Abstract
In the presence of cefoxitin, which inhibits septum formation during sporulation, Streptomyces griseus is unable to sporulate, retaining the sonication sensitivity of nonsporulating hyphae. Cefoxitin- and sonication-resistant mutant SKK2600 was isolated and showed many morphological differences from its parental strain. A 3.6-kb DNA fragment that complemented the mutations of SKK2600 contained two open reading frames (ORFs), either of which could complement SKK2600. One ORF, designated ssfR, encoded a protein containing a potential DNA-binding helix-turn-helix motif close to its N terminus. SsfR is similar to members of a large family of transcriptional regulators, particularly IclR of Escherichia coli. The second ORF was identified as ssgA, a previously described sporulation gene from S. griseus (S. Kawamoto and J. C. Ensign, Actinomycetology 9:136–151, 1995). A point mutation of C to T seven nucleotides upstream of the UGA stop codon of ssfR was responsible for the phenotype of isolated mutant strain SKK2600. Surprisingly, this mutation should not change the primary structure of SsfR. The ssfR and ssgA disruption mutants were constructed and showed the “white” mutant phenotype, with some growth medium dependence. In addition, the ssfR null mutant sporulated ectopically in phosphate starvation medium.
Streptomycetes are aerobic gram-positive soil bacteria that grow as multinucleoidal, multicellular, branched filaments and that undergo morphological and physiological differentiation in response to environmental factors (4–6, 20). The first sign of morphological differentiation is the formation of aerial sporogenic hyphae, which give a fuzzy, white appearance to the colonies. When these specialized hyphae have stopped growing, DNA segregates and sporulation septa form to generate the uninucleoidal compartments that become the spores. Sporulation septation occurs relatively synchronously throughout a single sporogenic hypha (22, 32).
Most studies of Streptomyces sporulation have been accomplished by the characterization of nonsporulating mutants of Streptomyces coelicolor. These mutants have been divided into bald (bld) strains, which appear not to form aerial hyphae, and white (whi) strains, which form aerial hyphae but which are blocked at various stages of sporulation. In S. coelicolor, 10 classes of bald mutants have been characterized: bld-261, bldA, bldB, bldC, bldD, bldF, bldG, bldH, bldI, and bldK (3, 8, 26, 27, 34, 35), and other constructed null mutants have displayed a bald phenotype (e.g., relA mutants [2]). In addition to their inability to produce aerial hyphae, many bald mutants are also defective in antibiotic production (3, 4), catabolite repression (29), and cell-cell signaling (34, 35). The highly pleiotropic phenotype of these mutants suggests that they identify genes involved at an early stage in the initiation of development and that the synthesis of antibiotics and the formation of aerial hyphae share some important regulatory links. Aerial mycelium formation and sporulation are partially restored to several bld mutants when they are grown on mannitol minimal medium (MM+M) rather than on complex or glucose minimal medium (MM+G) (3, 4, 6, 26). Several of the whi genes encode likely regulatory proteins (5). For example, whiG encodes a sigma factor (7, 25, 33) and whiH encodes a protein with similarity to members of a large family of bacterial regulatory proteins. Many proteins in this family are repressors of some genes involved in carbon metabolism (30).
We studied the sporulation of Streptomyces griseus, a species that can be induced to sporulate in submerged culture in response to phosphate or nutritional downshift (20, 21). With the submerged sporulation system of S. griseus, physiological and ultrastructural studies of rapidly sporulating cultures have been conducted (13, 22) and the time course of gene expression during sporulation has been determined (9). In previous studies, it was shown that cefoxitin completely prevented sporulation without affecting vegetative growth (13). Electron-microscopic examination of sporulating cultures that had been exposed to cefoxitin during 12 h of induction confirmed that this antibiotic inhibited sporulation septation (13). These results suggest that a sporulation-specific, penicillin-binding protein (PBP) is a target of cefoxitin during sporulation of S. griseus. To investigate this cefoxitin-binding protein, a cefoxitin-resistant mutant was isolated by a sonication enrichment method. This mutant showed not only cefoxitin resistance but also some other phenotypes that are different from those of the wild-type strain. To study this mutant further, a complementing DNA fragment has been cloned and characterized in the present study. It is shown to contain two genes (ssfR and ssgA) required for sporulation.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and microbiological techniques.
All of the bacterial strains and plasmids used in this study are listed in Tables 1 and 2, respectively. Escherichia coli strain DH5α was used for construction and propagation of plasmids. E. coli strain ET12567 (dam dcm) (24) was used when introducing plasmids from E. coli into S. griseus. Plasmid DNA isolated from E. coli ET12567 was methylated at 37°C for 2 h by mixing plasmid DNA with SstI and AluI methylase (New England Biolabs, Beverly, Mass.) in a methylase buffer containing 80 μM S-adenosylmethionine. S. griseus was induced to sporulate in submerged culture by phosphate starvation (20). The alkaline lysis method modified by Babcock and Kendrick (1) was used to isolate plasmids for restriction mapping, DNA fragment isolation, and transformation. Genomic DNA isolation from Streptomyces strains was carried out as described by Hopwood et al. (15). The DNA fragments used for ligation and as probes in hybridization analyses were prepared with the phenol-freeze-fracture method (16). DNA sequence was determined by using the exonuclease III-generated nested deletion method (14). Competent cells of E. coli strains were prepared and transformed with plasmid DNA as described by Sambrook et al. (31). Transformation of Streptomyces protoplasts with plasmid DNA was as described by Babcock and Kendrick (1) and Hopwood et al. (15). Microscopy, photography, and image processing were as described by Hao and Kendrick (13). All S. griseus cultures were incubated at 30°C. Minimal media supplemented with the appropriate carbon source were made as described previously (20).
TABLE 1.
Bacterial strains used in this work
| Strain | Genotype or phenotype | Source or reference |
|---|---|---|
| E. coli strains | ||
| DH5 | C. J. Daniels | |
| ET12567 | dcm dam | 24 |
| B. subtilis strain | ||
| ATCC 6633 | Streptomycin sensitive | |
| S. griseus strains | ||
| NRRL B-2682 | Wild type | 20 |
| SKK2600 | Spontaneous mutant of NRRL B-2682 | This study |
| SKK2663 | ssfR::aac(3)IV | This study |
| SKK2664 | ssgA::aac(3)IV | This study |
| SKK2668 | ssfR::aac(3)IV | This study |
| ssgA::aac(3)IV |
TABLE 2.
