OS? Additionally, with uncertainties about the definitive benefit of these drugs, should their prices be lower?
Limitations
First, we used the ESMO-MCBS as an assessment of clinical benefit, which inherently shares the same limitations as the trials being evaluated, especially for single-arm trials. Second, we only evaluated trials that supported regulatory approval, and outcomes of postapproval clinical studies were only included for accelerated approvals that were converted to regular approval during the study period. The postapproval study is especially important for drugs approved through the accelerated-approval pathway because these might lead to changes in ESMO-MCBS grades and ESCAT levels of evidence over time. Finally, for approved drugs, the ESCAT scale alone provides limited utility because once a drug is approved, it automatically implies that the target-genome-targeted drug pair is ready to use and achieves a tier I rating. However, in this context, the different effects on survival end points of target-drug pairs, along with existing variations in the magnitude of such benefit, can be indicative of their value as biomarkers in specific settings. For this reason, adding the ESMO-MCBS rating alongside the ESCAT tier when reporting the benefit of each target-drug combination would be more appropriate.
Conclusions
The results of this cohort study demonstrated that for new cancer drugs, clinical benefit frameworks like those developed by ESMO help to identify therapies and molecular targets that provide high clinical benefit. Although next-generation sequencing has helped personalize cancer therapy for a growing number of patients, robust trials supporting the drug approvals should still be preferred to retrospective, exploratory analyses, followed by required postapproval studies and monitoring. These data reinforce the need for continued engagement of all stakeholders in generating adequate data to enable high-value cancer care for patients.
eTable 1. Pivotal trials, Supplemental Trials, Subgroup Analyses, and Case Examples
eTable 2. ESCAT Levels of Evidence and Examples
eTable 3. ESMO-MCBS Version 1.1 Levels of Evidence and Examples
eTable 4. Clinical Benefits of Cancer Drugs Approved by Different Regulatory Agencies
eFigure. Molecular Targets Supporting Genome-Targeted Drugs Approved by the FDA 2015-2022
eReferences
Data Sharing Statement
References
- 1.Levêque D. Off-label use of targeted therapies in oncology. World J Clin Oncol. 2016;7(2):253-257. doi: 10.5306/wjco.v7.i2.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mateo J, Chakravarty D, Dienstmann R, et al. A framework to rank genomic alterations as targets for cancer precision medicine: the ESMO Scale for Clinical Actionability of Molecular Targets (ESCAT). Ann Oncol. 2018;29(9):1895-1902. doi: 10.1093/annonc/mdy263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veenstra DL, Mandelblatt J, Neumann P, Basu A, Peterson JF, Ramsey SD. Health economics tools and precision medicine: opportunities and challenges. Forum Health Econ Policy. 2020;23(1). doi: 10.1515/fhep-2019-0013 [DOI] [PubMed] [Google Scholar]
- 4.Weymann D, Pataky R, Regier DA. Economic evaluations of next-generation precision oncology: a critical review. JCO Precis Oncol. 2018;2:1-23. doi: 10.1200/PO.17.00311 [DOI] [PubMed] [Google Scholar]
- 5.Tan O, Shrestha R, Cunich M, Schofield DJ. Application of next-generation sequencing to improve cancer management: a review of the clinical effectiveness and cost-effectiveness. Clin Genet. 2018;93(3):533-544. doi: 10.1111/cge.13199 [DOI] [PubMed] [Google Scholar]
- 6.Phillips KA, Deverka PA, Marshall DA, et al. Methodological issues in assessing the economic value of next-generation sequencing tests: many challenges and not enough solutions. Value Health. 2018;21(9):1033-1042. doi: 10.1016/j.jval.2018.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Paggio JC, Berry JS, Hopman WM, et al. Evolution of the randomized clinical trial in the era of precision oncology. JAMA Oncol. 2021;7(5):728-734. doi: 10.1001/jamaoncol.2021.0379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cherny NI, Sullivan R, Dafni U, et al. A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS). Ann Oncol. 2015;26(8):1547-1573. doi: 10.1093/annonc/mdv249 [DOI] [PubMed] [Google Scholar]