Relevant features of plasmids used or constructed during this work
| Plasmid | Feature (reference) |
|---|---|
| pUC18 | Versatile E. coli vector (36) |
| pIJ2926 | pUC18 derivative with modified polylinker (17) |
| pKK2006 | pUC18 carrying tsr for selection in Streptomyces |
| pKK974 | 1.4-kb aac(3)IV-containing BamHI fragment in pIJ2925 (A. J. Dharmatilake, personal communication) |
| pKK1333 | pIJ2926 derivative containing 10-kb BamHI fragment from pKK1336 |
| pKK1334 | pIJ2926 derivative containing 10-kb BamHI fragment from pKK1337 |
| pKK1335 | pIJ2926 derivative containing 10-kb BamHI fragment from pKK1338 |
| pKK1336 | pXE4 derivative with 10-kb BamHI insert containing ssfR and ssgA |
| pKK1337 | pXE4 derivative with 10-kb BamHI insert containing ssfR and ssgA |
| pKK1338 | pXE4 derivative with 10-kb BamHI insert containing ssfR and ssgA |
| pKK1343 | pKK1333 derivative in which one BamHI site has been removed using KpnI digestion and religation |
| pKK1349 | pIJ2926 derivative containing 3.6-kb HincII insert from pKK1343 |
| pKK1351 | pIJ2926 derivative containing mutated version of 3.6-kb HincII insert |
| pKK1355 | pXE4 derivative containing 3.9-kb BglII insert from pKK1349 |
| pKK1356 | pXE4 derivative containing 3.9-kb BglII insert from pKK1351 |
| pKK1358 | pIJ2926 derivative containing 1.6-kb StyI insert from pKK1349 |
| pKK1359 | pIJ2926 derivative containing 1.2-kb MspA1I insert from pKK1349 |
| pKK1361 | pKK2006 derivative containing 3.9-kb BglII insert from pKK1349 |
| pKK1362 | pKK2006 derivative containing 3.9-kb BglII insert from pKK1351 |
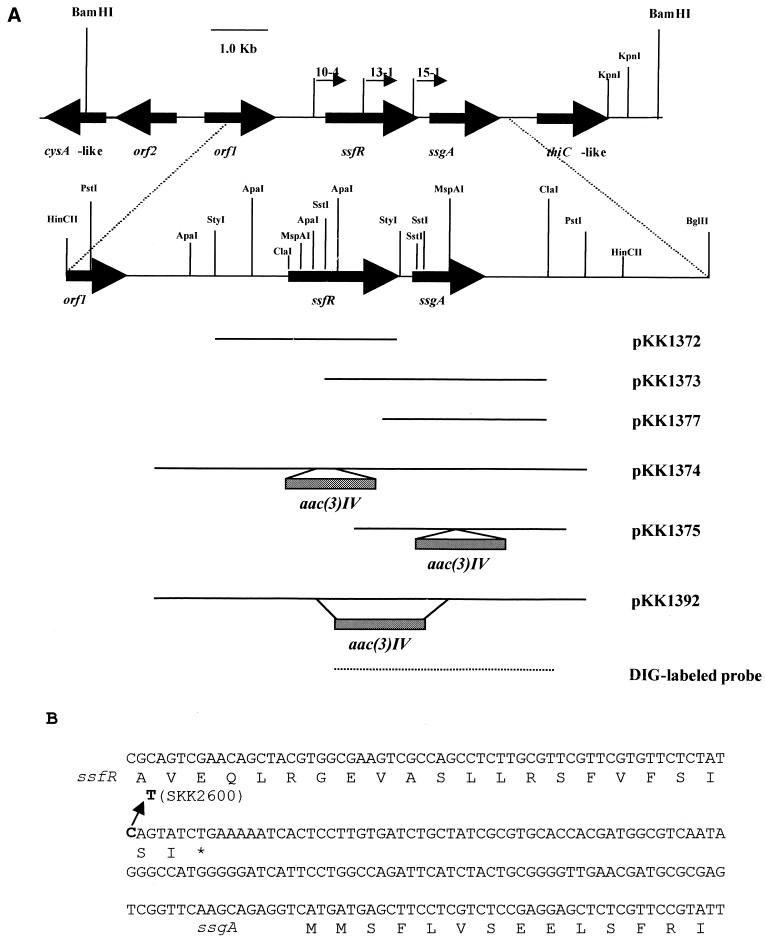
| pKK1367 | pIJ2926 derivative containing 2.5-kb PstI insert from pKK1343 ExoIII deletion fraction 10-4 (Fig. 1) |
| pKK1368 | pKK1343 ExoIII deletion fraction 13-1 derivative in which 5.7-kb ClaI-EcoRI fragment has been removed (Fig. 1) |
| pKK1369 | pKK1367 derivative in which 160-bp ApaI fragment has been removed |
| pKK1370 | pKK1369-based construct containing, within ssgR, a 1.4-kb BamHI fragment containing the aac(3)IV apramycin resistance marker, excised from pKK974 |
| pKK1371 | pKK1368-based construct containing, within ssgA, a 1.4-kb BamHI fragment containing the aac(3)IV apramycin resistance marker, excised from pKK974 |
| pKK1372 | pKK2006 derivative containing 1.5-kb BglII insert from pKK1358 |
| pKK1373 | pKK2006 derivative containing 1.4-kb BglII insert from pKK1368 |
| pKK1374 | pKK2006 derivative containing 3.9-kb BglII insert from pKK1370 |
| pKK1375 | pKK2006 derivative containing 2.8-kb BglII insert from pKK1371 |
| pKK1376 | pKK1343 ExoIII deletion fraction 15-1 derivative in which 5.7-kb ClaI-EcoRI fragment has been removed (Fig. 1) |
| pKK1377 | pKK2006 derivative containing 1.2-kb BglII insert from pKK1376 |
| pKK1385 | pKK1367 derivative in which EcoRI-BamHI fragment has been removed |
| pKK1391 | pKK1367-based construct in which 600-bp SstI fragment has been replaced with 1.4-kb aac(3)IV apramycin resistance marker excised from pKK974 |
| pKK1392 | pKK2006 derivative containing 3.4-kb BglII insert from pKK1391 |
Isolation of SKK2600, an S. griseus mutant.
Mutant SKK2600 was isolated by a sonication enrichment method in the presence of cefoxitin (13). Spores from a fresh colony were inoculated into liquid sporulation medium (SpM) containing 50 μg of cefoxitin per ml and incubated at 30°C with shaking at 250 rpm for 4 days. A 0.5-ml aliquot of sample was collected in a 5-ml polyethylene tube and subjected to sonication as described by Kwak and Kendrick (22). Then 30 μl of this sonicated sample was inoculated into 3 ml of SpM containing 50 μg of cefoxitin per ml. After incubation for 4 days, the culture was again treated with sonication and inoculated into 3 ml of fresh SpM containing cefoxitin. This procedure was repeated several times in the same manner. Each time, samples were taken, diluted, and plated on SpM in the presence of cefoxitin for viable counts and morphological analysis.
Library construction.
A library of S. griseus SKK2600 DNA fragments was constructed by isolating total genomic DNA, digesting it with BamHI, and ligating purified 9- to 12-kb fragments into the BamHI site of pIJ2926. The ligation mixture was then introduced to E. coli DH5α competent cells. After overnight incubation at 30°C, 960 white and ampicillin-resistant colonies were transferred with toothpicks into 10 96-well plates containing 450 μl of Luria-Bertani medium with 100 μg of ampicillin/ml in each well. After incubation at 30°C for 12 h, 50 μl of dimethyl sulfoxide (Sigma) was added to each well and the cells were stored at −70°C.
Southern and colony hybridization.
A nonradioactive DNA-labeling and detection kit (Boehringer Mannheim) was used for Southern hybridization. The genomic DNA was digested with AlwNI, fractionated on an agarose gel, and blotted to a nylon membrane (Hybond N; Amersham) by capillary transfer. The DNA was cross-linked by irradiation with UV light for 1.5 min. Labeling, hybridization, and detection were carried out according to the manufacturer's directions. Colony hybridization was the same as Southern hybridization except for the preparation of the membrane. Colonies were replicated to nitrocellulose membranes located on the surface of Luria-Bertani agar containing an appropriate antibiotic from a library constructed in an array of 96-well plates. After overnight incubation at 37°C, the membrane was transferred onto a 3-MM paper soaked with a solution containing 0.5 M NaOH and 1.5 M NaCl and incubated for 30 min. The membrane was acidified on a 3-MM paper soaked with 1.0 M Tris-HCl (pH 8.0)–1.5 M NaCl for 30 min. The DNA was fixed to the membrane by baking them at 80°C for 2 h.
Cloning of a region containing ssfR and ssgA.
S. griseus NRRL B-2682 genomic DNA was digested with BamHI. DNA fragments (2 to 10 kb) isolated from a 1% agarose gel were ligated with BamHI-digested low-copy-number shuttle vector pXE4 (isolated from S. griseus). The ligation mixture was introduced into SKK2600 protoplasts. The plates were overlaid with 5 μg of thiostrepton/ml after 17 h of incubation and incubated for four more days. The transformants were screened for restoration of the wild-type grey-colony phenotype. Grey patches were further examined for sporulation by phase-contrast microscopy. Plasmids isolated from selected transformants were used to transform E. coli DH5α competent cells. The BamHI inserts were isolated and ligated with BamHI-digested pIJ2926. The ligation mixtures were introduced into E. coli DH5α. White and ampicillin-resistant colonies were selected.
Construction of ssfR and ssgA null mutants.
pIJ2926 and pKK2006 were used to construct the ssfR null mutant, ssgA null mutant, and ssfR-ssgA double-null mutant. In pKK1374 (Fig. 1; Table 2), a 160-bp ApaI fragment was replaced with the 1.4-kb aac(3)IV cassette in the middle of ssfR. This construct was used to replace the chromosomal ssfR with the aac(3)IV-disrupted version, using SKK2600 (the transformation efficiency of this mutant was much higher than that of the wild-type stain [see Results]) as the recipient strain. Apramycin-resistant colonies were selected and screened for sensitivity to thiostrepton. These colonies were confirmed to be disruptants of ssfR by Southern hybridization. One resulting strain, SKK2663, was chosen for further morphological analysis. ssgA null mutant SKK2664 and ssfR-ssgA double-mutant SKK2668 were constructed in a similar manner. For the ssgA null mutant, SKK2600 protoplasts were transformed with pKK1375 (Fig. 1; Table 2), in which a 24-bp SstI fragment was replaced with the 1.4-kb aac(3)IV cassette at the N terminus of ssgA. For the ssfR and ssgA double-null mutant, SKK2600 was transformed with pKK1392 (Fig. 1; Table 2), in which a 0.6-kb SstI fragment containing portions of ssfR and ssgA was replaced with the 1.4-kb aac(3)IV cassette.
FIG. 1.
Restriction map of a 10-kb BamHI DNA segment of S. griseus containing ssfR and ssgA and nucleotide sequences of part of this region. (A) Segments of DNA subcloned into various plasmids are indicated by shaded bars. DIG, digoxigenin. (B) The nucleotide sequences of the part containing the intergenic region between ssfR and ssgA and deduced amino acid sequences. Arrow, point mutation of C to T; asterisk, stop codon of ssfR.
Nucleotide sequence accession number.
The DNA sequence data of ssfR gene have been deposited in GenBank with accession number AF239808.
RESULTS
Phenotype of the SKK2600 mutant strain.
Because cefoxitin inhibits sporulation-specific septum formation, S. griseus cannot form spores in its presence and remains in the form of a sonication-sensitive mycelium (13). Selection for resistance to sonication in the presence of cefoxitin could potentially yield mutants either with the cefoxitin resistance PBP or with sporulation-associated cell division altered in some other ways to bypass the effects of cefoxitin. After five cycles of cefoxitin and sonication treatment, the whole population of cells had become resistant to the selection regime, and one representative colony, designated SKK2600, was isolated and studied further. This strain not only showed cefoxitin resistance but also exhibited some other phenotypes that were different from those of the wild-type strain (following data not shown): (i) it underwent fragmentation and formed spore-like bodies in all growth media and conditions tested, some of which do not permit sporulation of the wild-type strain; (ii) a nucleoid was visualized in each cell fragment upon treatment with propidium iodide (PI); (iii) there was no grey spore pigment production; (iv) the colonies were soft in texture; (v) the enzymatic activities of at least three PBPs, which were detected by Flu-APA (13) increased two- to fourfold; and (vi) its protoplast-mediated transformation efficiency with DNA was much higher than that of the wild-type stain.
Cloning and sequencing of a 10-kb BamHI fragment that complemented SKK2600.
In order to clone the region which could complement the phenotype of the SKK2600 mutant, a ligation mixture of BamHI-digested S. griseus wild-type DNA fragments and plasmid pXE4 was introduced into SKK2600 protoplasts. Thiostrepton-resistant transformants were screened for their ability to restore the SKK2600 strain to the wild-type phenotype. Three clones, containing plasmids pKK1336, pKK1337, pKK1338, restored the sporulation, pigment production, and other wild-type phenotypes to the SKK2600 mutant. Microscopic examination confirmed the presence of abundant spores of normal appearance in the complemented mutant. For the purpose of sequencing, the inserts were cloned into pIJ2926, generating plasmids pKK1333, pKK1334, and pKK1335, respectively. The sequences of all three inserts in these plasmids were subsequently shown to be the same. Only pKK1333 was used in further experiments.