- 9.Cherny NI, Dafni U, Bogaerts J, et al. ESMO–Magnitude of Clinical Benefit Scale version 1.1. Ann Oncol. 2017;28(10):2340-2366. doi: 10.1093/annonc/mdx310 [DOI] [PubMed] [Google Scholar]
- 10.Wild C, Grössmann N, Bonanno PV, et al. Utilisation of the ESMO-MCBS in practice of HTA. Ann Oncol. 2016;27(11):2134-2136. doi: 10.1093/annonc/mdw297 [DOI] [PubMed] [Google Scholar]
- 11.Hammerman A, Greenberg-Dotan S, Feldhamer I, Birnbaum Y, Cherny NI. The ESMO–Magnitude of Clinical Benefit Scale for novel oncology drugs: correspondence with three years of reimbursement decisions in Israel. Expert Rev Pharmacoecon Outcomes Res. 2018;18(1):119-122. doi: 10.1080/14737167.2017.1343146 [DOI] [PubMed] [Google Scholar]
- 12.Gennari A, André F, Barrios CH, et al. ; ESMO Guidelines Committee . ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. 2021;32(12):1475-1495. doi: 10.1016/j.annonc.2021.09.019 [DOI] [PubMed] [Google Scholar]
- 13.Mosele F, Remon J, Mateo J, et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: a report from the ESMO Precision Medicine Working Group. Ann Oncol. 2020;31(11):1491-1505. doi: 10.1016/j.annonc.2020.07.014 [DOI] [PubMed] [Google Scholar]
- 14.Le Tourneau C, Delord JP, Gonçalves A, et al. ; SHIVA investigators . Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): a multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. 2015;16(13):1324-1334. doi: 10.1016/S1470-2045(15)00188-6 [DOI] [PubMed] [Google Scholar]
- 15.Massard C, Michiels S, Ferté C, et al. High-throughput genomics and clinical outcome in hard-to-treat advanced cancers: results of the MOSCATO 01 trial. Cancer Discov. 2017;7(6):586-595. doi: 10.1158/2159-8290.CD-16-1396 [DOI] [PubMed] [Google Scholar]
- 16.André F, Bachelot T, Commo F, et al. Comparative genomic hybridisation array and DNA sequencing to direct treatment of metastatic breast cancer: a multicentre, prospective trial (SAFIR01/UNICANCER). Lancet Oncol. 2014;15(3):267-274. doi: 10.1016/S1470-2045(13)70611-9 [DOI] [PubMed] [Google Scholar]
- 17.Tsimberidou AM, Wen S, Hong DS, et al. Personalized medicine for patients with advanced cancer in the phase I program at MD Anderson: validation and landmark analyses. Clin Cancer Res. 2014;20(18):4827-4836. doi: 10.1158/1078-0432.CCR-14-0603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Priestley P, Baber J, Lolkema MP, et al. Pan-cancer whole-genome analyses of metastatic solid tumours. Nature. 2019;575(7781):210-216. doi: 10.1038/s41586-019-1689-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.US Food and Drug Administration . New molecular entity (NME) drug and new biologic approvals. Accessed September 29, 2023. https://www.fda.gov/drugs/development-approval-process-drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products
- 20.Amir E, Seruga B, Martinez-Lopez J, et al. Oncogenic targets, magnitude of benefit, and market pricing of antineoplastic drugs. J Clin Oncol. 2011;29(18):2543-2549. doi: 10.1200/JCO.2011.35.2393 [DOI] [PubMed] [Google Scholar]
- 21.US Food and Drug Administration . List of Cleared or approved companion diagnostic devices (in vitro and imaging tools). Accessed September 29, 2023. https://www.fda.gov/medical-devices/in-vitro-diagnostics/list-cleared-or-approved-companion-diagnostic-devices-in-vitro-and-imaging-tools
- 22.List of targeted therapy drugs approved for specific types of cancer. Accessed September 29, 2023. https://www.cancer.gov/about-cancer/treatment/types/targeted-therapies/approved-drug-list
- 23.Strengthening the Reporting of Observational Studies in Epidemiology (STROBE). Accessed September 29, 2023. https://www.strobe-statement.org/
- 24.US Food and Drug Administration . Priority NDA and BLA approvals. Accessed September 29, 2023. https://www.fda.gov/drugs/nda-and-bla-approvals/priority-nda-and-bla-approvals
- 25.US Food and Drug Administration . Breakthrough therapy designated drugs. Accessed September 29, 2023. https://www.fda.gov/drugs/nda-and-bla-approvals/breakthrough-therapy-approvals
- 26.US Food and Drug Administration . Search Orphan Drug Act designation. Accessed September 29, 2023. https://www.accessdata.fda.gov/scripts/opdlisting/oopd/
- 27.US Food and Drug Administration . Accelerated Approval Program. Accessed September 29, 2023. https://www.fda.gov/drugs/nda-and-bla-approvals/accelerated-approval-program