The sequence of the 10-kb insert of pKK1333 revealed at least six open reading frames (ORFs) in this fragment (Fig. 1A). A comparison of the sequences to the database by using BLAST analysis suggested that three ORFs at one end of this fragment had high similarity to their ORF1, ORF2, and CysA gene-like homologues in S. coelicolor (23). Interestingly, in this S. griseus fragment there is no PtpA gene (23) homologue, which is located between ORF2 and the gene for the CysA-like protein in S. coelicolor. In the middle of the pKK1333 insert, there is an ORF that is the ssgA from S. griseus. This gene was first cloned by Kawamoto and Ensign (18) and encodes an acidic protein of approximately 15 kDa that shows no significant sequence homology to known proteins in the database. The ssgA gene, when present in high copy number in the wild-type strain, not only resulted in the suppression of sporulation but also caused the cells to grow in a fragmented rather than mycelial fashion (19). These results led us to hypothesize that ssgA might be responsible for the phenotype of mutant SKK2600.
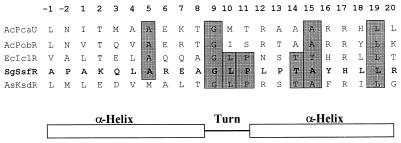
A 3.6-kb HincII fragment containing the entire ssgA gene was subcloned into pIJ2926 and pXE4, resulting in plasmids pKK1349 and pKK1355, respectively. SKK2600 protoplasts were transformed with in vitro-methylated pKK1355 DNA, and transformants were picked onto SpM medium for morphological analysis. All transformants showed the wild-type phenotype. This HincII fragment contains two ORFs, ORF1 and ORF2 (the ssgA gene). A search of the database by using the programs BLASTP and Psi-BLAST revealed that the ORF1 product was similar to members of a large family of transcriptional regulators, which are all involved in catabolic metabolism; the closest similarity was with IclR of E. coli. IclR is a repressor for the aceBAK operon, which is involved in the synthesis of the enzymes of the glyoxylate bypass when E. coli utilizes acetate as the sole carbon source (11, 12, 28). There is a putative helix-turn-helix (HTH) DNA-binding motif close to the N terminus of the ORF1 protein. Analysis for this motif using the Dodd and Egan weighted-matrix method (10) showed that the SD score was 3.8 (by this method, a SD score above 2.5 is considered strong evidence that a protein contains an HTH). The amino acid sequences forming the putative DNA-binding motifs of the product of ORF1 and IclR have very high similarity, as is found for other transcriptional regulators (Fig. 2). ORF1 was named the ssfR gene for reasons explained below.
FIG. 2.
Putative HTH motif and comparison with other DNA-binding proteins. For each protein, the 22-amino-acid alignment that constitutes the HTH motif is presented. The DNA-binding proteins which are selected for this alignment are PobR, the transcriptional activator of the Acinetobacter calcoaceticus pobA gene (AcPobR), IclR, the repressor of the E. coli aceBAK operon (EcIclR), PcaU, a regulatory protein of Acinetobacter sp. strain ADP1 (AcPcaU), and KsdR, a transcriptional regulator of Arthrobacter simplex (AsKsdR). Amino acids conserved with SsfR (SgSsfR) are shaded grey.
To verify that a mutation in the 3.6-kb HincII DNA fragment was responsible for the phenotype of mutant SKK2600, a library of SKK2600 chromosomal DNA was constructed in E. coli DH5α with plasmid pIJ2926. The 3.6-kb HincII fragment from the mutant strain was then cloned through colony hybridization and Southern hybridization, resulting in plasmid pKK1351. After the insert of pKK1351 was sequenced, a point mutation of C to T was identified seven nucleotides upstream of the UGA stop codon of ssfR and 138 nucleotides upstream of the putative AUG start codon of ssgA (Fig. 1B). This point mutation is located in the regulatory region of ssgA, which is required for the expression of ssgA, as described by Kawamoto and Ensign (18). Surprisingly, this mutation would not change the primary structure of SsfR protein, but it changes the isoleucine codon from AUC to AUU, which is a rare codon in Streptomyces. This point mutation also abolished one AlwNI digestion site, facilitating Southern analyses of chromosome structures during construction of ssfR and ssgA null mutants (see below). The mutant version of the 3.6-kb HincII fragment was subcloned into pXE4, resulting in plasmid pKK1356. SKK2600 was transformed with pKK1356, and transformants were picked onto SpM for morphological analysis. All transformants still showed the mutant phenotype and exhibited even more mycelial fragmentation than the SKK2600 strain.
It was considered possible that there was another mutation somewhere in the chromosome of mutant SKK2600 in addition to the point mutation identified above. To address this question, allele-exchange experiments were performed with plasmids pKK1361 and pKK1362 containing wild-type and mutated 3.6-kb HincII fragments, respectively, cloned in E. coli vector pKK2006, which cannot replicate in S. griseus but which contains the selectable Streptomyces marker tsr. Maintenance of the vector in S. griseus to confer thiostrepton resistance relies on homologous recombination between the plasmid insert and the host chromosome. The wild-type and mutant SKK2600 strains were transformed with pKK1362 and pKK1361, respectively. The thiostrepton-resistant transformants from both stains showed the wild-type phenotype. After several rounds of screening for double-crossover recombinants, it was found that two out of six thiostrepton-sensitive colonies isolated from the pKK1362-transformed wild-type strain showed the phenotype of the SKK2600 mutant strain. In the similar manner, one out of three thiostrepton-sensitive colonies isolated from pKK1361-transformed SKK2600 showed the wild-type phenotype. The arrangements of the chromosomal DNA in such strains were confirmed by Southern hybridization with AlwNI-digested genomic DNA to be those expected after excision of the plasmid by a second crossover (data not shown).
To test whether ssfR or ssgA alone could restore the wild-type phenotype to SKK2600, additional complementation experiments were carried out. First, a 1.6-kb StyI fragment containing only the wild-type ssfR gene was subcloned into pKK2006, resulting in plasmid pKK1372 (Fig. 1A). SKK2600 was transformed with pKK1372, and transformants screened were picked onto SpM for morphological analysis. All transformants showed the wild-type phenotypes. Second, SKK2600 was transformed with pKK1373 (Fig. 1A) containing the whole ssgA coding sequence and more than 0.8 kb of DNA from upstream. Fifty transformants were picked onto SpM for morphological analysis. All of them showed the wild-type phenotypes. When the insert was reduced to contain less than 15 nucleotides upstream of the mutation site, the resultant plasmid, pKK1377 (Fig. 1A), could not complement the colony morphology of the mutant SKK2600.
Disruption of ssfR.
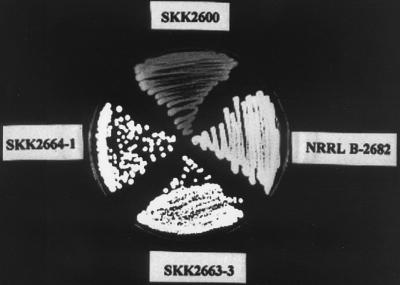
To further study the functions of these two genes, null mutants of both ssfR and ssgA were constructed (described in Materials and Methods). Screening for apramycin-resistant and thiostrepton-sensitive colonies resulted in the ssfR disruptant SKK2663. Since SKK2600 with a point mutation was used as recipient, the double crossover would be likely to occur on both sides of this point mutation to produce an ssfR disruptant without the C-T point mutation. The arrangement of chromosomal DNA in the SKK2663 mutant strain was confirmed by Southern hybridization with AlwNI-digested genomic DNA (data not shown). The colonies of the SKK2663 strain were white on SpM (Fig. 3). In contrast, the S. griseus wild-type colonies were grey on SpM. By using phase-contrast microscopy, it could be seen that the SKK2663 strain produced aerial hyphae but no spores.
FIG. 3.
Comparison of colony morphology between the wild-type strain and mutants grown on SpM. Wild-type strain NRRL B-2682 shows grey. Mutant SKK2600 shows light yellow. ssfR and ssgA null mutants SKK2663-3 and SKK2664-1 show white.
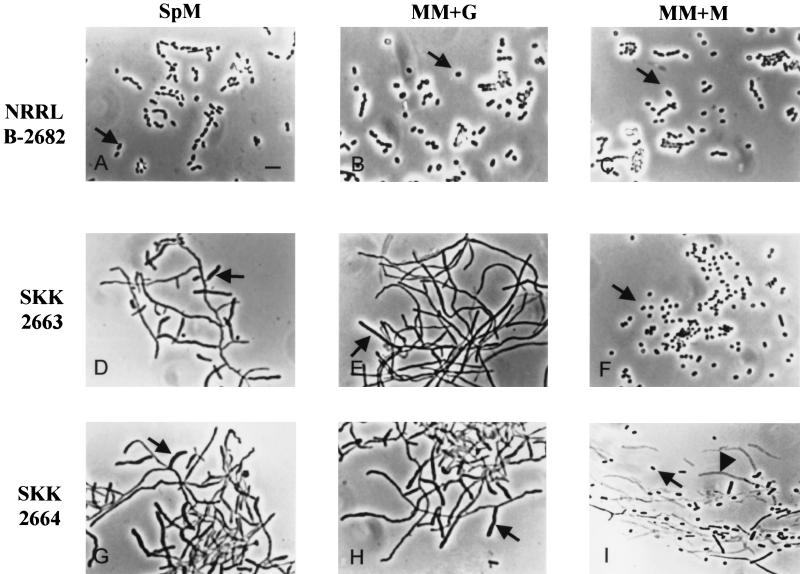
Since the ssfR gene product was similar to an array of catabolic regulators, SsfR might also be involved in the primary metabolism of S. griseus. To further characterize the ssfR mutant, MM+G, MM+M, and phosphate starvation medium were used. Compared to the wild-type strain, the SKK2663 strain could not sporulate in MM+G and SpM but sporulated normally in MM+M (Fig. 4D to F). SKK2663 showed a distinct phenotype in phosphate starvation medium, as described below.
FIG. 4.
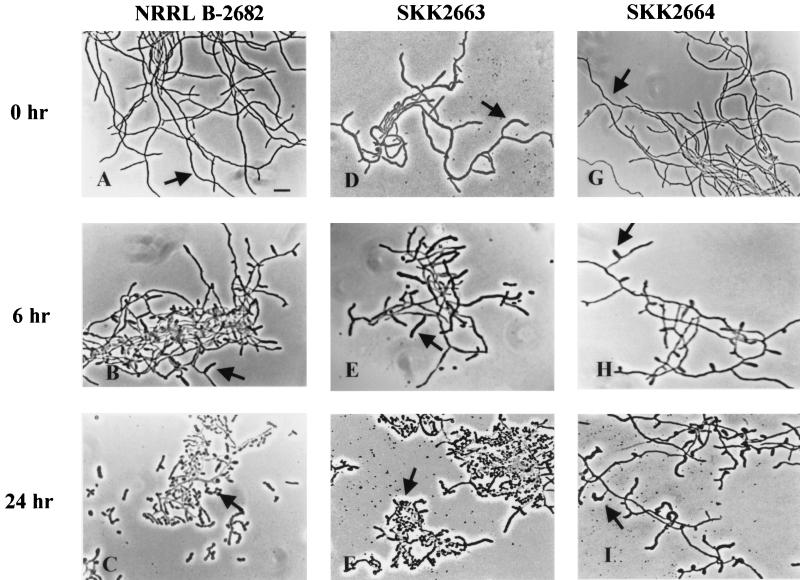
Phase-contrast micrographs of impression preparations of the wild-type strain and S. griseus mutants growing on different media. NRRL B-2682 (A to C), wild-type strain; SKK2663 (D to F), ssfR null mutant; SKK2664 (G to I), ssgA null mutant. Arrows in panels A to C, F, and I, spores; arrows in panels D, E, G, and H, aerial hyphae; arrowhead (I), empty mycelium shell. Bar, 4 μm.
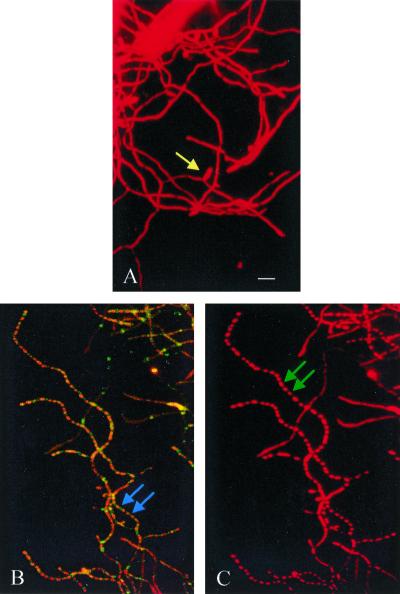
As reported before, S. griseus could be induced to sporulate in submerged culture by either phosphate starvation (20) or nutritional downshift (21). In the wild-type strain, sporogenic hyphae begin to emerge at 4 h of phosphate starvation, but sporulation septa are not formed at this time. After 10 h, the centripetal growth of the sporulation septa is visible. The formation of sporulation septa is complete by 12 h (13, 22). The morphogenesis of the SKK2663 strain was totally different from that of the wild-type strain. Even without phosphate starvation, fully extended sporogenic hyphae were evident and all hyphae appeared to be wider and thicker (Fig. 5D to F). To investigate whether septa were also formed earlier in the SKK2663 strain than in the wild-type strain, the cells were double labeled with Flu-APA (13) and PI. Flu-APA was shown to specifically target the sporulation septa (13), and PI could label both RNA and DNA. In PI-stained cells, SKK2663 (Fig. 6C) showed regular compartmentalization all over the mycelium after 4 h of induction. In contrast, there was no compartmentalization in the wild-type strain at this time (Fig. 6A). In Flu-APA-treated cultures, the septum was evident between two PI-labeled “beads” in SKK2663 (Fig. 6B) but not in the wild-type strain until 12 h of phosphate starvation (13). Complementation of the ssfR null mutant with pKK1372 confirmed that the phenotypes of the SKK2663 strain were caused by the absence of the SsfR protein. These results indicated that ssfR is required for sporulation in rich medium (SpM) or MM+G but not in MM+M. In phosphate starvation medium, this gene might be required for appropriate septum formation. Because the ssfR gene was involved in sporulation septum formation, it was named ssfR (sporulation septum formation regulator).