- 28.US Food and Drug Administration . Verified Clinical Benefit. Cancer Accelerated Approvals. Accessed September 29, 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/verified-clinical-benefit-cancer-accelerated-approvals
- 29.Tibau A, Molto C, Ocana A, et al. Magnitude of clinical benefit of cancer drugs approved by the US Food and Drug Administration. J Natl Cancer Inst. 2018;110(5):486-492. doi: 10.1093/jnci/djx232 [DOI] [PubMed] [Google Scholar]
- 30.Molto C, Hwang TJ, Borrell M, et al. Clinical benefit and cost of breakthrough cancer drugs approved by the US Food and Drug Administration. Cancer. 2020;126(19):4390-4399. doi: 10.1002/cncr.33095 [DOI] [PubMed] [Google Scholar]
- 31.Agrawal S, Arora S, Amiri-Kordestani L, et al. Use of single-arm trials for US Food and Drug Administration drug approval in oncology, 2002-2021. JAMA Oncol. 2023;9(2):266-272. doi: 10.1001/jamaoncol.2022.5985 [DOI] [PubMed] [Google Scholar]
- 32.Amatya AK, Fiero MH, Bloomquist EW, et al. Subgroup analyses in oncology trials: regulatory considerations and case examples. Clin Cancer Res. 2021;27(21):5753-5756. doi: 10.1158/1078-0432.CCR-20-4912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.ESMO-MCBS scorecards. Accessed September 29, 2023. https://www.esmo.org/guidelines/esmo-mcbs/esmo-mcbs-for-solid-tumours/esmo-mcbs-scorecards
- 34.ESMO-MCBS evaluation forms. Accessed September 29, 2023. https://www.esmo.org/guidelines/esmo-mcbs/esmo-mcbs-for-solid-tumours/esmo-mcbs-evaluation-forms
- 35.Zhong L, Li Y, Xiong L, et al. Small molecules in targeted cancer therapy: advances, challenges, and future perspectives. Signal Transduct Target Ther. 2021;6(1):201. doi: 10.1038/s41392-021-00572-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grössmann N, Del Paggio JC, Wolf S, et al. Five years of EMA-approved systemic cancer therapies for solid tumours-a comparison of two thresholds for meaningful clinical benefit. Eur J Cancer. 2017;82:66-71. doi: 10.1016/j.ejca.2017.05.029 [DOI] [PubMed] [Google Scholar]
- 37.Vivot A, Jacot J, Zeitoun JD, Ravaud P, Crequit P, Porcher R. Clinical benefit, price and approval characteristics of FDA-approved new drugs for treating advanced solid cancer, 2000-2015. Ann Oncol. 2017;28(5):1111-1116. doi: 10.1093/annonc/mdx053 [DOI] [PubMed] [Google Scholar]
- 38.Grössmann N, Wolf S, Rothschedl E, Wild C. Twelve years of European cancer drug approval—a systematic investigation of the ‘magnitude of clinical benefit’. ESMO Open. 2021;6(3):100166. doi: 10.1016/j.esmoop.2021.100166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adam R, Tibau A, Molto Valiente C, et al. Clinical benefit of cancer drugs approved in Switzerland 2010-2019. PLoS One. 2022;17(6):e0268545. doi: 10.1371/journal.pone.0268545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tibau A, Molto C, Borrell M, et al. Magnitude of clinical benefit of cancer drugs approved by the US Food and Drug Administration based on single-arm trials. JAMA Oncol. 2018;4(11):1610-1611. doi: 10.1001/jamaoncol.2018.4300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bujosa A, Moltó C, Hwang TJ, et al. Associations with definitive outcomes and clinical benefit of cancer drugs at the time of marketing approval and in the postmarketing period. J Natl Compr Canc Netw. 2021;19(13):1-9. doi: 10.6004/jnccn.2021.7003 [DOI] [PubMed] [Google Scholar]
- 42.Avorn J. Surrogate measures of drug efficacy—a finger pointing at the moon. JAMA Netw Open. 2023;6(4):e238835. doi: 10.1001/jamanetworkopen.2023.8835 [DOI] [PubMed] [Google Scholar]
- 43.Trapani D, Tay-Teo K, Tesch ME, et al. Implications of oncology trial design and uncertainties in efficacy-safety data on health technology assessments. Curr Oncol. 2022;29(8):5774-5791. doi: 10.3390/curroncol29080455 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Pivotal trials, Supplemental Trials, Subgroup Analyses, and Case Examples
eTable 2. ESCAT Levels of Evidence and Examples
eTable 3. ESMO-MCBS Version 1.1 Levels of Evidence and Examples
eTable 4. Clinical Benefits of Cancer Drugs Approved by Different Regulatory Agencies
eFigure. Molecular Targets Supporting Genome-Targeted Drugs Approved by the FDA 2015-2022
eReferences
Data Sharing Statement