FIG. 5.
Phase-contrast micrographs of the wild-type strain and S. griseus mutants during sporulation induced by phosphate starvation in submerged culture. NRRL B-2682 (A to C), wild-type strain; SKK2663 (D to F), ssfR null mutant; SKK2664 (G to I), ssgA null mutant. Cells were starved for 0 (A, D, and G); 6 (B, E, and H), and 24 h (C, F, and I). Arrows in panels A and G, vegetative hyphae; arrows in panels B, D, E, and H, sporogenic hyphae; arrows in panels C and F, spores; arrow in panel I, deformed sporogenic hypha. Bar, 5 μm.
FIG. 6.
Fluorescence micrographs of the wild-type strain and ssfR null mutant SKK2663 after phosphate starvation in submerged culture. Cells of S. griseus that had been induced to sporulate for 4 h were exposed to only PI or both Flu-APA and PI. (A) Wild-type strain treated with PI; (B) ssfR null mutant SKK2663 treated with both Flu-APA and PI; (C) ssfR null mutant SKK2663 treated with only PI. Yellow arrow (A), sporogenic hypha; blue arrows (B), sporulation septum labeled with Flu-APA; green arrows (C), sporulation compartments. Bar, 5 μm.
Disruption of ssgA.
The chromosomal ssgA gene was replaced with the aac(3)IV-disrupted version using SKK2600 as the recipient strain (described in Materials and Methods). One resulting strain, SKK2664, was chosen for further morphological analysis. The colonies of the SKK2664 strain were white on SpM and produced aerial hyphae but no spores (Fig. 3). Different growth media were also used to study whether the phenotype of the ssgA null mutant was conditional. SKK2664 showed almost the same phenotypes as on SpM (Fig. 4G) when it grew on MM+G (Fig. 4H) or phosphate starvation medium (Fig. 5G to I). No spores were produced in the cultures growing on the above media. Moreover, the sporogenic hyphae of SKK2664 were deformed in phosphate starvation medium. However, the SKK2664 strain sporulated poorly on MM+M despite the fact that the spores appeared to not be formed from aerial hyphae (Fig. 4I). There were many empty mycelium shells in the culture. These results indicated that ssgA is required for sporulation of S. griseus when it grows on media containing glucose as the sole carbon source. A complementation experiment with pKK1373 confirmed that the phenotype of the SKK2664 strain was caused by the disruption of ssgA. However, SKK2664 could not be complemented by pKK1377 (Fig. 1A), which contained a region upstream of the ssgA AUG start codon shorter than that contained in pKK1373. This result implies that a certain amount of the upstream region of the ssgA coding sequence was necessary for its expression.
ssfR and ssgA double mutant.
An ssfR and ssgA double-null mutant, strain SKK2668, was constructed in the same way as the ssfR and ssgA null mutants. Colonies of the SKK2668 strain were white on SpM because they formed aerial hyphae but did not produce mature spores, as is typical of a whi mutant. Different growth media were also examined to study whether the phenotypes of the SKK2668 strain were nutrient dependent. It could not sporulate on MM+G but sporulated poorly on MM+M; the culture in MM+M contained some spores, but most of them were unsporulated hyphae. The mycelium of the SKK2668 strain in MM+M was wider and thicker than that in MM+G. Strains SKK2663, SKK2664, and SKK2668 showed no difference in vegetative growth rate in rich media from the wild-type NRRL B-2682 strain. The sporulation behavior of the SKK2600 strain and the constructed mutant strains on different media is summarized in Table 3.
TABLE 3.
Sporulation comparison of mutants with wild-type strain on different media
| Strain | Genotype | Sporulationa on:
|
|||
|---|---|---|---|---|---|
| SpM | MM+G | MM+M | Pi starvationb | ||
| NRRL B-2682 | Wild type | ++ | ++ | ++ | ++ |
| SKK2600 | ssfR mutant | × | × | × | × |
| SKK2663 | ssfR::aac(3)IV | − | − | ++ | × |
| SKK2664 | ssgA::aac(3)IV | − | − | ± | − |
| SKK2668 | ssfR::aac(3)IV | − | − | ± | − |
| ssgA::aac(3)IV | |||||
++, sporulation; −, no sporulation; ±, poor sporulation; ×, ectopic sporulation.
Pi starvation, phosphate starvation medium.
Effect of disruption of ssfR, ssgA, or both genes on streptomycin production.
In addition to morphological differentiation, Streptomyces spp. undergo biochemical differentiation by synthesizing a wide variety of secondary metabolites such as antibiotics and antimetabolites. S. griseus produces streptomycin and cycloheximide. To investigate whether ssfR and ssgA were involved in the synthesis of streptomycin, streptomycin-sensitive strain Bacillus subtilis ATCC 6633 was used. Zones of inhibition surrounding the plugs of strains SKK2663, SKK2664, and SKK2668 were evident (data not shown). These results demonstrated that disruption of ssfR and ssgA did not affect the synthesis of streptomycin in S. griseus.
DISCUSSION
The pleiotropic phenotypes of the cefoxitin-resistant mutant strain SKK2600 suggest that this mutant is defective in the regulation of gene expression. It is noteworthy that the apparent transformation efficiency of this mutant is much higher than that of the wild-type strain. One possible explanation is that the peptidoglycan structure is changed in the SKK2600 strain, leading to more-complete removal of cell wall from the mutant than from the wild-type strain during protoplast preparation, or that the derepression of PBP synthesis may result in better regeneration of the cell wall after transformation. Changes of PBP profiles in SKK2600 (data not shown) support this explanation. Alternatively, a higher percentage of protoplasts made from the SKK2600 strain may be viable, because each spore-like body contains a nucleoid. The wild-type protoplasts might be less viable if the nucleoid has not segregated at the stage when the mycelia are collected for protoplast preparation, leading to a high percentage of anucleoidal and nonviable protoplasts.
The SKK2600 strain contains a point mutation of C to T seven nucleotides upstream of the UGA stop codon of ssfR and 138 nucleotides upstream of the predicted AUG start codon of ssgA. Complementation and allele-exchange experiments showed that this point mutation was solely responsible for the phenotype of this mutant. At this point, we do not have an explanation for the complex complementation phenomena observed with ssfR and ssgA clones and the SKK2600 mutant.
A disruption experiment demonstrated that the SsfR protein is required for sporulation in glucose-containing media but not in MM+M. This is consistent with the idea that there are at least two different pathways controlling sporulation in Streptomyces spp. (6, 29), one of which is repressed by glucose (3, 6). The ssfR null mutant SKK2663 is both temporally and spatially disordered in chromosome segregation, sporogenic hypha formation, and sporulation septum formation in phosphate starvation medium. It is noteworthy that these kinds of phenotypes are very similar to those of Streptomyces bld mutants (22). However, SsfR is apparently not involved in streptomycin production.
Remarkably, the SKK2663 strain had a whi mutant phenotype in SpM and MM+G, forming aerial hyphae but failing to sporulate or produce the grey pigment characteristic of a sporulating wild-type colony. However, the SKK2663 strain sporulated ectopically in phosphate starvation medium. This behavior is different from the phenotype of a whi mutant such as a whiG null S. griseus mutant, which could not sporulate in phosphate starvation medium (data not shown). Thus, the SKK2663 strain has some characteristics of both bld and whi S. griseus mutants.
As mentioned above, SsfR is similar to the E. coli IclR protein, which is involved in acetate catabolism. The expression of iclR is actively regulated by FadR (13), which is a transcriptional regulator of fatty acid metabolism and which shows high similarity to the S. coelicolor WhiH protein required for sporulation (30). Moreover, the ssfR null mutant showed a white phenotype. Therefore, it is possible that SsfR might be involved in the regulatory network controlling whi gene expression. On the other hand, SsfR might be involved in a pathway that senses the signals generated from glucose catabolism. In this way SsfR could transmit information about the metabolic or physiological state of cells to the machinery for transcription of genes involved in sporulation. Thus, in the SKK2663 strain the flow of this hypothetical signal would be blocked, leading to the SKK2663 phenotype. This is consistent with a proposal by Pope et al. (29) that, in bld mutants, the defect in the regulation of carbon utilization is epistatic to the defect in morphogenesis and hence that the inability of these mutants to initiate morphogenesis is a secondary consequence of their inability to sense and/or to signal starvation.
The ssgA null mutant SKK2664 also shows a whi mutant phenotype, like SKK2663, when grown on SpM and MM+G. However, there are two differences between the SKK2663 and SKK2664 strains. First, SKK2664 sporulated poorly and spores appear not to have formed from aerial hyphae when this strain grew on MM+M; but SKK2663 could sporulate normally on this medium. Second, SKK2664 showed the same developmental time course as the wild-type strain when grown submerged in phosphate starvation medium, except that the sporogenic hyphae did not develop into spores and had deformed shapes. In contrast, SKK2663 exhibited a phenotype similar to that of a bld mutant, such as S. griseus bldA, which undergoes temporally and spatially disordered septum formation and sporulation (22).
The ssfR and ssgA double-null mutant SKK2668 has almost the same phenotype as ssgA null mutant SKK2664. This result indicates that SsfR might function through SsgA during sporulation of S. griseus. Since the SKK2663 and SKK2664 strains show different phenotypes when grown on MM+M and phosphate starvation medium and the same phenotypes when growing on SpM and MM+G, SsfR, and SsgA might be involved in different sporulation pathways but also might share a common pathway.
Taking together the observations presented in the above discussion, we propose that SsfR functions as a transcriptional regulator, which could be negative or positive. It may be involved only in the sporulation pathway that is controlled by catabolite repression. If so, the expression of ssfR could be regulated by morphogenesis signals coming from nutrient limitation. The genes regulated directly or indirectly by SsfR may include cell division genes such as ftsZ and whiG. SsgA could be involved in two sporulation pathways, one SsfR dependent and the other SsfR independent and triggered by the metabolism of poorly used carbon sources such as mannitol. The genes for two S. coelicolor hypothetical proteins closely related to SsfR and SsgA have the same arrangement as ssfR and ssgA in S. griseus and are also separated by 131 nucleotides in S. coelicolor. This conservation of gene arrangement in the two species suggests that analysis of analogous mutants in S. coelicolor would be of interest.
ACKNOWLEDGMENTS
We are very grateful to Keith Chater, C. Richard Hutchinson, Tina Henkin, and Charles Daniels for critical reading and help in preparation of this manuscript. We thank Jangyul Kwak for helpful discussion.
This work was supported by a grant from the National Science Foundation (MCB-9724038).
REFERENCES
- 1.Babcock M J, Kendrick K E. Cloning of DNA involved in sporulation of Streptomyces griseus. J Bacteriol. 1988;170:2802–2808. doi: 10.1128/jb.170.6.2802-2808.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chakraburtty R, Bibb M. The ppGpp synthetase gene (relA) of Streptomyces coelicolor A3(2) plays a conditional role in antibiotic production and morphological differentiation. J Bacteriol. 1997;179:5854–5861. doi: 10.1128/jb.179.18.5854-5861.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Champness W C. New loci required for Streptomyces coelicolor morphological and physiological differentiation. J Bacteriol. 1988;170:1168–1174. doi: 10.1128/jb.170.3.1168-1174.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Champness W C, Chater K F. Regulation and integration of antibiotic production and morphological differentiation in Streptomyces spp. In: Piggot P Jr, Moran C P, Youngman P, editors. Regulation of bacterial differentiation. Washington, D.C.: American Society for Microbiology; 1994. pp. 61–94. [Google Scholar]
- 5.Chater K F. Taking a genetic scalpel to the Streptomyces colony. Microbiology. 1998;144:1456–1478. doi: 10.1099/00221287-144-6-1465. [DOI] [PubMed] [Google Scholar]
- 6.Chater K F. Morphological and physiological differentiation in Streptomyces. In: Losick R, Shapiro L, editors. Microbial development. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1984. pp. 89–115. [Google Scholar]
- 7.Chater K F, Bruton C J, Plaskitt K A, Buttner M J, Méndez C, Helmann J. The developmental fate of S. coelicolor hyphae depends upon a gene product homologous with the motility sigma factor of B. subtilis. Cell. 1989;59:133–143. doi: 10.1016/0092-8674(89)90876-3. [DOI] [PubMed] [Google Scholar]
- 8.Chater K F, Merrick M J. Approaches to the study of differentiation in Streptomyces coelicolor A3(2) In: MacDonald K D, editor. Second international symposium on the genetics of industrial microorganisms. London, United Kingdom: Academic Press; 1976. pp. 583–593. [Google Scholar]
- 9.Dharmatilake A J, Kendrick K E. Expression of the division-controlling gene, ftsZ, during growth and sporulation of the filamentous bacterium, Streptomyces griseus. Gene. 1994;147:21–28. doi: 10.1016/0378-1119(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 10.Dodd I B, Egan J B. Improved detection of helix-turn-helix DNA-binding motifs in protein sequences. Nucleic Acids Res. 1990;18:5019–5026. doi: 10.1093/nar/18.17.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gui L, Sunnarborg A, LaPorte D C. Regulated expression of a repressor protein: FadR activates iclR. J Bacteriol. 1996;178:4704–4709. doi: 10.1128/jb.178.15.4704-4709.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gui L, Sunnarborg A, Pan B, LaPorte D C. Autoregulation of iclR, the gene encoding the repressor of the glyoxylate bypass operon. J Bacteriol. 1996;178:321–324. doi: 10.1128/jb.178.1.321-324.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hao J, Kendrick K E. Visualization of penicillin-binding proteins during sporulation of Streptomyces griseus. J Bacteriol. 1998;180:2125–2132. doi: 10.1128/jb.180.8.2125-2132.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984;28:351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- 15.Hopwood D A, Bibb M J, Chater K F, Kieser T, Bruton C J, Kieser H M, Lydiate D J, Smith C P, Ward J M, Schrempf H, editors. Genetic manipulation of Streptomyces. A laboratory manual. Norwich, Great Britain: The John Innes Foundation; 1985. [Google Scholar]
- 16.Huff J P. Rapid isolation and purification of DNA from agarose gels: the phenol-freeze-fracture method. BioTechniques. 1991;10:724. [PubMed] [Google Scholar]
- 17.Janssen G R, Bibb M J. Derivatives of pUC18 that have BglII sites flanking a modified multiple cloning site and that retain the ability to identify recombinant clones by visual screening of Escherichia coli colonies. Gene. 1993;124:133–134. doi: 10.1016/0378-1119(93)90774-w. [DOI] [PubMed] [Google Scholar]
- 18.Kawamoto S, Ensign J C. Cloning and characterization of a gene involved in regulation of sporulation and cell division of Streptomyces griseus. Actinomycetology. 1995;9:136–151. [Google Scholar]
- 19.Kawamoto S, Watanabe H, Hesketh A, Ensign J C, Ochi K. Expression analysis of the ssgA gene product, associated with sporulation and cell division in Streptomyces griseus. Microbiology. 1997;143:1077–1086. doi: 10.1099/00221287-143-4-1077. [DOI] [PubMed] [Google Scholar]
- 20.Kendrick K E, Ensign J C. Sporulation of Streptomyces griseus in submerged culture. J Bacteriol. 1983;155:357–366. doi: 10.1128/jb.155.1.357-366.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroening T A, Kendrick K E. In vivo regulation of histidine ammonia-lyase activity from Streptomyces griseus. J Bacteriol. 1987;169:823–829. doi: 10.1128/jb.169.2.823-829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwak J, Kendrick K E. Bald mutants of Streptomyces griseus that prematurely undergo key events of sporulation. J Bacteriol. 1996;178:4643–4650. doi: 10.1128/jb.178.15.4643-4650.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y. Structure and function analysis of the asaA, ptpA, and cysA loci of Streptomyces coelicolor. Ph.D. dissertation. Columbus: The Ohio State University; 1995. [Google Scholar]
- 24.MacNeil D J, Gewain K M, Ruby C L, Dezeny G, Gibbons P H, MacNeil T. Analysis of Streptomyces avermitilis genes required for avermectin biosynthesis utilizing a novel integration vector. Gene. 1992;111:61–68. doi: 10.1016/0378-1119(92)90603-m. [DOI] [PubMed] [Google Scholar]
- 25.Méndez C, Chater K F. Cloning of whiG, a gene critical for sporulation of Streptomyces coelicolor A3(2) J Bacteriol. 1987;169:5715–5720. doi: 10.1128/jb.169.12.5715-5720.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merrick M J. A morphological and genetic mapping study of bald colony mutants of Streptomyces coelicolor. J Gen Microbiol. 1976;96:299–315. doi: 10.1099/00221287-96-2-299. [DOI] [PubMed] [Google Scholar]
- 27.Nodwell J R, McGovern K, Losick R. An oligopeptide permease responsible for the import of an extracellular signal governing aerial mycelium formation in Streptomyces coelicolor. Mol Microbiol. 1996;22:881–893. doi: 10.1046/j.1365-2958.1996.01540.x. [DOI] [PubMed] [Google Scholar]
- 28.Pan B, Unnikarishnan I, LaPorte D C. The binding site of the IclR repressor protein overlaps the promoter of aceBAK. J Bacteriol. 1996;178:3982–3984. doi: 10.1128/jb.178.13.3982-3984.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pope M K, Green B D, Westpheling J. The bld mutants of Streptomyces coelicolor are defective in the regulation of carbon utilization, morphogenesis and cell-cell signalling. Mol Microbiol. 1996;19:747–756. doi: 10.1046/j.1365-2958.1996.414933.x. [DOI] [PubMed] [Google Scholar]
- 30.Ryding N J, Kelemen G H, Whatling C A, Flardh K, Buttner M J, Chater K F. A developmentally regulated gene encoding a repressor-like protein is essential for sporulation in Streptomyces coelicolor A3(2) Mol Microbiol. 1998;29:343–357. doi: 10.1046/j.1365-2958.1998.00939.x. [DOI] [PubMed] [Google Scholar]
- 31.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 32.Schwedock J, McCormick J R, Angert E R, Nodwell J R, Losick R. Assembly of the cell division protein FtsZ into ladder-like structures in the aerial hyphae of Streptomyces coelicolor. Mol Microbiol. 1997;25:847–858. doi: 10.1111/j.1365-2958.1997.mmi507.x. [DOI] [PubMed] [Google Scholar]
- 33.Tan H, Chater K F. Two developmentally controlled promoters of Streptomyces coelicolor A3(2) that resemble the major class of motility-related promoters in other bacteria. J Bacteriol. 1993;175:933–940. doi: 10.1128/jb.175.4.933-940.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Willey J, Santamaria R, Guijarro J, Geistlich M, Losick R. Extracellular complementation of a developmental mutation implicates a small sporulation protein in aerial mycelium formation by S. coelicolor. Cell. 1991;65:641–650. doi: 10.1016/0092-8674(91)90096-h. [DOI] [PubMed] [Google Scholar]
- 35.Willey J, Schwedock J, Losick R. Multiple extracellular signals govern the production of a morphogenetic protein involved in aerial mycelium formation by Streptomyces coelicolor. Genes Dev. 1993;7:895–903. doi: 10.1101/gad.7.5.895. [DOI] [PubMed] [Google Scholar]
- 36.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]